Vibriosis is a common deadly hemorrhagic septicemic disease of marine and freshwater fish species, which has led to severe economic losses worldwide (Hyuk and Yue, 2016). Vibrio anguillarum, also known as Listonella anguillarum, is a main pathogen infecting a variety of marine fish and shellfish species (Michael et al., 2018). The virulence mechanisms, detection techniques and prevention strategies of V. anguillarum have been investigated previously (Tang et al., 2016; Khansari et al., 2017); however, V. anguillarum used in these studies have to be long-term preserved. As demonstrated early, the biological activities and characteristics of V. anguillarum change with environment factors and time (Kim et al., 2012; Ma et al., 2017). Therefore, the preservation methods maintaining the original characteristics of pathogen to the maximum extent are highly appreciated.
Continuous sub-culturing and cryopreserving are the common methods of microorganisms preservation. Continuous subculturing is usually used as a short-term preservation method, but it frequently causes contamination. The previous study indicated that, the pathogenicity and physiological and biochemical characteristics of Aeromonas hydrophila decreased after several sub-culturing, and the virulence factor ahpA of Aeromonas hydrophila was lost (Fu et al., 2011; Li et al., 2015). Therefore, the continuous sub-culturing is only a short-term method of preserving bacterial strains. Cryopreservation is the most common method for pathogen strains preservationr, and the strains usually need to be reactivated frequently. Both extracellular and intracellular components of most prokaryotes and eukaryotes are often damaged during freezing (Kenan et al., 2011; Fernanda et al., 2016; Julie et al., 2017). In addition, the age and concentration of bacteria, protectants and other factors should be considered (Jason et al., 2017).
Freeze-drying is a dehydrating process (Tasdemir et al., 2013; Chotiko and Sathivel, 2014; Miriam et al., 2014). After freeze-drying, the cells were fixed in powder and the cellular physiological metabolic activity tends to be stopped. Thus, this method is suitable for preserving a vast majority of microorganisms. During freeze-drying, cells have to survive a series of stimulations. A number of early studies demonstrated that bacterial species, damage mechanism, cell concentration, freeze-drying protectants, freeze-drying parameters, storage conditions, and others affect freeze-drying performance, but the most important factor is the protectants (Yang et al., 2007; Weng et al., 2017). The optimal protectant medium can improve the survival and storage stability of cells after freeze-drying (Chotiko and Sathivel, 2014; Wang et al., 2015).
In this study, the physiological and biochemical indexes and antibiotics resistance of V. anguillarum were analyzed after continuous sub-culturing, cryopreserving and freeze-drying, respectively. We also optimized the freeze-drying protectant for V. anguillarum. The results will provide references for the physiological and biochemical analysis and immunoprophylaxis of V. anguillarum, and the preservation method for marine microorganisms.
2 Materials and Methods 2.1 Bacteria RejuvenationV. anguillarum was identified and preserved in our laboratory, which can cause fin rot in cultured turbot (Scophthalmus maximus) (Zhang, 2004). The strain was inoculated on trypticase soy broth (TSB) medium for activation, and single colony was cultivated in 200 mL liquid TSB for 18 h. The culture was centrifuged and resuspended in 1.5% of NaCl to l08 CFU mL−1.
Turbot approximately 4-6 cm in body length and 9–12 g in body weight were obtained from a breeding farm in Weihai (Shandong, China). The fish were approved to be free of V. anguillarum infection. Fifteen fish were kept in an aquarium with 0.3 m3 seawater. The water and room temperatures varying between 16 and 19℃ and between 13 and 16℃, respectively. Fish were fed basal diet twice a day, and intramuscularly injected with 0.1 mL of 108 CFU mL−1 V. anguillarum per fish after two days acclimatization. For the control, 1.5% of NaCl was injected. When fin rot disease appear, V. anguillarum was isolated and injected with 107 CFU mL−1 and 106 CFU mL−1 as the second and third rejuvenations. Finally, V. anguillarum was isolated as the rejuvenated strain.
2.2 Continuous Passage TreatmentThe rejuvenated strain was inoculated on solid TSB for subculture. TSB medium was sterilized and cooled down to 60℃ for preparing the solid medium. Each plate contained 20 mL of medium and condensed for 30 min. The plates were pre-incubated at 28℃ for 1 h ahead of transferring. The interval each generation was 24 h, and the bacterial colony was between 0.8–1.0 mm in diameter. The 2nd (marked as primary 1) and 11th (marked as primary 11) generations of V. anguillarum were collected for further analysis.
2.3 Cryopreservation TreatmentThe rejuvenated V. anguillarum strains was re-suspended in 20% of glycerol to l08 CFU mL−1. They were kept in cryogenic vials with 1.5 mL volume and frozen at −80℃. The frozen cells were melted at room temperature for 2 h every 4 days to simulate the freeze-thaw phenomena. Two times later, the strain was activated and cultured with continuous passage method as described above. The 1st (marked as frozen 1) and 11th (marked as frozen 11) generations (corresponding to the second and eleventh generation in continuous passage treatment) was selected for the physicochemical property analysis.
2.4 Freeze-DryingTwelve protectants at different concentrations were tested. Skim milk and glycerol were set at 5 different concentrations, and the other 10 protectants were set at 6 concentrations. The protectants were dissolved in 1.5% NaCl. Skim milk was boiled to sterilize while others were sterilized at 106℃ for 30 min. The sterile rabbit serum was bought from Pingrui Biotechnology Co., Ltd. Freeze-drying and survival rate detection were done as previous described (Yu et al., 2017).
The freeze-dried cells in glycerol only or protectants contained glycerol were colloid or pasty, thus not conducive to long-term preservation. Accordingly, skim milk, serum, mannitol, sodium citrate, lactose and trehalose in 1.5% NaCl were chosen to precede orthogonal combination experiment. All the protectants were set at 4 different concentrations, and the L2546 orthogonal array was used (Table 1). Protectants before adding serum were sterilized at 106℃ for 30 min. Freeze-drying was carried out and the optimal combination of protectants was selected with the methods of Yu et al. (2017). The freeze-dried V. anguillarum was activated with continuous passage as described above, and the 1st (marked as dry 1) and 11th (marked as dry 11) generations were selected for further analysis.
|
|
Table 1 The survival rates of V. anguillarum during orthogonal test |
According to Bergey's manual of determinative bacteriology, 25 kinds of indexes were selected (Table 4). The bacterial biochemical identification tube was obtained from Reagent Company (Qingdao Hope Bio-Technology Co., Ltd.). V. anguillarum was prepared following the manufacturer's instructions.
|
|
Table 4 The results of physiological and biochemical test |
Different generations of V. anguillarum with different treatments were re-suspended in 1.5% NaCl to l07 CFU mL−1. The antibiotics resistance was detected by means of disc diffusion test (K-B method). The types and concentrations of antibiotics were listed in Table 5 (Hangzhou Microbial Reagent Co., Ltd.). Each group was replicate twice, and the mean of inhibition zone was used as the final result. The bacterial sensitivity was calculated by referring to the aquatic bacterial susceptibility test standard issued by the American association of clinical laboratory standardization.
|
|
Table 5 The results of drug sensitivity test |
V. anguillarum was cultivated in 200 mL liquid TSB for 18 h firstly. Then it was re-suspended in TSB to prepare high concentrations of bacteria with an absorbance value of about 1.9, and the cell concentration was about (1.0– 1.2)×109 CFU mL−1. Through 10-fold dilution, the bacterial suspension was diluted with TSB to 102–109 CFU mL−1. Then the prepared suspensions were inoculated in a sterile 96-well plates, 200 μL each well, 3 repeats each density. Ten microliters of 1% TTC (2, 3, 5-triphenyltetrazolium chloride) was added as growth indicator. Then the cells were incubated at 28℃ and the color change was observed every 1 h. The preliminary screening of the inoculation density was analyzed to decide whether it was suitable for drug susceptibility test.
After initial screening, three inoculation densities, 1×105, 1×106 and 1×107 CFU mL−1, were selected for further test. The chloramphenicol was selected as the indictor drug. Chloramphenicol solution (160 μg mL−1) was prepared and diluted to 80, 40, 20, 10, 5, 2.5 μg mL−1 in sterile 96-well microliters plate (A1-G6), 20 μL each well. Two parallel experiments were set. The first one with 10 μL TTC was indicator, and the other without TTC was used for OD value reading, and two replicates were set each experiment. Drug negative control wells (H1-H6) and blank control wells (A7-H7) were added the same volume of sterile water. One hundred and seventy microliters of bacterial suspension was added each drug well and the same volume of TSB was added into drug negative control and blank control wells. The samples were incubated at 28℃ and the color changes (contain TTC) were checked every 1.5 h. When the drug negative control turned to pink, the OD value at 595 nm of the TTC-absent plate was read. The bacterial survival rate was calculated by referring to the aquatic bacterial susceptibility test standard issued by the American association of clinical laboratory standardization (M49-A, 2006), and the lowest drug mass concentration within the survival rate ≤ 20% was regarded as the minimal inhibitory concentration (MIC).
2.7.2 MIC testBased on the commonly used drugs in aquaculture and their prohibition directory, eleven drugs at different concentrations were selected, which included chloramphenicol, ofloxacin, rifampicin, ampicillin, polymyxin B, doxycycline, kanamycin, neomycin, enrofloxacin, florfenicol and novobiocin, and the initial concentration was 160, 40, 40, 1280, 1280, 20, 1280, 1280, 20, 160, 80 μg mL−1, respectively (Fig. 1). The drug solutions were sterilized through filtration cross a sterile membrane with 0.22 nm pore size, and 2-fold serial dilution method was used to prepare a gradient of eight concentrations (Fig. 1A1–H11), and each hole contained 20 μL solution. The same volume of sterile water was added into blank control (Fig. 1A12–D12) and growth control wells (Fig. 1E12–H12).
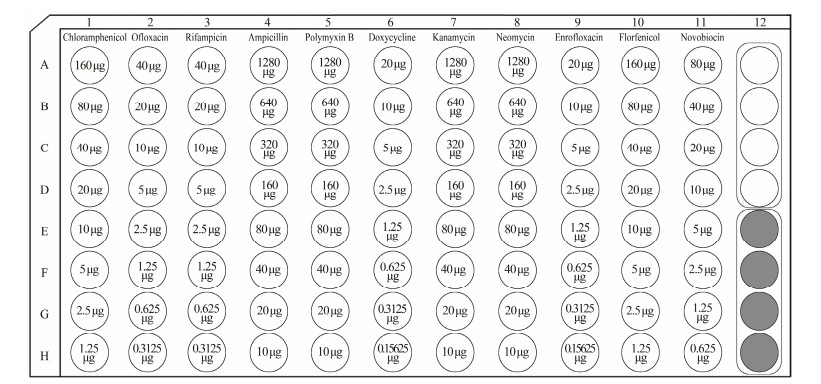
|
Fig. 1 The antibiotic types and concentrations in the drug-susceptibility test plate. 1–11, the types of antibiotics; A–H, the concentrations of antibiotics; A1–H11, the drug susceptibility test area; A12–D12, the blank control area; E12–H12, the growth control area. |
V. anguillarum with different treatments was re-suspended in TSB to (1.0–1.2)×106 CFU mL−1, which was added into testing wells, 170 μL each. The same volume of sterile TSB was added into growth control wells. TTC was employed as indicator. When the color of the growth control wells turned pink, the color changes of the rest wells were recorded. The MIC was the concentration corresponding to the colorless well which is adjacent to the pink well each column.
3 Results 3.1 Freeze-Drying ProtectantWith different protectants, the tendency of cell survival rate of V. anguillarum after freeze-drying changed with the type and concentration of protectants. The survival rate of V. anguillarum steadily reached the maximum and then declined. For skim milk, mannitol, fructose, trehalose, maltose, lactose, sodium citrate and glycerol in the selected concentration, the highest cell survival rate was 1.48%, 2.37%, 0.38%, 0.92%, 0.82%, 2.96%, 2.49% and 2.62%, and the corresponding protectant concentration was 20.0%, 10.0%, 5.0%, 10.0%, 7.5%, 7.5%, 5.0% and 10.0%, respectively (Fig. 2). For other four protectants, two peaks were observed, and the maximum survival rate reached in 15.0% serum, 7.5% PVP, 7.5% glucose and 5.0% sucrose (Fig. 2).
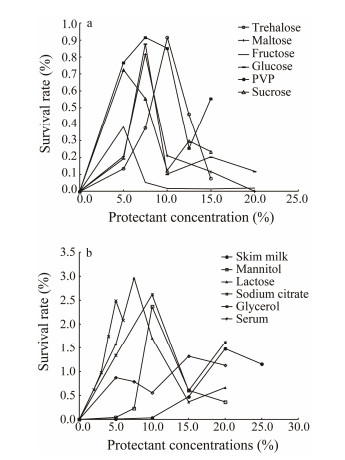
|
Fig. 2 The survival rates of V. anguillarum in different protectants. |
Orthogonal test showed that the protective effect of protectant mixture relied on the type and concentration of protectants. Among 25 tested groups, the survival rate varied between 0.01% and 11.40% (Table 1). The variance analysis indicated that, based on F value, trehalose has the highest impact on the survival rate of V. anguillarum, which was followed by lactose, serum, sodium citrate, skim milk and mannitol (Table 2). Based on the mean and significance analysis, the optimal protectant mixture for V. anguillarum was 8% trehalose (level 4), 12% skim milk (level 4), 8.0% lactose (level 4), 2.0% sodium citrate (level 3), 12.0% serum (level 4) and 8.0% mannitol (level 3) (Table 3). The verification test showed that the survival rate of V. anguillarum was more than 23.1%.
|
|
Table 2 The orthogonal analyses of different protectants |
|
|
Table 3 The mean and significance analyses of different protectants |
There were only a few changes in the physiological and biochemical indexes between the different treatments of V. anguillarum (Table 4). Compared with the primary 1, the mannitol metabolic and ornithine decarboxylase only changed in dry 1, and this change can restore to the initial by continuous passage. The urease reaction of V. anguillarum was transformed under three kinds of treatments, but the change for freeze-drying treatment can restored to the initial (same as primary 1) by continuous passage. The xylose and lysine decarboxylase activities merely changed in frozen 11 and primary 11, respectively.
3.3 Antibiotics Resistance PhenotypingThe antibiotics resistance phenotyping showed that the sensitivity of V. anguillarum to rifampicin (S to I), erythromycin (I to R), furazolidone (S to I), gentamicin (I to R), doxycycline (S to I) and co-trimoxazole (I to R) decreased after continuous passage. For freeze-drying, the sensitivity to erythromycin (I to R), furazolidone (S to I), gentamicin (I to R) and co-trimoxazole (I to R) showed a decreasing trend, and the sensitivity to ampicillin (R to I) increased. The sensitivity to co-trimoxazole (I to R) decreased after cryopreservation. For dry 11, the sensitivity to furazolidone (S to I), ceftazidime (S to I) and co-trimoxazole (I to R) decreased and the sensitivity to azithromycin (I to S) increased. For frozen 11, the sensitivity to furazolidone (S to I), lomefloxacin (S to I), gentamicin (I to R), azithromycin (I to R), co-trimoxazole (I to R) and cefoperazone (S to I) reduced (Table 5). Compared dry 11 and frozen 11 with primary 11, the sensitivity to rifampin (I to S), erythromycin (R to I), gentamicin (R to I), azithromycin (I to S) and doxycycline (I to S) increased and the sensitivity to ceftazidime (S to I) decreased for dry 11. The sensitivity to lomefloxacin (S to I), azithromycin (I to R) and cefoperazone (S to I) reduced while the sensitivity to rifampin (I to S), erythromycin (R to I) and doxycycline (I to S) increased for frozen 11 (Table 5). Therefore, compared with the original strains, the freeze-dried strains maintained the initial drug sensitivity of V. anguillarum to a greatest extent.
3.4 Determination of V. anguillarum Inoculation Concentration for MIC TestUnder TTC indicator, the color of the 102–109 CFU mL−1 groups turning from colorless to pink at 19 h, 16 h, 13 h, 10 h, 8 h, 6 h, 1 h and 0 h after inoculation, respectively. Based on the time span and the effects of drug, 1×105, 1×106 and 1×107 CFU mL−1 were selected for further tests.
For TTC tested, the minimal inhibitory concentration for 1×105, 1×106 and 1×107 CFU mL−1 was 5 μg mL−1, 5 μg mL−1 and 10 μg mL−1, respectively (Fig. 3). Furthermore, based on OD value, the minimal inhibitory concentration for 1×107 CFU mL−1 was 20 μg mL−1, and there was a certain deviation with the TTC color reaction (Table 6). In the test groups of 1×105 CFU mL−1 and 1×106 CFU mL−1, the minimal inhibitory concentration was 5 μg mL−1, which was consistent with the color reaction (Table 6). Because the time span of 1×105 CFU mL−1 was too long, 1×106 CFU mL−1 was selected for further test in order to ensure the intuitive and accuracy of the drug susceptibility results.
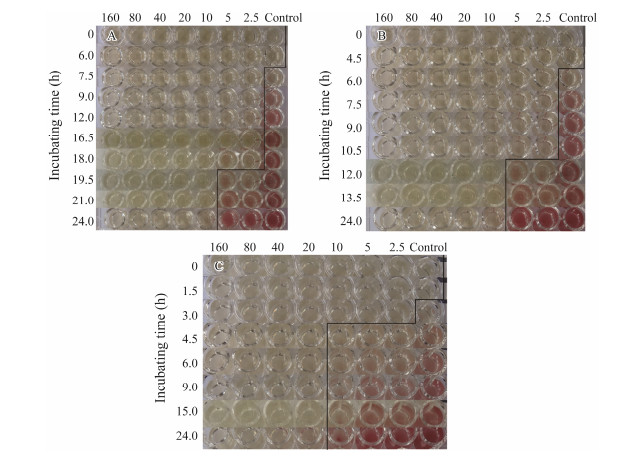
|
Fig. 3 The sensitivities of V. anguillarum to chloramphenicol with different concentrations. (A), 1×105 CFU mL−1; (B), 1×106 CFU mL−1; (C), 1×107 CFU mL−1. |
|
|
Table 6 The survival rates of V. anguillarum under different concentrations of chloramphenicol based on OD value |
The results of the colorimetric detection were shown in Fig. 4. Based on the TTC colorimetric detection and the distribution of drugs (Fig. 4), the MIC results of antibiotics were listed in Table 7.
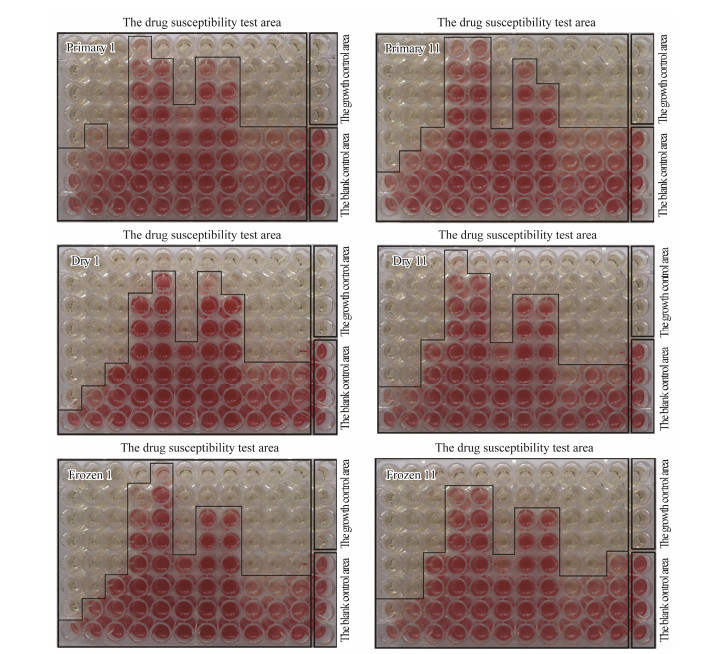
|
Fig. 4 The change of drug susceptibility of V. anguillarum under different treatments. |
|
|
Table 7 The MIC of eleven antibiotics against V. anguillarum under different treatments |
Compared with the primary strains, the MIC of chloramphenicol, ofloxacin, doxycycline and neomycin was decreased after different treatments (Table 7). The MIC of enrofloxacin and florfenicol unchanged after continuous passage, but decreased after freeze-dry and freeze-thaw treatments. For ampicillin, kanamycin and novobiocin, the MIC presented a downward trend after freeze-dry and freeze-thaw, and restored to the original sensitivity to a certain extent after re-activated and passage (except kanamycin). Compared with the primary 1, the MIC of V. anguillarum to rifampicin and polymyxin B was increased after continuous passage and freeze-thaw, but unchanged after freeze-drying.
Furthermore, compared the MIC testing results with those of K-B method, we found that the sensitivities of V. anguillarum to chloramphenicol, polymyxin B, kanamycin, etc. were changed (Table 5). The results further revealed that the sensitivity and accuracy of the disc diffusion method is lower than that of the MIC method.
4 DiscussionThe protective effects of different protectants during freeze-drying may vary for different cells. Trehalose and sodium citrate showed better protective effects than PVP and serum albumin during the freeze-drying of red blood cells (He et al., 2009). The protective effect of PVP on Saccharomyces cerevisiae was lower than that of trehalose and maltose during freeze-drying (Berny et al., 1991; Lodato et al., 1999). PVP offered a similar effect with that of trehalose on V. anguillarum during freeze-drying. The effect of disaccharides (non-reducing sugars) has been widely studied due to their interactions with cell membranes and prevention of intracellular ice-formation (Morgan et al, 2006; Heljo et al., 2011; Mimoza et al., 2014). In this study, the survival rate of V. anguillarum was higher in medium containing polyols or its mixture with other protectants than those containing PVP and saccharides (except lactose). Glucose offered a better protective effect than maltose and sucrose on V. anguillarum during freeze-drying. The results are opposite to that documented early, the protective effect of monosaccharide is lower than that of disaccharide (Abadias et al., 2001; Crowe et al., 2001). Furthermore, Mimoza et al. (2014) indicated that during the freeze-drying of Bifidobacterium infantis, the trehalose showed a better protective effect than lactose did at a concentration of 10.0%. However, when the concentration was 5.0%, the result was reversed. During the freeze-drying of Lactobacillus brevis and Oenococcus oeni, the protection effects were 10% of trehalose, 10.0% of lactose, 10.0% of glucose, 10.0% of fructose and 5.0% of mannitol from high to low (Zhao and Zhang, 2005). The previous studies also indicated that, by means of the statistical analysis or mathematical modeling (such as orthogonal analysis and response surface methodology), the optimal composition of protectant mixture can significantly improve the survival rate of bacteria after freeze-drying (Fatemeh et al., 2015; Zhang et al., 2015). In this study, by means of orthogonal test, the optimal composition of protectant mixture for V. anguillarum during freeze-drying was 8% of trehalose, 12% of skim milk, 8.0% of lactose, 2.0% of sodium citrate, 12.0% of serum and 8.0% of mannitol, and the cell survival rate was higher than 23.1%.
There are many factors influencing the bacterial physiological and biochemical indexes. Ryan et al. (2001) indicated that after cryopreservation and lyophilisation, the viability, secondary metabolite profiles and extracellular enzyme production of Metarhizium anisopliae, Fusarium oxysporum and Serpula lacrymans can be affected. After the cold, acid and ethanol shocks, the levels of saturated fatty acids/unsaturated fatty acids ratio and cyclopropane fatty acids in Oenococcus oeni were up-regulated, which leads to decreased membrane fluidity and better freezedrying viability and malic acid degradation ability (Zhang et al., 2013). The virulence of Streptococcus agalactiae in tilapia decreased significantly after continuous passage in vitro. The biological characteristics of leucine-arylamidase and D-ribose tests changed, and the bacterial strain grew slower and no hemolytic activity could be observed (Li et al., 2015). In this study, the mannitol metabolic, ornithine decarboxylase and urease reaction of V. anguillarum were transformed under different kinds of treatments, but this change for freeze-drying treatment can restored to the initial state by means of continuous passage. After continuous passage in vitro, the lysine decarboxylase test was changed. Moreover, as an important functional component of bacteria, the antibiotic sensitivity plays an important role in the disease prevention and treatment. The previous studies indicated that the drug sensitivity of bacteria can change accordingly with the environmental factors by means of mobile genetic elements and protein regulations (Levin et al., 2000; Qiu et al., 2014; Blair et al., 2015). Song et al. (2013) reported that with the SciTox™ direct toxicity assay, the EC50 values of 2, 4-dicholorophenol and 3, 5-dichlorophenol on freezedried Gram-negative bacteria (Acinetobacter calcoaceticus, Escherichia coli and Pseudomonas putida) were significantly lower than those of the initial bacteria. Our results indicated that the sensitivities of V. anguillarum to rifampicin, erythromycin, furazolidone, gentamicin, doxycycline and cotrimoxazole showed decreasing trends. Furthermore, the MIC test showed that the sensitivities of V. anguillarum to chloramphenicol, neomycin and polymyxin B changed, and such changes cannot be detected with K-B method. The results further revealed that compared with MIC method, K-B method showed a lower sensitivity and accuracy. At the same time, both freeze-drying and cryopreservation treatments can change the physiological and biochemical characteristics and drug sensitivity of V. anguillarum. However, the activated freeze-dried V. anguillarum has the best ability to maintain its initial characteristics.
The adaptation mechanism of V. anguillarum to salinity, low temperature, pH, starvation stress and other environmental stresses have been reported previously. The outer membrane proteins porins and efflux pumps play important roles in regulating drug sensitivity (Catel-Ferreira et al., 2011; Fernández-Cuenca et al., 2011; Bialek-Davenet et al., 2015). V. anguillarum can regulate the expression and conformation of membrane proteins to achieve the effect of environmental adaptation (Wang et al., 2003). The physicochemical characteristic changes may be caused by the changes of culture environment, and it may also be induced by the changes of cell structure and composition. Thus, the iTRAQ-based proteomic analysis of V. anguillarum under different preservation conditions has been detected and will be reported in another article.
5 ConclusionsIn conclusion, we found that the optimal composition of freeze-drying protectant mixture for V. anguillarum contained trehalose (8%), skim milk (12%), lactose (8.0%), sodium citrate (2.0%), serum (12.0%) and mannitol (8.0%). With the mixed protectants, the survival rate of V. anguillarum was significantly higher than those using individual protectant. After different treatments, the physicochemical properties of V. anguillarum changed. Some changes during freeze-dry treatment can be restored to the initial state by continuous passage. Therefore, compared with cryopreservation and continuous passage, the freeze-drying treatment is recommended for the long-term preservation of V. anguillarum.
AcknowledgementsThis work was funded by the Key Projects of Science and Technology Innovation of Shandong Province (No. 2018YFJH0703), the Central Public-Interest Scientific Institution Basal Research Fund, Yellow Sea Fisheries Research Institutes, CAFS (No. 20603022017004), the Projects of International Exchange and Cooperation in Agriculture, Ministry of Agriculture and Rural Affairs of China-Science, Technology and Innovation Cooperation in Aquaculture with Tropical Countries.
Abadias, M., Benabarre, A., Teixidó, N., Usall, J. and Viñas, I., 2001. Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake. International Journal of Food Microbiology, 65: 173-182. DOI:10.1016/S0168-1605(00)00513-4 (  0) 0) |
Berny, J. F. and Hennebert, G. L., 1991. Viability and stability of yeast cells and filamentous fungus spores during freeze-drying: Effects of protectants and cooling rates. Mycologia, 83: 805-815. DOI:10.1080/00275514.1991.12026086 (  0) 0) |
Bialek-Davenet, S., Lavigne, J. P., Guyot, K., Mayer, N., Tournebize, R., Brisse, S., Leflon-Guibout, V. and Nicolas-Chanoine, M. H., 2015. Differential contribution of AcrAB and OqxAB efflux pumps to multidrug resistance and virulence in Klebsiella pneumoniae. Journal of Antimicrobial Chemotherapy, 70: 81-88. DOI:10.1093/jac/dku340 (  0) 0) |
Blair, J. M. A., Webber, M. A., Baylay, A. J., Ogbolu, D. O. and Piddock, L. J. V., 2015. Molecular mechanisms of antibiotic resistance. Nature Reviews Microbiology, 13: 42-51. DOI:10.1038/nrmicro3380 (  0) 0) |
Catel-Ferreira, M., Coadou, G., Molle, V., Mugnier, P., Nordmann, P., Siroy, A., Jouenne, T. and Dé, E., 2011. Structure-function relationships of CarO, the carbapenem resistance-associated outer membrane protein of Acinetobacter baumannii. Journal of Antimicrobial Chemotherapy, 66: 2053-2056. DOI:10.1093/jac/dkr267 (  0) 0) |
Chotiko, A. and Sathivel, S., 2014. Effects of enzymatically-extracted purple rice bran fiber as a protectant of L. plantarum NRRL B-4496 during freezing, freeze drying, and storage. LWT–Food Science and Technology, 59: 59-64. DOI:10.1016/j.lwt.2014.05.056 (  0) 0) |
Crowe, J. H., Crowe, L. M., Oliver, A. E., Tsvekova, N., Wolkers, W. and Tablin, F., 2001. The trehalose myth revisited: Introduction to a symposium on stabilization of cells in the dry state. Cryobiology, 43: 89-105. DOI:10.1006/cryo.2001.2353 (  0) 0) |
Fatemeh, K. N., Reza, R. M., Mohammad, A. H., Babak, G., Mahmoud, S. K. and Khaled, Z. B., 2015. Optimization of the nanocellulose based cryoprotective medium to enhance the viability of freeze dried Lactobacillus plantarum using response surface methodology. LWT–Food Science and Technology, 64: 326-332. DOI:10.1016/j.lwt.2015.06.004 (  0) 0) |
Fernanda, F., Julie, M., Stéphanie, C., Stéphanie, P. and Morris, G. J., 2016. Determination of intracellular vitrification temperatures for unicellular microorganisms under conditions relevant for cryopreservation. PLoS One, 11: e0152939. DOI:10.1371/journal.pone.0152939 (  0) 0) |
Fernández-Cuenca, F., Smani, Y., Gómez-Sánchez, M. C., Docobo-Pérez, F., Caballero-Moyano, F. J., Domínguez-Herrera, J., Pascual, A. and Pachón, J., 2011. Attenuated virulence of a slow-growing pandrug-resistant Acinetobacter baumannii is associated with decreased expression of genes encoding the porins CarO and OprD-like. International Journal of Antimicrobial Agents, 38: 548-549. DOI:10.1016/j.ijantimicag.2011.08.002 (  0) 0) |
Fu, Q., Qiu, J., Hu, K., Yang, X. and An, J., 2011. Stability of virulence genes of Aeromonas hydrophila strains during subculture. Biotechnology Bulletin, 9: 130-135. (  0) 0) |
He, H., Liu, B. L., Hua, Z. Z., Li, C. and Wu, Z. Z., 2009. Intracellular trehalose improves the survival of human red blood cells by freeze-drying. Frontiers of Energy and Power Engineering in China, 1: 120-124. (  0) 0) |
Heljo, V. P., Jouppila, K., Hatanpää, T. and Juppo, A. M., 2011. The use of disaccharides in inhibiting enzymatic loss and secondary structure changes in freeze-dried galactosidase during storage. Pharmaceutical Research, 28: 540-552. DOI:10.1007/s11095-010-0300-x (  0) 0) |
Hyuk, C. K. and Yue, J. K., 2016. Effects of a subunit vaccine (FlaA) and immunostimulant (CpG-ODN 1668) against Vibrio anguillarum in tilapia (Oreochromis niloticus). Aquaculture, 454: 125-129. DOI:10.1016/j.aquaculture.2015.12.005 (  0) 0) |
Jason, S., Quinn, O., Wang, M., Aaron, C., Michael, A. M. and Nilay, C., 2017. Effect of trehalose as an additive to dimethyl sulfoxide solutions on ice formation, cellular viability, and metabolism. Cryobiology, 75: 134-143. DOI:10.1016/j.cryobiol.2017.01.001 (  0) 0) |
Julie, M., Stéphanie, P., Sébastien, D. and Fernanda, F., 2017. Biophysical characterization of the Lactobacillus delbrueckii subsp. bulgaricus membrane during cold and osmotic stress and its relevance for cryopreservation. Applied Microbiology and Biotechnology, 101: 1427-1441. DOI:10.1007/s00253-016-7935-4 (  0) 0) |
Kenan, C., Nuri, B., Mustafa, N. B. and Pınar, P. A., 2011. Effects of cysteine and ergothioneine on post-thawed Merino ram sperm and biochemical parameters. Cryobiology, 63: 1-6. DOI:10.1016/j.cryobiol.2011.04.001 (  0) 0) |
Khansari, A. R., Parra, D., Reyes-López, F. E. and Tort, L., 2017. Modulatory in vitro effect of stress hormones on the cytokine response of rainbow trout and gilthead sea bream head kidney stimulated with Vibrio anguillarum bacterin. Fish & Shellfish Immunology, 70: 736-749. (  0) 0) |
Kim, E. Y., Kim, Y. R., Kim, D. G. and Kong, I. S., 2012. A susceptible protein by proteomic analysis from Vibrio anguillarum under various environmental conditions. Bioprocess and Biosystems Engineering, 35: 273-282. DOI:10.1007/s00449-011-0636-6 (  0) 0) |
Levin, B. R., Perrot, V. and Walker, N., 2000. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics, 154: 985-997. (  0) 0) |
Li, L. P., Wang, R., Liang, W. W., Huang, T., Huang, Y., Luo, F. G., Lei, A. Y., Chen, M. and Gan, X., 2015. Development of live attenuated Streptococcus agalactiae vaccine for tilapia via continuous passage in vitro. Fish & Shellfish Immunology, 45: 955-963. (  0) 0) |
Lodato, P., Huergo, M. S. and Buera, M. P., 1999. Viability and thermal stability of a strain of Saccharomyces cerevisiae freeze-dried in different sugar and polymer matrices. Applied Microbiology and Biotechnology, 52: 215-220. DOI:10.1007/s002530051511 (  0) 0) |
Ma, Y., Wang, Q., Gao, X. and Zhang, Y., 2017. Biosynthesis and uptake of glycine betaine as cold-stress response to low temperature in fish pathogen Vibrio anguillarum. Journal of Microbiology, 55: 44-55. DOI:10.1007/s12275-017-6370-2 (  0) 0) |
Michael, E. H. and Lee, J. L., 2018. A comprehensive review of Vibrio (Listonella) anguillarum: Ecology, pathology and prevention. Reviews in Aquaculture, 10: 585-610. DOI:10.1111/raq.12188 (  0) 0) |
Mimoza, B. S., Monika, M., Sharareh, S. B., Frank, M. U. and Helmut, V., 2014. Effect of lyoprotectants on β-glucosidase activity and viability of Bifidobacterium infantis after freeze-drying and storage in milk and low pH juices. LWT–Food Science and Technology, 57: 276-282. DOI:10.1016/j.lwt.2014.01.011 (  0) 0) |
Miriam, D., Sylvia, W., Sascha, W., Franz, W., Hagen, V. B. and Jörg, K., 2014. Freeze-drying of HI-6-loaded recombinant human serum albumin nanoparticles for improved storage stability. European Journal of Pharmaceutics and Biopharmaceutics, 88: 510-517. DOI:10.1016/j.ejpb.2014.06.008 (  0) 0) |
Morgan, C. A., Herman, N., White, P. A. and Vesey, G., 2006. Preservation of microorganisms by drying. A review. Journal of Microbiological Methods, 66: 183-193. DOI:10.1016/j.mimet.2006.02.017 (  0) 0) |
Qiu, F. M., Mhanna, R., Zhang, L., Ding, Y., Fujita, S. and Bradley, J. N., 2014. Artificial bacterial flagella functionalized with temperature-sensitive liposomes for controlled release. Sensors and Actuators B: Chemical, 196: 676-681. DOI:10.1016/j.snb.2014.01.099 (  0) 0) |
Ryan, M. J., Bridge, P. D., Smith, D. and Jeffries, P., 2001. Developing cryopreservation protocols to secure fungal gene function. Cryo Letters, 22: 115-124. (  0) 0) |
Song, W. F., Ravi, G., Nicholas, G., Richard, J. W. and Neil, P., 2013. Appraising freeze-drying for storage of bacteria and their ready access in a rapid toxicity assessment assay. Applied Microbiology and Biotechnology, 97: 10189-10198. DOI:10.1007/s00253-013-4706-3 (  0) 0) |
Tang, L., Yue, S., Li, G. Y., Li, J., Wang, X. R., Li, S. F. and Mo, Z. L., 2016. Expression, secretion and bactericidal activity of type Ⅵ secretion system in Vibrio anguillarum. Archives of Microbiology, 198: 751-760. DOI:10.1007/s00203-016-1236-2 (  0) 0) |
Tasdemir, U., Büyükleblebici, S., Tuncer, P. B., Coskun, E., Özgürtas, T., Aydın, F. N., Büyükleblebici, O. and Gürcan, I. S., 2013. Effects of various cryoprotectants on bull sperm quality, DNA integrity and oxidative stress parameters. Cryobiology, 66: 38-42. DOI:10.1016/j.cryobiol.2012.10.006 (  0) 0) |
Wang, S. Y., Lauritz, J., Jass, J. and Milton, D. L., 2003. Role for the major outer-membrane protein from Vibrio anguillarum in bile resistance and biofilm formation. Microbiology, 149: 1061-1071. DOI:10.1099/mic.0.26032-0 (  0) 0) |
Wang, W. L., Chen, M. S., Wu, J. H. and Wang, S. Y., 2015. Hypothermia protection effect of antifreeze peptides from pigskin collagen on freeze-dried Streptococcus thermophiles and its possible action mechanism. LWT–Food Science and Technology, 63: 878-885. DOI:10.1016/j.lwt.2015.04.007 (  0) 0) |
Weng, L. D., Shannon, N. T., Anisa, S., Shannon, L. S. and Mehmet, T., 2017. Controlled ice nucleation using freeze-dried Pseudomonas syringae encapsulated in alginate beads. Cryobiology, 75: 1-6. DOI:10.1016/j.cryobiol.2017.03.006 (  0) 0) |
Yang, L., Ma, Y. and Zhang, Y. X., 2007. Freeze-drying of live attenuated Vibrio anguillarum mutant for vaccine preparation. Biologicals, 35: 265-269. DOI:10.1016/j.biologicals.2007.03.001 (  0) 0) |
Yu, Y. X., Zhang, Z., Wang, Y. G., Liao, M. J., Li, B. and Xue, L. Y., 2017. Optimization of protectant, salinity and freezing condition for freeze-drying preservation of Edwardsiella tarda. Journal of Ocean University of China, 16: 831-839. DOI:10.1007/s11802-017-3331-7 (  0) 0) |
Zhang, G. Q., Fan, M. T., Lv, Q., Li, Y. H., Liu, Y. L., Zhang, S. F. and Zhang, H., 2013. The effect of cold, acid and ethanol shocks on synthesis of membrane fatty acid, freeze-drying survival and malolactic activity of Oenococcus oeni. Annals of Microbiology, 63: 477-485. DOI:10.1007/s13213-012-0492-x (  0) 0) |
Zhang, Y., Ng, I. S., Yao, C. Y. and Lu, Y. H., 2015. Orthogonal array deciphering MRS medium requirements for isolated Lactobacillus rhamnosus ZY with cell properties characterization. Journal of Bioscience and Bioengineering, 118: 298-304. (  0) 0) |
Zhang, Z., 2004. Epizootic investigation and aetiological study on the bacterial diseases in cultured turbot (Scophthalmus maximus). Master thesis. Ocean University of China (in Chinese with English abstract).
(  0) 0) |
Zhao, G. and Zhang, G., 2005. Effect of protective agents, freezing temperature, rehydration media on viability of malolactic bacteria subjected to freeze-drying. Journal of Applied Microbiology, 99: 333-338. DOI:10.1111/j.1365-2672.2005.02587.x (  0) 0) |
 2019, Vol. 18
2019, Vol. 18


