Antarctic krill (Euphausia superba) is a shrimp-like invertebrate widely distributed in the Antarctic Ocean. It plays important roles as a basic supplier in the marine ecosystem and provides high-quality proteins with low-fat content to human consumers (Cavan et al., 2019). Recent studies show that the total capacity of Antarctic krill is approximately equal to the global fishery outputs (Zerbini et al., 2019). Its abundant availability has the potential to solve the shortage of natural resources for human consumption (Descamps et al., 2016; Cavan et al., 2019). Therefore, the potential value of Antarctic krill on commercial and biological scales have drawn considerable attention. Products and by-products of Antarctic krill were gradually developed. Initially, early products of Antarctic krill were boiled, frozen, and peeled. These low-value products were approved solely for and widely used in aquaculture as food additives (Bao et al., 2019; Ma et al., 2019; Xie et al., 2019). Recently, some attempts on the production of high-end products have been successfully applied. Most of these high-value products are prepared as bioactive peptides, with 10% of the total production being used for official fishing competitions (Ji et al., 2017; Lan et al., 2019; Zhao et al., 2019). Concurrently, many applications of Antarctic krill for human consumption have been reported by adding this seafood to soup. Many methodological studies are in progress to increase consumption and utilization of Antarctic krill as a portion of human food with higher nutritional value (Suzuki and Shibata, 1990; Kidd, 2007; Parolini et al., 2017).
Ready-to-eat roasted Antarctic krill, which imitates dried shrimp, has gained popularity with consumers in China on account of its taste. The products were made under low water content (28%). The low water content can lead to bacterial inhibition, as well as contribute to a crispy texture and colorful appearance with little changes in flavor. However, the low water content caused by thermal treatment may also lead to the growth of mold and lipid oxidation, which consequently leads to off-flavor development and rancidity, thereby directly impacting the shelf life of these products (Wang et al., 2019). Traditionally, adding antioxidants and acid, such as deoxidizers, citric acid, and sodium diacetate, during food processing are common strategies for inhibiting mold and overcoming lipid oxidation, particularly in ready-to-eat heat-treated products (Hidalgo and Zamora, 2017; Butts-Wilmsmeyer et al., 2018).
A high level of fluoride in Antarctic krill is a major concern for its utilization. The fluoride is a component in the exoskeleton of krill. The contents of it in Antarctic krill range between 500 – 2400 mg kg−1 in the whole body, 5 – 570 mg kg−1 in the muscle, and 1800 – 4260 mg kg−1 in the cuticle (Sands et al., 1998; Camargo, 2003; Peng et al., 2019). Fluoride is accumulated in the body of animals, although the toxic effects of accumulation are unknown. Considering that a large amount of Antarctic krill is consumed, and the harmful effects of fluoride is still uncertain, studies are required to address this controversy.
At present, the evaluation of shelf life and safety of ready-to-eat roasted Antarctic krill, particularly stored at 25℃, is limited. The aims of this study were 1) to investigate the quality changes of ready-to-eat roasted Antarctic krill during storage, 2) to analyze the advantage of using sodium diacetate and antioxidants during food processing on the quality of the products, and 3) to evaluate the safety of fluoride residue in ready-to-eat roasted Antarctic krill.
2 Materials and Methods 2.1 Sample CollectionAntarctic krill were captured at the FAO48.2 area in the Antarctic Ocean in March 2019, and rapidly frozen and stored at −20℃ in the polar research vessel. The Antarctic krill was delivered to the laboratory in May and stored at −80℃ before use.
2.2 Preparation of Ready-to-Eat Roasted Antarctic KrillFrozen Antarctic krill (1 kg) were thawed under running tap water at 25℃ until it completely separated. The Antarctic krill were manually selected and then cooked in boiling water for 10 min. Boiled Antarctic krill were cooled in ice-water for 15 min. The water from the boiled Antarctic krill was removed naturally at 4℃ for 2 h and Antarctic krill were subsequently divided into two groups. Each group was seasoned with sugar (12.0%), salt (2.0%) and monosodium glutamate (0.5%) correspondingly to the sample weights. One group with adding sodium diacetate (0.05%) was termed as SD group. Another group without sodium diacetate was employed as control group and was termed as CT group. Both of them were roasted at 155℃ until the water content of Antarctic krill reached 28%. All roasted Antarctic krill were sorted individually into 50 g portions, and immediately vacuum-sealed in a sterile package after cooling down at 25℃ for 30 min. The packaged samples were sterilized at 90℃ for 50 min and then cooled in running water for 20 min. Food grade iron powder organic deoxidizer oxygen absorber packets (20 mg) (Shenzhen Topcod Packing Materials Co., Ltd., China) was added into one-half of the SD samples, and this group was termed as SDD group. Three groups of ready-to-eat roasted Antarctic krill were stored at 25℃ for further study.
2.3 Microbiological AnalysisTotal viable count (TVC) and number of mold formed on ready-to-eat roasted Antarctic krill were determined during the storage at 25℃. In brief, the ready-to-eat roasted Antarctic krill (25 g) were aseptically transferred into sterile stomacher bags, diluted in 225 mL saline (0.85%) and homogenized in a Stomacher Masticator (IUL, Spain) for 30 s. A serial dilution (1:10) was prepared with saline and 100 μL of the solution was spread in triplicate onto the following agar plates: Plate count agar (PCA) (Oxoid, Germany) for TVC, and Rose Bengal Medium (RBM) (Solarbio, China) for mold detection. The TVC was recorded after 48 h of incubation at 30℃, and the number of mold on RBM was counted after 5 days of incubation at 28℃.
2.4 Total Volatile Basic Nitrogen (TVB-N) DeterminationApproximately 10 g of roasted Antarctic krill were blended with 100 mL distilled water and equilibrated at 25℃ for 30 min. The solution was filtered using filter paper (Ø11 cm, Hangzhou Xinhua Paper Industry Co., Ltd., China). The filtrate was used for TVB-N content measurement according to Xia et al. (2016). The amount of TVB-N was measured in duplicate and expressed as mg (100 g)−1.
2.5 Thiobarbituric Acid Reactive Substances (TBARS) DeterminationLipid oxidation of ready-to-eat roasted Antarctic krill was determined by analyzing the amount of TBARS described previously by Ghani et al. (2017). Approximately 10 g of roasted Antarctic krill were chopped and homogenized in 50 mL trichloroacetic acid (TCA) (7.5% with 0.1% EDTA) for 30 min. Then the solution was filtered twice by using filter paper (Ø11 cm, Hangzhou Xinhua Paper Industry Co., Ltd., China). The filtrate (5 mL) was blended with 5 mL of thiobarbituric acid (TBA) solution (0.02 mol L−1) and incubated at 90℃ for 40 min. The solution was centrifuged at 3000 r min−1 for 5 min after incubation. The supernatant (5 mL) was blended with 5 mL of chloroform and placed at room temperature for 10 min. The optical density of the supernatant was measured at 532 nm (A532) and 600 nm (A600). The value of TBA was calculated by using the following formulation:
| $T B A=\frac{A_{532}-A_{600}}{155 \times 0.1} \times 72.6 \times 100. $ |
The value of TBA was expressed as mg (100 g)−1. A532 and A600 represent the optical densities at 532 nm and 600 nm, respectively. The TBARS value converted the TBA values into the amounts of malondialdehyde (mg MA) per kg of roasted Antarctic krill.
2.6 Color PropertiesRoasted Antarctic krill (20 g) were cut and measured by using a colorimeter (Microptix Corporation S560, USA). The color parameter of roasted Antarctic krill was expressed by the b* (yellowness) values. Each sample was analyzed in triplicate.
2.7 Fluoride ContentFluoride contents for all samples were detected by ion chromatography. Samples (0.5 g) were dissolved with 8 mL of deionized water containing hydrochloric acid (v/v, 11:1), and subsequently digested for 1 h. Deionized water was added to the digestion solution to make up 100 mL. The mixture was kept at 25℃ for 10 min and then centrifuged at 4000 r min−1 for 15 min. The whole supernatant was collected successively via the aqueous phase membrane (0.22 μm) and IC-Ag10 phase (Pu'an Co., Ltd., Shanghai). Fluoride contents of all samples from the supernatant were detected following the method described by Li et al. (2018). The values of the fluoride content were expressed as mg kg−1.
2.8 Animal ExperimentA total of 50 female KunMing rats (15 – 21 g) were obtained from Fudan University (Shanghai, China). All animals were individually housed at 25℃. Rats were starved for at least 12 h prior to the commencement of the experiment, and they were randomly assigned into 5 groups (n = 10 per group). All groups were fed identically with fluoridefree rat food. Among all groups, one was intragastrically fed with 1 mL of sodium fluoride-containing deionization water (300 mg mL−1) daily, denoted as sodium fluoride group. Three groups of rats were intragastrically fed with different amounts of roasted Antarctic krill (converted as 100, 300, and 500 mg L−1 of fluoride, marked as T1, T2 and T3, respectively) in the deionization water. The rats fed with fluoride-free deionization water were considered as a control group. All rats were sacrificed following ethical protocols and procedures 10 days after fluoride-feeding treatment (Jech, 1979). Terminal blood samples from all rats were collected by heart puncture according to Sun et al. (2017) and the serum samples were stored at −80℃ before the investigation. The small intestine, liver, kidney, and thighbone of all rats were collected and stored at −20℃ before use. Approval was obtained from the Fudan University Institutional Review Board. All experiments with rats were approved by the Fudan University's Animal Care and Use Committee, and were performed following the Fudan University guidelines on animal care.
2.9 Sensory EvaluationSeven well-trained panelists volunteered to evaluate the ready-to-eat roasted Antarctic krill during the whole storage period. The samples with different treatments were randomly labeled and served to the panelists. All panelists were asked to score texture, color, odor, and flavor using a tenpoint scale, where point 10 was the highest quality score, 0 was the lowest, and point 5 was acceptable level according to Haouet et al. (2018).
2.10 Statistical AnalysisAll experiments were performed a minimum of three times. For each experiment, the results were recorded as mean ± standard deviation. A two-way analysis of variance (ANOVA) was carried out by using SPSS 24.0 (SPSS Inc., Chicago, IL, USA). Differences in the mean values of all results were calculated by using Duncan's multiple comparisons test. Statistical significance was characterized by P < 0.05.
3 Results 3.1 Shelf Life of Ready-to-Eat Roasted Antarctic Krill with Different TreatmentsSensory evaluation is one of the intuitive indicators of consumer acceptance of products. It can directly reflect consumers' demand and the quality changes of the products during storage. Therefore, sensory evaluation can be one of the effective indicators to evaluate the shelf life of products. Table 1 shows the sensory scores of the ready-to-eat roasted Antarctic krill with different treatments. In general, compared to the control group, the shelf life of the krill samples were extended by the treatments. The sensory score of CT decreased rapidly during the storage at 25℃, and it was close to the sensory rejection point at day 18. The sensory rejection point of the SD group could be extended to 40 days. A similar trend was found in the SDD group, while the sensory scores of SDD groups reached rejection point after approximately 70 days.
|
|
Table 1 Sensory evaluation of ready-to-eat roasted Antarctic krill with different treatments during storage |
Counts of the total bacterial population in ready-to-eat roasted Antarctic krill with different treatments are shown in Fig.1. While a significant difference between the control group (CT) and treated groups (SD and SDD) was noted (P < 0.05), the difference between the SD and SDD groups was not significant (P > 0.05). The bacterial residues were approximately (1.78 ± 0.13) log (CFU g−1) initially, indicating that the procedures were performed in a highly hygienic manner. The TVCs of all samples increased at similar rates, while the CT group increased faster compared to treated groups. The TVC of the CT group reached (4.62 ± 0.19) lg (CFU g−1) after 18 days. The SD and SDD groups demonstrated a slow logarithmic phase at approximately days 6 and 10, respectively. The TVCs of SD and SDD groups reached (4.58 ± 0.08) lg (CFU g−1) at day 33 and (4.52 ± 0.06) lg (CFU g−1) at day 85, respectively. According to the Chinese standard for roasted shrimp (SC/T 3305-2003), they were considered unacceptable once the TVC reaches 4.5 lg (CFU g−1). Hence, when they were stored at 25℃, the shelf life of CT, SD, and SDD groups were approximately 18, 33 and 70 days, respectively.
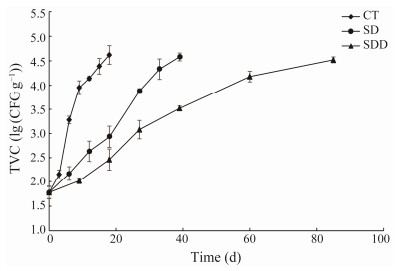
|
Fig. 1 Total viable count changes of ready-to-eat roasted Antarctic krill with different treatments during storage. |
Counts of mold in ready-to-eat roasted Antarctic krill with different treatments are shown in Table 2. Mold counts in CT showed a significant difference between SD and SDD groups (P < 0.05). No mold was found on all samples at the commencement of storage. The mold counts of CT increased from 1.9×102 to 4.7×105 on day 18, whereas mold on SD plates was found after 6 days of storage and increased to 5.2×106 by the end of its shelf life. Additionally, the mold of SDD was found after 12 days, and the number reached 1.6×106 on day 90.
|
|
Table 2 Numeral changes in mold of ready-to-eat roasted Antarctic krill with different treatments during storage |
In our study, Fig.2 shows the TVB-N values of ready-toeat roasted Antarctic krill with different treatments during the storage. The initial TVB-N value was approximately (5.76 ± 0.09) mg (100 g)−1. The values of TVB-N in the control group increased rapidly during the storage period, and reached (10.96 ± 0.33) mg (100 g)−1 at the end of its shelf life, while the TVB-N values in SD and SDD groups were maintained below 6.50 mg (100 g)−1. The TVB-N values from the SD group increased after 20 days and reached (12.11 ± 0.07) mg (100 g)−1 on day 33. TVB-N values from SDD groups increased linearly to (10.88 ± 0.15) mg (100 g)−1 at the end of its shelf life.
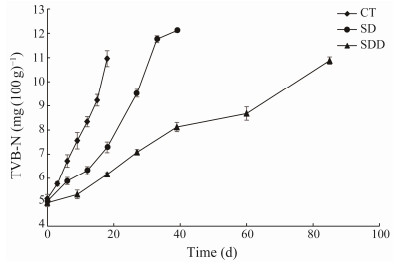
|
Fig. 2 Changes in TVB-N of ready-to-eat roasted Antarctic krill with different treatments during storage. |
Thiobarbituric acid reactive substances determination changes of TBARS values of ready-to-eat roasted Antarctic krill are shown in Fig.3. Thiobarbituric acid reactive substances values of CT and SD groups showed an increasing trend, changing from (22.85 ± 0.23) mg MA kg−1 (initial point) to approximately 95 mg MA kg−1 for the first 20 days. The TBARS values from CT and SD groups increased to approximately 120 mg MA kg−1 at 33 and 40 days, respectively. The TBARS values of SDD group changed slowly during the whole storage period. They increased from (22.85 ± 0.23) mg MA kg−1 to (43.30 ± 0.14) mg MA kg−1 by day 40, and eventually reached (91.88 ± 0.85) mg MA kg−1 at the end of its shelf life.
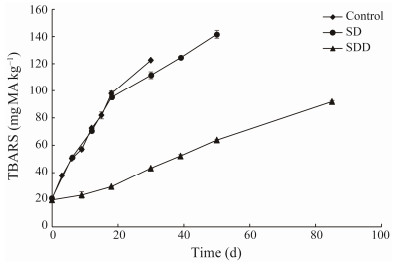
|
Fig. 3 Changes in TBARS of ready-to-eat roasted Antarctic krill with different treatments during storage. |
Color in seafood products is a critical characteristic that provokes consumer purchasing decisions. The enhancement of b* values lead to the stimulation of acceptability of the products' appearance (Shaviklo et al., 2011). The b* values were used to determine the color properties and quality changes of ready-to-eat roasted Antarctic krill in this study (Table 3). The b* values demonstrated increasing trend during storage, and varied from 11.63 to 27.48 for the CT groups. The b* values for SD and SDD groups increased gradually, and reached 29.23 and 28.60 at the end of their shelf life, respectively.
|
|
Table 3 Changes in b* value of ready-to-eat roasted Antarctic krill with different treatments during storage |
Table 4 shows the concentrations of fluoride residues remaining in different tissues of rats. The concentrations of fluoride residues in rats fed with ready-to-eat roasted Antarctic krill shows a concentration-dependent trend. The amounts of fluoride residues in the kidney, liver, blood and small intestine maintained a low dose (less than 1 mg kg−1) and showed no significant difference to the control group (P > 0.05). Conversely, the concentration of fluoride in the thighbone remained high level, while the fluoride content of the control group was (500.13 ± 5.02) mg kg−1. The tested groups showed a significantly higher concentration than the control group (P < 0.05). The concentration of fluoride in the sodium fluoride group reached (4621.01 ± 28.67) mg kg−1, and the concentrations of fluoride from Antarctic krill feeding groups were (1760.03 ± 38.21), (2371.52 ± 42.15), and (3615.44 ± 30.53) mg kg−1, respectively.
|
|
Table 4 Concentrations of fluoride residues in different tissue (blood, small intestine, liver, kidney and thighbone) of rats |
Ready-to-eat roasted Antarctic krill is a new approach in an attempt to increase the utilization and processing of this food source. The product was designed at low water content (28%), which can provide a crispy texture and inhibit bacterial proliferation. Concurrently, the usage of preservatives, such as sodium diacetate and food-graded deoxidizer, has been suggested as an antibacterial and anti-oxidation strategy. Particularly, the use of diacetate is an effective means to prevent the growth of bacteria in ready-to-eat foods. Furthermore, diacetate used in the processing of ready-to-eat products is active immediately and is effective while the product is at its freshest situation, which considerably hinders bacterial growth. Jiang et al. (2011) reported the inhibitory effect of diacetate for reducing the bacteria in readyto-eat smoked salmon. The results suggested that diacetate treatment at consumption level reduced the TVC throughout the shelf-life period, providing an important economic advantage of antimicrobial effect in the food industry. Alternatively, a high amount of lipid in Antarctic krill is vulnerable to high temperatures. Both flavor and color indicative of lipid oxidation are critical for the quality of ready-to-eat roasted Antarctic krill. The TBARS values were significantly decreased in the SDD group, even at 25℃. Low temperatures are predominantly used to restrain the lipid oxidation from seafood products. Since cost is essential for companies to consider, the use of preservatives are recommended to improve the quality of the products. Notably, combining sodium diacetate and food-graded deoxidizer offers potential for increasing the quality and prolonging the shelf life of ready-to-eat roasted Antarctic krill, and this combination is recommended in the treatment of ready-to-eat products.
A positive effect of deoxidizer on color changes of ready-to-eat roasted Antarctic krill was observed (Table 3). The effect indicates that deoxidizer slowed the oxidation process of ready-to-eat roasted Antarctic krill during the storage. Importantly, this effect maintains the red color of Antarctic krill per se. The color evaluated by b* value reflects the quality changes of ready-to-eat roasted Antarctic krill, showing quality changes are not subject to the L* (lightness) and a* (redness) values. Similar results showing a positive correlation between b* values and product quality were found by Wang et al. (2018).
It has been reported that consumption of Antarctic krill can cause the accumulation of fluoride in the body (Zuo et al., 2018). Some reports pertaining to krill-based dietary uptake by fish found fluoride accumulation in the muscle and bone of the fish (Yoshitomi and Nagano, 2012; Chen et al., 2013). Fluoride concentrations in vertebral bone of krill-eating fish increased due to the increasing proportion of Antarctic krill. The fluoride concentrations in vertebral bone from those fish remained as 33000 mg kg−1 for Champsocephalus gunnari, 15000 mg kg−1 for Notothenia rossii, and 2150 mg kg−1 for Seriola quinqueradiata (Yoshitomi and Nagano, 2012). The results indicate that the fluoride in fish bone likely depends on fish species and daily intake. The high concentration of fluoride was exclusively observ-ed in the thighbone and rare concentrations in other organs in our study. The fluoride may be accumulated in the muscle via a long-term dietary uptake to reach high values. Similar results have been found in Adélie penguins (Pygoscelis adeliae), whose femur possess a high concentration of accumulated fluoride (Chen et al., 2013). Simultaneously, fluoride concentration in the sodium fluoride treatment group was higher than that in all other tested groups, indicating the chemical form of fluoride in ready-to-eat roasted Antarctic krill is different from sodium fluoride. Tenuta-Filho and Alvarenga (1999) analyzed the distribution and chemical forms of fluoride in Antarctic krill. They noted that the exchangeable form of fluoride was a fundamental form in Antarctic krill. Importantly, the concentration of fluoride in all tested groups showed a linear relationship with ingested concentration. The results indicate that the concentration of fluoride is possibly predictable by monitoring the dietary uptake of Antarctic krill. FDA recommended an upper limit of 100 mg kg−1 as sodium fluoride for humans (Turner, 1996). Accordingly, 500 mg kg−1 of fluoride or less from ready-to-eat roasted Antarctic krill would be safe for human. Considering that Antarctic krill is not the primary food source for humans, the consumption of ready-to-eat roasted Antarctic krill may show less hazardous effects caused by fluoride accumulation. Further investigation is required to provide accurate limitations on the quantity of ready-to-eat roasted Antarctic krill for use.
5 ConclusionsIn this study, ready-to-eat roasted Antarctic krill (Euphausia superba) with SD and SDD treatment preserves the quality of this product by reducing microorganisms and inhibiting lipid oxidation. Compared with that of the CT groups, The usage of SD and SDD, in a meanwhile, allowed the products to maintain an attractive color and pleasing sensory quality during storage at 25℃, the TVC and number of mold maintain low values (P < 0.05) and TVB-N and TBARS values increased slowly (P < 0.05). The improvement in the quality of ready-to-eat roasted Antarctic krill extended its shelf life. Accordingly, the shelf life of ready-to-eat roasted Antarctic krill prolonged up to 15 days for SD treatment and 52 days for SDD treatment, respectively.
The fluoride residues from ready-to-eat roasted Antarctic krill were substantially accumulated in the thighbone of rats compared to other organs. The concentrations of fluoride residues remained less than 1 mg kg−1 in small intestine, blood, live and kidney. While the fluoride concentrations exclusively in thighbone showed a fluoride concentration-dependent phenomenon and to be less than sodium fluoride feeding group. The chemical form of fluoride residues from ready-to-eat roasted Antarctic krill is uncertain, although it is different from sodium fluoride commonly delimited by the FDA. Hence, further studies are required to evaluate the limitations on human consumption of ready-to-eat roasted Antarctic krill.
AcknowledgementsThis work was supported by the National Key R & D Program of China (Nos. 2020YFD0901204, 2017YFC1600706), and the Natural Science Foundation of Shanghai (No. 22ZR 1478500). We highly appreciated that the 36th Antarctic expedition of China supported the samples of Antarctic krill.
Bao, J., Chen, L., and Liu, T., 2019. Dandelion polysaccharide suppresses lipid oxidation in Antarctic krill (Euphausia superba). International Journal of Biological Macromolecules, 133: 1164-1167. DOI:10.1016/j.ijbiomac.2019.04.205 (  0) 0) |
Butts-Wilmsmeyer, C. J., Mumm, R. H., Rausch, K. D., Kandhola, G., Yana, N. A., Happ, M. M., et al., 2018. Changes in phenolic acid content in maize during food product processing. Journal of Agricultural and Food Chemistry, 66: 3378-3385. DOI:10.1021/acs.jafc.7b05242 (  0) 0) |
Camargo, J. A., 2003. Fluoride toxicity to aquatic organisms: A review. Chemosphere, 50: 251-264. DOI:10.1016/S0045-6535(02)00498-8 (  0) 0) |
Cavan, E. L., Belcher, A., Atkinson, A., Hill, S. L., Kawaguchi, S., McCormack, S., et al., 2019. The importance of Antarctic krill in biogeochemical cycles. Nature Communication, 10: 4742-4754. DOI:10.1038/s41467-019-12668-7 (  0) 0) |
Chen, J., Cao, J., Wang, J., Jia, R., Xue, R., Li, Y., et al., 2013. Effects of fluoride on growth, body composition, and serum biochemical profile in a freshwater teleost, Cyprinus carpio. Environmental Toxicology and Chemistry, 32: 2315-2321. DOI:10.1002/etc.2305 (  0) 0) |
Descamps, S., Tarroux, A., Cherel, Y., Delord, K., Godø, O. R., Kato, A., et al., 2016. At-sea distribution and prey selection of Antarctic petrels and commercial krill fisheries. PLoS One, 11: 1-18. (  0) 0) |
Ghani, M. A., Barril, C., Bedgood, D. R., and Prenzler, P. D., 2017. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chemistry, 230: 195-207. DOI:10.1016/j.foodchem.2017.02.127 (  0) 0) |
Haouet, M. N., Tommasino, M., Mercuri, M. L., Benedetti, F., Bella, S. D., Framboas, M., et al., 2018. Experimental accelerated shelf life determination of a ready-to-eat processed food. Italian Journal of Food Safety, 7: 189-192. (  0) 0) |
Hidalgo, F. J., and Zamora, R., 2017. Food processing antioxidants. Advances in Food and Nutrition Research, 81: 31-64. (  0) 0) |
Jech, J. A., 1979. Comparative uptake of fluoride from sodium fluoride, ammonium fluoride, and barium fluoride in rat teeth when predominantly administered in the pre-eruptive stage of development. Calcified Tissue International, 27: 117-119. DOI:10.1007/BF02441172 (  0) 0) |
Ji, W., Zhang, C., and Ji, L., 2017. Two novel bioactive peptides from Antarctic krill with dual angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Journal of Food Science, 82: 1742-1749. DOI:10.1111/1750-3841.13735 (  0) 0) |
Jiang, Z., Neetoo, H., and Chen, H., 2011. Control of Listeria monocytogenes on cold-smoked salmon using chitosan-based antimicrobial coatings and films. Journal of Food Science, 76: 22-26. DOI:10.1111/j.1750-3841.2010.01925.x (  0) 0) |
Kidd, P. M., 2007. Omega-3 DHA and EPA for cognition, behavior, and mood: Clinical findings and structural-functional synergies with cell membrane phospholipids. Alternative Medicine Review, 12: 207-227. (  0) 0) |
Lan, C., Zhao, Y. Q., Li, X. R., and Wang, B., 2019. High Fischer ratio oligopeptides determination from Antartic krill: Preparation, peptides profiles, and in vitro antioxidant activity. Journal of Food Biochemistry, 43: 1-12. (  0) 0) |
Li, X., Zhang, M., Wang, Y., Wang, X., Ma, H., Li, P., et al., 2018. Direct detection of fluoride ions in aquatic samples by surface-enhanced Raman scattering. Talanta, 178: 9-14. DOI:10.1016/j.talanta.2017.08.101 (  0) 0) |
Ma, X., Liu, C., Wang, C., Ma, X., Che, S., Feng, X., et al., 2019. Effects of three products from Antarctic krill on the nitrogen balance, growth, and antioxidation status of rats. Food Science and Nutrition, 7: 2760-2768. DOI:10.1002/fsn3.1140 (  0) 0) |
Parolini, C., Bjorndal, B., Busnelli, M., Manzini, S., Ganzetti, G. S., Dellera, F., et al., 2017. Effect of dietary components from Antarctic krill on atherosclerosis in apoE-deficient mice. Molecular Nutrition and Food Research, 61: 1700098-1700108. DOI:10.1002/mnfr.201700098 (  0) 0) |
Peng, Y., Ji, W., Zhang, D., Ji, H., and Liu, S., 2019. Composition and content analysis of fluoride in inorganic salts of the integument of Antarctic krill (Euphausia superba). Scientific Reports, 9: 7853. DOI:10.1038/s41598-019-44337-6 (  0) 0) |
Sands, M., Nicol, S., and McMinn, A., 1998. Fluoride in Antarctic marine crustaceans. Marine Biology, 132: 591-598. DOI:10.1007/s002270050424 (  0) 0) |
Shaviklo, G. R., Olafsdottir, A., Sveinsdottir, K., Thorkelsson, G., and Rafipour, F., 2011. Quality characteristics and consumer acceptance of a high fish protein puffed corn-fish snack. Journal of Food Science and Technology, 48: 668-676. DOI:10.1007/s13197-010-0191-1 (  0) 0) |
Sun, D., Zhang, L., Chen, H., Feng, R., Cao, P., and Liu, Y., 2017. Effects of Antarctic krill oil on lipid and glucose metabolism in C57BL/6J mice fed with high fat diet. Lipids in Health and Disease, 16: 218-225. DOI:10.1186/s12944-017-0601-8 (  0) 0) |
Suzuki, T., and Shibata, N., 1990. The utilization of Antarctic krill for human food. Food Reviews International, 6: 119-147. DOI:10.1080/87559129009540863 (  0) 0) |
Tenuta-Filho, A., and Alvarenga, R. C., 1999. Reduction of the bioavailability of fluoride from Antarctic krill by calcium. International Journal of Food Sciences and Nutrition, 50: 297-302. DOI:10.1080/096374899101184 (  0) 0) |
Turner, C. H., 1996. Fluoride and the FDA: A curious case. Journal of Bone and Mineral Research, 11: 1369-1371. (  0) 0) |
Wang, H., Wu, Y., Wang, N., Yang, L., and Zhou, Y., 2019. Effect of water content of high-amylose corn starch and glutinous rice starch combined with lipids on formation of starch-lipid complexes during deep-fat frying. Food Chemistry, 278: 515-522. DOI:10.1016/j.foodchem.2018.11.092 (  0) 0) |
Wang, Z. C., Yan, Y., Nisar, T., Sun, L., Su, P., Chen, D. W., et al., 2018. Influence of postmortem treatment with nitric oxide on the muscle color and color stability of tilapia (Oreochromis niloticus) fillets. Nitric Oxide, 76: 122-128. DOI:10.1016/j.niox.2017.09.009 (  0) 0) |
Xia, Z., Zhai, X., Liu, B., and Mo, Y., 2016. Conductometric titration to determine total volatile basic nitrogen (TVB-N) for post-mortem interval (PMI). Journal of Forensic and Legal Medicien, 44: 133-137. DOI:10.1016/j.jflm.2016.10.008 (  0) 0) |
Xie, D., Gong, M., Wei, W., Jin, J., Wang, X., Wang, X., et al., 2019. Antarctic krill (Euphausia superba) oil: A comprehensive review of chemical composition, extraction technologies, health benefits, and current applications. Comprehensive Reviews in Food Science and Food Safety, 18: 514-534. (  0) 0) |
Yoshitomi, B., and Nagano, I., 2012. Effect of dietary fluoride derived from Antarctic krill (Euphausia superba) meal on growth of yellowtail (Seriola quinqueradiata). Chemosphere, 86: 891-897. (  0) 0) |
Zerbini, A. N., Adams, G., Best, J., Clapham, P. J., Jackson J. A., and Punt, A. E., 2019. Assessing the recovery of an Antarctic predator from historical exploitation. Royal Society Open Science, 6: 190368-190389. (  0) 0) |
Zhao, Y. Q., Zhang, L., Tao, J., Chi, C. F., and Wang, B., 2019. Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (Euphausia superba): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs). Food Research International, 121: 197-204. (  0) 0) |
Zuo, H., Chen, L., Kong, M., Qiu, L., Lü, P., Wu, P., et al., 2018. Toxic effects of fluoride on organisms. Life Sciences, 198: 18-24. (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


