2) Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266100, China
Microorganisms in atmospheric aerosols not only potentially serve as cloud condensation nuclei (CCN) (Bauer et al., 2003) and ice nuclei (IN) (Christner, 2010) but also absorb or reflect incoming sunlight in the upper troposphere (Sesartic et al., 2012). Therefore, these microorganisms have large impacts on precipitation, radiative forcing, and global climate conditions. Several studies have reported that large proportions of microbes in atmospheric cloud water are metabolically active (Amato et al., 2007; Delort et al., 2010; Stopelli et al., 2017), and airborne microbes can change the multiphase chemistry in the atmosphere by using chemical species as nutrients (Ariya et al., 2002; Bauer et al., 2002; Amato et al., 2005; Fuzzi et al., 2006; Deguillaume et al., 2008). Biodegradation can compete with common radical chemistry in the atmosphere. However, biodegradation is highly variable because it depends on live microbes, which are affected by many environmental factors and are temporally and spatially variable.
Many researchers have focused on the microbial concentration (the number of airborne microorganisms per unit volume of air) over land and found that the concentrations of airborne microorganisms exhibit significant seasonal and spatial variations. Most studies observed high concentrations of airborne microbes in the summer or autumn (Kelly and Pady, 1954; Ho et al., 2005; Wu et al., 2007; Li et al., 2011; Hurtado et al., 2014) and in the winter in certain studies (Dong et al., 2016; Xie et al., 2018b). The concentration of airborne microbes varies greatly (even by one order of magnitude) in different regions (Burrows et al., 2009; Xie et al., 2018a). In addition, the microbial concentration is affected by foggy, hazy, and dusty weather conditions (Kellogg et al., 2004; Cao et al., 2014; Dong et al., 2016). The limited studies on atmospheric microbes over oceans have shown that airborne microbes vary from 10 to 105 cells m−3 (Burrows et al., 2009; Mayol et al., 2017); this range is much lower than the reported variation of 103-106 cells m−3 over land (Hara and Zhang, 2012; Murata and Zhang, 2014; Dong et al., 2016; Xie et al., 2018b; Maki et al., 2019). For example, the average concentrations of airborne microbes over the Caribbean Sea and the North Atlantic Ocean are 1.5×105 and 1.0×104 cells m−3, respectively (Mayol et al., 2014). Moreover, certain studies found that the total hydrocarbons (THCs) were statistically correlated with the fungal concentration (Ho et al., 2005; Wu et al., 2007). Overall, whether the microbial concentration is a key factor affecting the transformation of organic compounds in the atmosphere remains unclear.
Microbial activity, which is an indicator of the physiological state of microbes, represents the intensity of the metabolic activity of microbes (Qi et al., 2015). The microbial activity cannot be simply estimated from the microbial concentration, as the activity level has no correlation with the abundance of airborne microorganisms in the atmosphere (Zhong et al., 2016). Studies on microbes in soil have shown that microbial activity can affect the decomposition of organic matter (Rovira and Vallejo, 2002; Cui and Holden, 2015; Si et al., 2018); the production of dissolved organic carbon (DOC) (Balland-Bolou-Bi et al., 2019); and the C, N, and P stoichiometries in afforested ecosystems (Zhao et al., 2018). However, research on the microbial activity in atmospheric aerosols has been extremely limited. Some studies found that airborne microbes can biodegrade certain chemicals, such as dicarboxylic acids (DCAs), into volatile or toxic products via metabolism in the atmosphere (Ariya and Amyot, 2004). Zhong et al. (2016 studied the microbial activity in atmospheric aerosols in Qingdao by the fluorescein diacetate (FDA) hydrolysis method, and they found that the activity increases with increasing temperature within the range of −3.5℃ to 28.4℃ and can be inhibited at low temperatures (Zhong et al., 2016). However, the mechanism by which microbes are involved in atmospheric chemistry and the effect of microbial activity on the biodegradation of organic compounds remain unknown.
Previous research on the microbial concentration and activity in the atmosphere primarily focused on continental samples; thus, there is little information regarding the characteristics and influencing factors of microbes in the marine atmosphere. In the present study, we characterized the levels and size distributions of microbial concentration and activity over the South China Sea (SCS) and discussed the factors influencing the microbes. The correlations of the airborne microbial concentration and activity with the chemical species in aerosol particles were also analyzed.
2 Materials and Methods 2.1 Sampling SitesBioaerosol samples were collected on the research vessel R/V Dong Fang Hong 2 during cruises across the SCS in May-June 2016. Samplers were mounted onboard toward the front of the uppermost deck of the vessel at approximately 15 m above sea level. Sample collection was performed only when the ship was sailing and the wind direction relative to the bow ranged from −90˚ to 90˚ to avoid pollution from ship emissions. Thirteen sets of parallel bioaerosol samples (each set included two parallel samples for microbial activity determination and one sample for microbial concentration determination) were collected along the cruise trajectories. The sampling and meteorological information for the samples is provided in Table 1.
|
|
Table 1 Sampling and meteorological information for the bioaerosol samples collected over the SCS and in Qingdao in 2016 |
Bioaerosol samples were also collected at a coastal site in Qingdao (approximately 7.0 km away from the shore), adjacent to the Yellow Sea, to better understand the characteristics of the microbes in marine aerosols. Samplers were located on the roof of an academic building on the Lao-shan campus of the Ocean University of China (36˚10΄N, 120˚30΄E; 9.0 m above the ground). The bioaerosol samples were collected on May 11, 17, 20, and 30 and June 6, 2016, close to the SCS cruise periods.
2.2 Collection and Analysis of the Bioaerosol Samples 2.2.1 CollectionAll of the bioaerosol samples were collected on sterilized polycarbonate membranes by using a six-stage microorganism FA-1 cascade impactor (Applied Technical Institute of Liaoyang, China), which was placed on a tripod at a height of 1.5 m above the deck of the research vessel and roof in Qingdao. The polycarbonate membranes (pore size of 0.22 μm and a diameter of 80 mm) were sterilized by using an autoclave at 121℃ for 15 min before sampling and then placed in an oven at 60℃ for drying. One of these pretreated membranes was randomly selected as a blank. If the cells on the blank were not detected (using the same method as that in section 2.2.2), then the membranes were qualified to be used for sampling. The impactors of the samplers were wiped with 75% alcohol and placed on a clean bench for ultraviolet (UV) sterilization for 15 min before sampling. Subsequently, the sterilized polycarbonate membranes were placed in the impactors. All of the experimental apparatuses were sterilized prior to sampling. The bioaerosol particle sizes were divided into six categories based on the aerodynamic diameters: 0.65-1.1, 1.1-2.1, 2.1-3.3, 3.3-4.7, 4.7-7.0, and >7.0 μm. The air flow rate of the samplers was 28.3 L min−1 during sampling, with a duration of 30 min. One sampler was used to collect samples for microbial concentration, while two other samplers were used to collect parallel samples for microbial activity determination.
2.2.2 Analysis of the total airborne microbesThe concentration of total airborne microbes (including prokaryotes and unicellular eukaryotes) was determined by using an epifluorescence microscope equipped with a UV light source (Olympus BX51, Japan) after staining with 4', 6-diamidino-2-phenylindole (DAPI). The DAPI-stained cells in 10 randomly selected fields were counted at a magnification of 400 and then used to calculate the microbial concentrations in the bioaerosol samples. The detailed procedure can be found in the literature (Li et al., 2011; Dong et al., 2016). The mean relative standard deviation (RSD) of the counts in the 10 randomly selected fields was 36% (n = 488), and the cell number in each field varied from 0 to 21 depending on the sample and size range.
2.2.3 Analysis of the microbial activityThe microbial activity level in the bioaerosols was determined by the fluorescein diacetate (FDA) hydrolysis method, which has been widely used to measure microbial activity in soil and water samples (Fontvieille et al., 1992; Bjurman, 1993). This method can accurately quantify the overall metabolic activity of all microbial enzymes, including the ubiquitous lipases, proteases, and esterases (Stubberfield and Shaw, 1990; Green et al., 2006; Achuba and Peretiemo-Clarke, 2008; Yuan et al., 2017), because FDA can be hydrolyzed by the enzymes secreted by living bacteria and fungi (Adam and Duncan, 2001; Green et al., 2006) to produce a fluorescent compound. To eliminate the variation in fluorescence intensity due to differences in the fluorescence spectrophotometers used, sodium fluorescein (SF) salt was analyzed as a standard because it produces the same yellow hydrolysis product (fluorescein) as FDA does. Considering the dynamic measurement of microbial activity, the microbial activity in the aerosol particles is represented by the concentration of SF per hour in units of ng m−3 h−1 SF. Details of the method can be found in the literature (Qi et al., 2015; Zhong et al., 2016). The blank membranes and bioaerosol samples were treated following the same procedure. The fluorescence intensity determined from the analysis of nine blank sample sets (triplicate samples in each set) consisting of FDA (Sigma) and ultrapure water from different sources with varying fluorophotometers was less than 3 (A.U.). This intensity was used as the index for valid measurements in our study. If the fluorescence intensity of the blanks was less than 3 (A.U.), the samples were analyzed for microbial activity; otherwise, the samples were deemed invalid. To eliminate the influence of the membranes, reagents, and water on the sample fluorescence, we corrected the fluorescence intensity of valid samples by subtracting the blank values. The activity levels of the samples were calculated by using the standard curve of the sodium fluorescein concentration versus the fluorescence intensity (r2 = 0.99). The mean RSD among duplicates was 33% (n = 504). On the basis of the SD of parallel blank samples (σb, n = 25), the detection limit was estimated to be 0.072 ng m−3 h−1 SF by using the formula L = 4.6σb (Global Environmental Monitoring Service, GEMS).
2.3 Collection and Measurement of Aerosol Samples for Chemical AnalysisThe atmospheric aerosols used to determine ion concentrations were collected on Teflon membranes (80 mm in diameter) by using a cascade impactor (AN 200, Sibata Co. Inc., Japan) over the SCS. The aerosol particle sizes were divided into nine groups: < 0.43, 0.43 - 0.65, 0.65 - 1.1, 1.1 - 2.1, 2.1 - 3.3, 3.3 - 4.7, 4.7 - 7.0, 7.0 - 11, and > 11 μm. The size-segregated samples were collected at a flow rate of 28.3 L min−1, with a sampling duration of 48 h. The total suspended particle (TSP) samples for the organic carbon (OC) and water-soluble organic carbon (WSOC) analyses were collected on quartz microfiber filters (What- man QM-A) by using a high-volume air sampler (Model KC-1000, Qingdao Laoshan Electronic Instrument Complex Co., Ltd.) over the SCS. TSP samples were also collected in Qingdao during dust events in April, 2016 (Table 2). The quartz microfiber filters were heated at 450℃ for 4.5 h in an oven to remove organic compounds. TSP samples were collected at a flow rate of 1.05 m3 min−1, with a sampling duration of about 20 h. After sampling, the membranes were packaged and immediately stored at −20℃ until further analysis.
|
|
Table 2 Sampling information and concentration (μg m−3) of TSP, inorganic nitrogen, OC, and WSOC in aerosol samples collected over the SCS and in Qingdao in 2016 |
Half of the Teflon membranes were ultrasonically extracted with 10 mL of deionized water (specific resistivity of 18.2 MΩ cm−1) for 40 min in an ice-water bath. After filtration, the concentrations of NO2−, NO3−, NH4+, Na+, and Cl− in the extract were analyzed by using an ICS-1100 ion chromatograph (Thermo Fisher, USA). The measurement details can be found in the literature (Qi et al., 2011).
The quartz membranes used to collect the TSP samples were cut into several pieces to determine the OC and WS-OC contents. Two parallel pieces of each aerosol membrane were used to analyze the OC and EC contents (Elemental Carbon) by using a thermal/optical carbon analyzer (DRI 2001A, Atmoslytic Inc., USA). One piece of the sample membrane was ultrasonically extracted with ultrapure water (18.2 MΩ cm−1) in an ice-water bath for 40 min. After acidification, the solution was aerated with pure nitrogen to remove inorganic carbon. The extract was fil-tered through a GF-F membrane, and the WSOC concentration in the solution was measured by a total organic carbon analyzer (TOC-VCPN, Shimadzu Corporation, Japan).
2.4 Meteorological Data Sources and Statistical AnalysisThe meteorological parameters (temperature, relative humidity (RH), wind velocity, wind direction, and atmospheric pressure) over the SCS were measured via the meteorological observatory on the research vessel. The meteorological parameters in Qingdao were obtained from the Qingdao Meteorological Administration (http://qdqx.qingdao.gov.cn/). The 72 h air mass back trajectories at an altitude of 1000 m were calculated for each sample by TrajStat software (Wang et al., 2009) and National Oceanic and Atmospheric Administration Global Data Assimilation System (NOAA GDAS) archive data (http://www.arl.noaa.gov/ready/hysplit4.html). The horizontal offshore distance, transport distance over the sea, and transport speed were measured from the trajectory for each sample using TrajStat software (Wang et al., 2009). The correlations of the microbial concentration and activity with each of the meteorological parameters were examined via Spearman correlation analysis. Nonparametric tests were performed to examine the differences between land and oceansamples; P values less than 0.05 were considered significant.
3 Results and Discussion 3.1 Characteristics of the Microbial Concentration and Activity in the Atmospheric Aerosols over the SCSTo better characterize the microbial concentration and activity in the atmospheric aerosols over the ocean, we collected bioaerosol samples over the SCS and at a coastal site in Qingdao from May 11 to June 6, 2016, due to the lack of an available observation site in the coastal region of the SCS. The backward trajectories revealed that the air masses sampled over the SCS mainly came from the ocean and were less affected by land (Fig.1). However, the air masses sampled over Qingdao originated from the ocean for two samples and from the land for three samples. We divided the samples in Qingdao into two categories. Category 1 included the air mass from land, representing the land samples affected by terrestrial sources. Category 2 included the air mass from the ocean, representing the land samples affected by marine air masses. The average microbial concentration and activity in these two categories were used as relative references in the comparison with the samples taken over the SCS to determine the influence of air masses.
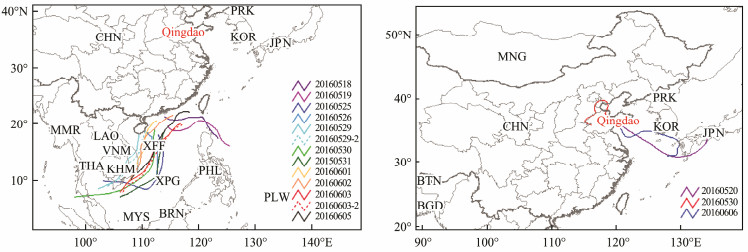
|
Fig. 1 The 72h air mass backward trajectories of the bioaerosol samples over the SCS (left) and in Qingdao (right) from May 18 to June 6, 2016. |
The magnitude and spatial distribution of the microbial concentration of the samples are shown in Table 3 and Fig.2, respectively. The concentration of total airborne microbes (TAMs) varied from 1.68 × 105 cells m−3 to 4.84 × 105 cells m−3 in the atmospheric aerosol particles over the SCS from May to June 2016, with an average of (2.92 ± 1.07) × 105 cells m−3. Studies on airborne microbes are extremely scarce. The concentration of airborne microbes in air has a range of 103-104 cells m−3 over the North Atlantic Ocean (Mayol et al., 2014) and 1.5 × 105 cells m−3 over the Caribbean Sea (DeLeon-Rodriguez et al., 2013). Moreover, the bacterial (part of TAM) concentrations in atmospheric aerosols are 0.7 × 105 - 1.2×105 cells m−3 in marine aerosols over the East Sea (Korea) (Cho and Hwang, 2011) and 1.0×104 - 2.5× 105 cells m−3 over the northwestern Pacific Ocean (Hu et al., 2017). Our results were in good agreement with most former studies but one order of magnitude higher than the results over the North Atlantic Ocean. This difference was probably because our samples were collected over the marginal sea and influenced by continental emissions.
|
|
Table 3 Measured and reported microbial concentration (TAM) and activity in bioaerosol samples taken over the SCS and in Qingdao and other regions |
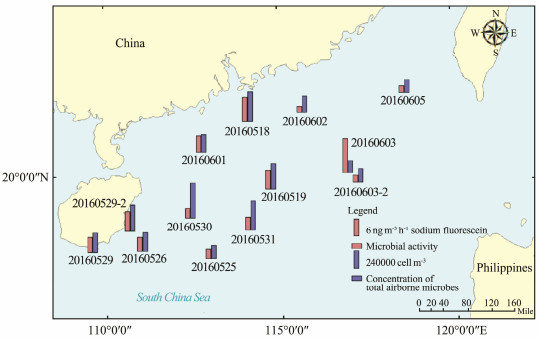
|
Fig. 2 Microbial concentrations and activity over the SCS from May 18 to June 6, 2016 (there is a bar at the midpoint of the sampling route for each sample). |
Table 3 shows that the TAM concentration over the SCS in 2016 was close to that in Xi'an and Qingdao in 2009-2010 but lower than that in Qingdao in other years (2014, 2016, and 2018) and other cities. The concentration difference between cities and the SCS was partially caused by the variations in sampling periods, geographical location, and climate. Subsequently, we compared the TAM concentration over the SCS and at the Qingdao coastal site during the same sampling period and found that the TAM concentration over the SCS was significantly different from that over Qingdao in 2016 (P < 0.05). The average microbial concentration over the SCS was decreased by 54% compared with the average concentration ((6.29 ± 1.29) × 105 cells m−3) in the Qingdao samples from land sources (Category 1) and by 40% compared with the average ((4.91 ± 1.66) × 105 cells m−3) in Qingdao samples with sea sources (Category 2). All these results showed that the concentration of airborne microbes over the ocean was lower than that over land. Here, the difference in study areas may affect the relative variation in the microbial concentration range over oceans compared with land; however, these observational data showed a similar tendency to higher values over cites on land than over the ocean (Table 3), even though these sites have different geographical and climate characteristics. In addition, the concentration of airborne microbes demonstrated a significant linear negative correlation (n = 13, **P < 0.01) with the horizontal offshore distance, the horizontal distance between the closest continental landmass, and a midpoint of the sampling route for each sample. When the horizontal offshore distance increased beyond 500 km, the microbial concentration decreased by 60% - 66%. Mayol et al. (2017 found that the bacterial concentration decreased exponentially with distance from the closest continental landmass. On the basis of the exponential relationships between the abundance of microbes and distance to land (Mayol et al., 2017), the microbial concentration decreased by 45% (55% for prokaryotes and 35% for eukaryotes) as the distance increased beyond 500 km. We found that the microbial concentration decreased slightly more with distance to land in the SCS than in the tropical and subtropical Atlantic, Indian, and Pacific Oceans (between 40˚S and 40˚N).
Oceans cover about 71% of the Earth's surface, and microbes can also enter the atmosphere from ocean surfaces via bubble bursts (Blanchard and Syzdek, 1982; Aller et al., 2005; Mayol et al., 2014). Therefore, we believe that the concentration of airborne microbes over oceans is influenced by the difference between the spray inputs and the losses caused by deposition during transport. The airborne microbes provided by ocean surfaces depend on the abundance of microbes in surface waters and wind speed (Mayol et al., 2017). Our samples were collected at locations less than 700 km away from land in the horizontal direction, where the average wind speed was 6.5 m s−1. Despite the open ocean conditions, the prokaryotic concentration from marine emissions was estimated at approximately 3 × 103 - 1.3 × 104 cells m−3 (Mayol et al., 2017), accounting for 1% - 3% of our measured concentration. The abundances of Na+ and Cl− in atmospheric aerosols over the ocean are typically employed to indicate the abundance of marine aerosols. From the size distributions of aerosols containing Na+ and Cl− over the SCS (Fig.3), we determined that the marine aerosols were mainly 3.3- 4.7 μm in size, in which an increase in the airborne microbe concentration was expected due to the supply of microbes from bubble bursts. However, the microbial concentration in aerosols of this size range decreased by 46% over the SCS compared with the Qingdao samples. All of these results suggested that the airborne microbes over the studied region were of both terrestrial and marine origins. Thus, the contribution of atmospheric transport is important in marginal seas that are less than 700 km away from a coast.
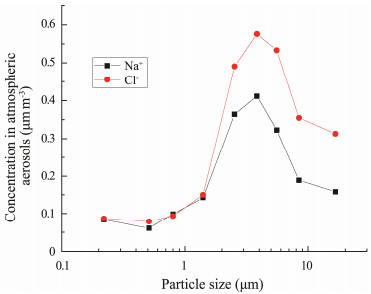
|
Fig. 3 Size distribution of Na+ and Cl− in atmospheric aerosols during an SCS cruise in 2016. |
Fig.4a shows the size distributions of airborne microbes in the aerosol particles collected on the SCS cruise, which indicated the proportion of airborne microbes in each size range to the sum of microbes in particles of all sizes. The size distributions of the microbes over the SCS presented three patterns (Fig.4a). Group 1, which includes 6 samples (20160519, 20160525, 20160529, 20160602, 20160603-2, and 20160605), showed high proportions of microbes in the particle size ranges of 2.1-3.3 μm and >7.0 μm, with average ratios of 24% and 23%, respectively. The microbes in the size range of 4.7-7.0 μm accounted for 8% of the microbes in all size ranges. Four samples in group 2 (20160518, 20160529-2, 20160530, and 20160531) showed similar size distributions to the samples in group 1, but the peak size of 2.1-3.3 μm for group 1 shifted to 3.3 - 4.7 μm for group 2. The proportion of microbes in the 3.3 - 4.7 μm range was 27%, which was 4% and 12% higher than those in the 2.1-3.3 and >7.0 μm ranges, respectively. The smallest proportion (10%) of microbes was in the 4.7-7.0 μm range. The samples in group 3 (20160526 and 20160603) had size distributions that differed from those of the other samples. The proportion of microbes in the 1.1-3.3 μm particles decreased to 11%- 12%, while that in the particles in the other size ranges varied between 17% and 21%, with the highest value in the >7.0 μm range. The source of the air mass was not a key factor influencing the size distribution of the airborne microbes (Fig.1). Correlation analysis showed that the microbial concentration in only the 1.1-2.1 μm range was positively correlated with wind speed (n = 13, **P < 0.01), indicating that the size distributions of the microbes were affected by wind speed during the sampling period. In addition, the size distributions of the microbes changed during transport, which was verified by the correlation between the microbial concentration and the horizontal offshore distance.
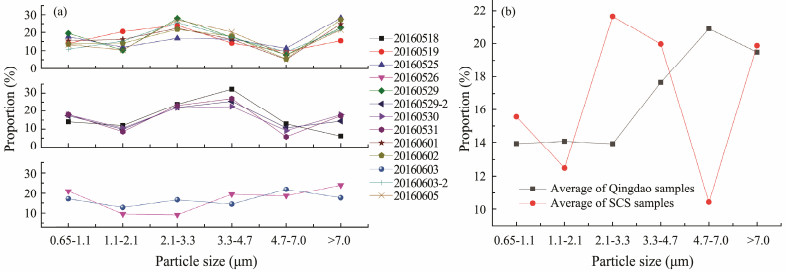
|
Fig. 4 Size distributions of airborne microbes over the SCS from May 18 to June 6, 2016. |
Overall, microbes were mainly present in the coarse particles (> 2.1 μm), with proportions of 63%-76% in all coarse samples. The results were in accordance with findings of previous studies (Lighthart and Mohr, 1994; Light-hart and Shaffer, 1995; Mori et al., 2003; Dong et al., 2016). Microbes attached to coarse particles are more likely to survive due to the better sanctuaries provided by coarse particles than fine particles (Bowers et al., 2013).
For the Qingdao samples, the average size distribution of the samples from land sources was similar to that of the samples taken over the SCS (Fig.4b). Thus, the airborne microbes over the SCS were partly affected by atmospheric transport. Compared with the airborne microbes in the samples from land in Qingdao, the airborne microbes in the SCS samples were present in significantly different size ranges: 0.65 - 2.1 and 4.7 - 7.0 μm (P0.01). The proportion of microbes in the 0.65 - 2.1 μm range increased from 16% in Qingdao to 28% over the SCS, while that in the 4.7 - 7.0 μm range decreased from 21% in Qingdao to 10% over the SCS. In marginal sea regions less than 700 km from the coast, atmospheric transport from land is more important than that from marine sources; therefore, we attributed the decrease in microbes in the 4.7 - 7.0 μm range to the deposition of coarse particles during transport. The microbes in the 0.65 - 2.1 μm range increased probably due to advection. All samples collected by Mayol et al. (2017 contained edeukaryotic cells at levels that greatly exceeded the calculated marine contributions, and 32% of their samples comprised prokaryotic cells at levels in excess of the calculated marine contributions, suggesting the advection of microbes from other locations. For the samples with sea sources collected over Qingdao, the size distribution with the highest proportion of microbes was 4.7 - 7.0 μm, and that with the lowest proportion was 2.1 - 3.3 μm, which was in contrast to the pattern for the samples with land sources. However, only the proportions of microbes in particles with sizes of 0.65 - 1.1 and 3.3 - 4.7 μm from sea sources were significantly different from those in the samples from land sources (P ≤ 0.05). These results showed that the difference in the size distribution of microbes between land and sea sources in Qingdao may be a result of the sources of microbes in the aerosols. However, the exact source of the microbes in the samples from Qingdao needs to be eludicated. Further research is needed to determine the characteristics of the airborne microbes produced by surface seawater, such as the size distribution, community, viability, and residence time in air.
3.1.2 Microbial activityThe microbial activity in the bioaerosol particles ranged from 2.09 ng m−3 h−1 SF to 11.97 ng m−3 h−1 SF over the SCS from May to June 2016, with an average of 5.75 ± 3.05 ng m−3 h−1 SF. The microbial activity over the SCS was significantly lower than that in Qingdao (mean: 15.55 ± 11.12 ng m−3 h−1 SF; P < 0.05). The average microbial activity over the SCS was 79% lower than that in samples from Qingdao with land sources (25.03 ± 0.79 ng m−3 h−1 SF) and 15% lower than that in Qingdao samples with sea sources (6.07 ± 3.27 ng m−3 h−1 SF). Although no reports of microbial activity over oceans have been made, previous studies observed that levels of viable airborne bacteria are higher over land than over oceans (Murata and Zhang, 2014; Hu et al., 2017). Microbial activity can decrease with the decline in microbial abundance and with the occurrence of death due to severe conditions in the atmospheric environment, such as low humidity (desiccation), high ultraviolet radiation, or other factors (Lighthart and Mohr, 1994), during transport.
The microbial activity in the size distributions of the bioaerosol particles varied greatly over the SCS and was classified into four groups, except for the activity in sample 20160526 (Fig.5a). The samples in group 1 (20160518 and 20160529-2) showed unimodal distributions of activity, and the maximum proportion (mean proportion of 34%) of the microbial activity occurred in the size range of > 7.0 μm. For the other samples, the microbial activity in the particles presented unimodal distributions, but the peak moved from the > 7.0 μm range to the 4.7-7.0, 3.3-7.0, and 0.65-7.0 μm ranges for group 2 (20160519, 20160525, 20160601, and 20160602), group 3 (20160529, 20160603, 20160603-2, and 20160605), and group 4 (20160530 and 20160531), respectively. The average maximum proportions were 35%, 39%, and 43% for groups 2, 3, and 4, respectively. Group 1 significantly differed from group 2 in terms of the activity in particles larger than 4.7 μm (P < 0.05) and from group 3 in terms of the activity in the 0.65-1.1 and >7.0 μm particles (P < 0.05). Group 2 significantly differed from group 3 in terms of the activity in the 1.1-2.1, 3.3-4.7, and 4.7-7.0 μm particles (P < 0.05). For sample 20160526, the peak in activity moved to the 2.1-3.3 μm range, with a proportion of 35% of the total microbial activity. This sample was significantly different from the samples in group 2 in terms of the 2.1-3.3 μm particles (P < 0.05), as well as from the samples in group 3 in terms of the 0.65-3.3 μm particles (P < 0.05). These results showed a more variable size distribution pattern of the microbial activity than of the microbial concentration, indicating that the microbial activity was affected by more factors than the microbial concentration. The source of the air mass had a minimal influence on the size distributions of the microbial activity in the samples in this study. However, the microbial activity in the 4.7-7.0 μm range was positively correlated with wind speed (n = 13, **P < 0.01), suggesting that wind speed affected the sizes with peak activity. Except in group 4, where 62% of the activity was detected in fine particles, microbial activity was mainly observed in coarse particles, containing 68% of the activity in the rest of the samples.
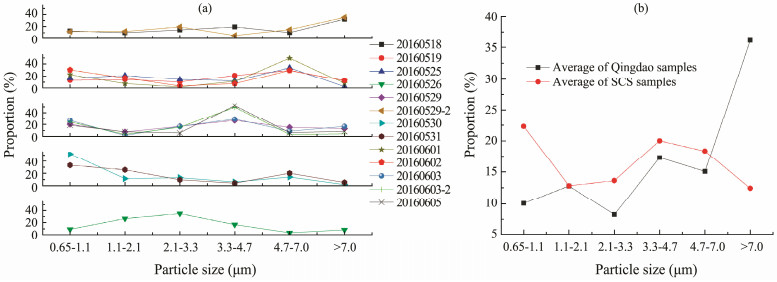
|
Fig. 5 Microbial activity in the size distributions of the bioaerosols over the SCS from May 18 to June 6, 2016. |
Fig.5b shows the average particle size distribution of the microbial activity over Qingdao. The size distribution of microbial activity in samples with land sources was similar to that in samples with sea sources (P > 0.05), with the highest proportion of activity in the particle size range of > 7.0 μm. This result was in accordance with findings of a previous study (Meng et al., 2016). The samples from Qingdao significantly differed (P < 0.05) from the samples obtained over the SCS in terms of the activity in the > 7.0 and 0.65-1.1 μm particles. Compared with the microbial activity in the samples from Qingdao with land sources, the microbial activity in the samples taken over the SCS decreased from 47% to 14% in the > 7.0 μm range and increased from 6% to 24% in the 0.65-1.1 μm range. The activity in the > 7.0 μm range decreased by 18%, whereas that in the 0.65-1.1 μm range increased by 15% over the SCS compared with that in the samples in Qingdao with sea sources. Regardless of whether the samples in the air mass were from the land or sea, the samples in Qingdao presented a higher proportion of activity in coarse particles than the samples over the SCS. The contribution of coarseness to the microbial activity in the aerosol particles collected over the sea was lower than that in particles collected over land. When the bioaerosols were transported from land to sea, the microbial activity decreased in the coarse particles due to particle deposition, but high microbial activity was observed over the SCS in fine particles, especially in particles smaller than 1.1 μm. Given that the majority of nutrients and organic compounds are present in fine particles (Qi et al., 2011; Bougiatioti et al., 2013; Deshmukh et al., 2015), the microbes in fine particles may have greater potential for biodegradation than those in coarse particles, especially over oceans. However, this speculation needs to be verified and studied through further observations and laboratory experiments.
3.2 OC and Inorganic Nitrogen Species and Their Correlations with Microbial Concentration and ActivityTo verify the influences of microbes on atmospheric chemical compounds, we analyzed the concentrations of OC, WSOC, and inorganic nitrogen ions in the samples (Table 2). The average concentrations of OC and WSOC were 1.23 ± 0.66 and 0.85 ± 0.41 μg m−3, respectively, over the SCS. The concentrations of OC and WSOC decreased with increasing offshore distance. The average OC/EC ratio was 3.44, which was higher than 2, thereby indicating that secondary organic carbon (SOC) contributed to the organic compounds in the atmosphere over the SCS (Chow et al., 1996). However, OC/EC was less than the average (7.04) in the coastal city of Qingdao, which showed that OC over the SCS was less affected by anthropogenic activity than OC over land. The high WSOC/ OC ratio of 72.8% showed that WSOC was the main component of OC in the SCS. The WSOC/OC over the SCS was 13.49% higher than that over Qingdao. This result showed that the formation of WSOC via the transformation of primary organic compounds or gas-to-particle conversion was more important over seas than over land.
The concentrations of NH4+, NO3−, and NO2− over the SCS were 0.39 ± 0.31, 3.85 ± 1.73, and 0.005 ± 0.003 μg m−3, respectively; these values were much lower than the concentrations over Qingdao (Qi et al., 2011). These results showed that the influence of anthropogenic emissions on the chemical characteristics of aerosols decreased during the transport of aerosol particles from land to sea. The concentrations of NH4+ and NO3− decreased and moved from high latitude to low latitude in the study area. The correlation analysis (Table 4) showed that the concentration of inorganic nitrogen was mainly affected by temperature and RH.
|
|
Table 4 Spearman correlation coefficients of NO3− and NH4+ with meteorological factors over the SCS (n = 8) |
The correlations of these compound concentrations with the microbial concentration and activity in the atmospheric aerosols over the SCS and Qingdao are shown in Table 5. The microbial concentration was positively correlated with the levels of OC, WSOC, and TSP but not with the concentration of inorganic nitrogen ions. Furthermore, the microbial activity in bioaerosols was positively correlated with the OC, WSOC, and TSP. These results indicated that the microbial concentration and activity increased with rising concentration of aerosol particles, which was in accordance with previous studies (Alghamdi et al., 2014; Dong et al., 2016; Xie et al., 2018b). Considering the above results and the modeled PM10 over the SCS during 2014 - 2015 (Sun et al., 2018; Fig.6), we presumed that the aerosol concentration was the main reason underlying the negative correlation between the microbial concentration and the horizontal offshore distance in marginal seas that are less than 700 km away from a coast.
|
|
Table 5 Spearman correlation coefficients for the relationships of the microbial concentration and activity with the OC, WSOC, and inorganic nitrogen levels over the SCS and Qingdao (n = 14 for OC, WSOC, and TSP; n = 7 for NO2−, NO3−, and NH4+) |
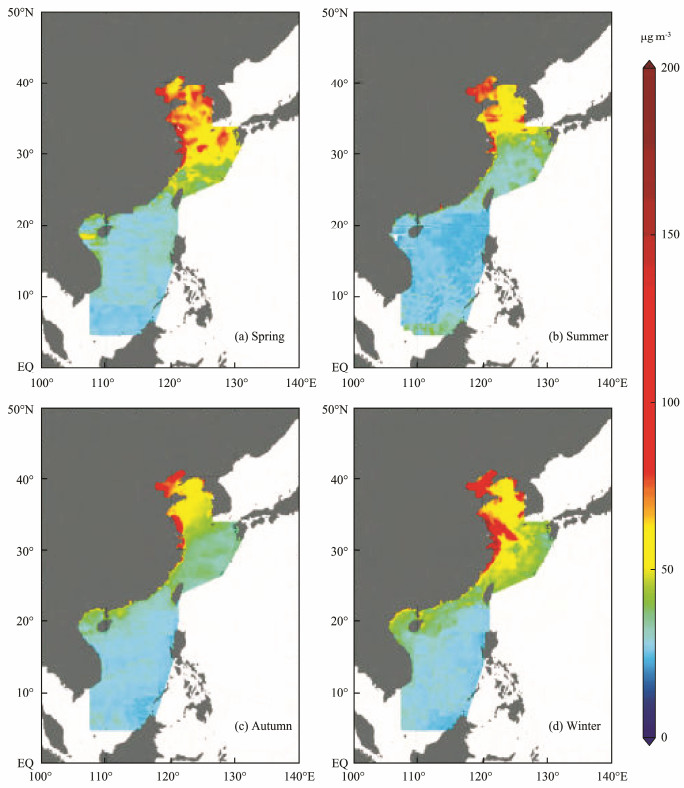
|
Fig. 6 Modeled PM10 mass concentration retrievals over the coastal seas of China in spring (a), summer (b), autumn (c), and winter (d) (adapted from Sun et al., 2018). |
The above results showed that microbes in the aerosols may be correlated with the reactions of certain water-soluble organic chemicals, and the ability of microbes to participate in reactions in the atmosphere can be indicated by the microbial activity and abundance. Ariya et al. (2002 provided evidence from laboratory experiments that DCAs can be efficiently transformed by existing airborne microbes (bacteria and fungi) in the boundary layer. Our results implied that certain organic compounds, especially water-soluble organic compounds, could be transformed by microbes in atmospheric aerosols. On the basis of linear regression [MC] = 7.0703 × 105*[WSOC]−4.4923 × 105 (r2 = 0.91, n = 14), when the WSOC increased by 1 μg m−3, the microbial concentration (MC) increased by 7.0703 × 105 cells m−3, while the microbial activity (MA) increased by 6.3196 ng m−3 h−1 SF ([MA] = 6.3196*[WSOC]−1.7006, r2 = 0.83, n = 14). In previous studies, 25% - 65% of the WSOC was observed in PM2.1 in the atmosphere (Timonen et al., 2008; Bougiatioti et al., 2013; Deshmukh et al., 2015). As discussed above, the microbial concentration and activity in PM1.1 were 8% and 15% higher over the SCS, respectively, than in the coastal samples. Thus, biodegradation via microbes may be an important atmospheric transformation pathway for the WSOC in the marine atmosphere. However, the microbial concentration and activity were averaged over the study duration for the analysis of the correlations with the OC and WSOC in each sample of TSP because of the different sampling periods used for the chemical components and microbes. Doing so possibly led to variations in the correlation analysis. Therefore, the correlations between airborne microbes and organic transformation reactions need further study.
3.3 Influence of Transport on the Microbial Concentration and ActivityTo investigate the influence of the transport path on the microbes in the atmospheric aerosols, we analyzed the correlations of the microbial concentration and activity with the parameters of the transport paths (Table 6). Spearman correlation analysis showed that the concentration of total microbes was significantly negatively correlated with the horizontal offshore distance over the SCS. These results verified that the MC in both fine and coarse particles decreased during transport from land to sea in this marginal sea. On the basis of linear regression [MC] = 4.2 × 105−341DH (r2 = 0.42, n = 13), when the horizontal offshore distance of the sampling site (DH) increased by 100 km, the microbial concentration decreased by 3.41 × 104 cells m−3, accounting for about 8% of the background concentration. From the intercept of the equation, we simply estimated the concentration of airborne microbes to be approximately 4.2 × 105 cells m−3 from May to June in the coastal region of the SCS, which could be verified by further research. Similarly, when the horizontal offshore distance increased by 100 km, the microbial concentration in the fine and coarse particles decreased by 9.9 × 103 and 1.1 × 104 cells m−3, respectively. These results showed that transport in the air mass had an important effect on the microbial concentration over the marginal sea. However, the microbial activity was not correlated with the horizontal offshore sampling distance, the transport distance over the sea, or the transport speed of the air mass, indicating that the transport path and average speed of the air mass had a minor influence on the microbial activity.
|
|
Table 6 Spearman correlation coefficients for the relationships of the microbial concentration and activity with the horizontal offshore distance, transport distance over the sea, and transport speed (n = 13) |
The microbes within atmospheric aerosols are affected by many atmospheric conditions, such as the air temperature, RH, and wind speed (Jones and Harrison, 2004). Correlation analysis revealed a significant positive correlation between wind speed and the concentration of airborne microbes in the 1.1-2.1 μm particles (Table 7). The wind speed was positively correlated with the microbial activity in the 4.7-7.0 μm particles (Table 8). These results showed that the wind speed increased the microbial abundance in some size ranges, probably due to bubble bursts. Except for the correlation of the atmospheric pressure with the microbial activity in the 0.65-1.1 μm particles, the other meteorological factors were not correlated with the microbial concentration or activity. Thus, the air temperature, RH, and atmospheric pressure had minor influences on the microbial concentration and activity during the study period.
|
|
Table 7 Spearman correlation coefficients for the relationships of the microbial concentration with the meteorological factors over the SCS (n = 13) |
|
|
Table 8 Spearman correlation coefficients of the microbial activity with meteorological factors over the SCS (n = 13) |
This study provided valuable information on the characteristics and factors influencing microbes in the marine atmosphere. The airborne microbes over the studied marginal sea were greatly affected by terrestrial sources, and the concentration of airborne microbes decreased by approximately 40%-54% during transport compared with the concentration in coastal samples. The horizontal offshore distance affected the microbial concentration, and the microbes in particles decreased by about 8% with an increase in distance of 100 km. Given the influences of wind and particle deposition during transport, the distributions of the particle sizes occupied by microbes over the SCS differed from those over the coastal region in the size ranges of 0.65-1.1 and >7.0 μm (P < 0.05).
The microbial concentration and activity in the bioaerosols were positively correlated with the particulates' WSOC and OC contents, implying that the microbes were involved in reactions of certain water-soluble organic chemicals in the atmosphere. The average microbial activity in the aerosol particles was 15%-79% lower over the SCS than in the continental samples. The size distributions of the microbial activity in the aerosol particles varied greatly over the SCS, and most samples showed high microbial activity in coarse particles, with an average proportion of 68% of the activity. Except for wind speed, which influenced the microbial activity in the 4.7-7.0 μm particles, the air temperature, RH, atmospheric pressure, and transport path-way had minor effects on the microbial activity during the study period.
The microbial activity did not vary synchronously with the abundance of airborne microbes. The limited data and correlation analysis showed that microbes may influence the transformation of certain organic compounds in the atmosphere. More attention should be given to the microbial activity and its role in the transformation reactions of organic compounds in the atmosphere. The kinds of organic compounds that can be degraded by microorganisms and how important biodegradation by microbes is relative to common radical chemistry in the atmosphere remain unknown. Further studies involving laboratory and field experiments are necessary to determine the effects of microbes on the transformation of atmospheric compounds.
AcknowledgementsThis project was financially supported by the National Natural Science Foundation of China (NSFC) (No. 4177 5148), the Fundamental Research Funds for the Central Universities (No. 201762006), and the Program for New Century Excellent Talents in University (No. NCET-13-0531). Data acquisition and sample collection were financially supported by the NSFC Open Research Cruise (Cruise no. NORC2015-05) and funded by the Ship Time Sharing Project of the NSFC. The cruises were conducted onboard the R/V Dong Fang Hong 2 of the Ocean University of China. We thank Prof. Jialiang Feng from Shang-hai University and Xiangbin Meng for their help in the determination of the OC and WSOC levels. We also thank Profs. Xiaohong Yao and Jing Gong for their valuable comments on this research.
Achuba, F. I. and Peretiemo-Clarke, B. O., 2008. Effect of spent engine oil on soil catalase and dehydrogenase activities. International Agrophysics, 22: 1-4. (  0) 0) |
Adam, G. and Duncan, H., 2001. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biology and Biochemistry, 33: 943-951. DOI:10.1016/S0038-0717(00)00244-3 (  0) 0) |
Alghamdi, M. A., Shamy, M., Redal, M. A., Khoder, M. and Awad, A. H., 2014. Microorganisms associated particulate matter: A preliminary study. Science of the Total Environment, 479-480: 109-116. DOI:10.1016/j.scitotenv.2014.02.006 (  0) 0) |
Aller, J. Y., Kuznetsova, M. R., Jahns, C. J. and Kemp, P. F., 2005. The sea surface microlayer as asource of viral and bacterial enrichment in marine aerosols. Journal of Aerosol Science, 36: 801-812. DOI:10.1016/j.jaerosci.2004.10.012 (  0) 0) |
Amato, P., Demeer, F., Melaouhi, A., Fontanella, S., Martin-Biesse, A. -S., Sancelme, M., Laj, P. and Delort, A. M., 2007. A fate for organic acids, formaldehyde and methanol in cloud water: Their biotransformation by micro-organisms. Atmospheric Chemistry and Physics, 7: 4159-4169. DOI:10.5194/acp-7-4159-2007 (  0) 0) |
Amato, P., MȦnager, M., Sancelme, M., Laj, P., Mailhot, G. and Delort, A. M., 2005. Microbial population in cloud water at the puy de dôme: Implications for the chemistry of clouds. Atmospheric Environment, 39: 4143-4153. DOI:10.1016/j.atmosenv.2005.04.002 (  0) 0) |
Ariya, P. A. and Amyot, M., 2004. New directions: The role of bioaerosols in atmospheric chemistry and physics. Atmospheric Environment, 38: 1231-1232. DOI:10.1016/j.atmosenv.2003.12.006 (  0) 0) |
Ariya, P. A., Nepotchatykh, O., Ignatova, O. and Amyot, M., 2002. Microbiological degradation of atmospheric organic com-pounds. Geophysical Research Letters, 29: 34-1-34-4. (  0) 0) |
Balland-Bolou-Bi, C., Bolou-Bi, E. B., Alphonse, V., Giusti-, Miller S., Jusselme, M. D., Livet, A., Grimaldi, M. and Bous-serhine, N., 2019. Impact of microbial activity on the mobility of metallic elements (Fe, Al and Hg) in tropical soils. Geoderma, 334: 146-154. DOI:10.1016/j.geoderma.2018.07.044 (  0) 0) |
Bauer, H., Giebl, H., Hitzenberger, R., Kasper-Giebl, A., Reischl, G., Zibuschka, F. and Puxbaum, H., 2003. Airborne bacteria as cloud condensation nuclei. Journal of Geophysical Research, 108: 4658-4663. (  0) 0) |
Bauer, H., Kasper-Giebl, A., Löflund, M., Giebl, H., Hitzenberger, R., Zibuschka, F. and Puxbaum, H., 2002. The contribution of bacteria and fungal spores to the organic carbon content of cloud water, precipitation and aerosols. Atmospheric Research, 64(1-4): 109-119. DOI:10.1016/S0169-8095(02)00084-4 (  0) 0) |
Bjurman, J., 1993. Determination of microbial activity in moulded wood by the use of Fluorescein diacetate. Material und Organismen, 28: 1-16. (  0) 0) |
Blanchard, D. C. and Syzdek, L. D., 1982. Water-to-air transfer and enrichment of bacteria in drops from bursting bubbles. Applied and Environmental Microbiology, 43: 1001-1005. DOI:10.1128/AEM.43.5.1001-1005.1982 (  0) 0) |
Bougiatioti, A., Zarmpas, P., Koulouri, E., Antoniou, M., Theodosi, C., Kouvarakis, G., Saarikoski, S., Mäkelä, T., Hillamo, R. and Mihalopoulos, N., 2013. Organic, elemental and water-soluble organic carbon in size segregated aerosols, in the marine boundary layer of the Eastern Mediterranean. Atmospheric Environment, 64: 251-262. DOI:10.1016/j.atmosenv.2012.09.071 (  0) 0) |
Bowers, R. M., Clements, N., Emerson, J. B., Wiedinmyer, C., Hannigan, M. and Fierer, N., 2013. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environmental Science and Technology, 47(21): 12097-12106. DOI:10.1021/es402970s (  0) 0) |
Burrows, S. M., Elbert, W., Lawrence, M. G. and Pöschl, U., 2009. Bacteria in the global atmosphere-Part 1: Review and synthesis of literature data for different ecosystems. Atmospheric Chemistry and Physics, 9: 9263-9280. DOI:10.5194/acp-9-9263-2009 (  0) 0) |
Cao, C., Jiang, W., Wang, B., Fang, J., Lang, J., Tian, G., Jiang, J. and Zhu, T. F., 2014. Inhalable microorganisms in Beijingos PM2.5 and PM10 pollutants during a severe smog event. Environmental Science and Technology, 48: 1499-1507. DOI:10.1021/es4048472 (  0) 0) |
Cho, B. C. and Hwang, C. Y., 2011. Prokaryotic abundance and 16S rRNA gene sequences detected in marine aerosols on the East Sea (Korea). FEMS Microbiology Ecology, 76: 327-341. DOI:10.1111/j.1574-6941.2011.01053.x (  0) 0) |
Chow, J. C., Watson, J. G., Lu, Z., Lowenthal, D. H., Frazier, C. A., Solomon, P. A., Thuillier, R. H. and Magliano, K., 1996. Descriptive analysis of PM2.5 and PM10 at regionally representative locations during SJVAQS/AUSPEX. Atmospheric Environment, 30(12): 2079-2112. (  0) 0) |
Christner, B. C., 2010. Bioprospecting for microbial products that affect ice crystal formation and growth. Applied Microbiology and Biotechnology, 85: 481-489. DOI:10.1007/s00253-009-2291-2 (  0) 0) |
Cui, J. and Holden, N. M., 2015. The relationship between soil microbial activity and microbial biomass, soil structure and grassland management. Soil and Tillage Research, 146: 32-38. DOI:10.1016/j.still.2014.07.005 (  0) 0) |
Deguillaume, L., Leriche, M., Amato, P., Ariya, P. A., Delort, A. M., Pöschl, U. and Morris, C. E., 2008. Microbiology and atmospheric processes: chemical interactions of primary biological aerosols. Biogeosciences Discussions, 5: 841-870. (  0) 0) |
Deleon-Rodriguez, N., Lathem, T. L., RodrȪguez-R,, L., M., Bara-, zesh, J., M., Anderson, B. E., Beyersdorf, A. J., Ziemba, L. D., Bergin, M., Nenes, A. and Konstantinidis, K. T., 2013. Microbiome of the upper troposphere: Species composition and prevalence, effects of tropical storms, atmospheric implications. PNAS, 110(7): 2575-2580. DOI:10.1073/pnas.1212089110 (  0) 0) |
Delort, A. M., Vaïtilingom, M., Amato, P., Sancelme, M., Parazols, M., Mailhot, G., Laj, P. and Deguillaume, L., 2010. A short overview of the microbial population inclouds: Potential roles in atmospheric chemistry and nucleation processes. Atmospheric Research, 98: 249-260. DOI:10.1016/j.atmosres.2010.07.004 (  0) 0) |
Deshmukh, D., Kawamura, K., Lazaar, M., Kunwar, B. and Suresh, Kumar Reddy B., 2015. Dicarboxylic acids, oxoacids, benzoic acid, ǁ-dicarbonyls, WSOC, OC, ions in spring aerosols from Okinawa Island in the western North Pacific Rim: Size distributions and formation processes. Atmospheric Chemistry and Physics, 16: 5263-5282. (  0) 0) |
Dong, L., Qi, J., Shao, C., Zhong, X., Gao, D., Cao, W., Gao, J., Bai, R., Long, G. and Chu, C., 2016. Concentration and size distribution of total airborne microbes in hazy and foggy weather. Science of the Total Environment, 541: 1011-1018. DOI:10.1016/j.scitotenv.2015.10.001 (  0) 0) |
Fontvieille, D. A., Outaguerouine, A. and Thevenot, D. R., 1992. Fluorescein diacetate hydrolysis as a measure of microbial activity in aquatic systems: Application to activated sludges. Environmental Technology, 13(6): 531-540. DOI:10.1080/09593339209385181 (  0) 0) |
Fuzzi, S., Andreae, M. O., Huebert, B. J., Kulmala, M., Bond, T. C., Boy, M., Doherty, S. J., Guenther, A., Kanakidou, M., Kawamura, K., Kerminen, V.-M., Lohmann, U., Russell, L. M. and Pschl, U., 2006. Critical assessment of the current state of scientific knowledge, terminology, research needs concerning the role of organic aerosols in the atmosphere, climate, global change. Atmospheric Chemistry and Physics, 6: 2017-2038. DOI:10.5194/acp-6-2017-2006 (  0) 0) |
Gong, J., Qi, J., Beibei, E., Yin, Y. and Gao, D., 2020. Concentration, viability and size distribution of bacteria in atmospheric bioaerosols under different types of pollution. Environmental Pollution, 257: 113485. DOI:10.1016/j.envpol.2019.113485 (  0) 0) |
Green, V. S., Stott, D. E. and Diack, M., 2006. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biology and Biochemistry, 38: 693-701. DOI:10.1016/j.soilbio.2005.06.020 (  0) 0) |
Hara, K. and Zhang, D., 2012. Bacterial abundance and viability in long-range transported dust. Atmospheric Environment, 47: 20-25. DOI:10.1016/j.atmosenv.2011.11.050 (  0) 0) |
Ho, H., Rao, C. Y., Hsu, H., Chiu, Y., Liu, C. and Chao, H. J., 2005. Characteristics and determinants of ambient fungal spores in Hualien, Taiwan. Atmospheric Environment, 39: 5839-5850. DOI:10.1016/j.atmosenv.2005.06.034 (  0) 0) |
Hu, W., Murata, K., Fukuyama, S., Kawai, Y., Oka, E., Uematsu, M. and Zhang, D., 2017. Concentration and viability of airborne bacteria over the Kuroshio extension region in the northwestern Pacific Ocean: Data from three cruises. Journal of Geophysical Research, 122: 12892-12905. (  0) 0) |
Hurtado, L., RodrȪguez,, G, ., LȮpez,, J, ., Castillo, J. E., Molina, L., Zavala, M. and Quintana, P. J. E., 2014. Characterization of atmospheric bioaerosols at 9 sites in Tijuana, Mexico. Atmospheric Environment, 96: 430-436. DOI:10.1016/j.atmosenv.2014.07.018 (  0) 0) |
Jones, A. M. and Harrison, R. M., 2004. The effects of meteorological factors on atmospheric bioaerosol concentrations-A review. Science of the Total Environment, 326: 151-180. DOI:10.1016/j.scitotenv.2003.11.021 (  0) 0) |
Kellogg, C. A., Griffin, D. W., Garrison, V. H., Peak, K. K., Royall, N., Smith, R. R. and Shinn, E. A., 2004. Characterization of aerosolized bacteria and fungi from desert dust events in Mali, West Africa. Aerobiologia, 20: 99-110. DOI:10.1023/B:AERO.0000032947.88335.bb (  0) 0) |
Kelly, C. and Pady, S., 1954. Microbiological studies of air masses over Montreal during 1950 and 1951. Canadian Journal of Botany, 32: 591-600. DOI:10.1139/b54-057 (  0) 0) |
Li, M., Qi, J., Zhang, H., Huang, S., Li, L. and Gao, D., 2011. Concentration and size distribution of bioaerosols in an outdoor environment in the Qingdao coastal region. Science of the Total Environment, 409: 3812-3819. DOI:10.1016/j.scitotenv.2011.06.001 (  0) 0) |
Lighthart, B. , and Mohr, A. J. , 1994. Atmospheric Microbial Aerosols: Theory and Applications. Chapman and Hall, London, 22-24.
(  0) 0) |
Lighthart, B. and Shaffer, B. T., 1995. Viable bacterial aerosol particle size distributions in the midsummer atmosphere at an isolated location in the high desert chaparral. Aerobiologia, 11: 19-25. DOI:10.1007/BF02136140 (  0) 0) |
Maki, T., Lee, K. C., Kawai, K., Onishi, K., Hong, C. S., Kurosaki, Y., Shinoda, M., Kai, K., Iwasaka, Y., Archer, S. D. J., Lacap-Bugler, D. C., Hasegawa, H. and Pointing, S. B., 2019. Aeolian dispersal of bacteria associated with desert dust and anthropogenic particles over continental and oceanic surfaces. Journal of Geophysical Research: Atmospheres, 124(10): 5579-5588. DOI:10.1029/2018JD029597 (  0) 0) |
Mayol, E., Arrieta, J. M., Jimenez, M. A., Martinez-Asensio, A., Garcias-Bonet, N., Dachs, J. and Duarte, C. M., 2017. Long-range transport of airborne microbes over the global tropical and subtropical ocean. Nature Communications, 201(8): 1-9. (  0) 0) |
Mayol, E., JimȦnez, M.A., Herndl, G. J., Duarte, C. M. and Arrieta, J. M., 2014. Resolving the abundance and air-sea fluxes of airborne microorganisms in the North Atlantic Ocean. Frontiers in Microbiology, 5: 557. (  0) 0) |
Meng, X. B., Li, M. Z., Li, H. T., Gao, D. M. and Qi, J. H., 2016. Microbial activity in bioaerosols in winter at the coastal region of Qingdao. Environmental Science, 37: 4147-4155. (  0) 0) |
Mori, I., Nishikawa, M., Tanimura, T. and Hao, Q., 2003. Change in size distribution and chemical composition of Kosa (Asian dust) aerosol during long-range transport. Atmospheric Environment, 37: 4253-4263. DOI:10.1016/S1352-2310(03)00535-1 (  0) 0) |
Murata, K. and Zhang, D., 2014. Transport of bacterial cells toward the Pacific in Northern Hemisphere westerly winds. Atmospheric Environment, 87: 138-145. DOI:10.1016/j.atmosenv.2013.12.038 (  0) 0) |
Qi, J., Gao, H., Yu, L. and Qiao, J., 2011. Distribution of inorganic nitrogen-containing species in atmospheric particles from an island in the Yellow Sea. Atmospheric Research, 101: 938-955. DOI:10.1016/j.atmosres.2011.06.003 (  0) 0) |
Qi, J., Zhong, X., Shao, Q., Gao, D., Wu, L., Huang, L. and Ye, Y., 2015. Microbial activity levels in atmospheric bioaerosols in Qingdao. Aerobiologia, 31: 353-365. DOI:10.1007/s10453-015-9369-3 (  0) 0) |
Rovira, P. and Vallejo, V. R., 2002. Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: An acid hydrolysis approach. Geoderma, 107: 109-141. DOI:10.1016/S0016-7061(01)00143-4 (  0) 0) |
Sesartic, A., Lohmann, U. and Storelvmo, T., 2012. Bacteria in the ECHAM5-HAM global climate model. Atmospheric Che-mistry and Physics, 11: 1457-1488. (  0) 0) |
Si, G., Yuan, J., Xu, X., Zhao, S., Peng, C., Wu, J. and Zhou, Z., 2018. Effects of an integrated rice-crayfish farming system on soil organic carbon, enzyme activity, microbial diversity in waterlogged paddy soil. Acta Ecologica Sinica, 38: 29-35. DOI:10.1016/j.chnaes.2018.01.005 (  0) 0) |
Stopelli, E., Conen, F., Guilbaud, C., Zopfi, J., Alewell, C. and Morris, C. E., 2017. Ice nucleators, bacterial cells and Pseudomonas syringae in precipitation at Jungfraujoch. Biogeosciences, 14(5): 1189-1196. DOI:10.5194/bg-14-1189-2017 (  0) 0) |
Stubberfield, L. and Shaw, P. A., 1990. A comparison of tetrazolium reduction and FDA hydrolysis with other measures of microbial activity. Journal of Microbiological Methods, 12(3): 151-162. (  0) 0) |
Sun, Z., Meng, L., Gao, H. and Wang, J., 2018. Retrieving aerosol concentration and dry deposition flux over coastal seas of China using satellite data. Periodical of Ocean University of China, 48(3): 30-40 (in Chinese with English abstract). (  0) 0) |
Timonen, H., Saarikoski, S., Tolonen-Kivimäki, O., Aurela, M., Saarnio, K., Petäjä, T., Aalto, P. P., Kulmala, M., Pakkanen, T. and Hillamo, R., 2008. Size distributions, sources and source areas of water-soluble organic carbon in urban background air. Atmospheric Chemistry and Physics, 8: 5635-5647. DOI:10.5194/acp-8-5635-2008 (  0) 0) |
Wang, Y., Zhang, X. and Draxler, R. R., 2009. TrajStat: GIS-based software that uses various trajectory statistical analysis methods to identify potential sources from long-term air pollution measurement data. Environmental Modelling and Software, 24: 938-939. DOI:10.1016/j.envsoft.2009.01.004 (  0) 0) |
Wei, K., Zou, Z., Zheng, Y., Li, J., Shen, F., Wu, C., Wu, Y., Hu, M. and Yao, M., 2016. Ambient bioaerosol particle dynamics observed during haze and sunny days in Beijing. Science of the Total Environment, 550: 751-759. DOI:10.1016/j.scitotenv.2016.01.137 (  0) 0) |
Wu, Y., Chan, C., Rao, C. Y., Lee, C., Hsu, H., Chiu, Y. and Chao, H. J., 2007. Characteristics, determinants, spatial variations of ambient fungal levels in the subtropical Taipei metropolis. Atmospheric Environment, 41: 2500-2509. DOI:10.1016/j.atmosenv.2006.11.035 (  0) 0) |
Xie, Z., Fan, C., Lu, R., Liu, P., Wang, B., Du, S., Jin, C., Deng, S. and Li, Y., 2018a. Characteristics of ambient bioaerosols during haze episodes in China: A review. Environmental Pollution, 243: 1930-1942. DOI:10.1016/j.envpol.2018.09.051 (  0) 0) |
Xie, Z., Li, Y., Lu, R., Li, W., Fan, C., Liu, P., Wang, J. and Wang, W., 2018b. Characteristics of total airborne microbes at various air quality levels. Journal of Aerosol Science, 116: 57-65. DOI:10.1016/j.jaerosci.2017.11.001 (  0) 0) |
Yuan, Z., Liu, H., Han, J., Sun, J., Wu, X. and Yao, J., 2017. Monitoring soil microbial activities in different cropping systems using combined methods. Pedosphere, 27: 138-146. DOI:10.1016/S1002-0160(15)60100-X (  0) 0) |
Zhao, F., Ren, C., Han, X., Yang, G., Wang, J. and Doughty, R., 2018. Changes of soil microbial and enzyme activities are linked to soil C, N and P stoichiometry in afforested ecosystems. Forest Ecology and Management, 427: 289-295. DOI:10.1016/j.foreco.2018.06.011 (  0) 0) |
Zhong, X., Qi, J., Li, H., Dong, L. and Gao, D., 2016. Seasonal distribution of microbial activity in bioaerosols in the outdoor environment of the Qingdao coastal region. Atmospheric Environment, 140: 506-513. DOI:10.1016/j.atmosenv.2016.06.034 (  0) 0) |
 2021, Vol. 20
2021, Vol. 20


