2) Shanghai Collaborative Innovation for Aquatic Animal Genetics and Breeding, Shanghai Ocean University, Shanghai 201306, China;
3) Higher Institution Center of Excellence (HICoE), Institute of Tropical Aquaculture and Fisheries, University Malaysia Terengganu, Terengganu 20000, Malaysia;
4) Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Kowloon 999077, China;
5) Beihai Product Quality Testing Institute, Beihai 536000, China
Horseshoe crabs are one of the most ancient marine organisms in the nature. They first appeared in the Devonian period of Paleozoic, and are known as 'living fossils' because their morphological structures have not changed much since then (Sekiguchi and Nakamura, 1979; Shuster, 1979; Chen et al., 2015). Tachypleus amebocyte lysate (TAL), which is made using the hemolymph of Tachypleus tridentatus, is internationally recognized as the most sensitive bacterial endotoxin detection product (Hong et al., 2016). At present, there are only three genera and four species of horseshoe crabs in the world. T. tridentatus is mainly distributed in Guangdong, Guangxi and Fujian provinces and region in China (Carmichael and Brush, 2012). Unfortunately, habitat destruction, production of TAL, and overfishing have caused a sharp decline in the T. tridentatus population (Shin et al., 2007; Liao et al., 2019), and it is now classified as a Class Ⅱ national protected species in China.
'Probiotics' are active microorganisms that contribute to the balance of gut microbes (Dai and Chen, 1995). Probiotics can significantly induce intestinal flora to produce a variety of metabolites-volatile short-chain fatty acids, which play key roles in maintaining intestinal health of aquatic species. Competition inhibition is an important mechanism of probiotics, and also the main viewpoint of microecological theory. Through competition for fixed sites in vivo, probiotics reduce the number and density of pathogenic bacteria, thereby contribute to disease prevention (Tian, 2011; Barton and Mary, 2014). In aquaculture, feeding probiotics such as Bacillus subtilis and its mixture can improve the activities of superoxide dismutase (SOD) and catalase (CAT), as well as total antioxidant capacity (T-AOC) in blood. It also can reduce malondialdehyde (MDA) content, improve lysozyme activity and antioxidant capacity, and increase immune function of the organism (Wang et al., 2021). Lactobacillus can regulate the normal intestinal flora of an organism, maintain microecological balance, enhance its immune function, improve disease resistance by producing antibacterial metabolites and inhibit the growth of Vibrio. Therefore, Bacillus subtilis and Lactobacillus were selected as experimental strains in this experiment.
The decline in the population of T. tridentatus is expected to exert ecological and social impacts. Raising offspring to increase the population is considered as an option for increasing the population of T. tridentatus, which will further support sustainable resource management and research activities. Hatchery and culture techniques for the species have been developed since the late 1980s, and large-scale farming is common in Japan (Iwaoka and Okayama, 2007). Previous studies mainly focused on the type of feed and feeding conditions to maximize the growth and survival of T. tridentatus juveniles (Kwan et al., 2017). Little is known about the practicality and effectiveness of probiotics on the gut microbiota and the health of horseshoe crabs. Miao et al. (2020) compared the intestinal microbes of the first and second instars of T. tridentatus, indicating that ecdysis exerted changes on the intestinal microbes of T. tridentatus, and Vibrio and Shewanella were genus-level biomarkers after molting. Vibrio is a kind of pathogenic bacteria and probiotics can reduce it. In the early stage, antibiotics were used as a reagent to eliminate pathogenic bacteria and achieved better results, which was later found that long-term use of antibiotics would destroy the intestinal ecological balance and lead to drug resistance of bacteria (Hashemi and Davoodi, 2011; Looft et al., 2014). Since the prosperity of biotechnology, probiotics are more beneficial for the development of aquaculture, especially for feeding applications and artificial culture technology (Yao et al., 2020). Therefore, the purpose of this study was to investigate whether probiotics (Bacillus subtilis and Lactobacillus) had a positive effect on intestinal microbes from the second instar to the third instar in a 21-day culture experiment. The results of this study provide a theoretical basis for the effect of probiotics on crustacean intestinal microbiome during instar developmental stages and spearhead further development of the artificial breeding technology of juvenile T. tridentatus.
2 Materials and Methods 2.1 Experimental Conditions and AnimalsJuveniles of T. tridentatus were artificially raised in the laboratory (Beihai Xinglong Biological Products Co., Ltd.). Wild T. tridentatus were stimulated to lay eggs and fertilized by simulating tidal changes during the breeding period. Egg incubation period was approximately 45-50 d. Newly hatched first instars were raised in a glass tank (1.5 m × 0.7 m × 0.3 m) with circulating water system at Shanghai Ocean University (Shanghai, China). Six baskets (0.35 m × 0.2 m × 0.1 m) covered with fine sands were placed in the glass tank, and each basket contained 100 first instars. This experiment was conducted in June, and the experimental conditions were maintained at temperature 29-30℃, pH 8.1-8.2, salinity 19-32, dissolved oxygen 6.0-8.0 mg L-1, and photoperiod 12 h: 12 h (darkness: light). Approximately one third of the total water volume was changed every three days. No feeding was required during the first instar stage. After about 60 d, the 1st instar T. tridentatus molted to become the 2nd instar T. tridentatus. Then they were transferred into experimental tanks (0.25 m × 0.165 m × 0.16 m, 6.6 L), with 10 instars per tank. Five treatments were used in this study, including control (Ctr) without any treatments, low concentration of Bacillus subtilis (1×107 CFU mL-1) (Lbs), high concentration of Bacillus subtilis (1×1012 CFU mL-1) (Hbs), low concentration of Lactobacillus (1×107 CFU mL-1) (L1), and high concentration of Lactobacillus (1×1012 CFU mL-1) (H1). One tank represented one treatment replicate, and triplicates were conducted for each treatment. We once took out the intestines of T. tridentatus and observed Artemia through microscope. So Artemia (approximately 1 g) was fed to juvenile horseshoe crab once a day. We mixed Artemia and probiotics to feed horseshoe crabs, and changed 1000 mL of configured seawater after 2 h of feeding. The culture experiments were conducted for a period of 21 d.
2.2 Sample CollectionJuvenile T. tridentatus of second instar were collected, dried on a test paper, disinfected with 70% alcohol, and re-washed with distilled water. After drying on a test paper, the complete intestines of 10 instars were collected individually, snap frozen with liquid nitrogen and stored at -80℃ until further analysis (Fig. 1).

|
Fig. 1 The gut of 2nd instar T. tridentatus. Scale bar length is 0.25 cm. |
The intestinal tissue specimens were crushed, and 0.4 g of the sample powder was placed in a 2 mL microcentrifuge tube. Subsequently, DNA was isolated with a MagPure Soil DNA LQ Kit (Magen, Guangdong, China) following the manufacturer's instructions. According to the method of Kumar et al. (2011), the target area of V3-V4 of bacterial 16S rRNA was used to compare the gut microbiome of juvenile HSC of different groups. Sequencing was performed on an Illumina NovaSeq6000 (Illumina, San Diego, CA, USA) at Shanghai OE Biotech. Co., Ltd. (Shanghai, China).
2.4 Data and Bioinformatic AnalysisData are presented as mean ± standard error (SE) for each group. Trimmomatic software was used to preprocess the paired-end reads (Bolger et al., 2014) for the detection and cutting off of ambiguous bases (N). Sequence denoising was further performed to remove reads with unclear sequence, homology or less than 200 bp. QIIME software (version 1.8.0) was used to retain 75% or more of the Q20 base reads (Caporaso et al., 2010). Cleaning reads were sequentially removed and clustered, and operational taxonomic units (OTUs) were generated using VSEARCH software with similarity truncation of 97% (Rognes et al., 2016). QIIME package was used to select a representative reading for each OTU. Unweighted Unifrac principal coordinate analysis (PCoA) and phylogenetic tree construction were performed using Unifrac distance matrix generated by QIIME software. Sequencing and analysis of 16S rRNA amplicon were performed by OE Biotechnology Co., Ltd. (Shanghai, China).
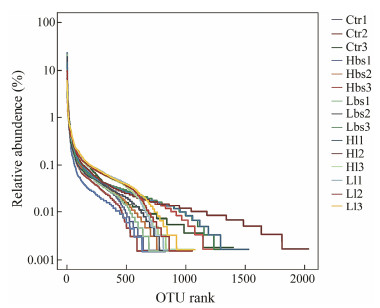
3 Results 3.1 Operational Taxonomic Units (OTUs) and Diversity AnalysesAfter 16S rRNA sequence analysis, Ctr (control), Hbs (high concentration of Bacillus subtilis), Lbs (low concentration of Bacillus subtilis), Hl (high concentration of Lactobacillus) and Ll (low concentration of Lactobacillus) were analyzed. The Alpha diversity analysis showed that Ctr, Hbs, Lbs and Ll dropped abruptly on the OTU rank graph while Hl smoothly declined, which indicated that the biodiversity of Ctr, Hbs, Lbs and Ll were not significantly different while the biodiversity of Hl was significantly higher than the other four groups (Table 1, Fig. 2).
|
|
Table 1 Statistics of the Alpha index of intestinal microbiome and water microbiome |
|
Fig. 2 Statistics of OTU rank and relative abundance of Alpha analysis. Ctr data were collected before the 21-d experiment. |
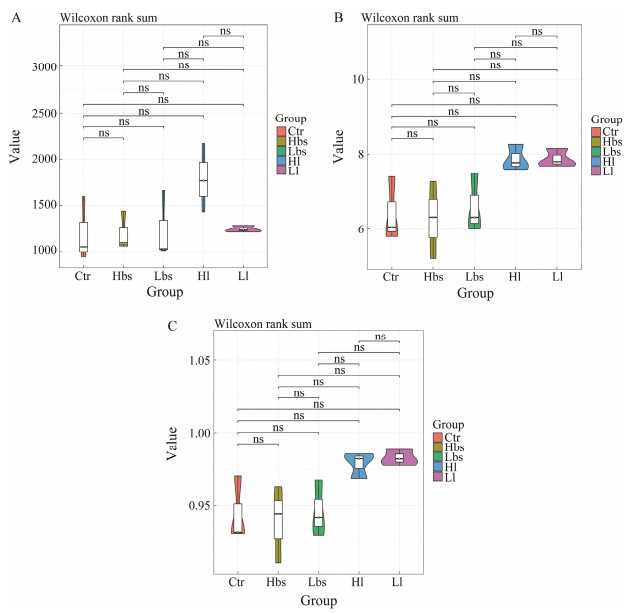
According to Alpha analysis, the chao1 (Fig. 3a) indices of Ctr, Hbs, Lbs, Hl and Ll had no significant difference, but the species richness of Hl was significantly higher than those of other groups. Shannon (Fig. 3b) and Simpson (Fig. 3c) indices of Ctr, Hbs, Lbs, Hl and Ll were not significantly different, but the biodiversity of Hl and Ll were significantly higher than those of other groups.
|
Fig. 3 Alpha metrics of intestinal microbiome: Chao1 index, and Shannon diversity. |
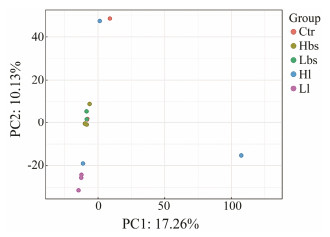
PCoA analysis of composition of three samples (Ctr, Hbs and Lbs) were mostly overlapped, and the difference between Hl and Ll samples was also not obvious. However, the bacterial compositions of Ctr, Hbs and Lbs were significantly different with Hl and Ll samples (Fig. 4).
|
Fig. 4 Principal component analysis of intestinal microbiome in HSC. |
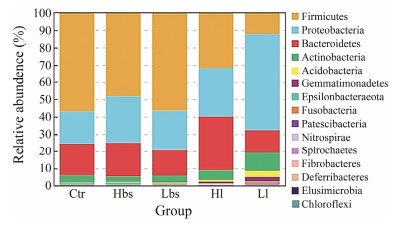
Cluster analysis was performed on the basis of OTUs and sequencing data to obtain a histogram of the microbial community of Ctr, Hbs, Lbs, Hl and Ll at the phylum level, showing the columnar distribution of the relative abundance of the top 15 phyla (Fig. 5). In general, the intestinal microbiota of the second instars was dominated by microbes from the category of Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, Acidobacteria, Gemmatimonadetes, Epsilonbacteraeota, Fusobacteria, Pa-Tescibacteria, Nitrospirae, Spirochaetes, Fibrobacteres, Deferribacteres, Elusimicrobia and Chloroflexi. In the five groups of the HSC gut microbiome (Ctr, Hbs, Lbs, Hl and Ll), Firmicutes, Proteobacteria and Bacteroidetes were always the dominant phyla. As for Ctr, Hbs and Lbs, Firmicutes was the most dominant phylum (56.6, 48.0% and 56.5%), followed by Proteobacteria (18.5%, 26.7% and 22.6%) and Bacteroidetes (18.4%, 19.6% and 14.6%). As for Hl, Firmicutes (31.5%), Bacteroidetes (31.1%) and Proteobacteria (28.0%) were still the three most advantageous categories, but the proportion of Firmicutes decreased and the proportion of Bacteroidetes increased compared with other groups. As for Ll, Proteobacteria was replaced Firmicutes as the most dominant phylum (55.3%) followed by Bacteroidetes (13.0%), Firmicutes (12.2%) and Actinobacteria (10.5%). Besides, it is worth noting that the proportion of Bacteroidetes in Ll had increased. The Ctr and Lbs abundances of other microorganisms were < 7%. The Hbs abundances of other microorganisms were < 6%. The Hl and Ll abundances of other microorganisms were < 10%.
|
Fig. 5 At the phylum level, the relative abundance of intestinal bacteria in 5 groups were in the top 15. |
The LEfSe analysis in Ctr, Hbs, Lbs and Ll revealed (Fig. 6) that Turicibacter was the genus-level biomarker in Ctr. Roseburia, Escherichia, Shigella and Fusicatenibacter were the genus-level biomarkers in Hbs; Gemmatimonadetes and Nitrospirae were phylum-level biomarkers in Lbs; and Chujaibacter, Sphingomonas, Rhodopseudomonas, Reyranella and Castellaniella were genus-level biomarkers in Ll.
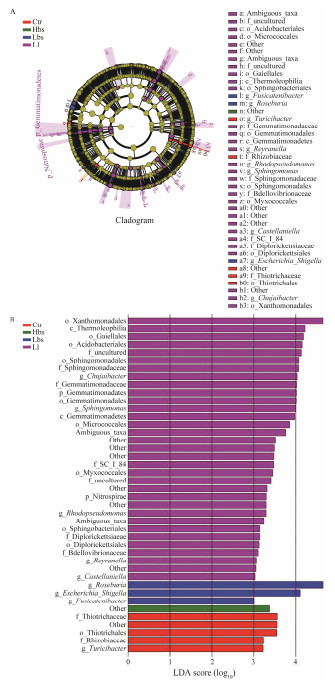
|
Fig. 6 LEfSe histogram of intestinal flora of Ctr (control), Hbs (high concentration of Bacillus subtilis), Lbs (low concentration of Bacillus subtilis) and Ll (low concentration of Lactobacillus) showed the log-Transform LDA score of bacterial groups appraised by LEfSe analysis (log-Transform LDA score 3.0 was the threshold). The single characters before the underlines are abbreviations: p, phylum; c, class; o, order; f, family; g, genus. |
The KEGG pathways related to the intestinal function of the second instars changed to a certain extent after adding different concentrations and different types of probiotics. As for Ll and Lbs, 'Cellular Processes' pathways, 'Metabolism' pathways and 'Organismal Systems' pathways were found more significantly different than those in Ctr, Hbs and Hl. In addition, 'Replication and Repair' pathways, 'Immune System' pathways and 'Environmental Adaptation' pathways were found more abundant in Lbs. 'Environmental Adaptation' pathways, 'Transport and Catabolism' pathways, 'Cell Growth and Death' pathways, 'Energy Metabolism' pathways and 'Metabolism' pathways were found more abundant in Ll (Fig. 7).
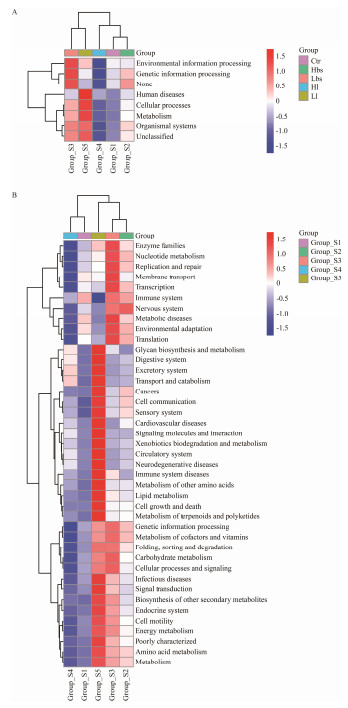
|
Fig. 7 Relative abundances of KEGG in Ctr, Hbs, Lbs, Hl (high concentration of Lactobacillus) and Ll. |
The composition of gut microbes is influenced by many factors, such as physiology, genetic inheritance, geography, life history and living environment (Yang and Shao, 2013). The formation and stability of the intestinal environment will not only affect the growth or death of the host, but also improve or reduce metabolic transformation, immunity and many other important physiological functions (Sun et al., 2020). The research of intestinal microbes has been a very popular topic in other aquatic organisms, but the research on the intestinal microbes of HSCs needs more attentions.
In recent years, probiotics have been widely favored by researchers due to their safety and ability to inhibit pathogenic bacteria. In the present study, the intestinal microbes of the second instars of HSCs are more abundant after the introduction of Lactobacillus, both in high and low concentrations (1 × 107, 1 × 1012 CFU mL-1). It is worth of noting that Actinobacteria shows significantly higher proportion than the other three groups (Kämpfer, 2010). However, the proportions of Actinobacteria in the groups treated with high and low concentrations of Bacillus subtilis were similar to that in Ctr. Actinobacteria is a biologically active substance that is more abundant than other microorganisms and has antibacterial activity as the main producer of antibiotics (Kämpfer, 2010). The results indicate that the introduction of Lactobacillus to the living environment of second instars can increase the proportion of actinomycetes in the intestinal microbes and reduce the proportion of pathogenic bacteria. This slight optimization may improve the health of the hosts' intestinal microbial community.
In the expression of the intestinal microbial richness, the intestinal microbes of the 5 groups of HSCs change with no significance after 21 days, but the intestinal microbial abundance of the two groups after treatment with Lactobacillus was still slightly higher than those of the other three groups. Firstly, the intestinal flora of aquatic animals will be affected by many factors, not only genetic and physiological differences, but also environmental factors (Nicholson et al., 2012). Research reports indicate that the number of bacteria in the intestine of freshwater fish is basically 105-108 cfu g-1, while the number of bacteria in the intestine of marine fish is 106-108 cfu g-1. Because of the great amount of intestinal microbes, the change of intestinal flora is a relatively long process (Sugita et al., 1983; Guo et al., 2019).
In the present research, the microbial composition of the intestinal tract has been greatly changed after feeding probiotics. KEGG prediction indicates that plenty of bacterial KEGG pathways significantly changed after feeding with low concentration of Bacillus subtilis (Lbs) and low concentration of Lactobacillus (Ll). For example, the 'Cellular Processes', 'Metabolism' and 'Organismal Systems' significantly increased in Lbs and Ll, but then significantly debased in Ctr, Hbs and Hl. Additionally, significant improvement of these pathways indicates that the metabolism and digestion capacity of juvenile HSCs can be improved by changing the breeding environment and adding appropriate amounts of probiotics, which can promote the growth and development of HSCs. However, different concentrations of the same type of probiotics may also lead to various results. The 'Replication and Repair' and 'Immune System' pathways have been significantly improved in Lbs, which indicates that feeding an appropriate amount of Bacillus subtilis can increase the immune system of juvenile HSCs. On the other hand, 'Digestive System', 'Transport and Catabolism', 'Energy Metabolism' and 'Metabolism' pathways significantly increase in Ll, which indicates that feeding an appropriate amount of Lactobacillus can increase metabolism and digestion of juvenile HSCs. Aquatic crustaceans will soon be affected by conditions such as temperature, light, and salinity when they molt. They will also be affected by conditions such as energy accumulation and adequate nutrition. When the accumulation of energy and nutrients is not sufficient, the time between molting periods will be prolonged, and the growth rate and weight gain rate of molting will decrease, even resulting death finally. Therefore, the present study shows that probiotics can improve digestion and metabolism which may accumulate enough energy for the next molting of horseshoe crabs and increase survival rate, which will be helpful for the follow-up studies to explore whether the molting cycle can be relatively reduced to achieve faster growth.
According to the result in the present study, Lactobacillus has a greater impact on the intestinal microbes, while Bacillus subtilis has less impact on the intestinal microbes. According to the LEfSe analysis, Gemmatimonadetes and Nitrospirae were phylum-level biomarkers in Lactobacillus groups. Gemmatimonadetes is a kind of gram-negative bacteria, which is related to the process of eliminating pathogenic bacteria, such as inhibiting the growth of pathogenic bacteria (Zeng et al., 2021). Roseburia is a genus-level biomarker in Bacillus subtilis groups, and a conditional pathogen that can reduce nitrate, meaning once the intestinal environment undergoes a major change it may become a pathogen (Shinohara et al., 2019).
In the present study, we find that two types of probiotics with different concentrations showed other effects on the structure, richness and function of intestinal microbes. Li et al. (2005) studied on the effect of Bacillus preparations on the intestinal microbes of Litopenaeus vannamei, showingthat the longer the cultivation time, the more energy the microbes use. Wang et al. (2009) used Lactobacillus to conduct experiments on the three common dominant microalgae in prawn farming ponds, showing that the inhibitory effect of Lactobacillus on the three microalgae was obvious, but microalgae would slowly appear after stopping the addition of Lactobacillus. Both experiments changed the concentration of probiotics and both showed that a higher concentration cannot have a better improvement. Similarly, our results show that the high concentration of Lactobacillus is not as effective as the low concentration on the second instars. In addition, the result with Bacillus subtilis treatment is similar to the control group. As Bacillus subtilis needs long period to have a function, our breeding cycle is too short to have a significant result. The low concentration of Lactobacillus in the present study had a positive effect on the intestinal microbial richness, community distribution and predicted function of juvenile HSCs. We speculate that selecting the appropriate types of probiotics and the appropriate dosage for delivery may be an effective way to improve the health and growth of juvenile HSCs. Therefore, on the basis of our research, further research can be carried out, such as selecting more strains or setting up different breeding cycles to find the optimal solution for probiotics to improve the health and growth of juvenile HSCs.
AcknowledgementsThis study was supported by the National Natural Science Foundation of China (No. 31872587), the CAS Key Laboratory of Coastal Environmental Processes and Ecological Remediation, and the YICCAS Grant (No. 2020 KFJJ11).
Barton M. D.. 2014. Impact of antibiotic use in the swine industry. Current Opinion in Microbiology, 19: 9-15. DOI:10.1016/j.mib.2014.05.017 (  0) 0) |
Bolger A. M., Lohse M., Usadel B.. 2014. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30: 2114-2120. DOI:10.1093/bioinformatics/btu170 (  0) 0) |
Caporaso J. G., Kuczynski J., Stombaugh J.. 2010. QⅡME allows analysis of high-throughput community sequencing data. Nature Methods, 7: 335-336. DOI:10.1038/nmeth.f.303 (  0) 0) |
Carmichael R. H., Brush E.. 2012. Three decades of horseshoe crab rearing: A review of conditions for captive growth and survival. Reviews in Aquaculture, 4: 32-43. DOI:10.1111/j.1753-5131.2012.01059.x (  0) 0) |
Chen Z. B., Fan H. Q., Liao Y. Y., Qiu G. L., Xie H. L., Lin W. Y.. 2015. A living animal facing a dilemma-horseshoe crab. Science (Shanghai), 67: 3 (in Chinese with English abstract). DOI:10.3969/j.issn.0368-6396.2015.03.015 (  0) 0) |
Dai Z. J., Chen Z. P.. 1995. Probiotics use prospects. Guide to Chinese Poultry, 1995(1): 46-47 (in Chinese). (  0) 0) |
Guo Q. Q., Hu J. N., Wei J. S., Zhang H.. 2019. Study method of intestinal microflora on aquatic animal. Henan Fisheries, 125(1): 17-20 (in Chinese with English abstract). (  0) 0) |
Hashemi S. R., Davoodi H.. 2011. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Veterinary Research Communications, 35: 169-180. DOI:10.1007/s11259-010-9458-2 (  0) 0) |
Hong J., Hu J. Y., Ke F.. 2016. Experimental induction of bacterial resistance to the antimicrobial peptide Tachyplesin Ⅰ and investigation of the resistance mechanisms. Antimicrobial Agents and Chemotherapy, 60: 6067-6075. DOI:10.1128/AAC.00640-16 (  0) 0) |
Iwaoka C., Okayama T.. 2007. Public awareness and community-based conservation for the horseshoe crab at Saikai National Park in Nagasaki Prefecture, Japan. International Symposium on the Science and Conservation of Horseshoe Crabs. Dowling Coll, Oakdale, NY, 571-575.
(  0) 0) |
Kämpfer, P., 2010. Actinobacteria. In: Handbook of Hydrocarbon and Lipid Microbiology. Timmis, K. N., ed., Springer, Berlin, Heidelberg, DOI: 10.1007/978-3-540-77587-4_133.
(  0) 0) |
Kumar P. S., Brooker M. R., Dowd S. E., Camerlengo T.. 2011. Target region selection is a critical determinant of community fingerprints generated by 16S pyrosequencing. PLoS One, 6(6): 1-8. DOI:10.1371/journal.pone.0020956 (  0) 0) |
Kwan B. K. Y., Chan A. K. Y., Cheung S. G., Shin P. K. S.. 2017. Marine microalgae as dietary supplements in the culture of juvenile Chinese horseshoe crabs, Tachypleus tridentatus (Xiphosura). Aquaculture Research, 48: 3910-3924. DOI:10.1111/are.13218 (  0) 0) |
Li Z. J., Lin L., Yang Y. Y.. 2005. Effect of Bacillus commercial probiotic on intestinal microflora of white shrmip, Litopenaeus vannamei. South China Fisheries Science, 1(3): 54-59 (in Chinese with English abstract). DOI:10.3969/j.issn.2095-0780.2005.03.009 (  0) 0) |
Liao Y. Y., Hsieh H. L., Xu S. Q., Kwan B. K. Y.. 2019. Wisdom of crowds reveals decline of Asian horseshoe crabs in Beibu Gulf, China. Oryx, 53: 222-229. DOI:10.1017/S003060531700117X (  0) 0) |
Looft T., Allen H., Cantarel B., Levine U. Y., Bayles D. O., Alt D. P., et al. 2014. Bacteria, phages and pigs: The effects of in-feed antibiotics on the microbiome at different gut locations. The ISME Journal, 8: 1566-1576. DOI:10.1038/ismej.2014.12 (  0) 0) |
Miao F. Z., Zhao Z. H., Li Q. Z., Song J., Wang Y. J., Hu M. H.. 2020. Impact of initial feeding and molting on Tachypleus tridentatus gut microbiota. Current Microbiology, 77: 2847-2858. DOI:10.1007/s00284-020-02108-x (  0) 0) |
Nicholson J. K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., et al. 2012. Host-Gut microbiota metabolic interactions. Science, 336: 1262-1267. DOI:10.1126/science.1223813 (  0) 0) |
Rognes T., Flouri T., Nichols B., Quince C.. 2016. VSEARCH: A versatile open source tool for metagenomics. PeerJ, 4: 2167-8359. DOI:10.7717/peerj.2584 (  0) 0) |
Sekiguchi K., Nakamura K.. 1979. Ecology of the extant horseshoe crabs. Progress in Clinical and Biological Research, 29: 37-45. (  0) 0) |
Shin P. K. S., Li H. Y., Cheung S. G.. 2007. Horseshoe crabs in Hong Kong: Current population status and human exploitation. International Symposium on the Science and Conservation of Horseshoe Crabs. Dowling Coll, Oakdale, NY, 347-351.
(  0) 0) |
Shinohara R., Sasaki K., Inoue J., Hoshi N.. 2019. ButyrylCoA: acetate CoA-transferase gene associated with the genus Roseburia is decreased in the gut microbiota of Japanese patients with ulcerative colitis. Bioscience of Microbiota Food and Health, 38: 159-163. DOI:10.12938/bmfh.18-029 (  0) 0) |
Shuster C. N.. 1979. Distribution of the American horseshoe 'crab, ' Limulus polyphemus (L.). Progress in Clinical and Biological Research, 29: 3-26. (  0) 0) |
Sugita H., Oshima K., Tamura M., Deguchi Y.. 1983. Bacterial flora of gastrointestine of freshwater fishes in river. Nihon-Suisan-Gakkai-Shi, 49: 1387-1395. DOI:10.2331/suisan.49.1387 (  0) 0) |
Sun F. L., Wang C. Z., Chen L. J., Weng G. Z., Zheng Z. P.. 2020. The intestinal bacterial community of healthy and diseased animals and its association with the aquaculture environment. Applied Microbiology and Biotechnology, 104: 775-783. DOI:10.1007/s00253-019-10236-z (  0) 0) |
Tian L.. 2011. Re-discussion on probiotics. Science and Technology Innovation Herald, 8(24): 251-252 (in Chinese with English abstract). (  0) 0) |
Wang H., Li Z. J., Li C. H., Cao Y. C., Yang Y. Y.. 2009. Effects of Lactobacillus spp. on three common microalgae in shrimp ponds. Journal of Applied Oceanography, 28(2): 223-227 (in Chinese with English abstract). DOI:10.3969/j.issn.1000-8160.2009.02.012 (  0) 0) |
Wang Y., Wang Q. K., Xing K. Z., Jiang P., Wang J.. 2021. Dietary cinnamaldehyde and Bacillus subtilis improve growth performance, digestive enzyme activity, and antioxidant capability and shape intestinal microbiota in tongue sole, Cynoglossus semilaevis. Aquaculture, 531: 75798. DOI:10.1016/j.aquaculture.2020.735798 (  0) 0) |
Yang B. B., Shao Q. J.. 2013. Research progress of fish intestinal microbes. China Feed, 5(23): 1-4 (in Chinese with English abstract). DOI:10.3969/j.issn.1004-3314.2013.23.001 (  0) 0) |
Yao Y. Y., Yang Y. L., Gao C. C.. 2020. Surface display system for probiotics and its application in aquaculture. Reviews in Aquaculture, 12: 2333-2350. DOI:10.1111/raq.12437 (  0) 0) |
Zeng Y. H., Wu N. C., Chen X. H.. 2021. Gemmatimonas groenlandica sp. nov. is an aerobic anoxygenic phototroph in the phylum Gemmatimonadetes. Frontiers in Microbiology, 11: 606612. DOI:10.3389/fmicb.2020.606612 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


