2) The Key Laboratory of Mariculture of Ministry of Education, Ocean University of China, Qingdao 266003, China
The spotted sea bass, Lateolabrax maculatus, belongs to Lateolabrax, Serranidae, and is a euryhaline teleost which can live in salinities ranging from freshwater to seawater and hypersaline environments. L. maculatus shows robust hyper-osmoregulatory and hypo-osmoregulatory abilities. To cope with the challenges of environmental salinities, euryhaline fishes have evolved sophisticated iono/osmoregulatory mechanisms. The gills, kidneys and intestine are important organs to maintain a stable internal body fluid homeostasis (Yang et al., 2016). Several other regulatory elements including Na+/K+-AT-Pase (NKA), Na+/K+/2Cl− cotransporter (NKCC), and the cystic fibrosis transmembrane conductance regulator (CFTR) were also proved to be important for NaCl secretion in gill (Evans et al., 2005; Hwang and Lee, 2007; Hwang et al., 2011).
Following the electrochemical gradient energized by NKA, ions are driven by a number of different transmembrane proteins (Hirose et al., 2003). NKCC and CFTR are two major ion-transport proteins within ionocytes. Generally, nkcc has two main isoforms including nkcc1 and nkcc2. NKCC1 is a member of cation-chloride cotransporter (CCC) family (Gamba, 2005), which is considered to be the secretory isoform. It participates in the simultaneous transport of Na+, K+ and Cl− into cells and is expressed ubiquitously and especially in ion-epithelial cells (Gamba et al., 1994; Marshall et al., 2002; Hiroi et al., 2008). NKCC1 is thought to play a crucial role in ionocytes during seawater acclimation because the elevated mRNA and/or protein levels of NKCC1 in gills have been reported in a variety of teleost including European eel (Anguilla anguilla), Atlantic killifish (Fundulus heteroclitus), brackish medaka (Oryzias melastigma), European sea bass (Dicentrarchus labrax), green sturgeon (Acipenser medirostris) and brown trout (Salmo trutta) when they are exposed to higher salinity (Cutler and Cramb, 2002; Tang and Lee, 2007; Hiroi et al., 2008; Flemmer et al., 2010; Hiroi and McCormick, 2012). Conversely, NKCC2 is the absorptive isoform which expresses specifically in intestine and kidney (Gamba, 2005). Similar to NKCC1, CFTR belongs to the cyclic adenosine monophosphate (cAMP)-activated Cl− channel (ClC), and also has an important role in NaCl secretion after salinity challenge (Hwang and Lee, 2007). Higher levels of CFTR protein and increased expression levels of cftr were identified in gills of different teleost after the fishes are transported from freshwater to seawater (McCormick et al., 2003; Scott et al., 2004; Nilsen et al., 2007; Shaw et al., 2008; Bodinier et al., 2009). The higher cftr expression is also found in seawater-adapted intestine of some species such as toadfish and killifish (Marshall et al., 2002; Ruhr et al., 2014).
L. maculatus is an ideal model for studying salinity adaptation as it can survive in both hyper-osmoregulatory and hypo-osmoregulatory environments. However, the gene features of cftr and nkccs and their functions in salinity regulation are currently unexplored. To fill in the gap, in this study, we reported the identification and characterization of one cftr and three nkcc in L. maculatus. Moreover, the expressions of these genes were determined in different tissues of L. maculatus under natural physiological condition and different salinities.
2 Materials and Methods 2.1 Ethics StatementAll animal experiments were conducted in accordance with the guidelines and approval of the respective Animal Research and Ethics Committees of Ocean University of China (Permit Number: 20141201. http://www.gov.cn/gongbao/content/2011/content_1860757.htm). The studies did not involve endangered or protected species.
2.2 Identification of cftr and nkccTo identify cftr and nkcc in L. maculatus, gene sequences of human (Homo sapiens), zebrafish (Danio rerio), Atlantic salmon (salmo salar), large yellow croaker (Larimichthys crocea), barramundi perch (Lates calcarifer) and European seabass (Dicentrarchus labrax) were retrieved from GenBank and were used as queries for TBLASTN (1e−5) search against the transcriptome database (accession numbers: SRR4409341 and SRR4409397) (Zhang et al., 2017) and the whole genome sequence database (unpublished data) of L. maculatus. TBLASTN was used to obtain the initial pool of cftr, nkcc1a, nkcc1b and nkcc2 transcript sequences in L. maculatus, and BLASTN was then used to verify the cDNA sequences through comparing the transcriptome sequences with the whole genome sequences. The ORFs (open reading frames) of those genes were searched from the retrieved transcript sequences by ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder/) and we are validated by using BLASTP against NCBI non-redundant protein sequence database.
The lengths of mRNA, 5'-untranslated region (5'-UTR), 3'-untranslated region (3'-UTR), and the number of amino acids translated by cftr and nkcc were obtained from the transcriptome database and genome database of L. maculatus. Molecular weight (MW, kDa) and isoelectric point (pI) of putative CFTR and NKCC proteins were calculated by using ExPASy Prot-Param tool (https://web.expasy.org/protparam/). Subcellular localization of all putative CFTR and NKCC proteins in L. maculatus was predicted by subcellular localization predictor (http://cello.life.nctu.edu.tw/) (Yu et al., 2006).
2.3 Phylogenetic Analysis of cftr and nkccTo investigate the phylogenetic relationship and classification of cftr, nkcc genes in L. maculatus, the amino acid sequences of these genes of several representative vertebrates including Mus musculus, H. sapiens, D. rerio, S. salar, L. crocea, L. calcarifer and D. labrax were selected and retrieved from the NCBI non-redundant protein sequence database for phylogenetic analysis. Multiple protein sequences were aligned by Clustal X1.83 Omega program (Goujon et al., 2010). Phylogenetic analyses were conducted using MEGA 7 with bootstrapping values taken from 1000 replicates by neighbor-joining method (Darriba et al., 2011; Kumar et al., 2016). The tree was displayed with Interactive Tree of Life (iTOL, http://itol.embl.de/).
2.4 Sequence Analysis of cftr and nkcc of L. maculatusMultiple protein sequence alignments of CFTR, NKCC were performed using DNAMAN 6.0 (Lynnon Biosoft, USA) with the default parameters. The species selected for CFTR sequence alignment included L. maculatus, H. sapiens, M. musculus, D. rerio, L. calcarifer and D. labrax. The predicted potential transmembrane (TM) domains of CFTR and NKCC were determined with Split 4.0 Server (http://split.pmsft.hr/split/4/). To confirm the presence of the conserved domains, all L. maculatus CFTR and NKCC proteins were analyzed with the Simple Modular Architecture Research Tool (SMART) (http://smart.emblheidelberg.de/). The DNA and cDNA sequences corresponding to each predicted cftr and nkcc genes in L. maculatus genome and transcriptome databases were used to determine the sizes of the exons and the positions of exon-intron boundaries. Exon-intron structure schematic diagrams of the cftr and nkcc genes were generated using the Gene Structure Display Server (GSDS, http://gsds.cbi.pku.edu.cn/). Conserved motifs among the CFTR and NKCC proteins in L. maculatus were predicted with MEME (Multiple Expectation Maximization for Motif Elicitation) software (http://memesuite.org/tools/meme) (Bailey et al., 2009). The parameters were set as follows: distribution of motif occurrences was zero or one per sequence; sizes of motifs were 6 to 50 residues; other parameters were default. The three-dimensional (3D) structures of CFTR and NKCC proteins were built by Swiss-Model (http://swissmodel.expasy.org/) and corrected by NCBI structure (https://www.ncbi.nlm.nih.gov/structure). The images were generated by PyMOL 2.2 (W.L. DeLano, The PyMOL Molecular Graphics System, 2002.)
2.5 Chromosomal Location Analysis of cftr and nkcc Genescftr, nkcc genes were mapped on chromosomes by identifying their chromosomal positions provided in L. maculatus genome database. The distribution map of cftr, nkcc genes throughout L. maculatus genome was protracted using MapDraw V2.1 software (Liu and Meng, 2003).
2.6 Salinity Challenge and Sample CollectionThe L. maculatus fingerlings (120.66 g ± 13.05 g) were obtained from Shuangying Aquaculture Farms (Dongying, Shandong Province, China). Prior to the experiment, fish were maintained under a 14 h:10 h light–dark photoperiod in a 5 m×5 m×1 m cement pond for one week. The water temperature was 25.3 ± 0.7℃; the concentration of dissolved oxygen was 7.01 ± 0.45 mg L−1; pH was 7.8 ± 0.5; and the salinity was 30. After an initial acclimation period, fish were exposed to gradually changing salinity over 12 h until it reached 0 (FW, fresh water group), 12 (IP, isotonic point group), 45 (HS, high salinity group). In control group (SW, sea water group) the salinity was kept at 30. Experiments were conducted in 12 cuboid tanks (120 L) with 12 fish per tank for one month and all treatment were conducted with triplicate. The fish were fed a commercial pellet and the water was replaced 50% once per day.
After 30 days of rearing, 3 fish in sea water group (SW) were collected to observe genes expression profiles in ten tissues (kidney, gonad, stomach, intestine, gill, muscle, heart, spleen, liver and brain). At the same time, three fish per tank of each salinity treatment group were treated with tricainemethane sulfonate (MS 222, 200 mg L−1) and the kidney, intestine and gill were sampled immediately. Then the samples were placed into 1.5 mL RNase-free tubes immediately and were quickly frozen by liquid nitrogen. Finally, they were stored at −80℃ for RNA extraction and further analyses.
2.7 Total RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)Quantitative real-time PCR (qRT-PCR) was used to detect the mRNA expressions of cftr and nkcc genes in different tissues of L. maculatus with different salinity treatments. Total RNA was isolated using TRIzol® reagent (Invitrogen, USA). The concentration and integrity of total RNA were assessed using the Biodropsis BD1000 nucleic acid analyzer (OSTC, Beijing). Any potential gDNA contamination was removed by using a PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Beijing, China) and the first-strand cDNA was synthesized using random primers and Reverse Transcriptase M-MLV (TaKaRa) according to the manufacturer's instructions. Gene-specific primers were listed in Table 1. 18S ribosomal RNA (18S rRNA) was used as the reference gene for qRT-PCR normalization as described in previous studies (Aitken, 2006). Each reaction for qRTPCR consisted of a total volume of 20 μL containing 10 μL of SYBR® FAST qPCR Master Mix (2X), 0.4 μL of ROX, 2 μL of template cDNA, 0.4 μL of each primer and 6.8 μL of nuclease-free water. The PCR amplification was in a 96-well optical plate at 95℃ for 30 s, followed by 40 cycles of 95℃ for 5 s, 60℃ for 30 s, and finally followed by a dissociation curve to verify the specificity of amplified products. qRT-PCR was performed using the StepOne Plus Real-Time PCR system (Applied Biosystems) and 2−ΔΔCT method was used to analyze the expression level of genes.
|
|
Table 1 Primers of cftr, nkcc1a, nkcc1b and nkcc2 and18S rRNA genes for qRT-PCR |
The data were further analyzed statistically using oneway ANOVA and Ducan's multiple range tests with SPSS 19.0 software (SPSS, Chicago, IL, USA). The values are presented as mean ± SEM (standard error of mean). Difference was considered significant at P < 0.05.
3 Results 3.1 Identification and Copy Numbers of cftr and nkcc GenesOne cftr and three nkcc genes were identified in L. maculatus genome including cftr, nkcc1a, nkcc1b and nkcc2. Their obtained amino acid sequences were named according to the BLASTP results based on several common fish species, such as D. rerio, S. salar, L. crocea, L. calcarifer and D. labrax. The cDNA sequences of the four genes have been submitted to GenBank. Their accession numbers and other sequence characteristics were presented in Table 2. The complete encoding sequences were obtained for cftr, nkcc1a and nkcc2 genes, and only partial sequence was generated for nkcc1b gene (Table 2). The transcript lengths of cftr, nkcc1a, nkcc1b and nkcc2 genes were from 2442 bp (nkcc1b) to 4868 bp (cftr), the number of amino acids in the predicted four proteins were from 813 (NKCC1B) to 1507 (CFTR), the putative MWs were from 87.64 (NKCC1B) to 169.70 kDa (CFTR), and the theoretical pI values were from 5.68 (NKCC1A) to 8.32 (CFTR) (Table 2). Subcellular location prediction indicated putative proteins of CFTR, NKCC1A, NKCC1B and NKCC2 were localized in the inner membrane (Im) (Table 2).
|
|
Table 2 Characteristics of cftr, nkcc1a, nkcc1b and nkcc2 genes in spotted sea bass |
The copy numbers of cftr, nkcc genes were investigated in L. maculatus and other vertebrates (Table 3). Among these genes, nkcc1 gene had two copies in L. maculatus genome, while both cftr and nkcc2 genes showed a single copy. The identities and copy numbers of cftr, nkcc1 and nkcc2 genes were relatively conservative among different species (Table 3). For cftr and nkcc2 genes, only one copy was identified in all tested species. However, nkcc1 gene had two copies in all the tested teleost including catfish (Ipu), medaka (Ola), fugu (Tru), zebrafish (Dre) and spotted sea bass (Lmu). It had a single copy in higher vertebrates including human (Hsa), mouse (Mmu) and chicken (Gga).
|
|
Table 3 Comparison of copy numbers of genesin vertebrate genomes |
To further confirm the annotations of cftr, nkcc1a, nkcc1b and nkcc2 genes in L. maculatus, phylogenetic analyses were conducted based on the amino acid sequences of several vertebrates including H. sapiens, M. musculus, G. gallus, D. rerio, S. salar, L. calcarifer, O. niloticus, L. crocea, C. semilaevis, T. rubripes and D. labrax. As shown in Fig.1, these genes of L. maculatus were clustered with respective counterparts consistent with their annotation. Additionally, the phylogenetic tree was divided into three groups (nkcc1, nkcc2, cftr) marked with covered lines, suggesting the conservation of the three genes in evolution. The annotations were further confirmed by the sizes of amino acid sequences out of this phylogenetic tree.
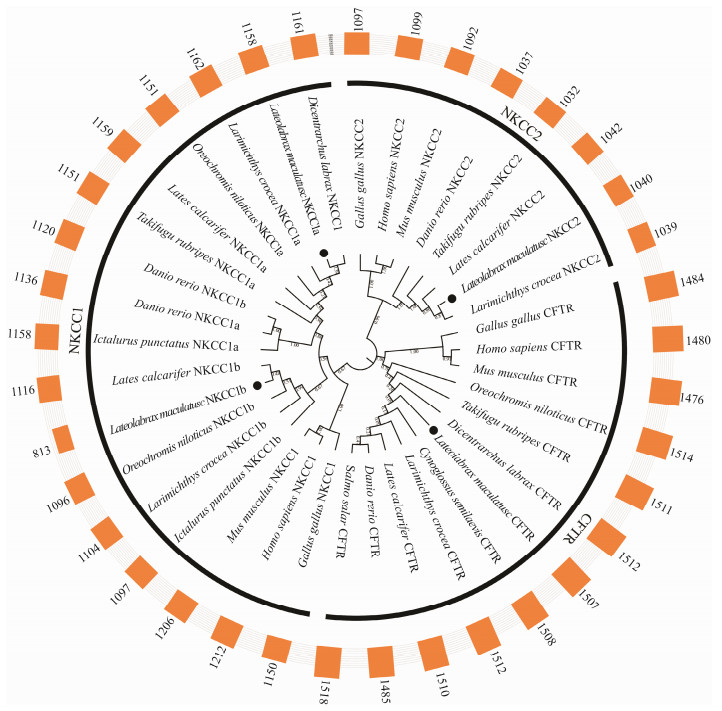
|
Fig. 1 Phylogenetic analyses of nkcc1, nkcc2 and cftr genes of L. maculatus. The phylogenetic tree was constructed by the amino acid sequences from several representative mammals and teleost with 1000 bootstrap replications in MEGA 7, ClustalX1.83 and iTOL online software. These genes of phylogenetic tree were marked with black dot. The phylogenetic tree was divided into three groups with covered lines. The simple bars outside phylogenetic tree stood for the sizes of amino acid sequences of these genes. |
The predicted amino acid sequences of CFTR in L. maculatus showed high similarity to CFTR proteins of teleost species (Fig.2): D. labrax (95.30%), L. calcarifer (91.60%), D. rerio (71.80%). The homology of CFTR to mammals and bird was relatively low: H. sapiens (human) (59.33%), G. gallus (chicken) (60.05%), M. musculus (mouse) (56.16%). The results suggested that the twelve predicted TM domains of CFTR protein were identified.
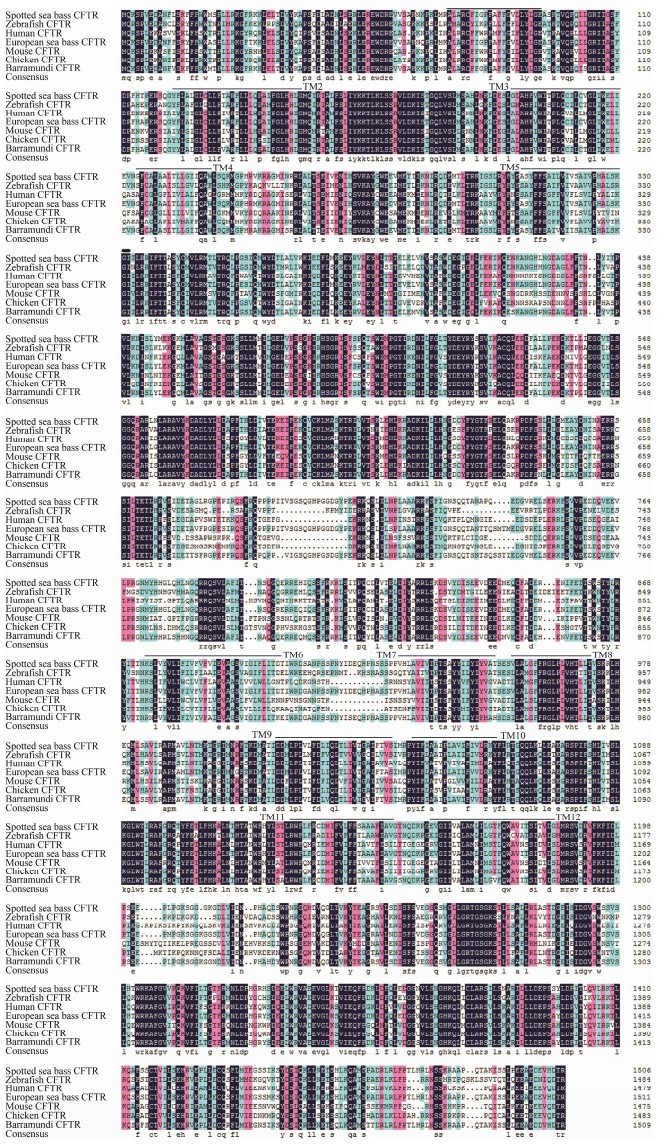
|
Fig. 2 Sequence alignment of CFTR protein of spotted sea bass, zebrafish, human, European sea bass, mouse, chicken and barramundi. Identical amino acid residues are shaded in black, while relatively conserved residues are shaded in pink and blue. The twelve transmembrane (TM) helices of the CFTR protein are marked with black lines. |
The predicted amino acid sequences showed a lower similarity among NKCC proteins in L. maculatus (Fig.3). Alignments of the deduced amino acid sequences of the NKCC proteins demonstrated that the similarity of NKCC proteins in L. maculatus was less than 55% to each other, while the similarity between NKCC1A and NKCC1B was 51.85%; the similarity between NKCC1A and NKCC2 was 52.09%; and the similarity between NKCC1B and NKCC2 was 41.29%. In addition, the eleven predicted TM domains (TM1-7, TM9-12) of NKCC proteins were identified. Compared with NKCC proteins, the amino acid sequences in the C-terminal were found to be highly homologous while the similarity was lower at N-terminal.
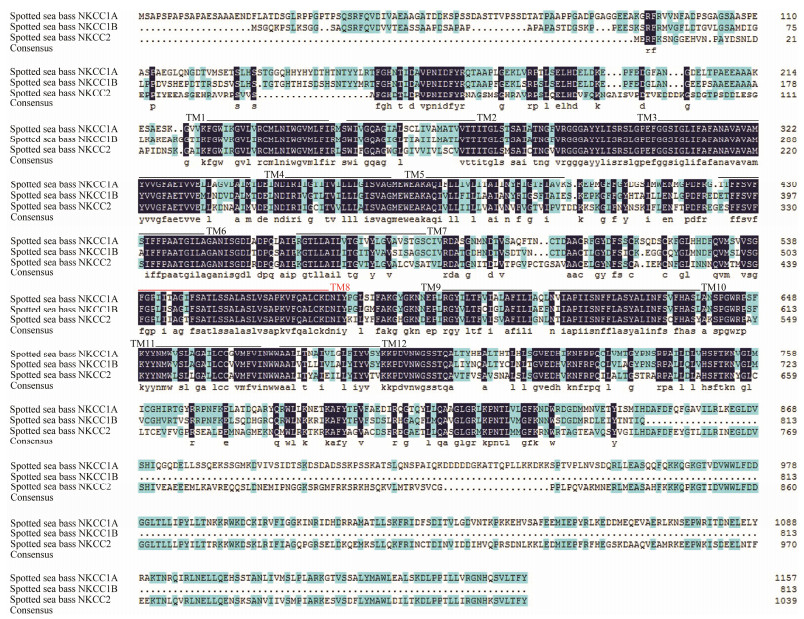
|
Fig. 3 Sequence alignment of spotted sea bass NKCC proteins. Identical amino acid residues are shaded in black while relatively conserved residues are shaded in pink and blue. The eleven transmembrane (TM) helices (TM1-7, TM9-12) of the NKCC proteins are marked with black lines. Compared with three-dimensional structure analysis, an unpredictable transmembrane helix (TM8) is marked with red line. |
The nkcc1a gene has twenty-one exons and twenty-two introns, while the nkcc1b gene has eighteen exons and seventeen introns. The nkcc2 gene has twenty-six exons and twenty-six introns and the cftr gene has twenty-five exons and twenty-six introns (Fig.4A). The conserved homeodomain of Pfam:AA_permease and Pfam:SLC12 was detected in each of the NKCC proteins. The low complexity, Pfam:AA_permease_N, Pfam:AA_permease and Pfam:SLC12 conserved homeodomain were detected in NKCC1A and NKCC1B proteins. The NKCC2 had Pfam: AA_permease_N, Pfam:AA_permease, Pfam:AA_permease_ N and Pfam:SLC12 domains. In addition, the CFTR protein had four domains including Pfam:ABC_ membrane, low complexity, AAA and Pfam:CFTR_R (Fig.4B). To explore the structural diversity and predict the functions of CFTR, NKCC1A, NKCC1B and NKCC2 proteins, only one motif in CFTR protein was identified by MEME software.
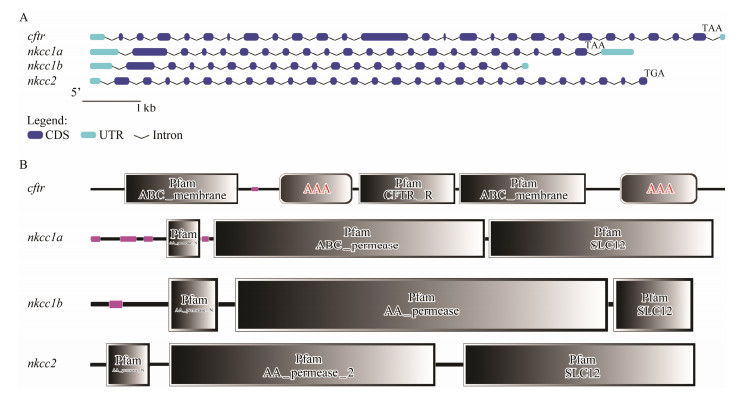
|
Fig. 4 Gene structure and homeodomain analyses of spotted sea bass cftr, nkcc1a, nkcc1b and nkcc2 genes. (A) Exon-intron structure analyses were performed using the Gene Structure Display Server database. The blue boxes indicate exons; the black lines indicate introns. The light blue boxes indicate UTR. The red arrows indicate the position of start and stop codons and black letters indicate the type of stop codon. (B) The domain analysis of CFTR, NKCC1A, NKCC1B and NKCC2 proteins was performed by the SMART analyses service. The low complexity domain was represented in pink. |
Four genes, cftr, nkcc1a, nkcc1b and nkcc2, were located on four different chromosomes of L. maculatus (Fig.5). The cftr gene was on chromosome 6, the nkcc1a gene was on chromosome 17, the nkcc1b gene was on chromosome 8 and the nkcc2 gene was on chromosome 1.
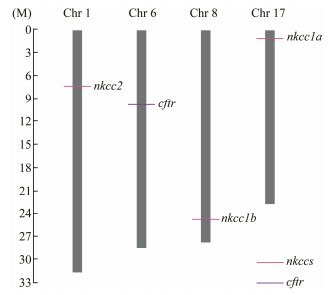
|
Fig. 5 Chromosomal locations of cftr, nkcc1a, nkcc1b and nkcc2 genes on different chromosomes of spotted sea bass. cftr and nkcc genes were localized to an exact position on a particular chromosome by different colors in the lower right corner, which can be calculated using the scale on the left. The chromosome number is shown at the top of each chromosome bar. |
The protein structure of CFTR, NKCC1A, NKCC1B and NKCC2 were predicted by homology modeling methods. The conserved 3D structure of L. maculatus CFTR contained two membrane-spanning domains (MSDs) including 6 TM helices in each MSDs (Fig.6A). The results are consistent with those of sequence alignment (Fig.2). Additionally, it contained two intracellular loops (ICLs) including 10 inner helices and β-sheets (Fig.6A). NKCC1A, NKCC1B and NKCC2 share highly conserved structures consisting of 12 TM helices (Figs. 6B, C and D).
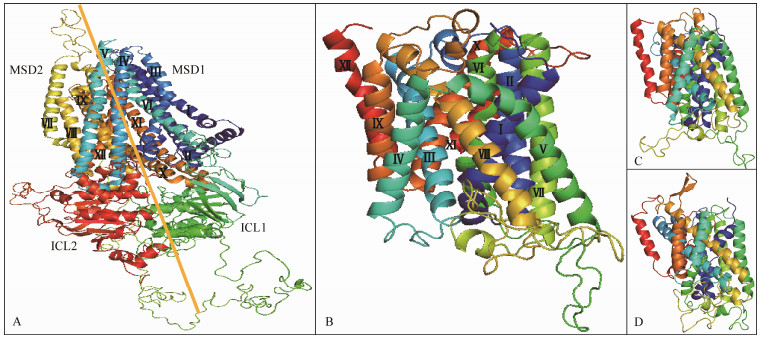
|
Fig. 6 The tertiary structures of CFTR, NKCC1A, NKCC1B and NKCC2 proteins from spotted sea bass. A) CFTR; B) NKCC1A; C) NKCC1B; D) NKCC2. The 12 TM helices are represented by Roman (Ⅰ-Ⅻ). |
qRT-PCR analysis was performed to analyze the expression of cftr, nkcc1a, nkcc1b and nkcc2 genes in ten tissues (kidney, gonad, stomach, intestine, gill, muscle, heart, spleen, liver and brain) of L. maculatus under nature seawater conditions. Results showed that cftr has the highest transcription level in heart, followed by brain, gill and intestine. The highest expression level of nkcc1a gene was detected in gill, which was at least 5-fold higher than in other tissues. Gene nkcc1b was primarily expressed in brain, while low level or no transcription was detected in other tissues. Gene nkcc2 showed the highest expression level in intestine, which was more than 10 times higher than in other tissues (Fig.7).
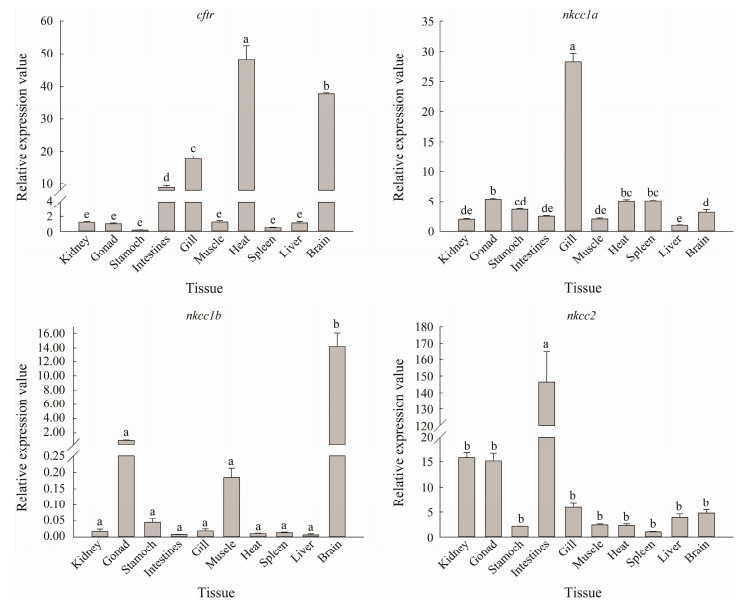
|
Fig. 7 The tissue distribution of cftr, nkcc1a, nkcc1b and nkcc2 gene transcripts. The results are shown as the means ± standard error of mean (SEM). Different letters in the different tissues indicate significant differences (P < 0.05). The graphs were made with SigmaPlot 14.0. |
According to the tissue distribution analysis, relatively high expression levels of cftr, nkcc1a and nkcc2 genes were detected in osmoregulatory tissues including gill, kidney and intestine. To further examine their potential involvement in osmo-regulation and salinity adaptation, their expressions in these three tissues after treatments with different salinities were examined by qRT-PCR (Fig.8). The mRNA levels of cftr in gill and intestine were higher in SW and HS groups, while the highest expression level was found in HS group. The expression level of nkcc1a in gill was significantly higher than those in kidney and intestine, which was consistent with the above results about its tissue distribution. There was obviously increasing trend of nkcc1a mRNA level in gill with the increasing salinity. Similarly, the mRNA level of nkcc2 gene in the intestine was significantly higher than those in gill and kidney, and increased with the increasing salinity, indicating its potential involvement in salinity regulation in L. maculatus (Fig.8).
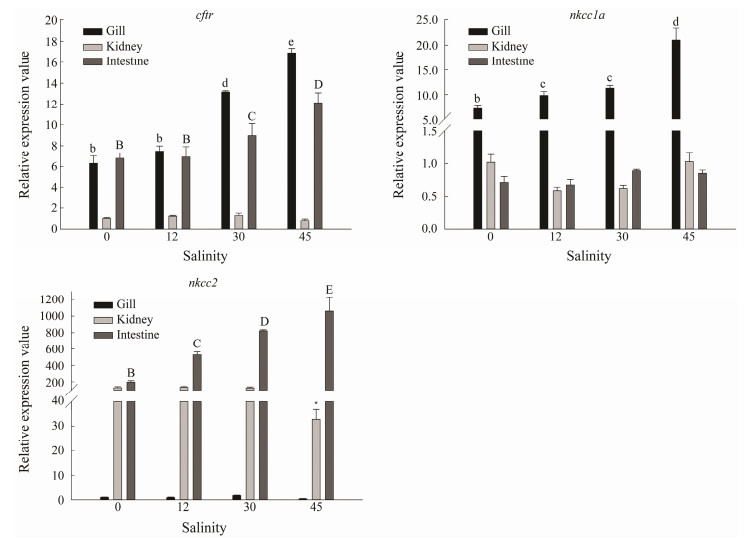
|
Fig. 8 The relative expression levels of cftr, nkcc1a and nkcc2 genes in gill, kidney and intestine of spotted sea bass under different salinity treatments. The significant differences (P < 0.05) in different tissues are represented by capital, lowercase letters and asterisks, respectively. The graphs were made with SigmaPlot 14.0. |
To cope with the challenges exerted by environmental salinities, NKCC and CFTR are proved to be the important proteins for osmoregulation and salinity adaptation in teleost. CFTR is a member of the ATP-binding cassette (ABC) transporter family of an ion channel (Gadsby et al., 2006; Cant et al., 2014), which is found in the apical membranes of epithelial cells (Anderson et al., 1992). NKCC is a member of the cation-chloride cotransporter family, localizing on the basolateral or apical plasma membranes of epithelial cells and associating with facilitation of the particular ion transporting activities (McCormick et al., 2003; Hiroi et al., 2008). In this paper, the identification and characterization of cftr and nkcc genes were reported in L. maculatus for the first time. Furthermore, its biological functions responding to salinity challenges were evaluated by their expression patterns.
We identified the correct annotations of one cftr gene and three nkcc genes (nkcc1a, nkcc1b and nkcc2) from the data of L. maculatus transcriptome and genome. The copy numbers of cftr and nkcc genes were relatively conserved in vertebrates, while some differences in the copy numbers of cftr gene were observed in teleost. Two copies of cftr gene, including cftra and cftrb (or cftrI and cftrII), are identified in Anguilla japonica (Wong et al., 2016) and Salmo salar (Nilsen et al., 2007). In addition, sequence analysis of CFTR and NKCC proteins showed that CFTR and NKCC proteins in L. maculatus shared the conserved domains with twelve predicted TM helices, consisting with previous results (Park and Saier Jr., 1996; Callebaut et al., 2016; Liu et al., 2017; Tordai et al., 2017; Callebaut et al., 2018). Therefore, the conserved domains and 3D structure further support the results of the phylogenetic analyses.
CFTR and NKCC proteins are involved in chloride secretion in teleost during salinity challenge (McCormick et al., 2003; Hiroi and McCormick, 2007; Tse et al., 2007; Yan et al., 2013). Wong et al. (2016) find that cftra is mainly expressed in intestine and kidney with decreased expression level when fish were transferred to SW. It was also reported that cftrb is the dominant isoform expressed in the gill and the higher expression is observed under osmotic stress (Wong et al., 2016). In our study, high mRNA expression levels of cftr were detected in gill and intestine. In addition, up-regulated mRNA expressions of cftr in gill and intestine were also observed with increased environmental salinity, suggesting it might play a role during salinity adaption. Similar results are reported in many teleost species, such as D. labrax (Bodinier et al., 2009), Fundulus heteroclitus (Scott et al., 2004), Juvenile anadromous and S. salar (Nilsen et al., 2007), Anabas testudineus (Ip et al., 2012), and O. latipes (Hsu et al., 2014). All these studies support the crucial function of cftr gene during NaCl secretion, suggesting a potential association between cftr and salinity adaption.
The nkcc1 gene is considered to be the secretory isoform with two copies (nkcc1a and nkcc1b). Indeed, nkcc1a is the main expressed isoform in gill of many teleost species including Anguilla Anguilla (Cutler and Cramb, 2002), Oreochromis mossambicus (Hiroi et al., 2008), Oryzias dancena (Kang et al., 2010). Similarly, high level of nkcc1a mRNA was detected in gill of L. maculatus in this study. There was obviously increasing trend of nkcc1a mRNA expression in gill with the increased salinity. Moreover, the expression level of nkcc1a gene in HS was the highest. These results strongly emphasized the important role of nkcc1a in ion/osmotic regulation. In contrast to the gill and kidney, the expression level of nkcc2 gene was the highest in intestine and significantly increased with increasing environmental salinity. The same results indicate that the expression of nkcc2 gene in the intestine is significantly increased from fresh water to seawater (Cutler and Cramb, 2008; Tresguerres et al., 2010). Moreover, previous studies also find that the expressions of nkcc genes in intestine are salinitydependent in fish (Kalujnaia et al., 2007; Gregório et al., 2013; Li et al., 2014). All these results indicate that nkcc1a and nkcc2 might play importance roles in ion/ osmotic regulation in gill and intestine.
In summary, the present study firstly reported the existence of cftr, nkcc1a, nkcc1b and nkcc2 genes in L. maculatus, including their identification, sequence analysis, expression profiles in tissues and direct responses to environmental salinity changes in essential osmoregulatory organs including gill, kidney and intestine. Our findings suggest that the expression patterns of cftr, nkcc1a, nkcc1b and nkcc2 genes are tissue-specific. In addition, expression profiles of cftr, nkcc1a and nkcc2 genes after salinity challenges showed that the expressions of cftr and nkcc1a in gill and the mRNA level of nkcc2 in intestine were induced by high salinity, indicating their potential involvement in response to salinity stress.
AcknowledgementsThis work was supported by the China Agriculture Research System (No. CARS-47) and the National Natural Science Foundation of China (No. 31602147). The authors thank Mr. Luoluo Chen and Mr. Zhicheng Chang for their assistance during sample collection.
Aitken, A., 2006. 14-3-3 proteins: A historic overview. Seminars in Cancer Biology, 16: 162-172. DOI:10.1016/j.semcancer.2006.03.005 (  0) 0) |
Anderson, M. P., Sheppard, D. N., Berger, H. A. and Welsh, M. J., 1992. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. American Journal of Physiology-Lung Cellular and Molecular Physiology, 263: L1-L14. DOI:10.1152/ajplung.1992.263.1.L1 (  0) 0) |
Bailey, T. L., Boden, M., Buske, F. A., Frith, M., Grant, C. E., Clementi, L., Ren, J., Li, W. W. and Noble, W. S., 2009. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Research, 37: 202-208. DOI:10.1093/nar/gkp335 (  0) 0) |
Bodinier, C., Boulo, V., Lorin-Nebel, C. and Charmantier, G., 2009. Influence of salinity on the localization and expression of the CFTR chloride channel in the ionocytes of Dicentrarchus labrax during ontogeny. Journal of Anatomy, 214: 318-329. DOI:10.1111/j.1469-7580.2009.01050.x (  0) 0) |
Callebaut, I., Chong, P. A. and Forman-Kay, J. D., 2018. CFTR structure. Journal of Cystic Fibrosis, 17: S5-S8. (  0) 0) |
Callebaut, I., Hoffmann, B., Lehn, P. and Mornon, J. P., 2016. Molecular modelling and molecular dynamics of CFTR. Cellular and Molecular Life Sciences, 74: 3-22. (  0) 0) |
Cant, N., Pollock, N. and Ford, R. C., 2014. CFTR structure and cystic fibrosis. International Journal of Biochemistry and Cell Biology, 52: 15-25. DOI:10.1016/j.biocel.2014.02.004 (  0) 0) |
Cutler, C. P. and Cramb, G., 2002. Two isoforms of the Na+/ K+/2Cl- cotransporter are expressed in the European eel (Anguilla anguilla). Biochimica et Biophysica Acta-Biomembranes, 1566: 92-103. DOI:10.1016/S0005-2736(02)00596-5 (  0) 0) |
Cutler, C. P. and Cramb, G., 2008. Differential expression of absorptive cation-chloride-cotransporters in the intestinal and renal tissues of the European eel (Anguilla anguilla). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 149: 63-73. DOI:10.1016/j.cbpb.2007.08.007 (  0) 0) |
Darriba, D., Taboada, G. L., Doallo, R. and Posada, D., 2011. ProtTest-3: Fast selection of best-fit models of protein evolution. Bioinformatics, 27: 1164-1165. DOI:10.1093/bioinformatics/btr088 (  0) 0) |
Evans, D. H., Piermarini, P. M. and Choe, K., 2005. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiological Reviews, 85: 97-177. DOI:10.1152/physrev.00050.2003 (  0) 0) |
Flemmer, A. W., Monette, M. Y., Djurisic, M., Dowd, B., Darman, R., Gimenez, I. and Forbush, B., 2010. Phosphorylation state of the Na+-K+-Cl- cotransporter (NKCC1) in the gills of Atlantic killifish (Fundulus heteroclitus) during acclimation to water of varying salinity. Journal of Experimental Biology, 213: 1558-1566. DOI:10.1242/jeb.039644 (  0) 0) |
Gadsby, D. C., Vergani, P. and Csanády, L., 2006. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature, 440: 477-483. DOI:10.1038/nature04712 (  0) 0) |
Gamba, G., 2005. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiological Reviews, 85: 423-493. DOI:10.1152/physrev.00011.2004 (  0) 0) |
Gamba, G., Miyanoshita, A., Lombardi, M., Lytton, J., Lee, W. S., Hediger, M. A. and Hebert, S. C., 1994. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)chloride cotransporter family expressed in kidney. Journal of Biological Chemistry, 269: 17713-17722. (  0) 0) |
Goujon, M., McWilliam, H., Li, W., Valentin, F., Squizzato, S., Paern, J. and Lopez, R., 2010. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Research, 38: W695-W699. DOI:10.1093/nar/gkq313 (  0) 0) |
Gregório, S. F., Carvalho, E. S. M., Encarnação, S., Wilson, J. M., Power, D. M., Canário, A. V. M. and Fuentes, J., 2013. Adaptation to different salinities exposes functional specialization in the intestine of the sea bream (Sparus aurata L.). Journal of Experimental Biology, 216: 470-479. DOI:10.1242/jeb.073742 (  0) 0) |
Hiroi, J. and McCormick, S. D., 2007. Variation in salinity tolerance, gill Na+/K+-ATPase, Na+/K+/2Cl- cotransporter and mitochondria-rich cell distribution in three salmonids Salvelinus namaycush, Salvelinus fontinalis and Salmo salar. Journal of Experimental Biology, 210: 1015-1024. DOI:10.1242/jeb.002030 (  0) 0) |
Hiroi, J. and McCormick, S. D., 2012. New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish. Respiratory Physiology and Neurobiology, 184: 257-268. DOI:10.1016/j.resp.2012.07.019 (  0) 0) |
Hiroi, J., Yasumasu, S., McCormick, S. D., Hwang, P. P. and Kaneko, T., 2008. Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. Journal of Experimental Biology, 211: 2584-2599. DOI:10.1242/jeb.018663 (  0) 0) |
Hirose, S., Kaneko, T., Naito, N. and Takei, Y., 2003. Molecular biology of major components of chloride cells. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 136: 593-620. DOI:10.1016/S1096-4959(03)00287-2 (  0) 0) |
Hsu, H. H., Lin, L. Y., Tseng, Y. C., Horng, J. L. and Hwang, P. P., 2014. A new model for fish ion regulation: Identification of ionocytes in freshwaterand seawater-acclimated medaka (Oryzias latipes). Cell and Tissue Research, 357: 225-243. DOI:10.1007/s00441-014-1883-z (  0) 0) |
Hwang, P. P. and Lee, T. H., 2007. New insights into fish ion regulation and mitochondrion-rich cells. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 148: 479-497. DOI:10.1016/j.cbpa.2007.06.416 (  0) 0) |
Hwang, P. P., Lee, T. H. and Lin, L. Y., 2011. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. AJP: Regulatory, Integrative and Comparative Physiology, 301: R28-R47. DOI:10.1152/ajpregu.00047.2011 (  0) 0) |
Ip, Y. K., Wilson, J. M., Loong, A. M., Chen, X. L., Wong, W. P., Delgado, I. L. S., Lam, S. H. and Chew, S. F., 2012. Cystic fibrosis transmembrane conductance regulator in the gills of the climbing perch, Anabas testudineus, is involved in both hypoosmotic regulation during seawater acclimation and active ammonia excretion during ammonia exposure. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 182: 793-812. DOI:10.1007/s00360-012-0664-9 (  0) 0) |
Kalujnaia, S., McWilliam, I. S., Zaguinaiko, V. A., Feilen, A. L., Nicholson, J., Hazon, N., Cutler, C. P., Balment, R. J., Cossins, A. R., Hughes, M. and Cramb, G., 2007. Salinity adaptation and gene profiling analysis in the European eel (Anguilla anguilla) using microarray technology. General and Comparative Endocrinology, 152: 274-280. DOI:10.1016/j.ygcen.2006.12.025 (  0) 0) |
Kang, C. K., Tsai, H. J., Liu, C. C., Lee, T. H. and Hwang, P. P., 2010. Salinity-dependent expression of a Na+, K+, 2Cl- cotransporter in gills of the brackish medaka Oryzias dancena: A molecular correlate for hyposmoregulatory endurance. Comparative Biochemistry and Physiology-A Molecular and Integrative Physiology, 157: 7-18. DOI:10.1016/j.cbpa.2010.05.013 (  0) 0) |
Kumar, S., Stecher, G. and Tamura, K., 2016. MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Molecular Biology and Evolution, 33: 1870-1874. DOI:10.1093/molbev/msw054 (  0) 0) |
Li, Z., Lui, E. Y., Wilson, J. M., Ip, Y. K., Lin, Q., Lam, T. J. and Lam, S. H., 2014. Expression of key ion transporters in the gill and esophageal-gastrointestinal tract of euryhaline mozambique tilapia oreochromis mossambicus acclimated to fresh water, seawater and hypersaline water. PLoS One, 9: e87591. DOI:10.1371/journal.pone.0087591 (  0) 0) |
Liu, F., Zhang, Z., Csanády, L., Gadsby, D. C. and Chen, J., 2017. Molecular structure of the human CFTR ion channel. Cell, 169: 85-95. DOI:10.1016/j.cell.2017.02.024 (  0) 0) |
Liu, R. H. and Meng, J. L., 2003. MapDraw: A microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Hereditas (Beijing), 25: 317-321. (  0) 0) |
Marshall, W. S., Howard, J. A., Cozzi, R. R. and Lynch, E. M., 2002. NaCl and fluid secretion by the intestine of the teleost Fundulus heteroclitus: involvement of CFTR. Journal of Experimental Biology, 205: 745-758. (  0) 0) |
McCormick, S. D., Sundell, K., Björnsson, B. T., Brown, C. L. and Hiroi, J., 2003. Influence of salinity on the localization of Na+/K+-ATPase, Na+/K+/2Cl- cotransporter (NKCC) and CFTR anion channel in chloride cells of the Hawaiian goby (Stenogobius hawaiiensis). The Journal of Experimental Biology, 206: 4575-4583. DOI:10.1242/jeb.00711 (  0) 0) |
Nilsen, T. O., Ebbesson, L. O. E., Madsen, S. S., McCormick, S. D., Andersson, E., Bjornsson, B. T., Prunet, P. and Stefansson, S. O., 2007. Differential expression of gill Na+, K+ATPase alphaand beta-subunits, Na+, K+, 2Cl- cotransporter and CFTR anion channel in juvenile anadromous and landlocked Atlantic salmon Salmo salar. Journal of Experimental Biology, 210: 2885-2896. DOI:10.1242/jeb.002873 (  0) 0) |
Park, J. H., Saier, Jr. and M., H., 1996. Phylogenetic, structural and functional characteristics of the Na-K-Cl cotransporter family. Journal of Membrane Biology, 149: 161-168. DOI:10.1007/s002329900016 (  0) 0) |
Ruhr, I. M., Bodinier, C., Mager, E. M., Esbaugh, A. J., Williams, C., Takei, Y. and Grosell, M., 2014. Guanylin peptides regulate electrolyte and fluid transport in the Gulf toadfish (Opsanus beta) posterior intestine. AJP: Regulatory, Integrative and Comparative Physiology, 307: R1167-R1179. DOI:10.1152/ajpregu.00188.2014 (  0) 0) |
Scott, G. R., Richards, J. G., Forbush, B., Isenring, P. and Schulte, P. M., 2004. Changes in gene expression in gills of the euryhaline killifish Fundulus heteroclitus after abrupt salinity transfer. AJP: Cell Physiology, 287: C300-C309. DOI:10.1152/ajpcell.00054.2004 (  0) 0) |
Shaw, J. R., Sato, J. D., VanderHeide, J., LaCasse, T., Stanton, C. R., Lankowski, A., Stanton, S. E., Chapline, C., Coutermarsh, B., Barnaby, R., Karlson, K. and Stanton, B. A., 2008. The role of SGK and CFTR in acute adaptation to seawater in Fundulus heteroclitus. Cellular Physiology and Biochemistry, 22: 69-78. DOI:10.1159/000149784 (  0) 0) |
Tang, C. H. and Lee, T. H., 2007. The effect of environmental salinity on the protein expression of Na+/K+-ATPase, Na+/K+/ 2Cl- cotransporter, cystic fibrosis transmembrane conductance regulator, anion exchanger 1, and chloride channel 3 in gills of a euryhaline teleost, Tetraodon nigroviri. Comparative Biochemistry and Physiology-A Molecular and Integrative Physiology, 147: 521-528. DOI:10.1016/j.cbpa.2007.01.679 (  0) 0) |
Tordai, H., Leveles, I. and Hegedűs, T., 2017. Molecular dynamics of the cryo-EM CFTR structure. Biochemical and Biophysical Research Communications, 491: 986-993. DOI:10.1016/j.bbrc.2017.07.165 (  0) 0) |
Tresguerres, M., Levin, L. R., Buck, J. and Grosell, M., 2010. Modulation of NaCl absorption by[HCO3-] in the marine teleost intestine is mediated by soluble adenylyl cyclase. AJP: Regulatory, Integrative and Comparative Physiology, 299: R62-R71. DOI:10.1152/ajpregu.00761.2009 (  0) 0) |
Tse, W. K. F., Au, D. W. T. and Wong, C. K. C., 2007. Effect of osmotic shrinkage and hormones on the expression of Na+/H+ exchanger-1, Na+/K+/2Cl- cotransporter and Na+/K+ATPase in gill pavement cells of freshwater adapted Japanese eel, Anguilla japonica. Journal of Experimental Biology, 210: 2113-2120. DOI:10.1242/jeb.004101 (  0) 0) |
Wong, M. K. S., Pipil, S., Kato, A. and Takei, Y., 2016. Duplicated CFTR isoforms in eels diverged in regulatory structures and osmoregulatory functions. Comparative Biochemistry and Physiology-Part A: Molecular and Integrative Physiology, 199: 130-141. DOI:10.1016/j.cbpa.2016.06.018 (  0) 0) |
Yan, B., Wang, Z. H. and Zhao, J. L., 2013. Mechanism of osmoregulatory adaptation in tilapia. Molecular Biology Reports, 40: 925-931. DOI:10.1007/s11033-012-2133-7 (  0) 0) |
Yang, W. K., Chung, C. H., Cheng, H. C., Tang, C. H. and Lee, T. H., 2016. Different expression patterns of renal Na+/K+-ATPase ǁ-isoform-like proteins between tilapia and milkfish following salinity challenges. Comparative Biochemistry and Physiology Part-B: Biochemistry and Molecular Biology, 202: 23-30. DOI:10.1016/j.cbpb.2016.07.008 (  0) 0) |
Yu, C. S., Chen, Y. C., Lu, C. H. and Hwang, J. K., 2006. Prediction of protein subcellular localization. Proteins: Structure, Function, and Bioinformatics, 64: 643-651. DOI:10.1002/prot.21018 (  0) 0) |
Zhang, X. Y., Wen, H. S., Wang, H. L., Ren, Y. Y., Zhao, J. and Li, Y., 2017. RNA-Seq analysis of salinity stress-responsive transcriptome in the liver of spotted sea bass (Lateolabrax maculatus). PLoS One, 12: 1-18. (  0) 0) |
 2019, Vol. 18
2019, Vol. 18


