2) Collaborative Innovation Center for Zhejiang Marine High-Efficiency and Healthy Aquaculture, Ningbo 315832, China
Diabetes mellitus is a metabolic disorder characterized by chronic hyperglycaemia with disturbances in carbohydrate metabolism, involving complications in glycol-lipid metabolism and insulin deficiency or insulin resistance leading to highly elevated glucose levels (Fiorino et al., 2012; Meng et al., 2019). Presently, the pharmacological agents used to treat diabetes mellitus include sulfonylurea, biguanide, and α-glycosidase inhibitors, which have been restricted use due to side effects and failing to significantly alter the course of diabetes (Balamurugan et al., 2014). The high prevalence of diabetes and the severity of diabetes-associated complications has led to an ongoing search for hypoglycaemic agents derived from natural sources.
Cephalopod ink is a great source for decreasing various health problems and could be used widely in both pharmaceuticals and food industries (Hossain et al., 2018). The ink contains melanin, proteins, peptidoglycans, amino acids, lipid, metals, tetrodotoxin (Li et al., 2018). Sepia ink melanin (SIM), a eumelanin extracted from Sepia ink, composed of an indole monomer 5, 6-dihydroxyindole (DHI), and 5, 6-dihydroxyindole-2 carboxylic acid (DHICA), might play an overall protective role against exogenous and endogenous oxidative stressors (Novellino et al., 1998; Borges et al., 2001; Xie et al., 2021). Melanin has also manifested antiinflammatory and anti-tumour properties (Sha et al., 2014), through scavenging free radicals (Chen et al., 2007) and enhancement of immune function (Lei et al., 2012), as demonstrated by the effect of squid ink melanin on regulation of lipid metabolism in hyperlipidaemia mice. Our previous study demonstrated that exposure to sepia melanin enhanced anti-oxidant action and regulated glycosylation end products accumulation in a model of D-galactose induced aging mice (Zhou et al., 2015), suggesting that melanin could affect carbohydrate metabolism.
The liver is an insulin sensitive tissue, which plays a vital role in maintaining energy homeostasis by providing balance to the pathways of gluconeogenesis and glycolysis (Prabakaran and Ashokkumar, 2012). Under the persistent state of hyperglycaemia, the activities of enzymes related to glycolysis and pentose phosphate pathway are impaired, while the activities of enzymes relevant to gluconeogenesis and glycogenolysis are increased, leading to a deterioration of the diabetic condition (Ferre et al., 2003). Moreover, persistent hyperglycaemia increases susceptibility to oxidative stress and can result in increased production of reactive oxygen species (ROS) through glucose autooxidation and non-enzymatic protein glycation, which disturbs enzymes involved in antioxidant defence (Hamed et al., 2018). Furthermore, hyperglycaemia-induced oxidative stress results in the activation of several damaging pathways involved in the pathogenesis of diabetes and related complications (Johansen et al., 2005).
A primary function of the hormone insulin is to decrease blood glucose concentration through the rapid promotion of liver glycogen synthesis and the inhibition of glycogen metabolism. However, deregulation of the insulin signalling pathway members PI3K/Akt can lead to the impaired insulin secretion, reduce glycogen synthesis and enhance glycogen breakdown (Zhang et al., 2020). Therefore, an approach to improve the control of blood glucose level in diabetes can involve in normalizing the activities of PI3K/ Akt in the insulin signalling pathway. This can stimulate or repair remaining pancreatic beta-cells for insulin secretion, which will enhance glycogen synthesis and inhibit gluconeogenesis. Furthermore, this may result in a reduction in the glycosylation end products accumulation suffering from the long-term elevated glucose level and improving diabetes complications resulting from oxidative damage (Chen et al., 2020).
The model of STZ-induced experimental hyperglycaemia has often been used to study the activity of hypoglycaemic agents (Abolfathi et al., 2012). The mechanism by which STZ brings about diabetic state including selective destruction of insulin-producing beta-cells in the pancreatic islets, leading to hyperinsulinemia, decreased glucose levels, and hyperglycaemia (Burns and Gold, 2007). In the present study, the STZ-induced diabetic mice were applied, we examined the effect and mechanisms of SIM on hepatic metabolism enzymes in diabetic mice, including enzymes involved in gluconeogenesis, glycolysis, transduction of the insulin signalling pathway, and anti-oxidized responses.
2 Methods 2.1 Chemicals and ReagentsStreptozotocin was purchased from Sigma-Aldrich (St. Louis, Mo, USA). RT-PCR and qRT-PCR reagents were purchased from Trans Co. (Beijing, China). All other chemicals and solvents were of analytical grade and purchased from Jiancheng Research Institute (Nanjing, China). Blood glucose meters and urine sugar tests were purchased from Qiangsheng Co. (USA).
2.2 Extraction of Sepia Ink MelaninWild sepias (Sepia esculenta) (258.4 g ± 18.6 g per sepia, total 5.3 kg) were purchased from Lulin aquatic products wholesale market (Ningbo, China). The ink melanin extraction method was based on our previous study (Zhou et al., 2015). Briefly, the sepia ink sac was squeezing into a three layer-gauze filter, repeated twice, and centrifuged at 8000 g for 10 min at 4℃, followed by freeze-drying of the melanin crude product. Alkaline protease extraction involved combining of 2% crude melanin and 1.5% alkaline protease at pH 10.3 and followed by holding at 50℃ for 4 – 5 h. The products of enzymolysis were centrifuged 6 times at 6000 g for 10 min at 4℃ to obtain highly purified melanin, the purity of extracted melanin was derived from the sum of DHI and DHICA contents measuring by CE analysis, which is similar to the method reported previously (Ito and Jimbow, 1983). The absorbance at 222.5 nm is used as a relative indicator for determining the SIM content. Detailed methods for the characterization of melanin were provided in the supplementary materials.
2.3 Experimental AnimalsAll experiments in this study were conducted in compliance with the Chinese legislation regarding the use and care of laboratory animals and were approved by the Animal Care and Use Committee of Ningbo University.
Eighty healthy male ICR strain mice were provided by the Zhejiang Province Animal Center, with the Certificate NO. SCXK 2014-0001 (Zhejiang, China). Animals were raised at the animal centre of Ningbo University for four weeks under standard conditions (12 h light and 12 h dark cycle, 25℃ ± 3℃) and were fed a diet of standard mouse chow with water ad lib until body weight reached 20.8 ± 3.6 g.
2.4 Experimental Induction of Diabetes in MiceThe diabetic model mice were induced using STZ as previously described (Wei et al., 2003). Briefly, diabetes was induced in mice that had fasted for 8 h intraperitoneal injection of STZ (40 mg (kg BW)−1), dissolved in freshly prepared citrate buffer (0.1 mol L−1, pH 4.5), for three consecutive days. STZ injected mice were allowed to drink 10% glucose solution to overcome drug-induced hypoglycaemic mortality. Control mice were injected with same volume of citrate buffer alone. After 72 h, plasma glucose levels were measured. Mice with fasting blood glucose more than 11.1 mmol L−1 were considered as diabetes and incorporated into the experimental groups used in this study.
2.5 Experimental DesignMice were randomly divided into 6 groups with 10 mice in each group: NC (Normal group), MG (STZ-induced model group), PG (Positive control group using metformin hydrochloride), LD (MG + Low dose of melanin group), MD (MG + Medium dose of melanin group), HD (MG + High dose of melanin group). Treatment vehicle was 0.9% saline. Single doses of melanin (120, 240, and 480 mg (kg BW)−1) (BW, body weight) and single doses of metformin hydrochloride (240 mg (kg BW)−1) were suspended in 0.9% saline and were administered orally every day for four weeks (Table 1).
|
|
Table 1 Experimental design |
Body weight, food and water intake, and glucose levels were measured weekly during the experiment. Four weeks after treatment, experimental mice were euthanized by decapitation, blood was collected, and serum was separated immediately.
2.6 Oral Glucose Tolerance Test (OGTT)After fasting 8 h in the final treatment cycle, a baseline 0 min blood sample was taken from all mice. Without delay, a glucose solution (2 g (kg BW)−1) was administered orally. Blood samples were taken at 30, 60, 90 and 120 min after glucose administration, respectively. Blood samples were measured with blood glucose meter.
2.7 Biochemical Evaluations 2.7.1 Serum biochemical parametersSerum biochemical parameters, including plasma liver marker enzymes, such as alanine aminotransaminase (ALT), aspartate aminotransferase (AST) activities, alkaline phosphatase (ALP), and lactate dehydrogenase (LD) were detected. Plasma insulin concentration was measured by the automated clinical analyzer (Cobas Integra 400) at the affiliated hospital of Ningbo University.
2.7.2 Estimation of glucokinase, glucose-6-phosphatase, and glycogen in liverGlucokinase and glucose-6-phosphatase were assayed by a human glucokinase ELISA kit (96T) and G6PT ELISA kit, respectively, according to the manufacturer's instructions (Yaji Biochemical Tech. Co., Shanghai, China). Glycogen was measured by the mouse glycogen ELISA (96T) kit according to the manufacturer's instructions (Yaji Biochemical Tech. Co., Shanghai, China).
2.7.3 Determination of key antioxidant enzymes in liverThe mice's liver was removed immediately after the death, and washed in normal saline, and homogenate was prepared in 1.15% (w/v) of potassium chloride. The homogenate was centrifuged at 7000 g for 10 min at 4℃ and supernatant was used for measurement of antioxidant enzymes' activities. Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and the concentration of malondialdehyde (MDA), were measured by the respective analysis kits according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.7.4 Hematoxylin and Eosin (H-E) stainingIsolated liver tissues were preserved in 10% formalin solution and embedded in paraffin. The tissues were sliced into 4 μm sections, dewaxed and rehydrated. Finally, samples were stained with H-E and examined under a standard light microscopy.
2.7.5 Transmission electron microscopy (TEM) analysisLiver tissues were isolated immediately and cut into several blocks of 1 mm3. The tissue blocks were fixed in the fixative solution overnight at 4℃. Following several washes in phosphate-buffered saline (PBS), the tissue blocks were postfixed in 1% (w/v) osmium tetroxide. The tissue blocks were dehydrated with a series of washing with different concentrations of ethanol as follows: 50% (v/v) ethanol for 15 min, 75% (v/v) ethanol for 15 min, 95% (v/v) ethanol for 15 min (twice) and absolute ethanol for 30 min (twice). The tissue blocks were finally dehydrated with absolute acetone for 10 min (twice). The tissue blocks were then embedded with Spur's resin and cured at 60℃ in an oven for 48 h. Ultrathin sections (90 nm thickness) of the tissue blocks were cut using Leica EMUC7 ultrathin slicer and the sections were then collected on a 150-mesh copper grid. The sections on the copper grid were double-stained with uranyl acetate and lead citrate. The ultrathin sections were viewed under H-7650 Transmission Electron Microscope (Hitachi-Science & Technology, Japan).
2.7.6 qRT-PCR analysisThe qRT-PCR experiment was performed as previously reported (Wang et al., 2020). Briefly, total RNA was extracted from frozen liver tissues using TRIZOL reagent (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instructions. Reverse transcription was performed using Trans RT-PCR reagents (Trans Co., Shanghai, China) according to the manufacturer's instructions. SYBR Green PCR Mater Mix reagent kits (Trans Co., Shanghai, China) were used according to the manufacturer's instructions. Primers specific for murine genes were designed for the genes of interest using Primer Express software-Primer5. β-actin was used as a housekeeping gene for normalization. Primer sequences, PCR product length, annealing temperature, and number of cycles are shown in Table 2. The active expression ratio (R) of a target gene was expressed for the sample versus the control in comparison to the β-actin gene. R was calculated based on the following equation: R = 2−∆∆Ct.
|
|
Table 2 The primer sequences, product length, annealing temperature and cycles of genes |
The Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA), version 19.0, was used for statistical analysis. All data were presented as mean ± SD. The data obtained were tested by ANOVA, followed by Duncan's post hoc multiple comparison test. P < 0.05 was considered statistically significant and P < 0.01 was considered highly significant.
3 Results 3.1 Effect of SIM on the Physiological IndexesAll groups were investigated for the physiological indexes, including body weight, food and fluid intake. Results were shown in Table 3. Compared with the control group, the fluid and food intake of diabetic model mice were significantly higher, yet with considerably lower body weight, which were in line with the typical diabetes symptoms. As we can see, the symptoms were improved after four-weeks melanin and metformin hydrochloride treatment (P < 0.05), respectively.
|
|
Table 3 Body weight, food, water intake and urine sugar in STZ-induced diabetic mice before and after treatment with melanin |
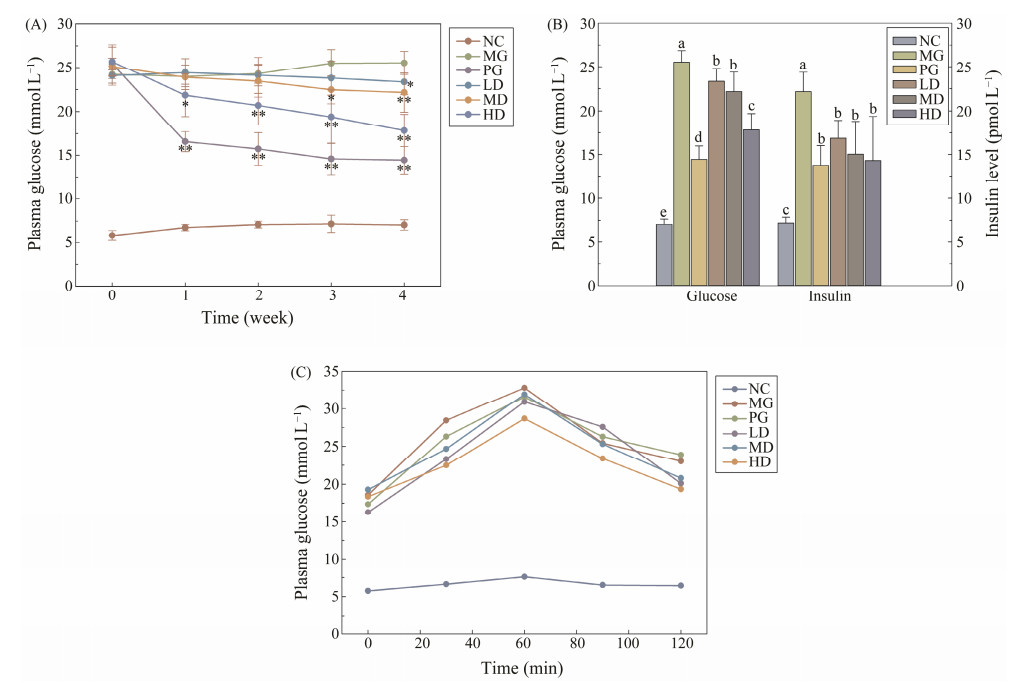
The FBG levels of diabetic mice were remarkably higher than the NC group, after the repeated treatment of lowdose STZ (Fig.1A). However, significant decrease of blood glucose levels were measured in the HD group and PG group after one-week SIM administration. Notably, it seemed that metformin took into effect more quickly than high dosage of SIM, as PG group demonstrated a significant decrease at the first week, while the HD group gradually restored to a comparable level with metformin after four weeks. Moreover, the blood glucose of LD and MD groups were also significantly lower than the MG group, which retained a considerably higher level. Similarly, the plasma insulin level of three SIM treated groups and PG group were markedly lower than the MG group by the end of the experiment (Fig.1B). The OGTT results were shown in Fig.1C, the peaks of all treated groups and NG group appeared at 60 min. Compared with the MG group, the AUC value of PG and SIM treated groups were significantly lower, with a considerably lower peak value. These results demonstrated that SIM exhibited the potency for diabetic treatment.
|
Fig. 1 The blood glucose and plasma insulin levels of all experimental mice. (A) The FBG levels of experimental mice under different repeated treatment in each week. * P < 0.05 vs. Model Group; ** P < 0.01 vs. Model Group; (B) The blood glucose level and plasma insulin level after four-week treatment. For each group, groups not sharing a common superscript letter (a – e) differ significantly at P < 0.05; (C) The OGTT results. Each value is mean ± S.D. for ten mice in each group. |
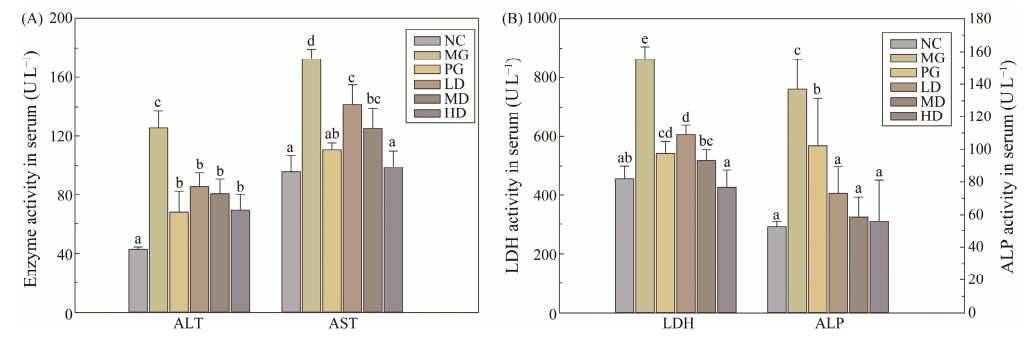
The liver has been confirmed as a sensitive organ to insulin, which can maintain the dynamic stability of glucose metabolism by regulating glycolysis and gluconeogenesis in vivo (Prabakaran and Ashokkumar, 2012). As an essential catalyst in metabolism, transaminase is one of the indices to reflect liver function. As shown in Fig.2A, compared with the NC group, the serum level of AST and ALT were significantly increased (P < 0.05). After four weeks of SIM administration, the AST and ALT levels in all the SIM treated groups were remarkably decreased when compared with the MG group, suggesting that the feeding of SIM could ameliorate the liver damage caused by diabetes.
|
Fig. 2 Effects of SIM on plasma liver enzyme activities in experimental mice. (A) ALT and AST activities; (B) ALP and LDH activities. Each value is mean ± S.D. for ten mice in each group. ALT-alanine aminotransaminase, AST-aspartate aminotransferase, ALP-alkaline phosphatase, LDH-Lactate dehydrogenase. For each measured group, groups not sharing a common superscript letter (a – e) differ significantly at P < 0.05. |
Moreover, the widely distributed ALP in the liver were used as the biomarker to evaluate the obstruction of bile duct. Due to the constantly high sugar environment, cardiovascular disease becomes the most common diabetic complication, then the LDH level was introduced to detect myocardial function. Fig.2B indicated that the ALP and LDH values of the MG group were significantly increased (P < 0.05) compared with the NC group, indicating the bile duct obstruction and cardiovascular damage. However, after four weeks SIM feeding, these indexes in all the SIM treated groups were significantly lower than that in the MG group and restored to the similar level with the NC group.
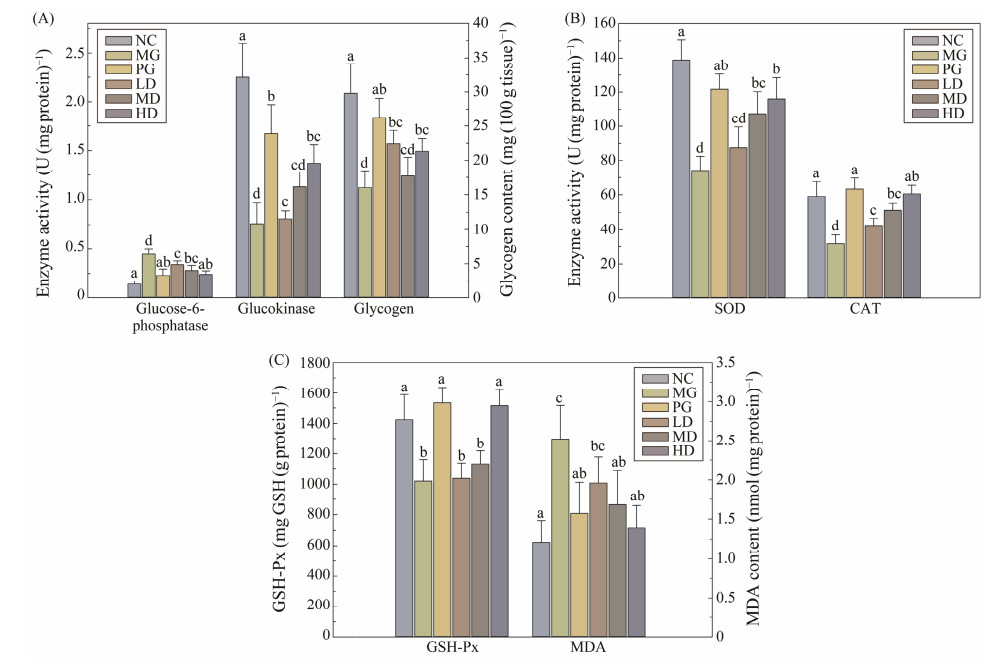
3.4 Effects of SIM on Carbohydrate Metabolism EnzymesGlucose-6-phosphatase and glucokinase are two hepatic glucometabolic related enzymes, which are responsible for the gluconeogenesis and glycolysis, respectively. The activities of glucose-6-phosphatase, glucokinase, and hepatic glycogen content in all groups were determined (Fig.3A). Compared to MG group, the activity of glucose-6-phosphatase was significantly decreased (P < 0.05), while the activity of glucokinase and hepatic glycogen levels were significantly increased (P < 0.05) in SIM treated groups.
|
Fig. 3 Effects of SIM on carbohydrate metabolism enzymes and antioxidant enzymes in experimental mice. (A) Glucose-6-phosphatase and glucokinase activities; (B) SOD and CAT activities; (C) GSH-Px activity and MDA content. For each measured group, groups not sharing a common superscript letter (a – d) differ significantly at P < 0.05. |
As shown in the Fig.3B, the activities of antioxidant enzymes, namely SOD, CAT and GSH-Px, were significantly lower in the MG group than the NC group, while the level of MDA was remarkably higher than that of the NC group. After SIM treatment, activities of all three determined antioxidant enzymes were significantly increased, and all SIM treated groups exhibited similar trends. Meanwhile, the MDA content in the liver was considerably (P < 0.05) decreased in all dosage of SIM feeding groups, compared with the MG group.
3.6 Effects of SIM on mRNA Expression of Genes Regulating Glucose Metabolism Signal PathwaysTo investigate the effect of SIM on the pentose-glucuronate interconversion pathway, we examined the relative expression levels of Dcxr and Ugdh, which were responsible for two vital rate-limiting enzymes in this pathway. As shown in Fig.4A, the expression levels of two genes were significantly up-regulated in three SIM treated groups, compared with the MG group. Meanwhile, SIM seemed to exhibit dose-independent effect, while the increased level sequentially varied from the SIM feeding dosage.

|
Fig. 4 Effects of melanin on the expression of pentose-glucuronate interconversion pathway genes and insulin signal transduction pathway related genes. (A) Expression levels of Dcxr, Ugdh, Akt and Gsk-3β genes; (B) Expression levels of Insr, Irs-2 and PI3K genes. Values are given as mean ± S.D. for ten mice in each group. For each gene, groups with different superscript letters (a – c) differ significantly at P < 0.05 in mRNA expression. |
The expression levels of related genes involved in the insulin signal transduction pathway were also determined, in which PI3K and Akt represented two major enzymes. As shown in Fig.4B, expression level of PI3K increased 0.34-, 1.16-, and 1.61-fold (P < 0.05) in the livers of SIM treated group with the dosage of 120, 240, or 480 mg (kg BW)−1, respectively. With the increased expression of PI3K, the expression of Insr and Irs-2 were improved correspondingly (Fig.4B). Compared with the NC group, the expression level of Akt was significantly decreased in the MG group. After four weeks SIM feeding, the Akt expression level was increased by 0.83 times (P < 0.01) in the HD group, which was restored to the normal level. Meanwhile, the expression level of Akt in MD and LD groups were significantly upregulated, compared with that of MG group. Moreover, under the same treatment, the expression level of Gsk-3β, a downstream gene of insulin signaling pathway, were 27%, 43% and 52% lower in the LD, MD and HD groups, respectively, compared with that of the MG group.
3.7 Histological Observation on Mice Liver TissueTo further explore the potential beneficial effects of SIM on hepatic tissue, as well as verifying the improvement corresponding to the biochemical parameters, we performed the histological observation. As shown in Fig.5A, the liver histology results indicated that the NC group exhibited normal hepatic cell architecture and morphology. However, severe liver damages were observed in the MG group, including fibrosis and cell deformation. After four weeks of SIM treatment, the intercellular space became slightly filled, fibrosis and hepatocytes deformation were alleviated, thus the liver function might be improved.

|
Fig. 5 Effects of SIM on hepatic microstructure and ultrastructure changes of experimental mice observed by optical microscope (A) and electron microscope (B), respectively. NC, normal control group; MG, model samples; HD, model samples with high dosage of SIM treatment. |
As shown in the electron microscope results in Fig.5B, in the MG group, apoptosis of mitochondria and other organelles were observed, the apoptosis products were around the nucleus, and the chromatin accumulated in the nucleus, without observable fat particles. After four weeks of SIM feeding, the nucleus was filled with many clear tubular mitochondria, with the observable endoplasmic reticulum, the nuclear content was clear, and there was no significant difference in nuclear shape between the SIM treated group and the NC group. Therefore, it is suggested that SIM can enhance liver function by preventing nuclear deformation and organelle dissolution.
4 DiscussionDiabetes mellitus is a disease characterized by abnormal carbohydrate metabolism and mainly linked with chronic hyperglycaemia and insensitivity of target organs to insulin (Hamed et al., 2018). Currently available drug regimens for treating and improving type I diabetes mellitus have certain drawbacks, thus it is urgent to find safer and more effective antidiabetic treatments (Grover et al., 2000). In this study, we characterized the role of cuttlefish ink melanin in protecting against the death of pancreatic betacells caused by STZ-induced diabetes. Moreover, treatment with melanin significantly ameliorated STZ-induced alterations, indicating the utility of SIM as an antioxidant. In addition, our results demonstrated that melanin might be able to modulate key hepatic enzymes function involved in carbohydrate metabolism, and improve the insulin signalling pathways in STZ-induced diabetic mice.
STZ-induced diabetic model mice is also characterized by severe dehydration, increased water and food intake, and weight loss (Wei et al., 2003). Body weight loss could be attributed to the protein wasting causing by the unavailability of carbohydrates for energy metabolism (Balamurugan, 2014). Oral administration of SIM improved body weight and decreased food and water intake in diabetic model mice. The result might be ascribed to the improved cellular glucose utilization, which can alleviate the loss of glucose in the urine and mitigate the stimulus for food and water intake. Moreover, SIM feeding effectively prevented the increasing glucose level in the fasting blood and avoided the hyperglycaemic state, possibly resulting from the restoration of damaged pancreatic beta-cells or stimulus for the secretion of insulin from the remaining betacells. Therefore, SIM treatment demonstrates an improvement in glycemic control.
The liver plays an important role in glucose homeostasis by regulating glucose uptake and glucose storage, however, STZ-induced diabetic mice tend to suffer from liver necrosis (Ohaeri, 2001). Activities of the AST, ALT, and ALP enzymes are indicators for liver damage, and the increased activities of AST and ALT indicate hepatic dysfunction caused by diabetes. In this study, increased activities of serum enzymes including AST, ALT, ALP, and LD in STZ-induced diabetic mice may induce the breakdown of protein, which lead to the enhanced amino acid catabolism and provided substrates for gluconeogenesis. Our results demonstrated that oral administration of SIM significantly reduced the activities of these enzymes in diabetic model mice, which could be attributed to the alleviation of liver damage and simultaneous restoration of liver function.
Long-lasting hyperglycaemia is associated with susceptibility to elevated free radical levels, followed by production of reactive oxygen species (ROS), which could lead to increased lipid peroxidation, alter antioxidant defences, and further impair glucose metabolism (Choudhary et al., 2012). Chronic treatment with melanin significantly improved the levels of endogenous antioxidant enzymes (SOD, CAT and GSH-Px) and prevented membrane damage by decreasing lipid peroxidation, which indicated that melanin might demonstrate the antioxidant properties by reducing free radicals (Dong et al., 2016). Recently, melanin has been used as an antioxidant against the inflammatory liver damage, oxidative stress, and lipid peroxidation induced intraperitoneally by gold nanoparticles (GNPs) in vivo, and the results verified the beneficial use of melanin together with GNPs for alleviating its toxicity (Abdelhalim et al., 2018). Moreover, considering their o-diphenolic structures, DHI and DHICA are highly oxidizable, and can provide an efficient barrier against a broad range of cytotoxic oxidants, which are produced during chronic hyperglycaemia (Jiang et al., 2010). Therefore, melanin might serve as an anti-oxidant to prevent the destruction of beta cells from the peroxidation chain reaction and might be helpful in the management and restoration of diabetes.
The liver is the primary site of endogenous glucose production through gluconeogenesis or glycogenolysis, with a minor contribution from the kidneys (Meyer et al., 2004). Glycolysis and gluconeogenesis are the two primary complementary events balancing glucose levels. A partial or total deficiency and over-reliance on insulin cause derangement in carbohydrate metabolism and decrease the activity and amount of several key enzymes, including glucokinase and glucose-6-phosphatase (Choudhary et al., 2012), which play pivotal roles in the deregulation of glucose metabolism leading to imbalance of systemic glucose. In the present study, glucokinase activity decreased in the liver of diabetic mice possibly due to insulin dependence, and treatment with melanin increased insulin secretion, which elevated the activity of glucokinase. Increased glucokinase activity can decrease the glucose level in blood by increasing glucose utilization. On the other hand, decreased activity of glucose-6-phosphatase, a key rate-limiting enzyme in glycolysis, leads to a severe metabolic disorder mainly characterized by hypoglycaemia; its activity is stimulated by cAMP (cyclic adenosine monophosphate) and repressed by insulin (Kurosaki et al., 2003; Soty et al., 2016). Insulin deficiency or insulin dependence in experimental diabetic mice increases glucose-6-phosphatase activity, which in turn increases blood glucose. Here we demonstrated that administration of melanin diminished the activities of glucose-6-phosphatase in STZ-induced diabetic mice. Therefore, we concluded that the reduced glucose-6-phosphatase activity might lead to decreased gluconeogenesis, hence reducing the endogenous glucose production.
Hepatic glycogen deposition is a physiological response to an increase in blood glucose concentration after a meal (Gomis et al., 2003). Glycogen content plays an important role in balancing glucose metabolism and blood glucose stability, and serves as the reflection of insulin activity, as insulin promotes intracellular glycogen deposition by stimulating glycogen synthesis and inhibiting glycogen phosphorylase. Diabetes mellitus impairs the normal capacity of the liver to synthesize glycogen, and glycogen levels are significantly decreased in STZ-induced diabetic mice. Oral administration of melanin for four weeks significantly improved liver glycogen content, indicating an improved insulin activity in the diabetic mice treated with melanin.
Dcxr is a highly conserved and phylogenetically widespread enzyme that converts l-xylulose into xylitol and reduces highly reactive α-dicarbonyl compounds (DCs), thus performing a dual role in carbohydrate metabolism and detoxification in the pentose-glucuronate interconversion pathway. Xylitol is an intermediate carbohydrate, which can be absorbed through cells in the absence of insulin to affect glucose metabolism (Lee et al., 2013). DCs are routinely generated during various normal metabolic reactions, widely reactive and tend to be converted into advanced glycation end-products (AGEs) (Bohlender et al., 2005), which are frequently accumulated in the plasma proteins and tissues of diabetics, and are associated with kidney failure and advanced aging. Dcxr effectively reduces DCs and detoxifies endogenous and xenobiotic carbonyl compounds. Thus, deficiency of the Dcxr gene causes human clinical condition, and low Dcxr activity is implicated in age-related diseases including cancers and diabetes. Here, we demonstrated that SIM treatment could up-regulate the expression level of Dcxr in diabetic mice. After four weeks of melanin administration, the expression levels of Dcxr increased 1.40-fold, 1.62-fold, and 2.45-fold in LD, MD, and HD groups, respectively. Our results are consistent with prior reports that up-regulation of the Dcxr gene can regulate glucose metabolism in diabetic mice in a positive way (Edhager et al., 2018).
Ugdh is another key enzyme in carbohydrate metabolism in the pentose-glucuronate interconversion pathway (Roman et al., 2003). Up-regulation of the Ugdh gene can promote glycolysis and induce galactose metabolism, thereby resulting in a decrease in blood glucose level in diabetic mice. Melanin treatment mediated the pentose-glucuronate interconversion pathway and resulted in a 1.06-fold, 1.16-fold, and 2.16-fold increase in Ugdh expression in LD, MD, and HD groups, respectively, compared to the MG group. Therefore, we speculated that the reduction in blood glucose by melanin treatment could be attributed to the increased expression of the Ugdh gene.
Moreover, we further studied the insulin signalling pathway genes PI3K/Akt/Gsk-3β, which mediate liver glycogen synthesis (Wang et al., 2013). Insulin signalling is initiated through activation of insulin receptor (InsR), and excess InsR expression can enhance the sensitivity of insulin target cells and lead to the increased insulin levels. The insulin receptor substrate (IRS), downstream of InsR, is a tyrosine kinase receptor, which is constituted of two isoforms, namely, IRS-1 and IRS-2. IRS-1/2 expression is involved in insulin-mediated glucose metabolism in skeletal muscle and liver. Additionally, IRS is susceptible to tyrosine phosphorylation when insulin is bound to InsR, leading to downstream activation of the PI3K/Akt signal pathway, which can further promote glycogen synthesis. PI3K and Akt are two key protein kinases and play a vital role in activating Gsk-3β in signal transduction pathways (Yu et al., 2011). The activation of Akt might be affected by the upstream PI3K signalling cascade and can mediate downstream inhibitory phosphorylation of Gsk-3β, which is involved in glycogen synthesis. Conversely, when the activity of Akt is inhibited, gene expression level of GSK-3β is elevated, leading to the blockage of glycogen synthesis (Xia et al., 2011). Our data indicated that the expression levels of InsR, IRS-2, PI3K, and Akt in liver were significantly increased in diabetic mice in response to melanin treatment, while gene expression of Gsk-3β was decreased, suggesting that SIM inhibited the GSK-3β by activating and up-regulating the PI3K/Akt insulin signal transduction pathway. This promoted the conversion and storage of glycogen, further ameliorating plasma glucose levels in STZ-induced diabetic mice.
5 ConclusionsIn this study, the protective effects of Sepia melanin ink on STZ-induced diabetic mice and alleviation of liver tissue damage were studied for the first time. Results demonstrated that typical diabetes symptoms, such as excess food and water intake, and severe body weight loss were improved. Biomarkers in the serum and liver were ameliorated, indicating the restored body functioning. Meanwhile, enhanced antioxidant enzyme activities alleviated the oxidant stress damage. Up-regulated transcription factors in PI3K/Akt, as well as pentose-glucuronate interconversion pathways in hepatocytes indicated the positive regulation of SIM in treating diabetes. Moreover, the histological examination demonstrated the repairment of damaged liver cells, which is consistent with the biochemical investigation results. Cumulatively, these data indicated that SIM exhibited beneficial aspects to alleviate diabetes and demonstrated reservoir of heuristic therapeutic value in the future.
AbbreviationsSIM: sepia ink melanin;
Dcxr: dicarbonyl/l-xylulose reductase;
Ugdh: UDP-glucose dehydrogenase;
DHI: 5, 6-di-hydroxyindole;
DHICA: 5, 6-dihydroxyindole-2 carboxylic acid;
ROS: reactive oxygen species;
STZ: streptozotocin;
ALT: alanine aminotransaminase;
AST: aspartate aminotransferase;
ALP: alkaline phosphatase;
LD: lactate dehydrogenase;
SOD: superoxide dismutase;
GSH-Px: glutathione peroxidase;
CAT: catalase;
MDA: malondialdehyde;
TEM: transmission electron microscopy;
FBG: fasting blood glucose;
OGTT: oral glucose tolerance test;
cAMP: cyclic adenosine monophosphate;
DCs: dicarbonyl compounds;
AGEs: advanced glycation end-products.
AcknowledgementsThis research was supported by the Natural Science Foundation of Zhejiang Province (No. LY18C190006) and sponsored by K. C. Wong Magna Fund in Ningbo University.
Abdelhalim, M. A. K., Moussa, S. A. A., Qaid, H. A., and Al-Ayed, M. S., 2018. Effect of melanin on gold nanoparticle-induced hepatotoxicity and lipid peroxidation in rats. International Journal of Nanomedicine, 13: 5207-5213. DOI:10.2147/IJN.S170758 (  0) 0) |
Abolfathi, A. A., Mohajeri, D., Rezaie, A., and Nazeri, M., 2012. Protective effects of green tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats. Evidence-Based Complementary and Alternative Medicine, 2012: 740671. (  0) 0) |
Balamurugan, R., Duraipandiyan, V., and Ignacimuthu, S., 2014. Antidiabetic activity of γ-sitosterol isolated from Lippia nodiflora L. in streptozotocin induced diabetic rats. European Journal of Pharmacology, 30: 410-418. (  0) 0) |
Bohlender, J. M., Franke, S., Stein, G., and Wolf, G., 2005. Advanced glycation end products and the kidney. European Journal of Clinical Investigation, 40: 742-755. (  0) 0) |
Borges, C. R., Roberts, J. C., Wilkins, D. G., and Rollins, D. E., 2001. Relationship of melanin degradation products to actual melanin content: Application to human hair. Analytical Biochemistry, 290: 116-125. DOI:10.1006/abio.2000.4976 (  0) 0) |
Burns, N., and Gold, B., 2007. The effect of 3-methyladenine DNA glycosylase-mediated DNA repair on the induction of toxicity and diabetes by the beta-cell toxicant streptozotocin. Toxicological Science, 95: 391-400. (  0) 0) |
Chen, S. G., Xue, C. H., Xue, Y., Li, Z. J., and Ma, Q., 2007. Studies on the free radical scavenging activities of melanin from squid ink. Chinese Journal of Marine Drugs, 26: 24-27. (  0) 0) |
Chen, T. S., Lai, C. H., Shen, C. Y., Pai, P. Y., Chen, R. J., PadmaViswanadha, V., et al., 2020. Orally administered resveratrol enhances the therapeutic effect of autologous transplanted adipose-derived stem cells on rats with diabetic hepatopathy. Biotechnic & Histochemistry, 95: 37-45. (  0) 0) |
Choudhary, S. K., Chhabra, G., Sharma, D., Vashishta, A., Ohri, S., and Dixit, A., 2012. Comprehensive evaluation of anti-hyperglycemic activity of fractionated Momordica charantia seed extract in alloxan-induced diabetic rats. Evidence-Based Complementary and Alternative Medicine, 2012: 293650. (  0) 0) |
Dong, H., Wang, L. D., Wang, C. L., Song, W. W., Mu, C. K., and Li, R. H., 2016. Immunomodulatory effects of melanin from Sepiella maindroni ink on hypoimmune mice. Journal of Biology, 33: 27-30. (  0) 0) |
Edhager, A. V., Povlsen, J. A., Løfgren, B., Bøtker, H. E., and Palmfeldt, J., 2018. Proteomics of the rat myocardium during development of type 2 diabetes mellitus reveals progressive alterations in major metabolic pathways. Journal of Proteome Research, 17: 2521-2532. DOI:10.1021/acs.jproteome.8b00276 (  0) 0) |
Ferre, T., Riu, E., Franckhauser, S., Agudo, J., and Bosch, F., 2003. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia, 46: 1662-1668. DOI:10.1007/s00125-003-1244-z (  0) 0) |
Fiorino, P., Evangelista, F. S., Santos, F., Motter Magri, F. M., Delorenzi, J. C., Ginoza, M., et al., 2012. The effects of green tea consumption on cardiometabolic alterations induced by experimental diabetes. Experimental Diabetes Research, 2012: 309231. (  0) 0) |
Gomis, R. R., Favre, C., Garcı́a-Rocha, M., Fernández-Novell, J. M., Ferrer, J. C., and Guinovart, J. J., 2003. Glucose 6-phosphate produced by gluconeogenesis and by glucokinase is equally effective in activating hepatic glycogen synthase. Journal of Biological Chemistry, 278: 9740-9746. DOI:10.1074/jbc.M212151200 (  0) 0) |
Grover, J. K., Vats, V., and Rathi, S. S., 2000. Anti-hyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. Journal of Ethnopharmacology, 73: 461-470. DOI:10.1016/S0378-8741(00)00319-6 (  0) 0) |
Hamed, A. E., Elsahar, M., Elwan, N. M., El-Nakeep, S., Naguib, M., Soliman, H. H., et al., 2018. Managing diabetes and liver disease association. Arab Journal of Gastroenterology, 19: 166-179. DOI:10.1016/j.ajg.2018.08.003 (  0) 0) |
Hossain, M. P., Rabeta, M. S., and Husnal, A. T., 2018. Medicinal and therapeutic properties of cephalopod ink: A short review. Food Research, 3: 188-198. DOI:10.26656/fr.2017.3(3).201 (  0) 0) |
Ito, S., and Jimbow, K., 1983. Quantitative analysis of eumelanin and pheomelanin in hair and melanomas. Journal of Investigative Dermatology, 80: 268-272. DOI:10.1111/1523-1747.ep12534616 (  0) 0) |
Jiang, S., Liu, X. M., Dai, X., Zhou, Q., Lei, T. C., Beermann, F., et al., 2010. Regulation of DHICA-mediated antioxidation by dopachrome tautomerase: Implication for skin photoprotection against UVA radiation. Free Radical Biology & Medicine, 48: 1144-1151. (  0) 0) |
Johansen, J. S., Harris, A. K., Rychly, D. J., and Ergul, A., 2005. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovascular Diabetology, 4: 5. DOI:10.1186/1475-2840-4-5 (  0) 0) |
Kurosaki, E., Nakano, R., Momose, K., Shimaya, A., Suzuki, T., Shibasaki, M., et al., 2003. Hypoglycemic agent YM440 suppresses hepatic glucose output via gluconeogenesis by reducing glucose-6-phosphatase activity in obese Zucker rats. European Journal of Pharmacology, 468: 151-158. DOI:10.1016/S0014-2999(03)01670-4 (  0) 0) |
Lee, S. K., Le, T. S., Choi, H. J., and Ahnn, J., 2013. Dicarbonyl/ L-xylulose dehydrogenase (DCXR): The multifunctional pentosuria enzyme. International Journal of Biochemistry & Cell Biology, 45: 2563-2567. (  0) 0) |
Lei, M., Zhao, M., and Liu, Q., 2012. Immunomodulatory effects of sepia melanin on hypoimmune mice. Science and Technology of Food Industry, 33: 397-400. (  0) 0) |
Li, F., Luo, P., and Liu, H., 2018. A potential adjuvant agent of chemotherapy: Sepia ink polysaccharides. Marine Drugs, 16: 106. DOI:10.3390/md16040106 (  0) 0) |
Meng, J. M., Cao, S. Y., Wei, X. L., Gan, R. Y., Wang, Y. F., Cai, S. X., et al., 2019. Effects and mechanisms of tea for the prevention and management of diabetes mellitus and diabetic complications: An updated review. Antioxidants (Basel), 8: 170. DOI:10.3390/antiox8060170 (  0) 0) |
Meyer, C., Woerle, H. J., Dostou, J. M., Welle, S. L., and Gerich, J. E., 2004. Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes. American Journal of Physiology Endocrinology and Metabolism, 287: E1049-1056. DOI:10.1152/ajpendo.00041.2004 (  0) 0) |
Novellino, L., d'Ischia, M., and Prota, G., 1998. Nitric oxide-induced oxidation of 5, 6-dihydroxyindole and 5, 6-dihydroxyindole-2-carboxylic acid under aerobic conditions: Non-enzymatic route to melanin pigments of potential relevance to skin (photo)protection. Biochimica et Biophysica Acta – General Subjects, 1425: 27-35. DOI:10.1016/S0304-4165(98)00060-9 (  0) 0) |
Ohaeri, O. C., 2001. Effect of garlic oil on the levels of various enzymes in the serum and tissue of streptozotocin diabetic rats. Bioence Reports, 21: 19-24. (  0) 0) |
Prabakaran, D., and Ashokkumar, N., 2012. Antihyperglycemic effect of esculetin modulated carbohydrate metabolic enzymes activities in streptozotocin induced diabetic rats. Journal of Functional Foods, 4: 776-783. DOI:10.1016/j.jff.2012.05.005 (  0) 0) |
Roman, E., Roberts, I., Lidholt, K., and Kusche-Gullberg, M., 2003. Overexpression of UDP-glucose dehydrogenase in Escherichia coli results in decreased biosynthesis of K5 polysaccharide. Biochemical Journal, 374: 767-772. DOI:10.1042/bj20030365 (  0) 0) |
Sha, L., Wu, W. H., Wu, H. B., Hou, Y. Y., and Xu, J. F., 2014. Anti-inflammatory effect of the extract from Sepiella maindroni visceral organs. Journal of Shanghai Ocean University, 23: 629-633. (  0) 0) |
Soty, M., Chilloux, J., Delalande, F., Zitoun, C., Bertile, F., Mithieux, G., et al., 2016. Post-translational regulation of the glucose-6-phosphatase complex by cyclic adenosine monophosphate is a crucial determinant of endogenous glucose production and is controlled by the glucose-6-phosphate transporter. Journal of Proteome Research, 15: 1342-1349. DOI:10.1021/acs.jproteome.6b00110 (  0) 0) |
Wang, C. C., Shi, H. H., Xu, J., Yanagita, T., Xue, C. H., Zhang, T. T., et al., 2020. Docosahexaenoic acid-acylated astaxanthin ester exhibits superior performance over non-esterified astaxanthin in preventing behavioral deficits coupled with apoptosis in MPTP-induced mice with Parkinson's disease. Food & Function, 11: 8038-8050. (  0) 0) |
Wang, X., Wang, Z., and Chen, Y., 2013. The functions of PI3K/ AKT signaling pathway in glucose homeostasis. Chinese Bulletin of Life Sciences, 25: 133-139. (  0) 0) |
Wei, M., Ong, L., Smith, M. T., Ross, F. B., Schmid, K., Hoey, A. J., et al., 2003. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung and Circulation, 12: 44-50. DOI:10.1046/j.1444-2892.2003.00160.x (  0) 0) |
Xia, X., Yan, J., Shen, Y., Tang, K., Yin, J., Zhang, Y., et al., 2011. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One, 6: e16556. DOI:10.1371/journal.pone.0016556 (  0) 0) |
Xie, J. W., Li, H. Y., Che, H. X., Dong, X. F., Yang, X. H., and Xie, W. C., 2021. Extraction, physicochemical characterisation, and bioactive properties of ink melanin from cuttlefish (Sepia esculenta). International Journal of Food Science and Technology, 56: 3627-3640. DOI:10.1111/ijfs.14992 (  0) 0) |
Yu, X., Shen, N., Zhang, M. L., and Pan, F. Y., 2011. Egr-1 decreases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice. Embo Journal, 30: 3754-3765. DOI:10.1038/emboj.2011.277 (  0) 0) |
Zhang, H. J., Chen, C., Ding, L., Shi, H. H., Wang, C. C., Xue, C. H., et al., 2020. Sea cucumbers-derived sterol sulfate alleviates insulin resistance and inflammation in high-fat-highfructose diet-induced obese mice. Pharmacological Research, 160: 105191. (  0) 0) |
Zhou, Y. Y., Wang, C. L., Mu, C. K., Li, R. H., and Song, W. W., 2015. Research on the extraction method of the melanin from Sepiella maindroni ink. Journal of Biology, 2: 28-32. (  0) 0) |
Zhou, Y. Y., Wang, L. D., Du, M. F., Wang, C. L., Mu, C. K., Li, R. H., et al., 2015. Antioxidant effects of melanin from Sepiella maindroni on subacute aged model mice. Natural Product Research and Development, 27: 1664-1667. (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


