2) National Innovation Center for Digital Fishery, China Agricultural University, Beijing 100083, China;
3) Key Laboratory of Smart Farming Technologies for Aquatic Animals and Livestock, Ministry of Agriculture and Rural Affairs, China Agricultural University, Beijing 100083, China;
4) Beijing Engineering and Technology Research Center for Internet of Things in Agriculture, Beijing 100083, China;
5) College of Information and Electrical Engineering, China Agricultural University, Beijing 100083, China;
6) Function Laboratory for Marine Fisheries Science and Food Production Processes, Laoshan Laboratory, Qingdao 266237, China
Light plays a pivotal role in fish growth and development, serving as a key environmental factor with diverse components such as spectrum (quality), intensity (quantity), and photoperiod (duration) (Villamizar et al., 2011; Zhang et al., 2020). Fish opsins include short-wave sensitive (SWS), medium-wave sensitive (MWS), and long-wave sensitive (LWS) components, which are specifically sensitive to blue, green and red lights, respectively (Ward et al., 2008). Most fish species can perceive light in the spectrum range from 40 to 750 nm, with different fish selectively adapting to the different spectrums (Nguyen and Winger, 2018). This adaptation involves both retinal and non-retinal photoreceptors, influencing growth, reproduction, immunity, and behavior through the central nervous and endocrine systems (Villamizar et al., 2011). Optimal light environments are thus crucial for promoting fish welfare (Ruchin, 2021).
The impact of light intensity on fish growth has been well-documented (Wei et al., 2019; Ruchin, 2021). For instance, Wang et al. (2013) identified an optimal light intensity range of 320 – 1150 lx for promoting growth in grouper (Epinephelus coioides, average weight 22.5 g ± 0.6 g), whereas Tian et al. (2015) noted the highest growth rate in blunt snout bream (Megalobra Amblycephala, average weight 18.04 g ± 0.22 g) under 400 lx light intensity. However, both excessively high and low light intensities can inhibit growth (Tian et al., 2015). Similarly, the photoperiod significantly affects growth, with varying effects observed in different species. A photoperiod of 24 h light: 0 h dark (24L: 0D) significantly promoted somatic growth in Nile tilapia (Oreochromis niloticus L., average weight 0.06 g) during the fingerling stage (Rad et al., 2006). Meanwhile, an 18L: 6D photoperiod enhanced the healthy growth of Mickey mouse platy (Xiphophorus maculatus, average weight 0.41 g ± 0 g) (Singh and Zutshi, 2020), but exhibited a negative effect on growth performance in rohu (Labeo rohita, average weight 2.39 g ± 0.35 g) (Shahjahan et al., 2020).
Diverse light colors affect physiological properties differently, reflecting the environmental characteristics of fish habitats and their visual functions (Baekelandt et al., 2019; Noureldin et al., 2021; Wei et al., 2021; Wu et al., 2021; Yang et al., 2021; Zou et al., 2022). Studies indicate that deep-sea teleost fishes are more sensitive to blue light, while shallow coastal water species are more responsive to red light (Carleton et al., 2020). The effects of light color on steelhead trout growth and metabolism are less clear, while different results have been reported. Karakatsouli et al.(2007, 2008) reported that red light color was beneficial to increase the growth of rainbow trout (Oncorhynchus mykiss), while blue light color can inhibit their growth but reduce the stress response of the body. Similarly, Guller et al. (2020) found that the blue color can enhance the antioxidant enzyme synthesis of rainbow trout. Moreover, Heydarnejad et al. (2013) reported that the yellow light color can improve the growth performance of rainbow trout, while reducing the metabolic level of plasma cortisol. Luchiari and Pirhonen (2008) found that rainbow trout favored the green environment, and avoided the red environment. In our previous research (unpublished), we found that steelhead trouts are insensitive to green lights and more susceptible to the effects of blue and red lights. However, to date, studies have mostly focused on the effects of single light color on the growth, immunity, and behavior of fish, and few studies have been conducted regarding the physiology and biochemistry of fish under changing light colors, which may be more conducive to fish growth.
Considering the diurnal changes in the sun's altitude, which alter the light spectrum in natural water bodies, it is imperative to understand the effects of these changing light conditions on fish, particularly on species like steelhead trout that experience different light environments during their life stages (Wetzel, 1983; Kendall et al., 2015; Busserolles et al., 2020; Ruchin, 2021). This study aims to investigate the growth performance, daily activity, and energy budget of juvenile steelhead trout under various light color conditions, offering insights into their optimal light requirements.
2 Materials and Methods 2.1 AnimalsThis experiment involved the use of triploid steelhead trout eyed eggs, sourced from Troutlodge Inc., Washington, USA. Conducted in a controlled environment at Wanzefeng Fishery Company's laboratory in Rizhao, Shandong, China, the study involved 1200 juvenile steelhead trout. These fish, averaging 36.24 g ± 5.76 g, were acclimatized for two weeks in brackish saltwater (salinity 14.2 ± 0.7) before the trial. During acclimation, they were fed a commercial diet from Greatseven Inc., Qingdao, China, to apparent satiation. Feedings occurred twice daily at 8:00 and 18:30. The environment provided 24-h oxygenation and replicated the trout's natural light conditions, ensuring optimal adjustment prior to the experiment.
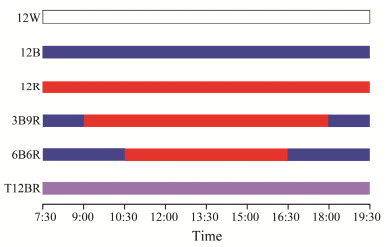
2.2 Experimental DesignIn this trial, illuminance values were measured for the surface, middle, and bottom water layers, and the illuminance was varied by adjusting the height of the light over the water surface to keep the mean illuminance values at 150 lx for each group. Six distinct color light treatments were examined (refer to Fig.1):
|
Fig. 1 Illustration of experimental light regimes. This figure graphically represents various experimental light conditions used in the study. The regimes are categorized based on the duration and type of light exposure, followed by a consistent period of darkness. 12W, 12 h continuous white light followed by 12 h darkness; 12B, 12 h under blue light followed by 12 h darkness; 12R, 12 h red light followed by 12 h darkness; 3B9R, 1.5 h blue light, 9 h red light, 1.5 h blue light followed by 12 h darkness; 6B6R, 3 h blue light, 6 h red light, 3 h blue light followed by 12 h darkness; T12BR, 12 h mixed blue and red lights followed by 12 h darkness. |
1) 12W (full spectrum, range: 350 – 800 nm, (148 ± 4) lx): 12 h white light followed by 12 h darkness.
2) 12B (blue light, rang: 406 – 504 nm, λ = 454.9 nm, (151 ± 5) lx): 12 h blue light followed by 12 h darkness.
3) 12R (red light, rang: 575 – 655 nm, λ = 614.8 nm, (149 ± 4) lx): 12 h red light followed by 12 h darkness.
4) 3B9R (combined light, λ = 454.9 nm and 614.8 nm, (152 ± 3) lx): 1.5 h blue light, 9 h red light, 1.5 h blue light followed by 12 h darkness.
5) 6B6R (combined light, λ = 454.9 nm and 614.8 nm, (151 ± 4) lx): 3 h blue light, 6 h red light, 3 h blue light followed by 12 h darkness.
6) T12BR (mixed light, λ = 454.9 nm + 614.8 nm, (75 ± 3) lx + (74 ± 3) lx): 12 h mixed blue and red light followed by 12 h darkness.
The photoperiod followed a 12L: 12D cycle, with lights being turned on and off at 7:30 and 19:30, respectively. The illuminance and spectral distribution were quantified using a waterproof handheld illuminometer (PLA-30, Everfine Inc., China). The light source was a chip-on-board (COB) LED, which was custom-designed and produced by Qingdao Lanchi Technology Company.
Each treatment had four replicate tanks with 20 fish per tank, totally 480 fish with similar size. The breeding tanks were circular (0.95 m diameter, 0.72 m high, 380 L capacity, white background) and shielded with black light-absorbing cloth to prevent inter-treatment light contamination. Additionally, 20 fish were randomly selected for initial sampling in subsequent energy budget analysis.
Before the experiment, steelhead trout were subjected to a 36-h fasting period to ensure an empty digestive tract. The fish were then anesthetized using a 30 mg L−1 tricaine methane sulfonate (MS-222, Sigma Chemicals Inc., USA) solution, blotted dry, and weighed on an electronic balance (Mettler-Toledo International, Inc., Switzerland). The initial average body weight was 34.67 g ± 2.69 g.
During the experiment, fish were fed twice daily (8:00 and 18:30) with commercial trout feed. Unconsumed feed was collected after 30 min, dried, weighed, and adjusted for moisture content and leaching loss to accurately calculate daily intake. Fecal collection was performed three times a day using a dual-mesh siphon system, and the feed remnants and feces were seperated. The materials were dried at 65℃ for 48 h, weighed, and stored in a drying dish for further analysis.
Tanks were filled with brackish water in a single-flow system with a water flow rate of 1.15 L min−1. Environmental parameters, including temperature, salinity, dissolved oxygen and pH value, were monitored thrice daily using a YSI Professional Plus multiparameter meter (YSI Inc., USA). For analyzing total ammonia nitrogen, phosphate, nitrite, and nitrate levels, water was collected from the tanks every three days and processed using an automatic chemical analyzer (Cleverchem 380, DeChem-Tech Inc., Germany).
Throughout the experiment, water was rigorously controlled with temperature at 16.5℃ ± 0.2℃, salinity at 14.2 ± 0.7, dissolved oxygen at (8.7 ± 0.3) mg L−1, pH at 7.3 ± 0.1, total ammonia nitrogen (TAN) at (0.03 ± 0.03) mg N L−1, phosphate at (0.11 ± 0.08) mg P L−1, nitrite nitrogen at (0.09 ± 0.05) mg N L−1, and nitrate nitrogen at (3.43 ± 1.9) mg N L−1 (mean ± standard deviation).
A 1080P infrared night vision video webcam (DS-IPC-T12, HikVison Inc., China) was installed 1.5 meters above each tank to capture daily activity data at a frame rate of 25 frames s−1. Data were recorded daily from 7:30 to 19:30.
The experiment lasted for 16 weeks, and all fish were weighed every four weeks to determine growth rates. At the end of the experiment, whole fish (three fish per tank) were selected to be weighed and then anesthetized with 100 mg L−1 MS-222, oven-dried at 65℃ for 72 h to a constant weight, and preserved in a drying dish for energy budget analyses.
2.3 Energy and Nitrogen Contents AnalysesIn this study, we meticulously processed and analyzed whole fish, feed, surplus feed, and feces to determine their energy and nitrogen contents. Initially, these samples were desiccated and pulverized to a fine consistency. The resultant dry powder was then meticulously sieved through a 60-mesh screen to ensure uniformity. Weights of the samples were weighed using a microbalance (Model MYA 5.4Y, Radwag Wagi Elektroniczne, Radom, Poland) with an exceptional reading accuracy of 1 µg.
To determine nitrogen content, an elemental analyzer (Vario EL III, Elementar Inc., Hanau, Germany) was employed. The gross energy contents were quantified using an automatic adiabatic oxygen bomb calorimeter (Model PA-RR6400, Parr Inc., Moline, IL, USA). To ensure the robustness and reproducibility, each sample underwent quadruplicate measurements, and the mean values were subsequently recorded.
The research further studied the energy associated with various physiological processes in the fish, namely feeding (C), growth (G), fecal production (F), excretion (U), and respiration (R). The calculations were based on the following formula, as described by Ye et al. (2009):
| $ C = G + F + U + R, $ | (1) |
where C is the energy consumed in feed, G is the energy deposited for growth, F is the energy lost in feces, U is the energy in excretion, and R is the energy lost through respiration.
To accurately calculate the energy of excretion, we used the formula outlined by Zhu et al. (2004):
| $ U = ({C_{\text{N}}}-{G_{\text{N}}}-{F_{\text{N}}}) \times 24.83, $ | (2) |
where CN is the nitrogen contained in the feed intake, GN is the nitrogen stored in the fish, FN is the nitrogen excreted in feces, and 24.83 represents the energy value contained in ammonia nitrogen (kJ g−1).
Respiratory energy was calculated using the equation:
| $ R = C-G-F-U. $ | (3) |
To accurately quantify the total daily activity (TDA) of fish, our methodology encompassed two pivotal phases, detection and tracking (Cai et al., 2020; Liu et al., 2020). In the detection phase, leveraging the robust capabilities of the You Only Look Once, version 4 (YOLOv4) algorithm, we efficiently identified and localized the head of each fish within the image. This phase was enhanced by training the YOLOv4 model with a diverse dataset comprising images of various growth stages and living conditions, thus augmenting its generalizability in precisely pinpointing the fish.
The subsequent tracking phase was crucial in maintaining consistent identification of individual fish across successive frames. By incorporating the principles of the assignment problem and utilizing key parameters such as swimming velocity and posture, we integrated the Kalman filter and Kuhn-Munkres algorithm. This approach enabled us to allocate a unique identity to each steelhead trout within each group, thereby facilitating the generation of accurate trajectory coordinates over the experimental periods.
Our system was designed to ensure that once a fish was detected, it was assigned as a unique identification number, which remained constant unless the tracker lost its tracking information. In instances where the tracker lost its target, a new identification number was allocated to any unmarked fish. The total activity of the school was calculated by aggregating the activities of all individual fish, thereby minimizing errors associated with identification changes.
The computation of TDA was formulated as follows (Barry, 2012; Papadakis et al., 2012):
| $ TDA = \sum\limits_{i = 1}^{{T_n}} {{a_i}{v_i}}, $ | (4) |
where vi is the velocity of motion of the ith individual, calculated using displacement and time, ai is the proportion of time when the tracker ID remains constant at the time of calculation, and Tn is the total identification number of fish.
To ensure the statistical robustness of our daily activity data, we accounted for variations induced by feeding and changes in light intensity. The daily activity data were aggregated over an 11-h period, intentionally excluding the first 30 min of post-light activation and the 15 min pre and post-feeding intervals. The data were initially averaged per day with each tank and subsequently across treatments.
2.5 Data CalculationsSpecific growth rate (SGR), feed conversion efficiency (FCE), average daily feed intake (ADFI), and survival rate (SR) were calculated as follows:
| $ SGR (\% {{\text{d}}^{ {\text{1}}}}) = 100 \times (\ln {W_t} \ln {W_0})/t, $ | (5) |
| $ FCE (\%) = 100 \times ({W_t} {W_0})/{C_w}, $ | (6) |
| $ ADFI ({\text{g }}{{\text{d}}^{ {\text{1}}}}) = {C_w}/t, $ | (7) |
| $ SR (\%) = 100 \times {F_t}/{F_0}, $ | (8) |
where Wt and W0 are the final and initial wet body masses (g) of fish, respectively; t is the feeding duration (days); Cw is the feed intake (g); and Ft and F0 are the numbers of fishtail at the end and beginning of the trial, respectively.
2.6 Statistical AnalysisAll data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). The correlation analysis was carried out using Origin Pro 2022 (OriginLab, Northampton, MA, USA). Data distribution normality and homogeneity of variance were assessed using the Shapiro-Wilk and Levene tests, respectively. When the data satisfied the normal distribution and homogeneity of variance criteria, a one-way analysis of variance (ANOVA) was conducted, and the average values were further compared using Tukey's multiple comparison tests. Nonparametric tests, including the Mann-Whitney Rank Sum test and a Kruskal-Wallis ANOVA on ranks, were applied to the abnormally distributed data. Differences were considered significant at P < 0.05, and the results are expressed as the mean ± standard deviation.
2.7 Ethics Approval StatementAll procedures were performed under the Regulations of the Administration of Affairs Concerning Experimental Animals of China as well as the Regulations of the Administration of Affairs Concerning Experimental Animals of Shandong Province.
3 Results 3.1 Growth PerformanceThe 16-week growth data for juvenile steelhead trout are detailed in Table 1. Notably, the specific growth rates (SGRs) in the 3B9R and 12R treatment groups were significantly superior to those in the 6B6R group (P < 0.05). Additionally, feed conversion efficiency (FCE) values varied across treatments, ranking from the highest to the lowest as follows: 3B9R, 12R, 12B, 12W, T12BR, and 6B6R. The FCE of the 3B9R group was markedly higher compared to that of the 6B6R group (P < 0.05). Contrasts in feed intake (FI), average daily feed intake (ADFI), and survival rate (SR) among the treatment groups did not reveal any significant differences (P > 0.05).
|
|
Table 1 Effects of different light colors on growth performance of juvenile steelhead trout during the 16-week trial |
Table 2 encapsulates the energy budget for the juvenile steelhead trout. The 6B6R group exhibited a significantly elevated feeding energy (C) compared to the 3B9R group. Considering feeding energy (C%) as a reference, the growth energy (G) in the 3B9R group was significantly higher than in the 6B6R and 12W groups (P < 0.05). Furthermore, the fecal energy (F) was substantially lower in the 6B6R and 12R groups compared to those in other treatments (P < 0.05). The excretory and respiratory energies in the 6B6R group were notably higher than those in the 3B9R group (P < 0.05).
|
|
Table 2 Effects of different light conditions on energy budgets of juvenile steelhead trout during the 16-week trial |
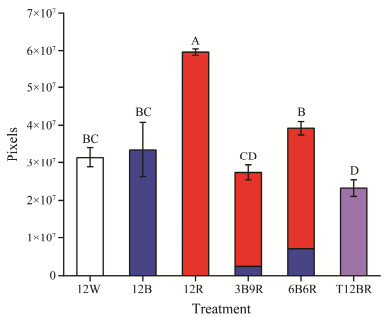
Fig.2 presents the total daily activity (TDA) of juvenile steelhead trout under varying treatment conditions. There were significant differences in TDA levels among the groups (P < 0.05). The 12R group demonstrated a notably higher TDA compared to other groups (P < 0.05), followed by the 6B6R group. The T12BR group's TDA was significantly lower than all other groups, except for the 3B9R group (P < 0.05).
|
Fig. 2 Total daily activity of juvenile steelhead trout. This figure illustrates the total quantity of daily activity observed in juvenile steelhead trout under different experimenttal light conditions. Each condition combines varying durations of light exposure with a consistent period of darkness. The different colors in the bars of 3B9R and 6B6R indicate the proportion of trout activity to the total quantity of daily activity during blue and red lights. 12W, 12 h continuous white light followed by 12 h darkness; 12B, 12 h under blue light followed by 12 h darkness; 12R, 12 h red light followed by 12 h darkness; 3B9R, 1.5 h blue light, 9 h red light, 1.5 h blue light followed by 12 h darkness; 6B6R, 3 h blue light, 6 h red light, 3 h blue light followed by 12 h darkness; T12BR, 12 h mixed blue and red light followed by 12 h darkness. |
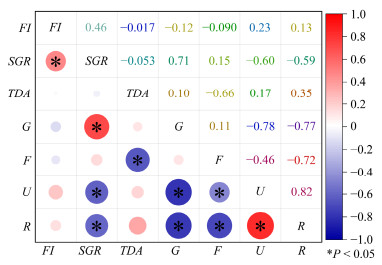
Pearson correlations for growth performance, total daily activity (TDA), and energy allocation were shown in Fig.3. Significant positive correlations (P < 0.05) were found between specific growth rate (SGR) with food intake (FI) and growth energy (G), as well as between excretion energy (U) and respiration energy (R). Significant negative correlations (P < 0.05) were found between SGR, G, and fecal energy (F) with U and R, as well as between TDA and F. The Pearson correlation results indicated a direct correlation between growth performance, TDA and energy allocation. The increased SGR and G were positively correlated with higher FI and negatively correlated with lower U and R, reflecting the efficiency of energy allocation.
|
Fig. 3 Pearson correlations between feed intake, specific growth rate, energy budget and total daily activity. FI, feed intake; SGR, specific growth rate; TDA, total daily activity; G, energy deposited for growth; F, energy lost in feces; U, energy in excretion; R, energy lost through respiration. |
Water depth considerably influences the intensity and spectral composition of light in aquatic environments (Liu et al., 2016; Hou et al., 2019). Specifically, blue light penetrates up to 200 m, while red light is rapidly absorbed within the top 20 m in unadulterated water (Sanchez-Vazquez et al., 2019; Ruchin, 2021). The steelhead trout, as an anadromous migratory species, spends its early life stages in pristine freshwater streams before ocean migration (Sobocinski et al., 2020; Xiong et al., 2020). Therefore, it is plausible to suggest that steelhead trout exhibit adaptability to both red and blue light.
During sunrise and sunset, the sunlight's low angle yields a lengthy path length, allowing short-wavelength blue light to penetrate deeper compared to long-wavelength red light in clear water bodies like pools. Conversely, at midday, the sunlight's path is shorter, leading to a higher proportion of red light in the water (Wetzel, 1983; Ruchin, 2021). It is likely that steelhead trout juveniles have evolved strategies to thrive in these shifting light conditions.
Therefore, our experiment selected white light (12W) to simulate natural light, along with blue light (12B) and red light (12R), as underwater light conditions at different times. We also examined combinations of these lights (3B9R and 6B6R) to study their effects on the growth and metabolism of steelhead trout.
In our experiment, the 3B9R light environment, which mimics daily light color variation rhythms, appears to promote juvenile steelhead trout growth. A similar observation was made in our recent study under the same light conditions, where we noted significant increases in GH/IGF-1 axis gene expression and immune performance in steelhead trout (Chen et al., 2022a).
Arechavala-Lopez et al. (2022) argued that light environments closely mirroring natural conditions are optimal for fish growth. In our study, growth was significantly lower in the 6B6R steelhead trout group. Considering the trouts have adapted to a particular light color environment, when the light changes, it possibly induces stress to the fish. In turn, the stress inhibited digestive enzyme activity, consequently impacting growth (Chen et al., 2022b). The growth of other steelhead trout groups remained unaffected, suggesting they may possess adaptability to these light conditions, which needs further investigation.
This study's primary objective was to examine the effects of various light conditions, particularly the 3B9R combination, on the growth and metabolic processes of juvenile steelhead trout. Our results indicate that the 3B9R lighting, simulating natural light cycles, can notably enhance the specific growth rate and feed conversion efficiency without significantly increasing the TDA of the fish.
The intriguing aspect of the 3B9R group was their lower TDA coupled with a higher feed conversion efficiency. This result suggests a more efficient energy utilization that is beneficial for growth. Conversely, the 12R lighting condition, though promoting rapid growth, led to increased daily activity and energy expenditure for respiration. This observation underscores a potential trade-off between growth rate and energy utilization efficiency. Similarly, Owen et al. (2010) found that red light caused an increase in captive trench (Tinca tinca) activity, and Luchiari and Pirhonen (2008) reported that rainbow trout (Oncorhynchus mykiss) showed behavioral avoidance to red light. In addition, Chen et al. (2022a) reported that oxidative stress existed in the 12R light environment for steelhead trout. Consequently, further study is required as why the 12R light environment was beneficial to the growth of steelhead trout.
However, the study also observed that under the 6B6R lighting conditions, the growth of the trout was noticeably impeded. This finding underscores the criticality of a harmonized light environment conducive to the development of these aquatic species. The 6B6R lighting spectrum necessitated an increased metabolic expenditure for respiretion and excretion, indicating a substantial influence of light color on the metabolic processes and the distribution of energy resources. This, in turn, exerted a considerable impact on their growth rates. Parallel research conducted by Guo et al. (2011) on Litopenaeus vannamei, a species of shrimp, further substantiates this phenomenon. Their findings highlighted that light color markedly alters the percentage of energy allocated toward metabolic activities. This evidence coherently aligns with the notion that the spectral quality of light is a pivotal factor in shaping the energy budget patterns in aquatic organisms.
Our findings contribute significantly to understanding of the impact of light on aquaculture practices. While both 3B9R and 12R lighting conditions show potential for trout cultivation, pinpointing the most effective lighting regime for maximizing production efficiency requires further investigation. This study serves as a foundation for future research in this area, potentially revolutionizing aquaculture methodologies and outcomes.
Overall, our research suggests that both the 3B9R and 12R light environments are conducive to the cultivation needs of steelhead trout. However, the optimal lighting condition for enhancing trout production remains an open question. Our study lays the groundwork for further research in this area, which is essential for refining aquaculture practices and improving production efficiency.
5 ConclusionsThis investigation explored the influences of varying light conditions, specifically the 3B9R light combination, on the growth and metabolic efficiency of juvenile steelhead trout. Our findings reveal that the 3B9R light regime, mimicking diurnal rhythmic variations in light color, was associated with an upward trend in specific growth rate, feed conversion efficiency, and growth energy allocation ratio. Notably, this light condition did not significantly increase the total daily activity levels in steelhead trout.
The 3B9R group exhibited a lower TDA yet higher FCE, which appears to be advantageous for the growth of trout. In contrast, under the 12R light conditions, although a rapid growth in trout was observed, it was accompanied by increased daily activity and greater respiratory energy expenditure. Importantly, the growth of steelhead trout was markedly hindered under the 6B6R lighting conditions.
AcknowledgementsThis research was supported by the Shandong Postdoctoral Science Foundation (No. SDCX-ZG-202302007), the National Key Research and Development Program of China (No. 2019YFD0901000), the National Natural Science Foundation of China (Nos. U1906206 and 31872575), and the Major Science and Technology Innovation Project of Shandong Province (No. SD2019YY006).
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Xueweijie Chen. Research progress, proposed research methods, proposed research ideas, and edited manuscripts were performed by Yangen Zhou and Shuanglin Dong. Data analysis was performed by Jinze Huang. Dong An, Li Li, Yunwei Dong, Qinfeng Gao, and Shuanglin Dong reviewed and edited the draft. The draft of the manuscript was written by Xueweijie Chen, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
The data and references presented in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval and Consent to Participate
All methods used in this study were conducted according to the guiding principles of the Chinese Legislation on the Use and Care of Laboratory Animals. The Academic Council approved the animal protocol of the Ocean University of China.
Consent for Publication
Informed consent for publication was obtained from all participants.
Conflict of Interests
The authors declare that they have no conflict of interests.
Arechavala-Lopez, P., Cabrera-Álvarez, M. J., Maia, C. M., and Saraiva, J. L., 2022. Environmental enrichment in fish aquaculture: A review of fundamental and practical aspects. Reviews in Aquaculture, 14(2): 704-728. DOI:10.1111/raq.12620 (  0) 0) |
Baekelandt, S., Mandiki, S. N. M., Schmitz, M., and Kestemont, P., 2019. Influence of the light spectrum on the daily rhythms of stress and humoral innate immune markers in pikeperch Sander lucioperca. Aquaculture, 499: 358-363. DOI:10.1016/j.aquaculture.2018.09.046 (  0) 0) |
Barry, M. J., 2012. Application of a novel open-source program for measuring the effects of toxicants on the swimming behavior of large groups of unmarked fish. Chemosphere, 86(9): 938-944. DOI:10.1016/j.chemosphere.2011.11.011 (  0) 0) |
Busserolles, F. D., Fogg, L., Cortesi, F., and Marshall, J., 2020. The exceptional diversity of visual adaptations in deep-sea teleost fishes. Seminars in Cell & Developmental Biology, 106: 20-30. DOI:10.1016/j.semcdb.2020.05.027 (  0) 0) |
Cai, K. W., Miao, X. Y., Wang, W., Pang, H. S., Liu, Y., and Song, J. Y., 2020. A modified YOLOv3 model for fish detection based on MobileNetv1 as backbone. Aquacultural Engineering, 91: 102117. DOI:10.1016/j.aquaeng.2020.102117 (  0) 0) |
Carleton, K. L., Escobar-Camacho, D., Stieb, S. M., Cortesi, F., and Marshall, N. J., 2020. Seeing the rainbow: Mechanisms underlying spectral sensitivity in teleost fishes. Journal of Experimental Biology, 223(8): 193334. DOI:10.1242/jeb.193334 (  0) 0) |
Chen, X. W. J., Zhou, Y. G., Huang, J. Z., An, D., Li, L., Dong, Y. W., et al., 2022a. The effects of blue and red light color combinations on the growth and immune performance of juvenile steelhead trout, Oncorhynchus mykiss. Aquaculture Reports, 24: 101156. DOI:10.1016/j.aqrep.2022.101156 (  0) 0) |
Chen, X. W. J., Zhou, Y. G., Huang, J. Z., An, D., Li, L., Dong, Y. W., et al., 2022b. Blue and red light color combinations can enhance certain aspects of digestive and anabolic performance in juvenile steelhead trout Oncorhynchus mykiss. Frontiers in Marine Science, 9: 853327. DOI:10.3389/fmars.2022.853327 (  0) 0) |
Guller, U., Onalan, S., Arabaci, M., Karatas, B., Yasar, M., and Kufrevioglu, O. I., 2020. Effects of different LED light spectra on rainbow trout (Oncorhynchus mykiss): In vivo evaluation of the antioxidant status. Fish Physiology and Biochemistry, 46(6): 2169-2180. DOI:10.1007/s10695-020-00865-x (  0) 0) |
Guo, B., Wang, F., Dong, S. L., and Gao, Q. F., 2011. The effect of rhythmic light color fluctuation on the molting and growth of Litopenaeus vannamei. Aquaculture, 314(1-4): 210-214. DOI:10.1016/j.aquaculture.2011.02.023 (  0) 0) |
Heydarnejad, M. S., Parto, M., and Pilevarian, A. A., 2013. Influence of light colours on growth and stress response of rainbow trout (Oncorhynchus mykiss) under laboratory conditions. Journal of Animal Physiology and Animal Nutrition, 97(1): 67-71. DOI:10.1111/j.1439-0396.2011.01243.x (  0) 0) |
Hou, Z. S., Wen, H. S., Li, J. F., He, F., Li, Y., Qi, X., et al., 2019. Effects of photoperiod and light spectrum on growth performance, digestive enzymes, hepatic biochemistry and peripheral hormones in spotted sea bass (Lateolabrax maculatus). Aquaculture, 507: 419-427. DOI:10.1016/j.aquaculture.2019.04.029 (  0) 0) |
Karakatsouli, N., Papoutsoglou, S. E., Panopoulos, G., Papoutsoglou, E. S., Chadio, S., and Kalogiannis, D., 2008. Effects of light spectrum on growth and stress response of rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquacultural Engineering, 38(1): 36-42. DOI:10.1016/j.aquaeng.2007.10.006 (  0) 0) |
Karakatsouli, N., Papoutsoglou, S. E., Pizzonia, G., Tsatsos, G., Tsopelakos, A., Chadio, S., et al., 2007. Effects of light spectrum on growth and physiological status of gilthead seabream Sparus aurata and rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquacultural Engineering, 36(3): 302-309. DOI:10.1016/j.aquaeng.2007.01.005 (  0) 0) |
Kendall, N. W., McMillan, J. R., Sloat, M. R., Buehrens, T. W., Quinn, T. P., Pess, G. R., et al., 2015. Anadromy and residency in steelhead and rainbow trout (Oncorhynchus mykiss): A review of the processes and patterns. Canadian Journal of Fisheries and Aquatic Sciences, 72(3): 319-342. DOI:10.1139/cjfas-2014-0192 (  0) 0) |
Liu, L., Ouyang, W. L., Wang, X. G., Fieguth, P., Chen, J., Liu, X. W., et al., 2020. Deep learning for generic object detection: A survey. International Journal of Computer Vision, 128: 261-318. DOI:10.1007/s11263-019-01247-4 (  0) 0) |
Liu, X. H., Zhang, Y. L., Shi, K., Lin, J. F., Zhou, Y. Q., and Qin, B. Q., 2016. Determining critical light and hydrologic conditions for macrophyte presence in a large shallow lake: The ratio of euphotic depth to water depth. Ecological Indicators, 71: 317-326. DOI:10.1016/j.ecolind.2016.07.012 (  0) 0) |
Luchiari, A. C., and Pirhonen, J., 2008. Effects of ambient colour on colour preference and growth of juvenile rainbow trout Oncorhynchus mykiss (Walbaum). Journal of Fish Biology, 72(6): 1504-1514. DOI:10.1111/j.1095-8649.2008.01824.x (  0) 0) |
Nguyen, K. Q., and Winger, P. D., 2018. Artificial light in commercial industrialized fishing applications: A review. Reviews in Fisheries Science & Aquaculture, 27(1): 106-126. DOI:10.1080/23308249.2018.1496065 (  0) 0) |
Noureldin, S. M., Diab, A. M., Salah, A. S., and Mohamed, R. A., 2021. Effect of different monochromatic LED light colors on growth performance, behavior, immune-physiological responses of gold fish, Carassius auratus. Aquaculture, 538: 736532. DOI:10.1016/j.aquaculture.2021.736532 (  0) 0) |
Owen, M. A. G., Davies, S. J., and Sloman, K. A., 2010. Light colour influences the behaviour and stress physiology of captive tench (Tinca tinca). Reviews in Fish Biology and Fisheries, 20(3): 375-380. DOI:10.1007/s11160-009-9150-1 (  0) 0) |
Papadakis, V. M., Papadakis, I. E., Lamprianidou, F., Glaropoulos, A., and Kentouri, M., 2012. A computer-vision system and methodology for the analysis of fish behavior. Aquacultural Engineering, 46: 53-59. DOI:10.1016/j.aquaeng.2011.11.002 (  0) 0) |
Rad, F., Bozaoğlu, S., Ergene Gözükara, S., Karahan, A., and Kurt, G., 2006. Effects of different long-day photoperiods on somatic growth and gonadal development in Nile tilapia (Oreochromis niloticus L.). Aquaculture, 255(1-4): 292-300. DOI:10.1016/j.aquaculture.2005.11.028 (  0) 0) |
Ruchin, A. B., 2021. Effect of illumination on fish and amphibian: Development, growth, physiological and biochemical processes. Reviews in Aquaculture, 13(1): 567-600. DOI:10.1111/raq.12487 (  0) 0) |
Sanchez-Vazquez, F. J., Lopez-Olmeda, J. F., Vera, L. M., Migaud, H., Lopez-Patino, M. A., and Miguez, J. M., 2019. Environmental cycles, melatonin, and circadian control of stress response in fish. Frontiers in Endocrinology, 10: 279. DOI:10.3389/fendo.2019.00279 (  0) 0) |
Shahjahan, M., Al-Emran, M., Majharul Islam, S. M., Abdul Baten, S. M., Rashid, H., and Mahfuzul Haque, M., 2020. Prolonged photoperiod inhibits growth and reproductive functions of rohu Labeo rohita. Aquaculture Reports, 16: 100272. DOI:10.1016/j.aqrep.2019.100272 (  0) 0) |
Singh, A., and Zutshi, B., 2020. Photoperiodic effects on somatic growth and gonadal maturation in Mickey Mouse platy, Xiphophorus maculatus (Gunther, 1866). Fish Physiology and Biochemistry, 46: 1483-1495. DOI:10.1007/s10695-020-00806-8 (  0) 0) |
Sobocinski, K. L., Kendall, N. W., Greene, C. M., and Schmidt, M. W., 2020. Ecosystem indicators of marine survival in Puget Sound steelhead trout. Progress in Oceanography, 188: 102419. DOI:10.1016/j.pocean.2020.102419 (  0) 0) |
Tian, H. Y., Zhang, D. D., Xu, C., Wang, F., and Liu, W. B., 2015. Effects of light intensity on growth, immune responses, antioxidant capability and disease resistance of juvenile blunt snout bream Megalobrama amblycephala. Fish & Shellfish Immunology, 47(2): 674-680. DOI:10.1016/j.fsi.2015.08.022 (  0) 0) |
Villamizar, N., Blanco-Vives, B., Migaud, H., Davie, A., Carboni, S., and Sánchez-Vázquez, F. J., 2011. Effects of light during early larval development of some aquacultured teleosts: A review. Aquaculture, 315(1-2): 86-94. DOI:10.1016/j.aquaculture.2010.10.036 (  0) 0) |
Wang, T., Cheng, Y. Z., Liu, Z. P., Yan, S. H., and Long, X. H., 2013. Effects of light intensity on growth, immune response, plasma cortisol and fatty acid composition of juvenile Epinephelus coioides reared in artificial seawater. Aquaculture, 414-415: 135-139, https://doi.org/10.1016/j.aquaculture.2013.08.004.
(  0) 0) |
Ward, M. N., Churcher, A. M., Dick, K. J., Laver, C. R. J., Owens, G. L., Polack, M. D., et al., 2008. The molecular basis of color vision in colorful fish: Four Long Wave-Sensitive (LWS) opsins in guppies (Poecilia reticulata) are defined by amino acid substitutions at key functional sites. BMC Evolutionary Biology, 8: 210. DOI:10.1186/1471-2148-8-210 (  0) 0) |
Wei, H., Li, H. D., Xia, Y., Liu, H. K., Han, D., Zhu, X. M., et al., 2019. Effects of light intensity on phototaxis, growth, antioxidant and stress of juvenile gibel carp (Carassius auratus gibelio). Aquaculture, 501: 39-47. DOI:10.1016/j.aquaculture.2018.10.055 (  0) 0) |
Wei, J., Tian, L., Wang, Y. K., Yu, L. Y., and Zhu, X. P., 2021. Effects of salinity, photoperiod, and light spectrum on larval survival, growth, and related enzyme activities in the giant freshwater prawn, Macrobrachium rosenbergii. Aquaculture, 530: 735794. DOI:10.1016/j.aquaculture.2020.735794 (  0) 0) |
Wetzel, R. G., 1983. Limnology. 2nd edition. CBS College Publishing, New York, 767pp.
(  0) 0) |
Wu, L. L., Wang, Y. N., Li, J., Song, Z. C., Xu, S. H., Song, C. B., et al., 2021. Influence of light spectra on the performance of juvenile turbot (Scophthalmus maximus). Aquaculture, 533: 736191. DOI:10.1016/j.aquaculture.2020.736191 (  0) 0) |
Xiong, Y. H., Dong, S., L., Huang, M., Li, Y., Wang, X., Wang, F., et al., 2020. Growth, osmoregulatory response, adenine nucleotide contents, and liver transcriptome analysis of steelhead trout (Oncorhynchus mykiss) under different salinity acclimation methods. Aquaculture, 520: 734937. DOI:10.1016/j.aquaculture.2020.734937 (  0) 0) |
Yang, M. F., Hu, F. Y., Leng, X. F., Chi, X. M., Yin, D. H., Ding, J. Y., et al., 2021. Long-term effects of light spectra on fitness related behaviors and growth of the sea urchin Strongylocentrotus intermedius. Aquaculture, 537: 736518. DOI:10.1016/j.aquaculture.2021.736518 (  0) 0) |
Ye, L., Jiang, S. G., Zhu, X. M., Yang, Q. B., Wen, W. G., and Wu, K. C., 2009. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture, 290(1-2): 140-144. DOI:10.1016/j.aquaculture.2009.01.028 (  0) 0) |
Zhang, X. Y., Bian, Z. H., Yuan, X. X., Chen, X., and Lu, C. G., 2020. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends in Food Science & Technology, 99: 203-216. DOI:10.1016/j.tifs.2020.02.031 (  0) 0) |
Zhu, C. B., Dong, S. L., Wang, F., and Huang, G. Q., 2004. Effects of Na/K ratio in seawater on growth and energy budget of juvenile Litopenaeus vannamei. Aquaculture, 234(1-4): 485-496. DOI:10.1016/j.aquaculture.2003.11.027 (  0) 0) |
Zou, Y. X., Peng, Z. Z., Wang, W. X., Liang, S. S., Song, C. B., Wang, L. J., et al., 2022. The stimulation effects of green light on the growth, testicular development and stress of olive flounder Paralichthys olivaceus. Aquaculture, 546: 737275. DOI:10.1016/j.aquaculture.2021.737275 (  0) 0) |
 2025, Vol. 24
2025, Vol. 24


