2) Key Laboratory of Aquatic Biodiversity and Conservation of Chinese Academy of Sciences, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China;
3) Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao 266003, China;
4) College of Fisheries, Ocean University of China, Qingdao 266003, China
The Diophrys-complex is a common group of order Eu plotida and belongs to the most morphologically complex class Spirotrichea. These species have dominant oral field and strong cirri on ventral side, and generally exist in marine and estuarine biotopes (Song et al., 2007, 2009a; Lynn, 2008; Hu et al., 2019). In the past 40 years, this com plex group was clarified many times and include six genera now. Forexample, Jankowski (1979) divided it into Diophrys and Paradiophrys; Hill and Borror (1992) established the third genus, Diophryopsis; Jankowski (2007) reassigned these three genera in Diophryinae. Hereafter, three more genera, Apodiophrys, Heterodiophrys and Pseu dodiophrys, have been reported and added to this subfa mily (Jiang and Song, 2010; Jiang et al., 2011). Subsequently, Huang et al. (2012) and Fan et al. (2013) reveal ed some opinions based on molecular and morphogenetic characters, and some of them did not completely support the classification of the subfamily, i.e., Paradiophrys and Apodiophrys were not included in this group.
After several revisions and divestitures, several species have been removed from the genus Diophrys. The remain ing species are mainly characterized by the combined fea tures, namely three caudal cirri located in a prominent pos terior concave area, oral area with prominent adoral zone of membranelles and distinct paroral and endoral membranes, ventral side arranged with five frontal, two ventral, five transverse, and one or two left marginal cirri. So far, only ten species still remain in genus Diophrys: D. salina Ruinen, 1938; D. peloetes Borror, 1965; D. oligothrix Borror, 1965; D. apoligothrix Song et al., 2009a; (D. scutum Dujardin, 1841) Kahl, 1932; D. appendiculata (Ehrenberg, 1838) Schewiakoff, 1893; D. parappendiculata Shen et al., 2011; D. japonica Hu, 2008; D. blakeneyensis Hu et al., 2012; D. peculiaris Luo et al., 2014 (Dujardin, 1841; Kahl, 1932; Curds and Wu, 1983; Hill and Borror, 1992; Song and Packroff, 1997; Song and Wilbert, 1994, 2002; Song et al., 2007, 2009a, b; Hu, 2008; Shen et al., 2011; Hu et al., 2012; Fan et al., 2013; Luo et al., 2014). Till now, seven out of ten species have been investigated based on both mor phological descriptions and molecular data.
As a new contribution, the present work describes three Diophrys ciliates collected from subtropical brackish waters, namely D. quadrinucleata n. sp., D. oligothrix and D. scutum. Moreover, the phylogeny of all Diophrys-com-plex species based on smallsubunit rDNA (SSU rDNA) sequences and a key to all valid species of Diophrys according to morphological characters are provided.
2 Material and Methods 2.1 Sample Collection, Observation and Terminology (Figs. 1A–D1)

|
Fig. 1 Map and sampling sites. A, Map of the four seas of China, red asterisk indicates the location of Ningbo. B, Coastal beach of Songlanshan at Xiangshan Island. B1, Diophrys quadrinucleata n. sp. collected from site (B). C, C', A brackish water lake which connected with the Xiangshan Bay, Ningbo. C1, D. oligothrix collected from site (C). D, D', A sandy beach at Meishan island, Ningbo. D1, D. scutum collected from site (D). |
Diophrys quadrinucleata n. sp. (Fig. 1B1) was collected on August 23, 2014 from Songlanshan beach, Xiangshan Island, Ningbo (29˚26΄22΄΄E, 121˚58΄24΄΄N), China (Figs. 1A, B). The particle size of sand was 0.15–0.25 mm. The upper 10–20 cm layer of sand was collected with seawater from the site. The water temperature was about 30℃ and the salinity was 20.
Diophrys oligothrix (Fig. 1C1) was collected on 15 November 2018 from a brackish water lake (Figs. 1A, C, C') which is connected with the Xiangshan Bay, Ningbo (29˚45΄28΄΄E, 121˚54΄53΄΄N), China. The water temperature was about 19℃ and the salinity was about 22. Samples were taken from the surface layer of lake-bed sediment using a Pasteur pipette and then diluted with untreated water from the collection site.
Diophrys scutum (Fig. 1D1) was collected on November 15, 2018 from a sandy beach at Meishan Island, Ningbo (29˚46΄28΄΄E, 121˚55΄53΄΄N), China. The location and habitats of sampling are shown in Figs. 1D, D'. The water temperature was about 18℃, salinity was about 18. Samples were taken from intertidal puddles using a sterile sy ringe.
Specimens were maintained in the laboratory as raw cultures for one to two weeks at room temperature (about 25℃), and rice grains were employed to promote the growth of bacteria as food for the ciliates. Living cells were observed using bright field and differential interference contrast microscopy. The protargol silver staining method from Wilbert (1975) was used to reveal the infraciliature and nuclear apparatus. Counts and measurements of stained specimens were performed at a magnification of 1000×. All drawings were made with the help of a drawing attachment. Terminology and systematics mainly follow Lynn (2008), Song et al. (2009a) and Gao et al. (2016).
2.2 DNA Extraction, PCR Amplification and SequencingA single cell of each species was isolated from the ori ginal sample and washed four times with filtered habitat water (0.22 µm-pore size membrane, Millipore, USA) and two times with ultrapure water. Then the cells were trans ferred to a 1.5 mL microfuge tube with a minimum vo lume of water, respectively. Genomic DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen, CA) according to the manufacturer's instructions, modified according to Sheng et al. (2018). PCR amplifications of the SSU rDNA were performed with the primers 18SF (5'-AACCTGGTTGATCCTGCCAGT-3') and 18SR (5'-TGATCCTTCTGCAGGTTCACCTAC-3') (Medlin et al., 1988). Cycling parameters were as follows: initial denaturation of 94℃ for 5 min, followed by 18 cycles of amplification (94℃, 1 min; 66–49℃ touch down, 40 s; 72℃, 2 min) and another 18 cycles of amplification (94℃, 30 s; 48℃, 40 s; 72℃, 2 min), with a final extension of 72℃ for 7 min. The PCR pro ducts of the new species, D. oligothrix and D. scutum, were sequenced directly in both directions using primers 18SF, 18SR and two internal primers (Zhao et al., 2016; Lian et al., 2019; Wang et al., 2019) at GENEWIZ (Beijing, China, incorporated company) and Tsingke Biotechnology (Hang zhou, China), respectively. Contigs were assembled into one consensus sequence by Seqman (DNAStar).
2.3 Molecular Phylogenetic AnalysesThe SSU rDNA sequences of the three newly isolated species and 47 Diophryinae ciliates downloaded from the GenBank database were aligned using MUSCLE v3.7 (Edgar, 2004) on the website (URL: http://www.ebi.ac.uk/Tools/msa/muscle/). Uronychia, Paradiophrys and Apodiophrys were selected as the outgroup taxa. Ambiguous columns were removed with default parameters using Gblock v. 0.91b, resulting in a nucleotide matrix of 1730 sites. Maximum likelihood (ML) analysis, with 1000 bootstrap replicates, was carried out using RAxML-HPC2 on XSEDE v. 8.2.10 (Stamatakis et al., 2014) of the CIPRES Science Gateway (URL: http://www.phylo.org/sub_sec tions/portal). The program MrModeltest v.2.3 (Nylander, 2008) selected the GTR + I + G as the best model with Akaike information criterion (AIC). Bayesian inference (BI) analysis was performed with MrBayes 3.2.6 on XSEDE (Ronquist et al., 2012), with 1000000 generations, a sampling frequency of 100, and a burn-in of 2500 trees. Tree topologies were displayed with MEGA X (Kumar et al., 2018).
3 Results 3.1 Diophrys quadrinucleata n. sp. (Fig. 2, Table 1)
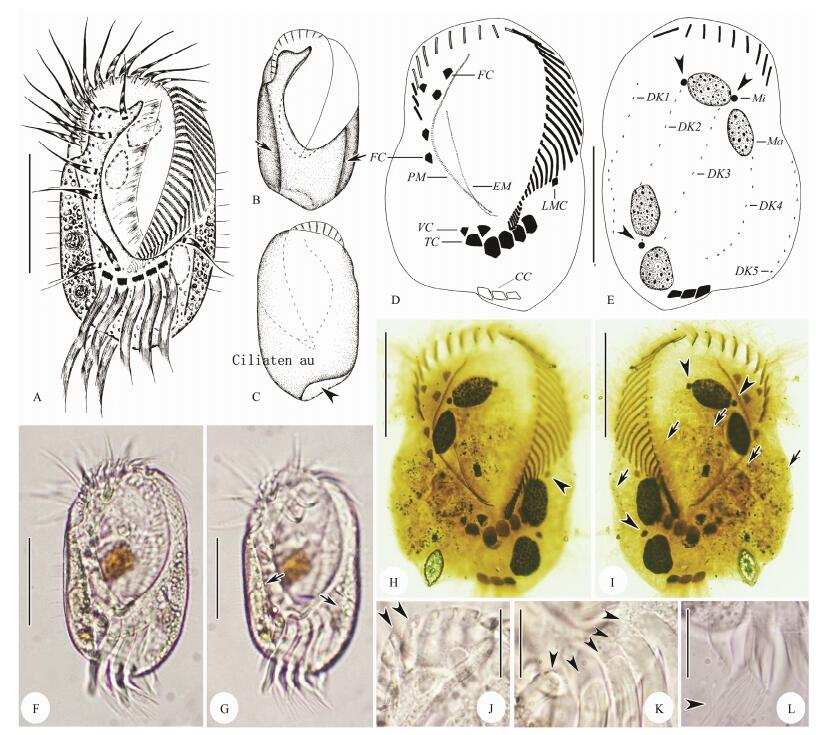
|
Fig. 2 Diophrys quadrinucleata n. sp. from life (A–C, F, G, J–L) and after protargol impregnation (D, E, H, I). A–C, F, G, Ventral and dorsal views of different body shapes and wide oral area, short arrows in (B, G) show two longitudinal ribs on ventral side; arrowhead in (C) indicates the concave area where caudal cirri located. D, E, H, I, Ventral (D, H) and dorsal (E, I) views of the holotype specimen noting infraciliature and nuclear apparatus: arrowheads in (E, I) show the micronucleus; arrowhead in (H) indicates the left marginal cirrus; short arrows in (I) show densely arranged dikinetids within dorsal kineties. J–L, Amplification of some structures: arrowheads in (J) mark the conspicuous and ridged collar, arrowheads in (K) showing the five strong transverse cirri; arrowhead in (L) shows the brush-like end of caudal cirri. CC, caudal cirri; DK1–5, dorsal kinety 1–5; EM; endoral membrane; FC, frontal cirri; LMC, left marginal cirrus; Ma, macronucleus; Mi, micronuclei; PM, paroral membrane; TC, transverse cirri; VC, ventral cirri. Scale bars = 50 μm (A–I), 20 μm (J–L). |
|
|
Table 1 Morphometric characterization of Diophrys quadrinucleata n. sp., D. oligothrix, and D. scutum in the present work |
Cell size (100–140) μm × (60–80) μm in vivo; body elliptical, slightly greyish to yellowish in color; adoral zone comprising about 42 membranelles; five frontal, two ventral, five transverse, one left marginal and three caudal cirri; five continuous dorsal kineties with densely arranged dikinetids; four ellipsoidal macronuclear nodules; brackish habitat.
3.1.2 Type localityIntertidal beach near Songlanshan (29˚26΄22΄΄E, 121˚ 58΄24΄΄N), Xiangshan Island, Ningbo, China. The water temperature was approximately 30℃, and the salinity was about 20.
3.1.3 EtymologyThis species has four macronuclear nodules; therefore, its name recalls this feature: quadri-(four), nucleate.
3.1.4 Type materialThe slide (registration number: LBR20140823-02-01) with protargol impregnated holotype specimen (Figs. 2H, I) is deposited in the Laboratory of Protozoology, Ocean University of China (OUC), China. Two paratype slides (re gistration numbers: LBR20140823-02-02, 03) are deposited in the Ningbo University.
3.1.5 ZooBank accession number of the new speciesUrn:lsid:zooban.org:pub:DDB8B90C-B727-4022-BC74-D1D5E5FACA66.
3.1.6 Deposition of SSU rDNA sequence dataThe SSU rDNA sequence is deposited in GenBank with accession number MT109370; its length and GC content are 1736 bp and 44.59%, respectively.
3.1.7 DescriptionCell size (100–140) μm × (60–80) μm in vivo, ratio of length to width about 2:1; body elliptical with both sides straight (Figs. 2A–C, F). Dorsoventrally flattened with ventral side flat and dorsal side bulging. Apparently sculptured with two longitudinal ribs on ventral side, and obviously depression in central region of body between ribs (Figs. 2A, B, G). Anterior end with conspicuous thin, ridged collar and cilia of apical adoral membranelles emerging between ridges (Fig. 2J). Posterior end rounded with right lateral concave area on dorsal side where caudal cirri located (Figs. 2A, F). Body color slightly greyish to yellowish at low magnification, cytoplasm colorless and transparent, usually packed with numerous granules (about 1– 5 μm in diameter) and food vacuoles (containing brownish diatoms). Contractile vacuole and cortical granules not recognized. Four macronuclear nodules, spherical to ellipsoidal in shape, each about 20 μm × 15 μm in size, two in a group and diagonally distributed (Figs. 2E, H). About four ellipsoidal micronuclei adjacent to macronuclear nodules (Figs. 2E, I). Locomotion by rapid crawling on substrate or freely swimming in water. Feeding on bacteria and diatoms.
Cilia of distal membranelles approximately 25–35 μm long. Frontal, ventral and left marginal cirri about 25 μm in length. Transverse and caudal cirri very strong with cilia about 40 μm and 30 μm long, respectively (Figs. 2K, L). Dorsal cilia conspicuous in vivo, about 8 μm long (Figs. 2A, F).
Buccal field extending about 2/3 of body length, widest part occupying about 3/5 of body width (Figs. 2A, B, F, G). Adoral zone composed of 38–51 membranelles, of which four or five membranelles at distal end reached right side of body; eight to ten membranelles located on dorsal side (Fig. 2E). Paroral membrane (PM) curved and long, generally composed of two lines of kinetosomes. Endoral mem brane (EM) about 3/5 of the length of PM, single-rowed. PM and EM optically intersect near their posterior ends (Figs. 2D, H). Five frontal cirri grouped in anterior region of frontal area and always two ventral cirri located together as 'pre-transverse cirri'. Invariably five strong transverse cirri aligned in a row (Figs. 2D, H, K); one thin left marginal cirrus positioned behind proximal end of adoral zone of membranelles (Figs. 2D, H). Always three caudal cirri at right cell margin with brush-like ends (Figs. 2E, I, L). Five dorsal kineties with densely arranged kinetosomes, leftmost and rightmost kineties usually on ventral side (Figs. 2E, I).
3.2 Diophrys oligothrix Borror, 1965 (Figs. 3, 4, Table 1)

|
Fig. 3 Diophrys oligothrix from life (A–G) and after protargol impregnation (H, I). A, F, G, Ventral (A, F) and dorsal (G) views to show different body shapes. B, The lateral view showing the sculptured body margin. C–E, Ventral (C, D) and dorsal (E) views to show numerous tiny cortical granuleswhich distribute around the cirri and along the paroral membrane on the ventral surface (C, D), arranging in irregular lines and around every dorsal cilium in a rosette-pattern on the dorsal side (arrowheads in E to show the granules around the dorsal cilium). H, I, Ventral (H) and dorsal (I) views of a typical specimen noting the infraciliature and nuclear apparatus: arrowheads in (H) indicate two left marginal cirri. CC, caudal cirri; DK1– 4, dorsal kinety 1– 4; EM, endoral membrane; FC, frontal cirri; Ma, macronucleus; Mi, micronuclei; PM, paroral membrane; TC, transverse cirri; VC, ventral cirri. Scale bars = 30 μm. |

|
Fig. 4 Diophrys oligothrix from life (A, D from bright field; B, C, E from differential interference contrast microscopy) and after protargol impregnation (F–H). A, Ventral view of a representative individual. B, Ventral view of a slightly squashed specimen: arrowheads to show two widely separated left marginal cirri. C, D, Ventral (C) and dorsal (D) views to show numerous tiny yellow-greenish cortical granules: arrowheads in (C) indicate the cortical granules distributed around the cirri and along the right edge of oral area; circles in (D) to show the cortical granules arranged irregular lines and around every dorsal cilium. E, Lateral view showing the sculptured body margin. F, G, Ventral (F) and dorsal (G) views of a typical specimen showing infraciliature and nuclear apparatus: arrowheads in (F) indicate two left marginal cirri; arrowheads in (G) show regularly arranged dikinetids within dorsal kineties. H, Detail of nuclear apparatus: arrowhead in (H) show micronuclei. Scale bars = 30 μm. |
Cell size (60–80) μm × (40–45) μm in vivo, body shape elliptical to oval with left side nearly straight and right margin convex (Figs. 3A, F, G, 4A). Anterior end with conspicuous ridged collar accompanied by ridges, cilia of apical adoral membranelles emerge near these ridges (Fig. 3A). Posterior end rounded with right concave area on dorsal side where caudal cirri located. Bright greenish spherical cortical granules approximately 0.5 μm across, on dorsal surface, densely arranged in irregular lines and about seven to nine granules packed together around every dorsal cilium in a rosette-pattern (Fig. 3E, arrowheads, 4D, circles); on ventral surface, packed around the cirri and along the right edge of oral area (Figs. 3C, D, 4C). Cytoplasm colorless and transparent, usually packed with numerous granules (3–7.5 μm in diameter) and food vacuoles (containing diatoms, some big diatom almost as long as body length) (Fig. 4D). Contractile vacuole not recognized. Always two sausage-shaped macronuclear nodules with each one located in anterior and posterior half of cell (Figs. 3I, 4F). One to four spherical or ellipsoidal micronuclei adjacent to macronuclear nodules (Figs. 3I, 4H). Locomotion by rapid crawling on substrate or freely swimming in water. Feeding on bacteria and diatoms.
Cilia of distal adoral membranelles approximately 25 μm long. Frontal, ventral and marginal cirri thin and about 20 μm in length. Transverse and caudal cirri very strong, 30 μm and 20 μm long, respectively. Dorsal cilia about 6 μm long and obviously detectable in vivo.
Buccal field extending about 1/2 of body length and adoral zone composed of 28–39 membranelles. Paroral membbrane (PM) and endoral membrane (EM) optically intersecting near their posterior ends. PM almost the same length as buccal field, slightly curved, extending near the first frontal ventral cirrus, EM about 3/5 of PM in length. Five frontal cirri grouped in frontal area of cell and always two ventral cirri located together as 'pre-transverse cirri' (Fig. 4F). Invariably five strong transverse cirri aligned in a row; three caudal cirri at right cell margin. Two left marginal cirri, of which one beneath proximal end of AZM, the other near the leftmost transverse cirrus. Four dorsal kineties with loosely arranged dikinetids. (Figs. 3H, I, 4G).
3.2.2 Deposition of SSU rDNA sequence dataThe SSU rDNA sequence is deposited in GenBank with accession number MT109371. Its size and GC content are 1656 bp and 44.32% respectively.
3.2.3 Ecology and distributionNingbo population of Diophrys oligothrix was collected on 15 November 2018. Water temperature was 19℃, salinity was about 22.
New Hampshire, USA (Borror, 1965b; tidal marsh pools); Coastal of UK (Carey, 1992, marine interstitial area; Hu et al., 2012, a salt marsh near Blakeney village); Qingdao, China (Song and Wilbert, 1994, seawater ponds; Song and Packroff, 1997, coastal waters; Chen and Song, 2002, shrimp and shellfish farming ponds; Song et al., 2009a, coastal waters); King George Island, Antarctica (Song and Wilbert, 2002, seawater ponds); Guangdong, China (Chen and Song, 2002, coastal waters of Zhanjiang); Nagasaki, Japan (Luo et al., 2014, a fishing port).
3.3 Diophrys scutum (Dujardin, 1841) Kahl, 1932 (Fig. 5, Table 1)

|
Fig. 5 Diophrys scutum from life (A–C, F, G, J from bright field; K from differential interference contrast microscopy) and after protargol impregnation (D, E, H, I). A–C, F, G, Ventral (A, B, F) and dorsal (C, G) views to show different body shapes: arrowheads in (G) show numerous granules. D, E, H, I, Ventral (D, H) and dorsal (E, I) views of a typical specimen showing infraciliature and sausage-shaped nuclear apparatus: arrowheads in (D, H) indicate the two left marginal cirri; arrowheads in (I) show densely arranged dikinetids within dorsal kineties; arrows in (H) to show micronuclei. J, Dorsal side of slightly squashed specimen: arrowheads to show the densely distributed symbiotic bacteria/archaeobacteria (?). K, Ventral view showing strong transverse cirri: arrowheads indicate two left marginal cirri; short arrows denote two ventral cirri. CC, caudal cirri; DK1– 6, dorsal kinety 1– 6; EM, endoral membrane; FC, frontal cirri; Ma, macronucleus; PM, paroral membrane; TC, transverse cirri; VC, ventral cirri. Scale bars = 50 μm (A, D, E, F–I), 20 μm (J, K). |
Elliptical cell (150–170) μm × (70–85) μm in vivo (Figs. 5A–C, F). Ventral side sculptured with two longitudinal ribs (Fig. 5B). Anterior end with conspicuous, large collar; posterior end lightly pointed with right concave area on dorsal side where caudal cirri located (Figs. 5A, F). Cytoplasm colorless and transparent, packed with granules (3– 8.5 μm in diameter) and food vacuoles (Figs. 5F, G). Contractile vacuole not recognized. Dorsal side densely covered with symbiotic bacteria/archaeobacteria (?) (Fig. 5J). Two sausage-shaped macronuclear nodules (Figs. 5E, H). One to five spherical micronuclei adjacent to macronuclear nodules (Fig. 5H, arrows).
Buccal field extending about 2/3 of body length. Adoral zone composed of 67–87 membranelles. Paroral membrane (PM) and endoral membrane (EM) optically intersecting near middle part of EM. PM long, slightly curved, extending almost to anterior end of cell, while EM about three quarters of PM in length (Figs. 5D, H). Five frontal cirri approximately in a line along anterior right margin, two strong and developed ventral cirri located together as 'pretransverse cirri'; five strong transverse and three caudal cirri; two thin left marginal cirri widely separated (Figs. 5H, K). Six dorsal kineties with densely distributed dikinetids on dorsal and lateral ventral sides (Figs. 5E, I).
3.3.2 Deposition of SSU rDNA sequence dataThe SSU rDNA sequence is deposited in GenBank with accession number MT109372; its size and GC content are 1669 bp and 44.46% respectively.
3.3.3 Ecology and distributionThe Ningbo population of Diophrys scutum was collected on November 15, 2018. Water temperature was 18℃, salinity was about 18.
New Hampshire, USA (Borror, 1965b, tidal marsh pools); Japan Sea (Raikov and Kovaleva, 1968, sandy sediments); Caspian Sea (Agamaliev, 1971, western coast); UK (Carey, 1992, marine interstitial area); Qingdao, China (Song and Packroff, 1997, coastal waters; Chen and Song, 2002, shrimp farming pond; Song et al., 2009a, coastal waters).
3.4 SSU rDNA Sequence and Phylogenetic Analyses (Fig. 6)

|
Fig. 6 The maximum-likelihood (ML) tree inferred from the small subunit rDNA sequences of 50 species in the family Uronychiidae, showing the positions of Diophrys quadrinucleata n. sp., D. oligothrix and D. scutum. Numbers near nodes are non-parametric bootstrap values for ML and posterior probability values for Bayesian inference (BI). Solid circles represent 100% for ML and 1.00 for BI. The scale bar corresponds to 1 substitution per 100 nucleotide positions. All branches are drawn to scale. |
The BI and ML trees inferred from SSU rDNA sequences had similar topologies for the Diophrys-complex group regardless of the number of taxa included in the preliminary analyses. As shown in Fig. 6, Diophrys quadrinucleata n. sp. grouped with D. apoligothrix and D. japonica in both methods while the support values are variable (53% ML, 0.96 BI). Our population of D. scutum falls within a big cluster of D. scutum sequences (99% ML, 1.00 BI). In contrast, species of D. oligothrix do not form a monophyletic lineage, as sequences under the names of Diophrys cf. oligothrix, D. blakeneyensis (JN172996), D. appendiculata (MG603601) are nested within it. However, their internal relationships are far from robust as indicated by the poor support values. The newly isolated population of D. oligothrix grouped well with a British population (86% ML, 1.00 BI), followed by a sequence under the name of D. appendiculata (MG603601), although this grouping only receives very low support (21% ML, 0.55 BI). The other two sequences of D. appendiculata clustered with D. parappendiculata, forming the sister clade to the big cluster of D. oligothrix and several congeners.
4 DiscussionThe genus Diophrys is characterized by having stable front-ventral cirri, that is, five front and two ventral cirri; one or two left marginal cirri near the proximal portion of the adoral zone, two separated undulating membranes and three posterior-laterally located caudal cirri (Hu et al., 2012). Till now, only ten nominal species mainly from marine habitat have been reported. Two species of them lack infraciliature and/or molecular data, namely Diophrys salina Ruinen, 1938 and D. peloetes Borror, 1965. Based on the previous researches and present findings, nine Diophrys morphospecies with infraciliature are currently well recognized: D. quadrinucleata n. sp.; D. apoligothrix Song et al., 2009a; D. appendiculata (Ehrenberg, 1838) Schewiakoff, 1893; D. blakeneyensis Hu et al., 2012; D. japonica Hu, 2008; D. oligothrix Borror, 1965; D. parappendiculata Shen et al., 2011; D. peculiaris Luo et al., 2014; D. scutum (Dujardin, 1841) Kahl, 1932. Detailed comparisons of morphological characteristics are listed in Table 2. We here supply a key for the identification of nine welldescribed species (for illustrations of selected key characters, see Fig. 7).
|
|
Table 2 Morphometrical and morphological comparison of nine related and well-described Diophrys species |

|
Fig. 7 Illustrated key to nine Diophrys species with infraciliature information. AZM, adoral zone of membranelles; DK, dorsal kinety; Ma, macronucleus. Scale bars = 50 μm. |
The key for the identification of nine well-described species
1 One left marginal cirrus…………………………………………………………………………………………………2
1.1 Two left marginal cirri…………………………………………………………………………………………………4
2 Four macronuclear nodules……………………………………………………………D. quadrinucleata n. sp.
2.1 Two macronuclear nodules………………………………………………………………………………………………3
3 Dorsal kineties with densely distributed dikinetids, fragment dorsal kinety present………………D. japonica
3.1 Dorsal kineties with sparsely distributed dikinetids…………………………………………………………D. apoligothrix
4 Moniliform macronucleus…………………………………………………………………………………D. blakeneyensis
4.1 Two macronuclear nodules………………………………………………………………………………………………5
5 Ovoid to ellipsoidal macronuclear nodules……………………………………………………………………D. peculiaris
5.1 Sausage-shaped macronuclear nodules……………………………………………………………………………………6
6 Huge size body……………………………………………………………………………………………………D. scutum
6.1 Normal size body…………………………………………………………………………………………………………7
7 Bipartite adoral zone of membranelles.............................................................…………………………D. parappendiculata
7.1 Continuous adoral zone of membranelles……………………………………………………………………………………8
8 Continuous dorsal kineties………………………………………...……………….….…………………………D. oligothrix
8.1 Fragmented dorsal kineties………………………….………………………...…..…………………………D. appendiculata
4.1 Diophrys quadrinucleata n. sp.Before the present work, most of known Diophrys species have two ellipsoidal or sausage-shaped macronuclear nodules. Hu et al. (2012) described a multi-macronucleus species, D. blakeneyensis, which has 7–23 macronuclear nodules. It cannot be confused with D. quadrinucleata n. sp. according to the character of macronucleus. Additionally, D. blakeneyensis can be distinguished from the new species by the number of left marginal cirri (one vs. two). Kattar (1970) reported a Diophrys with four macronuclear nodules and named it as D. tetramacronucleata. Except for the number of macronuclear nodules, it is very similar to D. scutum in other morphological features. Indeed the validity of this species was even doubted by the original author. We agree with Song et al. (2007) that D. tetrama cronucleata is a special population of D. scutum. In fact, it can be easily distinguished from D. quadrinucleata n. sp. by the number of left marginal cirri (two vs. one in new species).
Although Diophrys salina is a rare species that has never been described with infraciliature and molecular information since it was originally reported by Ruinen (1938), it can be well distinguished from the new species by some characteristics, such as smaller body size (30–40 μm vs. 100–140 μm), well-developed anterior adoral membranelles (vs. normal frontal adoral membranelles size), the short and fine frontal-ventral-transverse cirri (vs. normal frontalventral cirri size, developed and strong transverse cirri), and the absence of the left marginal cirri (vs. presence).
The information of Diophrys peloetes is also incomplete and it has never been redescribed since the original description by Borror (1965a). However, this understudied species can also be clearly distinguished from D. quadrinucleata n. sp. by having two left marginal cirri (vs. single left marginal cirrus), two macronuclear nodules (vs. four), and eight dorsal kineties (vs. five).
The remaining well-described eight species can be divided into two groups based on the number of left marginal cirri. D. appendiculata, D. blakeneyensis, D. oligothrix, D. parappendiculata, D. peculiaris and D. scutum have two left marginal cirri, while D. apoligothrix and D. japonica only possess single left marginal cirrus (Fig. 7). Our new species D. quadrinucleata n. sp. can be easily distinguished from the majority of its congeners by having single left marginal cirrus. Thus, only two species with single left marginal cirrus need to be compared with. However, the new species clearly differs from D. apoligothrix and D. japonica as it has four spherical to ellipsoidal macronuclear nodules (vs. two ellipsoidal nodules). Moreover, the distinctions between D. quadrinucleata n. sp. and its two congeners are also demonstrated by the divergence of the SSU rDNA sequences. There are seven and 20 nucleotide differences between the new species and D. apoligothrix and D. japonica, respectively. Therefore, Diophrys quadrinucleata n. sp. is identified as a well-outlined and distinctive member of the genus Diophrys.
4.2 Diophrys oligothrix Borror, 1965Diophrys oligothrix was discovered by in a tidal marsh pool in New Hampshire, USA. In the following half century, it was isolated and identified from a variety of habitats all over the world, such as marine interstitial area or salt marsh in UK, coastal waters or farming ponds in China, seawater ponds in Antarctica, and fishing port in Japan (Carey, 1992; Song and Wilbert, 1994, 2002; Song and Packroff, 1997; Chen and Song, 2002; Hu et al., 2012; Luo et al., 2014). Our population isolated from the brackish-water lake corresponds well with previously published populations in terms of living morphology and ciliary pattern, especially the continuous dorsal kineties with loosely arranged cilia. The slightly dissimilar number of adoral membranelles between the Ningbo population and the original description was considered as a difference between populations (Curds and Wu, 1983; Song et al., 2007). Thus, the Ningbo population was identified as Diophrys oligothrix.
In the phylogenetic trees (Fig. 6), the Ningbo population grouped well with the Blakeney population from UK (JN172995, Hu et al., 2012), supporting the morphological identification of it as a population of D. oligothrix. How ever, these two populations did not form a monophyletic clade with the Qingdao population isolated from China (DQ353850, Song et al., 2009a). The above three populations cannot be further distinguished from each other based on morphological characters, although their sequence divergences of the SSU rDNA range from 11 to 18 nucleotide sites (Table 3). Thus, it is reasonable to deduce that there might be cryptic species in D. oligothrix, which need further information to distinguish and define them.
|
|
Table 3 SSU rDNA sequence similarities between three Diophrys oligothrix populations and D. blakeneyensis |
Diophrys scutum is the largest species in the genus and has been reported several times (Borror, 1965b; Agamaliev, 1971; Song and Packroff, 1997; Song et al., 2007). The detailed morphological characteristics were provided based on a Qingdao population in Song and Packroff (1997). Our population resembles with the previous populations very well in both living morphology and ciliary patterns. The only difference is that we found densely distributed symbiotic bacteria/archaeobacteria (?) on the dorsal surface of the Ningbo population, which was never mentioned previously (Agamaliev, 1971; Song and Packroff, 1997). The symbiotic bacteria/archaeobacteria (?) covering the dorsal surface are very similar to cortical granules in the living normal species. However, these particles can be easily separated from the dorsal surface in the slightly squeezed cells. Recently, Luo et al. (2014) described a Japanese population of Diophrys cf. scutum with numerous tiny, colourless, rice-shaped cortical granules densely arranged on dorsal side. It is highly possible that these particles are symbiotic bacteria/archaeobacteria (?) and are misinterpreted as cortical granules. Furthermore, all sequences of Diophrys scutum form a well-supported monophyletic cluster in the present phylogenetic trees and the sequence divergences of the SSU rDNA are less than four nucleotide sites each other, justifying the morphological identification of this organism.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (No. 31970398), the National Key Research and Development Program of China (No. 2018YFD0900701), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 2019 333), the Technology Innovation Team of Ningbo City (No. 2015C110018), and the K. C. Wong Magna Fund in Ningbo University.
Agamaliev, F. G., 1971. Complements to the fauna of psammophilic ciliates of the Western coast of the Caspian Sea. Acta Protozoologica, 8: 379-404. (  0) 0) |
Borror, A. C., 1965a. Morphological comparison of Diophrys scutum (Dujardin, 1841) and Diophrys peloetes n. sp. (Hypotrichida, Ciliophora). Journal of Protozoology, 12: 60-66. (  0) 0) |
Borror, A. C., 1965b. New and little-known tidal marsh ciliates. Transactions of the American Microscopical Society, 84: 550-565. DOI:10.2307/3224801 (  0) 0) |
Carey, P. G., 1992. Marine Interstitial Ciliates. An Illustrated Key. Chapman & Hall, London, 351pp.
(  0) 0) |
Chen, Z. G. and Song, W. B., 2002. Characterization and identification of the Diophrys species (Protozoa, Ciliophora, Hypotrichida) based on RAPD fingerprinting and ARDRA riboprinting. European Journal of Protistology, 38: 383-391. DOI:10.1078/0932-4739-00884 (  0) 0) |
Curds, C. R. and Wu, I. C. H., 1983. A review of the Euplotidae (Hypotrichida, Ciliophora). Bulletin of the British Museum (Natural History), 44: 191-247. (  0) 0) |
Dujardin, F., 1841. Histoire naturelle des zoophytes. Infusoires, comprénant la physiologie et la classification de ces animaux, et la manière de les étudier al'aide du microscope. Libraire Encyclopédique de Roret, Paris.
(  0) 0) |
Edgar, R. C., 2004. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32: 1792-1797. DOI:10.1093/nar/gkh340 (  0) 0) |
Fan, Y. B., Warren, A., Al-Rasheid, K. A. S., Chen, X. and Shao, C., 2013. Morphology and SSU rRNA gene-based phylogeny of two Diophrys-like ciliates from northern China, with notes on morphogenesis of Pseudodiophrys nigricans (Protozoa, Ciliophora). Journal of Morphology, 274: 395-403. DOI:10.1002/jmor.20097 (  0) 0) |
Gao, F., Warren, A., Zhang, Q. Q., Gong, J., Miao, M., Sun, P., Xu, D. P., Huang, J., Yi, Z. Z. and Song, W. B., 2016. The all- data-based evolutionary hypothesis of ciliated protists with a revised classification of the phylum Ciliophora (Eukaryota, Alveolata). Scientific Reports, 6: 24874. DOI:10.1038/srep24874 (  0) 0) |
Hill, B. F. and Borror, A. C., 1992. Redefinition of the genera Diophrys and Paradiophrys and establishment of the genus Diophryopsis n. g. (Ciliophora, Hypotrichida): Implication for the species problem. Journal of Protozoology, 39: 144-153. (  0) 0) |
Hu, X. Z., 2008. Cortical structure in non-dividing and dividing Diophrys japonica spec. nov. (Ciliophora, Euplotida) with notes on morphological variation. European Journal of Protistology, 44: 115-129. (  0) 0) |
Hu, X. Z., Huang, J. and Warren, A., 2012. The morphology and phylogeny of two euplotid ciliates, Diophrys blakeneyensis spec. nov. and Diophrys oligothrix Borror, 1965 (Protozoa, Ciliophora, Euplotida). International Journal of Systematic and Evolutionary Microbiology, 62: 2757-2773. (  0) 0) |
Hu, X. Z., Lin, X. F., and Song, W. B., 2019. Ciliate Atlas: Species Found in the South China Sea. Science Press, Beijing, 936pp.
(  0) 0) |
Huang, J., Dunthorn, M. and Song, W. B., 2012. Expanding character sampling for the molecular phylogeny of euplotid ciliates (Protozoa, Ciliophora) using three markers, with a fo- cus on the family Uronychiidae. Molecular Phylogenetics and Evolution, 63: 598-605. DOI:10.1016/j.ympev.2012.02.007 (  0) 0) |
Jankowski, A. W., 1979. Revision of the order Hypotrichida Stein, 1859. Generic catalogue, phylogeny, taxonomy. Proceedings of the Academy of Sciences of the USSR, 86: 48-85. (  0) 0) |
Jankowski, A. W., 2007. Phylum Ciliophora Doflein, 1901. In: Protista. Part 2. Handbook on Zoology. Alimov, A. F., ed.. St. Petersburg, Nauka, 415-993.
(  0) 0) |
Jiang, J. M. and Song, W. B., 2010. Two new Diophrys-like genera and their type species, Apodiophrys ovalis n. g., n. sp. and Heterodiophrys zhui n. g., n. sp. (Ciliophora: Euplotida), with notes on their molecular phylogeny. Journal of Eukaryotic Microbiology, 57: 354-361. (  0) 0) |
Jiang, J. M., Warren, A. and Song, W. B., 2011. Morphology and molecular phylogeny of two new marine euplotids, Pseudodiophrys nigricans n. g., n. sp., and Paradiophrys zhangi n. sp. (Ciliophora: Euplotida). Journal of Eukaryotic Microbiology, 58: 437-445. (  0) 0) |
Kahl, A., 1932. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria). 3. Spirotricha. Die Tierwelt Deutshlands und der Angrenzenden Meeresteile, 25: 399-650. (  0) 0) |
Kattar, M. R., 1970. Estudo dos protozoarios ciliados psamofilos do litoral Brasileiro. Boletim de Zoologia e Biologia Marinha (Nova Serie), Sao Paulo, 27: 123-206. (  0) 0) |
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Mentjies, P. and Drummond, A., 2012. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647-1649. DOI:10.1093/bioinformatics/bts199 (  0) 0) |
Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K., 2018. Mega X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35: 1547-1549. DOI:10.1093/molbev/msy096 (  0) 0) |
Lian, C. Y., Zhang, T. T., Al-Rasheid, K. A. S., Yu, Y. H., Jiang, J. M., and Huang, J., 2019. Morphology and SSU rDNA-based phylogeny of two Euplotes species from China: W. wuhanensis sp. n. and E. muscicola Kahl, 1932 (Ciliophora, Euplotida).European Journal of Protistology, 6: 1-14.
(  0) 0) |
Luo, X. T., Hu, X. Z. and Suzuki, T., 2014. Microscopic investigation of three species of Diophrys (Ciliophora, Euplotida, Uronychiidae) from Japan, including Diophrys peculiaris nov. spec. European Journal of Protistology, 50: 496-508. DOI:10.1016/j.ejop.2014.08.003 (  0) 0) |
Lynn, D. H., 2008. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. 3rd edition. Springer Press, Dordrecht, 605pp.
(  0) 0) |
Medlin, L., Elwood, H. J., Stickel, S. and Sogin, M. L., 1988. The characterization of enzymatically amplified eukaryotic 16S- like rRNA-coding regions. Gene, 71: 491-499. DOI:10.1016/0378-1119(88)90066-2 (  0) 0) |
Nylander, J. A. A., 2008. MrModeltest 2.3. Department of Systematic Zoology, Uppsala University, Uppsala, Sweden.
(  0) 0) |
Raikov, I. B. and Kovaleva, V. G., 1968. Complements to the fauna of psammobiotic ciliates of the Japan Sea (Posjet Gulf). Acta Protozoologica, 6: 309-333. (  0) 0) |
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M. A. and Huelsenbeck, J. P., 2012. MrBayes 3. 2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Society of Systematic Biologists, 61: 539-542. (  0) 0) |
Ruinen, J., 1938. Notizenüber Ciliaten aus konzentrierten Salzgewässern. Zoologische Mededelingen, 20: 243-256. (  0) 0) |
Shen, Z., Yi, Z. Z. and Warren, A., 2011. The morphology, ontogeny, and small subunit rRNA gene sequence analysis of Diophrys parappendiculata n. sp, (Protozoa, Ciliophora, Euplotida), a new marine ciliate from coastal waters of southern China. Journal of Eukaryotic Microbiology, 58: 242-248. (  0) 0) |
Sheng, Y. L., He, M., Zhao, F. Q., Shao, C. and Miao, M., 2018. Phylogenetic relationship analyses of complicated class Spirotrichea based on transcriptomes from three diverse microbial eukaryotes: Uroleptopsis citrina, Euplotes vannus and Protocruzia tuzeti. Molecular Phylogenetics and Evolution, 129: 338-345. DOI:10.1016/j.ympev.2018.06.025 (  0) 0) |
Song, W. B. and Packroff, G., 1997. Taxonomische Untersuchungen an marinen Ciliaten aus China mit Beschreibungen von zwei neuen Arten, Strombidium globosaneum nov. spec. und S. platum nov. spec. (Protozoa, Ciliophora). Archiv FürProtistenkunde, 147: 331-360. (  0) 0) |
Song, W. B. and Wilbert, N., 1994. Morphogenesis of the marine ciliate Diophrys oligothrix Borror, 1965 during the cell division (Protozoa, Ciliophora, Hypotrichida). European Journal of Protistology, 30: 38-44. (  0) 0) |
Song, W. B. and Wilbert, N., 2002. Faunistic studies on marine ciliates from the Antarctic benthic area, including descriptions of one epizoic form, 6 new species and 2 new genera (Protozoa: Ciliophora). Acta Protozoologica, 41: 23-61. (  0) 0) |
Song, W. B., Shao, C., Yi, Z. Z., Li, L. Q., Warren, A., Al-Rasheid, K. A. S. and Yang, J. P., 2009a. The morphology, morphogenesis and SSU rRNA gene sequence of a new marine ciliate, Diophrys apoligothrix spec. nov. (Ciliophora; Euplotida). European Journal of Protistology, 45: 38-50. (  0) 0) |
Song, W. B., Warren, A., and Hu, X. Z., 2009b.Free-Living Ciliates in the Bohai and Yellow Seas, China. Science Press, Beijing, 518pp.
(  0) 0) |
Song, W. B., Wilbert, N., Al-Rasheid, K. A. S., Warren, A., Shao, C., Long, H. A., Yi, Z. Z. and Li, L. Q., 2007. Redescriptions of two marine hypotrichous ciliates, Diophrys irmgard and D. hystrix (Ciliophora, Euplotida), with a brief revision of the genus Diophrys. Journal of Eukaryotic Microbiology, 54: 283-296. (  0) 0) |
Stamatakis, A., 2014. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312-1313. (  0) 0) |
Wang, Y. R., Wang, C. D., Jiang, Y. H., Katz, L. A., Gao, F. and Yan, Y., 2019. Further analyses of variation of ribosome DNA copy number and polymorphism in ciliates provide insights relevant to studies of both molecular ecology and phylogeny. Science China Life Sciences, 62: 203-214. (  0) 0) |
Wilbert, N., 1975. Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos, 64: 171-179. (  0) 0) |
Zhao, Y., Yi, Z. Z., Gentekaki, E., Zhan, A., Al-Farraj, S. A. and Song, W. B., 2016. Utility of combining morphological characters, nuclear and mitochondrial genes: An attempt to resolve the conflicts of species identification for ciliated protists. Molecular Phylogenetics and Evolution, 94: 718-729. (  0) 0) |
 2020, Vol. 19
2020, Vol. 19


