2) School of Biological and Chemical Engineering, Panzhihua University, Panzhihua 617000, China;
3) Earth & Life Institute, Bioengineering Laboratory, Catholic University of Louvain, Louvain-La-Neuve 1348, Belgium;
4) Key Laboratory of Applied Marine Biotechnology, Ministry of Education of China, Ningbo University, Ningbo 315832, China
The increased emission of CO2 has led to various environmental issues, including global warming and ocean acidification. Microalgae, as a vast group of photosynthetic microorganisms widely distributed in diverse habitats, are well known for their major roles in carbon recycling in natural ecosystem. Additionally, they have a remarkable ability to convert CO2 into various high-value bioproducts, such as carotenoids, polyunsaturated fatty acids (PUFAs), vitamins and proteins, which can be widely used as biofuels, functional foods, and pharmaceuticals (Xu et al., 2023). Hence, the cultivation of microalgae has emerged as an exceptional strategy for both mitigating CO2 and producing bio-based materials with diverse applications.
Coccolithophores, belonging to the Haptophyta, are a group of unicellular flagellate microalgae known for their unique capacity to form intricate inorganic calcite scales (termed as coccoliths) around their cells, made of calcium carbonate (CaCO3). Their dual carbon fixation systems, combining photosynthesis and biocalcification, make them significant contributors to marine carbon sink, thereby attracting considerable attention in the field of marine carbon cycling (Claxton et al., 2022). Recent studies have highlighted the efficient accumulation of various bioactive compounds by several coccolithophores species. For example, Emiliania huxleyi, one of the most abundant coccolithophores, was found to accumulate a high percentage (60.8%) of polyunsaturated fatty acids (PUFA) of the total fatty acid (TFA) and substantial total alkenone content (2.5%) of the total biomass (Vicente et al., 2021). In another study, Zhang et al. (2023) reported that the biomass content of E. huxleyi reached 864.2 mg L−1 with a fucoxanthin concentration around 0.2 mg L−1 when this microalga was cultivated using red light. In addition, studies have shown that coccoliths from coccolithophores could be utilized as novel feedstock for biomaterials production and have diverse applications in the biomedical field (Moheimani et al., 2012). All these studies indicate that coccolithophores can be considered a promising feedstock of the bioactive substances.
Chrysotila (formerly known as Pleurochrysis) is another representative genus of Haptophyta that has been extensively studied in the field of global biogeochemical cycle (Meng et al., 2022). Research has also indicated the significant potential of Chrysotila as a resource for biofuels and bioactive compounds. For example, one study has shown that Chrysotila spp. can accumulate a substantial amount of PUFA, accounting for 30% – 60% of the TFA (Moreira et al., 2022). Additionally, studies also reported that fucoxanthin is the most abundant carotenoid in Chrysotila. C. carterae, when cultured in a semi-continuous cultivation mode, exhibited a high fucoxanthin content up to 6.88% of dry cell weight (DCW) (Okcu et al., 2021). Except exhibiting high growth rates and rich bioactive substances, Chrysotila spp. show additional characteristics essential for large-scale cultivation. Long-term (13 months) outdoor raceway pond cultivation of C. carterae in Western Australia demonstrated a wide range of temperatures tolerance (3 – 41℃) and an average biomass productivity of 0.19 g L−1 d−1, with significant lipid and CaCO3 contents (Moheimani and Borowitzka, 2006). In a studied conducted by Rezvani et al. (2019) the biomass of C. carterae was found to contain 6% – 11% coccoliths and around 34% lipids. The authors also assessed the economic viability of C. carterae cultivation based on various biorefinery strategies (including bio-oil based on total lipids, biogas from wastes, animal feed and fishmeal from proteins and carbohydrates, biomaterial from coccoliths, etc.), and concluded that the multiproduction of fishmeal, bio-oil, and biomaterial based on C. carterae biomass is economically attractive. Collectively, these studies demonstrate that Chrysotila is a versatile genus with great potential for both commercial application and CO2 bioremediation.
To enhance the commercial exploitation of Chrysotila spp., the development of efficient cultivation strategy is essential. Microalgae can be cultivated under three major modes, photoautotrophy, mixotrophy, and heterotrophy, each with distinct metabolic pathways. In general, achieving optimal light supply in photoautotrophy poses a significant challenge in mass cultivation of microalgae, making mixotrophy and heterotrophy more favorable due to higher cell growth rates, biomass accumulation rates, and productivities of bioactive substances (Hu et al., 2018). Although heterotrophic cultivation offers high growth rates, it heavily relies on the assimilation of organic carbon, making it less suitable for mitigating global CO2 emissions. On the other hand, mixotrophy allows for simultaneous assimilation of both organic carbon and inorganic carbon, making it a preferred process for microalgae-based high-value substances production.
At an earlier time, we isolated a strain of C. roscoffensis from coastal waters in Xiangshan Bay, China, and performed comprehensive studies relevant to its biological properties. This strain demonstrated the ability to bloom under stress conditions and exhibited a remarkable capacity for accumulating fucoxanthin and PUFA (Zhou et al., 2022). Furthermore, we successfully developed a bacteria-removal method, through which an axenic C. roscoffensis strain was obtained (Liu et al., 2023). The axenic strain allows us to study the influences of external organic carbon on microalga without the interference of other organisms. Recognizing the potential advantages of C. roscoffensis in both CO2 mitigation and bioactive compounds production, we conducted a series of trials with the following objectives: 1) to verify the mixotrophic and heterotrophic capacities of C. roscoffensis by testing its response to three organic carbon sources; 2) to compare the growth performances and bioactive substances productivities among different cultivation modes. Based on these trials, we aim to gain a comprehensive understanding about the growth potential and bioactive compounds production of C. roscoffensis under different cultivation modes. This information will be valuable for optimizing the cultivation strategy of this microalga and exploring its potential application in CO2 mitigation and bio-based materials production.
2 Materials and Methods 2.1 Microalgae Strain and General Cultivation ConditionsBoth xenic and axenic C. roscoffensis NMBjih026-8 strains were preserved in the Microalgae Collection Center of Ningbo University using antibiotic-free medium. For all experiments, axenic cells in the early stationary phase (the optical density at 750 nm, OD750, at around 0.15) were inoculated into fresh medium with an inoculation ratio of 1:9 (cultures volume: medium volume). The initial cell density was maintained at around 0.015 of OD750. In photoautotrophy experiments, cells were cultured using NMB3# liquid medium (each liter containing 100.0 mg KNO3, 10.0 mg KH2PO4, 10.0 mg EDTA-Na2, 2.5 mg FeSO4·7H2O, 250.0 µg MnSO4·H2O, 6.0 µg VB1, and 0.05 µg VB12) prepared with artificial seawater (Liu et al., 2023). The initial pH and salinity were adjusted to 8.30 and 25‰, respectively (Meng et al., 2022). The temperature was then maintained at 23℃ ± 1℃, and the illumination intensity and the light/dark cycle were set to 60 μmol photons m−2 s−1 and 12 h: 12 h, respectively.
2.2 Determination of Optimal Organic Carbon Under Mixotrophic and Heterotrophic ConditionsTo screen the suitable organic carbon source for C. roscoffensis, sterilized glucose, glycerol, and sodium acetate (NaAc) at gradient concentrations (carbon concentration: 0.1, 0.2, 0.3, 0.4, and 0.5 mol L−1) were supplemented into NMB3# liquid medium to prepare organic carbon-containing media. Before inoculation, the axenicity of C. roscoffensis was confirmed using SYBI Green I, based on a previously reported method (Liu et al., 2023). For the heterotrophy experiments, cells were cultivated in dark conditions, while for mixotrophy, the light intensity was same as the photoautotrophy. For this batch, all experiments were conducted in 100 mL conical flasks containing 30 mL of medium, and the photoautotrophic C. roscoffensis was taken as the control group. During the cultivation, the cell density (represented by OD750) was measured every two days by Thermo Fisher Scientific Microplater Reader (Varioskan LUX, Finland). At the final day of cultivation, 10 mL of C. roscoffensis cultures were collected using pre-weighted Whatman GF/F microfiber filter (pore size 0.7 µm). The collected cells were then washed with 30 mL of ultrapure water and dried to a constant weight to calculate the biomass content.
2.3 Comparative Analysis of C. roscoffensis Under Different Cultivation ModesTo comprehensively evaluate the effects of organic carbon on C. roscoffensis, additional cultivation trials of photoautotrophy and mixotrophy were conducted with the axenic strain.
Throughout the entire cultivation period of 12 d, OD750 were determined every two days, and photosynthetic parameters, including the maximal photosystem Ⅱ (PSⅡ) quantum yield (Fv/Fm), relative electron transfer rate at PSII (ETR), and photochemical quenching coefficient (qP), were measured by WATER-PAM (WALZ, Germany). The biomass content was determined at the end of cultivation. The specific growth rate (μ, d−1) was calculated based on OD750 using the Eq. (1):
| $ \mu {\text{ (}}{{\text{d}}^{ -{\text{1}}}}{\text{) = (ln }}{N_{{t_1}}} -{\text{ln }}{N_{{t_2}}}{\text{) / }}{t_1} -{t_2}, $ | (1) |
in which t1 and t2 are the initial and ending time of the cultivation period, and
Biomass compositions (refers to lipid, protein, carbohydrates, and coccoliths), fatty acids (FAs) and pigments were measured at day 6 and day 12. The coccolith was determined based on 10 mL of microalga cultures, following the protocol reported by Moheimani and Boro-witzka (2007). The carbohydrate was extracted using phenol-sulfuric acid method (DuBois et al., 2002), and the content was calculated based on a glucose standard curve. With homogenized microalga powder, the lipid and protein were determined using chloroform-methanol method (Bligh and Dyer, 1959) and Coomassie brilliant blue staining method (Sedmak and Grossberg, 1977), respectively. For FA analysis, lyophilized microalga powder was used for lipid extraction, and FA components were determined by gas chromatography-mass spectrometry (GC-MS). Pigments were extracted using 5 mL of microalga cultures with chloroform-methanol method, and then quantified by liquid chromatography-mass spectrometry (LC-MS). The experimental procedures and related details for FAs and pigments were the same as our previous reports (Sun et al., 2022; Zhang et al., 2022).
3 Results and Discussion 3.1 Identifying the Heterotrophic and Mixotrophic Capacities of C. roscoffensisIt is well known that certain microalgae under heterotrophy and mixotrophy can achieve much higher growth and biomass accumulation rates than autotrophy. Studies also showed that both the carbon concentration and carbon type are important factors underlying cultivation efficiency (Castillo et al., 2021). Hence, three organic carbon sources with gradient concentrations, all of which have been widely used for microalgae cultivation, were tested to verify the performance of axenic C. roscoffensis (Fig.1) under heterotrophic and mixotrophic conditions.

|
Fig. 1 Axenicity verification of Chrysotila roscoffensis based on SYBR Green I. Red and orange arrows indicate the microalga and bacteria, respectively. |
In darkness, the OD750 of C. roscoffensis in all treatments displayed almost even curves during the whole cultivation period, whereas that of the photoautotrophy group increased from 0.015 to 0.170 (Figs.2a – c). Such results proved that C. roscoffensis could not grow solely on external organic carbon. Due to no observable cell density enhancement in heterotrophic cultures, the biomass was not measured further.
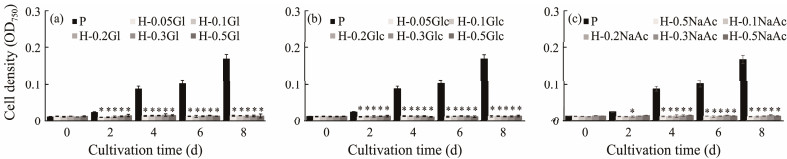
|
Fig. 2 Growth performance of Chrysotila roscoffensis under heterotrophic conditions based on glycerol (a), glucose (b), and NaAc (c). '*' indicates significant differences. P, photoautotrophy; H, heterotrophy; Gl, glycerol; Glc, glucose; NaAc, sodium acetate. |
Under illuminated condition, the OD750 values of C. roscoffensis varied great along with both carbon sources and carbon concentrations (Figs.3a – c). The final OD750 of autotrophy group was 0.146, while those of the glycerol addition groups varied from 0.189 (0.05 mol L−1) to 0.303 (0.5 mol L−1) (Fig.3a), significantly higher than that of the autotroph. With regard to glucose groups, all concentrations resulted in OD750 values ranging from 0.146 to 0.168, close to the photoautotrophy group, suggesting that the addition of external glucose had no appreciable influence on C. roscoffensis growth (Fig.3b). Contrary to glucose and glycerol, the addition of NaAc (even at 0.05 mol L−1) induced growth inhibition of C. roscoffensis, and when the NaAC was further increased to 0.5 mol L−1, the final OD750 sharply reduced to 0.98, around 30% lower than that of the autotrophy group (Fig.3c). The influences of external organic carbon on C. roscoffensis growth were further consolidated by biomass data (Figs.3d –f). The autotrophy group reached a biomass content of 210.0 mg L−1 on the final day, while those of the glycerol addition groups ranged from 226.7 mg L−1 to 323.3 mg L−1, around 7.9% – 54.0% higher than the autotrophy group (Fig.3d). The DCW values of glucose groups (ranging from 196.7 – 213.3 mg L−1) showed no significant differences compared to that of the autotrophy group (Fig.3e). The lowest DCW of 126.7 mg L−1 was obtained from the group supplemented with 0.5 mol L−1 NaAC, accounting for only 60% of the autotrophy group (Fig.3f). All the results above demonstrated that only glycerol significantly promoted C. roscoffensis growth under illuminated conditions, and the facilitating effects enhanced with the increase of glycerol concentrations.
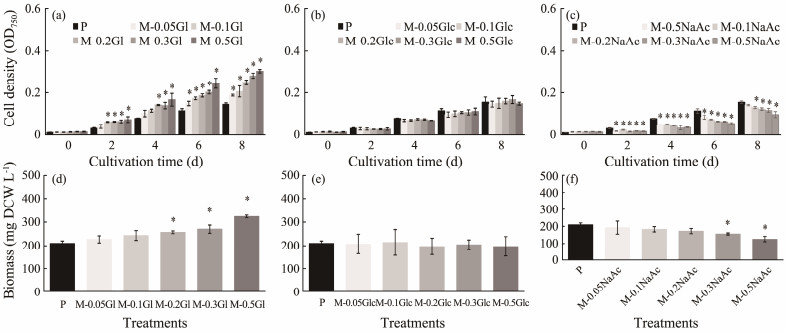
|
Fig. 3 Cell densities (a–c) and biomass (d–f) of Chrysotila roscoffensis under mixotrophic conditions based on glycerol (a, d), glucose (b, e), and NaAc (c, f). '*' indicates significant differences. P, photoautotrophy; M, mixotrophy; Gl, glycerol; Glc, glucose; NaAc, sodium acetate. |
It has been reported that only some microalgae (e.g., Chlorella spp., Nitzschia laevis, Cyclotella cryptica) can grow by assimilating organic carbon in the absence of light (Perez-Garcia et al., 2011). For obligate autotrophy microalgae, the lack of heterotrophic capacity can be attributed to various reasons, including the absence of sugar transporter in the outer membrane (Zhang et al., 2023), the low levels or insufficiency of enzymes related to carbon dissimilation (Perez-Garcia et al., 2011), and the inhibition of carbon assimilation pathway in the dark (Wang et al., 2023).
The transmembrane uptake of glucose is hexose transporter-dependent. In this study, the addition of glucose did not result in any variation in the growth performance of C. roscoffensis, either under heterotrophic or mixotrophic conditions, demonstrating that this microalga lacks glucose transporters. Such an observation also has been reported in other microalgae, e.g., Phaeodactylum tricornutum and Volvox carteri (Hallmann and Sumper, 1996; Zaslavskaia et al., 2001).
With respect to acetate, the failure of heterotrophy based on external NaAC might not be simply attributed to the lack of monocarboxylic acid/proton transporter because a concentration dependent inhibition effect can be observed in the mixotrophic groups. It is clear that the addition of NaAC can lead to increased pH, which may affect the growth of C. roscoffensis in turn. Indeed, the NaAC groups did show elevated pH in this study, albeit the initial pH had been adjusted to 8.3. Moheimani and Borowitzk (2011) have reported that another species of Chrysotila (C. carterae) can grow fast within a diurnal pH range of 8.3 – 10.9, suggesting that the potential influence of elevated pH on C. roscoffensis may be negligible. Studies have found that acetate can suppress the ammonium uptake and nitrogen assimilation by Scenedesmus, which can impose negative effects on the growth and biomass accumulation rates (Combres et al., 1994; Ogbonna et al., 2000). Nevertheless, related information has never been reported in Chrysotila or other closely related species, thus more research is still needed to disclose the mechanism behind the inhibition effect of acetate on C. roscoffensis in future.
Glycerol can enter cells by passive diffusion and almost show no toxicity even at high concentrations. However, the addition of glycerol resulted in no growth enhancement of C. roscoffensis in darkness, suggesting this microalga can only utilize glycerol in the presence of light. Such an observation has been reported in many microalgae species, e.g., Nannochloropsis sp., Chlorella sp., and Cylindrotheca sp. (Poddar et al., 2018; Wang et al., 2023). However, it is worthy to note that the mechanism underlying the incapacity of these microalgae to metabolize glycerol in the dark is still elusive.
3.2 Comprehensive Analysis of Organic Carbon on C. roscoffensis 3.2.1 Growth performance and biomass accumulationIn this section, for the purpose of obtaining sufficient microalgae biomass for comprehensive analyses, we conducted experiments with an increased cultivation volume of 300 mL and an extended cultivation period to 12 d. Despite these changes, the results obtained from this batch of experiments were basically in line with those observed in the previous batches (Fig.4). For example, the OD750 values of mixotrophy were higher than those of corresponding photoautotrophy throughout the entire cultivation period (Fig.4a). The specific growth rate and biomass content of mixotrophy were 40% and 60% higher than those of photoautotrophy, respectively (Figs.4b, c). These data once again confirmed that the addition of external glycerol could significantly stimulate the cell density and biomass accumulation of C. roscoffensis.
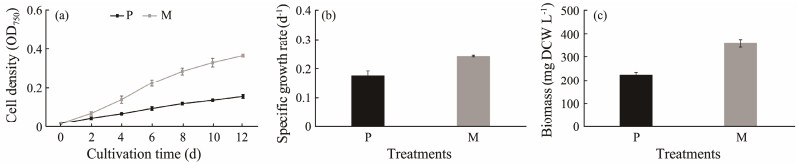
|
Fig. 4 Cell density (a), specific growth rate (b), and biomass (c) of Chrysotila roscoffensis under different cultivation modes. P and M represent photoautotrophy and mixotrophy, respectively. |
Previous studies have reported that the addition of external organic carbon can disturb the formation of thylakoids, leading to a significant decrease of photosynthetic capacity of microalgae (Grama et al., 2016; Ma et al., 2022). However, other reports indicated that microalgae cells cultivated under optimal conditions can achieve enhanced photosynthetic activities, generating increased biomass levels (Villanova et al., 2021). To reveal the actual situation of external organic carbon induced in C. roscoffensis, photophysiological parameters such as Fv/Fm, qP, and ETR, which have been extensively used to investigate the physiological status of microalgae cells, were investigated and compared between photoautotrophy and mixotrophy.
Fv/Fm indicates the photosynthetic activity and corresponds to the maximum quantum efficiency of PSII. In this study, the Fv/Fm of photoautotrophy increased from 0.43 at the initial cultivation day to around 0.55 at the fourth day, and then began to decrease gradually to 0.41 at the end of cultivation (Fig.5a). As for mixotrophy, its Fv/Fm presented similar values and followed the same variation pattern as the photoautotrophic group during the early stage of cultivation (Fig.5a), implying that the addition of glycerol displayed no negative effect on the photosynthetic apparatus of C. roscoffensis. However, the Fv/Fm of glycerol addition group began to drop sharply from the eighth day and achieved a value of 0.28 at the final day, around 30% lower than that of the photoautotrophy. The observation that mixotrophy reached much higher cell densities and biomass contents than photoautotrophy suggested that microalgal cells of the former group consumed more nutrients during cultivation. Additionally, our previous report has demonstrated that the nitrate in the NMB3# can be rapidly consumed up by microalgae within 5 – 7 d, thereby generating a nutrients deficiency condition (Zhang et al., 2023). Thus, it can be speculated that the low Fv/Fm values of mixotrophy at the later cultivation phase are probably caused by severe nutrient deficiency. Such an observation was consistent with previous studies, in which the Fv/Fm of microalgae dropped under nutrient limitation conditions and rose back to normal level when nutrients were supplemented (Young and Beardall, 2003; Tan et al., 2019).
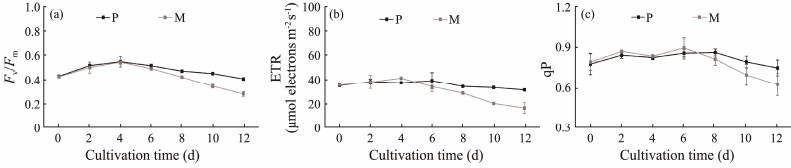
|
Fig. 5 Chlorophyll fluorescence parameters of Chrysotila roscoffensis under different cultivation modes. P and M represent photoautotrophy and mixotrophy, respectively. |
ETR indicates the electrons transported for energy production. Under autotrophic conditions, an increase in ETR often means an enhancement of biomass accumulation, while a decline in ETR represents a reduction of biochemical processes related to photosynthesis (Mogany et al., 2022). Under autotrophic conditions, the ETR remained between 31.5 µmol electrons m−2 s−1 (day 12) and 39.2 µmol electrons m−2 s−1 (day 6), displaying a relative flat curve during the whole cultivation period (Fig.4b). However, the glycerol addition group exhibited a dramatic decline in ETR since the sixth day (34.3 µmol electrons m−2 s−1) and minimized at the final day (16.7 µmol electrons m−2 s−1) (Fig.5b). The observation of the low ETR and high biomass showed in mixotrophic group indicated that glycerol assimilation played an important role in the biomass accumulation of C. roscoffensis.
qP reflects the probability that a singlet-excited chlorophyll in PSII drives photochemistry and has been widely used as an important parameter describing the percentage of light energy absorbed by the PSII for photosynthetic electron transfer (Yu et al., 2022). In this study, all of qP values of different groups were around 0.8 during days 0 – 8, suggesting that external glycerol did not influence the capacity of cells to direct light energy to photochemistry (Fig.5c). However, mixotrophy group displayed downward qP values at days 10 and 12, implying that the electron transport activity of C. roscoffensis cells was restrained, probably due to nutrient deficiency (Mogany et al., 2022).
In summary, the chlorophyll fluorescence parameters obtained in this study demonstrated that the addition of glycerol showed no negative effects on the photosynthetic capacity of C. roscoffensis under nutrients repletion conditions. These observations were consistent with Villanova et al. (2021) but contrary to others (Grama et al., 2016; Ma et al., 2022).
3.2.3 Biomass composition analysisCoccolithophores are well known for their unique ability of forming CaCO3 scales, whereas their coccolith contents varied great among different species. Using an outdoor raceway pond, Moheimani and Borowitzka (2006) conducted a long-term cultivation trial of C. carterae and found the coccolith contents of this microalga were 6.8% – 7.9%, showing no significant seasonal change. In another study, Moheimani et al. (2011) tested the feasibility of producing C. carterae, Emiliania huxleyi, and Gephyrocapsa oceanica in different types of closed photobioreactors and illustrated that the coccoliths of different species range between 1.0% and 18.5%. In this study, the coccoliths content of C. roscoffensis under photoautotrophy increased from 6.37% at day 6 to 9.73% at day 12. The situation of mixotrophy group, increasing from 5.79% to 10.21 along with the cultivation period extension, resembled that of photoautotrophy (Table 1). Moreover, no significant differences were observed between different treatments. All these results demonstrated that coccoliths biosynthesis in C. roscoffensis is inducible, while nutrients level rather than external organic carbon maybe the key regulatory factor.
|
|
Table 1 Biochemical compositions of Chrysotila roscoffensis under different modes |
Although coccolithophores are generally considered as lipid-rich microalgae (Fernández et al., 1994; Moheimani et al., 2011; Rezvani et al., 2019), other studies have suggested that the lipid contents of coccolithophorids are only around 10.0% (Kanda et al., 2020; Moreira et al., 2022). In this study, the lipid contents of C. roscoffensis varied substantially between different cultivation phases (Table 1). Under photoautotrophic conditions, the lipid content at day 6 was only 15.00%, while reached as high up to 32.26% at day 12. The same variation trend was also observed from mixotrophic group. Availabilities of nitrogen and phosphorous are important factors affecting the lipid contents of microalgae. Under nutrients-deficient conditions, most microalgal species tend to promote lipid biosynthesis by modulating biochemical pathways (Khoo et al., 2023). Our results suggested that this was also the case for C. roscoffensis. Additionally, it has been widely reported that the addition of external organic carbon will lead to an increase in lipid contents of microalgae, such as Phaeodactylum tricornutum, Nitzschia laevis, and Chlorella sorokiniana (Cecchin et al., 2018; Lu et al., 2019). The lipid contents of mixotrophic C. roscoffensis, ranging between 20.21% and 39.67%, were significantly higher than those of corresponding photoautotrophy and aligned well with previous reports (Cecchin et al., 2018; Lu et al., 2019).
In comparison with rich lipid contents, C. roscoffensis contained relatively small amounts of carbohydrates. For example, the carbohydrate content of photoautotrophy at day 6 was only 17.85%, which further declined to10.62% at day 12. In the presence of external glycerol, the carbohydrate content increased only at the end of cultivation (Table 1). These values agreed with previous reports, in which the carbohydrate contents of Chrysotila spp. were found to be around 10% – 20% (Rezvani et al., 2019; Moreira et al., 2022; ).
Contrary to the lipid, C. roscoffensis exhibited a decrease in protein along with cultivation process. For instance, the protein of photoautotrophic group decreased from 34.74% at day 6 to 26.05% at day 12. Mixotrophic group displayed the same variation trend (Table 1). Additionally, significant differences in protein contents were only observed from the ending time of cultivation period between different treatments.
3.2.4 Fatty acids profile and contentThe FA profiles of Chrysotila species other than C. roscoffensis have been investigated in several studies, revealing that the palmitic acid (PA, C16:0), oleic acid (OA, C18:1), α-linolenic acid (ALA, C18:3), and stearic acid (SA, C18:4) are commonly predominant FAs of Chrysotila, with proportions of PUFAs ranging between 30% and 60% (Kanda et al., 1996; 2020; Moreira et al., 2022). In this study, a total of 11 types of FAs were identified in C. roscoffensis, with the proportions of PUFA, monounsaturated fatty acid (MUFA), and saturated fatty acid (SFA) found to be 14.12% – 28.12%, 15.54% – 30.10%, and 55.78% – 59.54%, respectively (Table 2). FAs such as ALA, OA, PA, linoleic acid (LA, C18:2), and SA (5.88% – 19.50%) were relatively abundant (Table 2). Additionally, C. roscoffensis also accumulated considerable amount of docosahexaenoic acid (DHA, C22:6) (7.07% – 7.96%). These data suggest that the FAs profile of C. roscoffensis is generally consistent with that of other Chrysotila species (Kanda et al., 1996, 2020; Moreira et al., 2022).
|
|
Table 2 Fatty acid profiles and contents of Chrysotila roscoffensis under different modes |
It has been reported that external organic carbon can modify the FAs contents and compositions of microalgae. In one study conducted by Su et al. (2020), the SFA ratio and TFA content of Phaeodactylum tricornutum were found to increase under mixotrophic conditions, but the proportion of unsaturated fatty acid (UFA) declined largely. Similar conclusions have also been reached in other microalgal species, such as Thalassiosira pseudonana, Chlamydomonas reinhardtii, and Micractinium reisseri (Baldisserotto et al., 2021; Cao et al., 2022). Unexpectedly, our data revealed a different pattern from above observations. At day 6, the SFA ratio of photoautotrophy group was 28.12%, remarkably higher than that of mixotrophy (20.32%). However, the photoautotrophic group displayed much lower MUFA proportion (15.54%) than the glycerol addition group (23.71%). As for PUFA, the ratios showed no significant differences between different cultivation modes. The significant influences of glycerol on FA were further evident in specific FAs. At day 6, C16:0 with a proportion of 25.29% was the most dominant FA in photoautotrophy. However, its ratio decreased to 18.51% in mixotrophy. On the contrary, C18:3 increased from 15.74% to 21.32%, becoming the predominant FA in mixotrophy group. Besides that, the addition of glycerol also significantly enhanced TFA contents. For example, the TFA content of the photoautotrophy group at day 6 was 99.32 mg g−1 DCW, whereas that of mixotrophy group was 124.93 mg g−1 DCW, showing a remarkable increase of around 25%. Considering that all groups showed no significant differences related to the chlorophyll fluorescence parameters at day 6, it can be concluded that the reduced ratios of SFA as well as the enhanced TFA contents and MUFA ratios during the early cultivation stage were a result of glycerol addition rather than nutrient deficiency. Additionally, day 12 displayed a similar variation pattern to that of day 6, featured by increased TFA and MUFA ratios, as well as decreased MUFA ratio in mixotrophic conditions. Taking the significant enhancements of mixotrophy on biomass accumulation, TFA production and proportion of PUFAs into consideration, mixotrophy displays enormous advantages in prompting FAs productivities in C. roscoffensis.
Our results also indicated that both the FAs compositions and contents of C. roscoffensis were heavily dependent on the cultivation stages. For example, with the prolonged cultivation time, the PA and SA ratios in the photoautotrophic group declined from 25.29% and 19.34% to 14.34% and 9.84%, respectively. In contrast, OA and ALA ratios increased from 13.80% and 15.74% to 20.00% and 26.14%, respectively. With regard to TFA contents, photoautotrophy displayed TFA value of 167.74 mg g−1 DCW at day 12, around 1.5-times higher than that of day 6. In comparison with the photoautotrophy group, mixotrophic C. roscoffensis exhibited the same variation trend but with much higher variation magnitude, ranging from 124.93 mg g−1 DCW at day 6 to 232.84 mg g−1 DCW at day 12. Researchers have claimed that nutrients limitation is capable of inducing an increase in TFA contents of microalgae such as Chlamydomonas sp., Nannochloropsis sp., Scenedesmus sp., and Chlorella sp. (Mandal and Mallick, 2009; Can et al., 2017). As mentioned in Section 3.2.2, a phenomenon of nutrients deficiency reflected by the declining values of Fv/Fm and ETR was observed during the later stage of cultivation period, which possibly contributed to the remarkable increase of TFA contents of C. roscoffensis at day 12.
3.2.5 Pigments contentsTo further clarify the effects of glycerol on photosynthesis system and evaluate the potential of C. roscoffensis for fucoxanthin production, three types of pigments with relatively high amounts were analyzed by LC-MS. At day 6, the chlorophyll a (Chl-a) of two groups remained around 20.0 mg g−1 DCW, showing no significant differences (Table 3). These findings further supported the conclusions deduced from the chlorophyll fluorescence parameters, suggesting that the glycerol addition imposed no significant impacts on photosynthetic capacity of C. roscoffensis. However, as the cultivation time was prolonged, the Chl-a of two groups decreased sharply. Among them, the mixotrophic group exhibited relatively large descending values, resulting in much lower Chl-a contents (8.32 mg g−1 DCW) compared to photoautotrophic group (13.05 mg g−1 DCW). Previous studies demonstrated that the Chl-a content of microalgae is closely related to nutrient availability. For example, Ikaran et al. (2015) investigated the effect of nitrogen limitation on the physiology of Chlorella vulgaris and found that the cellular Chl-a content was twice as high under nitrogen repletion conditions compared to nitrogen depletion conditions. Studies conducted with other microalgae such as Chlamydomonas sp., Coccomyxa sp., and even Spirulina sp. (Li et al., 2018; Seth et al., 2021) also reached the same conclusion. The trends in fucoxanthin closely resembled those of Chl-a. Compared to day 6, the values of day 12 displayed a sharp decrease of 26.57% in autotrophic group and 58.16% in mixotrophic group, respectively (Table 3).
|
|
Table 3 Pigments of Chrysotila roscoffensis under different modes |
Fucoxanthin is attracting intensive attention due to its multiple health benefits, and majority of previous studies related to its industrial production focused on diatoms (Seth et al., 2021). Until recently, several studies have evaluated the feasibility of producing fucoxanthin in coccolithophores (Ishika et al., 2017; Okcu et al., 2021).
In this study, the fucoxanthin of C. roscoffensis peaked at day 6 with a value of around 7.0 mg g−1 DCW. Compared to other known microalgae species, such a value of C. roscoffensis seems moderate (Seth et al., 2021). Additionally, it has been reported that the specific growth rate and biomass productivity of coccolithophores can be largely enhanced when cultivated using photobioreactors with a semi-continuous cultivation method (Moheimani et al., 2011). Thus, it can be expected that mixotrophic C. roscoffensis cultivated using semi-continuous cultivation mode, in which microalgal cells can be maintained at exponential phase with relatively high fucoxanthin content, maybe the optimal choice for the purpose of producing fucoxanthin from C. roscoffensis.
4 ConclusionsUnder heterotrophic cultivation conditions, none of glucose, glycerol, or NaAC was found to be able to promote cell growth, confirming that C. roscoffensis lacks the ability of heterotrophy. Under illuminated conditions, the addition of glucose showed no effects on C. roscoffensis either, demonstrating the absence of a hexose transporter in this microalga. External NaAC resulted in reduced cell density of C. roscoffensis even in the presence of light supply, whereas the underlying mechanism behind this phenomenon requires further exploration. On the other hand, only glycerol, when added under illuminated conditions, facilitated C. roscoffensis growth, and its promoting effects were enhanced with the increase of carbon concentrations (within the range of 0.05 – 0.5 mol L−1). Moreover, mixotrophy significantly modified the biochemical composition of C. roscoffensis biomass, characterized by increased lipids and TFA contents as well as altered FAs profile. However, the photosynthetic capacity, biocalcification process, and pigments biosynthesis of C. roscoffensis seemed unaffected by glycerol addition. Considering the multiple biotechnological advantages of C. roscoffensis, such as its unique biocalcification ability, rich lipids content with high PUFA ratios, moderate fucoxanthin contents, and high growth rates under mixotrophy, it can be concluded that this microalga deserves more attention for the purpose of CO2 mitigation and high-value substances production.
AcknowledgementsThis work was supported by the Ningbo Science and Technology Project (No. 2024J176), the Key Program of Science and Technology Innovation in Ningbo (No. 2023 Z118), the Zhejiang Provincial Natural Science Foundation of China (No. LY22C190001).
Author Contributions
Luyao Zhang: methodology, raw material preparation, writing-original draft. Zhichao He: methodology, data curation, raw material preparation. Yan Sun: methodology, software, surveys. Jian Li and Pengfei Cheng: software, data analysis. Spiros N. Agathos: conceptualization, investigation. Chengxu Zhou and Lin Zhang: administration and supervision, conceptualization. Jichang Han: writing-review and editing, funding acquisition, project administration.
Data Availability
The data and references presented in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
Informed consent for publication was obtained from all participants.
Conflict of Interests
The authors declare that they have no conflict of interests.
Baldisserotto, C., Sabia, A., Guerrini, A., Demaria, S., Maglie, M., Ferroni, L., et al., 2021. Mixotrophic cultivation of Thalassiosira pseudonana with pure and crude glycerol: Impact on lipid profile. Algal Research, 54: 102194. DOI:10.1016/j.algal.2021.102194 (  0) 0) |
Bligh, E. G., and Dyer, W. J., 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8): 911-917. DOI:10.1139/o59-099 (  0) 0) |
Can, S. S., Koru, E., and Cirik, S., 2017. Effect of temperature and nitrogen concentration on the growth and lipid content of Spirulina platensis and biodiesel production. Aquaculture International, 25(4): 1485-1493. DOI:10.1007/s10499-017-0121-6 (  0) 0) |
Cao, Y. Q., Xu, J. M., Tong, Y. X., Xie, Z. J., and Kong, W. B., 2022. Sodium acetate promotes the production of biomass and intracellular biochemical components by Micractinium reisseri FM1 under batch and fed-batch cultivation. Journal of Applied Phycology, 34(6): 2857-2868. DOI:10.1007/s10811-022-02851-7 (  0) 0) |
Cecchin, M., Benfatto, S., Griggio, F., Mori, A., Cazzaniga, S., Vitulo, N., et al., 2018. Molecular basis of autotrophic vs. mixotrophic growth in Chlorella sorokiniana. Scientific Reports, 8: 6465. DOI:10.1038/s41598-018-24979-8 (  0) 0) |
Claxton, L. M., McClelland, H. L. O., Hermoso, M., and Rickaby, R. E. M., 2022. Eocene emergence of highly calcifying coccolithophores despite declining atmospheric CO2. Nature Geoscience, 15(10): 826-831. DOI:10.1038/s41561-022-01006-0 (  0) 0) |
Combres, C., Laliberté, G., Reyssac, J. S., and Noüe, J., 1994. Effect of acetate on growth and ammonium uptake in the microalga Scenedesmus obliquus. Physiologia Plantarum, 91(4): 729-734. DOI:10.1111/j.1399-3054.1994.tb03012.x (  0) 0) |
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F., 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3): 350-356. DOI:10.1021/ac60111a017 (  0) 0) |
Fernández, E., Balch, W. M., Marañón, E., and Holligan, P. M., 1994. High rates of lipid biosynthesis in cultured, mesocosm and coastal populations of the coccolithophore Emiliania huxleyi. Marine Ecology Progress Series, 114: 13-22. DOI:10.3354/meps114013 (  0) 0) |
Hallmann, A., and Sumper, M., 1996. The Chlorella hexose/H+ symporter is a useful selectable marker and biochemical reagent when expressed in Volvox. Proceedings of the National Academy of Sciences of the United States of America, 93(2): 669-673. DOI:10.1073/pnas.93.2.669 (  0) 0) |
Hu, J. J., Nagarajan, D., Zhang, Q. G., Chang, J. S., and Lee, D. J., 2017. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnology Advances, 36(1): 54-67. DOI:10.1016/j.biotechadv.2017.09.009 (  0) 0) |
Ikaran, Z., Suárez-Alvarez, S., Urreta, I., and Castañón, S., 2015. The effect of nitrogen limitation on the physiology and metabolism of Chlorella vulgaris var L3. Algal Research, 10: 134-144. DOI:10.1016/j.algal.2015.04.023 (  0) 0) |
Ishika, T., Moheimani, N. R., Bahri, P. A., Laird, D. W., Blair, S., and Parlevliet, D., 2017. Halo-adapted microalgae for fucoxanthin production: Effect of incremental increase in salinity. Algal Research, 28: 66-73. DOI:10.1016/j.algal.2017.10.002 (  0) 0) |
Kanda, H., Hoshino, R., Murakami, K., Zheng, Q. X., and Goto, M., 2020. Lipid extraction from microalgae covered with biomineralized cell walls using liquefied dimethyl ether. Fuel, 262: 116590. DOI:10.1016/j.fuel.2019.116590 (  0) 0) |
Khoo, K. S., Ahmad, I., Chew, K. W., Iwamoto, K., Bhatnagar, A., and Show, P. L., 2023. Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: A review. Progress in Energy and Combustion Science, 96: 101071. DOI:10.1016/j.pecs.2023.101071 (  0) 0) |
Li, X. T., Li, W., Zhai, J., and Wei, H. X., 2018. Effect of nitrogen limitation on biochemical composition and photosynthetic performance for fed-batch mixotrophic cultivation of microalga Spirulina platensis. Bioresource Technology, 263: 555-561. DOI:10.1016/j.biortech.2018.05.046 (  0) 0) |
Liu, J. J., Sun, Y., Zhang, L., Li, X. H., He, Z. C., Zhou, C. X., et al., 2023. Screening of antibiotics to obtain axenic cell cultures of a marine microalga Chrysotila roscoffensis. Frontiers in Bioengineering and Biotechnology, 11: 1218031. DOI:10.3389/fbioe.2023.1218031 (  0) 0) |
Lu, X., Liu, B., He, Y. J., Guo, B. B., Sun, H., and Chen, F., 2019. Novel insights into mixotrophic cultivation of Nitzschia laevis for co-production of fucoxanthin and eicosapentaenoic acid. Bioresource Technology, 294: 122145. DOI:10.1016/j.biortech.2019.122145 (  0) 0) |
Ma, X. M., Mi, Y. W., Zhao, C., and Wei, Q., 2022. A comprehensive review on carbon source effect of microalgae lipid accumulation for biofuel production. Science of the Total Environment, 806: 151387. DOI:10.1016/j.scitotenv.2021.151387 (  0) 0) |
Mandal, S., and Mallick, N., 2009. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Applied Microbiology and Biotechnology, 84(2): 281-291. DOI:10.1007/s00253-009-1935-6 (  0) 0) |
Meng, R., Zhang, L., Zhou, C. X., Liao, K., Xiao, P., Luo, Q. J., et al., 2022. Genome sequence of Chrysotila roscoffensis, a coccolithphore contributed to global biogeochemical cycles. Genes, 13(1): 40. DOI:10.3390/genes13010040 (  0) 0) |
Mogany, T., Bhola, V., Ramanna, L., and Bux, F., 2022. Photosynthesis and pigment production: Elucidation of the interactive effects of nutrients and light on Chlamydomonas reinhardtii. Bioprocess and Biosystems Engineering, 45(1): 187-201. DOI:10.1007/s00449-021-02651-2 (  0) 0) |
Moheimani, N. R., and Borowitzka, M. A., 2006. The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. Journal of Applied Phycology, 18(6): 703-712. DOI:10.1007/s10811-006-9075-1 (  0) 0) |
Moheimani, N. R., and Borowitzka, M. A., 2007. Limits to productivity of the alga Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. Biotechnology and Bioengineering, 96(1): 27-36. DOI:10.1002/bit.21169 (  0) 0) |
Moheimani, N. R., and Borowitzka, M. A., 2011. Increased CO2 and the effect of pH on growth and calcification of Pleurochrysis carterae and Emiliania huxleyi (Haptophyta) in semicontinuous cultures. Applied Microbiology and Biotechnology, 90(4): 1399-1407. DOI:10.1007/s00253-011-3174-x (  0) 0) |
Moheimani, N. R., Isdepsky, A., Lisec, J., Raes, E., and Borowitzka, M. A., 2011. Coccolithophorid algae culture in closed photobioreactors. Biotechnology and Bioengineering, 108(9): 2078-2087. DOI:10.1002/bit.23161 (  0) 0) |
Moheimani, N. R., Webb, J. P., and Borowitzka, M. A., 2012. Bioremediation and other potential applications of coccolithophorid algae: A review. Algal Research, 1(2): 120-133. DOI:10.1016/j.algal.2012.06.002 (  0) 0) |
Moreira, A. S. P., Gonçalves, J., Conde, T. A., Couto, D., Melo, T., Maia, I. B., et al., 2022. Chrysotila pseudoroscoffensis as a source of high-value polar lipids with antioxidant activity: A lipidomic approach. Algal Research, 66: 102756. DOI:10.1016/j.algal.2022.102756 (  0) 0) |
Ogbonna, J. C., Yoshizawa, H., and Tanaka, H., 2000. Treatment of high strength organic wastewater by a mixed culture of photosynthetic microorganisms. Journal of Applied Phycology, 12(3): 277-284. DOI:10.1023/A:1008188311681 (  0) 0) |
Okcu, G. D., Eustance, E., Lai, Y. S., and Rittmann, B. E., 2021. Evaluation of co-culturing a diatom and a coccolithophore using different silicate concentrations. Science of the Total Environment, 769: 145217. DOI:10.1016/j.scitotenv.2021.145217 (  0) 0) |
Perez-Garcia, O., Escalante, F. M. E., de-Bashan, L. E., and Bashan, Y., 2011. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Research, 45(1): 11-36. DOI:10.1016/j.watres.2010.08.037 (  0) 0) |
Poddar, N., Sen, R., and Martin, G. J. O., 2018. Glycerol and nitrate utilisation by marine microalgae Nannochloropsis salina and Chlorella sp. and associated bacteria during mixotrophic and heterotrophic growth. Algal Research, 33: 298-309. DOI:10.1016/j.algal.2018.06.002 (  0) 0) |
Rezvani, S., Kennedy, C., and Moheimani, N. R., 2019. Techno-economic study of multi-product resource scenarios for Pleurochrysis carterae grown in open ponds in Western Australia. Algal Research, 39: 101456. DOI:10.1016/j.algal.2019.101456 (  0) 0) |
Sedmak, J. J., and Grossberg, S. E., 1977. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Analytical Biochemistry, 79(1): 544-552. DOI:10.1016/0003-2697(77)90428-6 (  0) 0) |
Seth, K., Kumar, A., Rastogi, R. P., Meena, M., Vinayak, V., and Harish., 2021. Bioprospecting of fucoxanthin from diatoms – Challenges and perspectives. Algal Research, 60: 102475. DOI:10.1016/j.algal.2021.102475 (  0) 0) |
Su, M., D'Imporzano, G., Veronesi, D., Afric, S., and Adani, F., 2020. Phaeodactylum tricornutum cultivation under mixotrophic conditions with glycerol supplied with ultrafiltered digestate: A simple biorefinery approach recovering C and N. Journal of Biotechnology, 323: 73-81. DOI:10.1016/j.jbiotec.2020.07.018 (  0) 0) |
Sun, Y., Xin, Y., Zhang, L. Y., Wang, Y., Liu, R. L., Li, X. H., et al., 2022. Enhancement of violaxanthin accumulation in Nannochloropsis oceanica by overexpressing a carotenoid isomerase gene from Phaeodactylum tricornutum. Frontiers in Microbiology, 13: 942883. DOI:10.3389/fmicb.2022.942883 (  0) 0) |
Tan, L. J., Xu, W. J., He, X. L., and Wang, J. T., 2019. The feasibility of Fv/Fm on judging nutrient limitation of marine algae through indoor simulation and in situ experiment. Estuarine, Coastal and Shelf Science, 229: 106411. DOI:10.1016/j.ecss.2019.106411 (  0) 0) |
Vicente, B., Matos, J., Gomes, R., Sapatinha, M., Afonso, C., Rodrigues, T., et al., 2021. Production and bioaccessibility of Emiliania huxleyi biomass and bioactivity of its aqueous and ethanolic extracts. Journal of Applied Phycology, 33(6): 3719-3729. DOI:10.1007/s10811-021-02551-8 (  0) 0) |
Villanova, V., Singh, D., Pagliardini, J., Fell, D., Le Monnier, A., Finazzi, G., et al., 2021. Boosting biomass quantity and quality by improved mixotrophic culture of the diatom Phaeodactylum tricornutum. Frontiers in Plant Science, 12: 642199. DOI:10.3389/fpls.2021.642199 (  0) 0) |
Wang, S., Zhou, X. Y., Wu, S., Zhao, M. K., and Hu, Z. L., 2023. Transcriptomic and metabolomic analyses revealed regulation mechanism of mixotrophic Cylindrotheca sp. glycerol utilization and biomass promotion. Biotechnology for Biofuels and Bioproducts, 16(1): 84. DOI:10.1186/s13068-023-02338-8 (  0) 0) |
Xu, P., Li, J., Qian, J., Wang, B., Liu, J., Xu, R., et al., 2023. Recent advances in CO2 fixation by microalgae and its potential contribution to carbon neutrality. Chemosphere, 319: 137-987. DOI:10.1016/j.chemosphere.2023.137987 (  0) 0) |
Young, E. B., and Beardall, J., 2003. Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. Journal of Phycology, 39(5): 897-905. DOI:10.1046/j.1529-8817.2003.03042.x (  0) 0) |
Yu, H. F., Zhuang, L. L., Zhang, M., and Zhang, J., 2022. The mechanism study of attached microalgae cultivation based on reverse osmosis concentrated water (WROC). Resources, Conservation and Recycling, 179: 106066. DOI:10.1016/j.resconrec.2021.106066 (  0) 0) |
Zaslavskaia, L. A., Lippmeier, J. C., Shih, C., Ehrhardt, D., Grossman, A. R., and Apt, K. E., 2001. Trophic obligate conversion of a photoautotrophic organism through metabolic engineering. Science, 292(5524): 2073-2075. DOI:10.1126/science.160015 (  0) 0) |
Zhang, J., Liu, F. L., Wang, Q. H., Gong, Q. L., and Gao, X., 2023. Effect of light wavelength on biomass, growth, photosynthesis and pigment content of Emiliania huxleyi (Isochrysidales, Cocco-Lithophyceae). Journal of Marine Science and Engineering, 11(2): 456. DOI:10.3390/jmse11020456 (  0) 0) |
Zhang, L., Han, J. C., Ma, S. N., Zhang, Y. B., Wang, Y. M., and Xu, J. L., 2023. Comprehensive evaluation of growth characteristics, nitrogen removal capacity, and nutritional properties of three diet microalgae. Frontiers in Marine Science, 10: 1117043. DOI:10.3389/fmars.2023.1117043 (  0) 0) |
Zhang, L., Wang, S., Han, J. C., Yang, G. P., Pan, K. H., and Xu, J. L., 2022. Manipulation of triacylglycerol biosynthesis in Nannochloropsis oceanica by overexpressing an Arabidopsis thaliana diacylglycerol acyltransferase gene. Algal Research, 61: 102590. DOI:10.1016/j.algal.2021.102590 (  0) 0) |
Zhang, W. L., Zhou, W. J., Jiang, S., Wang, Y. Y., Chen, L., Yang, G. P., et al., 2023. Heterotrophic modification of Phaeodactylum tricornutum Bohlin. Algal Research, 72: 103137. DOI:10.1016/j.algal.2023.103137 (  0) 0) |
Zhou, D. H., Meng, R., Xiao, P., Chang, T., Li, Y. R., Han, J. C., et al., 2022. Frequent antibiotic exposure stabilized the associated bacterial community while altering physiological and biochemical characteristics of the coccolithophore Chrysotila roscoffensis. Algal Research, 67: 102807. DOI:10.1016/j.algal.2022.102807 (  0) 0) |
 2025, Vol. 24
2025, Vol. 24


