2) Key Laboratory of Marine Eco-Environmental Science and Technology, First Institute of Oceanography, Ministry of Natural Resources, Qingdao 266061, China;
3) Qingdao Key Laboratory of Analytical Technology Development and Offshore Eco-Environment Conservation, Qingdao 266061, China;
4) The Yellow River Delta Sustainable Development Institute of Shandong Province, Dongying 257100, China
Per- and polyfluoroalkyl substances (PFASs) are a class of synthetic fluorinated alkane compounds defined as emerging persistent organic pollutants (POPs) that are currently of great concern and intensive research interests (Yu et al., 2024). The characteristics of the molecular structure determine that PFASs are chemically and thermally stable, hydrophobic and oleophobic with high surface energy. Therefore, they are widely used in consumer products and industrial production, such as food packaging, non-stick coatings, flame retardants, papermaking, mist suppressants in the metal plating industry, fire-fighting foams, and textile surface coatings (Renfrew and Pearson, 2021). Due to their high production and widespread usage, PFASs are currently detected in a wide variety of environmental matrices, including water (Yi et al., 2022), soil (Xing et al., 2023), sediment (Zhong et al., 2021), air and atmospheric particulates (Wang et al., 2022b), plants and animals (Liu et al., 2017), and even human blood (Joudan et al., 2020). The stability of PFASs allows them to be persistent and gradually accumulated in the environment once they enter the environmental matrix. PFASs can be bioaccumulated and biomagnified in food webs, exerting a latent threat to ecosystems and human being (Chen et al., 2018; Xie et al., 2024). In vivo and in vitro studies have shown that exposure to PFASs can result in adverse health effects such as hepato, nephron, pneumo, neuro, immuno, reproductive and cardiovascular toxicities, and thyroid disruption (Zeng et al., 2019). Perfluorooctanoic acid (PFOA, C8) and perfluorooctanesulfonic acid (PFOS, C8), listed and regulated under the Stockholm Convention in 2019 and 2009 respectively, are two typical PFASs that belong to the classes of perfluoroalkylcarboxylic acids (PFCAs) and perfluoro alkane sulfonic acids (PFSAs), respectively. Perfluorohexanesulfonic acid (PFHxS), its salts and related chemicals have also been proposed for listing under the Convention (Fiedler et al., 2022).
With the restriction of production and usage of the abo-ve PFASs, some alternative chemicals, such as perfluoro-butanoic acid (PFBA, C4), perfluorobutane sulfonic acid (PFBS, C4), polyfluoroalkyl ether sulfonic acid, fluoro-telomer carboxylic acid, perfluorosulfonamides, and so on, have emerged as emerging PFASs. Hexafluoropropy-lene oxide dimer acid (HFPO-DA) is an alternative to PFOA as a processing aid in the fluoropolymer manufacturing industry, while 6:2 chlorinated polyfluoroalkyl ether sulfonic acid (6:2 Cl-PFESA) is an alternative to PFOS that is commonly used as an acid mist inhibitor in the chrome plating industry in China (He et al., 2022). Previous studies have suggested that HFPO-DA may not be a safe alternative (Wang et al., 2023a), and that Cl-PFESA is more biopersistent than the PFOS (Shi et al., 2016).
Sediment is an important sink for POPs, and the partitioning of PFASs between water and sediment affects their transport and fate in the environment (Zhong et al., 2021). In the Shaying River Basin, total PFASs concentrations ranged from 5.07 to 20.32 ng L−1 in surface water and 6.46 to 20.05 ng g−1 dry weight (dw) in sediment, with the presence of short-chain PFBS being the most prominent (Zhang et al., 2023). The total concentration of PFASs in the sediments of the inflow rivers of Bohai Sea ranged from 0.40 to 61.4 ng g−1 dw, with PFOA as the pre-dominant compound, while 6:2 FTSA and 6:2 Cl-PFESA were also widely detected (Meng et al., 2021). Legacy PFASs such as PFOA, PFOS and PFHxS, and emerging PFASs such as 6:2 Cl-PFESA and PFOSA were detected in surface water samples from two major tributaries (Fen and Wei Rivers) of the Yellow River (Zhou et al., 2019). PFASs have also been widely detected in surface sediments and water samples from the upper, middle and lower reaches of the Yellow River (Pan et al., 2014b; Zhao et al., 2016; Li et al., 2019).
The Yellow River Delta wetland (YRDW) is located along the Bohai Sea, with many tributary rivers in the region, forming a vast and complete estuarine wetland ecosystem, and the 'National Nature Reserve of Yellow River Delta' in Shandong Province was established to protect the local eco-environment. The neighboring cities of the Yellow River Basin (e.g., Zibo, Jinan, etc.) are the home to a number of fluoride plants. During the production of fluoropolymers, PFASs are transferred to the surrounding and transported to the downstream environment through river runoff and atmospheric transport (Feng et al., 2021; Wang et al., 2022a). To the authors' knowledge, there is no systematic report about PFASs in sedimentary environment of the YRDW area until recently. It is therefore of great significance to study the contamination and potential ecological risk of PFASs in sediments of the YRDW.
In this study, 26 targeted PFASs in 47 surface sediments from the YRDW were qualitatively and quantitatively determined with the objectives of 1) investigating the concentration levels, compound compositions, and spatial distribution characteristics of PFASs in the surface sediments of the YRDW, 2) identifying the potential sources of PFASs in the region, and 3) assessing the ecological risk of PFASs in the YRDW. The results will contribute to the understanding of PFAS pollution status in the area and similar wetlands.
2 Materials and Methods 2.1 Chemicals and ReagentsTwenty-six PFASs were selected as target compounds in this study, including 13 PFCAs: PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, PFHxDA, and PFODA; 4 PFSAs: PFBS, PFHxS, PFOS and PFDS; and 9 emerging PFASs: HFPO-DA, FBSA, 6:2 FTCA, 6:2 FTSA, PFOSA, 6:2 Cl-PFESA, 8:2 Cl-PFESA, N-MeFOSAA and N-EtFOSAA. Nine isotopically labeled PFASs were used as internal standards, namely 13C4-PFBA (MPFBA), 13C2-PFHxA (MPFHxA), 18O2-PFHxS (MPFHxS), 13C4-PFOA (MPFOA), 13C5-PFNA (MPFNA), 13C4-PFOS (MPFOS), 13C2-PFNA (PFNA), 13C2-PFUnDA (MPFUnDA), and 13C2-PFDoDA (MPFDoDA). All of these standards were purchased from Wellington Laboratories, Canada. Detailed information on the names, acronyms, classifications, and molecular formulas of the 26 PFASs is provided in Supporting Information (SI) Table S1. PFCAs with 7 or more perfluorinated carbons and PFSAs with 6 or more perfluorinated carbons are defined as long-chain PFASs, and other PFASs are also referred to as long-chain PFASs if the perfluoroalkyl chain has 7 or more carbon atoms (Buck et al., 2011). FBSA, PFOSA, N-MeFOSAA, N-EtFOSAA, 6:2 FTCA and 6:2 FTSA are defined as precursors of PFASs (Chen et al., 2024).
Methanol (LC-MS grade) and formic acid (HPLC grade) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. Ammonia (guaranteed reagent containing 28.0% – 30.0% of NH3) was purchased from Sigma-Aldrich, USA. Ammonium formate (LC-MS grade with purity higher than 99.9%) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Pure water was then prepared using an ultrapure water meter (UNIQUE-R20 laboratory pure water system, Xiamen Ruisijie Scientific Instrument Co., Ltd., China). The 26 PFASs were prepared into a mixed standard solution of 0.5 mg mL−1 for each with methanol as a stock solution and stored at − 4℃, and then diluted stepwise to the required concentration when used. The mixed standard solution of the isotopically labeled internal standards was also diluted with methanol.
2.2 SamplingThe sampling area was located in the Hekou and Kenli districts of Dongying City, Shandong Province (37˚40´ – 38˚10´N, 118˚30´ – 119˚20´E). Forty-seven stations denoted as YH1 – YH14, YR1 – YR9, SX1 – SX14, and GD1 – GD10, were selected (Fig.1) along the banks of the three tributary rivers and the coast of Bohai Sea. Surface sediment samples within the depth of 5 cm were collected in May 2022 with a stainless steel blade and stored in polypropylene zip bags. The samples were kept refrigerated on ice and transported to the laboratory as soon as possible, where they were kept frozen at − 20℃ until analyzed.
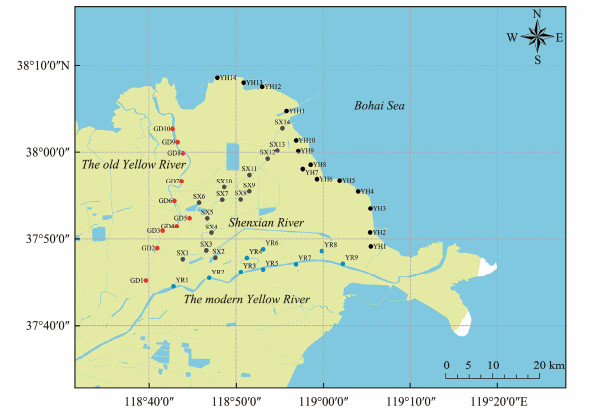
|
Fig. 1 Map of the sampling stations in the YRDW area. |
Sediment samples were processed based on previous studies (Gosetti et al., 2010; Meng et al., 2021) with minor modifications. The samples were lyophilized in a vacuum frozen dryer and then ground in an agate mortar and passed through an 80 mesh sieve. Lyophilized sediment sample (2.00 g) was added to a 50 mL polypropylene centrifuge tube with 1 ng of mixed internal standard added, then vortexed and mixed and left overnight. Methanol of 5 mL was then added to the tube and vortexed for 1 more minute to mix them well. The samples were then ultrasonically extracted for 30 min, centrifuged for 8 min at 9000 r min−1 and the supernatant was transferred to a separate tube. The extraction steps were repeated twice with 5 mL and 3 mL of methanol, respectively. All supernatants were combined, concentrated to dryness with a termovap concentrator under mild nitrogen stream and reconstituted to 500 µL with methanol. The extract was filtrated into a 2 mL sample vial through a 0.22 μm nylon needle filter and stored at − 4℃ until analysis.
PFASs were analyzed by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry (Online SPE-HPLC-MS/MS), consisting of an Agilent 1290 Infinity II high-performance liquid chromatography (HPLC) system coupled with a 6470 LC/TQ triple quadrupole mass spectrometer (Agilent, USA). The online SPE column was Oasis® WAX (Waters, USA), and the analytical column was Infinity Lab Poroshell HPH-C18 (3.0 mm × 30 mm, 1.9 μm, Agilent, USA), with a column temperature of 30℃ and an injecttion volume of 10 μL. More detailed information on instrument parameters and mobile phases can be found in SI Text S1.
A series of standards with concentration range from 0.50 ng mL−1 to 10.00 ng mL−1 for each targeted compound were dispensed with addition of isotopically labelled internal standards (1.00 ng mL−1 for each) and analyzed. Quantification of PFASs was based on an internal standard method, relative to isotopically labelled PFASs. The standard curve for each targeted PFASs showed good linearity with correlation coefficients of > 0.99. PFAS concentrations were expressed on a dry weight (dw) base, unless otherwise noted. Concentrations below the limit of detection (LOD) are reported as N.D. (not detected) and those below the limit of quantification (LOQ) are granted as half of the LOQ. The LOD and LOQ were determined as 3- and 10-fold of the signal-to-noise ratio (S/N), respectively, and are listed in Table S4.
2.4 Determination of Sediment Total Organic Carbon (TOC), Grain Size and pHThe total organic carbon (TOC) of the sediments was determined using an elemental analyzer (vario MACRO cube, Elementar, Germany). The grain size of the sediments was determined using a laser particle size analyzer (Mastersizer 3000, Malver, UK) (Li et al., 2022), and the pH of the sediments was determined using a pen-type pH meter (PH200, Sanliang, Japan).
2.5 Quality Assurance and Quality ControlTo avoid any potential contamination, contact to fluorine-containing materials was avoided during the whole process of sample collection, handling and analysis. Containers used in experiments were rinsed with ultrapure water and methanol prior to use.
To trace potential contamination, a procedural blank was added every 10 samples and followed the same steps for extraction and analysis as the sample analysis. The procedural blank sample was a quartz sand with a mesh size of 25 – 50, baked at 450℃ for 4 h prior to use. All targeted compounds were below the LOD (limit of detection). During the instrumental analysis, a solvent blank (methanol) and a standard sample of known concentration were added every 20 samples to monitor potential contamination and the stability of the instrumental conditions. The concentrations of the targeted compounds in all solvent blanks were below the corresponding detection limits. The average matrix spike drecoveries of 26 PFASs ranged from 61% to 99% and are also listed in Table S4.
2.6 Statistical AnalysisData were compiled for calculation and statistical analysis using Excel and IBM SPSS statistic 25, respectively. Spearman rank correlation analysis was used to test for possible correlations among PFASs in the samples, and data were visualized using OriginPro 2024 (10.1.0.178). Sampling station and spatial distribution maps were created using the ArcMap component of ArcGIS v10.7 (ESRI, Redland, CA) (Wang et al., 2019; Zhong et al., 2021).
2.7 Positive Matrix Factorization (PMF) Model for PFASs Source AnalysisIn addition to the correlation analysis, a positive matrix factorization (PMF) receptor model was employed for source identification of PFASs in surface sediments of the YRDW by using a PMF 5.0 software (USEPA, Environmental Protection Agency of the USA). Briefly, the model factorizes (or decomposes) the component concentration matrix of the samples into a factor contribution matrix (sources' contribution) and a factor spectrum matrix (sources' profile), both of them are non-negatively constrained, as well as a residual matrix. Details about the principle of PMF model is provided in SI Text S2.
2.8 Risk AssessmentThe risk quotient (RQ), defined as the ratio of the measured environmental concentration (MEC) to the predicted no effect concentration (PNEC) of a contaminant, was used to assess the risk of PFAS. PNEC is a toxicity threshold suggested by the EU Technical Guidance Document (TGD) (Leeuwen, 2003) for assessing the environmental risks of existing and new chemicals. PNEC is generally derived using the species sensitivity distribution and assessment factor method. Toxicity testing of compounds is typically performed on organisms in aqueous environments and terrestrial soil matrices. Based on the value of RQ, the risk classification can be categorized as 'unlikely to pose a risk' (RQ < 0.01), 'low risk' (0.01 ≤ RQ < 0.1), 'medium risk' (0.1 ≤ RQ < 1) and 'high risk' (RQ ≥ 1) (Tsui et al., 2014).
Ecotoxicological data on PFAS in sediments are relatively scarce, and PNEC values for PFAS in sediments are less commonly reported. Therefore, we referred to the equilibrium partitioning method (EqP) proposed in the TGD to extrapolate the sediment PNECsediment (μg kg−1) from the PNECwater (μg L−1) of the aquatic environment (Leeuwen, 2003; Zhang et al., 2023). Details about EqP are provided in SI Text S3.
3 Results and Discussion 3.1 Concentrations and Compositional Characteristics of PFASs in Surface Sediments from the YRDWTwenty-three out of 26 targeted PFASs were detected in surface sediment samples from the YRDW, with detection frequencies (DFs) ranging from 2% to 100%. FBSA, 6:2 FTCA and N-MeFOSAA were not detected in any of the 47 samples. The DFs of 6:2 FTSA, PFOSA, N-EtFOSAA, PFDS, 8:2 Cl-PFESA, and PFODA were less than 20%, while those of the remaining 17 PFASs were greater than 50%. The DFs of four short-chain PFCAs (PFBA, PFPeA, PFHxA, and PFHpA) and five long-chain PFCAs (PFOA, PFNA, PFDA, PFUnDA, and PFTeDA) were 100%.
PFASs concentrations are summarized in Table 1, and characterized in Fig.2 along with the composition of PFASs. The total concentration of the 23 detected PFASs (∑23PFASs) in 47 sediment samples ranged from 0.23 to 16.30 ng g−1 dw with an average and a median value of 2.27 and 1.38 ng g−1, respectively. The top five detected PFASs with high concentration levels were PFOA, PFBA, PFOS, PFNA and PFUnDA. PFOA was found to have the highest concentration levels with a range of 0.10 ng g−1 to 10.79 ng g−1. The mean concentration of PFOA was 1.57 ng g−1, which was 1 – 3 orders of magnitude higher than those of the other compounds. PFOA was the largest contributor to the total concentration with an average contribution of 60.8% and more than 80% at some stations. The next largest contributors to the total concentration were PFBA and PFOS, with concentration ranges of 0.03 – 1.08 ng g−1 and N.D. – 2.22 ng g−1, and mean contributions of 10.2% and 5.4%, respectively.
|
|
Table 1 Concentrations (ng g−1 dw) of PFASs in YRDW surface sediments |
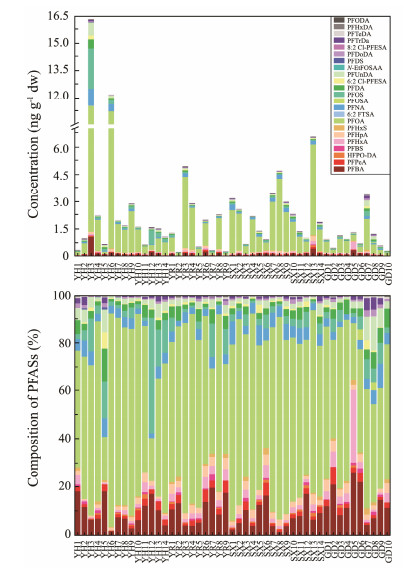
|
Fig. 2 Concentrations and composition of PFASs in the surface sediments of the YRDW. |
The mean concentrations of the 13 PFCAs and the 4 PFSAs were 7.59 ng g−1 and 1.64 ng g−1, dominated by PFOA and PFOS, respectively. The mean concentrations of the 17 legacy PFASs and the 6 emerging PFASs were 6.19 ng g−1 and 0.24 ng g−1, dominated by PFOA and 6:2 Cl-PFESA with mean contributions of 98.6% and 1.4% to the ∑23PFASs, respectively. The average concentrations of the 16 long-chain and 7 short-chain PFASs were 5.81 ng g−1 and 1.95 ng g−1, dominated by PFOA and PFBA, respectively. The average concentration of long-chain PFASs was about three times higher than that of short-chain PFASs, with contributions of 92.0% and 6.6% to the ∑23PFASs, respectively. PFOA and PFOS are two widely used and easily detected PFASs that have been identified in the environment as targets with high concentration levels in several previous studies (Pan et al., 2014a; Meng et al., 2021; Antonopoulou et al., 2024). Due to the restriction on the production and usage of PFOA and PFOS, the production of a number of short-chain PFASs and alternatives has gradually increased, contributed to their frequent detection in a wide range of environmental matrices such as snow, ice, freshwater, seawater, sediments, and soil (Ma et al., 2022; Ahrens et al., 2023; Xie and Kallenborn, 2023). Examples include short-chain PFASs (C4 – C6) as well as HFPO-DA, 6:2 FTSA and 6:2 Cl-PFESA. It is known from the physicochemical properties of PFASs that, in general, the shorter the alkyl chain of a PFAS, the lower is the value of the equilibrium partitioning coefficient (Kd). Therefore, short-chain PFASs tend to be retained in aqueous phase, compared to long-chain PFASs that are more easily bound to particulates and sediments (Gao et al., 2020).
It is therefore speculated that short-chain PFASs, such as PFBA, would also be prevalent in the aquatic phase in the YRDW area. In this study, PFBA was the short-chain PFAS with the highest DF and the highest concentration in sediment. PFBA is mainly used in stain-resistant fabrics, food packaging papers and carpets, as well as in the manufacture of films. It has now been found to be accumulated in crops and detected in household dust, food, drinking water, surface water, ground water, and soil (Ahrens et al., 2023; Zheng et al., 2023; Yuan et al., 2024).
HFPO-DA is one of the common alternatives to PFOA. The concentration of HFPO-DA in YRDW sediment was at an intermediate level, with a DF greater than 50%. The ∑PFOA was 175 times higher than ∑HPFO-DA. It has been shown that exposure of zebrafish to HFPO-DA and HFPO-TA (hexafluoropropylene oxide trimeric acid) induces oxidative damage, inflammation, apoptosis, metabolic disorders, and changes in the intestinal flora (Wang et al., 2023a); and affects the heart rate and motility of zebrafish embryos, altering enzyme activity, and expression of genes related to lipid metabolism (Wang et al., 2023b). Therefore, HFPO-DA may not be a safe alternative to PFOA. Its persistence and environmental hazards are not negligible due to its anti-biodegradability and high adsorption affinity (Harfmann et al., 2021).
Chlorinated polyfluoroalkyl ether sulfonic acids (Cl-PFESAs) are a class of PFOS alternatives used as chrome mist inhibitors in the metal plating industry. The major component of a commercial product, F-53B, is 6:2 Cl-PFESA (Cheng et al., 2023). In this study, 6:2 Cl-PFESA was detected in 89% of the sediment samples with a 10 times higher DF than that of 8:2 Cl-PFESA (DF = 8%). The concentration ranges of these two Cl-PFESA were N.D. – 0.22 ng g−1 and N.D. – 0.17 ng g−1, with mean values of 19.66 pg g−1 and 0.40 pg g−1, respectively. As alternatives to PFOS, both compounds were present at lower concentrations than PFOS at the majority of sites. However, at three sites, YR8, SX5, and GD4, the concentration of 6:2 Cl-PFESA was slightly higher than that of PFOS, indicating that a significant amount of Cl-PFESA is currently used as a substitute for PFOS in production.
6:2 FTCA, 6:2 FTSA, N-EtFOSAA and N-MeFOSAA are precursors of PFASs. Fluorotelomers are the most common alternative to PFOS and are commonly used in metal plating and production of firefighting foam. N-EtFOSAA and N-MeFOSAA are commonly used in surface treatments for textiles, carpets, paper and packaging materials, and in products such as pesticides. They are less stable than other PFASs and are subject to transformation by abiotic or biotic processes under certain conditions (Jiang et al., 2023). For example, 6:2 FTSA can undergo aerobic degradation in soil to produce short-chain PFCAs (mainly PFPeA and PFHxA) and 5:3 FTCA. The degradation of 6:2 FTSA is therefore one of the potential sources of short-chain PFCAs in the environment (Chen et al., 2019). It has been reported that the rhizomes of several plants, including corn, soybean, alfalfa and lettuce, can uptake N-EtFOSAA and degrade in soils and plants to four products, including N-ethyl perfluorooctane sulfonamide (N-EtFOSA), perfluorooctane sulfonamide acetate (FOSAA), perfluorooctane sulfonamide (FOSA) and PFOS (Wen et al., 2018). In this study, only 6:2 FTSA and N-EtFOSAA, out of the four above-mentioned targeted PFASs, were detected in small amounts at individual stations. This phenomenon might be related to their susceptibility to transformation and degradation in the environment.
In summary, PFASs are commonly found in the surface sediments of the YRDW area, with PFOA having the highest DF and concentration level. PFCAs were more frequently detected and widespread than PFSAs, precursors of PFASs and alternative PFASs. The contribution of long-chain PFASs to the ∑23PFASs was higher than that of short-chain PFASs, predominantly legacy PFASs. In addition to PFOA, PFBA is also present in surface sediments of the region with a high DF and relatively high concentration. Therefore, the same attention should be paid to short-chain and emerging PFASs as legacy PFASs.
Comparison of this study with others in surface sediments is shown in Table 2. The total PFAS concentrations in the present study area were higher than those in sediments from the Pearl River Delta region and the Qiantang River Basin, and also higher than those reported from the coastal areas of Korea, Tokyo Bay in Japan, and the rivers of southeastern North Carolina in the United States, but lower than those in the Shaying River Basin and the area of the inflow rivers of the Bohai Sea. The concentration levels in this study were at an intermediate level. The compositional characteristics of PFASs were similar to other studies, mostly PFOA as the main compound.
|
|
Table 2 Comparison of the present results with studies related to PFASs in sediments from other regions |
The correlation analysis between the concentration of PFAS and TOC content are shown in Table S5 (only 17 PFASs with a DF greater than 50% were included in the correlation analysis). ∑23PFASs showed a significantly positive correlation with TOC (r = 0.60, p < 0.001), suggesting that the distribution of total PFASs was to some extent related to the TOC content in sediments. A similar finding was reported by Zhao et al. in the riverine and near-shore sediments of Laizhou Bay (Zhao et al., 2013). Significantly positive correlations between concentrations of individual PFCAs and TOC content (r = 0.35 – 0.70, p < 0.05) were also observed for 10 of the 11 PFCAs, except for PFHxDA. On the other hand, PFOS concentration was significantly and positively correlated with TOC content (r = 0.66, p < 0.001), contrast to the cases of the remaining PFSAs, which showed no correlation with TOC content. For 6:2 Cl-PFESA, a significantly positive correlation (r = 0.74, p < 0.001) was also observed.
However, concentrations of PFAS showed essentially no significant correlation with sediment grain size and pH. To further quantitatively determine the effect of TOC content on PFAS concentrations, a one-way linear regression analysis was performed. As shown from Fig.3, TOC content was positively correlated with ∑23PFASs and three monomeric PFASs, and the logarithm of TOC content explains 37%, 26%, 23% and 33% of the variances in the logarithm of ∑23PFASs, PFOA, PFBA, and PFOS concentrations, respectively. We therefore propose that the distribution of PFASs is significantly linked to TOC content, which is one of the important physicochemical properties of a sediment sample.
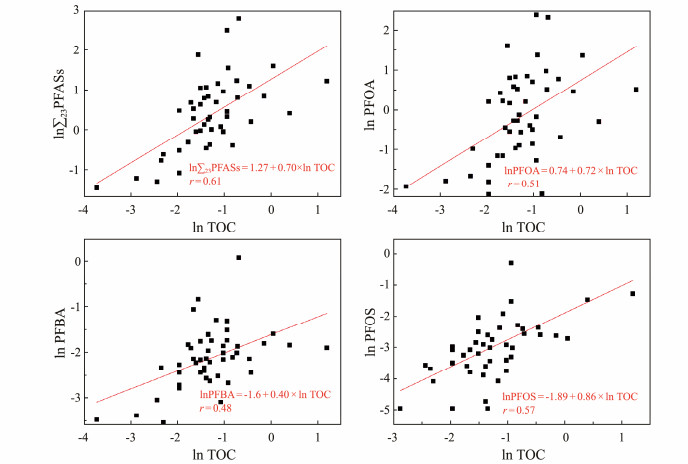
|
Fig. 3 One-way linear regression analysis of TOC content with ∑23PFASs, PFOA, PFBA and PFOS concentrations. |
Fig.4 illustrates the spatial distribution of PFASs in sediments of the YRDW area. The average ∑23PFASs along the coast of Bohai Sea, the Shenxian River, the modern Yellow River, and the old Yellow River were 3.26, 2.52, 1.64 and 1.10 ng g−1 dw, respectively. Overall, the concentration levels of PFASs were lower along the modern Yellow River and the old Yellow River. The lower concentration levels of PFASs are probably attributed to the fact that the two sampling areas are located in ecological preservation areas and natural landscapes, which are less affected by industrial and human activities.
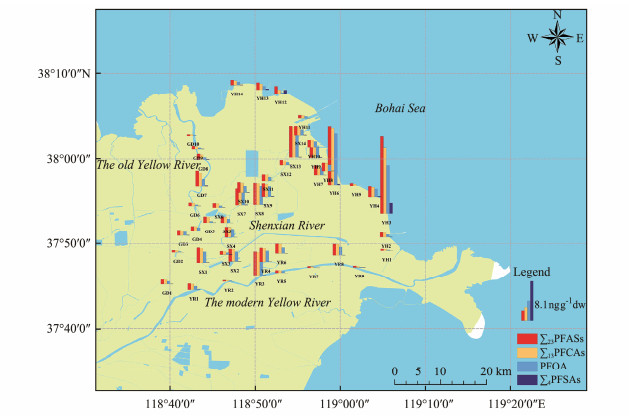
|
Fig. 4 Spatial distribution of PFASs in surface sediments of the YRDW area. |
It is also seen that higher values of ∑23PFASs tended to be concentrated at individual stations in the present study, with the highest at station YH3 (16.30 ng g−1), followed by station YH6 (12.17 ng g−1). And the values of ∑23PFASs at the remaining stations ranged from 0.23 to 6.56 ng g−1, which is significantly lower than the maximum value. Similar characteristics exist in the distributions of ∑13PFCAs, PFOA and ∑4PFSAs. The highest concentrations of several PFASs, including PFBA, PFOA, PFOS, PFNA, PFUnDA, and 6:2 Cl-PFESA, were found at the same station YH3. This station is located near a bathing beach of the Yellow River estuary, and the intensive human activities may be one of the reasons for the high PFASs concentration. Release of PFASs from the use of household items, such as water- and oil-repellent fabrics and disposable food packaging materials, are important potential sources of PFASs in the environment. Aqueous film-forming foam (AFFF) containing PFASs used in activities such as fire suppression and fire drills is a continuous source of PFASs in the ambient environment (Pozo et al., 2022). Therefore, the fire stations around YH3 are also an important part of the PFASs sources at the site. SX13, YR3, and GD7 are adjacent to villages where domestic sewage (laundry wastewater et al.) and solid wastes (e.g., compost and landfill wastes) are important sources of PFASs in the environment (Reinhart et al., 2023). It has been suggested that PFASs contained in fabrics, such as carpets and clothing, are released to the environment during use, cleaning and recycling (Chen et al., 2020). High concentrations of PFASs (28.7 – 75.9 μg kg−1) have been detected in organic solid waste compost containing food packaging materials (Choi et al., 2019). Physical, chemical, and biological processes in composting promote the transfer of PFASs to leachates and landfill gases over the time. These PFASs are subsequently transferred to other environmental matrices, leading to higher DFs and concentrations of PFASs near those sites.
3.3 Source Analysis of PFASs in the Surface Sediments from the YRDWThe strong correlation between the concentrations of the compounds suggests that they may have similar sources or environmental behaviors (Ahrens et al., 2009; Meng et al., 2021). The correlation heat-map among the PFASs in this study is shown in Fig.5 (only 17 PFASs with a DF greater than 50% are used in the correlation analysis).
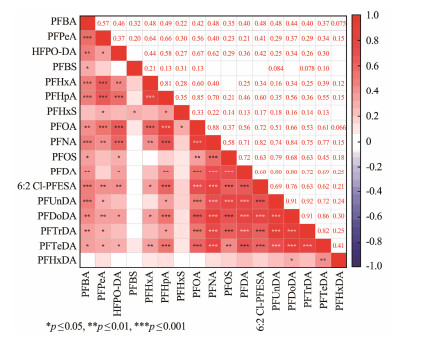
|
Fig. 5 The heat-map of correlation between targeted PFASs. |
PFOA showed significantly positive correlations with all of the 15 PFAS (r = 0.37 – 0.88, p < 0.05), except for no significant correlation with PFBS and PFHxDA. The strongest correlation was found between PFOA and PFNA (r = 0.88, p < 0.001), suggesting that the two compounds may share the same sources or migration pathways, which was also observed in a previous study (Meng et al., 2021). Similarly, PFBA showed a significantly positive correlation with all of the PFASs except PFHxS and PFHxDA, with the strongest correlation observed between PFBA and PFPeA (r = 0.57, p < 0.001), which is also a short-chain PFCAs.
On the other hand, PFOS showed a significantly positive correlation mainly with long-chain PFASs. Significant correlations (r = 0.51 – 0.92, p < 0.01) were also found between all of the long chain PFASs (C8 – C14) except PFHxDA. The fact that they all have high KOC values is most probably the reason, which makes them susceptible to be trapped in sediment and thus have similar environmental behaviors (Meng et al., 2021).
The potential sources of PFASs in the sedimentary environment of the YRDW were further analyzed with a PMF model by using the EPA PMF 5.0 software. In this study, Qtrue = 693.5, Qrobust = 692.2, and Qtrue/Qrobust is less than 1.5, suggesting that the modelling results are reasonable. Combining the results of Q-value, residual analysis and error analysis, three factors were finally identified to explain the sources of PFASs. The spectrum of the source components of PFASs and the contribution percentage of each factor are shown in Fig.6.
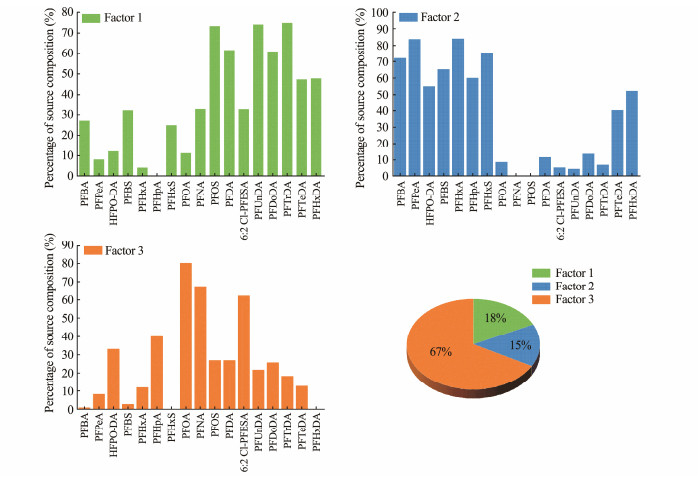
|
Fig. 6 Source profiles of PFAS in surface sediments of the YRDW obtained from PMF model. |
Factor 1 is dominated by PFOS and long-chain PFCAs (C11 – 16). PFOS is widely used in industries such as metal plating and textile treatment (Xie et al., 2013), and it can also be detected in aqueous film-forming foam for fire extinguishing products in China (Mumtaz et al., 2019). Long-chain PFCAs are commonly used in water- and oil-repellent processing of daily life consumer products such as textiles, carpets, leather and other items (Kotthoff et al., 2015; Xie et al., 2024). Therefore, Factor 1 was defined as metal plating industry, firefighting foams and textile treatment sources. Factor 2 consists primarily of short-chain PFCAs such as PFBA, PFPeA, PFHxA, and PFHpA, as well as PFBS and PFHxS. These compounds are alternatives to traditional PFAS such as PFOA and PFOS. It has been noted that short-chain PFCAs are also commonly used in moisture- and oil-resistant paper making for food packaging (Kotthoff et al., 2015; Zabaleta et al., 2017; Glenn et al., 2021). Thus, Factor 2 was defined as the source of food packaging material. Factor 3 is characterized by PFOA, PFNA and 6:2 Cl-PFESA. PFOA and PFNA are commonly used as processing aids in the manufacture of fluoropolymers (Qi et al., 2017; Shi et al., 2021; Li et al., 2023); and 6:2 Cl-PFESA is widely used as the main component of chromium mist inhibitors in metal plating industry (Wang et al., 2013; Cheng et al., 2023). Therefore, the fluoropolymer manufacturing and metal plating industry were assigned as the sources of Factor 3. Overall, the PMF model indicated that Factor 3 contributed the most to the sources of PFAS (67%) in surface sediments of the YRDW, while Factors 1 and 2 contributed 18% and 15%, respectively.
3.4 Risk Assessment of PFASs in the YRDWFOC, susp was set as the arithmetic mean of TOC in surface sediments in the area (0.0039 kg kg−1 dw). KOC values for PFBA, PFPeA, PFBS, PFHxA, PFNA, and PFDA were calculated using the EPI SuiteTMv4.11 software. The logKOC values for PFOA, PFOS and 6:2 Cl-PFESA were based on measured values from other studies, which were 2.11, 2.68 (Higgins and Luthy, 2006) and 4.35 (Gao et al., 2020), respectively. For the remaining parameters, default values suggested in TGD were used in the calculation. The PNECwater (μg L−1) value of each compound can be obtained referring to the relevant studies on ecotoxicology and ecological risk of PFASs. The PNECsediment, dw (ng g−1) was calculated using the equilibrium partitioning method, as described in SI Text S3. The PNECwater and the corresponding PNECsediment, dw values of each compound are listed in Table 3.
|
|
Table 3 PNECwater and PNECsediment values for each PFAS |
The results of risk assessment show that PFOS poses a moderate risk with RQ values greater than 0.1 at two stations, YH3 and YH12 (0.37 and 0.12, respectively), and a low risk or unlikely to pose a risk at the remaining stations. PFOA is unlikely to pose a risk at stations other than YH3, YH6 and SX13, where PFOA exerts a low risk. The remaining seven PFASs are unlikely to pose a risk at any station, with RQ values < 0.01.
Overall, RQ values were less than 1 at all stations in the study area, indicating that the current concentrations of PFASs in surface sediments of the YRDW do not pose a significant threat to benthic organisms. However, PFOA, PFBA and PFOS were widely detected at high concentrations, and ∑23PFASs showed high values at some stations, suggesting that attentions may still need to be paid for point source pollution by PFASs. Although 6:2 Cl-PFESA was found to be unlikely to pose a risk overall, it should not be overlooked, since its use as an alternative to PFAS continues to increase.
4 ConclusionsIn this study, 23 out of 26 PFASs were detected in surface sediments of the YRDW, and the total concentrations of PFASs (0.23 ng g−1 – 16.30 ng g−1) were at an intermediate level compared with reports from other regions. PFCAs, PFOA representatively, were the main PFASs in the area, and the contribution of long-chain PFASs to the total concentrations was higher than that of short-chain PFASs. Emerging PFASs such as 6:2 Cl-PFESA and HFPO-DA were also detected, indicating that these alternative PFASs are now being used in the market. Sediment TOC content is an important factor influencing the distribution of PFASs. Spatially, the highest concentration of PFASs was found at station YH3 (near a bathing beach and fire-fighting stations). Higher concentration levels have also been observed at several stations near the neighboring villages, where wastewaters and solid wastes from human activities are most probably the major sources of PFASs. PMF receptor modeling further revealed that metal plating, firefighting foam and textile treatment, food packaging material, fluoro polymer manufacturing are the major sources of PFASs in surface sediments of the YRDW. Risk assessment showed that the environmental and ecological risks of PFASs in the region are not significant, but continuous concerns are still needed to be paid for PFOA, PFBA, PFOS and the emerging PFASs.
This study provided a clearer understanding of the presence, sources, and ecological risk of PFASs in YRDW region. We recommend future studies on the environmental behavior and the health risk of PFASs to local populations.
AcknowledgementsThis study was financially supported by the National Natural Science Foundation of China (NSFC) (No. 42377217), and the Cooperation Fund between Dongying City and Universities (No. SXHZ-2023-02-6).
Supplementary Materials
Supplementary material is available in the online version of this article at https://doi.org/10.1007/s11802-024-5931-3.
Ahrens, L., Barber, J. L., Xie, Z., and Ebinghaus, R., 2009. Longitudinal and latitudinal distribution of perfluoroalkyl compounds in the surface water of the Atlantic Ocean. Environmental Science & Technology, 43: 3122-3127. DOI:10.1021/es803507p (  0) 0) |
Ahrens, L., Rakovic, J., and Ekdahl, S., 2023. Environmental distribution of per- and polyfluoroalkyl substances (PFAS) on Svalbard: Local sources and long-range transport to the Arctic. Chemosphere, 345: 140463. DOI:10.1016/j.chemosphere.2023.140463 (  0) 0) |
Ahrens, L., Taniyasu, S., Yeung, L. W. Y., Yamashita, N., Lam, P. K. S., and Ebinghaus, R., 2010. Distribution of polyfluoroalkyl compounds in water, suspended particulate matter and sediment from Tokyo Bay, Japan. Chemosphere, 79: 266-272. DOI:10.1016/j.chemosphere.2010.01.045 (  0) 0) |
Antonopoulou, M., Spyrou, A., Tzamaria, A., Efthimiou, I., and Triantafyllidis, V., 2024. Current state of knowledge of environmental occurrence, toxic effects, and advanced treatment of PFOS and PFOA. Science of the Total Environment, 913: 169332. DOI:10.1016/j.scitotenv.2023.169332 (  0) 0) |
Buck, R. C., Franklin, J., Berger, U., Conder, J. M., Cousins, I. T., de Voogt, P., et al., 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated Environmental Assessment and Management, 7: 513-541. DOI:10.1002/ieam.258 (  0) 0) |
Chen, H., Zhao, L., Wang, N., Yao, Y., and Sun, H., 2019. Aerobic biotransformation of 6: 2 fluorotelomer sulfonic acid in soil. Chinese Science Bulletin, 64: 3441-3448 (in Chinese with English abstract). (  0) 0) |
Chen, J., Tang, L., Chen, W. Q., Peaslee, G. F., and Jiang, D., 2020. Flows, stock, and emissions of poly- and perfluoroalkyl substances in California carpet in 2000-2030 under different scenarios. Environmental Science & Technology, 54: 6908-6918. DOI:10.1021/acs.est.9b06956 (  0) 0) |
Chen, M., Wang, Q., Shan, G., Zhu, L., Yang, L., and Liu, M., 2018. Occurrence, partitioning and bioaccumulation of emerging and legacy per- and polyfluoroalkyl substances in Taihu Lake, China. Science of the Total Environment, 634: 251-259. DOI:10.1016/j.scitotenv.2018.03.301 (  0) 0) |
Chen, Y., Zhang, H., Liu, Y., Bowden, J. A., Townsend, T. G., Solo-Gabriele, H. M., et al., 2024. Evaluation of per- and polyfluoroalkyl substances (PFAS) released from two Florida landfills based on mass balance analyses. Waste Management, 175: 348-359. DOI:10.1016/j.wasman.2023.12.054 (  0) 0) |
Cheng, X., Liu, L., Ge, Y., Weber, R., and Huang, J., 2023. Target and non-target analysis of per- and polyfluoroalkyl substances in representative chrome mist suppressants on the Chinese market. Chemosphere, 337: 139419. DOI:10.1016/j.chemosphere.2023.139419 (  0) 0) |
Choi, Y. J., Kim Lazcano, R., Yousefi, P., Yousefi, P., Trim, H., Lee, L. S., et al., 2019. Perfluoroalkyl acid characterization in U. S. municipal organic solid waste composts. Environmental Science & Technology Letters, 6: 372-377. DOI:10.1021/acs.estlett.9b00280 (  0) 0) |
Feng, X., Chen, X., Yang, Y., Yang, L., Zhu, Y., and Shan, G., 2021. External and internal human exposure to PFOA and HFPOs around a mega fluorochemical industrial park, China: Differences and implications. Environment International, 157: 106824. DOI:10.1016/j.envint.2021.106824 (  0) 0) |
Fiedler, H., Sadia, M., Krauss, T., Baabish, A., and Yeung, L. W. Y., 2022. Perfluoroalkane acids in human milk under the global monitoring plan of the stockholm convention on persistent organic pollutants (2008-2019). Frontiers of Environmental Science & Engineering, 16: 132. DOI:10.1007/s11783-022-1541-8 (  0) 0) |
Gao, L., Liu, J., Bao, K., Chen, N., and Meng, B., 2020. Multicompartment occurrence and partitioning of alternative and legacy per- and polyfluoroalkyl substances in an impacted river in China. Science of the Total Environment, 729: 138753. DOI:10.1016/j.scitotenv.2020.138753 (  0) 0) |
Glenn, G., Shogren, R., Jin, X., Orts, W., Hart-Cooper, W., Olson, L., et al., 2021. Per‐ and polyfluoroalkyl substances and their alternatives in paper food packaging. Comprehensive Reviews in Food Science and Food Safety, 20: 2596-2625. DOI:10.1111/1541-4337.12726 (  0) 0) |
Gosetti, F., Chiuminatto, U., Zampieri, D., Mazzucco, E., Robotti, E., Calabrese, G., et al., 2010. Determination of perfluorochemicals in biological, environmental and food samples by an automated on-line solid phase extraction ultra high performance liquid chromatography tandem mass spectrometry method. Journal of Chromatography A, 1217: 7864-7872. DOI:10.1016/j.chroma.2010.10.049 (  0) 0) |
Harfmann, J. L., Tito, K., Kieber, R. J., Avery, G. B., Mead, R. N., Shimizu, M. S., et al., 2021. Sorption of hexafluoropropylene oxide dimer acid to sediments: Biogeochemical implications and analytical considerations. ACS Earth and Space Chemistry, 5: 580-587. DOI:10.1021/acsearthspacechem.0c00323 (  0) 0) |
He, Y., Lv, D., Li, C., Liu, X., Liu, W., and Han, W., 2022. Human exposure to F-53B in China and the evaluation of its potential toxicity: An overview. Environment International, 161: 107108. DOI:10.1016/j.envint.2022.107108 (  0) 0) |
Higgins, C. P., and Luthy, R. G., 2006. Sorption of perfluorinated surfactants on sediments. Environmental Science & Technology, 40: 7251-7256. DOI:10.1021/es061000n (  0) 0) |
Hoke, R. A., Bouchelle, L. D., Ferrell, B. D., and Buck, R. C., 2012. Comparative acute freshwater hazard assessment and preliminary PNEC development for eight fluorinated acids. Chemosphere, 87: 725-733. DOI:10.1016/j.chemosphere.2011.12.066 (  0) 0) |
Jiang, T., Pervez, M. N., Quianes, M. M., Zhang, W., Naddeo, V., and Liang, Y., 2023. Effective stabilization of per- and polyfluoroalkyl substances (PFAS) precursors in wastewater treatment sludge by surfactant-modified clay. Chemosphere, 341: 140081. DOI:10.1016/j.chemosphere.2023.140081 (  0) 0) |
Joudan, S., Liu, R., D'Eon, J. C., and Mabury, S. A., 2020. Unique analytical considerations for laboratory studies identifying metabolic products of per- and polyfluoroalkyl substances (PFASs). TrAC Trends in Analytical Chemistry, 124: 25-32. DOI:10.1016/j.trac.2019.02.032 (  0) 0) |
Kotthoff, M., Müller, J., Jürling, H., Schlummer, M., and Fiedler, D., 2015. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environmental Science and Pollution Research, 22: 14546-14559. DOI:10.1007/s11356-015-4202-7 (  0) 0) |
Lee, J. W., Lee, H. K., Lim, J. E., and Moon, H. B., 2020. Legacy and emerging per- and polyfluoroalkyl substances (PFASs) in the coastal environment of Korea: Occurrence, spatial distribution, and bioaccumulation potential. Chemosphere, 251: 126633. DOI:10.1016/j.chemosphere.2020.126633 (  0) 0) |
Leeuwen, K. V., 2003. Technical Guidance Document on Risk Assessment. Institute for Health and Consumer Protection, Italy, 1-22.
(  0) 0) |
Li, H., Zhu, X., Zhang, J., Wang, Z., and Li, R., 2023. Characterizing the long-term occurrence and anthropogenic drivers of per- and polyfluoroalkyl substances in surface water of the Rhine River. Water Research, 245: 120528. DOI:10.1016/j.watres.2023.120528 (  0) 0) |
Li, Q., Cheng, X., Zhao, Z., Guo, M., Yuan, M., Hua, X., et al., 2019. Distribution and fluxes of perfluoroalkyl and polyfluoroalkyl substances in the middle reaches of the Yellow River (Weinan-Zhengzhou section). Environmental Science, 40: 228-238. DOI:10.13227/j.hjkx.201805240 (  0) 0) |
Li, Z., Liu, Y., Zhang, D., Feng, L., He, X., Duan, X., et al., 2022. Distribution and environmental risk assessment of microplastics in continental shelf sediments in the southern East China Sea: A high-spatial-resolution survey. Marine Pollution Bulletin, 177: 113548. DOI:10.1016/j.marpolbul.2022.113548 (  0) 0) |
Liu, Y., Ruan, T., Lin, Y., Liu, A., Yu, M., Liu, R., et al., 2017. Chlorinated polyfluoroalkyl ether sulfonic acids in marine organisms from Bohai Sea, China: Occurrence, temporal variations, and trophic transfer behavior. Environmental Science & Technology, 51: 4407-4414. DOI:10.1021/acs.est.6b06593 (  0) 0) |
Liu, Z., Zhou, J., Xu, Y., Lu, J., Chen, J., and Wang, J., 2022. Distributions and sources of traditional and emerging per- and polyfluoroalkyl substances among multiple environmental media in the Qiantang River watershed, China. RSC Advances, 12: 21247-21254. DOI:10.1039/d2ra02385g (  0) 0) |
Ma, D., Zhong, H., Lv, J., Wang, Y., and Jiang, G., 2022. Levels, distributions, and sources of legacy and novel per- and perfluoroalkyl substances (PFAS) in the topsoil of Tianjin, China. Journal of Environmental Sciences, 112: 71-81. DOI:10.1016/j.jes.2021.04.029 (  0) 0) |
Meng, L., Song, B., Zhong, H., Ma, X., Wang, Y., Ma, D., et al., 2021. Legacy and emerging per- and polyfluoroalkyl substances (PFAS) in the Bohai Sea and its inflow rivers. Environment International, 156: 106735. DOI:10.1016/j.envint.2021.106735 (  0) 0) |
Mumtaz, M., Bao, Y., Liu, L., Huang, J., Cagnetta, G., Yu, G., et al., 2019. Per- and polyfluoroalkyl substances in representative fluorocarbon surfactants used in Chinese film-forming foams: Levels, profile shift, and environmental implications. Environmental Science & Technology Letters, 6: 259-264. DOI:10.1021/acs.estlett.9b00154 (  0) 0) |
Pan, C. G., Ying, G. G., Liu, Y. S., Zhang, Q. Q., Chen, Z. F., Peng, F. J., et al., 2014a. Contamination profiles of perfluoroalkyl substances in five typical rivers of the Pearl River Delta region, South China. Chemosphere, 114: 16-25. DOI:10.1016/j.chemosphere.2014.04.005 (  0) 0) |
Pan, C. G., Ying, G. G., Zhao, J. L., Liu, Y. S., Liu, S. S., Du, J., et al., 2014b. Spatial distribution of perfluoroalkyl substances in surface sediments of five major rivers in China. Archives of Environmental Contamination and Toxicology, 68: 566-576. DOI:10.1007/s00244-014-0113-8 (  0) 0) |
Pozo, K., Moreira, L. B., Karaskova, P., Přibylová, P., Klánová, J., de Carvalho, M. U., et al., 2022. Using large amounts of firefighting foams releases per- and polyfluoroalkyl substances (PFAS) into estuarine environments: A baseline study in Latin America. Marine Pollution Bulletin, 182: 113938. DOI:10.1016/j.marpolbul.2022.113938 (  0) 0) |
Qi, Y., He, Z., Huo, S., Zhang, J., Xi, B., and Hu, S., 2017. Source apportionment of perfluoroalkyl substances in surface sediments from lakes in Jiangsu Province, China: Comparison of three receptor models. Journal of Environmental Sciences, 57: 321-328. DOI:10.1016/j.jes.2016.12.007 (  0) 0) |
Reinhart, D. R., Bolyard, S. C., and Chen, J., 2023. Fate of per- and polyfluoroalkyl substances in postconsumer products during waste management. Journal of Environmental Engineering, 149: 03123002. DOI:10.1061/joeedu.Eeeng-7060 (  0) 0) |
Renfrew, D., and Pearson, T. W., 2021. The social life of the 'forever chemical'. Environment and Society, 12: 146-163. DOI:10.3167/ares.2021.120109 (  0) 0) |
Shi, B., Wang, T., Yang, H., Zhou, Y., Bi, R., Yang, L., et al., 2021. Perfluoroalkyl acids in rapidly developing coastal areas of China and South Korea: Spatiotemporal variation and source apportionment. Science of the Total Environment, 761: 143297. DOI:10.1016/j.scitotenv.2020.143297 (  0) 0) |
Shi, Y., Vestergren, R., Xu, L., Zhou, Z., Li, C., Liang, Y., et al., 2016. Human exposure and elimination kinetics of chlorinated polyfluoroalkyl ether sulfonic acids (Cl-PFESAs). Environmental Science & Technology, 50: 2396-2404. DOI:10.1021/acs.est.5b05849 (  0) 0) |
Shimizu, M. S., Garcia, R. S., Avery, G. B., Kieber, R. J., Skrabal, S. A., and Mead, R. N., 2022. Distribution of legacy and emerging per- and polyfluoroalkyl substances in riverine and coastal sediments of southeastern North Carolina, USA. Environmental Science: Processes & Impacts, 24: 2119-2128. DOI:10.1039/d2em00246a (  0) 0) |
Tsui, M. M. P., Leung, H. W., Wai, T. C., Yamashita, N., Taniyasu, S., Liu, W., et al., 2014. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Research, 67: 55-65. DOI:10.1016/j.watres.2014.09.013 (  0) 0) |
Wang, Q., Tsui, M. M. P., Ruan, Y., Lin, H., Zhao, Z., Ku, J. P. H., et al., 2019. Occurrence and distribution of per- and polyfluoroalkyl substances (PFASs) in the seawater and sediment of the South China Sea coastal region. Chemosphere, 231: 468-477. DOI:10.1016/j.chemosphere.2019.05.162 (  0) 0) |
Wang, R., Zhang, J., Yang, Y., Chen, C. E., Zhang, D., and Tang, J., 2022a. Emerging and legacy per- and polyfluoroalkyl substances in the rivers of a typical industrialized province of China: Spatiotemporal variations, mass discharges and ecological risks. Frontiers in Environmental Science, 10: 986719. DOI:10.3389/fenvs.2022.986719 (  0) 0) |
Wang, S., Huang, J., Yang, Y., Hui, Y., Ge, Y., Larssen, T., et al., 2013. First report of a Chinese PFOS alternative overlooked for 30 years: Its toxicity, persistence, and presence in the environment. Environmental Science & Technology, 47: 10163-10170. DOI:10.1021/es401525n (  0) 0) |
Wang, S., Lin, X., Li, Q., Li, Y., Yamazaki, E., Yamashita, N., et al., 2022b. Particle size distribution, wet deposition and scavenging effect of per- and polyfluoroalkyl substances (PFASs) in the atmosphere from a subtropical city of China. Science of the Total Environment, 823: 153528. DOI:10.1016/j.scitotenv.2022.153528 (  0) 0) |
Wang, Y., Chen, X., Wang, B., Lu, G., Liu, J., Wu, D., et al., 2023a. Toxicity comparison of perfluorooctanoic acid (PFOA), hexafluoropropylene oxide dimer acid (HFPO-DA), and hexafluoropropylene oxide trimer acid (HFPO-TA) in zebrafish gut. Aquatic Toxicology, 262: 106655. DOI:10.1016/j.aquatox.2023.106655 (  0) 0) |
Wang, Y., Jiang, S., Wang, B., Chen, X., and Lu, G., 2023b. Comparison of developmental toxicity induced by PFOA, HFPO-DA, and HFPO-TA in zebrafish embryos. Chemosphere, 311: 136999. DOI:10.1016/j.chemosphere.2022.136999 (  0) 0) |
Wen, B., Pan, Y., Shi, X., Zhang, H., Hu, X., Huang, H., et al., 2018. Behavior of N-ethyl perfluorooctane sulfonamido acetic acid (N-EtFOSAA) in biosolids amended soil-plant microcosms of seven plant species: Accumulation and degradation. Science of the Total Environment, 642: 366-373. DOI:10.1016/j.scitotenv.2018.06.073 (  0) 0) |
Xie, S., Wang, T., Liu, S., Jones, K. C., Sweetman, A. J., and Lu, Y., 2013. Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environment International, 52: 1-8. DOI:10.1016/j.envint.2012.11.004 (  0) 0) |
Xie, X., Lu, Y., Wang, P., Lei, H., Chen, N., Liang, Z., et al., 2024. Per- and polyfluoroalkyl substances in a subtropical river-mangrove estuary-bay system. Journal of Hazardous Materials, 464: 132937. DOI:10.1016/j.jhazmat.2023.132937 (  0) 0) |
Xie, Z., and Kallenborn, R., 2023. Legacy and emerging per- and poly-fluoroalkyl substances in polar regions. Current Opinion in Green and Sustainable Chemistry, 42: 100840. DOI:10.1016/j.cogsc.2023.100840 (  0) 0) |
Xing, Y., Li, Q., Chen, X., Huang, B., Ji, L., Zhang, Q., et al., 2023. PFASs in soil: How they threaten human health through multiple pathways and whether they are receiving adequate concern. Journal of Agricultural and Food Chemistry, 71: 1259-1275. DOI:10.1021/acs.jafc.2c06283 (  0) 0) |
Yi, S., Morson, N., Edwards, E. A., Yang, D., Liu, R., Zhu, L., et al., 2022. Anaerobic microbial dechlorination of 6: 2 chlorinated polyfluorooctane ether sulfonate and the underlying mechanisms. Environmental Science & Technology, 56: 907-916. DOI:10.1021/acs.est.1c05475 (  0) 0) |
Yu, Y., Wang, S., Yu, P., Wang, D., Hu, B., Zheng, P., et al., 2024. A bibliometric analysis of emerging contaminants (ECs) (2001-2021): Evolution of hotspots and research trends. Science of the Total Environment, 907: 168116. DOI:10.1016/j.scitotenv.2023.168116 (  0) 0) |
Yuan, W., Song, S., Lu, Y., Shi, Y., Yang, S., Wu, Q., et al., 2024. Legacy and alternative per- and polyfluoroalkyl substances (PFASs) in the Bohai Bay Rim: Occurrence, partitioning behavior, risk assessment, and emission scenario analysis. Science of the Total Environment, 912: 168837. DOI:10.1016/j.scitotenv.2023.168837 (  0) 0) |
Zabaleta, I., Negreira, N., Bizkarguenaga, E., Prieto, A., Covaci, A., and Zuloaga, O., 2017. Screening and identification of per- and polyfluoroalkyl substances in microwave popcorn bags. Food Chemistry, 230: 497-506. DOI:10.1016/j.foodchem.2017.03.074 (  0) 0) |
Zeng, Z., Song, B., Xiao, R., Zeng, G., Gong, J., Chen, M., et al., 2019. Assessing the human health risks of perfluorooctane sulfonate by in vivo and in vitro studies. Environment International, 126: 598-610. DOI:10.1016/j.envint.2019.03.002 (  0) 0) |
Zhang, Y., Cao, Y., Zhou, T., Wang, Y., and Liu, Z., 2013. Predicted non-effect concentrations for PFOS of environment in China. China Environmental Science, 33: 1670-1677 (in Chinese with English abstract). (  0) 0) |
Zhang, Y., Ding, T., Huang, Z., Liang, H., Du, S., Zhang, J., et al., 2023. Environmental exposure and ecological risk of perfluorinated substances (PFASs) in the Shaying River Basin, China. Chemosphere, 339: 139537. DOI:10.1016/j.chemos-phere.2023.139537 (  0) 0) |
Zhao, P., Xia, X., Dong, J., Xia, N., Jiang, X., Li, Y., et al., 2016. Short- and long-chain perfluoroalkyl substances in the water, suspended particulate matter, and surface sediment of a turbid river. Science of the Total Environment, 568: 57-65. DOI:10.1016/j.scitotenv.2016.05.221 (  0) 0) |
Zhao, Z., Tang, J., Xie, Z., Chen, Y., Pan, X., Zhong, G., et al., 2013. Perfluoroalkyl acids (PFAAs) in riverine and coastal sediments of Laizhou Bay, North China. Science of the Total Environment, 447: 415-423. DOI:10.1016/j.scitotenv.2012.12.095 (  0) 0) |
Zheng, G., Eick, S. M., and Salamova, A., 2023. Elevated levels of ultrashort- and short-chain perfluoroalkyl acids in US homes and people. Environmental Science & Technology, 57: 15782-15793. DOI:10.1021/acs.est.2c06715 (  0) 0) |
Zhong, H., Zheng, M., Liang, Y., Wang, Y., Gao, W., Wang, Y., et al., 2021. Legacy and emerging per- and polyfluoroalkyl substances (PFAS) in sediments from the East China Sea and the Yellow Sea: Occurrence, source apportionment and environmental risk assessment. Chemosphere, 282: 131042. DOI:10.1016/j.chemosphere.2021.131042 (  0) 0) |
Zhou, J., Li, Z., Guo, X., Li, Y., Wu, Z., and Zhu, L., 2019. Evidences for replacing legacy per- and polyfluoroalkyl substances with emerging ones in Fen and Wei River Basins in central and western China. Journal of Hazardous Materials, 377: 78-87. DOI:10.1016/j.jhazmat.2019.05.050 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


