2) Department of Animal Production, Faculty of Agriculture, Al-Azhar University, Nasr City, Cairo 11884, Egypt;
3) CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
4) School of Life Sciences, East China Normal University, Shanghai 200241, China;
5) Research Institute for Aquaculture No.1, Dinh Bang-Tu Son-Bac Ninh 16352, Vietnam;
6) East China Sea Fishery Research Institute, Chinese Academy of Fishery Sciences, Shanghai 200090, China
Plastic are organic polymer synthesized from fossil materials such as natural gas, oil, and coal, and have been mass-produced since the middle of the last century (Thompson et al., 2009). Up to now, they have been widely used in all aspects of our daily lives. With the global population growing at an average rate of 1.68% and the large consumption of plastic products, the total global production of plastic had reached 368 million tons since its birth (Verla et al., 2019; PlasticsEurope, 2020). Due to the lack of timely recovery, neglect, and contingency, an inevitable consequence was the release of plastic products into the environment (Wagner et al., 2014). Studies have shown that about 10% of the plastic entered the sea and remained there for an extended period because of its persistence and difficulty in degradation (Thompson et al., 2009). Photodegradation, thermal oxidation degradation, hydrolysis, and microbial biodegradation can eventually decompose plastic wastes to generate small plastic particles (Browne et al., 2008; Canesi et al., 2015). Plastic particles created in this way that were smaller than 5 mm in size were called microplastics (Thompson et al., 2009). More importantly, they can be further degraded to nanoscale plastics less than 1 µm (Koelmans et al., 2015; Mattsson et al., 2015). While the amount of nanoplastics in the aquatic environment is unknown, microplastics can be found along coastlines, water columns, sediments, beaches, and even remote areas (Yu et al., 2016; Waller et al., 2017; Jian et al., 2020).
Consequently, microplastics in the ocean pose threats to the aquatic environment and marine life (Guzzetti et al., 2018). The accidental ingestion of microplastics was pervasive, and researches had reported traces of them in marine creatures such as seaweed, zooplankton, shellfish, shrimp, crab, and fish (Browne et al., 2008; Foekema et al., 2013; Sundbæk et al., 2018; Savoca et al., 2019; Hossain et al., 2020). Sjollema et al. (2016) measured the photosynthesis capacity and the development of Dunaliella tertiolecta in nanoscale and microplastic environments. They found that the enhancement of the negative effect was related to reducing of the plastic to the nano-level. In zooplankton, nanoscale polystyrene plastic significantly weakened the viability and reproduction of Daphnia galeata. The latter was characterized by reduced embryos and abnormal development of mortality-prone embryos (Cui et al., 2017). In shellfish, relevant experimental results showed that larger particles may be filtered by the oysters whereas, rather than ingested, but remained in the shell cavity by adhesion (Graham et al., 2019). In grass shrimp, acute exposures to various shapes and sizes increased mortality (Gray and Weinstein, 2017). Like the above results, Murray and Cowie (2011) found that lobsters were incapable of completely passing microplastic. Hence, over time, residual fibers probably became entangled inside the digestive tract, leading to biodegradable failure, causing intestinal obstruction and even mortality (Besseling et al., 2013). In crabs, an investigation reported that microparticles were detected in their gills and that crabs consumed less oxygen after exposure to PS beads (Watts et al., 2016). In one inquiry, the fish that were given food containing microplastic swam and hunted to a slower degree, displaying significant behavior contrasts to the control. There was an interference of the lipid metabolism because of microplastic uptake (Cedervall et al., 2012). Despite the growing concerns about biological consumption of microplastic, few studies had probed interactions between microplastic and zooplankton. The size and extent of microplastics' potential effects on zooplankton are yet not well understood (Desforges et al., 2015).
As an intermediate between micro food chain and traditional food chain, rotifers are one of the most important secondary producers in marine ecosystems and play a pivotal role in species circulation and energy transfer in the aquatic ecosystem (Scheda and Cowell, 1988; Arndt, 1993; Devetter and Seďa, 2006; Xie et al., 2009). On account of feeding on algae, detritus, or other microorganisms with high assimilation efficiency, their rapid population growth rate makes rotifers the primary food source for many fish and aquatic invertebrates (Snell and Janssen, 1995; Ortaz et al., 2006). Due to their high protein content, some species are used as starter feeds and have a good attraction to a variety of economically farmed animals (Yoshimura et al., 1996; Wang and Mai, 2005). Even so, their value is not only in terms of specific economic efficiency, but also in terms of toxicology as a model organism (Yúfera, 2001). Rotifers, usually less than 200 microns, are common zooplankton. Their culture medium volume is small, and they even can be cultured with microliter (Dahms et al., 2011). Therefore, the amplification effect of rotifer on the test substance is conducive to toxicological evaluation. The internal organs of the rotifer are relatively complete, with nervous, digestive, excretory, reproductive, and other organ systems (Wang, 1961). Hence, toxic and harmful substances in water can reflect obviously by the growth and reproduction of invertebrate rotifers (Preston et al., 1999; Radix et al., 2002; Faggio et al., 2018). The resting eggs of the rotifer can be easily stored and hatched at the same time when needed for experiments (Preston and Snell, 2001; Zhang et al., 2016). In this way, the physiological conditions of test materials are consistent, and experimental errors are reduced. Meanwhile, the shorter life cycle simplifies the experiment, dramatically shortens the observation time, and reduces the experimental cost (Sarma and Rao, 1991; Snell and Moffat, 1992; Luna-Andrade et al., 2002; Zhang et al., 2016).
In order to further advance our understanding of the nanoscale and microplastics potential effects on zooplankton, this study aimed to evaluate the effects of different nominal concentrations (0.5, 2, 8, 32 μg mL−1 (Lee et al., 2013)) and sizes (0.08 (Gaspar et al., 2018), 0.5 (Farrell and Nelson, 2013), 6 μm (Deng et al., 2017)) of microplastics on the longevity of rotifer zooplankton, as the influences of microplastics on rotifers were reflected in body type, reproduction, as well as average life span (Besseling et al., 2014). This study, simultaneously, will offer a better understanding of the effects of microplastics on marine organisms (Fig.1).
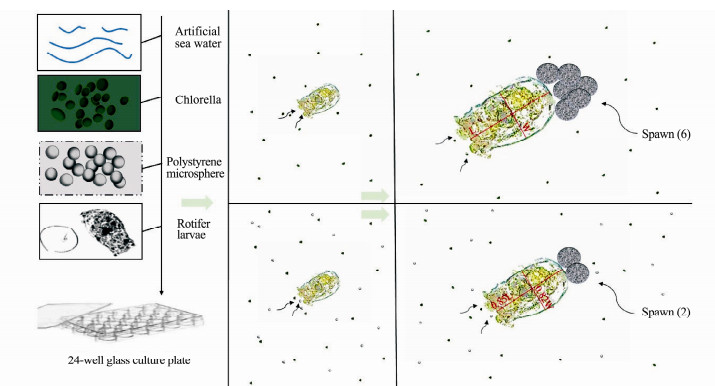
|
Fig. 1 Graphic abstract. |
The rotifer (Brachionus plicatilis) was purchased from Dashiqiao City Xi Lin Aquarium Shop (Liaoning, China). These experimental animals were raised in the laboratory at 25℃ under light: dark 12:12 h photoperiod with a 20‰ concentration of artificial seawater. The microalgae algae Chlorella vulgaris (approximately 1.0 × 106 cells mL−1) were fed daily. Nonfunctionalized polystyrene microspheres with three sizes of 0.08, 0.5, and 6 μm were purchased from BaseLine Chrom Tech Research Centre (Tianjin, China). The shape and size of the experimental microplastics for testing B. plicatilis were observed and measured under a scanning electron microscope (SEM). It was confirmed that the microplastics particles used in the experiment were spherical, with sizes ranging from nanometer to micron (Fig.2).
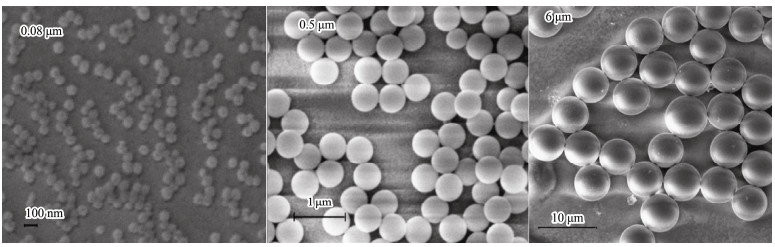
|
Fig. 2 The SEM images showed the shape and size of the nanoscale (0.08 and 0.5 μm) and microscale (6 μm) plastic. |
As concerns about microplastic particles of small size was increasing, we used nanosized (defined here as particles less than 1 µm; 0.08 and 0.5 μm) and micro-sized (6 μm) polystyrene microspheres, as their widespread presence in the ocean (Browne et al., 2008; Canesi et al., 2015; Da Costa et al., 2016). Polystyrene was used since it was the one of the most plentiful high molecular compounds in ocean garbage (Andrady, 2011). To examine the effects of microplastic exposure on B. plicatilis lifehistory parameters, one rotifer larva less than 6 h and 1 mL of artificial seawater containing microalgae C. vulgaris (approximately 1.0 × 106 cells mL−1) were placed into one well in a 24-well culture plate at 25℃ (n = 10 or 12 per replicate). This based on the report that zooplankton can mistake microplastics for food (Desforges et al., 2015). The culture medium was renewed daily. The experimental animals were exposed to a fully crossed design: 1) five nominal concentrations (0, 0.5, 2, 8, 32 µg mL−1) within the range in experiments on the size effects of microplastic particles on marine zooplankton and the corresponding particle numbers were given in Table 1 (Lee et al., 2013); and 2) three sizes (0.08, 0.5 and 6 μm), ranging from those similar in the size of the microalgae C. vulgaris. They were placed in an incubator at 25℃. All exposures were conducted over 20 d.
|
|
Table 1 The relationship between the size, concentration, and number of polystyrene microspheres |
Life-history parameters were counted every 12 h under an optical microscope until the death of each capped female rotifer, including the removal of newborn offspring after each recording. The whole experiment started with newly born rotifers less than 6 h old, randomly selected under an optical microscope. The experiments were incubated at 25℃ under light: dark 12:12 h photoperiod in 4000 LX with a 20‰ concentration of artificial seawater.
2.3 Measurement of Life-History ParametersTo inspect the effects of microplastics on B. plicatilis body sizes, three parameters were measured for each rotifer (n = 10 per replicate) after the first brood under the treatments: 1) the length of the body, 2) the length of the lorica, 3) the width of the lorica.
To measure the effects of microplastics on B. plicatilis life-history parameters, four parameters were measured for each rotifer (n = 12 per replicate) every 12 h until the rotifer died: 1) pre-reproductive period–the time from the start of the experiment to the first spawning, 2) reproductive period–the time required for the rotifers to lay their first eggs until their last laying before death, 3) post-reproductive period–the time from the last spawning to death, 4) oviposition amount–the number of the initial maternal rotifers producing offspring. The average lifespan was calculated from the sum of the pre-reproductive period, reproductive period, and post-reproductive period.
2.4 Statistical AnalysisAll averages were presented with the standard deviation of the mean (± SD). All the statistical analyses were carried out using the statistical software SPSS 26.0. For each measured parament, the difference between the effect of size and nominal concentration was tested using a two-way analysis of variance (ANOVA). When a significant difference was detected, a post-hoc test was carried out. For all analyses, P < 0.05 was considered significant. The results were expressed as the means ± SD. All data were checked for normal distribution and homoscedasticity.
3 Results 3.1 Effect of Microplastics on the Individual Size 3.1.1 Body lengthTwo-way ANOVA based on the growing data after the first brood displayed that 0.08 μm microplastics had a more apparent adverse effect on body length than the other two sizes of microplastics (Fig.3, Table 2). Furthermore, there was a remarkable interaction between microplastics sizes and nominal concentrations on the rotifer body length (P = 0.010) (Fig.3, Table 2). The body length of rotifers (165.6 μm ± 8.3 μm) exposed to 32 μg mL−1 microplastics of 0.08-μm size exerted the most deleterious effects compared to the other nominal concentrations in identical size. Specifically, it was 14% shorter than those exposed to the control (192.8 μm ± 6.6 μm) (Fig.3). Different nominal concentration treatments of 0.5 and 6 μm microplastics had no noticeable effect on body length (Fig.3).
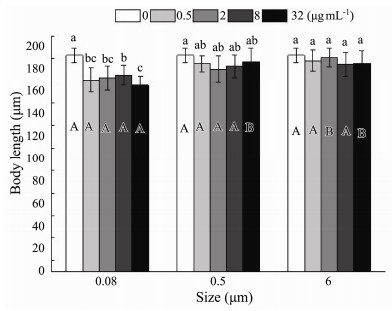
|
Fig. 3 Body length of B. plicatilis exposed to five microplastic concentration and three microplastic size treatments (n = 10 per replicate). Different capital letters denote significant differences among three microplastic size treatments at each microplastic concentration. Different small letters denote significant differences among five microplastic concentration treatments at each microplastic size. The figure was made by Origin Pro 9 software. |
|
|
Table 2 Experimental data |
Both sizes (0.08, 0.5 and 6 μm) (F = 7.541, P = 0.001) and nominal concentrations (0.5, 2, 8, 32 µg mL−1) (F = 6.211, P < 0.001) had evident effects on lorica length, and there was no interaction of microplastic size and nominal concentration (P = 0.076) (Table 2). Lorica length was the longest in control, and microplastics did not exhibit overt size-dependent and nominal concentration-dependent toxicity (Fig.4). What was worth noting was that in 2 µg mL−1 treatment, the inhibitory effect of small size MPs on lorica length was more significant in 0.08 and 0.5 than in 6 μm. In 32 µg mL−1 treatment, microplastics significantly inhibited the rotifers' lorica length for both 0.08 and 6 µm exposure, whereas the lowest suppression was found in 0.5 µm exposure (Fig.4). The lorica length in 0.08 μm under any nominal concentration was distinctly different from the control, whereas there was no difference among disparate nominal concentration treatments of 0.5 and 6 μm microplastics (Fig.4).
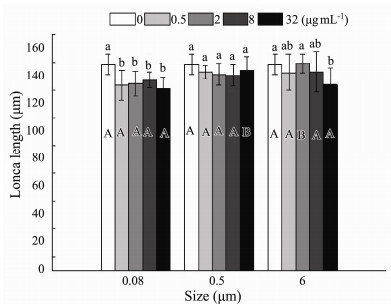
|
Fig. 4 Lorica length of B. plicatilis exposed to five microplastic concentration and three microplastic size treatments for 19 d (n = 12 per replicate). Different capital letters denote significant differences among three microplastic size treatments at each microplastic concentration. Different small letters denote significant differences among five microplastic concentration treatments at each microplastic size. The figure was made by Origin Pro 9 software. |
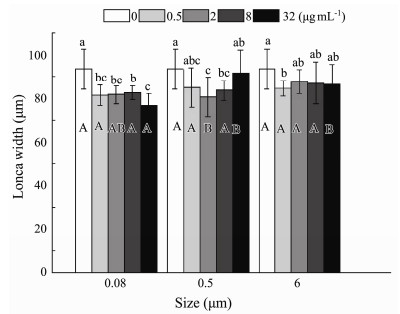
The influence of microplastics on the width of the lorica B. plicatilis was examined under the five different nominal concentrations and three sizes. There was a significant interaction between MP sizes and nominal concentrations (P = 0.048) (Table 2). The average shortened size of lorica width ranged from a tenth to a fifth of the original lorica width, with the shortest observed width recorded in 32 µg mL−1 0.08-μm treatment (Fig.5). Nonsignificant effects were observed in two other microplastic sizes (0.5 and 6 μm), except for noticeable change–only 81% of the controlled width–residing in one example in 0.08 μm at 32 µg mL−1 (Fig.5).
|
Fig. 5 Same as those of Fig. 4 but for lorica width of B. plicatilis. |
Significant effects appeared only at five nominal concentrations (P < 0.001) but were not observed at three sizes (P = 0.050), including their interactions (P = 0.656) (Table 3). Demonstrable time extension was observed in 0.5 µg mL−1 0.5-μm and 8 µg mL−1 0.5-μm treatments ranging from 136.6% of the controlled pre-reproductive period to 154.6% of the controlled pre-reproductive period. Similarly, all sizes at the nominal concentration of 8 µg mL−1 significantly lengthen the pre-reproductive period (Fig.6). In contrast, a non-significant impact on the pre-reproductive period was examined in different nominal concentration treatments of 0.08 and 6 μm plastic beads (Fig.6).
|
|
Table 3 Experimental data |

|
Fig. 6 Same as those of Fig. 4 but for pre-reproductive period of B. plicatilis. |
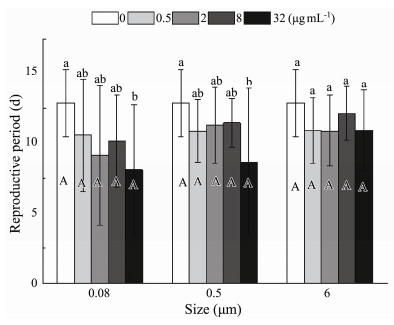
The nominal concentration of MPs, as the only factor, significantly shortened the reproductive period (P < 0.001) (Table 3). In 0.08 μm and 0.5 μm treatments, a significantchange was noticed in the reproductive at the maximum nominal concentration (32 µg mL−1), and non-significant changes were observed at 0.5 µg mL−1, 2 µg mL−1, and 8 µg mL−1 (Fig.7). Interestingly, the time required for the rotifers to lay their first eggs until their last laying before death decreased slightly with increasing MP nominal concentrations ranging from 0.5 to 8 µg mL−1. In 6 μm, the non-significant influence was displayed in all exposure nominal concentrations (Fig.7).
|
Fig. 7 Same as those of Fig. 4 but for reproductive period of B. plicatilis. |
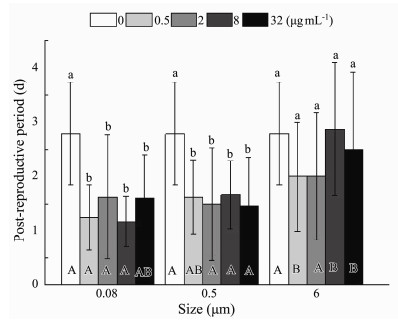
Relevant life history data suggested that there was anoticeable effect of size (P < 0.001) and nominal concentrations (P < 0.001) on the post-reproductive period, the time from the last spawning to death, without observing an interaction between size and nominal concentrations (P < 0.172) (Table 3). The time from the last spawning to death was shortened significantly in the smaller size of 0.08 and 0.5 μm than in 6 μm at total experimental exposure nominal concentrations (Fig.8). In 0.08 and 0.5 μm treatment, the process of death after the last spawning was conspicuously accelerated by different exposure nominal concentrations. However, the non-significant influence was presented at any nominal concentrations in 6 μm treatment (Fig.8).
|
Fig. 8 Same as those of Fig. 4 but for post-reproductive period of B. plicatilis. |
Statistical analysis calculated by the sum of the prereproductive period, reproductive period, and post-reproductive period presented evidence that size (P = 0.008) and nominal concentration (P < 0.001) had a significant effect on average lifespan (Table 3). At 0.5 µg mL−1, the existence of plastic exerted significant deleterious effects, which accelerated the progress towards death (Fig.9). At the highest nominal concentration of 32 µg mL−1, less apparent virulence was only discovered in maximum size. The distinct toxicity presented in 0.08 and 0.5 μm, while similar phenomena were observed in 6 μm with 0.5 and 2 µg mL−1 (Fig.9). In terms of rotifers' average lifespan, there was no interaction between the size and nominal concentration of microplastic (P = 0.437) (Table 3).

|
Fig. 9 Same as those of Fig. 4 but for average lifespan of B. plicatilis. |
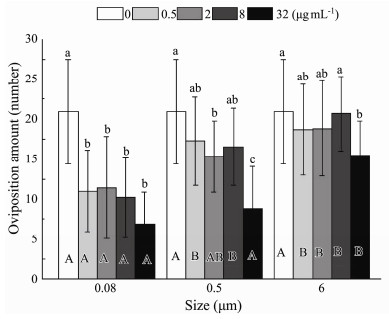
Effects of size (P < 0.001) and nominal concentration (P < 0.001) on oviposition amount were detected (Table 2). Compared to the control, the ability to lay eggs was significantly inhibited at four nominal concentrations with the minimum size (Fig.10). Different nominal concentrations in the other two sizes displayed non-significant prohibitive capability. The only exception was the impact on the number of the initial maternal rotifers producing offspring significantly in 32 µg mL−1 0.5 μm treatment. At four different nominal concentrations, the oviposition amount was different between the experimental and control groups, decreasing with increasing microplastic sizes ranging from 0.08 to 6 μm (Fig.10). The interaction of plastic size and nominal concentration was not associated with decreased oviposition amount (P = 0.068) (Table 2).
|
Fig. 10 Same as those of Fig. 4 but for the number of eggs laid by B. plicatilis. |
Plastic in marine, as a persistent and increasing contaminant, eventually break down into particulate pieces after undergoing solar radiation, biological degradation, or mechanical forces processes, which can inflict damage of individual growth among aquatic organisms, especially in zooplankton after ingestion (Rummel et al., 2017; Sun et al., 2019). Rotifers are filter-feeding zooplankton, and they can ingest MPs within the size of their food particles. The current study aimed to widen our understanding of the effect of MPs on a primary marine food source and a model species for ecotoxicological studies (Rico-Martínez et al., 2016). Here, we examined the responses of the rotifer B. plicatilis exposed to MPs at different sizes and nominal concentrations, which were similar to their feeding prey. The results showed that high nominal concentrations of small nano-plastics could negatively affect the life history parameters of B. plicatilis.
The current study showed that microplastics surrounding rotifers suppressed growth, and this was reflected in rotifers' bodies and lorica. Compared with the control treatment, 32 µg mL−1 0.08 μm treatment showed apparent growth inhibition. Microplastics of high nominal concentration significantly decreased body and lorica size, as measured when the spawn was first hatched, but had non-significant effects in fair-sized treatments. These results are identical to previous studies suggesting toxicity of nanoand micro-plastic act on the physical development of organisms Daphnia magna (Besseling et al., 2014) and Caenorhabditis elegans (Lei et al., 2018), whose body size respectively decreased about 3.1% and 4.89%. Moreover, the lorica length defined the range of particles ingested, which was attributed to a regression line Y = 0.0896X − 0.033 (r = 0.94), which obtained between the lorica length (X) of the B. plicatilis and the maximum size (Y) of particles ingested, allowing B. plicatilis (X = 93.5) to ingest granule with size under the 8.34 μm (Hino and Hirano, 1980). Therefore, the microspheres' sizes used in the current study were within the feeding range of B. plicatilis cultivated. Since filter feeders in the oceanic surroundings cannot successfully digest and absorb consumed microplastics, which may cause the inhibited intestine digestion and insufficient nutrition (Andrady, 2011). A widely applicable theory deciphered the dynamic energy budget framework in which the metabolic energy stored from comestible was used primarily for the primary functions of somatic, structural maintenance of life, and the like (Kooijman, 2001). Therefore, it was likely that energy shortage after microplastic ingestion would reduce the type of body, concretely in body length and lorica size. Insufficient energy for growth also probably arose from a drop in feeding rates due to microplastic ingestion, as found in D. magna (Ogonowski et al., 2016; Rist et al., 2017). Furthermore, inhibition in the body size may be due to the partial incorporation of microplastic into the lorica cuticle, based on the discovery about mussel that some tiny particles of polystyrene microspheres could infiltrate the structure of byssus in singles (Li et al., 2019).
4.2 Effects of Microplastics on Life CycleIn the life cycle parameter determination section, we found that the time from the start of the experiment to the first spawning had been lengthened. In contrast, microplastic exposure shortened the time required for the rotifers to lay their first eggs until their last laying before death, as well as the time from the last spawning to death. Specifically, nano-plastic (0.08 μm) ingested by rotifers postponed the maturity, reduced reproduction, and expedited the death process by about a third. Likewise, during the chronic toxicity of polystyrene, a decline in dry weight and a considerable loss of fecundity was observed in the freshwater benthic invertebrates microspheres (Au et al., 2015). Of greatest concern, however, were the phenomena mentioned in some studies in which short-size plastic microspheres had been found ingested in the cytoplasm by marine organisms, indicating their ability to enter multifarious cells (Browne et al., 2008; Von Moos et al., 2012). What is more, nanoparticles with a size of 0.08 μm in our tests were well-known to interfere with cell metabolism and activate cytotoxicity, and may cause functional interference in specific cells, such as a finding that transfer of diminutive Carboxyl Polystyrene (CPS) latex beads to the insect endoplasmic reticulum impair the catalytic activity of CYP450 enzyme (Fröhlich et al., 2010). Hence, the adverse effects of small size can be interpreted as prolonged chemical reaction time in critical and biochemical reactions, because plastic microspheres can easily diffuse from milieu interieur into cells when the size is reduced. Microplastic may also damage the cytoskeleton and lead cellular deformation, which are fundamental to life. Posteriorly, the ability to filtering algae can be weakened because of the presence of polystyrene beads (Cole et al., 2013). Another research on four freshwater invertebrates showed that the number of 1 and 10 µm particles plastic microspheres ingested by D. magna increased steadily increased with age (Scherer et al., 2017). These observations indicated that several stages of invertebrates development did vary in terms of energy intake. Specifically, the consumption of nanoscale and microplastic increases as the larvae mature. The possible reason may be that it is difficult for the larvae to effectively filter C. vulgaris that provided energy for individual growth and development from the microplastics-rich environment, leading to a delay in the emergence of the adults. More importantly, in other studies, some species had exhibited the act of giving up fecundity, which was thought to be in order to continue to survive in such harsh environmental conditions that they must rationally utilize low energy, resulting in a shortened breeding period (Won and Lee, 2014; Han et al., 2015). Thus, it is interesting to note that it is more important to be aware of the increase in virulence, which was corresponded to the growth of B. plicatilis during pre-reproductive, reproductive and post-reproductive periods, with life-history parameters changing 31.14%, 37.11% and 42.65%, respectively.
4.3 Effects of Microplastics on Reproduction and SpawnOwing to reducing the energy intake and then depriving the breeding time, this brought to a usually worse state, with the cumulative number of neonates significantly reduced on nanoscale by about 70% at higher nominal concentrations treated with control offspring. Similarly, research on the progeniture index of microplastic about pacific oysters suggested a 41% decrease in Dlarval yield born from plastic-treated parents (Sussarellu et al., 2016). However, the middle-large size plastic microspheres displayed a slim impact, only 87.48% of the control group (Canniff and Hoang, 2018). Microplastics tend to gather in the artificial seawater, which cause agglomeration to form, making B. plicatilis less likely to filter them and weaken toxicity. As a final result, including the aforementioned premature death, these poor performances will greatly reduce the natural feed intake of zooplankton, allowing the algae to obtain greater abundance. Therefore, the occurrence frequency of marine red tides may be closely related to the microplastics in seawater. Primary producers and creatures that prey on zooplankton and consumers with higher nutritional levels may also be negatively affected through the food chain (Cedervall et al., 2012; Mattsson et al., 2015). Perhaps in the future, nanoplastic-rich waters will continue to expand, because uncontrollable natural decomposition process will still occur in marine plastic debris, leaving currently damaged marine ecosystems in a state of devastation (Mattsson et al., 2015).
5 ConclusionIn summary, we showed here that individual size at maturity, different breeding periods, average lifetime, and generative yield of B. plicatilis were on a sticky wicket during exposure to polystyrene microspheres, especially the smaller nano-sized plastics. Proceeding from the whole physiological reaction, the behavior of rotifers exposed to 0.08-μm nanoplastic at a nominal concentration of 32 µg mL−1 was greatly poor. At the same time, the larger microspheres slightly altered the rotifers' performance. Another observation was that the toxicity of experimental polystyrene microspheres to life-history parameters was positively correlated with the increase in the nominal concentration of microplastics and the decrease in microplastics size. In addition, the developmental toxicity of microplastics to organisms is of concern. These experimental date complement the understanding of the interactions between microplastic and zooplankton, which are usually hard to observe, and provide a reference for future researchers.
AcknowledgementsThis study was funded by research grants from the Natural Science Foundation of China (Nos. 41706142 and 619360 14), the National Modern Agricultural Industry Technology System Construction Project (No. CARS-49) and the National Key R & D Program during the 13th Five-Year Plan Period (No. 2018YFD900603). Dr. Yanming Sui is supported by a fellowship from China Scholarship Council.
Andrady, A. L.. 2011. Microplastics in the marine environment. Marine Pollution Bulletin, 62(8): 1596-1605. DOI:10.1016/j.marpolbul.2011.05.030 (  0) 0) |
Arndt, H.. 1993. Rotifers as predators on components of the microbial web (bacteria, heterotrophic flagellates, ciliates)-a review. Hydrobiologia, 255(1): 231-246. DOI:10.1007/BF00025844 (  0) 0) |
Au, S. Y., Bruce, T. F., Bridges, W. C., and Klaine, S. J.. 2015. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environmental Toxicology and Chemistry, 34(11): 2564-2572. DOI:10.1002/etc.3093 (  0) 0) |
Besseling, E., Wang, B., Lürling, M., and Koelmans, A. A.. 2014. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environmental Science & Technology, 48(20): 12336-12343. DOI:10.1021/es503001d (  0) 0) |
Besseling, E., Wegner, A., Foekema, E. M., Van Den Heuvel-Greve, M. J., and Koelmans, A. A.. 2013. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environmental Science & Technology, 47(1): 593-600. DOI:10.1021/es302763x (  0) 0) |
Browne, M. A., Dissanayake, A., Galloway, T. S., Lowe, D. M., and Thompson, R. C.. 2008. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environmental Science & Technology, 42(13): 5026-5031. DOI:10.1021/es800249a (  0) 0) |
Canesi, L., Ciacci, C., Bergami, E., Monopoli, M. P., Dawson, K. A., Papa, S., et al.. 2015. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Marine Environmental Research, 111: 34-40. DOI:10.1016/j.marenvres.2015.06.008 (  0) 0) |
Canniff, P. M., and Hoang, T. C.. 2018. Microplastic ingestion by Daphnia magna and its enhancement on algal growth. Science of the Total Environment, 633: 500-507. DOI:10.1016/j.scitotenv.2018.03.176 (  0) 0) |
Cedervall, T., Hansson, L. A., Lard, M., Frohm, B., and Linse, S.. 2012. Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PLoS One, 7(2): e32254-e32254. DOI:10.1371/journal.pone.0032254 (  0) 0) |
Cole, M., Lindeque, P., Fileman, E., Halsband, C., Goodhead, R., Moger, J., et al.. 2013. Microplastic ingestion by zooplankton. Environmental Science & Technology, 47(12): 6646-6655. DOI:10.1021/es400663f (  0) 0) |
Cui, R., Kim, S. W., and An, Y. J.. 2017. Polystyrene nanoplastics inhibit reproduction and induce abnormal embryonic development in the freshwater crustacean Daphnia galeata. Scientific Reports, 7(1): 12095. DOI:10.1038/s41598-017-12299-2 (  0) 0) |
Da Costa, J. P., Santos, P. S. M., Duarte, A. C., and Rocha-Santos, T.. 2016. (Nano)plastics in the environment-sources, fates and effects. Science of the Total Environment, 566-567: 15-26. DOI:10.1016/j.scitotenv.2016.05.041 (  0) 0) |
Dahms, H. U., Hagiwara, A., and Lee, J. S.. 2011. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquatic Toxicology, 101(1): 1-12. DOI:10.1016/j.aquatox.2010.09.006 (  0) 0) |
Deng, Y., Zhang, Y., Lemos, B., and Ren, H.. 2017. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Scientific Reports, 7(1): 46687. DOI:10.1038/srep46687 (  0) 0) |
Desforges, J. P. W., Galbraith, M., and Ross, P. S.. 2015. Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Archives of Environmental Contamination and Toxicology, 69(3): 320-330. DOI:10.1007/s00244-015-0172-5 (  0) 0) |
Devetter, M., and Seďa, J.. 2006. Regulation of rotifer community by predation of Cyclops vicinus (Copepoda) in the Rimov Reservoir in spring. International Review of Hydrobiology, 91(1): 101-112. DOI:10.1002/iroh.200510810 (  0) 0) |
Faggio, C., Tsarpali, V., and Dailianis, S.. 2018. Mussel digestive gland as a model tissue for assessing xenobiotics: An overview. Science of the Total Environment, 636: 220-229. DOI:10.1016/j.scitotenv.2018.04.264 (  0) 0) |
Farrell, P., and Nelson, K.. 2013. Trophic level transfer of microplastic: Mytilus edulis (L. ) to Carcinus maenas (L. ). Environmental Pollution, 177: 1-3. DOI:10.1016/j.envpol.2013.01.046 (  0) 0) |
Foekema, E. M., De Gruijter, C., Mergia, M. T., Van Franeker, J. A., Murk, A. J., and Koelmans, A. A.. 2013. Plastic in North Sea fish. Environmental Science & Technology, 47(15): 8818-8824. DOI:10.1021/es400931b (  0) 0) |
Fröhlich, E., Kueznik, T., Samberger, C., Roblegg, E., Wrighton, C., and Pieber, T. R.. 2010. Size-dependent effects of nanoparticles on the activity of cytochrome P450 isoenzymes. Toxicology and Applied Pharmacology, 242(3): 326-332. DOI:10.1016/j.taap.2009.11.002 (  0) 0) |
Gaspar, T., Chi, R., Parrow, M., and Ringwood, A.. 2018. Cellular bioreactivity of microand nano-plastic particles in oysters. Frontiers in Marine Science, 5: 345. DOI:10.3389/fmars.2018.00345 (  0) 0) |
Geyer, R., Jambeck, J. R., and Law, K. L.. 2017. Production, use, and fate of all plastics ever made. Science Advances, 3(7): e1700782-e1700782. DOI:10.1126/sciadv.1700782 (  0) 0) |
Graham, P., Palazzo, L., Andrea De Lucia, G., Telfer, T. C., Baroli, M., and Carboni, S.. 2019. Microplastics uptake and egestion dynamics in pacific oysters, Magallana gigas (Thunberg, 1793), under controlled conditions. Environmental Pollution, 252: 742-748. DOI:10.1016/j.envpol.2019.06.002 (  0) 0) |
Gray, A. D., and Weinstein, J. E.. 2017. Sizeand shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environmental Toxicology and Chemistry, 36(11): 3074-3080. DOI:10.1002/etc.3881 (  0) 0) |
Guzzetti, E., Sureda, A., Tejada, S., and Faggio, C.. 2018. Microplastic in marine organism: Environmental and toxicological effects. Environmental Toxicology and Pharmacology, 64: 164-171. DOI:10.1016/j.etap.2018.10.009 (  0) 0) |
Han, J., Won, E. J., Lee, M. C., Seo, J. S., Lee, S. J., and Lee, J. S.. 2015. Developmental retardation, reduced fecundity, and modulated expression of the defensome in the intertidal copepod Tigriopus japonicus exposed to BDE-47 and PFOS. Aquatic Toxicology, 165: 136-143. DOI:10.1016/j.aquatox.2015.05.022 (  0) 0) |
Hino, A., and Hirano, R.. 1980. Relationship between body size of the rotifer Brachionus plicatilis and the maximum size of particles ingested. Bulletin of the Japanese Society of Scientific Fisheries, 46(7): 1217-1222. DOI:10.2331/suisan.46.1217 (  0) 0) |
Hossain, M. S., Rahman, M. S., Uddin, M. N., Sharifuzzaman, S. M., Chowdhury, S. R., Sarker, S., et al.. 2020. Microplastic contamination in penaeid shrimp from the northern Bay of Bengal. Chemosphere, 238: 124688. DOI:10.1016/j.chemosphere.2019.124688 (  0) 0) |
Jian, M., Zhang, Y., Yang, W., Zhou, L., Liu, S., and Xu, E. G.. 2020. Occurrence and distribution of microplastics in China's largest freshwater lake system. Chemosphere, 261: 128186. DOI:10.1016/j.chemosphere.2020.128186 (  0) 0) |
Koelmans, A. A., Besseling, E., and Shim, W. J., 2015. Nanoplastics in the aquatic environment. Critical review. In: Marine Anthropogenic Litter. Bergmann, M., and Gutow, L., eds., Springer International Publishing, Cham, 325-340, https://doi.org/10.1007/978-3-319-16510-3_12.
(  0) 0) |
Kooijman, S. A.. 2001. Quantitative aspects of metabolic organization: A discussion of concepts. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences, 356(1407): 331-349. DOI:10.1098/rstb.2000.0771 (  0) 0) |
Lee, K. W., Shim, W. J., Kwon, O. Y., and Kang, J. H.. 2013. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environmental Science & Technology, 47(19): 11278-11283. DOI:10.1021/es401932b (  0) 0) |
Lei, L., Wu, S., Lu, S., Liu, M., Song, Y., Fu, Z., et al.. 2018. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Science of the Total Environment, 619-620: 1-8. DOI:10.1016/j.scitotenv.2017.11.103 (  0) 0) |
Li, Q., Sun, C., Wang, Y., Cai, H., Li, L., Li, J., et al.. 2019. Fusion of microplastics into the Mussel byssus. Environmental Pollution, 252: 420-426. DOI:10.1016/j.envpol.2019.05.093 (  0) 0) |
Luna-Andrade, A., Aguilar-Duran, R., Nandini, S., and Sarma, S. S. S.. 2002. Combined effects of copper and microalgal (Tetraselmis suecica) concentrations on the population growth of Brachionus plicatilis Müller (Rotifera). Water, Air, and Soil Pollution, 141(1): 143-153. DOI:10.1023/A:1021346512560 (  0) 0) |
Mattsson, K., Ekvall, M. T., Hansson, L. A., Linse, S., Malmendal, A., and Cedervall, T.. 2015. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environmental Science & Technology, 49(1): 553-561. DOI:10.1021/es5053655 (  0) 0) |
Mattsson, K., Hansson, L. A., and Cedervall, T.. 2015. Nanoplastics in the aquatic environment. Environmental Science Processes & Impacts, 17(10): 1712-1721. DOI:10.1039/c5em00227c (  0) 0) |
Murray, F., and Cowie, P. R.. 2011. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Marine Pollution Bulletin, 62(6): 1207-1217. DOI:10.1016/j.marpolbul.2011.03.032 (  0) 0) |
Ogonowski, M., Schür, C., Jarsén, Å., and Gorokhova, E.. 2016. The effects of natural and anthropogenic microparticles on individual fitness in Daphnia magna. PLoS One, 11(5): e0155063. DOI:10.1371/journal.pone.0155063 (  0) 0) |
Ortaz, M., González, E., and Peñaherrera, C.. 2006. Depredación de peces sobre el zooplancton entres embalses neotropicales con distintos estados tróficos. Interciencia, 31(7): 517-524. (  0) 0) |
PlasticsEurope, 2020. Plastics–The facts 2020: An analysis of european plastic production, demand and waste data. PlasticsEurope, https://www.plasticseurope.org/en.
(  0) 0) |
Preston, B. L., and Snell, T. W.. 2001. Full life-cycle toxicity assessment using rotifer resting egg production: Implications for ecological risk assessment. Environmental Pollution, 114(3): 399-406. DOI:10.1016/S0269-7491(00)00232-3 (  0) 0) |
Preston, B. L., Snell, T. W., and Kneisel, R.. 1999. UV-B exposure increases acute toxicity of pentachlorophenol and mercury to the rotifer Brachionus calyciflorus. Environmental Pollution, 106(1): 23-31. DOI:10.1016/S0269-7491(99)00065-2 (  0) 0) |
Radix, P., Severin, G., Schramm, K. W., and Kettrup, A.. 2002. Reproduction disturbances of Brachionus calyciflorus (Rotifer) for the screening of environmental endocrine disrupters. Chemosphere, 47(10): 1097-1101. DOI:10.1016/S0045-6535(01)00335-6 (  0) 0) |
Rico-Martínez, R., Arzate-Cárdenas, M. A., Robles-Vargas, D., Pérez-Legaspi, I. A., Jesús, A. F., and Santos-Medrano, G. E., 2016. Rotifers as models in toxicity screening of chemicals and environmental samples. In: Invertebrates–Experimental Models in Toxicity Screening. Larramendy, M. L., and Soloneski, S., eds., IntechOpen, London, 57-99, https://doi.org/10.5772/61771.
(  0) 0) |
Rist, S., Baun, A., and Hartmann, N. B.. 2017. Ingestion of microand nanoplastics in Daphnia magna–quantification of body burdens and assessment of feeding rates and reproduction. Environmental Pollution, 228: 398-407. DOI:10.1016/j.envpol.2017.05.048 (  0) 0) |
Rummel, C. D., Jahnke, A., Gorokhova, E., Kühnel, D., and Schmitt-Jansen, M.. 2017. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environmental Science & Technology Letters, 4(7): 258-267. DOI:10.1021/acs.estlett.7b00164 (  0) 0) |
Sarma, S. S. S., and Rao, T. R.. 1991. The combined effects of food and temperature on the life history parameters of muller Brachionus patulus (Rotifera). Internationale Revue der gesamten Hydrobiologie und Hydrographie, 76(2): 225-239. DOI:10.1002/iroh.19910760207 (  0) 0) |
Savoca, S., Capillo, G., Mancuso, M., Bottari, T., Crupi, R., Branca, C., et al.. 2019. Microplastics occurrence in the Tyrrhenian waters and in the gastrointestinal tract of two congener species of seabreams. Environmental Toxicology and Pharmacology, 67: 35-41. DOI:10.1016/j.etap.2019.01.011 (  0) 0) |
Scheda, S. M., and Cowell, B. C.. 1988. Rotifer grazers and phytoplankton: Seasonal experiments on natural communities. Archiv für Hydrobiologie, 114(1): 31-44. (  0) 0) |
Scherer, C., Brennholt, N., Reifferscheid, G., and Wagner, M.. 2017. Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Scientific Reports, 7(1): 17006. DOI:10.1038/s41598-017-17191-7 (  0) 0) |
Sjollema, S. B., Redondo-Hasselerharm, P., Leslie, H. A., Kraak, M. H. S., and Vethaak, A. D.. 2016. Do plastic particles affect microalgal photosynthesis and growth. Aquatic Toxicology, 170: 259-261. DOI:10.1016/j.aquatox.2015.12.002 (  0) 0) |
Snell, T. W., and Janssen, C. R.. 1995. Rotifers in ecotoxicology: A review. Hydrobiologia, 313(1): 231-247. DOI:10.1007/BF00025956 (  0) 0) |
Snell, T. W., and Moffat, B. D.. 1992. A 2-d life cycle test with the rotifer Brachionus calyciflorus. Environmental Toxicology and Chemistry, 11(9): 1249-1257. DOI:10.1897/1552-8618(1992)11[1249:ADLCTW]2.0.CO;2 (  0) 0) |
Sun, Y., Xu, W., Gu, Q., Chen, Y., Zhou, Q., Zhang, L., et al.. 2019. Small-sized microplastics negatively affect rotifers: Changes in the key life-history traits and rotifer-phaeocystis population dynamics. Environmental Science & Technology, 53(15): 9241-9251. DOI:10.1021/acs.est.9b02893 (  0) 0) |
Sundbæk, K. B., Koch, I. D. W., Villaro, C. G., Rasmussen, N. S., Holdt, S. L., and Hartmann, N. B.. 2018. Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. Journal of Applied Phycology, 30(5): 2923-2927. DOI:10.1007/s10811-018-1472-8 (  0) 0) |
Sussarellu, R., Suquet, M., Thomas, Y., Lambert, C., Fabioux, C., Pernet, M. E. J., et al.. 2016. Oyster reproduction is affected by exposure to polystyrene microplastics. Proceedings of the National Academy of Sciences of the United States of America, 113(9): 2430-2435. DOI:10.1073/pnas.1519019113 (  0) 0) |
Thompson, R. C., Moore, C. J., Saal, F. S. V., and Swan, S. H.. 2009. Plastics, the environment and human health: Current consensus and future trends. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1526): 2153-2166. DOI:10.1098/rstb.2009.0053 (  0) 0) |
Thompson, R. C., Swan, S. H., Moore, C. J., and Vom Saal, F. S.. 2009. Our plastic age. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1526): 1973-1976. DOI:10.1098/rstb.2009.0054 (  0) 0) |
Verla, A. W., Enyoh, C. E., Verla, E. N., and Nwarnorh, K. O.. 2019. Microplastic-toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Applied Sciences, 1(11): 1400. DOI:10.20944/preprints201908.0260.v1 (  0) 0) |
Von Moos, N., Burkhardt-Holm, P., and Köhler, A.. 2012. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environmental Science & Technology, 46(20): 11327-11335. DOI:10.1021/es302332w (  0) 0) |
Wagner, M., Engwall, M., and Hollert, H.. 2014. Editorial: (Micro) plastics and the environment. Environmental Sciences Europe, 26(1): 16. DOI:10.1186/s12302-014-0016-3 (  0) 0) |
Waller, C. L., Griffiths, H. J., Waluda, C. M., Thorpe, S. E., Loaiza, I., Moreno, B., et al.. 2017. Microplastics in the Antarctic marine system: An emerging area of research. Science of the Total Environment, 598: 220-227. DOI:10.1016/j.scitotenv.2017.03.283 (  0) 0) |
Wang, J. J., 1961. Freshwater Rotifers Records of China. Science Press, Beijing, 38-41, http://ir.ihb.ac.cn/handle/342005/10660 (in Chinese).
(  0) 0) |
Wang, X., and Mai, K.. 2005. A successful microbound diet for the larval culture of Chinese shrimp Fenneropenaeus chinensis. Journal of Ocean University of China, 4(3): 267-271. DOI:10.1007/s11802-005-0046-y (  0) 0) |
Watts, A. J. R., Urbina, M. A., Goodhead, R., Moger, J., Lewis, C., and Galloway, T. S.. 2016. Effect of microplastic on the gills of the shore crab Carcinus maenas. Environmental Science & Technology, 50(10): 5364-5369. DOI:10.1021/acs.est.6b01187 (  0) 0) |
Won, E. J., and Lee, J. S.. 2014. Gamma radiation induces growth retardation, impaired egg production, and oxidative stress in the marine copepod Paracyclopina nana. Aquatic Toxicology, 150: 17-26. DOI:10.1016/j.aquatox.2014.02.010 (  0) 0) |
Xie, Z., Xiao, H., Tang, X., and Cai, H.. 2009. Experimental study on the interspecific interactions between the two bloomforming algal species and the rotifer Brachionus plicatilis. Journal of Ocean University of China, 8(2): 203-208. DOI:10.1007/s11802-009-0203-9 (  0) 0) |
Yoshimura, K., Hagiwara, A., Yoshimatsu, T., and Kitajima, C.. 1996. Culture technology of marine rotifers and the implications for intensive culture of marine fish in Japan. Marine and Freshwater Research, 47(2): 217-222. DOI:10.1071/mf9960217 (  0) 0) |
Yu, X., Peng, J., Wang, J., Wang, K., and Bao, S.. 2016. Occurrence of microplastics in the beach sand of the Chinese inner sea: The Bohai Sea. Environmental Pollution, 214: 722-730. DOI:10.1016/j.envpol.2016.04.080 (  0) 0) |
Yúfera, M.. 2001. Studies on Brachionus (Rotifera): An example of interaction between fundamental and applied research. Hydrobiologia, 446(1): 383-392. DOI:10.1007/978-94-010-0756-6_49 (  0) 0) |
Zhang, L., Niu, J., and Wang, Y.. 2016. Full life-cycle toxicity assessment on triclosan using rotifer Brachionus calyciflorus. Ecotoxicology and Environmental Safety, 127: 30-35. DOI:10.1016/j.ecoenv.2015.12.043 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


