2) Laboratory for Marine Drugs and Bioproducts, Qingdao Marine Science and Technology Center, Qingdao 266237, China;
3) Department of Stomatology, Qingdao Women and Children's Hospital, Qingdao 266034, China;
4) Department of Stomatology, Qingdao Special Servicemen Recuperation Center of PLA Navy, Qingdao 266071, China
Uncontrollable hemorrhaging is the main cause of casualties in accidents, while wounds in these cases are often irregular and incompressible, which greatly aggravates the difficulty of hemostasis and wound management (Pourshahrestani et al., 2020; Ma et al., 2022). Johnson et al. reported that more than 5.8 million people die from uncontrollable hemorrhaging every year, even the number is still increasing (Li et al., 2022b). Therefore, there has been a focus on the development of inexpensive, effective, portable, easy-to-use, and easily stored hemostatic materials (Johnson et al., 2014).
Currently, various hemostatic materials for hemorrhage control can be divided into two categories: inorganic materials and polymeric materials (Chen et al., 2018). Although many hemostatic materials have been used as commodities, there are still many shortcomings that make them unable to meet the needs of first aid (Donaldson et al., 2021; Tao et al., 2021; Yang et al., 2021b). Traditional hemostatic materials such as gauze and tourniquet can stop bleeding by filling the wound and exerting pressure on the surrounding tissue (Kragh et al., 2015). Although traditional hemostatic materials can control bleeding, they are difficult to go deep into the wound and have no effect on irregular and incompressible wounds (Yang et al., 2021a). Silicate hemostatic agents, including zeolite, kaolin and montmorillonite, have been widely used because of their high efficiency, maneuverability, low cost and low tissue reactivity (Margolis, 1958; Devlin et al., 2011). However, silicate hemostatic agents still have many shortcomings, such as inflammation, difficulty in debridement, heat production, and limited surface area (Feng et al., 2022). Composite hydrogels and multifunctional sponges can absorb water quickly, expand rapidly, keep the wound moist, prevent cell dehydration, and show good effects in hemostasis of irregular deep wounds (Li et al., 2022a; Wu et al., 2022). However, there are also problems such as difficult operation, poor absorption, weak mechanical properties, and easy to cause tissue adhesion (Singh et al., 2022; Weng et al., 2022). In clinic, injectable blood products including coagulation factors, platelets and red blood cells are usually used for systemic hemostasis against incompressible bleeding in the body, but the application is seriously limited due to limited shelf life, high pollution risk, low targeting efficiency, unstable blood clots and other reasons (Kelly et al., 2020; Capraru et al., 2021; Ding et al., 2022). In addition, patients with coagulation dysfunction cannot simply use traditional concentrated blood to stop bleeding, and more ideal hemostatic materials need to be developed (Bennett and Littlejohn, 2014; Schulick et al., 2020).
Recently, the application of mesoporous silicon in hemostatic materials has been developed because of the excellent water absorption capacity due to the good porous structure, large specific surface area, and the ability to induce the intrinsic coagulation pathway because of the polar surface (Ostomel et al., 2007; Wu et al., 2009; Wu et al., 2016; Zhang et al., 2022a). Furthermore, the application of frustules in hemostatic materials has caused great concern (Delalat et al., 2015; Feng et al., 2016). Diatom's siliceous cell wall has regular multi-layered porous structure, uniform pore size, large specific surface area, good biocompatibility and biodegradability, which is unmatched by man-made mesoporous silicon, showing excellent performance as a hemostatic material (Bennett and Littlejohn, 2014; Maher et al., 2015; Lee et al., 2020). The number of diatom species is estimated to be 200000, accounting for 20% of the global primary productivity (Fu et al., 2022). Despite the wide distribution and variety of diatoms, only a few species are currently used in the research of hemostatic materials, among which Coscinodiscus sp. is especially outstanding (Feng et al., 2016; Li et al., 2018; Wang et al., 2021). Among the known diatoms, Coscinodiscus sp. is relatively large (maximum diameter is 100 μm), symmetrical in shape, highly ordered multi-level pore structure and regular circular pores (the diameters of the three layers of pores from the outside to the inside are 45, 200 and 1150 nm respectively), which can shorten the coagulation time by about half, and have good biocompatibility (Losic et al., 2007; Feng et al., 2016; Xing et al., 2017). However, the harvest rate of Coscinodiscus sp. cultured by traditional methods is very low, which seriously limits the production and application. Coscinodiscus sp. tends to sink and gather at the bottom, which is not conducive to large-scale cultivation. The current culture method could not reach a high density of diatom culture, which seriously limits the harvest rate. Coscinodiscus sp. is prone to pollution in long-term culture, and the current single culture mode cannot achieve the circular culture of Coscinodiscus sp.. There is no relevant optimization strategy of enhancing the productivity of Coscinodiscus sp.. Changing the culture method can improve biomass and harvest rate of Coscinodiscus sp., but what effect such a change in culture method will have on the porous structure and hemostatic performance remains to be further studied.
Herein, the culture medium of Coscinodiscus sp. was adjusted to improve the yield. The suspension culture of Coscinodiscus sp. was realized by adding agar to the culture medium. High nutrient concentration culture under semi-continuous culture mode improved the yield of Coscinodiscus sp. and shortened the culture cycle (Fig.1). In addition, to explore the effect of different concentrations of agar or nutrition on the hemostatic effect of diatoms, the surface structure, coagulation activity and biocompatibility of diatoms harvested under different culture conditions were compared.
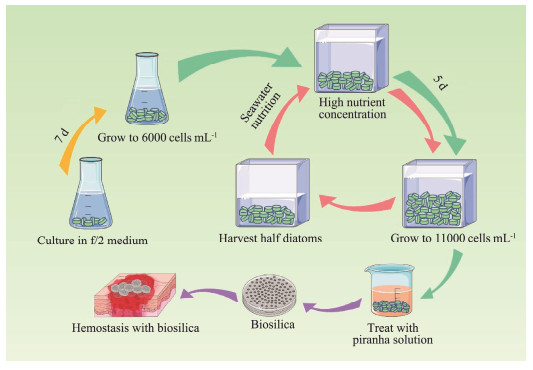
|
Fig. 1 Schematic illustration of diatom culture modes. |
In order to obtain strains that can be rapidly propagated, Coscinodiscus sp. was cultured on f/2 solid medium with 1% agar and penicillin-streptomycin solution at 21℃ under light-dark cycles of 12 – 12 h using light of intensity 5000 lux (Kourtchenko et al., 2018). After one week of cultivation, single spots of the alga were picked and cultured in f/2 seawater medium. Strains capable of rapid propagation were selected for subsequent experiments.
2.2 Restriction of Nutrient ElementsIn order to find out the key nutrients that limit the growth of Coscinodiscus sp., nutrients in the culture medium were supplemented separately. Diatoms were divided into 4 groups (n = 3/group) and cultured in f/2 medium at 21℃ under light-dark cycles of 12 – 12 h using light of intensity 5000 lux with an initial density of about 1000 cells mL−1 (Lananan et al., 2013). After five days of culture, the diatoms grew to the plateau stage. Each group was supplemented with the same amount of N, P and Si nutrition as the beginning respectively every three days. The control group was not supplemented with nutrition. The growth of each group was recorded by counting every day.
2.3 Suspension Culture of Coscinodiscus sp.To achieve suspension culture of Coscinodiscus sp., different concentrations of agar were added to the f/2 medium (Iwasaki et al., 2021). The concentrations of agar were 0, 0.05%, 0.1% and 0.2% respectively, and the initial density of diatoms was about 1000 cells mL−1, each group had three replicates. Other culture conditions were the same as in Section 5.2.
Moreover, for the effect of the addition of small molecule organics on the growth of Coscinodiscus sp., mannitol at concentrations of 0.125%, 0.25% and 0.5% was added to the medium. In addition, the effect of media salinity on diatom growth was also studied. The salinity of the medium was adjusted to 23.3‰, 30‰, 38.7‰ and 50‰ by adding water or sodium chloride. The growth curve was recorded every day.
2.4 High Nutrient Concentration Culture of Coscinodiscus sp.In order to realize semi-continuous culture of Coscinodiscus sp. under high nutrient concentrations, the nutrient concentration of the medium was increased 5, 10 and 20 folds, respectively (Saxena et al., 2022). Other culture conditions were the same as in Section 5.2. After the diatom grew to a plateau, half of the diatom was removed and nutrients were replenished on the seventh day. The growth status of the diatom was expressed by growth curves.
To explore the effect of timing of nutrient supplementation in Coscinodiscus sp. culture, diatoms were divided into three groups that were supplemented at different times with equal amounts of nutrients to the culture medium every day. The supplementation time was 08:00, 20:00 and half at each time point. The growth status of Coscinodiscus sp. was recorded daily.
2.5 Collection and Treatment of FrustulesCoscinodiscus sp. cultured in different concentrations of agar or nutrients was collected for the following experiments respectively. In the treatment of frustules, the surface sugars were removed by lye and organic matter was removed by piranha solution. Fresh diatoms were collected by centrifugation at 3000 r min−1 for 5 min. The preparation of lye was down by mixing potassium hydroxide, urea and water in proportion of 16:8:76. Then 10 g of fresh diatoms added 20 mL of lye, repeated freezing and thawing for 6 – 7 h. The frustules after lye treatment were washed with deionized water. The formula of piranha solution is mixed with H2SO4 and H2O2 at 7:3 ratio on ice (Al-Gharabli and Kujawa, 2021). Next, frustules were added to 25 mL piranha solution at 75℃ for 60 min. Finally, the purified frustules were obtained by washing and drying.
2.6 Characterization of FrustulesThe morphology of frustules was observed by a Scanning Electron Microscope after coating the gold powder (Lananan et al., 2013; Leynaert et al., 2018; Meng et al., 2023). A Malvern Zetasizer (Zetasizer Nano, Malvern, England) was adopted to measure the zeta potential of frustules.
2.7 In vitro Coagulation Effect of FrustulesCoagulation experiments with whole blood were used to compare the differences in hemostatic efficacy of frustules obtained from Coscinodiscus sp. cultured in different concentrations of agar or nutrients (Wu et al., 2015; Feng et al., 2016; Sun et al., 2017). Blood was obtained from the heart of New Zealand white rabbit and stored in sodium citrate anticoagulant tubes (3.8% sodium citrate: blood = 1: 9). Five mg frustules were incubated for one h in two mL centrifuge tubes for 37℃. Then 500 μL anticoagulated blood were added to each centrifuge tube. Timing was started immediately after the addition of 20 μL of 0.2 mol L−1 CaCl2. The tubes were placed in a 37℃ water bath and inverted every 10 s to check whether the blood clotted. Blood clots were washed with PBS and fixed with 2.5% glutaraldehyde. The samples were dried using a critical point dryer before being observed with a scanning electron microscope.
2.8 TEG AnalysisTEG evaluates blood coagulation by measuring blood viscoelasticity (Sulaiman et al., 2014; Sun et al., 2019). In this study, a thromboelastograph was used to compare the performance of frustules cultured in different concentrations of agar or nutrients in promoting blood clotting. 500 μL of sodium citrate treated whole blood (obtained from New Zealand white rabbits) was pipetted into the test cup after mixing with 5 mg of frustules, after the TEG had been preheated at 37℃. 20 μL of 0.2 mol L−1 CaCl2 was then added, and the test was initiated. Blood without the addition of frustules was used as a control. The coagulation mechanisms of different samples were compared by 4 factors: R (reaction time), K (clot formation time), angle α (rate of clot formation), and MA (maximum clot strength) (Pretorius et al., 2017).
2.9 Blood Plasma Coagulation TestsTo compare the differences in coagulation mechanism between frustules cultured in different concentrations of agar or nutrients, a semi-automatic hemagglutinator was used to test prothrombin time (PT) and activated partial thromboplastin time (APTT) (Chen et al., 2017). The blood was taken from New Zealand white rabbits, mixed with 0.2 mol L−1 sodium citrate in the ratio of 1:9, then centrifuged at 3000 r min−1 for 15 min, and the upper plasma was taken for detection within 2 h. For PT testing, 100 μL of plasma was taken, one pellet of test beads was added, incubated at 37℃ for 3 min, 100 μL of PT reagent containing frustules that had been preheated to 37℃ was added, and the clotting time was recorded. For the APTT test, 100 μL of plasma to be measured, 100 μL of APTT reagent containing frustules was added, and one test bead was added, 100 μL of CaCl2 that had been preheated to 37℃ was added, and the clotting time was recorded.
2.10 Hemocompatibility EvaluationFrustules cultured in different concentrations of agar or nutrients were added to 1 mL physiological saline at concentrations of 0.625, 1.25, 2.5, 5 and 10 mg mL−1 respectively. Heparinized anticoagulated blood was obtained from the heart of New Zealand white rabbit and diluted at a ratio of 4:5 for blood: physiological saline. Deionized water and physiological saline were added with 20 μL diluted blood respectively as positive and negative controls. Each sample was added 20 μL diluted blood after prewarmed at 37℃, then incubated at 37℃ for one hour. After that, the sample was centrifuged at 2000 r min−1 for 10 min, and the absorbance value of 545 nm was detected by Microplate Reader (Ooi et al., 2019). The hemolysis ratio (HR, %) was calculated through Eq. (1):
| $ H R(\%)=(D s-D n)/(D p-D n) \times 100 \% \text {, } $ | (1) |
where Ds, Dp, and Dn were the absorbance value of the sample, the positive and the negative control, respectively.
2.11 Cytotoxicity EvaluationThe cytotoxicity of frustules cultured in different concentrations of agar or nutrients was tested on the mouse fibroblast L-929 cells using CCK-8 assays (Zhang et al., 2022b). Briefly, L929 cells were inoculated in 96 well plates at a concentration of about 1×105 cells per well, and cultured for 12 h, each well containing 100 μL DMEM high glucose medium. Sterilized frustules were resuspended with medium to make a 10, 5, 2.5, 1.25, and 0.625 mg mL−1 suspension (medium without frustule served as a control), and the original medium was replaced. Then the plates were incubated for 24, 48, and 72 h, respectively. At each time point, the medium in the plate was removed, and the residual frustules was removed by washing with PBS. Subsequently, 100 μL medium containing 5% CCK-8 was added, while the well without cells was used as blank well. The incubation was continued for 2 h, then the absorbance at 450 nm was measured using a microplate reader. The cell viability (%) was calculated by following Eq. (2):
| $ \text { Cell viability }(\%)=[(A s-A b)/(A c-A b)] \times 100 \% \text {, } $ | (2) |
where As, Ac, Ab were the absorbance of experimental well, absorbance of control well and absorbance of blank well, respectively.
3 Results and Discussion 3.1 Restriction of Nutrient ElementsThe impact of the three main nutrients (N, P, and Si) in the f/2 medium on the growth of Coscinodiscus sp. were demonstrated. As shown in the results, Coscinodiscus sp. reached the plateau stage on the fifth day in f/2 medium, with cell density of 4100 cells mL−1 (Fig.2A). Once Coscinodiscus sp. reach the plateau stage in the f/2 medium, only the group supplemented with silicon continued to grow. The density of diatoms in the Si group reached 6000 cells mL−1 after nutrition supplement on the sixth day and reached 8000 cells mL−1 after the second supplementation of nutrition on the ninth day. In contrast, Coscinodiscus sp. from other groups did not grow after reaching the plateau stage on the fifth day. This indicated that Coscinodiscus sp. could have a higher density of growth than in the medium, silicon was an essential nutrient element for Coscinodiscus sp. high-density culture, and periodic silicon supplement was an effective means to achieve high density cultivation of Coscinodiscus sp..
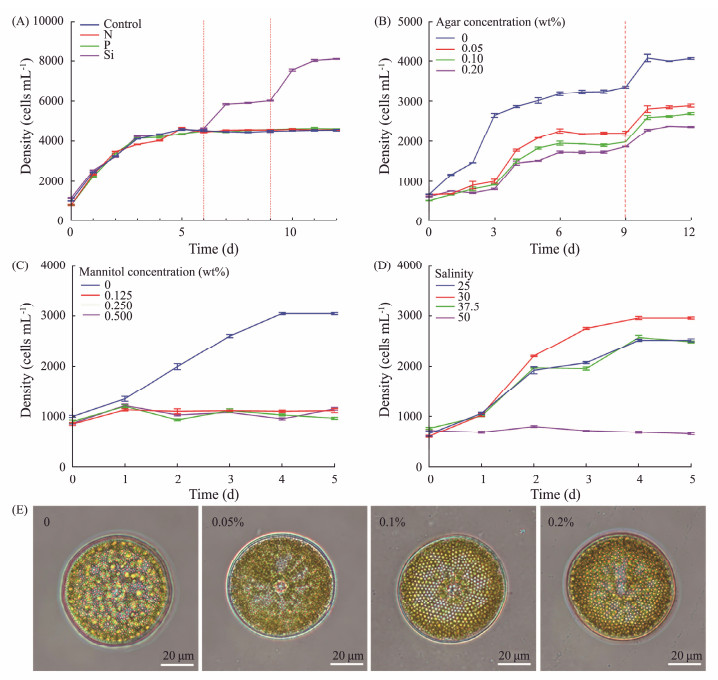
|
Fig. 2 Growth curve of Coscinodiscus sp. supplemented with single nutrient elements (A), cultured with different concentrations of agar (B), mannitol (C), and salinity (D). Photographs of Coscinodiscus sp. cultured with different concentrations of agar (E). |
Because Coscinodiscus sp. could easily sink to the bottom when cultured, which was not conducive to the utilization of nutrients, space and light in the culture medium. Agar could not be utilized by diatoms, and the formed gel could change the fluidity of the medium, so agar was selected as the suspending agent for suspension culture. The concentration of agar was set to 0.05%, 0.1% and 0.2%, the growth status of Coscinodiscus sp. in different concentrations of agar was shown by growth curve (Fig.2B). The addition of agar made Coscinodiscus sp. evenly suspended in the culture medium, and realized the suspension culture of Coscinodiscus sp.. The groups added with agar reached a plateau on the fourth day. With increasing agar concentration, the density of Coscinodiscus sp. decreased from 2200 cells mL−1 to 1800 cells mL−1. The group without agar reached the plateau stage on the third day, the density was 3200 cells mL−1. When the nutrition was supplemented again on the ninth day, the density of diatoms cultured in 0.05% agar reached 3000 cells mL−1 on the tenth day, while that of the group without agar reached 4000 cells mL−1. This suggested that high concentration of agar was conducive to the suspension of Coscinodiscus sp., while low concentration of agar is conducive to the growth of Coscinodiscus sp.. In addition, through the observation of optical microscope, the cell morphology of Coscinodiscus sp. cultured in different agar concentrations showed no significant difference (Fig.2E).
To reduce the viscosity of the suspension agent, neutral small molecular mannitol was used instead of agar for diatom culture in this study. The results showed that diatoms could survive in different concentrations of mannitol, but could not be suspended, and the growth rate was very slow compared with the control group (Fig.2C). This result was probably due to the fact that Coscinodiscus sp. could grow only under suitable conditions and was sensitive to changes in osmotic pressure. Mannitol may be used in aquaculture as an inhibitor of diatom growth or in diatom product transport to inhibit diatom growth.
Diatoms have a wide range of adaptability to salinity according to different species, and are distributed in seawater, fresh water and wet soil. Therefore, the growth status of Coscinodiscus sp. in different salinities was evaluated. Diatoms cultivated in salinity 30 exhibited the fastest growth rate and the largest density (Fig.2D). The growth rate and maximum cell density of diatoms in other salinity were significantly lower than that in salinity 30. When the salinity of the medium reached 50, the growth of diatom stopped. All these indicated that Coscinodiscus sp. were sensitive to the change of salinity, and the salinity 30 was the most suitable condition for growth.
3.3 High Nutrient Concentration Culture of Coscinodiscus sp.In order to realize the large-scale production of Coscinodiscus sp., improve the yield and shorten the production cycle, a semi-continuous high nutrient concentration culture mode is used to culture Coscinodiscus sp.. After increasing the nutrient amount by 5, 10, and 20 folds, the diatom was inoculated at an initial density of 3000 cells mL−1, and the growth rate of the diatom was significantly improved compared with diatoms cultured in f/2 medium (Fig.3A). The group cultured in 20 times nutrient concentration reached the plateau stage on the fifth day, and the other groups reached the plateau stage on the third day. After the diatom reached a plateau, the density reached 11000 cells mL−1 in the group with a 20 times nutrient concentration, the density of diatoms cultured in 1, 5 and 10 times nutrient concentration was 6000 cells mL−1, 8000 cells mL−1 and 9000 cells mL−1 respectively. To realize semi-continuous cultivation of Coscinodiscus sp., at the seventh day, half of the diatoms were removed and the cultures were continued with supplemental nutrition. Diatom was able to continue growing and to achieve the same density at the same time as before. This illustrated that increasing the concentration of nutrients up to 20 fold could significantly enhance the growth rate and yield of Coscinodiscus sp.. The rapid harvest of Coscinodiscus sp. can be realized through the semi-continuous high-nutrient concentration culture mode. Moreover, the morphology of diatoms cultured in different nutrient concentrations did not show significant differences (Fig.3C).
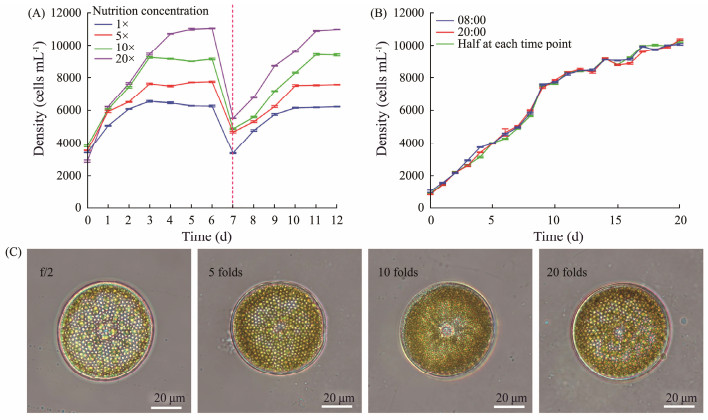
|
Fig. 3 Growth curve of Coscinodiscus sp. cultured in different nutrient concentrations (A), cultured with supplementary nutrition at different times (B), and photographs of Coscinodiscus sp. cultured in different nutrient concentrations (C). |
Due to the existence of light-dark cycle, Coscinodiscus sp. was in different physiological states during the day and night. The effect of nutritional supplement time on the growth of Coscinodiscus sp. was explored. Although the timing of nutritional supplements was divided into 08:00, 20:00 and half at each time point, the nutrition supplement time did not affect the growth speed and density of Coscinodiscus sp. (Fig.3B). Once the density of Coscinodiscus sp. reached 10000 cells mL−1, the supplement of nutrients could not make Coscinodiscus sp. continue to grow, and Coscinodiscus sp. reached the maximum density in this culture mode.
3.4 Characterization of FrustulesScanning electron microscopy (SEM) images showed that frustules had intact pore structure and uniform pore size without malformation or other problems after treatment. The structure of frustules harvested from Coscinodiscus sp. cultured in different concentrations of agar did not exhibit any obvious differences (Figs.4A – D). The diatoms cultured in different nutrient concentration had no obvious difference in microstructure (Figs.4F – I). Because the diameter of diatoms would be smaller and smaller in asexual reproduction, and the diatoms cultured in high nutrition had more generations of reproduction, the average diameter of the harvested frustules was slightly smaller than that of other frustules harvested in the same batch. In addition, through the comparison of frustules harvested in different batches, there was no obvious difference in the phenotype of frustules (Figs.4E and J), indicating that the culture method and treatment method were stable, no significant differences will be expected among different batches.
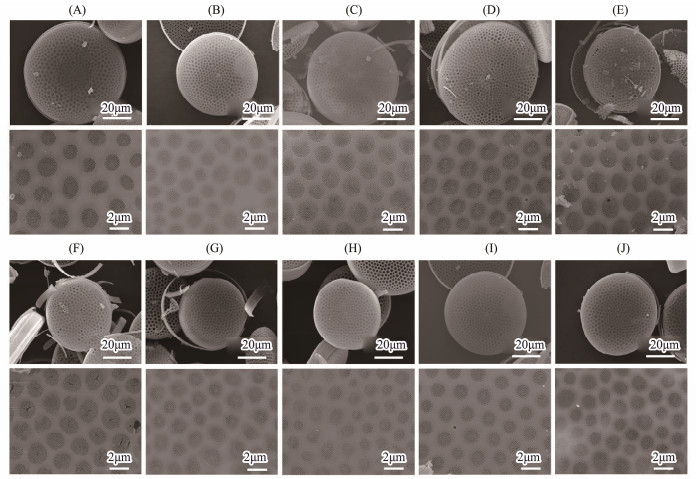
|
Fig. 4 SEM images of frustules cultured in 0 (A), 0.05% (B), 0.1% (C), 0.2% (D) agar concentration, frustules cultured in 1 (F), 5 (G), 10 (H), 20 (I) times nutrient concentration, different batches of frustules cultured in 0.2% agar concentration (E), and cultured in 20 times nutrient concentration (J). |
Since a negatively charged surface of frustules could activate the endogenous coagulation pathway, the effect of the two cultivation modes on the surface charge of frustules was compared by measuring the zeta potential (Table 1). The zeta potential of frustules cultured in agar with different concentrations was not significantly different from frustules cultured without agar (− 24.31 ± 1.23 mV). The charge of frustules cultured in different concentrations of nutrients was also not different from that of frustules cultured in f/2 medium (− 22.26 ± 1.65 mV). The results showed that the addition of agar or the change of nutrient concentration during Coscinodiscus sp. culture will not change the zeta potential on the material surface.
|
|
Table 1 The zeta potential of frustules harvested by different culture methods |
In order to test the effect of Coscinodiscus sp. cultured in different agar concentrations or different nutrient concentrations on the hemostatic effect of frustules, the coagulation time in vitro was measured (Figs.5A – B). In frustules cultivated with agar, the coagulation time of the groups with the addition of frustules was reduced by almost half compared to the control group (503.33 ± 20.82 s), the result of each group containing frustules was not significantly different. The clotting time of frustules obtained from medium without agar was 283.33 ± 15.28 s, the hemostasis times of frustules obtained from media containing 0.05%, 0.1% and 0.2% agar were 270.00 ± 10.00, 253.33 ± 15.28 and 260.00 ± 10.00 s. In the diatoms cultured at different nutrient concentrations, there was no significant difference among the groups with frustules. The shortest coagulation time (243.33 ± 5.77 s) was obtained from diatoms cultured in f/2 medium, while the longest coagulation time (273.33 ± 12.58 s) was obtained from diatoms cultured at five times of the nutrient concentration. The coagulation time of control group without frustules was 513.33 ± 15.28 s, twice that of groups with frustules added.
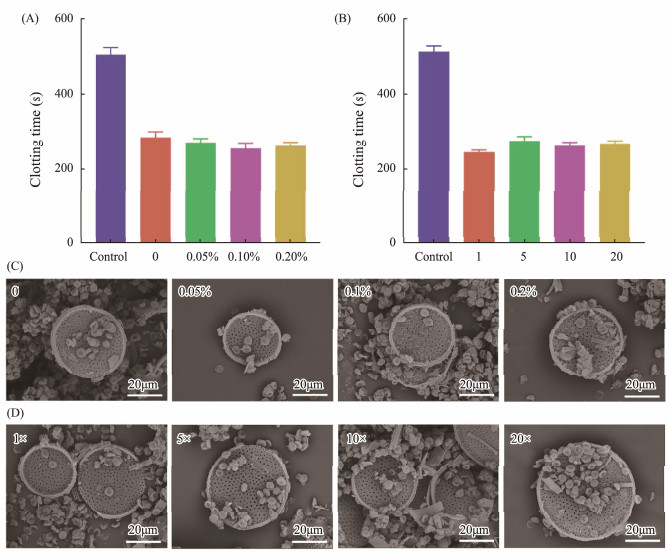
|
Fig. 5 In vitro blood clotting time of frustules obtained from Coscinodiscus sp. cultured in different agar concentrations (A), in different nutrient concentrations (B), and SEM images of interfacial interaction between RBCs and diatom frustules harvested from Coscinodiscus sp. cultured in different agar concentrations (C), and cultured in different nutrient concentrations (D). |
Moreover, SEM images showed morphology of blood cells in blood clot after contacting with frustules (Figs.5C and D). Red blood cells were concentrated and closely attached to the surface of frustules. Although different concentrations of agar or nutrients were added to the culture medium, there was no effect on the coagulation activity of frustules. The SEM images of blood clot also confirmed the effect of frustules under different culture conditions on blood cell morphology was not different.
3.6 Thromboelastography (TEG) AnalysisTEG, as an important method to evaluate the coagulation process, has been widely applied in the study of hemostatic materials. TEG was used to test the frustules cultured in different concentrations of agar or nutrients. In this study, the reaction time (R) and clot formation time (K) of the group with the addition of frustules were significantly decreased compared with the control group, the rate of clot formation (angle α) were markedly increased (Table 2, Figs.6A – B). This illustrated that the frustules could accelerate the initiation time of the coagulation reaction and the rate of blood clot formation. On the other hand, although the agar concentration of the medium was different, the TEG results of frustules were similar. The R value of the control group was 6.1 min, the R value of the frustules cultured without agar was 1.8 min, which was about 70% shorter than the control. The R values of the frustules cultured with 0.05%, 0.1% and 0.2% agar were 1.5, 1.6 and 1.7 min respectively, which was not much different from the frustules cultured without agar. The K value of frustules cultured without agar is 2.1 min, and the angle α is 64.3 deg. There is no significant difference between the results of frustules cultured in different concentrations of agar, while the K value of the control group without frustules is larger (2.6 min), and the angle α is smaller (54.7 deg). This further illustrated that the addition of agar during Coscinodiscus sp. culture did not affect the hemostatic effect of frustules, which was consistent with the previous experimental results. Similarly, the TEG results of Coscinodiscus sp. cultured in medium with different nutrient concentrations (1.5 – 1.8 min for K value, 1.5 – 1.7 min for R value, 68.8 – 69.2 deg for angle α) also showed no obvious difference with f/2 medium (1.5 min for K value, 1.7 min for R value, 66.9 deg for angle α), which illustrated that the raising of nutrient concentration only improved the yield of frustules and did not affect the hemostatic effect of frustules. Additionally, the MA values of the harvested frustules of diatoms cultured on different agar concentrations and those cultured on different nutrient concentrations varied from 48.9 mm to 52.7 mm, and from 52.7 mm to 56.8 mm, respectively. This also suggested that the frustules of Coscinodiscus sp. had little effect on the strength of the blood clot and that the addition of agar at different concentrations or changes in nutrient concentrations did not alter this property of the frustules.
|
|
Table 2 TEG results of frustules harvested by different culture methods |
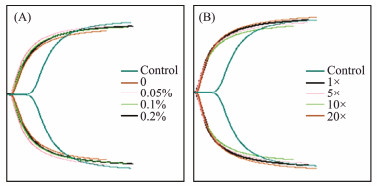
|
Fig. 6 TEG curves of diatom frustules obtained from Coscinodiscus sp. cultured in different agar concentrations (A) and in different nutrient concentrations (B). |
Due to the negatively charged surface, frustules could activate endogenous coagulation pathways to accelerate hemostasis (Xu et al., 2002; Ostomel et al., 2006). APTT and PT measurements were used to compare the coagulation activity of frustules harvested from Coscinodiscus sp. cultured in different concentrations of agar or nutrients. For APTT test, the addition of frustules shortened the coagulation time to about 70%, and there was no significant difference in the results of frustules harvested in the medium containing different concentrations of agar (from 67.8% to 69.1%), the change of nutrient concentration also had no impact on this performance of frustules (Figs. 7A and B). For PT test, the change of agar or nutrient concentration in diatom culture had no effect on coagulation time (Figs.7C and D). The results illustrated that the addition of agar or the change of nutrient concentration during Coscinodiscus sp. culture will not change APTT and PT.
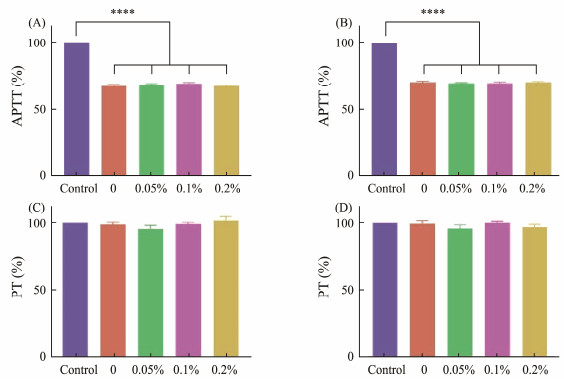
|
Fig. 7 APTT measurements of frustules obtained from Coscinodiscus sp. cultured in different agar concentrations (A), in different nutrient concentrations (B), and PT measurements of frustules obtained from Coscinodiscus sp. cultured in different agar concentrations (C), and in different nutrient concentrations (D). ****p < 0.0001 (n = 5). |
Hemocompatibility was compared between frustules obtained by cultured in different concentrations of agar or nutrients (Figs.8A – D). The hemolysis rate of frustules showed an obvious positive correlation with their concentration. Although diatoms grown in different concentrations of agar or nutrients, the hemolysis rates of harvested frustules did not exhibit obvious differences. On the other hand, the hemolysis rate was positively correlated with the concentration of frustules. The hemolysis rate of diatoms cultured in different concentrations of agar varied from 7.50% (0.2% agar) to 8.22% (0% agar) at the concentration of 10 mg mL−1. When the concentration of frustules was 5 mg mL−1, the hemolysis rate was about 4%; when the concentration was 2.5 mg mL−1, the hemolysis rate was about 2%; and when the concentration was less than 1.25 mg mL−1, the hemolysis rate was less than 1%. Similar results were obtained for diatoms cultured in different nutrient concentrations. The hemolysis rate of frustules decreased from 8% at 10 mg mL−1 to 3% at 2.5 mg mL−1. When the concentration of frustules was less than 1.25 mg mL−1, the hemolysis rate was less than 1%. The results showed that the change of agar or nutrient concentration would not affect the blood compatibility of frustules.
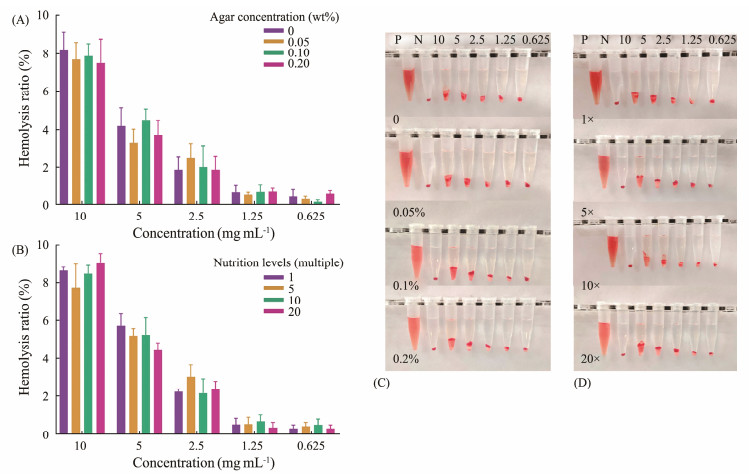
|
Fig. 8 In vitro hemolysis ratio of frustules obtained from Coscinodiscus sp. cultured in different agar concentrations (A), in different nutrient concentrations (B) (n = 5), and photos of hemocompatibility of diatom frustules harvested from Coscinodiscus sp. cultured in different agar concentrations (C), and cultured in different nutrient concentrations (D). |
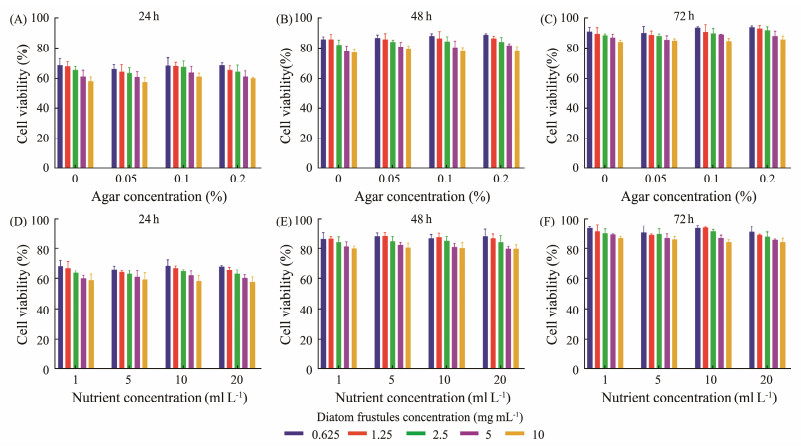
As a necessary indicator to evaluate the biosafety of hemostatic materials, the cytotoxicity of frustules harvested from different agar or nutrient concentrations against L929 cells was measured using CCK-8. After co-cultured with frustules for 24 h, the cell viability was 68% to 58%, decreased with the increase of frustules concentration (Fig.9A). This showed that the cytotoxicity of frustules was related to the concentration of frustules, which may be due to the damage of silanol groups on the surface of frustules and the sedimentation of frustules during incubation. In addition, the cytotoxicity of frustules was also affected by shape, charge, surface chemistry and other factors. Then, the viability of cells gradually increased with the extension of incubation time, reaching about 80% after 48 h of incubation. After 72 h of incubation, the group with 0.0625 mg mL−1 frustules concentration reached about 90%, while the group with 10 mg mL−1 frustules concentration was about 85% (Figs.9B and C). Although diatoms were grown in various agar concentrations, the cytotoxicity of frustules was not affected. As for the frustules harvested from Coscinodiscus sp. cultured in different concentrations of nutrients, the result of cytotoxicity was the same as that of frustules harvested from different agar concentrations (Figs.9D, E and F). The above results showed that the addition of agar in the culture medium or the change of nutrient concentration would not affect the cytocompatibility of frustules. Although the frustules of Coscinodiscus sp. showed some cytotoxicity in 24 h, the vitality of cells could be restored with incubation.
|
Fig. 9 Cytotoxicity of frustules obtained from Coscinodiscus sp. cultured in different agar concentrations at different frustules concentrations after 24 h (A), 48 h (B), 72 h (C) incubation, and in different nutrient concentrations at different frustules concentrations after 24 h (D), 48 h (E), 72 h (F) incubation. |
The exploration of marine derived biomedical materials is an important aspect in developing the utilization of marine resources (Deng et al., 2020). As a novel marine inorganic biomaterial, frustules have excellent performance in hemostasis, wound repair, tissue repair and other fields (Feng et al., 2016; Li et al., 2018; Cao et al., 2022; Zhang et al., 2022a). Despite the wide variety of diatoms, only a few species are currently used in the research of hemostatic materials. Coscinodiscus sp. has shown excellent hemostatic performance, but the low yield is the greatest difficulty hindering the application. Coscinodiscus sp. sinks faster in water than other diatoms and needs more nutrition because of the larger volume (Du et al., 2019). Coscinodiscus sp. gathered at the bottom cannot make full use of the nutrition and light in the culture medium, and the space of the culture container is not fully utilized. In the large-scale cultivation of Coscinodiscus sp., how to prevent sinking and how to improve the yield are the key issues to be solved.
The traditional culture for Coscinodiscus sp. uses f/2 medium, and aeration is adopted to replenish carbon sources as well as prevent Coscinodiscus sp. from sinking (Orefice et al., 2019). The nutrition was replenished on the 7th day, and all diatoms were harvested and reinoculated on the 12th day. In this way, Coscinodiscus sp. can only be cultured to 4000 cells mL−1, far from reaching the biomass required for production. In the large-scale cultivation of algae, the factors that usually restrict the growth of algae are carbon, nitrogen, light and temperature (Jiang et al., 2020). When Coscinodiscus sp. is cultured using f/2 medium, silicon limitations precede these factors. Although aeration can prevent the Coscinodiscus sp. from sinking, the amount of aeration is difficult to control, the suspension of Coscinodiscus sp. is unstable, and the disturbance of bubbles seems to be detrimental to the growth of Coscinodiscus sp. (Xiao et al., 2021).
In this study, suspension culture of Coscinodiscus sp. was realized by adding 0.05% agar to the culture medium. The agar suspension culture mode of Coscinodiscus sp. replaced the aeration method. Because Coscinodiscus sp. could be evenly and stably suspended in the culture medium, the space of the culture container, the nutrition of the culture medium and the light could be fully utilized. By increasing the amount of nutrients added in the culture medium by 20 times, the cell density of Coscinodiscus sp. was increased to 11000 cells mL−1. During the cultivation of Coscinodiscus sp., a semi-continuous culture method was adopted. When Coscinodiscus sp. culture reaches the platform stage, half of the diatom would be harvested, and new seawater and nutrients would be added to continue the cultivation. Through this culture mode, the harvest cycle of diatoms was shortened from 12 days to 5 days. The detailed comparison between suspension culture, high nutrient concentration culture and traditional culture methods is listed in Table 3.
|
|
Table 3 Comparison of suspension culture, high nutrient concentration culture and traditional culture methods |
Environmental conditions such as nutrition supply and light would affect the expression of genes and molecules related to silica formation in diatoms (De et al., 2017). The morphological structure of frustules was the main factor affecting their hemostatic effect, whether the change of culture mode could change the hemostatic effect of frustules needed further exploration to ensure the stable quality of harvested materials (Zgłobicka and Kurzydłowski, 2022). By evaluating the characterization of frustules and the hemostatic effect, the addition of agar and the change of nutrient concentration were proved not to affect the coagulation activity of frustules.
Although the suspension culture of Coscinodiscus sp. and the increase of production have been achieved through the change of culture mode, there are still many deficiencies to be improved. Agar suspension culture mode proves the feasibility of adding macromolecular suspension agent to the culture medium to achieve suspension culture, more convenient and efficient suspension agent needs to be explored. To realize the industrial production of Coscinodiscus sp., further improvement of culture density and pilot testing are necessary. The proportion of nutrient elements in the medium also needs to be further optimized for Coscinodiscus sp. to find a suitable medium formula. Additionally, domestication of diatom species to adapt to high-density cultivation under artificial culture conditions is a feasible measure.
5 ConclusionsIn this study, the method of increasing the yield of Coscinodiscus sp. was explored through the improvement of the culture medium, and the properties of the harvested frustules as a hemostatic material were compared to test the impact of changes in culture methods. The results showed that the suspension culture of Coscinodiscus sp. could be realized by adding 0.05% agar to the culture medium. The semi-continuous culture mode at 20 times nutrient concentration significantly increased the cell density of Coscinodiscus sp. and shortened the culture cycle. A salinity of 30‰ was found to be the most suitable for Coscinodiscus sp. growth by cultivation in different salinities. The silicon element was the main factor limiting the growth of Coscinodiscus sp.. On the other hand, the addition of frustules could shorten the in vitro coagulation time to 243.33± 5.77 s. The results illustrated that frustules can shorten the initiation time of blood clot, accelerate the formation of blood clot, and reduce the APTT to about 70% of the control. More importantly, addition of agar or modification of nutrient concentrations did not change the surface structure, hemostatic performance, and biosafety of frustules. This study provided two new culture modes to increase the production of Coscinodiscus sp. without changing the hemostatic performance, established the basis for large-scale cultivation of Coscinodiscus sp..
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (No. U22A20588), the Sanya Science and Technology Project (No. 2022KJCX57), and the Qingdao National Laboratory for Marine Science and Technology (No. 12-04), and the Project supported by the Education Department of Hainan Province (No. Hnjg2024-276).
Animal and Human Rights Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Al-Gharabli, S., and Kujawa, J., 2021. Molecular activation of fluoropolymer membranes via base piranha treatment to enhance transport and mitigate fouling-new materials for water purification. Journal of Membrane Science, 624: 119105. DOI:10.1016/j.memsci.2021.119105 (  0) 0) |
Bennett, B. L., and Littlejohn, L., 2014. Review of new topical hemostatic dressings for combat casualty care. Military Medicine, 179(5): 497-514. DOI:10.7205/MILMED-D-13-00199 (  0) 0) |
Capraru, A., Jalowiec, K. A., Medri, C., Daskalakis, M., Zeerleder, S. S., and Taleghani, B. M., 2021. Platelet transfusion – insights from current practice to future development. Journal of Clinical Medicine, 10(9): 1990. DOI:10.3390/jcm10091990 (  0) 0) |
Cao, Z., Su, C., Sun, X., Shao, K., Wang, X., Mu, Y., et al., 2022. Enhanced mechanical properties of hydroxybutyl chitosan hydrogel through anchoring interface effects of diatom biosilica. Carbohydrate Polymers, 296: 119975. DOI:10.1016/j.carbpol.2022.119975 (  0) 0) |
Chen, Z., Yao, X., Liu, L., Guan, J., Liu, M., Li, Z., et al., 2017. Blood coagulation evaluation of N-alkylated chitosan. Carbohydrate Polymers, 173: 259-268. DOI:10.1016/j.carbpol.2017.05.085 (  0) 0) |
Chen, Z., Han, L., Liu, C., Du, Y., Hu, X., Du, G., et al., 2018. A rapid hemostatic sponge based on large, mesoporous silica nanoparticles and N-alkylated chitosan. Nanoscale, 10: 20234-20245. DOI:10.1039/c8nr07865c (  0) 0) |
De Tommasi, E., Gielis, J., and Rogato, A., 2017. Diatom frustule morphogenesis and function: A multidisciplinary survey. Marine Genomics, 35: 1-18. DOI:10.1016/j.margen.2017.07.001 (  0) 0) |
Delalat, B., Sheppard, V. C., Rasi Ghaemi, S., Rao, S., Prestidge, C. A., McPhee, G., et al., 2015. Targeted drug delivery using genetically engineered diatom biosilica. Nature Communications, 6: 8791. DOI:10.1038/ncomms9791 (  0) 0) |
Deng, Z., Wang, T., Chen, X., and Liu, Y., 2020. Applications of chitosan-based biomaterials: A focus on dependent antimicrobial properties. Marine Life Science & Technology, 2: 398-413. DOI:10.1007/s42995-020-00044-0 (  0) 0) |
Devlin, J. J., Kircher, S., Kozen, B. G., Littlejohn, L. F., and Johnson, A. S., 2011. Comparison of ChitoFlex®, CELOXTM, and QuikClot® in control of hemorrhage. Journal of Emergency Medicine, 41(3): 237-245. DOI:10.1016/j.jemermed.2009.02.017 (  0) 0) |
Ding, Y. F., Huang, Q., Quan, X., Cheng, Q., Li, S., Zhao, Y., et al., 2022. Supramolecularly functionalized platelets for rapid control of hemorrhage. Acta Biomaterialia, 149: 248-257. DOI:10.1016/j.actbio.2022.07.007 (  0) 0) |
Donaldson, R. I., Zimmermann, E. M., Fisher, T. C., Buchanan, O. J., Armstrong, J. K., Cambridge, J. S., et al., 2021. Thermoreversible reverse-phase-shift foam for treatment of noncompressible torso hemorrhage. Journal of Surgical Research, 259: 175-181. DOI:10.1016/j.jss.2020.11.039 (  0) 0) |
Du Clos, K. T., Karp-Boss, L., Villareal, T. A., and Gemmell, B. J., 2019. Coscinodiscus wailesii mutes unsteady sinking in dark conditions. Biology Letters, 15: 3. DOI:10.1098/rsbl.2018.0816 (  0) 0) |
Feng, C., Li, J., Wu, G. S., Mu, Y. Z., Kong, M., Jiang, C. Q., et al., 2016. Chitosan-coated diatom silica as hemostatic agent for hemorrhage control. ACS Applied Materials and Interfaces, 8(50): 34234-34243. DOI:10.1021/acsami.6b12317 (  0) 0) |
Feng, Y., Luo, X., Wu, F., Liu, H., Liang, E., He, R. R., et al., 2022. Systematic studies on blood coagulation mechanisms of halloysite nanotubes-coated PET dressing as superior topical hemostatic agent. Chemical Engineering Journal, 428: 132049. DOI:10.1016/j.cej.2021.132049 (  0) 0) |
Fu, W., Shu, Y., Yi, Z., Su, Y., Pan, Y., Zhang, F., et al., 2022. Diatom morphology and adaptation: Current progress and potentials for sustainable development. Sustainable Horizons, 2: 100015. DOI:10.1016/j.horiz.2022.100015 (  0) 0) |
Iwasaki, K., Evenhuis, C., Tamburic, B., Kuzhiumparambil, U., O'Connor, W., Ralph, P., et al., 2021. Improving light and CO2 availability to enhance the growth rate of the diatom, Chaetoceros muelleri. Algal Research, 55: 102234. DOI:10.1016/j.algal.2021.102234 (  0) 0) |
Jiang, A., Ji, H., Liu, H., Zhu, H., Ai, G., and Guo, X., 2020. Culture of benthic diatom Nitzschia sp. with macroalgae carriers and its application as feed of juveniles Stichopus japonicus. Helgoland Marine Research, 74: 11. DOI:10.1186/s10152-020-00544-7 (  0) 0) |
Johnson, D., Bates, S., Nukalo, S., Staub, A., Hines, A., Leishman, T., et al., 2014. The effects of QuikClot Combat Gauze on hemorrhage control in the presence of hemodilution and hypothermia. Annals of Medicine and Surgery, 3(2): 21-25. DOI:10.1016/j.amsu.2014.03.001 (  0) 0) |
Kelly, K., Cancelas, J. A., Szczepiorkowski, Z. M., Dumont, D. F., Rugg, N., and Dumont, L. J., 2020. Frozen platelets – Development and future directions. Transfusion Medicine Reviews, 34(4): 286-293. DOI:10.1016/j.tmrv.2020.09.008 (  0) 0) |
Kourtchenko, O., Rajala, T., and Godhe, A., 2018. Growth of a common planktonic diatom quantified using solid medium culturing. Scientific Reports, 8: 1-10. DOI:10.1038/s41598-018-28129-y (  0) 0) |
Kragh, J. F., Aden, J. K., Steinbaugh, J., Bullard, M., and Dubick, M. A., 2015. Gauze vs XSTAT in wound packing for hemorrhage control. The American Journal of Emergency Medicine, 33(7): 974-976. DOI:10.1016/j.ajem.2015.03.048 (  0) 0) |
Lananan, F., Jusoh, A., Ali, N., Lam, S. S., and Endut, A., 2013. Effect of Conway medium and f/2 medium on the growth of six genera of South China Sea marine microalgae. Bioresource Technology, 141: 75-82. DOI:10.1016/j.biortech.2013.03.006 (  0) 0) |
Lee, J., Lee, H. A., Shin, M., Juang, L. J., Kastrup, C. J., Go, G. M., et al., 2020. Diatom frustule silica exhibits superhydrophilicity and superhemophilicity. ACS Nano, 14(4): 4755-4766. DOI:10.1021/acsnano.0c00621 (  0) 0) |
Leynaert, A., Fardel, C., Beker, B., Soler, C., Delebecq, G., Lemercier, A., et al., 2018. Diatom frustules nanostructure in pelagic and benthic environments. Silicon, 10: 2701-2709. DOI:10.1007/s12633-018-9809-0 (  0) 0) |
Li, H., Zhou, X., Luo, L., Ding, Q., and Tang, S., 2022a. Bioorthogonally crosslinked catechol-chitosan hydrogel for effective hemostasis and wound healing. Carbohydrate Polymers, 281: 119039. DOI:10.1016/j.carbpol.2021.119039 (  0) 0) |
Li, J., Han, J., Sun, Q., Wang, Y., Mu, Y., Zhang, K., et al., 2018. Biosynthetic calcium-doped biosilica with multiple hemostatic properties for hemorrhage control. Journal of Materials Chemistry B, 6: 7834-7841. DOI:10.1039/c8tb00667a (  0) 0) |
Li, P., Cao, L., Sang, F., Zhang, B., Meng, Z., Pan, L., et al., 2022b. Polyvinyl alcohol/sodium alginate composite sponge with 3D ordered/disordered porous structure for rapidly controlling noncompressible hemorrhage. Biomaterials Advances, 134: 112698. DOI:10.1016/j.msec.2022.112698 (  0) 0) |
Losic, D., Pillar, R. J., Dilger, T., Mitchell, J. G., and Voelcker, N. H., 2007. Atomic force microscopy (AFM) characterisation of the porous silica nanostructure of two centric diatoms. Journal of Porous Materials, 14: 61-69. DOI:10.1007/s10934-006-9009-y (  0) 0) |
Ma, C., Zhao, J., Zhu, C., Jiang, M., Ma, P., Mi, Y., et al., 2022. Oxidized dextran crosslinked polysaccharide/protein/polydopamine composite cryogels with multiple hemostatic efficacies for noncompressible hemorrhage and wound healing. International Journal of Biological Macromolecules, 215: 675-690. DOI:10.1016/j.ijbiomac.2022.06.130 (  0) 0) |
Maher, S., Alsawat, M., Kumeria, T., Fathalla, D., Fetih, G., Santos, A., et al., 2015. Luminescent silicon diatom replicas: Self-reporting and degradable drug carriers with biologically derived shape for sustained delivery of therapeutics. Advanced Functional Materials, 25(32): 5107-5116. DOI:10.1002/adfm.201501249 (  0) 0) |
Margolis, J., 1958. The kaolin clotting time. Journal of Clinical Pathology, 11: 406-409. DOI:10.1136/jcp.11.5.406 (  0) 0) |
Meng, F., Zheng, Y., Wang, H., and Chen, L., 2023. Study on acoustic performance for diatom frustule with nanoporous structure. Journal of Bionic Engineering, (4): 1656-1669. DOI:10.1007/s42235-023-00337-x (  0) 0) |
Ooi, C. H., Ling, Y. P., Abdullah, W. Z., Mustafa, A. Z., Pung, S. Y., and Yeoh, F. Y., 2019. Physicochemical evaluation and in vitro hemocompatibility study on nanoporous hydroxyapatite. Journal of Materials Science: Materials in Medicine, 30: 44. DOI:10.1007/s10856-019-6247-5 (  0) 0) |
Orefice, I., Musella, M., Smerilli, A., Sansone, C., Chandrasekaran, R., Corato, F., et al., 2019. Role of nutrient concentrations and water movement on diatom's productivity in culture. Scientific Reports, 9: 1479. DOI:10.1038/s41598-018-37611-6 (  0) 0) |
Ostomel, T. A., Stoimenov, P. K., Holden, P. A., Alam, H. B., and Stucky, G. D., 2006. Host-guest composites for induced hemostasis and therapeutic healing in traumatic injuries. Journal of Thrombosis and Thrombolysis, 22: 55-67. DOI:10.1007/s11239-006-7658-y (  0) 0) |
Ostomel, T. A., Shi, Q., Stoimenov, P. K., and Stucky, G. D., 2007. Metal oxide surface charge mediated hemostasis. Langmuir, 23(22): 11233-11238. DOI:10.1021/la701281t (  0) 0) |
Pourshahrestani, S., Zeimaran, E., Kadri, N. A., Mutlu, N., and Boccaccini, A. R., 2020. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Advanced Healthcare Materials, 9(20): 2000905. DOI:10.1002/adhm.202000905 (  0) 0) |
Pretorius, E., Swanepoel, A. C., DeVilliers, S., and Bester, J., 2017. Blood clot parameters: Thromboelastography and scanning electron microscopy in research and clinical practice. Thrombosis Research, 154: 59-63. DOI:10.1016/j.thromres.2017.04.005 (  0) 0) |
Saxena, A., Mishra, B., Sindhu, R., Binod, P., and Tiwari, A., 2022. Nutrient acclimation in benthic diatoms with adaptive laboratory evolution. Bioresource Technology, 351: 126955. DOI:10.1016/j.biortech.2022.126955 (  0) 0) |
Schulick, A. C., Moore, H. B., Walker, C. B., Yaffe, H., Pomposelli, J. J., Azam, F., et al., 2020. A clinical coagulopathy score concurrent with viscoelastic testing defines opportunities to improve hemostatic resuscitation and enhance blood product utilization during liver transplantation. The American Journal of Surgery, 220(6): 1379-1386. DOI:10.1016/j.amjsurg.2020.07.034 (  0) 0) |
Singh Chandel, A. K., Ohta, S., Taniguchi, M., Yoshida, H., Tanaka, D., Omichi, K., et al., 2022. Balance of antiperitoneal adhesion, hemostasis, and operability of compressed bilayer ultrapure alginate sponges. Biomaterials Advances, 137: 212825. DOI:10.1016/j.bioadv.2022.212825 (  0) 0) |
Sulaiman, O. M., Pabón, G. A., Cortés, C. C., Munozmumunoz, L. A., Reyes, L. E., and Arevalo, J. J., 2014. An overview of thrombelastography research Un resumen de la investigación en tromboelastografía. Colombian Journal of Anesthesiology, 42(4): 302-308. DOI:10.1016/j.rcae.2014.06.005 (  0) 0) |
Sun, X., Tang, Z., Pan, M., Wang, Z., Yang, H., and Liu, H., 2017. Chitosan/kaolin composite porous microspheres with high hemostatic efficacy. Carbohydrate Polymers, 177: 135-143. DOI:10.1016/j.carbpol.2017.08.131 (  0) 0) |
Sun, X., Fang, Y., Tang, Z., Wang, Z., Liu, X., and Liu, H., 2019. Mesoporous silica nanoparticles carried on chitosan microspheres for traumatic bleeding control. International Journal of Biological Macromolecules, 127: 311-319. DOI:10.1016/j.ijbiomac.2019.01.039 (  0) 0) |
Tao, Q., Guo, L., Diao, H., and Feng, L., 2021. Facile antibacterial materials with turbine-like structure for: P. aeruginosa infected scald wound healing. Biomaterials Science, 9(10): 3830-3837. DOI:10.1039/d1bm00483b (  0) 0) |
Wang, L., Pan, K., Zhang, L., Zhou, C., Li, Y., Zhu, B., et al., 2021. Tentative identification of key factors determining the hemostatic efficiency of diatom frustule. Biomaterials Science, 9(6): 2162-2173. DOI:10.1039/d0bm02002h (  0) 0) |
Weng, H., Jia, W., Li, M., and Chen, Z., 2022. New injectable chitosan-hyaluronic acid based hydrogels for hemostasis and wound healing. Carbohydrate Polymers, 294: 119767. DOI:10.1016/j.carbpol.2022.119767 (  0) 0) |
Wu, C., Ramaswamy, Y., Zhu, Y., Zheng, R., Appleyard, R., Howard, A., et al., 2009. The effect of mesoporous bioactive glass on the physiochemical, biological and drug-release properties of poly(DL-lactide-co-glycolide) films. Biomaterials, 30(12): 2199-2208. DOI:10.1016/j.biomaterials.2009.01.029 (  0) 0) |
Wu, S., Huang, Z., Yue, J., Liu, D., Wang, T., Ezanno, P., et al., 2015. The efficient hemostatic effect of Antarctic krill chitosan is related to its hydration property. Carbohydrate Polymers, 132: 295-303. DOI:10.1016/j.carbpol.2015.06.030 (  0) 0) |
Wu, T., Cheng, N., Xu, C., Sun, W., Yu, C., and Shi, B., 2016. The effect of mesoporous bioglass on osteogenesis and adipogenesis of osteoporotic BMSCs. Journal of Biomedical Materials Research Part A, 104(12): 3004-3014. DOI:10.1002/jbm.a.35841 (  0) 0) |
Wu, B., Du, F., Liu, F., Liu, Y., Zheng, W., Li, G., et al., 2022. Graphene-ophicalcite heterogeneous composite sponge for rapid hemostasis. Colloids and Surfaces B: Biointerfaces, 216: 112596. DOI:10.1016/j.colsurfb.2022.112596 (  0) 0) |
Xiao, W., Xu, G., and Li, G., 2021. Role of shear stress in biological aerated filter with nanobubble aeration: Performance, biofilm structure and microbial community. Bioresource Technology, 325: 124714. DOI:10.1016/j.biortech.2021.124714 (  0) 0) |
Xing, Y., Yu, L., Wang, X., Jia, J., Liu, Y., He, J., et al., 2017. Characterization and analysis of Coscinodiscus genus frustule based on FIB-SEM. Progress in Natural Science: Materials International, 27(3): 391-395. DOI:10.1016/j.pnsc.2017.04.019 (  0) 0) |
Xu, C. Q., Zeng, Y. J., and Gregersen, H., 2002. Dynamic model of the role of platelets in the blood coagulation system. Medical Engineering & Physics, 24(9): 587-593. DOI:10.1016/S1350-4533(02)00047-4 (  0) 0) |
Yang, W., Song, J., Zhu, Y., Ye, Z., Wang, M., Fang, Y., et al., 2021a. Application of chain-based sponge dressing for gunshot wounds in the groin. The American Journal of Emergency Medicine, 39: 24-27. DOI:10.1016/j.ajem.2020.09.006 (  0) 0) |
Yang, Y., Han, P., Xie, X., Yin, X., Duan, G., and Wen, L., 2021b. Protein corona reduced graphene oxide cytotoxicity by inhibiting endocytosis. Colloid and Interface Science Communications, 45: 100514. DOI:10.1016/j.colcom.2021.100514 (  0) 0) |
Zgłobicka, I., and Kurzydłowski, K. J., 2022. Multi-length scale characterization of frustule showing highly hierarchal structure in the context of understanding their mechanical properties. Materials Today Communications, 33: 104741. DOI:10.1016/j.mtcomm.2022.104741 (  0) 0) |
Zhang, Y., Liu, J., Wu, Z., Mei, X., Zhu, W., and Wang, A., 2022a. Rapid promoting thrombus formation and fibrin cross-linked Bi-doped mesoporous bioglass for hemostatic agent. Materials Today Chemistry, 25: 100980. DOI:10.1016/j.mtchem.2022.100980 (  0) 0) |
Zhang, Z., Liu, T., Qi, Z., Li, F., Yang, K., Ding, S., et al., 2022b. Fabrication of effective mesoporous silica materials for emergency hemostasis application. Silicon, 14: 10521-10534. DOI:10.1007/s12633-021-01648-6 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


