2) Laboratory for Marine Ecology and Environmental Science, Qingdao Marine Science and Technology Center, Qingdao 266237, China;
3) Frontiers Science Center for Deep Ocean Multispheres and Earth System, Ocean University of China, Qingdao 266100, China
Since the Industrial Revolution, the amount of CO2 emitted into the atmosphere by human activities has increased dramatically. As a 'carbon sink', the ocean has absorbed this additional CO2, resulting in both a rise in dissolved CO2 and a decreasing trend in seawater pH (Sabine et al., 2004). By the end of this century, it is anticipated that atmospheric CO2 concentrations will reach 1000 ppm, resulting in a decrease of 0.3 to 0.5 pH units in seawater (Lacoue-Labarthe et al., 2016). Ocean acidification (OA) induces alterations in the marine carbonate system, characterized by a reduction in carbonate ion (CO32−) concentration and a corresponding increase in bicarbonate ion (HCO3−) concentration (Koch et al., 2013; Wei et al., 2020). These chemical shifts have profound implications for the physiological activities of marine phy-toplankton.
Ocean acidification impacts the assimilation of carbon and nitrogen by marine microalgae, subsequently affecting their physiological and biochemical activities. For carbon assimilation, these microorganisms possess unique CO2 concentrating mechanisms (CCMs). When the availability of free CO2 in seawater is insufficient for the carboxylation reaction catalyzed by ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO), CCMs can significantly elevate CO2 concentration around RuBisCO, ensuring the continuity of the photosynthetic process (Spalding, 2008). Although essential for the photosynthetic carbon sequestration pathway in algae, CCMs require considerable energy to operate. Consequently, the down-regulation of CCMs in response to ocean acidification could theoretically conserve energy for algal growth and metabolism (Kim et al., 2013). Moreover, the regulation of CCMs is intricately connected to nitrogen metabolism. Research indicates that under ocean acidification, the downregulation of CCMs in Skeletonema costatum can reduce metabolic costs by enhancing amino acid synthesis and nitrogen assimilation. This energy-saving adaptation allows active protein processing machinery to adjust to environmental change (Thangaraj and Sun, 2021).
Marine microalgae primarily assimilate nitrogen through the nitrogen metabolism pathway. This metabolic process involves the conversion of various chemical forms of nitrogen into biologically usable nitrogen, which then supports the energy requirements and growth of these organisms (Dagenais-Bellefeuille and Morse, 2013). Currently, omics-based analysis of gene expression in key metabolic pathways of microalgae is a prevalent approach to uncover the molecular response mechanisms under ocean acidification conditions. For instance, Zhang et al. (2022) conducted a transcriptome study on Karenia mikimotoi and discovered that 1121 genes exhibited up-regulated expression in the acidified group compared to the control group. Notably, among these, key genes associated with nitrogen metabolism were significantly up-regulated. Similarly, Zhang et al. (2021) employed transcriptomic and proteomic analyses to investigate the effects of seawater acidification on Skeletonema marinoi. They found that S. marinoi up-regulated the expression of genes and proteins related to the nitrate transporter, nitrate reductase, nitrite reductase, and glutamine synthetase under acidified conditions. These findings suggest that seawater acidification may enhance the nitrogen metabolism pathway in S. marinoi. In conclusion, acidification of seawater can significantly influence the nitrogen and carbon metabolic processes in phytoplankton.
S. costatum is a ubiquitous marine diatom found across the global ocean, which flourishes across a broad spectrum of temperatures and salinities (Tada et al., 2001). Notably, S. costatum is also a primary species associated with red tides along the coast of China. Its presence was first noted in a red tide event in Chinese waters in 1933. According to the Bulletin of China Marine Disaster, this species has been responsible for annual red tides up to 2022 (Chen et al., 2023). Consequently, examining the nitrogen metabolism of S. costatum in response to ocean acidification is of significant importance. Such research can offer vital insights for forecasting the effects of ocean acidification on marine diatoms and provide scientific backing for the mitigation and management of algal blooms.
2 Materials and Methods 2.1 Skeletonema costatum Strain CultureSkeletonema costatum stain used in this study was cultivated in the Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China. The cells were nurturedin f/2 medium (Guillard, 1975), and the culture medium was prepared using seawater from the coastal region of Qingdao, which was initially filtered through a 0.45-micron filter membrane and subsequently sterilized via autoclaving. The environmental conditions were meticulously regulated, with the temperature maintained at 20 ± 1℃ and the light exposure set at a rate of 80 – 100 μmol photons m−2 s−1 under a 12 h/12 h light/dark cycle.
Since aerating the seawater would lead to the accumulation of organic matter released by phytoplankton at the surface (Gattuso et al., 2010), potentially impacting the experimental outcomes, the semi-continuous culture was employed. The culture medium was refreshed with a fresh solution every 24 h, and the initial density of algal cells was adjusted to 1×104 cells mL−1, ensuring that the microalgae had access to ample nutrients for growth.
Four distinct culture conditions were meticulously established to replicate the existing pH and pCO2 levels in seawater, which are (pH 8.1, pCO2 400 ppm), as well as the predicted levels by the Intergovernmental Panel on Climate Change for the close of this century, anticipated to be (pH 7.8, pCO2 1000 ppm). The specific conditions included a control group maintained at (pH 8.1, pCO2 400 ppm), an acidification group set at (pH 7.8, pCO2 1000 ppm), a pH-decreasing group with conditions of (pH 7.8, pCO2 400 ppm), and a pCO2-increasing group with elevated levels at (pH 8.1, pCO2 1000 ppm).
The acidification was induced by blending a high concentration of CO2 gas with ambient air, utilizing a CO2 enrichment apparatus (SFO-E04, Qingdao Haixing Instrument Co., Ltd.). To ensure the stability of the carbonate system, 2 mmol L−1 of 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid (HEPPS) was incorporated into the culture medium. Furthermore, after the microalgae had acclimated to the various experimental conditions for a minimum of 25 generations, the subsequent parameters were assessed.
2.2 Growth Rate MeasurementDaily, 1 mL of the algal culture was carefully transferred to a centrifuge tube, and 30 mL of Lugol's iodine solution were added to facilitate fixation. Following this, 100 μL of the fixed sample were carefully dispensed onto a plankton counting frame with a capacity of 0.1 mL for microscopic enumeration using a microscope (BX51, Olympus, Japan). The specific growth rate (µ) of the algae was subsequently calculated using the following formula:
| $ \mu = \frac{{\ln ({N_t}/{N_0})}}{t}, $ | (1) |
where Nt represents the cell density at time t, and N0 is the initial cell density.
2.3 Intracellular Organic Nitrogen MeasurementThe concentrations of particulate organic nitrogen (PON) within the intracellular particles were measured using an elemental analyzer (FLASH 2000, Thermo Fisher Scientific, USA). A 100 mL volume of algal culture in the exponential growth phase was filtered through GF/F membranes (Whatman) that had been pre-combusted at 450℃ for 2 h. Control filters, which were free of algae, were also processed with an equal volume of culture medium to act as blanks. Immediately after filtration, the collected membranes were sealed in pre-combusted tin foil and stored at − 80℃. Before analysis, the samples were taken out and dried at 50℃ in an oven (LS-O410, Thermo Fisher Scientific, USA) for 72 h. The dried samples were then subjected to acid fumigation with concentrated hydrochloric acid (HCl) for 24 h to eliminate inorganic carbon. After the acid fumigation was complete, the samples were placed in an oven set at 60℃ until they were thoroughly dry. Finally, the samples were transferred into tin cups, compacted with a press mold, and made ready for analysis.
2.4 RNA Extraction and Reverse TranscriptionFor each collected S. costatum sample, 1 mL TRIzol reagent (Invitrogen, USA) was added to lyse the cells, and total RNA was extracted according to the manufacturer's protocol. The RNA concentration and quality were determined by 1% agarose gel electrophoresis and spectrophotometer (DS-11, Denovix, USA). Then, 400 ng of qualified total RNA was reverse transcribed to cDNA using the PrimeScript RT Reagent Kit with the gDNA Eraser Kit (Takara, China).
For each S. costatum sample collected, 1 mL of TRIzol reagent (Invitrogen, USA) was applied to lyse the cells, followed by total RNA extraction in accordance with the manufacturer's guidelines. The concentration and integrity of the RNA were assessed using a 1% agarose gel electrophoresis and a spectrophotometer (DS-11, Denovix, USA). Subsequently, 400 ng of the qualified total RNA was reverse transcribed to cDNA using the PrimeScript RT Reagent Kit with the gDNA Eraser Kit (Takara, China).
2.5 RNA-Seq AnalysisThe cDNA was sent to OE Biotech Co., Ltd. (Shanghai, China) for transcriptome sequencing using the Illumina HiSeq X Ten platform. Raw image data files from sequencing were analyzed for base recognition, which were then preprocessed and quality assessed using Trimmomatic software to obtain clean reads (Bolger et al., 2014). Furthermore, Trinity software was utilized to perform de novo splicing to obtain transcripts (Grabherr et al., 2011). Subsequently, the transcripts were clustered and de-duplicated using the CD-HIT software to get a final set of ungenes. Then, the unigenes were compared to the NR, KOG, GO, SWISS-Prot, Eggnog, and KEGG databases using Diamond software (Buchfink et al., 2015), and matched to the Pfam database through HMMER software for functional analysis of unigenes (Mistry et al., 2013). Eventually, the nitrogen metabolism pathway of S. costatum was obtained by functional annotation of the KEGG database (Kanehisa et al., 2008).
All reads from this study were deposited in the Short Reads Archive (SRA) database of GenBank with the accession number PRJNA675179.
2.6 Quantitative Expression of Target GenesFive key genes related to nitrate reductase (NR), nitrite reductase (NIR), glutamate dehydrogenase (GD), glutamate synthase (GOGAT) and glutamine synthetase (GS) were selected for fluorescence RT-qPCR reactions, respectively. Their primers were separately designed by Primer Premier 5.0 software and synthesized by BGI Genomics Co., Ltd. In the meantime, the primer amplification efficiencies (E) were all in the range of 80-120%. The 7500 Real-Time qPCR System (Applied Biosystems, USA) was used to perform the fluorescence qPCR reaction. Besides, qPCR reaction conditions were as follows: 50℃ for 2 min, 95℃ for 10 min, followed by 40 cycles of 95℃ for 15 s and a certain annealing temperature for 1 min; the annealing temperatures of the different primers were shown in Table 1. Also, the 20 μL qPCR reaction contained 10 μL of ROX, 0.2 μL of bovine serum albumin (20 mg mL−1), 2 μL of cDNA, 0.6 μL of forward primer (10 μmol L−1) and 0.6 μL of reverse primer (10 μmol L−1). Each set of experiments was performed in triplicate and included a negative control.
|
|
Table 1 List of nitrogen metabolism gene primers in this study |
The 2−∆∆Ct method was employed to assess the relative expression levels of the target genes. Furthermore, the 18S rRNA gene was selected as the reference gene for the analysis, given its consistent expression across various conditions in S. costatum (Liu et al., 2013). Additionally, gene expression levels observed in the control group on the 2nd day were utilized as the baseline for comparison to evaluate the expression differences among the four experimental groups: the control group, acidification group, the pH-decreasing group, and the pCO2-increasing group.
2.7 Nitrate Reductase Activity (NRA) AssayThe cells of S. costatum in each group were sampled at two-day intervals. And the nitrate reductase activity assay kit (Jiancheng Bioengineering Institute, Nanjing, China) was used to determine NRA.
2.8 Statistical AnalysisData were all analyzed with SPSS 26.0 and were presented as mean ± SD (standard deviation). Student's t-test was applied to compare differences between two pCO2 levels at each identical pH level. And the significance of the two variables (pH and pCO2) as well as the interaction between the two variables was analyzed using two-way ANOVA, and the results of the interaction that were significant were subjected to a simple effect analysis. A 95% confidence interval was set for all data analysis.
3 Results 3.1 Growth Rates and Intracellular Particulate Organic Nitrogen ContentsThe current study meticulously tracked the specific growth rates of S. costatum under a range of pH and pCO2 conditions throughout a preliminary 10-day experimental phase (Fig.1A). After a brief one-day acclimation period, all experimental groups entered the phase of exponential growth by the next day. On the first day of the experiment, the specific growth rates for the control and pH-decreasing groups were documented at 0.49 d−1 and 0.31 d−1, respectively, and later stabilized at approximately 1.03 d−1. The acidification and pCO2-increasing groups initially showed negative growth rates of −0.18 d−1 and −0.03 d−1, respectively, but these rates rebounded to stabilize around 0.98 d−1. The study's results indicate that the influence of elevated pCO2 levels on the growth of S. costatum is more pronounced than the impact of lowered pH. Although the initial rise in pCO2 led to a temporary decrease in algal density, the species quickly adjusted to the higher pCO2 conditions, highlighting its remarkable adaptive capabilities in the face of ocean acidification.

|
Fig. 1 The specific growth rate and intracellular particulate organic nitrogen contents of S. costatum. A, specific growth rate; B, intracellular particulate organic nitrogen contents. |
The particulate organic nitrogen (PON) contents per cell of S. costatum within the different experimental groups were depicted in Fig.1B. The PON content per cell was measured at 0.07 pmol N cell −1 for the control group, 0.10 pmol N cell −1 for the acidified group, 0.08 pmol N cell −1 for the pH-decreasing group, and 0.09 pmol N cell −1 for the pCO2-increasing group. Upon analyzing the effect of pCO2 on the cellular PON contents of the algae, it was observed that at pH levels of 7.8 and 8.1, an increase in pCO2 was associated with a 20% and 26.8% rise in PON contents, respectively (P < 0.05). This finding underscores the substantial influence that pCO2 levels have on the cellular PON contents of S. costatum. The statistically significant increases in PON content with elevated pCO2 suggest a direct response in the cellular nitrogen quota, potentially reflecting an adaptation mechanism to the altered carbonate chemistry in the marine environment.
Further analysis, conducted under conditions of constant pCO2, indicated that pH levels did not exert a significant influence on the PON content (P > 0.05). However, when comparing the acidified group with the control group, it was found that the PON content in the acidified group was notably higher, with an increase of 43.3% (P < 0.05). This significant enhancement in PON accumulation in S. costatum under acidified conditions is primarily attributed to the elevated levels of pCO2. This observation highlights the critical role of CO2 in the biogeochemical cycling of nitrogen within marine microalgae, especially in the context of ocean acidification (Liu et al., 2013; Chi et al., 2023).
3.2 Transcriptome AnalysisThe clean reads were assembled, yielding a total of 93459 unigene entries, encompassing a cumulative length of 79433647 bp, with an average length of 849 bp, a maximum length reaching 20750 bp, and a minimum of 301 bp. Among these, 58086 entries, representing 62.15%, were annotated successfully in the NR database, with the highest percentage of annotations among the seven major databases (Table 2).
|
|
Table 2 Annotation rates of RNA-seq in databases |
Functional annotation and analysis of the S. costatum transcriptome data identified a total of 17 enzymes linked to nitrogen metabolism, participating in three principal metabolic processes: nitrate assimilation, glutamate metabolism, and the urea cycle. Specifically, the nitrate as similation pathway includes the nitrate transport protein (NRT), the nitrate reductase (NR), and nitrite reductase (NIRA, NIT6); glutamate metabolism encompasses glutamine synthetase (GLNA), glutamate synthetase (GLT1, GLTD), and glutamate dehydrogenase (GDHA, GDH2); the urea cycle features ornithine carbamoyltransferase (OTC), argininosuccinate synthetase (ARGG), argininesuccinate lyase (ASL), arginase (ROCF), and urease (URE).
The nitrogen metabolism pathway of S. costatum was delineated through KEGG metabolic pathway enrichment analysis (Fig.2). As depicted in the figure, S. costatum imports two prevalent nitrogen sources (NO3− and NO2−) into the algal cell through the NRT and integrates them into ammonium for symbiotic use via the action of NR and NIR. NR facilitates the conversion of NO3− to NO2− (Joseph and Villareal, 1998), marking it as a pivotal enzyme in nitrate assimilation. NIR, another crucial enzyme in this assimilatory process, is capable of reducing NO2− to NH4+.

|
Fig. 2 Nitrogen metabolism pathway delineated based on S. costatum transcriptome. |
In S. costatum, the primary destination of NH4+ is its involvement in the glutamate metabolism process. Glutamine synthetase (GLNA) plays a role in the glutamate cycle by converting ammonia to glutamine, a reaction that is subsequently catalyzed by glutamate synthetase (GLT1, GLTD). This enzyme incorporates the carbon skeleton from α-ketoglutarate to produce glutamate (Muro-Pastor et al., 2005). Ultimately, glutamate is converted back to ammonia by the action of glutamate dehydrogenase (GDHA, GDH2), thereby completing the GS-GOGAT cycle.
As illustrated in the figure, S. costatum possesses an incomplete urea-ornithine metabolic pathway. This pathway is deemed 'incomplete' because the enzymes carbamoyl-phosphate synthase (CPS) and carbamate kinase (CK), which are involved in the conversion of ammonia to carbamoylphosphate, are not identified in this study. However, the subsequent metabolic pathway for carbamoylphosphate is more extensively characterized. Ornithine carbamoyltransferase (OTC) catalyzes the transfer of a carbamoyl group from carbamoylphosphate to ornithine, resulting in the formation of citrulline. Citrulline is then converted into argininosuccinic acid by argininosuccinate synthase (ARGG), which is subsequently cleaved by argininosuccinate lyase (ASL) into arginine and fumaric acid. Fumaric acid serves as an intermediary, connecting the urea cycle with the tricarboxylic acid (TCA) cycle, while arginine is further metabolized into ornithine or directly into urea by the enzyme arginase (ROCF). Ornithine, once produced, is recycled back into the urea cycle through the catalytic action of ornithine carbamoyltransferase (OTC), initiating a new cycle; in contrast, urea is either excreted directly from the cell or converted into CO2 by the enzyme urease (URE).
In the nitrogen metabolic pathways of S. costatum, there are five distinct sources of ammonia. Beyond the ammonia generated through nitrate assimilation and the glutamate-glutamine cycle, three additional endogenous pathways contribute to ammonia replenishment. These include the catabolism of urea, the catabolism of cyanate (Mao et al., 2022), and the catalytic conversion of formamide. Each of these processes serves to sustain the nitrogen metabolic processes by providing a necessary source of ammonia (Li et al., 2019).
3.4 Expression of Key Genes Associated with Nitrate Assimilation 3.4.1 Expression of the gene encoding nitrate reductase (NR)The expression levels of the NR-encoding gene in S. costatum exhibited a significant upregulation in response to elevated pCO2 concentrations, irrespective of pH levels (Fig.3). Under conditions of low pH, the gene expression escalated from 1.083 ± 0.065 at 400 ppm to 1.838 ± 0.083 at 1000 ppm. Similarly, at a higher pH, the expression rose from 1.034 ± 0.029 at 400 ppm to 1.412 ± 0.075 at 1000 ppm.

|
Fig. 3 Expression of NR-encoding gene of S. costatum in different experimental conditions. Asterisks indicate significant differences between the two pCO2 levels under the same pH conditions. |
Two-way ANOVA indicated that both pH and pCO2 alone had an extremely significant positive effect on the expression of NR-encoding gene (P < 0.001, Table 3). Besides, further use of partial Eta squares indicated that pCO2 level had a greater effect on the data. Furthermore, the analysis also showed an extremely significant interaction between pH and pCO2 in promoting the expression of NR-encoding gene.
|
|
Table 3 Results of analysis of variance (ANOVA) between pH and pCO2 on NR, NIR, GD, GOGAT and GS mRNA relative expression as well as the NRA in S. costatum |
Two-way ANOVA revealed that both pH and pCO2 alone exerted a highly significant positive influence on the expression of the NR-encoding gene (P < 0.001, Table 3). Additionally, the application of partial Eta squared analysis suggested that the level of pCO2 had a more pronounced impact on the observed data. Moreover, the analysis highlighted a highly significant interactive effect between pH and pCO2 in enhancing the expression of the NR-encoding gene (P < 0.001, Table 3).
3.4.2 Expression of the gene encoding nitrite reductase (NIR)As illustrated in Fig.4, the expression of the NIR-encoding gene showed a significant increase at both low and high pH levels. At the lower pH, the expression rose from 1.088 ± 0.157 at 400 ppm to 1.695 ± 0.185 at 1000 ppm, which was statistically significant (P < 0.05). Similarly, at the higher pH, there was a notable increase in expression from 1.237 ± 0.047 at 400 ppm to 1.415 ± 0.036 at 1000 ppm, indicating a highly significant difference (P < 0.01).

|
Fig. 4 Expression of NIR-encoding gene of S. costatum in different experimental conditions. Asterisks indicate significant differences between the two pCO2 levels under the same pH conditions. |
Unlike the effects on the NR-encoding gene, the twoway ANOVA indicated that only pCO2 alone had a significant main effect on the expression of NIR-encoding gene (P < 0.001, Table 3). Moreover, the interaction between pH and pCO2 was also statistically significant.
Unlike the impact on the NR-encoding gene, two-way ANOVA revealed that it was solely the pCO2 that exerted a significant main effect on the expression of the NIR-encoding gene (P < 0.001, Table 3). Additionally, the interaction between pH and pCO2 was found to be statistically significant (P < 0.05, Table 3).
3.5 Expression of Key Genes Associated with Glutamate Metabolism 3.5.1 Expression of the gene encoding glutamate dehydrogenase (GD)The expression of GD-encoding gene of S. costatum ranged from 0.600 ± 0.036 to 0.855 ± 0.019 under different experimental conditions. Elevated pCO2 showed no significant effects at low pH (P > 0.05); however, it had a remarkable influence on the expression of GD-encoding gene at high pH (P < 0.01).
The expression levels of the GD-encoding gene in S. costatum varied from 0.600 ± 0.036 to 0.855 ± 0.019 across various experimental conditions (Fig.5). An increase in pCO2 did not yield significant effects at a low pH level (P > 0.05). In contrast, at a high pH level, elevated pCO2 had a pronounced effect on the expression of the GD-encoding gene (P < 0.01).

|
Fig. 5 Expression of GD-encoding gene of S. costatum in different experimental conditions. Asterisks indicate significant differences between the two pCO2 levels under the same pH conditions. |
Based on the results of the ANOVA analysis, both pH and pCO2 had an effect on the expression of GD-encoding gene (P < 0.05, Table 3). Besides, further use of partial Eta squares indicated that pCO2 level had a greater effect on the data. However, the results showed that there was no significant interaction between the pH and pCO2 level on the expression of GD-encoding gene.
The ANOVA analysis revealed that both pH and pCO2 significantly influenced the expression of the GD-encoding gene (P < 0.05, Table 3). Moreover, the application of partial Eta squared statistics highlighted that the pCO2 level exerted a more substantial impact on the gene expression data. Nonetheless, the findings indicated that there was no significant interactive effect between pH and pCO2 levels regarding the expression of the GD-encoding gene (P > 0.05, Table 3).
3.5.2 Expression of the gene encoding glutamate synthase (GOGAT)Across a range of experimental conditions, the expression levels of the GOGAT-encoding gene in S. costatum ranged from 0.804 ± 0.042 to 0.934 ± 0.042. Notably, elevated pCO2 did not significantly alter gene expression at low pH levels (P > 0.05). However, at high pH levels, an increase in pCO2 significantly influenced the expression of the GOGAT-encoding gene (P < 0.05) (Fig.6).

|
Fig. 6 Expression of GOGAT-encoding gene of S. costatum in different experimental conditions. Asterisks indicate significant differences between the two pCO2 levels under the same pH conditions. |
The ANOVA analysis indicated that the primary effect of pCO2 level was statistically significant (P < 0.05, Table 3). In contrast, neither the main effect of pH level nor the interactive effect between pH and pCO2 levels had a significant impact on the expression of the GOGAT-encoding gene (P > 0.05, Table 3).
3.5.3 Expression of the gene encoding glutamine synthetase (GS)As depicted in Fig.7, under low pH conditions, the expression of the GS-encoding gene significantly rose from 1.064 ± 0.046 at 400 ppm to 1.678 ± 0.047 at 1000 ppm (P < 0.001). Conversely, at elevated pH levels, the gene expression also showed an increase, from 1.150 ± 0.030 at 400 ppm to 1.332 ± 0.055 at 1000 ppm (P < 0.01).

|
Fig. 7 Expression of GS-encoding gene of S. costatum in different experimental conditions. Asterisks indicate significant differences between the two pCO2 levels under the same pH conditions. |
The Two-way ANOVA results demonstrate that both pH and pCO2 significantly and positively influence the expression of the GS-encoding gene (P < 0.001, Table 3). The partial Eta squared analysis further indicates that pCO2 exerts a more substantial effect on the gene expression levels. Moreover, the analysis reveals a significant interactive effect between pH and pCO2 in enhancing the expression of the GS-encoding gene (P < 0.001, Table 3).
3.6 Changes in NRA of S. costatum Under Ocean Acidification ConditionsThe NRA expression levels significantly increased from 0.090 ± 0.023 pmol min−1 cell−1 at 400 ppm to 0.160 ± 0.012 pmol min−1 cell−1 at 1000 ppm under low pH conditions (P < 0.01). However, at high pH levels, the increase from 0.093 ± 0.017 pmol min−1 cell−1 at 400 ppm to 0.130 ± 0.022 pmol min−1 cell−1 at 1000 ppm did not yield a statistically significant effect (P > 0.05) (Fig.8).
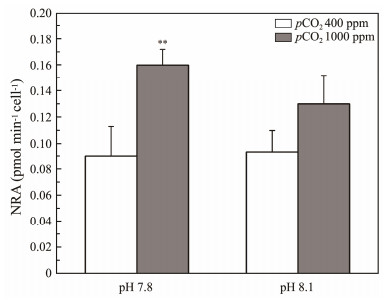
|
Fig. 8 Effects of different experimental conditions on nitrate reductase activity of S. costatum. Asterisks indicate significant differences between the two pCO2 levels under the same pH conditions. |
The ANOVA analysis results indicated that the primary effect of the pCO2 level was statistically significant (P < 0.001, Table 3). In contrast, neither the main effect of the pH level nor the interaction between pH and pCO2 levels had a significant impact on NRA (P > 0.05, Table 3).
4 Discussion 4.1 Comparison of Nitrogen Metabolism Pathways in Two DiatomsTo further explore the nitrogen metabolism of S. costatum, we conducted a comparative analysis of its nitrogen metabolic pathway with that of the diatom S. marinoi (Genbank accession number: GSE77468) (Jing et al., 2016) (Fig.9). Both S. costatum and S. marinoi are classified within the diatom phylum Bacillariophyta, order Centricae, and the genus Skeletonema, which suggests a close evolutionary relationship. S. marinoi was originally isolated and described by Sarno et al. (2005). Like S. costatum, it is a cosmopolitan, eurythermal and euryhaline planktonic diatom. In summary, by examining the similarities and differences in the nitrogen metabolic pathways of these two diatoms, our understanding of nitrogen metabolism in diatoms can be significantly advanced.

|
Fig. 9 Nitrogen metabolism pathway delineated based on S. marinoi transcriptome. Using S. costatum as a reference point, gene expression differences are marked with red boxes. Genes with reduced expression levels in S. marinoi relative to S. costatum are accentuated in green, whereas genes with increased expression in S. marinoi are presented in red. |
The comparison of nitrogen metabolic pathways between the two Skeletonema species reveals numerous similarities. For instance, the molecular mechanisms underlying nitrate assimilation and glutamate metabolism are fundamentally the same. The distinction lies in the more comprehensive nitrogen metabolic pathway of S. marinoi, particularly in its more fully realized urea-ornithine metabolic pathway. In comparison to S. marinoi, S. costatum exhibits no annotation for two pivotal genes of the urea-ornithine pathway: carbamoyl-phosphate synthase (CPS) and carbamate kinase (CK). Zhang et al. (2015) reveals the presence of CPS using the proteome analysis of S. costatum, but the absence of its transcripts in the transcriptome in this study. The long half-life of CPS within the cell can account for its detection in proteomic studies, even when its mRNA is not captured due to low expression or sampling limitations (Schwanhäuser et al., 2011). This highlights the need to consider protein stability and turnover in the interpretation of proteomic data. CPS, a crucial enzyme in the regulation of the urea cycle, primarily facilitates the conversion of NH4+ or glutamine to carbamate, which is then transformed into carbamoyl phosphate. This compound subsequently engages in arginine and proline metabolism through a cascade of enzymatic reactions (Allen et al., 2006). It has been theorized that CPS may also play a role in driving and regulating the flows of carbon and nitrogen within the diatom's urea cycle (Holden et al., 1999). Furthermore, CK in S. marinoi operates in a manner analogous to CPS, predominantly catalyzing the formation of carbamate from ATP to carbamoyl phosphate.
The scarcity of intracellular CPS and CK in S. costatum restricts the synthesis of carbamoyl phosphate, hindering the initiation of the urea cycle. Additionally, S. marinoi cells are annotated with nitric-oxide synthase (NOS1), an enzyme that mediates the cyclic interconversion of citrulline and arginine. In the realm of glutamate metabolism, S. costatum possesses a greater diversity of glutamate dehydrogenase variants and a lesser array of GOGAT types compared to S. marinoi. Moreover, the two diatoms exhibit slight variations in their nitrite reductase enzymes, both of which are characterized by the use of ferredoxin and NADH as coenzymes.
4.2 Analysis of Differentially Expressed Genes in Nitrogen Metabolism PathwayIn this study, we explored the effects of ocean acidification on gene expression within the nitrogen metabolic pathways of S. costatum. The results demonstrated a strong adaptive capacity of S. costatum to ocean acidification, evidenced by a notable enhancement in particulate organic nitrogen and an upregulation of key genes associated with nitrate assimilation and glutamate metabolism. Elevated pCO2 levels were identified as the primary factor driving these responses.
Nitrate assimilation is the biological process by which nitrate is converted into ammonium, integral to the synthesis of organic matter and energy storage (Gao et al., 1993). Nitrate reductase, the enzyme that catalyzes the first step of nitrogen metabolism, is a key player in this metabolic pathway and thus a focal point in related research (Hong et al., 2007). In our study, we observed that the acidification of seawater significantly upregulated the expression of nitrate reductase and enhanced its activity, with pCO2 identified as the principal factor driving this increase. While some research has reported a decrease in the nitrate reductase activity with rising pCO2 levels (Xia and Gao, 2005; Ma et al., 2019), it is important to recognize that the enzyme's properties can differ among algal species. Consequently, its sensitivity to environmental changes and its role in nitrate utilization can also differ (Berges and Harrison, 1995). Furthermore, nitrate reductase activity is induced by the intracellular nitrate content and is not dependent on extracellular nitrate levels (Joseph and Villareal, 1998). This allows S. costatum to maintain a certain level of nitrate reductase activity using its intracellular nitrate reserves, even when external nitrate availability is low (Dortch et al., 1979).
The gene expression alterations related to nitrate assimilation are indicative of the molecular responses of S. costatum to fluctuations in external nitrogen levels. The expression levels and enzymatic activities of the NR-encoding (nitrate reductase) and NIR-encoding (nitrite reductase) genes are telling indicators of the nitrate assimilation rate. In the conducted experiment, it was observed that the expression of the NIR-encoding gene was influenced solely by pCO2 levels under low pH conditions, unlike the NR-encoding gene, which may be affected by a combination of factors. This specificity in gene response underscores the complexity of the molecular mechanisms underlying the organism's adaptation to environmental changes.
The shifts in the expression of genes tied to glutamate metabolism mirror the molecular reactions of S. costatum to internal nitrogen level variations. The acidification of seawater poses a risk of disrupting the internal cellular environment, potentially disrupting cellular homeostasis (Flynn et al., 2012). As a result, S. costatum may need to expend more energy to sustain appropriate ion transport mechanisms. Given that reduced nitrogen can be directly converted into amino acids through transamination when enzymes like glutamine synthetase (GS) and glutamate synthase (GOGAT) are present, the use of reduced nitrogen forms such as NH4+ becomes an energetically favorable pathway for nitrogen uptake and utilization in algae (Clayton and Ahmed, 1986; Capone et al., 2008). Hence, the upregulation of genes associated with glutamate metabolism in S. costatum serves as an energy conservation strategy, crucial for preserving the homeostatic balance within algal cells amidst the challenges posed by ocean acidification.
Furthermore, the impacts of ocean acidification on algal nitrogen metabolism are indeed multifaceted. It has been established that iron serves as a functional cofactor in numerous algal metabolic pathways (Boyd et al., 2000; van Oijen et al., 2004). Key enzymes for nitrogen metabolism in S. costatum, such as nitrite reductase and glutamate synthetase, also rely on ferredoxin as a coenzyme (Takahashi et al., 2001; Lancien et al., 2002). In marine environments, only the dissolved and non-chelated iron (Fe) is accessible to algae. The bioavailability of Fe in seawater is influenced by pH levels; specifically, the concentrations of Fe(Ⅱ) and Fe(Ⅲ), along with the stability of Fe(Ⅱ), are known to increase at lower pH levels. Additionally, under conditions of elevated CO2, the concentration of Fe(Ⅱ) rises due to reduced oxidation (Gerringa, 2000; Breitbarth et al., 2010). This suggests that under ocean acidification, the availability of dissolved iron to marine algae is likely to increase, which can, in turn, affect the efficiency of nitrogen metabolic processes. Consequently, the heightened availability of iron can enhance the cellular uptake of nitrate (Torres et al., 2023), potentially contributing to the pronounced effects of CO2 levels on nitrogen metabolism.
Algal cells necessitate an ample supply of carbon and nitrogen to sustain the synthesis of proteins and pigments, with a pivotal interplay between CO2 and nitrogen utilization (Giordano et al., 2003). When the pCO2 is heightened in the culture medium, it disrupts the inherent C/N balance within algal cells. To preserve a state of relative equilibrium, these cells must adapt to the surge in carbon concentration. In our study, the upregulation of nitrate reductase and nitrite reductase genes in S. costatum was potentially a cellular response to the increased nitrogen intake, aimed at preserving the intracellular carbon-to-nitrogen balance (Zhang et al., 2021). This finding aligns with similar results reported by Zhang et al. (2022). Furthermore, Montechiaro et al. (2010) noted that Protoceratium reticulatum adjusted to higher environmental pCO2 by maintaining a stable carbon-to-nitrogen ratio, underscoring the strategic cellular adjustments made to counterbalance changes in CO2 levels.
In our study, pCO2 emerged as the predominant factor influencing the nitrogen metabolic pathway in S. costatum. This is likely due to the synergistic relationship between carbon and nitrogen metabolism within the microalgae cells. It is well-established that carbon metabolism can supply the ATP required for nitrogen metabolism (Falkowski and Stone, 1975), and these two processes reciprocally support each other, sustaining the growth and development of living organisms (Wang et al., 2020). In the face of ocean acidification, the intracellular CO2-concentrating mechanisms (CCMs) in diatoms are capable of modifying energy storage and reallocating it to other cellular processes by reducing metabolic expenses (Wu et al., 2014; Valenzuela et al., 2018). Thangaraj et al. (2021) delved deeper into the molecular mechanisms behind this adaptive strategy in diatoms. They demonstrated that alterations in the transcriptional regulation of CCMs could stimulate amino acid synthesis and nitrogen metabolism, decrease metabolic costs, and consequently conserve energy. These findings are in harmony with the outcomes of our experimental study, reinforcing the intricate interplay between carbon and nitrogen metabolic pathways and their adaptation to environmental changes.
The study's findings underscore the remarkable adaptive capacity of S. costatum to the challenges posed by ocean acidification. The significant upregulation of genes that play a crucial role in the nitrogen metabolic process indicates a proactive response to the changing conditions of seawater chemistry. The heightened expression of genes related to nitrate assimilation and glutamate metabolism points to a strategic biological adjustment by S. costatum, allowing it to continue nitrogen uptake and utilization efficiently despite the acidification. Moreover, the study emphasizes the critical role of pCO2 as a key modulator of gene expression, revealing the intricate interplay between the physiological responses of the algae and the chemical composition of the marine environment. This insight into the molecular mechanisms at work provides a deeper understanding of how microalgae like S. costatum might cope with the environmental pressures of increasing CO2 levels and decreasing pH, offering valuable knowledge for predicting the impacts of ocean acidification on algal physiology and marine ecosystems.
The upregulation of genes associated with nitrogen metabolism in S. costatum in response to increased pCO2 levels indeed offers valuable insights into the molecular strategies that could contribute to the species' ecological resilience amidst ocean acidification. This adaptive response suggests a sophisticated biochemical flexibility that enables the algae to maintain essential metabolic functions, even as the marine environment becomes more acidic. The ability to enhance the expression of these key genes may allow S. costatum to more efficiently assimilate nitrogen. This could potentially give the species a competitive edge, enabling it to thrive in an increasingly acidic ocean. Furthermore, such genetic plasticity might also be a factor in the species' capacity to acclimate to other environmental stressors, reinforcing its role in marine ecosystems. Understanding these mechanisms is not only important for the conservation of marine biodiversity but also for predicting broader ecological shifts and the impacts of ocean acidification on primary productivity and the marine food web. This knowledge can aid in developing a more comprehensive assessment of how phytoplankton, as a foundation of marine food chains, will respond to the changing conditions of our oceans.
AcknowledgementsThis work was supported by the Scientific and Technological Innovation Project of the Laoshan Laboratory (No. LSKJ202203700), the National Key Research and Development Program of China (No. 2022YFC3105202) and the National Natural Science Foundation of China (No. 41976133).
Allen, A. E., Vardi, A., and Bowler, C., 2006. An ecological and evolutionary context for integrated nitrogen metabolism and related signaling pathways in marine diatoms. Current Opinion in Plant Biology, 9(3): 264-273. DOI:10.1016/j.pbi.2006.03.013 (  0) 0) |
Berges, J. A., and Harrison, P. J., 1995. Nitrate reductase activity quantitatively predicts the rate of nitrate incorporation under steady state light limitation: A revised assay and characterization of the enzyme in three species of marine phytoplankton. Limnology and Oceanography, 40(1): 82-93. DOI:10.4319/lo.1995.40.1.0082 (  0) 0) |
Bolger, A. M., Lohse, M., and Usadel, B., 2014. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30(15): 2114-2120. DOI:10.1093/bioinformatics/btu170 (  0) 0) |
Boyd, P. W., Watson, A. J., Law, C. S., Abraham, E. R., Trull, T., Murdoch, R., et al., 2000. A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature, 407(6805): 695-702. DOI:10.1038/35037500 (  0) 0) |
Breitbarth, E., Bellerby, R. J., Neill, C. C., Ardelan, M. V., Meyerhöfer, M., Zöllner, E., et al., 2010. Ocean acidification affects iron speciation during a coastal seawater mesocosm experiment. Biogeosciences, 7(3): 1065-1073. DOI:10.5194/bg-7-1065-2010 (  0) 0) |
Buchfink, B., Xie, C., and Huson, D. H., 2015. Fast and sensitive protein alignment using DIAMOND. Nature Methods, 12(1): 59-60. DOI:10.1038/nmeth.3176 (  0) 0) |
Capone, D. G., Bronk, D., Mulholland, M., and Carpenter, E. J.,, 2008. Nitrogen in the Marine Environment. 2nd edition. Academic Press, San Diego, 1385-1444.
(  0) 0) |
Chen, N., Zhang, M., Liu, S., and Cui, Z., 2023. Diversity of HAB species in coastal regions of China. Oceanologia et Limnologia Sinica, 54(3): 599-624. (  0) 0) |
Chi, X., Zhu, J., Mi, T., Zhen, Y., and Wang, J., 2023. Effects of seawater acidification on Skeletonema costatum. Marine Environmental Science, 42(5): 758-765. (  0) 0) |
Clayton, J., and Ahmed, S., 1986. Detection of glutamate synthase (GOGAT) activity in phytoplankton: Evaluation of cofactors and assay optimization. Marine Ecology Progress Series, 32: 115-122. DOI:10.3354/meps032115 (  0) 0) |
Dagenais-Bellefeuille, S., and Morse, D., 2013. Putting the N in dinoflagellates. Frontiers in Microbiology, 4: 369. (  0) 0) |
Dortch, Q., Ahmed, S. I., and Packard, T. T., 1979. Nitrate reductase and glutamate dehydrogenase activities in Skeletonema costatum as measures of nitrogen assimilation rates. Journal of Plankton Research, 1(2): 169-186. DOI:10.1093/plankt/1.2.169 (  0) 0) |
Falkowski, P. G., and Stone, D. P., 1975. Nitrate uptake in marine phytoplankton: Energy sources and the interaction with carbon fixation. Marine Biology, 32(1): 77-84. DOI:10.1007/BF00395161 (  0) 0) |
Flynn, K. J., Blackford, J. C., Baird, M. E., Raven, J. A., Clark, D. R., Beardall, J., et al., 2012. Changes in pH at the exterior surface of plankton with ocean acidification. Nature Climate Change, 2(7): 510-513. DOI:10.1038/nclimate1489 (  0) 0) |
Gao, Y., Smith, G. J., and Alberte, R. S., 1993. Nitrate reductase from the marine diatom Skeletonema costatum (biochemical and immunological characterization). Plant Physiology, 103(4): 1437-1445. DOI:10.1104/pp.103.4.1437 (  0) 0) |
Gattuso, J. P., Lee, K., Rost, B., Schulz, K., and Gao, K., 2010. Approaches and Tools to Manipulate the Carbonate Chemistry. Publications Office of the European Union, Luxembourg, 41-52.
(  0) 0) |
Gerringa, L. J. A., de Baar, H. J. W., and Timmermans, K. R., 2000. A comparison of iron limitation of phytoplankton in natural oceanic waters and laboratory media conditioned with EDTA. Marine Chemistry, 68(4): 335-346. DOI:10.1016/S0304-4203(99)00092-4 (  0) 0) |
Giordano, M., Norici, A., Forssen, M., Eriksson, M., and Raven, J. A., 2003. An anaplerotic role for mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiology, 132(4): 2126-2134. DOI:10.1104/pp.103.023424 (  0) 0) |
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., et al., 2011. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nature Biotechnology, 29(7): 644-652. DOI:10.1038/nbt.1883 (  0) 0) |
Guillard, R. R. L., 1975. Culture of Phytoplankton for Feeding Marine Invertebrates. Springer, Boston, MA, 1-22.
(  0) 0) |
Holden, H. M., Thoden, J. B., and Raushel, F. M., 1999. Carbamoyl phosphate synthetase: An amazing biochemical odyssey from substrate to product. Cellular and Molecular Life Sciences, 56(5): 507-522. (  0) 0) |
Hong, H., Wang, Y., and Wang, D., 2007. Study on process of nitrate reductase (NR) in marine phytoplankton. Marine Sciences, 10: 4-10. (  0) 0) |
Jing, X., Mi, T., Zhen, Y., Fu, B., Li, C., and Yu, Z., 2016. Description of nitrogen metabolism pathway based on Skeletonema marinoi transcriptome. Marine Sciences, 35(5): 703-711. (  0) 0) |
Joseph, L., and Villareal, T. A., 1998. Nitrate reductase activity as a measure of nitrogen incorporation in Rhizosolenia formosa (H. Peragallo): Internal nitrate and diel effects. Journal of Experimental Marine Biology and Ecology, 229 (2): 159-176.
(  0) 0) |
Kanehisa, M., Araki, M., Goto, S., Hattori, M., Hirakawa, M., Itoh, M., et al., 2008. KEGG for linking genomes to life and the environment. Nucleic Acids Research, 36(suppl.1): D480-D484. (  0) 0) |
Kim, J. H., Kim, K. Y., Kang, E. J., Lee, K., Kim, J. M., Park, K. T., et al., 2013. Enhancement of photosynthetic carbon assimilation efficiency by phytoplankton in the future coastal ocean. Biogeosciences, 10(11): 7525-7535. DOI:10.5194/bg-10-7525-2013 (  0) 0) |
Koch, M., Bowes, G., Ross, C., and Zhang, X. H., 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology, 19(1): 103-132. DOI:10.1111/j.1365-2486.2012.02791.x (  0) 0) |
Lacoue-Labarthe, T., Nunes, P. A. L. D., Ziveri, P., Cinar, M., Gazeau, F., Hall-Spencer, J. M., et al., 2016. Impacts of ocean acidification in a warming Mediterranean Sea: An overview. Regional Studies in Marine Science, 5: 1-11. DOI:10.1016/j.rsma.2015.12.005 (  0) 0) |
Lancien, M., Martin, M., Hsieh, M. H., Leustek, T., Goodman, H., and Coruzzi, G. M., 2002. Arabidopsis glt1-T mutant defines a role for NADH-GOGAT in the non-photorespiratory ammonium assimilatory pathway. Plant Journal, 29(3): 347-358. DOI:10.1046/j.1365-313X.2002.01218.x (  0) 0) |
Li, W., Jaroszewski, L., and Godzik, A., 2001. Clustering of highly homologous sequences to reduce the size of large protein databases. Bioinformatics, 17(3): 282-283. DOI:10.1093/bioinformatics/17.3.282 (  0) 0) |
Li, Y., Mi, T., Qiao, L., and Zhen, Y., 2019. Nitrogen metabolism pathway of Minutocellus polymorphus under two nitrogen nutrition conditions based on transcriptome. Oceanologia et Limnologia Sinica, 50(6): 1241-1251. (  0) 0) |
Liu, Y., Song, X., Han, X., and Yu, Z., 2013. Influences of external nutrient conditions on the transcript levels of a nitrate transporter gene in Skeletonema costatum. Acta Oceanologica Sinica, 32(6): 82-88. DOI:10.1007/s13131-013-0324-2 (  0) 0) |
Ma, J., Wang, P., Wang, X., Xu, Y., and Paerl, H. W., 2019. Cyanobacteria in eutrophic waters benefit from rising atmospheric CO2 concentrations. Science of the Total Environment, 691: 1144-1154. DOI:10.1016/j.scitotenv.2019.07.056 (  0) 0) |
Mao, X., Chen, J., van Oosterhout, C., Zhang, H., Liu, G., Zhuang, Y., et al., 2022. Diversity, prevalence, and expression of cyanase genes (cynS) in planktonic marine microorganisms. ISME Journal, 16(2): 602-605. DOI:10.1038/s41396-021-01081-y (  0) 0) |
Mistry, J., Finn, R. D., Eddy, S. R., Bateman, A., and Punta, M., 2013. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Research, 41(12): e121. DOI:10.1093/nar/gkt263 (  0) 0) |
Montechiaro, F., and Giordano, M., 2010. Compositional homeostasis of the dinoflagellate Protoceratium reticulatum grown at three different pCO2. Journal of Plant Physiology, 167(2): 110-113. DOI:10.1016/j.jplph.2009.07.013 (  0) 0) |
Muro-Pastor, M. I., Reyes, J. C., and Florencio, F. J., 2005. Ammonium assimilation in cyanobacteria. Photosynthesis Research, 83(2): 135-150. DOI:10.1007/s11120-004-2082-7 (  0) 0) |
Sabine, C. L., Feely, R. A., Gruber, N., Key, R. M., Lee, K., Bullister, J. L., et al., 2004. The oceanic sink for anthropogenic CO2. Science, 305(5682): 367-371. DOI:10.1126/science.1097403 (  0) 0) |
Sarno, D., Kooistra, W. H. C. F., Medlin, L. K., Percopo, I., and Zingone, A., 2005. Diversity in the genus Skeletonema (Bacillariophyceae). Ⅱ. An assessment of the taxonomy of S. costatum-like species with the description of four new species. Journal of Phycology, 41(1): 151-176. DOI:10.1111/j.1529-8817.2005.04067.x (  0) 0) |
Schwanhäuser, B., Busse, D., Li, N., Dittmar, G., Schuchhardt, J., Wolf, J., et al., 2011. Global quantification of mammalian gene expression control. Nature, 473(7347): 337-342. DOI:10.1038/nature10098 (  0) 0) |
Spalding, M. H., 2008. Microalgal carbon-dioxide-concen-trating mechanisms: Chlamydomonas inorganic carbon transporters. Journal of Experimental Botany, 59(7): 1463-1473. (  0) 0) |
Tada, K., Morishita, M., Hamada, K., Montani, S., and Yamada, M., 2001. Standing stock and production rate of phytoplankton and a red tide outbreak in a heavily eutrophic embayment, Dokai Bay, Japan. Marine Pollution Bulletin, 42(11): 1177-1186. DOI:10.1016/S0025-326X(01)00136-9 (  0) 0) |
Takahashi, M., Sasaki, Y., Ida, S., and Morikawa, H., 2001. Nitrite reductase gene enrichment improves assimilation of NO2 in arabidopsis. Plant Physiology, 126(2): 731-741. DOI:10.1104/pp.126.2.731 (  0) 0) |
Thangaraj, S., and Sun, J., 2021. Transcriptomic reprogramming of the oceanic diatom Skeletonema dohrniiunder warming ocean and acidification. Environmental Microbiology, 23(2): 980-995. DOI:10.1111/1462-2920.15248 (  0) 0) |
Torres, R., Reid, B., Pizarro, G., Frangópulos, M., Alarcón, E., Márquez, M., et al., 2023. Iron and silicic acid addition effects on early spring macronutrient drawdown and biogenic silica production of Patagonia estuarine waters. Progress in Oceanography, 214: 102982. DOI:10.1016/j.pocean.2023.102982 (  0) 0) |
Valenzuela, J. J., López García de Lomana, A., Lee, A., Armbrust, E. V., Orellana, M. V., and Baliga, N. S., 2018. Ocean acidification conditions increase resilience of marine diatoms. Nature Communications, 9(1): 2328. DOI:10.1038/s41467-018-04742-3 (  0) 0) |
van Oijen, T., van Leeuwe, M. A., Gieskes, W. W., and de Baar, H. J., 2004. Effects of iron limitation on photosynthesis and carbohydrate metabolism in the Antarctic diatom Chaetoceros brevis (Bacillariophyceae). European Journal of Phycology, 39(2): 161-171. DOI:10.1080/0967026042000202127 (  0) 0) |
Wang, F., Li, Y., Zhen, Y., and Liu, Q., 2020. Transcriptome analysis of carbon fixation pathway of Minutocellus polymorphus under different nitrogen source conditions and the comparison with other microalgae. Oceanologia et Limnologia Sinica, 51(5): 1127-1135. (  0) 0) |
Wei, Z., Long, C., Yang, F., Long, L., Huo, Y., Ding, D., et al., 2020. Increased irradiance availability mitigates the physiological performance of species of the calcifying green macroalga Halimeda in response to ocean acidification. Algal Research, 48: 101906. DOI:10.1016/j.algal.2020.101906 (  0) 0) |
Wu, Y., Campbell, D. A., Irwin, A. J., Suggett, D. J., and Finkel, Z. V., 2014. Ocean acidification enhances the growth rate of larger diatoms. Limnology and Oceanography, 59(3): 1027-1034. DOI:10.4319/lo.2014.59.3.1027 (  0) 0) |
Xia, J. R., and Gao, K. S., 2005. Impacts of elevated CO2 concentration on biochemical composition, carbonic anhydrase, and nitrate reductase activity of freshwater green algae. Journal of Integrative Plant Biology, 47(6): 668-675. DOI:10.1111/j.1744-7909.2005.00114.x (  0) 0) |
Zeebe, R. E., 2012. History of seawater carbonate chemistry, atmospheric CO2, and ocean acidification. Annual Review of Earth and Planetary Sciences, 40(1): 141-165. DOI:10.1146/annurev-earth-042711-105521 (  0) 0) |
Zhang, H., Wang, D. Z., Xie, Z. X., Zhang, S. F., Wang, M. H. and Lin, L., 2015. Comparative proteomics reveals highly and differentially expressed proteins in field-collected and laboratory-cultured blooming cells of the diatom Skeletonema costatum. Environmental Microbiology, 17(10): 3976-3991. DOI:10.1111/1462-2920.12914 (  0) 0) |
Zhang, J., Yang, Q., Liu, Q., Liu, S., Zhu, Y., Yao, J., et al., 2022. The responses of harmful dinoflagellate Karenia mikimotoi to simulated ocean acidification at the transcriptional level. Harmful Algae, 111: 102167. DOI:10.1016/j.hal.2021.102167 (  0) 0) |
Zhang, M., Zhen, Y., Mi, T., and Lin, S., 2021. Integrated transcriptome sequencing (RNA-seq) and proteomic studies reveal resource reallocation towards energy metabolism and defense in Skeletonema marinoi in response to CO2 increase. Applied and Environmental Microbiology, 87(5): e02614-2. (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


