2) College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China;
3) College of Marine Geosciences, Ocean University of China, Qingdao 266100, China
Long-term power supply for supporting continuous working of marine instruments has not been well-solved. Marine sediment microbial fuel cells (MSMFCs) may provide an alternate solution on ocean floor for in-situ power supply. An MSMFCs, with cathode in overlying aerobic water, anode buried in the anaerobic sediments, and sediment-water interface as the natural proton exchange membrane, is used to harvest electricity from the microbial anaerobic oxidation of sediment organics and reduction of inorganics across a closed circuit with an external loading (Bond et al., 2002).
Marine sediment is abundant with organic matter that can provide nutrients as fuels for bacteria metabolism at the anode of MSMFCs, with CO2, protons, and electrons produced. The cathode reaction often uses dissolved oxygen in sea water as electron acceptors during reduction processes by consumption of electrons which are migrated from anode (Tender et al., 2008). The renewable MSMFCs with mild reaction inside allow a long-term power supply. One application example is that the MS MFCs continuously drive the Temperature Depth instrument (TD 5V) and Conductivity Temperature Depth (CTD 12V) for more than 17 months of continuous operations (Fu et al., 2012, 2018). However, the low output power still limits its further applications on ocean floor.
Currently much effort is put on to increase the output power of microbial fuel cell (MFC), for example, anode modification, structure design (Zhao et al., 2019; Asim et al., 2020), electricity producing cable bacteria and its conducting filamentous nanowires (Liu et al., 2020; Wang et al., 2021), cathode materials (Liu et al., 2018). Among them, anode modifications are used most to enhance the MFCs electricity generation efficiency, by either increasing the electron transfer rate through electron transfer mediator or increasing power density output on an modified anode materials (Chen et al., 2017; Zhang et al., 2018). It is reported that naturally occurring humic acid (HA) can serve as electron shuttles that increases electron transfer rate (Carlos and Garcia, 2003) and it could potentially be utilized to modify the anode to increase power density of the MSMFCs.
Humic acid is relatively less labile fraction of organic matter but is one of the most important components in marine environment. It uses aromatic rings as a skeleton and contains a variety of active functional groups such as carboxyl, phenolic hydroxyl and quinone groups (Yang et al., 2017). On the one hand, the quinone group of humic acid is redox active and can serve as the electron transfer mediator. On the other hand, HA has the chelation characteristic with cationic metal ions, such as Fe and Mn ions (Yang et al., 2017). As Fe oxides has high affinity with the electricity producing bacteria, it can be recognized by the cytochrome in outer membrane of bacteria as a natural electron acceptor for direct extracellular electron transfer (Gaye et al., 2013; Marian et al., 2015). Therefore, both HA and Fe ion may facilitate the electron transfer if they were present at anode of the MSMFCs.
Herein, HA-Fe modified electrode were prepared and utilized as the marine anode of the MSMFCs for the first time to prove that the HA and Fe ion chelation has a synergistic effect to facilitate electron transfer from microbial to anode and obtain a higher power output.
2 Materials and Methods 2.1 Electrode PreparationCarbon felt electrodes and HA solution was prepared for further modification of MSMFCs anodes. Carbon felts (thickness 2 mm, 3 cm length, 3 cm width) were sequentially immersed in acetone and ethanol for 30 min, then rinsed with deionized water several times, and finally dried at 90℃. To prepare blank anodes, the dried carbon felts were immersed in concentrated nitric acid in a water bath at 90℃ for 2 h, followed by deionized water until its pH reached neutral, and finally dried at 90℃. A concentration of HA at 5 g L−1 was prepared by dissolving HA (A.R.) reagent into NaOH solution, adjusting the final pH to 12.
HA modified anodes were obtained by using electrophoretic deposition (Carlos and Garcia, 2003). Specifically, by connecting the pretreated carbon felt as the anode and the platinum plate as the cathode in HA solution, a closed circuit was built under DC voltage of 2.0 V. The electrophoresis was continuously running for 5 min, then anodes were rinsed with deionized water and dried at 90℃ to obtain HA modified anodes. Similar steps were used for making HA-Fe modified anodes whereas the HA-Fe complex dispersion solution was essentially prepared first by dissolving 0.5 g of HA into 100 mL of 0.1 mol L−1 Fe2(SO4)3, adjusting the final pH 6.5 at room temperature, and diluting to 500 mL under ultrasound dispersion.
2.2 MFC ConstructionThe MSMFC was built by using natural marine sediment and seawater fetched from Jiaozhou Bay, Qingdao, China. Specifically, 600 g of marine sediment was put into a 500 mL beaker, with one anode inserted into the sediment, and 200 mL of overlaying seawater, in which a big sized cathode was positioned to keep its ready state. The anode and cathode were connected through a salt bridge with 10 kΩ of external resistance. After standing for about two weeks until the anode potential became stable, the electrochemical performance of MSMFCs was tested. For each type of anode, MSMFCs was built in triplicates.
2.3 Characterization of Modified AnodesThe morphology of the anode surface was characterized by scanning electron microscope (SEM, S-4, 800, Hitachi, Japan). Surface composition of modified anodes was analyzed by Fourier transfer infrared spectrometer (FT-IR, TENSOR27, Bruker, Germany) and these samples were prepared using the KBr tableting method. The contact angle measuring instrument (JC2, 000DM, Shanghai Zhongchen Digital Technology Equipment Co., Ltd.) was used to test the wettability of modified and blank anodes.
2.4 Quantitative Characterization of Microorganisms on AnodeMicroorganisms were concentrated through a microporous membrane by filtering a 50 mL solution, which contained 10 g of the sediment from the anode surface. The number of bacteria on the anode surface were observed and calculated by an upright fluorescence microscope (DM2, 500, Leica, Germany) after 4, 6-diamidino-2-phenylindole (4, 6-diamidino-2-phenylindole (DAPI) staining.
2.5 Electrochemical Performance TestAn electrochemical workstation (CHI-660E, Shanghai Chenhua Instrument Co., Ltd.) was used to measure the cyclic voltammetry (CV) curve, Tafel curve, and electrochemical impedance spectroscopy (EIS). The CV curve is calculated by Eq. (1). The test uses a three-electrode system: the carbon felt anode served as the working electrode, the Pt plate (3 cm × 3 cm Shanghai Yidian Scientific Instrument Co., Ltd.) as the auxiliary electrode, and the saturated calomel electrode (SCE, Shanghai Yidian Scientific Instrument Co., Ltd.) as the reference electrode. The scan interval of CV test is −0.1 to 1.0 V, and the scan speed 1.0 mV s−1. The Tafel scan range is ± 0.2 V of the open-circuit potential and its scan speed 1 mV s−1. The test range of electrochemical impedance spectroscopy is 10 mHz-20 kHz with a 5 mV of amplitude. The power density curve and polarization curve were calculated by continuously adjusting the external resistance value to measure the corresponding current, voltage, and anode potential (vs. SCE). Data analysis was performed in OriginLab 2017 (® Origin, USA).
| $C = S/2v\Delta U, $ | (1) |
where S is the integrated area of CV, ν is the sweep speed, and ΔU the potential scanning range.
3 Results and Discussion 3.1 Characteristic Analysis of Modified Anode Surface 3.1.1 Surface microstructureAs shown in Fig.1, the surface of blank carbon felt is relatively clean and smooth (Fig.1a) whereas the surface of the carbon felt after HA modification is grafted or attached with flakes and particulate matter, suggesting partly agglomeration (Fig.1b). A film-like and fine granular substances are deposited on the surface of the HA-Fe modified anode, and its distribution is relatively uniform (Fig.1c). This indicates that HA and HA-Fe modified anodes were successfully prepared by electrophoretic deposition methods.

|
Fig. 1 SEM images on different electrodes. |
The FT-IR spectra of different electrodes are shown in Fig.2. After nitric acid oxidation, the typical absorption peaks of carboxylic acid (C=O) appeared at about 1720 cm−1 (dotted red line) in both blank and modified carbon felt, but the peak intensity on HA modified carbon felt peak presented the largest. The modified carbon felts all had broad γO-H peaks around 3470 cm−1 (dotted red line), and typical bands of aromatic ring stretching vibrations were observed in the range of 1600–1400 cm−1. However, for HA-Fe modified anode, the intensity of the C=O absorption peak around 1720 cm−1 weakened, which suggested that the HA-Fe complex was formed on the carbon felt surface.
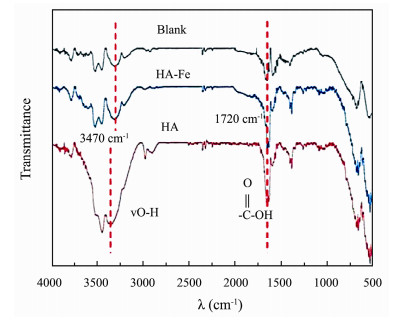
|
Fig. 2 Fourier transfer infrared (FT-IR) spectra of different electrodes. |
The contact angle of the electrode surface is shown in Fig.3 and their values were 119.5˚ (blank) and 78.12˚ (HA-Fe) respectively. The contact angle of HA modified carbon felt was too small to measure. Since the HA surface is rich in a large number of hydrophilic groups such as −OH and −COOH, the contact angle of the modified electrode decreased significantly. When the HA-Fe complex is formed, part of the hydrophilic groups is complexed, and the contact angle is expected smaller compared to blank.

|
Fig. 3 Contact angles of different electrodes. |
The quantitative statistics of microbial on the surface of each anode by fluorescence stain method are shown in Table 1, respectively. The number of microorganisms on the surface of HA-Fe modified anode was 27445 cfu m−2, which were 1.80-folds of blank anode (15304 cfu m−2) and 1.16-folds the HA modified anode (23724 cfu m−2). This indicated that the HA modified anode has enhanced hydro-philicity, or HA itself acts as nutrients for bacteria metabolism, which makes it beneficial for microorganisms to attach and grow, thus resulting the significant increase in the number of microorganisms on modified anode surface.
|
|
Table 1 Amount of microorganisms on different anodic surfaces |
The CV curves are shown in Fig.4. Both HA and HA-Fe modified anodes showed two oxidation peaks. One oxidation peak appeared around 0.70 V in all anodes, indicating that microorganisms attached to the anode surface could catalyze the oxidation of organics in the sediment. The oxidation peak of HA and HA-Fe modified anode at 0.20 V may be caused by the redox reaction of quinone groups in HA [16]. The maximum oxidation peak current density of the HA-Fe modified anode is 0.61 A m−2, which is 6.1 folds of the blank anode, indicating that the HA-Fe modified anode has the highest redox electrochemical activity.
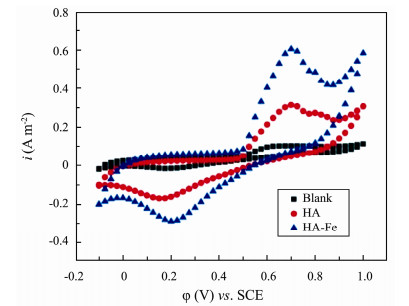
|
Fig. 4 Cyclic voltammetry (CV) curves of different anodes. |
By integrating the CV curves, the capacitance of different anodes was calculated (Table 2). The capacitance characteristics of the modified anode were significantly improved, following the order of CHA-Fe > CHA > CBlank. The capacitances of HA-Fe modified anode are 4.2 times that of blank, indicating the best performance of HA-Fe modified anode.
|
|
Table 2 Parameters of anodic cyclic voltammetry (CV) curves |
From Fig.5, the initial stage current density increased rapidly with the increase of overpotential, and then changed linearly. The blank, HA, and HA-Fe modified anode exchange current densities were 4.8 mA m−2, 21.0 mA m−2, and 62.9 mA m−2, respectively. The exchange current density of HA-Fe modified anode was the largest, which was 13.1 folds and 3.0 folds of blank and HA modified anode, respectively, which indicated that the HA-Fe modified anode had the highest electron transfer kinetic activity (Table 3).
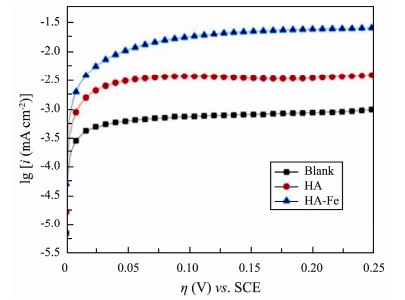
|
Fig. 5 Tafel curves of different anodes. |
|
|
Table 3 Parameters of anodic Tafel curves |
The fitting curves and equivalent circuit of different anode electrochemical impedances are shown in Fig.6 and Table 4. The resistance (Rs) of different anodes differed insignificantly, indicating that the modification of anode by HA and HA-Fe will not increase the internal resistance of the material itself. However, the charge transfer resistance (Rct) of HA and HA-Fe modified anodes were significantly smaller than that of blank anodes, which were 0.33 folds and 0.28-folds of blank anodes, respectively. This was likely attributable to that HA as an electron transfer mediator reduced the anode Rct, leading the anode exchange current density to increase significantly, which was consistent with the Tafel test results (Fig.5).
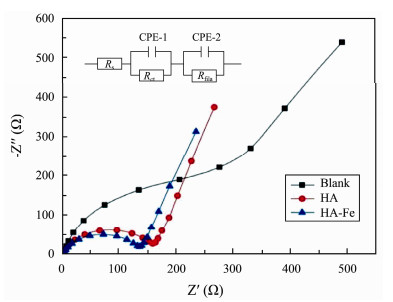
|
Fig. 6 Electrochemical impedance spectra (EIS) fitting curves and the equivalent circuit diagram. |
|
|
Table 4 Parameters of electrochemical impedance spectra (EIS) fitting values |
The modified anode electric double layer capacitor CPE-1 did not change significantly, but the biofilm capacitor CPE-2 increased significantly. It might be attributed to the increase in the number of microorganisms on the surface of the modified anode. The biofilm resistance (Rfilm) of the HA-Fe anode was the largest, due to the largest number of microorganisms attached on its surface, which is consistent with the fluorescence stain results (Table 1).
3.2.4 Polarization Performance AnalysisAs shown in Fig.7, the slope of the polarization curves positively correlates to the anti-polarization ability of the anodes. The larger the slope is, the worse the anti-polarization ability is. Anti-polarization capability of different anodes follows the order of HA-Fe > HA > Blank. When the Blank anode potential is polarized to 0 mV, the HA and HA-Fe modified anode potentials are polarized to −320 mV and −339 mV, respectively, and when the HA-Fe anode potential is polarized to 0 mV, the current is about 0.853 mA, which is about 6-folds of blank electrode. It suggested that the HA-Fe anode had better anti-polarization performance and electrochemical activity, meaning that MSMFCs constructed by the HA-Fe anode would have a higher power density.
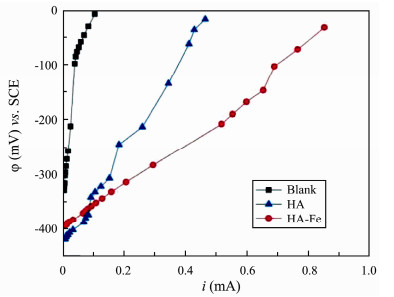
|
Fig. 7 Polarization curves of different anodes. |
As shown in Fig.8, the maximum power densities of MSMFCs constructed with Blank, HA modified, and HA-Fe modified anodes are 25.4 mW m−2, 84.9 mW m−2, and 165.3 mW m−2, respectively, in which the HA-Fe modified cell is 6.5- and 1.95-folds power density of Blank and HA MSMFCs respectively. This suggests that the HA-Fe modified anode significantly improves the output power.
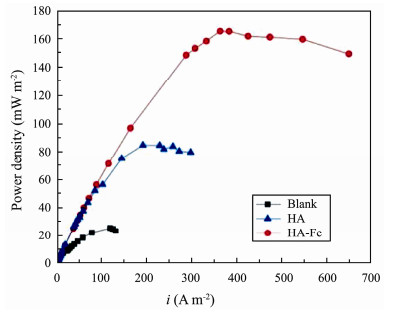
|
Fig. 8 Power density curves of MSMFCs. |
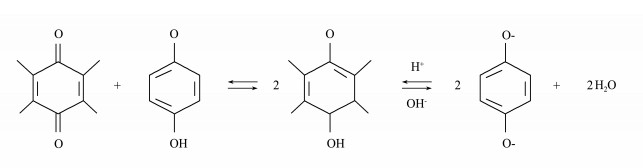
|
Fig. 9 1-quinone and phenols group interchange with each other. |
The higher power of HA-Fe modified MSMFCs was likely attributed to 1) a large number of hydrophilic groups such as –OH and −COOH on HA surface, which facilitated the adhesion of microorganisms (Hao et al., 2017); 2) the quinone group of HA could serve as an electron transfer mediator because quinone-phenol conversion can not only increase the electron transfer rate (Liu et al., 2014. seen in supporting information), but also reduce the charge transfer resistance; 3) the valence conversion between Fe(Ⅱ) and Fe(Ⅲ) occurring as electron transfer shuttle to accelerate electron transfer (Fu et al., 2013). On account of the synergistic effect of HA and Fe, HA-Fe modified anode performed best and its cell had the highest power output.
3.4 DiscussionsMore than 99.9% of dissolved Fe(Ⅲ) in seawater is present as organic complex and its content in sediments is as high as 10.03 mg L−1 (Yang et al., 2017). The active Fe (Ⅲ) as electron acceptors plays a vital role in the organic matter bio-mineralization process in bio-geology evolution (Lu et al., 2011), thus Fe existence on the MSMFCs anode may affect its electrochemical performance in marine sediments. The positive result in this study suggests that we can use the naturally occurring HA chelate the ubiquitous Fe ions in ambient environment on the anode to improve MCMFCs performance. The HA can be used as a nutrient substrate on the electrode surface to promote the adhesion of a variety of bacteria. Meanwhile, the naturally occurring Fe ions and their chelation make the synergy interactions between HA and Fe(Ⅲ) ions, so as to accelerate the electron transfer efficiency and improve the electron transfer kinetics at the anode / biofilm interface.
The HA and Fe synergistic interaction on the MSMFC anode surface in the marine sediment would make the modified anode have a 'renewable' advantage (sustainable supplement of dissolved Fe complex and humic acids from marine sediment and seawater into the natural anode chamber for lasting power), which is conducive to continuously improving the electrochemical stability of the anode and the steady high power output for a long term. Therefore, the high power MSMFCs will have great feasibility by utilizing the HA-Fe modified anode.
4 ConclusionsThe electrochemical performance of the HA-Fe modified anode is better than that of Blank and HA modified anode. The redox activity and anti-polarization ability of the HA-Fe modified anode are 6.1- and 4.2-folds of blank anode, respectively; its exchange current density reaches 62.9 mA m−2, and the relative kinetic activity is 13.1 times of the blank anode; the maximum power density of the constructed MSMFC is 165.3 mA m−2, which is 6.5 times that of the blank MSMFCs. The synergistic effect of HA and Fe greatly improves the electron transfer kinetics and energy harvest of HA-Fe modified anodes. The novel anodic modification design makes the MSMFCs much more suitable to marine sediment environment to develop a higher and longer lasting power source.
AcknowledgementThis work was financially supported by the National Natural Science Foundation of China (No. 22075262).
Asim, A. Y., Mohamad, N. M. I., and Susana, R. C.. 2020. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochemical Engineering Journal, 164: 107779. DOI:10.1016/j.bej.2020.107779 (  0) 0) |
Bond, D. R., Holmes, D. E., Tender, L. M., and Lovley, D. R.. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science, 295: 483-485. DOI:10.1126/science.1066771 (  0) 0) |
Carlos, D., and Garcia, P. I. O.. 2003. Characterization and application of humic acid modified carbon electrodes. Talanta, 61(4): 547-556. DOI:10.1016/S0039-9140(03)00321-7 (  0) 0) |
Chen, W., Liu, Z., Su, G., Fu, Y. B., Zai, X. R., Zhou, C. Y., et al.. 2017. Composite-modified anode by MnO2/polypyrrole in marine benthic microbial fuel cells and its electrochemical performance. International Journal of Energy Research, 41: 845-853. DOI:10.1002/er.3674 (  0) 0) |
Fu, Y. B., Li, J. H., Zhao, Z. K., and Xu, Q.. 2012. Application research of marine sediment microbial fuel cell as power supply to drive small electronic equipment. Periodical of Ocean University of China, 42(6): 93-98 (in Chinese with English abstract). (  0) 0) |
Fu, Y. B., Xu, Q., Zai, X. R., Liu, Y. Y., and Lu, Z. K.. 2013. Low electrical potential anode modified with Fe/ferric oxide and its application in marine benthic microbial fuel cell with higher voltage and power output. Applied Surface Science, 289(2): 472-477. (  0) 0) |
Gaye, F. W., Zhi, S., Liang, S., Wang, Z. M., Dohnalkova, M. J., Marshall, J. K., et al.. 2013. Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(Ⅲ) minreals. Proceedings of the National Academy of Sciences, 110(16): 6346-6351. DOI:10.1073/pnas.1220074110 (  0) 0) |
Hao, X. D., Zhou, P., and Cao, Y. L.. 2017. Origins and evolution processes of humic substances in waste water treatment. Chinese Journal of Environmental Engineeering, 10(1): 1-11. (  0) 0) |
Liu, L. D., Xiao, Y., Wu, Y. C., Chen, B. L., and Zhao, F.. 2014. Electron transfer mediators in microbial electrochemical systems. Progress in Chemistry, 26(11): 1859-1866. (  0) 0) |
Liu, W., Cheng, S., Yin, L., Sun, Y., and Yu, L. L.. 2018. Influence of soluble microbial products on the long-term stability of air cathodes in microbial fuel cells. Electrochimica Acta, 261: 557-564. DOI:10.1016/j.electacta.2017.12.154 (  0) 0) |
Liu, X. M., Gao, H. Y., Joy, E. W., Liu, X. R., Yin, B., Fu, T. D., et al.. 2020. Power generation from ambient humidity using protein nanowires. Nature, 578: 550-554. DOI:10.1038/s41586-020-2010-9 (  0) 0) |
Lu, R. Y., Zhu, M. X., Li, T., Yang, G. P., and Deng, F. F.. 2011. Specification of solid-phase iron in mud sediments collected from the shelf of the East China Sea: Constraints on diagenetic pathways of organic matter, iron, and sulfur. Geochimica, 40(4): 363-371 (in Chinese with English abstract). (  0) 0) |
Marian, B., Kevin, M. R., Osso, J. B., and Julea, N. B.. 2015. Multi-heam cytochromes in Shewanella Oneidensis MR-1: Structures, functions, and opportunities. Journal of the Royal Society Interface, 12(2): 1117-1144. (  0) 0) |
Tender, L. M., Gray, S. A., Groveman, E., Lowy, D. A., Kauffman, P., Melhado, J., et al.. 2008. The first demonstration of a microbial fuel cell as a viable power supply: Powering a meterological buoy. Journal of Power Sources, 179: 571-575. DOI:10.1016/j.jpowsour.2007.12.123 (  0) 0) |
Wang, B., Zhang, H., Yang, Y. G., and Xu, M. Y.. 2021. Diffusion and filamentous bacteria jointly govern the spatiotemporal process of sulfide removal in sediment microbial fuel cells. Chemical Engineering Journal, 405: 126680. DOI:10.1016/j.cej.2020.126680 (  0) 0) |
Yang, R. J., Su, H., Qu, S. L., and Wang, X. C.. 2017. Capacity of humic substances to complex with iron at different salinities in the Yangtze River Estuary and East China Sea. Scientific Reports, 7: 1381-1390. DOI:10.1038/s41598-017-01533-6 (  0) 0) |
Zhang, H. S., Fu, Y. B., Zhou, C. Y., Liu, S., Zhao, M. G., Chen, T. L., et al.. 2018. A novel anode modified by 1, 5-dihydroxyanthraquinone/multiwalled carbon nanotubes composite in marine sediment microbial fuel cell and its electrochemical performance. International Journal of Energy Research, 42: 2574-2582. DOI:10.1002/er.4034 (  0) 0) |
Zhao, Y. G., Ying, M., Fu, Y. B., and Chen, W.. 2019. Improving electrochemical performance of carbon felt anode by modifying with arkagneite in marine sediment microbial fuel cells. Fuel Cells, 19(2): 190-199. DOI:10.1002/fuce.201800112 (  0) 0) |
Zhou, C. Y., Fu, Y. B., Zhang, H. S., Chen, W., Liu, Z., Liu, Z. H., et al.. 2018. Structure design and performance comparison of large-scale marine sediment microbial fuel cells in lab and real sea as power source to drive monitoring instruments for long-term work. Ionics, 24(3): 797-805. DOI:10.1007/s11581-017-2251-2 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


