2) Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
3) National Oceanographic Center, Qingdao 266071, China
Photobacterium damselae subsp. damselae (PDD) is a subspecies of P. damselae belonging to Vibrionaceae family. As an important pathogen of marine animals, it is widely distributed in the seawater worldwide. The bacterium is firstly reported in 1981, which was isolated from skin ulcers of damselfish (Chromis punctipinnis), and originally was named as Vibrio damsel (Love et al., 1981). Subsequently, there are successive reports on PDD infection in different animals around the world, including fish (Ketterer et al., 1992; Labella et al., 2006), crustaceans (Song et al., 1993; Vaseeharan et al., 2007), mollusks (Hanlon et al., 1984; Lozano-León et al., 2003), sea turtles (Obendorf et al., 1987), mammals (Fujioka et al., 1988; Lee et al., 2018). In 1982, the first case of PDD infection in human was reported (Morris et al., 1982). Symptoms of human PDD infection usually appear as severe fasciitis (Yuen et al., 1993) and septicemia (Perez-Tirse et al., 1993). In 2004, Japanese scientists reported two cases of human PDD infection, causing severe fasciitis to acute death (Yamane et al., 2004). In 2015, a child was infected with PDD and died in Saudi Arabia (Alhemairi et al., 2015). These cases have confirmed that the bacteria are highly pathogenic to humans.
Usually PDD is studied with heterogeneously isolated strains according to their phenotypic characteristics and virulence (Pedersen et al., 1997; Terceti et al., 2016; Terceti et al., 2018). Some of them show strong hemolytic activity and high pathogenicity, while others exhibit weak hemolytic activity and lower pathogenicity, or even no pathogenicity. Early studies have confirmed that the pathogenic strains of PDD can produce a large amount of cytolytic toxin, which is named as damselysin (Dly) (Kothary et al., 1985; Kreger et al., 1987; Cutter et al., 1990). Subsequently, more researches revealed that a pore-forming toxin with hemolytic activity, named as phobalysin P (PhlyP), is another important virulence factor of the strain (Rivas et al., 2011, 2013a, 2015; Vences et al., 2017). These two toxins are encoded by dly gene and hlyApl gene, respectively, which are located on the virulence plasmid named as pPHDD1 (Rivas et al., 2011). In 2013, a study reported the third virulence gene on chromosome encoding another hemolysin (HlyA) (Rivas et al., 2013a). To date, adequate studies have supported that the conjunction of Dly, PhlyP and HlyA cytotoxins constitutes the virulence system of highly virulent lineages of PDD (Rivas et al., 2015; Rivas et al., 2013b). In fact, different strains of the same bacteria with significant differences in pathogenicity are common in nature (Saroj et al., 2008; Cheng et al., 2019).
Until now, infections of mariculture animals by PDD have been rarely reported, and PDD is considered as a newly discovered pathogen in China. Nevertheless, according to available studies, it can infect different marine fishes living in different environments, such as Epinephelus lanceolatus (Zhang et al., 2009), Cynoglossus semilaevis (Yan et al., 2018), Sebastes schlegeli (Zhang et al., 2019b) and so on. There seems to be no obvious regional specificity of this bacterium though the seawater temperatures in different environments are quite different. In 2016, we isolated a PDD strain from black rockfish S. schlegeli with the symptom of skin ulceration, which were cultured in net-cage of Changdao County, Shandong Province. The experiment has confirmed its pathogenicity to black rockfish (Zhang et al., 2019b). Its whole-genome sequencing has also been completed (Yu et al., 2019). Together with other 24 strains isolated previously, there are totally 25 PDD strains in our laboratory. These strains have significantly different physiological and biochemical characteristics as well as hemolytic activity and drug resistance (Shi et al., 2019). When 108 cfu mL-1 live bacterial suspension was injected into the fish, it can cause the death of experimental fish in 2 days, while no death was observed in the fish injected with non-pathogenic strain. In addition, our previous research has confirmed that PDD is widely distributed along China offshore seawater, posing a potential public health risk to fishermen, offshore workers and tourists. Therefore, in order to control PDD infection in animals and humans, it is necessary to establish a rapid detection method to distinguish these strains with different levels of virulence.
2 Material and Methods 2.1 Experimental StrainsA total of 20 strains belonging to different bacterial species and 25 different strains of PDD were selected in this study. The 20 different bacterial species included three V. rotiferianus, three V. harveyi, two V. anguillarum, two V. splendidus, two V. parahaemolyticus, two V. alginolyticus, one Aliivibrio fischeri, one V. scophthalmi, one V. cyclitrophicus, one P. damselae subsp. piscicida (PDP) and two Escherichia coli. Among them, seven strains of V. rotiferianus, V. harveyi, V. splendidus, V. parahaemolyticus, V. alginolyticus, A. fischeri and E. coli were purchased from China General Microbiological Culture Collection Center (CG-MCC) as standard strains. The remained 13 strains were respectively isolated from different diseased animals in the laboratory of Yellow Sea Fishery Research Institute (YSFRI). More detailed information of these 20 strains was listed in Table 1. The genomic sequences of two strains were detected and submitted to NCBI (Yu et al., 2019; Zhang et al., 2019a).
|
|
Table 1 The 23 bacteria strains for primers specific detection of PDD mcp gene fragment |
All the 25 PDD strains (Fig. 4) were isolated from different diseased marine animals along China coastline. They were all stored in YSFRI. These strains showed significant differences in terms of hemolytic phenotype and pathogenicity to black rockfish (Shi et al., 2019). Two of these strains, Pdd1601 (α-hemolysis) and Pdd1605 (β-hemolysis), had completed whole-genome sequencing data. One of them has been submitted to NCBI (Yu et al., 2019).
2.2 Specific Primer DesignBased on the whole-genome sequencing data of two strains, Pdd1601 and Pdd1605, a highly conserved sequence in mcp gene with a length of 348 bp and a highly conserved sequence in dly gene with a length of 695 bp were respectively selected as the targets to design specific primers. The mcp gene was used to distinguish PDD from other different bacteria, and the dly gene was used to distinguish PDD strains with different pathogenicity. Specific primers were designed through Primer Premier 5.0 software and synthesized in Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). The mcp gene primer sequences were as follows: (5'-3') TGAAATTGCCCAACTGTCCC (forward) and TCACTTACTTGGGCCACATC (reverse). The dly gene primer sequences were as follow: (5'-3') TTTGGACGAGCGGTCCATTT (forward) and GGAG CCCAATCTTGACCAGG (reverse).
2.3 Preparation of StandardsThe 348-bp target fragment in mcp gene was amplified using the designed primers, and the PCR product was ligated to PMD18-T vector, and transformed into DH-5α competent cells. The positive clones were enriched to extract plasmids, which were then digested and identified through sequencing. The correctly recombined plasmid was named Plmcp-Pdd and used as the positive standard in the present study. The copy number of recombinant plasmids was calculated using a previously established method (Raymond et al., 2004). Briefly, the OD260nm was firstly determined to calculate the plasmid concentration. Then the copy number was calculated according to the formula as follows:
| $ {\rm{ Copies }} = \frac{{{\rm{ Plasmid}}\;{\rm{concentration }}\left({{\rm{g}}\;{\rm{ \mathtt{ μ} }}{{\rm{L}}^{ - 1}}} \right) \times 6.02 \times {{10}^{23}}}}{{660 \times {\rm{ Total}}\;{\rm{length}}\;{\rm{of}}\;{\rm{plasmid }}}} $ |
The genomic DNA of bacterial strains was extracted using TIANamp Bacterial DNA kit (TIANGEN Biotech Co., Ltd., Beijing, China). The qRT-PCR was conducted using Realplex model quantitative real-time PCR instrument (Eppendorf Co., Ltd., Hamburg, Germany) in a 20-μL reaction system consisting of 10 μL of 2×SYBR Green Pro Taq HS PREMIX (Takara Biomedical Technology Co., Ltd., Beijing, China), 0.4 μL of 10 μmol L-1 mcp gene forward primer, 0.4 μL of 10 μmol L-1 mcp gene reverse primer, 0.2 μL of 20 μmol L-1 ROX Reference Dye, 2 μL of DNA template and 7 μL of RNase-free water. Briefly, after an initial denaturation step at 95℃ for 30 s, the amplifications were carried out with 40 cycles at a melting temperature of 95℃ for 10 s, an annealing temperature 60℃ for 20 s and an extension temperature of 72℃ for 20 s, followed by a melting curve analysis (95℃ for 15 s, 60℃ for 15 s, 60℃ to 95℃ for 20 min, 95℃ for 15 s).
The prepared standard plasmid Plmcp-Pdd was diluted into different gradient concentrations using 10-fold serial dilution, and a qRT-PCR was performed according to the optimized reaction conditions to draw a standard curve.
2.5 The Primer Specificity and UniversalityThe specificity of designed primers of mcp gene fragment was verified by qRT-PCR under the optimized conditions using the genomic DNA of 23 bacterial strains (Table 1) as the template, and RNase-free water was used as a negative control.
The universality of mcp gene primers was verified using the genomic DNA of 25 PPD strains as the template, and RNase-free water was used as a negative control.
The specificity of designed primers of dly gene fragment was verified by qRT-PCR using the genomic DNA of 25 PPD strains as template, and RNase-free water as a negative control.
2.6 The Sensitivity of mcp Gene PrimersThe standard plasmid Plmcp-Pdd was serially diluted into a concentration gradient consisting of 1.0×101-1.0×107 copies μL-1 using 10-fold serial dilution, which was used as templates. The primer sensitivity was verified by qRT-PCR under the reaction conditions in Section 2.4. Meanwhile, ordinary PCR was carried out to compare the sensitivity for designed primers of mcp gene.
2.7 Establishment of a Microfluidics-Based Real-Time PCR Method for PDD DetectionGENECHECKER UF-150 Ultra-Fast qRT-PCR instrument (CHK Biotech Co., Ltd., Shanghai, China) was used to establish a microfluidics-based qRT-PCR method for PDD detection. The PCR was conducted in a 10-μL reaction system consisting of 5 μL of 2 × ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China), 1 μL of 10 μmol L-1 forward primer, 1 μL of 10 μmol L-1 reverse primer, 2 μL of bacterial DNA template and 1 μL of RNase-free water. Briefly, after an initial denaturation step at 95℃ for 30 s, the amplifications were carried out for 30 cycles with a melting temperature of 95℃ for 5 s, an annealing temperature of 60℃ for 20 s, and an extension temperature of 72℃ for 5 s, followed by melting curve analysis (95℃ for 5 s, 60℃ for 40 s, and 95℃ for 5 s). Fluorescence detection was performed after the qRT-PCR was finished. The amplified products using specific primers of mcp and dly genes in different channels of the chip were required by synchronous detection.
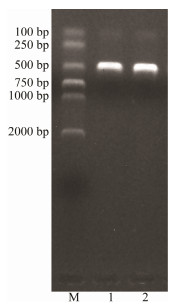
3 Results 3.1 The Recombinant Plasmid Plmcp-PddIn the present study, we successfully amplified the target sequence with 348 bp in length using the Pdd1601 strain DNA as template and mcp-F/R primer pairs. The PCR product was recovered and ligated, and the constructed recombinant standard plasmid Plmcp-Pdd (Fig. 1) was transformed into DH-5α competent cells. The recombinant plasmid was used as the positive standard, and its concentration was detected as 67.70 ng μL-1, which was converted into 2.03×1010 copies μL-1.
|
Fig. 1 Verification of ligation and transformation of PDD mcp gene target fragment with electrophoresis. M, DNA molecular quality standards (DL 2000); 1, 2, Enzyme-digested products. |
The standard plasmid Plmcp-Pdd was diluted to 2.03× 102-2.03×108 copies μL-1. Using it as a template, the amplification was performed under the optimized qRT-PCR conditions mentioned in section 2.4. The amplification curve (Fig. 2Ⅰ) and standard curve (Fig. 2Ⅱ) were obtained based on the results, indicating that there was a good linear relationship between the amount of Plmcp-Pdd plasmid copies and Ct value. The formula was y = -3.012x + 36.70, and the correlation coefficient R2 was 0.998.

|
Fig. 2 Amplification curve and standard curve of standard plasmid Plmcp-Pdd. Ⅰ, Amplification curve of different concentrations of plasmid Plmcp-Pdd. A, 2.03×108 copies μL-1; B, 2.03×107 copies μL-1; C, 2.03×106 copies μL-1; D, 2.03×105 copies μL-1; E, 2.03×104 copies μL-1; F, 2.03×103 copies μL-1; G, 2.03×102 copies μL-1. Ⅱ, Standard curve of plasmid Plmcp-Pdd. |
The designed primers for mcp gene were used to amplify the DNA fragments of 23 strains listed in Table 1. The results showed that only three PDD strains were successfully amplified. The target sequence was about 348 bp in length (Fig. 3), confirming the specificity of the designed primers to PDD strains.

|
Fig. 3 Specific detection of the designed primers for PDD mcp gene fragment. M, DNA molecular quality standards (DL 2000); 1, V. rotiferianus (VR 1601); 2, V. rotiferianus (VR 1602); 3, V. rotiferianus (VRST-01); 4, PDD (Pdd0905); 5, PDD (Pdd 1601); 6, PDD (Pdd 1605); 7, V. harveyi (VHST-01); 8, V. harveyi (VH0207); 9, V. harveyi (VH 1809); 10, V. anguillarum (VA1012); 11, V. anguillarum (VA0531); 12, V. splendidus (VSST-01); 13, V. splendidus (VS 1805); 14, V. parahaemolyticus (VPST-01); 15, V. parahaemolyticus (VP0531); 16, V. alginolyticus (VAlST-01); 17, V. alginolyticus (VAl 1811); 18, A. fischeri (AFST-01); 19, V. scophthalmi (VSc0531); 20, V. cyclitrophicus (VC0406); 21, PDP (Pdp 1810); 22, E. coli (EC0701); 23, E. coli (EC ST-01); 24, RNase-free H2O. |
Moreover, qRT-PCR was performed to amplify the plasmid Plmcp-Pdd and the other 10 strains using the designed primers for mcp gene. These 10 strains were chosen from Table 1, including V. rotiferianus (VRST-01), V. harveyi (VHST-01), V. splendidus (VSST-01), V. parahaemolyticus (VPST-01), V. alginolyticus (VAlST-01), A. fischeri (AFST-01), V. anguillarum (VA0531), V. cyclitrophicus (VC0406), PDP (Pdp 1810) and E. coli (ECST-01). The amplification curve was only observed from the plasmid Plmcp-Pdd, while negative results were found in other 10 strains. The results reconfirmed the specificity of the designed primers to PDD mcp gene.
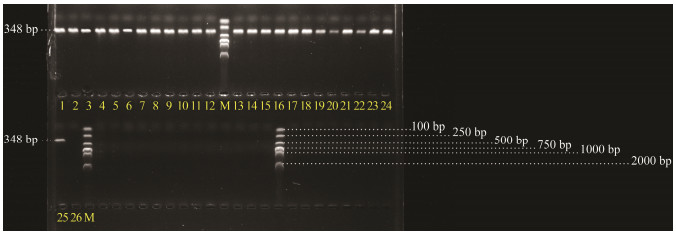
Amplification results of the 25 PDD strains showed that all these strains harbored the target fragment (Fig. 4), demonstrating the good intraspecific universality of the designed mcp gene primers in PDD strains.
|
Fig. 4 Intra-specific universality detection of the designed primers for PDD mcp gene fragment. M, DNA molecular quality standards (DL 2000); 1, Pdd0210; 2, Pdd0905; 3, Pdd0906; 4, Pdd0907; 5, Pdd0908; 6, Pdd0909; 7, Pdd1208; 8, Pdd1308; 9, Pdd 1605; 10, Pdd 1606; 11, Pdd 1607; 12, Pdd 1608; 13, Pdd 1609; 14, Pdd 1611; 15, Pdd 1612; 16, Pdd 1613; 17, Pdd 1614; 18, Pdd 1615; 19, Pdd 1616; 20, Pdd 1617; 21, Pdd 1701; 22, Pdd 1706; 23, Pdd 1807; 24, Pdd 1809; 25, Pdd 1808; 26, RNase-free H2O. |
The 25 PDD strains were amplified with the designed dly gene primers. The results showed that the target fragment of 695 bp in length was only detected in two strains with high hemolytic activity (Fig. 5), confirming the good specificity of the designed primers to PDD dly gene.

|
Fig. 5 Specific detection of the designed primers for PDD dly gene fragment. M, DNA molecular quality standards (DL-2000); 1, Pdd 1605; 2, Pdd 1608; 3, Pdd0210; 4, Pdd- 0905; 5, Pdd0906; 6, Pdd0907; 7, Pdd0908; 8, Pdd0909; 9, Pdd1208; 10, Pdd1308; 11, Pdd 1606; 12, Pdd 1607; 13, Pdd 1609; 14, Pdd 1611; 15, Pdd 1612; 16, Pdd 1613; 17, Pdd 1614; 18, Pdd 1615; 19, Pdd 1616; 20, Pdd 1617; 21, Pdd 1701; 22, Pdd 1706; 23, Pdd 1807; 24, Pdd 1809; 25, Pdd 1808; 26, RNase-free H2O. |
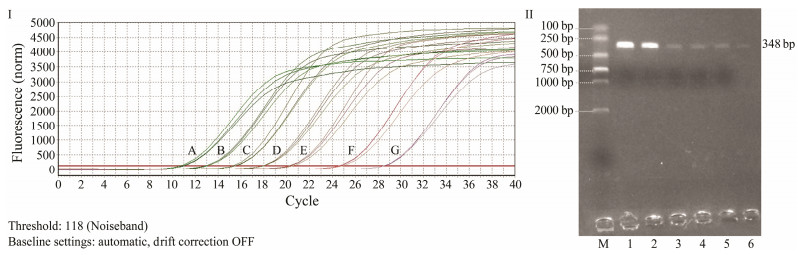
Comparing the results of qRT-PCR and ordinary PCR amplifications using the designed mcp gene primers showed that the detection limit of qRT-PCR was as low as 1.0× 101 copies μL-1, while it was 1.0×104 copies μL-1 for ordinary PCR (Fig. 6). The sensitivity of qRT-PCR was 1000 times higher compared with the ordinary PCR.
|
Fig. 6 Detection sensitivity comparison of qRT-PCR and ordinary PCR using the primers of mcp gene. I, Amplification curve of qRT-PCR; Ⅱ, Gel detection of ordinary PCR; A, 1.0×107 copies μL-1; B, 1.0×106 copies μL-1; C, 1.0×105 copies μL-1; E, 1.0×104 copies μL-1; E, 1.0×103 copies μL-1; F, 1.0×102 copies μL-1; G, 1.0×101 copies μL-1; M, DNA molecular quality standards (DL 2000); 1, 1.0×105 copies μL-1; 2, 1.0×105 copies μL-1; 3, 1.0×103 copies μL-1; 4, 1.0×102 copies μL-1; 5, 1.0×101 copies μL-1; 6, RNase-free H2O. |
The microfluidics-based ultra-fast PCR method for PDD detection was established using the specific primers of mcp gene and dly gene under the optimized reaction conditions, and GENECHECKER UF-150 was used as the instrument platform. Into two channels in the chip, mcp gene primers and dly gene primers added respectively. After the PCR amplification, detection results were visually judged through fluorescence signal. If fluorescence signal simultaneously appeared in both two channels, the test object could be confirmed as the highly pathogenic PDD strain. If fluorescence signal appeared in the channel with mcp gene primers and no fluorescence signal was detected in the channel with dly gene primers, the test object could be confirmed as the low or non-pathogenic PDD strain. If no fluorescence signal was detected in both two channels, or if fluorescence signal was only detected in the channel with dly gene primers, the test object was not a PDD strain (Fig. 7). The reaction time of qRT-PCR was reduced to about 17 min. To obtain the Ct value in the amplification curve based on the standard curve, the quantitative detection of PDD strains was performed. The lowest limit detection was 1.0×101 copies μL-1, with extremely high sensitivity. Due to the outstanding portability of the instrument, this method could be used for field testing.

|
Fig. 7 Detection results of different bacterial strains using GENECHECKER UF-150 qRT-PCR instrument. The chip has 10 reaction channels, of which the 1st, 3rd, 5th and 7th channels are added with mcp gene primers, the 2nd, 4th, 6th and 8th channels are added with dly gene primers, and the 9th and 10th channels are added with RNase-free H2O. In chip I, the 1st, 2nd, 3rd, 5th and 7th channels appear fluorescence, 1st and 2nd channels are highly pathogenic PDD strain (Pdd 1605), 3rd and 4th channels are lowly pathogenic PDD strain (Pdd0906), 5th and 6th channels are non-pathogenic PDD strain (Pdd0909), 7th and 8th channels are lowly pathogenic PDD strain (Pdd0210), 9th and 10th channels are RNase-free H2O. In chip Ⅱ, the 1st, 2nd, 3rd, 5th and 7th channel appear fluorescence, 1st and 2nd channels are highly pathogenic PDD strain (Pdd 1608), 3th and 4th channels are lowly pathogenic PDD strain (Pdd1208), 5th and 6th channels are lowly pathogenic PDD strain (Pdd 1611), 7th and 8th channels are lowly pathogenic PDD strain (Pdd 1616), 9th and 10th channels are RNase-free H2O. In chip Ⅲ, the 1st, 2nd and 3rd channel appear fluorescence, 1st and 2nd channels are highly pathogenic PDD strain (Pdd 1605), 3rd and 4th channels are lowly pathogenic PDD strain (Pdd 1809), 5th and 6th channels are PDP (Pdp 1810), 7th and 8th channels are V. parahaemolyticus (VPST-01), and 9th and 10th channels are RNase-free H2O. |
As a pathogenic bacterial strain, which is widely distributed in the marine environment worldwide, PDD is also an important zoonotic pathogen. PDD has no obvious host specificity, and it can infect not only poikilotherm, but also homotherm (Rivas et al., 2013), or even cause acute death of humans (Yamane et al., 2004). Sufficient attention has to be paid to this pathogen. From the existing studies, there are changeable phenotypes among different PDD strains. They show diversities in pathogenicity, hemolysis, antibiotic resistance, physiological and biochemical characteristics (Takahashi et al., 2008). Environmental stress factors, such as temperature and salinity, can also affect the virulence of PDD strains (Vences et al., 2017; Matanza et al., 2018). Our previous research has also confirmed that the strains isolated from the same mariculture environment have significantly different pathogenicities to black rockfish, S. schlegeli. Therefore, it is important to quickly detect the PDD strains with different pathogenicities to prevent and control the infections of such bacteria.
The classical method of bacterial detection usually uses 16S rDNA gene (Cole et al., 2009) and gyrB gene (Sun et al., 2019). Many bacterial PCR-based classification and identification methods have been successfully established according to these genes. However, it is difficult to accurately distinguish PDD species due to the extremely high homology of Vibrionaceae 16S rDNA. For example, the homology between V.cholerae and V. mimicus is 99.6%, while it is 99.8% between V. alginolyticus and V. parahaemolyticus (Wen et al., 2009). The gyrB gene also needs to cooperate with other genes to achieve higher inter-species discrimination (Teh et al., 2010). The highly virulent plasmid pPHDD1 encoding damselysin (Dly) and phobalysin P (PhlyP), two key virulence factors of highly virulent PDD strains, has been reported in 2011 (Rivas et al., 2011). Moreover, the third hemolysin encoding gene of hlyAch located on PDD chromosome has been found in 2013 (Rivas et al., 2013). From the existing researches, dly and hlyApl on the plasmid pPHDD1 and hlyAch on the chromosome constitute the virulence system of PDD together. Their synergy and participation of some functional genes create the high pathogenicity and strong hemolytic activity. The absence or silence of key virulence factors can cause a significant decrease of pathogens' virulence (Rivas et al., 2013; Rivas et al., 2015; Luo et al., 2019). It offers a new possible method for distinguishing PDD strains with different virulence through detecting the existence of virulence factors or functional genes.
In the present study, we collected 25 different PDD strains along the coast of China. These strains exhibited significant differences in pathogenicity to black rockfish. We completed the whole-genome sequencing of two strains, which displayed notable difference in hemolytic phenotype and pathogenicity. From the results, both dly gene on plasmid and mcp gene on chromosome were selected together as target sequences for PDD detection. The mcp gene was employed to distinguish PDD from other different bacteria, while the dly gene was used to identify PDD strains with high pathogenicity. It has been confirmed that Dly is one of the most important virulence factors encoded by plasmid pPHDD1, which can vanish with the loss of the plasmid. Methyl-accepting chemotaxis proteins (Mcps) are the most common receptors widely found in bacteria and archaea. They not only participate in various physiological activities of cells, but also play an important role in pathogenicity of many bacteria (Ud-Din et al., 2017). Gene mcp is usually considered to be a housekeeping gene, while its sequence greatly varies in different species. Through genome-wide analysis, we screened out the mcp gene of PDD and found that this gene was ubiquitous in PDD strains with excellent specificity. It can be employed to distinguish PDD from other strains, including subsp. piscicida, and can be used as a target gene for PDD detection. Moreover, such gene might also be a potential indicator to identify the two subspecies of P. damselae.
Notably, the two strains that were detected to harbor dly gene showed strong hemolytic activity on sheep blood plate and high pathogenicity to black rockfish, whereas the other stains without dly gene exhibited weak hemolytic ability and low or non-pathogenicity. From these results, we speculated that dly gene is directly associated with the strong hemolytic activity and high pathogenicity of PDD. The sequence of dly gene in this study was derived from a megaplasmid (374 kb) of a highly virulent PDD strain, which was much larger than the 153-kb virulent plasmid pPHDD1. This finding confirmed the existence of other highly virulent plasmids in addition to pPHDD1 in PDD.
Another aim of this study was to establish a microfluidics-based qRT-PCR technique for the detection of PDD strains. As an emerging technology of molecular biology, microfluidics-based PCR, with the advantages of shorttime consumption and low cost, has been widely used in medical application and research areas (De Paz et al., 2014; Park et al., 2011). We successfully established a microfluidics-based qRT-PCR method for PDD detection through specific primers. The test results can also be qualitatively judged by visualization. Quantitative detection would be achieved through the standard curve calculation. The minimum detection limit was as low as 1.0×101 copies μL-1, and the detection time was shortened to less than 20 min. This method has the feasibility of on-site detection, which is suitable to develop fast and accurate PDD detection for field research.
AcknowledgementsThis work was supported by the National Key Research and Development Program of China (No. 2019YFD0900 104), and the Key Projects of Science and Technology Innovation of Shandong Province (No. 2018YFJH0703).
Alhemairi, M., Alghanmi, F. and Alshamrani, A. S., 2015. Child death due to infection with Photobacterium damselae subs. damselae, a new case. Journal of Medical Sciences, 23(3): 176-178. (  0) 0) |
Cheng, G., Hussain, T., Sabir, N., Ni, J., Li, M., Zhao, D. and Zhou, X., 2019. Comparative study of the molecular basis of pathogenicity of M. bovis strains in a mouse model. International Journal of Molecular Sciences, 20(1): 5. (  0) 0) |
Cole, J. R., Wang, Q., Cardenas, E., Fish, J., Chai, B., Farris, R. J., Kulam-Syed-Mohideen, A. S., McGarrell, D. M., Marsh, T., Garrity, G. M. and Tiedje, J. M., 2009. The ribosomal database project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Research, 37(Sup l): D141-D145. (  0) 0) |
Cutter, D. L. and Kreger, A. S., 1990. Cloning and expression of the damselysin gene from Vibrio damsela. Infection and Immunity, 58(1): 266-268. DOI:10.1128/IAI.58.1.266-268.1990 (  0) 0) |
De Paz, H. D., Brotons, P. and Muñoz-Almagro, C., 2014. Molecular isothermal techniques for combating infectious diseases: Towards low-cost point-of-care diagnostics. Expert Review of Molecular Diagnostics, 14(7): 827-843. DOI:10.1586/14737159.2014.940319 (  0) 0) |
Fujioka, R. S., Greco, S. B., Cates, M. B. and Schroeder, J. P., 1988. Vibrio damsela from wounds in bottlenose dolphins Tursiops truncatus. Diseases of Aquatic Organisms, 4(1): 1-8. (  0) 0) |
Hanlon, R. T., Forsythe, J. W., Cooper, K. M., Dinuzzo, A. R., Folse, D. S. and Kelly, M. T., 1984. Fatal penetrating skin ulcers in laboratory-reared octopuses. Journal of Invertebrate Pathology, 44(1): 67-83. DOI:10.1016/0022-2011(84)90047-8 (  0) 0) |
Ketterer, P. J. and Eaves, L. E., 1992. Deaths in captive eels (Anguila reinhardtii) due to Photobacterium (Vibrio) damsela. Australian Veterinary Journal, 69(8): 203-204. DOI:10.1111/j.1751-0813.1992.tb07528.x (  0) 0) |
Kothary, M. H. and Kreger, A. S., 1985. Purification and characterization of an extracellular cytolysin produced by Vibrio damsela. Infection and Immunity, 49(1): 25-31. DOI:10.1128/IAI.49.1.25-31.1985 (  0) 0) |
Kreger, A. S., Bernheimer, A. W., Etkin, L. A. and Daniel, L. W., 1987. Phospholipase D activity of Vibrio damsela cytolysin and its interaction with sheep erythrocytes. Infection and Immunity, 55(12): 3209-3212. DOI:10.1128/IAI.55.12.3209-3212.1987 (  0) 0) |
Labella, A., Vida, M., Alonso, M. C., Infante, C., Cardenas, S., Lopez-Romalde, S., Manchado, M. and Borrego, J. J., 2006. First isolation of Photobacterium damselae ssp. damselae from cultured redbanded seabream, Pagrus auriga Valenciennes, in Spain. Journal of Fish Diseases, 29(3): 175-179. DOI:10.1111/j.1365-2761.2006.00697.x (  0) 0) |
Lee, K., Kim, H. K., Sohn, H., Cho, Y., Choi, Y. M., Jeong, D. G. and Kim, J. H., 2018. Genomic insights into Photobacterium damselae subsp. damselae strain KC-Na-1, isolated from the finless porpoise (Neophocaena asiaeorientalis). Marine Genomics, 37: 26-30. DOI:10.1016/j.margen.2017.09.004 (  0) 0) |
Love, M., Teebken-Fisher, D., Hose, J. E., Farmer, J. J., Hickman, F. W. and Fanning, G. R., 1981. Vibrio damsela, a marine bacterium, causes skin ulcers on the damselfish Chromis punctipinnis. Science, 214(4525): 1139-1140. DOI:10.1126/science.214.4525.1139 (  0) 0) |
Lozano-León, A., Osorio, C. R., Nuñez, S., Martínez-Urtaza, J. and Magariños, B., 2003. Occurrence of Photobacterium damselae subsp. damselae in bivavlve molluscs from Northwest Spain. Bulletin of the European Association of Fish Pathologists, 23(1): 40-44. (  0) 0) |
Luo, G., Xu, X., Zhao, L., Qin, Y., Huang, L., Su, Y. and Yan, Q., 2019. clpV is a key virulence gene during in vivo Pseudomonas plecoglossicida infection. Journal of Fish Diseases, 42(7): 991-1000. (  0) 0) |
Matanza, X. M. and Osorio, C. R., 2018. Transcriptome changes in response to temperature in the fish pathogen Photobacterium damselae subsp. damselae: Clues to understand the emergence of disease outbreaks at increased seawater temperatures. PLoS One, 13(12): e0210118. DOI:10.1371/journal.pone.0210118 (  0) 0) |
Morris, J. G., Wilson, R., Hollis, D. G., Weaver, R. E., Miller, H. G., Tacket, C. O., Hickman, F. W. and Blake, P. A., 1982. Illness caused by Vibrio damsela and Vibrio hollisae. The Lancet, 319(8284): 1294-1297. DOI:10.1016/S0140-6736(82)92853-7 (  0) 0) |
Obendorf, D. L., Carson, J. and McManus, T. J., 1987. Vibrio damsela infection in a stranded leatherback turtle (Dermochelys coriacea). Journal of Wildlife Diseases, 23(4): 666-668. DOI:10.7589/0090-3558-23.4.666 (  0) 0) |
Park, S., Zhang, Y., Lin, S., Wang, T. H. and Yang, S., 2011. Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnology Advances, 29(6): 830-839. DOI:10.1016/j.biotechadv.2011.06.017 (  0) 0) |
Pedersen, K., Dalsgaard, I. and Larsen, J. L., 1997. Vibrio damsela associated with diseased fish in Denmark. Applied and Environmental Microbiology, 63(9): 3711-3715. DOI:10.1128/AEM.63.9.3711-3715.1997 (  0) 0) |
Perez-Tirse, J., Levine, J. F. and Mecca, M., 1993. Vibrio damsela: A cause of fulminant septicemia. Archives of Internal Medicine, 153(15): 1838-1840. DOI:10.1001/archinte.1993.00410150128012 (  0) 0) |
Raymond, C. R. and Wilkie, B. N., 2004. Th-1/Th-2 type cytokine profiles of pig T-cells cultured with antigen-treated monocyte-derived dendritic cells. Vaccine, 22(8): 1016-1023. DOI:10.1016/j.vaccine.2003.08.026 (  0) 0) |
Rivas, A. J., Balado, M., Lemos, M. L. and Osorio, C. R., 2011. The Photobacterium damselae subsp. damselae hemolysins damselysin and HlyA are encoded within a new virulence plasmid. Infection and Immunity, 79(11): 4617-4627. DOI:10.1128/IAI.05436-11 (  0) 0) |
Rivas, A. J., Balado, M., Lemos, M. L. and Osorio, C. R., 2013a. Synergistic and additive effects of chromosomal and plasmidencoded hemolysins contribute to hemolysis and virulence in Photobacterium damselae subsp. damselae. Infection and Immunity, 81(9): 3287-3299. DOI:10.1128/IAI.00155-13 (  0) 0) |
Rivas, A. J., Lemos, M. L. and Osorio, C. R., 2013b. Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Frontiers in Microbiology, 4: Article 283. (  0) 0) |
Rivas, A. J., Vences, A., Husmann, M., Lemos, M. L. and Osorio, C. R., 2015. Photobacterium damselae subsp. damselae major virulence factors Dly, plasmid-encoded HlyA, and chromosome-encoded HlyA are secreted via the Type Ⅱ scretion system. Infection and Immunity, 83(4): 1246-1256. DOI:10.1128/IAI.02608-14 (  0) 0) |
Rivas, A. J., Von Hoven, G., Neukirch, C., Meyenburg, M., Qin, Q., Füser, S., Boller, K., Lemos, M. L., Osorio, C. R. and Husmann, M., 2015. Phobalysin, a small β-pore-forming toxin of Photobacterium damselae subsp. damselae. Infection and Immunity, 83(11): 4335-4348. DOI:10.1128/IAI.00277-15 (  0) 0) |
Saroj, S. D., Shashidhar, R., Karani, M. and Bandekar, J. R., 2008. Distribution of Salmonella pathogenicity island (SPI)-8 and SPI-10 among different serotypes of Salmonella. Journal of Medical Microbiology, 57(4): 424-427. DOI:10.1099/jmm.0.47630-0 (  0) 0) |
Shi, L. N., Yu, Y. X., Jiang, Y., Zhang, Z., Wang, Y. G., Liao, M. J. and Rong, X. J., 2019. Studies on the phenotypic differences of different Photobacterium damselae subsp. damselae strains. Marine Sciences, 43(6): 15-24 (in Chinese with English abstract). (  0) 0) |
Song, Y. L., Cheng, W. and Wang, C. H., 1993. Isolation and characterization of Vibrio damsela, infectious for cultured shrimp in Taiwan. Journal of Invertebrate Pathology, 61(1): 24-31. DOI:10.1006/jipa.1993.1005 (  0) 0) |
Sun, Y., Zhuang, Z., Wang, X., Huang, H., Fu, Q. and Yan, Q., 2019. Dual RNA-seq reveals the effect of flgM gene of Pseudomonas plecoglossicida on immune response of Epinephelus coioides. Fish & Shellfish Immunology, 87: 515-523. (  0) 0) |
Takahashi, H., Miya, S., Kimura, B., Yamane, K., Arakawa, Y. and Fujii, T., 2008. Difference of genotypic and phenotypic characteristics and pathogenicity potential of Photobacterium damselae subsp. damselae between clinical and environmental isolates from Japan. Microbial Pathogenesis, 45(2): 50-158. (  0) 0) |
Terceti, M. S., Ogut, H. and Osorio, C. R., 2016. Photobacterium damselae subsp. damselae, an emerging fish pathogen in the Black Sea: Evidence of a multiclonal origin. Applied and Environmental Microbiology, 82(13): 3736-3745. DOI:10.1128/AEM.00781-16 (  0) 0) |
Terceti, M. S., Vences, A., Matanza, X. M., Dalsgaard, I., Pedersen, K. and Osorio, C. R., 2018. Molecular epidemiology of Photobacterium damselae subsp. damselae outbreaks in marine rainbow trout farms reveals extensive horizontal gene transfer and high genetic diversity. Frontiers in Microbiology, 9: Article 2155. DOI:10.3389/fmicb.2018.02155 (  0) 0) |
The, C. S. J., Chua, K. H. and Thong, K. L., 2010. Simultaneous differential detection of human pathogenic and nonpathogenic Vibrio species using a multiplex PCR based on gyrB and pntA genes. Journal of Applied Microbiology, 108(6): 1940-1945. (  0) 0) |
Ud-Din, A. I. M. S. and Roujeinikova, A., 2017. Methyl-accepting chemotaxis proteins: A core sensing element in prokaryotes and archaea. Cellular and Molecular Life Sciences, 74(18): 3293-3303. DOI:10.1007/s00018-017-2514-0 (  0) 0) |
Vaseeharan, B., Sundararaj, S., Murugan, T. and Chen, J. C., 2007. Photobacterium damselae ssp. damselae associated with diseased black tiger shrimp Penaeus monodon Fabricius in India. Letters in Applied Microbiology, 45(1): 82-86. DOI:10.1111/j.1472-765X.2007.02139.x (  0) 0) |
Vences, A., Rivas, A. J., Lemos, M. L., Husmann, M. and Osorioa, C. R., 2017. Chromosome-encoded hemolysin, phospholipase, and collagenase in plasmidless isolates of Photobacterium damselae subsp. damselae contribute to virulence for fish. Applied and Environmental Microbiology, 83(11): e00401-17. (  0) 0) |
Wen, W. Y., Xie, Z. Y., Xu, X. D., Zhang, X. Z., Zhang, S. X. and Zhou, Y. C., 2009. Establishment of a rapid PCR detection method for Vibrio fluvialis based on toxR gene. Fisheries Science, 28(10): 575-578 (in Chinese with English abstract). (  0) 0) |
Yamane, K., Asato, J., Kawade, N., Takahashi, H., Kimura, B. and Arakawa, Y., 2004. Two cases of fatal necrotizing fasciitis caused by Photobacterium damsela in Japan. Journal of Clinical Microbiology, 42(3): 1370-1372. DOI:10.1128/JCM.42.3.1370-1372.2004 (  0) 0) |
Yan, N., Zhang, Z. Q., Wu, T. L., Zhu, J. X., Fu, Y. F., Han, H. S., Wang, H. B., Shi, Q. M. and Gao, G. S., 2018. Isolation and identification of Photobacterium damselae subsp. damselae (PDD) from Tongue Sole. Animal Husbandry and Feed Science, 10(2): 99-114. (  0) 0) |
Yu, Y. X., Zhang, Z., Wang, Y. G., Liao, M. J., Rong, X. J., Li, B., Wang, K., Chen, J. and Zhang, H., 2019. Complete genome sequence of Photobacterium damselae subsp. damselae strain SSPD1601 isolated from deep-sea cage-cultured Sebastes schlegelii with septic skin ulcer. International Journal of Genomics, 2019: Article 4242653. (  0) 0) |
Yuen, K. Y., Ma, L., Wong, S. S. Y. and Ng, W. F., 1993. Fatal necrotizing fasciitis due to Vibrio damsel. Scandinavian Journal of Infectious Diseases, 25(5): 659-661. DOI:10.3109/00365549309008557 (  0) 0) |
Zhang, X. J., Qin, G. M., Chen, C. Z., Fang, G. and Yan, B. L., 2009. Biological characterization and phylogenetic analysis of Photobacterium damselae subsp. damselae from diseased Epinephelus lanceolatus L.. Progress in Fishery Science, 30(3): 38-43 (in Chinese with English abstract). (  0) 0) |
Zhang, Z., Yu, Y. X., Jiang, Y., Wang, Y. G., Liao, M. J., Rong, X. J., Wang, K., Zhang, H. and Chen, J., 2019a. First report of isolation and complete genome of Vibrio rotiferianus strain SSVR1601 from cage-cultured black rockfish (Sebastes schlegelii) associated with skin ulcer. Journal of Fish Diseases, 42(5): 623-630. DOI:10.1111/jfd.12963 (  0) 0) |
Zhang, Z., Yu, Y. X., Wang, K., Wang, Y. G., Jiang, Y., Liao, M. J. and Rong, X. J., 2019b. First report of skin ulceration caused by Photobacterium damselae subsp. damselae in net-cage cultured black rockfish (Sebastes schlegeli). Aquaculture, 503: 1-7. DOI:10.1016/j.aquaculture.2018.12.088 (  0) 0) |
 2021, Vol. 20
2021, Vol. 20


