2) Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao Marine Science and Technology Center, Qingdao 266237, China;
3) Key Laboratory of Tropical Aquatic Germplasm of Hainan Province, Sanya Oceanographic Institution, Ocean University of China, Sanya 572024, China
Aquaculture represents the most rapid expanding sector within food production, progressively emerging as the predominant source of premium animal protein for human consumption (Gratacap et al., 2019). However, it is noteworthy that the majority of farmed aquatic species are either still sourced directly from wild populations or are undergoing the initial stages of domestication, which contributed to unstable production and serious problems restricted by environmental factors. In comparison to terrestrial livestock, only a small percentage of aquaculture production is reinforced by a selective breeding program, and there is a substantial potential for genetic improvement in aquatic species (Gjedrem, 2012; Gjedrem and Rye, 2018). The utilization of selective breeding programs in aquaculture has been commercial success in enhancing various traits such as growth, disease resistance, morphology, and yield across several species including Atlantic salmon, rainbow trout, various carps, shrimp, and others (Gjedrem and Baranski, 2009; Chavanne et al., 2016). The bivalve mollusk stands as a principal commodity within the global aquaculture sector, with numerous selective breeding initiatives focused on enhancing traits in clams (Zhao et al., 2012), scallops (Zheng et al., 2012), and mussels (Nguyen et al., 2014), oysters (Langdon et al., 2003) species have been initiated worldwide.
The Pacific oyster (Crassostrea gigas), renowned for its prolific reproduction and short reproductive cycle, adaptability to diverse habitats, and nutritional richness, has emerged as a pivotal shellfish species with substantial economic significance on a global scale. In China, C. gigas production constituted around 31.18% of the country's total oyster output in 2022, underscoring its considerable economic importance (China Fishery Statistical Yearbook, 2023). Previous studies have shown that considerable progress has been completed in a series of selective breeding programs for C. gigas phenotypic character. For example, the growth rate (Langdon et al., 2003; Li et al., 2011), disease resistance (Dégremont et al., 2007; Zhai et al., 2021), thermal resistance (Chi et al., 2024), shell color (Xu et al., 2019). Similar to numerous cultivated species, the growth and survival traits are paramount phenotypic characteristics of C. gigas, given their direct impact on yield and economic viability of production (Dégremont et al., 2005). Genetic improvement of these crucial phenotypic traits proves to be an effective approach for augmenting production, as it enables the accumulation of favorable genes from various individuals, thereby ensuring their inheritance in subsequent generations (Gjedrem and Baranski, 2009).
Polyploidy technology is also an effective way to improve the growth and survival rate of oysters. The development of diploid gonads requires significant nutrient and energy expenditure in the breeding season, resulting in slower growth and increased mortality. However, triploid can mitigate these drawbacks owing to their sterility and rapid growth. The efficacy of these advantages is influenced by environmental conditions and geographical location (Francesc et al., 2009; Zhou et al., 2023). Triploid can be generated through the hybridization of tetraploid males and diploid females resulting in 100% triploid offspring (Guo and Allen, 1994a; Zhang et al., 2016). Tetraploid oysters play a crucial role in this method. The induced methods of tetraploid are akin to the methods of induced triploid (Guo and Allen, 1994b; Guo et al., 2009; Benabdelmouna and Ledu, 2015). Tetraploid can subsequently reproduce sexually, yielding large populations of tetraploid individuals. However, ploidy increase does not always result in enhanced characteristics in polyploids. The tetraploid oysters had poor performance which is different from triploid oysters. For example, the growth and survival rate of tetraploid larvae and adult oysters were lower than those of diploid and triploid (Li et al., 2022; Qin et al., 2022; Zhang et al., 2022; Zhou et al., 2023), which made it difficult to obtain. This problem can be solved by tetraploidization of the selected breeding diploid line or by selective breeding of the ordinary tetraploid (Wan et al., 2023; Zhou et al., 2023).
The existence of additive genetic variation in the current population is a prerequisite for the effectiveness of selective breeding. Heritability (h2) quantifies the proportion of overall phenotypic variation attributable to additive genetic effect, thereby serving as a pivotal metric in forecasting selection responses, evaluating selection techniques, and formulating selective breeding strategies (Falconer and Mackay, 1996). Previous studies have shown that heritability estimates for many important traits in molluscan shellfish such as growth (Guo et al., 2018; Wu et al., 2022), disease resistance (Dietrich et al., 2022), survival (Dégremont et al., 2007, 2010; Chi et al., 2020), meat composition (Wan et al., 2019), shell shape and color (Evans et al., 2009; Nguyen et al., 2014; Hu et al., 2021). Heritability is affected by traits, genotypes, environmental conditions, and various development stages. Hence, obtaining a reliable estimate of heritability is imperative for devising a well-founded selective breeding program. The heritability of growth and survival, as the most important traits for diploid and tetraploid C. gigas are generally medium to high (Dégremont et al., 2010; Kong et al., 2015; Divilov et al., 2021; Wan et al., 2023).
In this study, the 28 full-sib and half-sib families were generated by the use of the parents of tetraploid C. gigas and estimated heritability for larvae growth and survival traits, along with their correlations at various developmental stages. We aimed to furnish valuable insights into breeding strategies tailored for tetraploid C. gigas. These findings serve as foundational data for the design and refinement of a selective breeding regimen, aimed at cultivating tetraploid C. gigas line characterized by both rapid growth and high survival rates.
2 Materials and Methods 2.1 Family ConstructionThe acquisition of progenitors and rearing condition of tetraploid oysters followed the instructions of Bai et al. (2024). Using the matching method of one male with two females, the appropriate amount of sperm and eggs were mixed in 5 L plastic buckets, and a total of 25 half-sib families and 50 full-sib families were established. The 28 fullsib families were cultivated to the juvenile stage. The survival rate of tetraploids was 56%. At the same time, two unbred ordinary diploid females and one unbred ordinary diploid male were selected as parents to establish a control group.
2.2 Larvae CultureLarvae culture followed the protocols outlined by Fang et al. (2021), and the culture conditions were consistent across all families. Every family underwent individual hatching in separate containers, and was maintained in the same condition until 24 h of post-fertilization. Subsequently, Dlarvae from each family were gathered and individually relocated into 150-L plastic buckets. Initially, stocking densities were adjusted to 10 larvae mL−1. The water temperature was 24℃ ± 1℃ and salinity was 30 ± 1.
2.3 Measurement of Growth Rate and Survival RateSamples were collected from replicates every 5 days, spanning from day 3 to day 23 post fertilization. Three random 100 mL samples were randomly gathered and preserved by the addition of 1% Lugol's solution. The total quantity of intact larval shells (differentiated from abnormal and empty shells) was counted thrice for each family with a 40× magnification. The survival of larvae was assessed on days 3 to 23 post fertilization as the ratio of living larvae in each bucket relative to the initial number of D-larvae, respectively. The shell length and shell height of 30 randomly chosen larvae from each family at each sampling time were measured using a light microscope (100×) equipped with an ocular micrometer (Han and Li, 2018).
2.4 Genetic and Statistical AnalysisThe shell height, shell length, and survival rate of larvae were separately analyzed at different ages of days by SPSS 23.0 software. Genetic analyses were conducted following the methods described by Chi et al. (2021). For shell height and shell length, the model was applied using the Restricted Maximum Likelihood (REML) algorithm, employing a univariate animal model formulated as follows:
| $ {Y_{jkl}} = \mu + {\alpha _{jkl}} + {e_{jkl}} . $ | (1) |
In the model, the different variable representations were referred the methods of Chi et al. (2021). The heritability of the shell height and shell length is estimated with the following formula by using univariate animal model:
| $ {h^2} = \frac{{\sigma _a^2}}{{\sigma _a^2 + \sigma _e^2}}. $ | (2) |
In this formula,
For survival rate, a sire-dam threshold model was employed to estimate the genetic parameters. In this model, deceased individuals were recorded as 0, while surviving individuals were recorded as 1. The model is as follows:
| $ \Pr ({Y_{ijk}} = 1) = \frac{{\exp (\mu + {a_i} + {c_j})}}{{1 + \exp (\mu + {a_i} + {c_j})}}. $ | (3) |
In this model, the different variable representations were referred to in the description of Chi et al. (2022). The heritability of survival is evaluated using formula (2) as described above. The genetic correction and phenotypic correlation between shell height and shell length were assessed utilizing a bivariate animal model, as expressed by the following formula:
| $ r = \frac{{{\sigma _a}(x, y)}}{{\sqrt {\sigma _{a(x)}^2\sigma _{a(y)}^2} }}. $ | (4) |
In this model, the different variable representations were referred to the methods of Chi et al. (2021). The SPSS 23.0 was used to calculate by Pearson correlation for the genetic and phenotypic correlations between growth traits and survival. The significance of these genetic and phenotypic correlations was assessed using the t test (Liu et al. 2005).
3 Results 3.1 Growth and Survival of LarvalTable 1 presents the sample size, mean value, maximum value, minimum value, standard deviation, and coefficient of variation pertaining to the growth and survival traits of tetraploid larvae of C. gigas. At day 3 of age, the mean shell height (SH) and shell length (SL) were 118.17 μm, and 119.90 μm, respectively. The mean survival rate (S) was 86.69%, with coefficients of variation ranging from 3.77% to 5.35%. At day 8 of age, the shell height, shell length, and survival rate were 130.76 μm, 124.14 μm, and 78.08%, respectively, with coefficients of variation ranging from 6.20% to 7.56%. At day 13 of age, the shell height, shell length, and survival rate were 168.23 μm, 159.34 μm, and 64.65%, respectively, with coefficients of variation ranging from 7.80% to 13.95%. At day 18 of age, the shell height, shell length, and survival rate were 223.55 μm, 201.08 μm, and 53.26%, respectively, with coefficients of variation ranging from 8.38% to 17.69%. At day 23 of age, the shell height, shell length, and survival rate were 295.09 μm, 260.10 μm, and 42.49%, respectively, with coefficients of variation ranging from 7.26% to 12.05%. At day 18 of age, the coefficient of variation of larvae survival traits was the largest (17.69%), indicating that the survival rate of different families was significantly different.
|
|
Table 1 The growth and survival traits in tetraploid larvae of Crassostrea gigas at different ages |
The shell height and survival rate of tetraploid larvae are shown in Fig.1. At day 3 of age, there was no significant difference in survival rate between tetraploid families and control families (P > 0.05); With the increase of larvae incubation time, the survival rate of each family showed a downward trend, and some families such as T1, T2, T4, T7 and T8 showed a significant decrease at days 8 and 13 of age. In the whole stage of larval culture, at day 13, the average survival rate of all tetraploid families was lower than that of the control group, and the survival rates of T7, T11, T25, T27, T29, and other families remained at a low level. The survival rate at day 23 decreased to 31.77%, 37.57%, 37.43%, 33.17%, and 36.67%, respectively. The T3, T16, T10, T15 and T17 families maintained a higher survival rate at day 23, which were 52.27%, 49.70%, 49.17%, 48.23% and 51.00%, respectively, and were significantly lower than that of the control group (P < 0.05, Fig.1A). At days 3 and 8, the shell height of all tetraploid families was significantly higher than that of control group (P < 0.05). However, with the longer larval incubation time, the growth rate of tetraploid families decreased compared with that of the control group (Fig.1B). Some families such as T17, T13, T16, and T7 maintained a high growth rate throughout the whole stage of larval cultivation, and the shell heights at day 23 were 317.11 μm, 310.92 μm, 310.62 μm, and 310.56 μm, respectively. There was no significant difference in shell height between the T17 family and control group (P > 0.05). Some families, such as T8, T6, and T12, had lower growth rates, while their average shell heights at day 23 were 278.63 μm, 277.74 μm, and 276.59 μm, respectively, which were significantly lower than that of the control group (P < 0.05, Fig.1B).
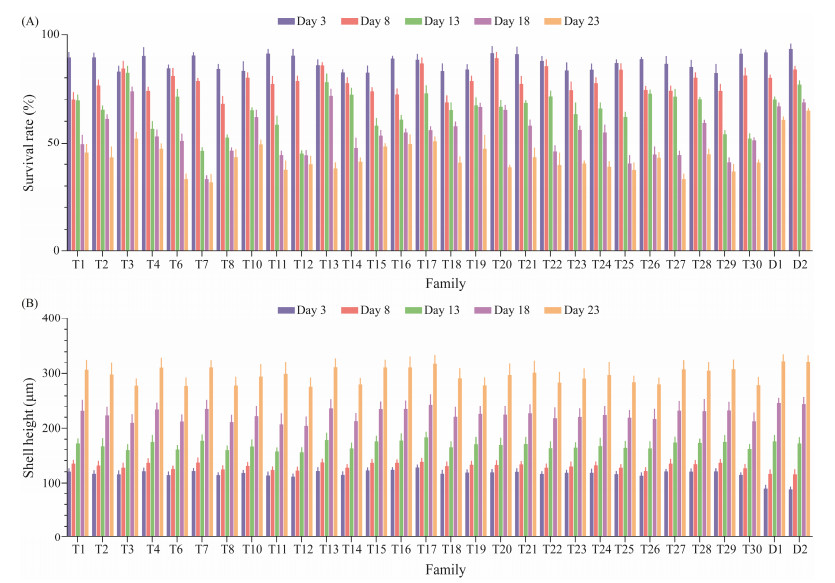
|
Fig. 1 Survival rate (A) and shell height (B) of each full-sib family in tetraploid larvae of C. gigas. T, the tetraploid family; D, the diploid control family. |
Table 2 displays the variance components and heritability estimates associated with the growth and survival traits of tetraploid C. gigas. The heritability of shell height and shell length were 0.50 – 0.71 and 0.44 – 0.71, respectively. The heritability of survival traits of larvae in different periods ranged from 0.40 to 0.64. The genetic and phenotypic correlations of growth and survival traits are shown in Table 3. The genetic correlations of shell height and shell length were higher (0.959 – 0.999, P < 0.01) than phenotypic correlations (0.187 – 0.340, P < 0.01). The shell height and shell length showed positive correlations in different periods. From day 8 to day 18, there were low and negative genetic correlations between growth traits and survival traits (−0.149 – −0.028). At day 23, there were significant negative correlations between growth traits and survival traits (−0.198 – 0.293, P < 0.01). In terms of phenotypic correlations, the correlations were different in different periods. There were very low negative correlations between shell height and survival traits at days 3 and 8 (−0.011 – −0.026), and very low but not significant positive correlations between growth and survival traits at other periods (0.044 – 0.171).
|
|
Table 2 Variance components and heritability for growth and survival traits in tetraploid larvae of C. gigas |
|
|
Table 3 Genetic and phenotypic correlations between growth and survival traits in tetraploid larvae of C. gigas |
In the previous studies, the biological characteristics of tetraploid oysters were mainly focused on the first-generation offspring, and there were few studies on their genetic improvement (Guo and Allen, 1994b; McCombie et al., 2005a; Matt and Allen, 2014; Li and Li, 2022; Li et al., 2022; Qin et al., 2022; Zhou et al., 2023). As the parents of the triploid industry, tetraploid oysters have a growth and survival disadvantage that makes tetraploid difficult to obtain and increases the breeding cost (Qin et al., 2022; Zhang et al., 2022; Zhou et al., 2023). To improve the performance of tetraploid oysters, some researchers hybridize different species of tetraploid oysters, such as C. gigas and C. angulata, to improve their growth and survival performance (Jiang et al., 2024a; Yue et al., 2024). However, due to its characteristics of producing normal gametes and self-breeding, some researchers have also carried out selective breeding on the tetraploid of C. gigas. Wan et al. (2023) selected the tetraploid of C. gigas for four generations through population selection, which improved the growth rate and ploidy stability of the tetraploid of C. gigas. Selective breeding techniques are currently widely used to improve the growth, survival, meat quality, and resistance to disease of selected organisms, and to deal with problems such as germplasm degradation and environmental deterioration (Jiang et al., 2024b). Tetraploid oysters have high fertility, and can produce a large number of functional gametes, short reproductive cycle, and large genetic variability, so they are very suitable for selective breeding methods (Newkirk and Haley, 1982; Qin et al., 2022; Wan et al., 2023; Zhou et al., 2023). As an important method of selective breeding, family selection can effectively use the genetic variation between families to make the target genes controlling important economic traits homozygous quickly and finally get the breeding materials required for the characteristics of selection. Growth and survival, as two important indexes affecting the final yield of oysters, can be used to obtain tetraploid families with high growth rates and high survival rates of oysters. In this study, the difference in survival between tetraploid families of C. gigas was similar to the results of other studies (Degremont et al., 2007; Chi et al., 2021), which also presents a strong genetic basis for tetraploid survival traits in C. gigas. In this study, the living conditions of larvae were the same, so the main factors affecting the survival of tetraploid larvae of C. gigas were gamete quality, inter-larval competitiveness, and viability (Kong et al., 2015).
4.1 Heritability of Growth and Survival TraitsHeritability is a crucial genetic parameter to be assessed during selection. It can infer potential selection responses for specific traits in a population (Chi et al., 2021). The size of heritability can be divided into lower (h2 ≤ 0.30), medium (0.30 < h2 < 0.50), and high levels (h2 ≥ 0.50) (Falconer and Mackay, 1996). In this study, the heritability of growth traits of larvae in different periods was medium to high (0.44 – 0.71), indicating that the first-generation tetraploid families have the potential for genetic improvement through selection, which is consistent with previous studies on diploid bivalve. For example, Chi et al. (2021) found that the heritability of growth traits in the larval stage was 0.30 – 0.86 in families constructed using fast-growing strains of C. gigas selected by multigenerational populations. The heritability of the growth rate of C. virginica larvae ranged from 0.25 to 0.50 (Newkirk et al., 1977). The heritability of the larval shell height of Mytilus chilensis was 0.38 (Toro et al., 2004). The realized heritability of larval shell length in the third generation of Argopecten irradians selected populations introduced from the United States was 0.511 (Zheng et al., 2004). In this study, the heritability of shell height and shell length showed a similar increase or decrease with the increase in age. Factors affecting heritability at various ages encompass maternal effects and the influence of shared environmental conditions (Gjedrem and Baranski, 2009; Brokordt et al., 2015). In addition, if full-sib or half-sib families share the same breeding environment, their shared environmental effects will increase over time (Gjedrem and Baranski, 2009). However, the maternal effects and common environmental effects shared by all full-sib families in this study were not significant. Thus, the changes in the heritability of growth traits observed during larval development may be related to individual factors (Barros et al., 2018).
Survival often represents an individual's ability to adapt to the environment. The heritability of survival traits of tetraploid C. gigas in this study was medium to higher (0.40 – 0.64), which was consistent with previous studies. The heritability of larvae survival traits assessed by Ernande et al. (2003) was 0.55. Evans and Langdon (2006) assessed the generalized heritability of survival rates of juvenile and adult larvae in 34 full-sib families at four breeding sites, ranging from 0.49 to 0.71. Degremont et al. (2007) found that the narrow heritability of the survival rate of C. gigas with 6 – 8 months old at three different sites ranged from 0.49 to 1.08. Wan et al. (2023) evaluated the realistic heritability of the four-generation selected population of tetraploid C. gigas and found that the average realistic heritability of the four-generation selected population was 0.39. In this study, the heritability of tetraploid larval survival traits was high, indicating a high potential for improving larval survival through direct selection. In addition, in the process of family construction, tetraploid families usually had a low survival rate (56%). Some families had a so low survival rate (< 30%) that the larvae were unable to reach the normal breeding density during attachment. Thus only 28 tetraploid families were preserved for subsequent breeding work.
4.2 Genetic and Phenotypic CorrelationsIn the process of selecting target traits, it is essential to assess the genetic correlation between two traits. In previous studies, Barros et al. (2018) found that the genetic correlation between shell height and shell length of Argopecten nucleus was 0.983 – 0.988. There was also a mediumhigh positive genetic and phenotypic correlation between shell height and shell length of diploid larvae of C. gigas (0.466 – 0.964) (Chi et al., 2021). In this study, the genetic correlation between shell height and shell length of tetraploid larvae at different periods was highly positive (0.959 – 0.999), which was consistent with previous reports. These results suggest that the shell height and shell length of tetraploid C. gigas may be controlled by the same set of genes, and the shell length can be increased synchronously during the process of shell height selection, showing strong potential for correlation response in the selection of growth traits.
To enhance the efficacy of breeding programs, it is imperative to understand the genetic correlations among key economic traits under selection (Kenway et al., 2006). Growth and survival traits should be considered in combination, and in this study, the genetic correlation between growth and survival was extremely low, suggesting that the genes controlling the two traits are likely to be different and unrelated. Chi et al. (2021) found that the genetic correlation between larval growth and survival rates of C. gigas was low and negative (−0.057 – −0.210), which was consistent with the results of this study. Degremont et al. (2007) found that the genetic correlation between growth and survival rates of one-age C. gigas cultured at three different sites was −0.17 ± 0.14. From the perspective of resource allocation theory, one can anticipate a negative correlation between growth and survival. This expectation arises because these traits represent competing demands for resources, and indiscriminate selection for a single trait may disrupt the overall balance of the organism (Rauw et al., 1998). However, the negative correlation between growth and survival makes it difficult to improve both of them at the same time. The results showed that improving survival rate during larva is not the best selection strategy. Chi et al. (2021) found that the genetic correlation between growth and survival rate of C. gigas in the juvenile stage was positive, and the heritability was moderate. Therefore, the best selection period to improve the survival rate of C. gigas should be in the juvenile stage, and the selection for survival could also have a positive impact on growth, thereby increasing the harvest yield (Chi et al., 2021). In the next study, it is necessary to evaluate the genetic parameters related to growth and survival rates in juvenile and adult stages of tetraploid families of C. gigas, which can provide further guidance for the selection of tetraploid C. gigas.
5 ConclusionsOur results show that there is still extensive genetic variation in the tetraploid of C. gigas and that it is feasible to genetically improve growth and survival rates through targeted selective breeding efforts. The medium and high heritability of related characteristics suggests that selective breeding for these characteristics can be successfully applied to the tetraploid of C. gigas. However, improving the survival rate of tetraploid of C. gigas through genetic selection without affecting growth still needs further evaluation because of their antagonistic relationship in the larval stage. The results provided guiding evidence for the selective breeding program of tetraploid of C. gigas.
AcknowledgementsThis work was supported by grants from the National Natural Science Foundation of China (No. 32373115), the Earmarked Fund for Agriculture Seed Improvement Project of Shandong Province (Nos. 2022LZGCQY010, 2021ZLGX03 and 2021TSGC1240), and the China Agriculture Research System Project (No. CARS-49).
Author Contributions
Xianchao Bai: Completion of the experiment, data analysis, and manuscript drafting. Yuanxin Liang: Experimental coordination. Geng Cheng: Experimental coordination. Ziheng Wang: Experimental coordination and Oyster farming. Chengxun Xu: data analysis. Qi Li: Experimental design, coordination, and manuscript revision.
Data Availability
The data and references presented in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval and Consent to Participate
The present study was performed according to the standard operation procedures (SOPs) of the Guide for the Use of Experimental Animals of the Ocean University of China. All animal care and use procedures were approved by the Institutional Animal Care and Use Committee of Ocean University of China.
Consent for Publication
Informed consent for publication was obtained from all participants.
Conflict of Interests
The authors declare that they have no conflict of interests.
Bai, X., Liang, Y., Zhang, H., Cheng, G., Xu, C., and Li, Q., 2024. Combined effects of temperature, salinity and rearing density on the larval growth and survival of the diploid, triploid and tetraploid of the Pacific oyster Crassostrea gigas. Aquaculture, 585: 740690. DOI:10.1016/j.aquaculture.2024.740690 (  0) 0) |
Benabdelmouna, A., and Ledu, C., 2015. Autotetraploid Pacific oysters (Crassostrea gigas) obtained using normal diploid eggs: Induction and impact on cytogenetic stability. Genome, 58: 333-348. DOI:10.1139/gen-2015-0014 (  0) 0) |
Brokordt, K. B., González, R. C., Farías, W. J., and Winkler, F. M., 2015. Potential response to selection of HSP70 as a component of innate immunity in the abalone Haliotis rufescens. PLoS One, 10: e0141959. DOI:10.1371/journal.pone.0141959 (  0) 0) |
Chavanne, H., Janssen, K., Hofherr, J., Contini, F., Haffray, P., Komen, H., et al., 2016. A comprehensive survey on selective breeding programs and seed market in the European aquaculture fish industry. Aquaculture International, 24: 1287-1307. DOI:10.1007/s10499-016-9985-0 (  0) 0) |
Chi, Y., Jiang, G., Liang, Y., Xu, C., and Li, Q., 2022. Selective breeding for summer survival in Pacific oyster (Crassostrea gigas): Genetic parameters and response to selection. Aquaculture, 556: 738271. DOI:10.1016/j.aquaculture.2022.738271 (  0) 0) |
Chi, Y., Li, Q., Liu, S., and Kong, L., 2021. Genetic parameters of growth and survival in the Pacific oyster Crassostrea gigas. Aquaculture Research, 52: 282-290. DOI:10.1111/are.14891 (  0) 0) |
Chi, Y., Li, Q., Xu, C., Liu, W., and Liu, H., 2024. Heritability of chronic thermal tolerance and genetic correlations with growth traits in the Pacific oyster (Crassostrea gigas). Journal of Applied Genetics, 65: 155-165. DOI:10.1111/are.14891 (  0) 0) |
Dégremont, L., Bédier, E., and Boudry, P., 2010. Summer mortality of hatchery-produced Pacific oyster spat (Crassostrea gigas). Ⅱ. Response to selection for survival and its influence on growth and yield. Aquaculture, 299: 21-29. DOI:10.1016/j.aquaculture.2009.11.017 (  0) 0) |
Dégremont, L., Bédier, E., Soletchnik, P., Ropert, M., Huvet, A., Moal, J., et al., 2005. Relative importance of family, site, and field placement timing on survival, growth, and yield of hatchery-produced Pacific oyster spat (Crassostrea gigas). Aquaculture, 249: 213-229. DOI:10.1016/j.aquaculture.2005.03.046 (  0) 0) |
Dégremont, L., Ernande, B., Bédier, E., and Boudry, P., 2007. Summer mortality of hatchery-produced Pacific oyster spat (Crassostrea gigas). Ⅰ. Estimation of genetic parameters for survival and growth. Aquaculture, 262: 41-53. DOI:10.1016/j.aquaculture.2006.10.025 (  0) 0) |
Dietrich, J. P., Hicks, M. B. R., Hard, J. J., Nichols, K. M., Langdon, C. J., Divilov, K., et al., 2022. Heritability estimates of disease resistance to Vibrio coralliiyticus in Pacific oyster (Crassostrea gigas) larvae from a selective broodstock program. Aquaculture, 560: 738492. DOI:10.1016/j.aquaculture.2022.738492 (  0) 0) |
Divilov, K., Schoolfield, B., Cortez, D. M., Wang, X., Fleener, G., Jin, L., et al., 2021. Genetic improvement of survival in Pacific oysters to the Tomales Bay strain of OsHV-1 over two cycles of selection. Aquaculture, 543: 737020. DOI:10.1016/j.aquaculture.2021.737020 (  0) 0) |
Ernande, B., Clobert, J., McCombie, H., and Boudry, P., 2003. Genetic polymorphism and trade-offs in the early life-history strategy of the Pacific oyster, Crassostrea gigas (Thunberg, 1795): A quantitative genetic study. Journal of Evolutionary Biology, 16: 399-414. DOI:10.1046/j.1420-9101.2003.00543.x (  0) 0) |
Evans, S., and Langdon, C., 2006. Effects of genotype × environment interactions on the selection of broadly adapted Pacific oysters (Crassostrea gigas). Aquaculture, 261: 522-534. DOI:10.1016/j.aquaculture.2006.07.022 (  0) 0) |
Falconer, D. S., and Mackay, T. F. C., 1996. Introduction to Quantitative Genetics. 4th edition, Pearson Education Ltd., Essex, 464pp.
(  0) 0) |
Fang, J., Han, Z., and Li, Q., 2021. Effect of inbreeding on performance and genetic parameters of growth and survival traits in the Pacific oyster Crassostrea gigas at larval stage. Aquaculture Reports, 19: 100590. DOI:10.1016/j.aqrep.2021.100590 (  0) 0) |
Francesc, P., Andy, B., Jeanclaude, F. R., Martin, F. H., Pierrick, H., and Lorenzo, C., 2009. Polyploid fish and shellfish: Production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture, 293: 125-156. DOI:10.1016/j.aquaculture.2009.04.036 (  0) 0) |
Gjedrem, T., 2012. Genetic improvement for the development of efficient global aquaculture: A personal opinion review. Aquaculture, 344-349(2012): 12-22. DOI:10.1016/j.aquaculture.2012.03.003 (  0) 0) |
Gjedrem, T., and Baranski, M., 2009. Selective Breeding in Aquaculture: An Introduction. Springer, Dordrecht, 221pp.
(  0) 0) |
Gjedrem, T., and Rye, M., 2018. Selection response in fish and shellfish: A review. Reviews in Aquaculture, 10: 168-179. DOI:10.1111/raq.12154 (  0) 0) |
Gratacap, R. L., Wargelius, A., Edvardsen, R. B., and Houston, R. D., 2019. Potential of genome editing to improve aquaculture breeding and production. Trends in Genetics, 35: 672-684. DOI:10.1016/j.tig.2019.06.006 (  0) 0) |
Guo, H., Zeng, Q., Li, Y., Wang, Y., Chen, Z., Lin, P., et al., 2018. Estimating realized heritability for growth in Zhikong scallop (Chlamys farreri) using genome-wide complex trait analysis. Aquaculture, 497: 103-108. DOI:10.1016/j.aquaculture.2018.07.046 (  0) 0) |
Guo, X., and Allen, S. K., 1994a. Reproductive potential and ge-netics of triploid Pacific oysters, Crassostrea gigas (Thunberg). Biology Bulletin, 187: 309-318. DOI:10.2307/1542288 (  0) 0) |
Guo, X., and Allen, S. K., 1994b. Viable tetraploid Pacific oyster (Crassostrea gigas Thunburg) produced by inhibiting polar body Ⅰ in eggs of triploids. Molecular Marine Biology and Biotechnology, 3: 42-50. (  0) 0) |
Guo, X., Wang, Y., Xu, Z., and Yang, H., 2009. Chromosome set manipulation in shellfish. New Technologies in Aquaculture: 165-194. DOI:10.1533/9781845696474.1.165 (  0) 0) |
Hu, H., Sun, C., Bai, Z., and Li, J., 2021. Genotype by environment interactions for inner shell color and growth traits in the purple freshwater pearl mussel, Hyriopsis cumingii, reared with different water depths and mud substrates. Aquaculture, 531: 735942. DOI:10.1016/j.aquaculture.2020.735942 (  0) 0) |
Jiang, G., Xu, C., and Li, Q., 2024a. Establishment of four types of allotetraploids derived from Crassostrea gigas and C. angulata and their breeding potential. Aquaculture International, 32: 4971-4989. DOI:10.1007/s10499-024-01411-9 (  0) 0) |
Jiang, K., Chen, C., Jiang, G., Chi, Y., Xu, C., Kong, L., et al., 2024b. Genetic improvement of oysters: Current status, challenges, and prospects. Reviews in Aquaculture, 16: 796-817. DOI:10.1111/raq.12868 (  0) 0) |
Kenway, M., Macbeth, M., Salmon, M., McPhee, C., Benzie, J., Wilson, K., et al., 2006. Heritability and genetic correlations of growth and survival in black tiger prawn Penaeus monodon reared in tanks. Aquaculture, 259: 138-145. DOI:10.1016/j.aquaculture.2006.05.042 (  0) 0) |
Kong, N., Li, Q., Yu, H., and Kong, L., 2015. Heritability estimates for growth-related traits in the Pacific oyster (Crassostrea gigas) using a molecular pedigree. Aquaculture Research, 46: 499-508. DOI:10.1111/are.12205 (  0) 0) |
Langdon, C., Evans, F., Jacobson, D., and Blouin, M., 2003. Yields of cultured Pacific oysters Crassostrea gigas Thunberg improved after one generation of selection. Aquaculture, 220: 227-244. DOI:10.1016/S0044-8486(02)00621-X (  0) 0) |
Li, Q., Wang, Q., Liu, S., and Kong, L., 2011. Selection response and realized heritability for growth in three stocks of the Pacific oyster Crassostrea gigas. Fisheries Science, 77: 643-648. DOI:10.1007/s12562-011-0369-0 (  0) 0) |
Li, Y., and Li, Q., 2022. The growth, survival and ploidy of dip-loid, triploid and tetraploid of the Pacific oyster (Crassostrea gigas) in larval and juvenile stages. Aquaculture, 553: 738083. DOI:10.1016/j.aquaculture.2022.738083 (  0) 0) |
Li, Y., Xu, C., and Li, Q., 2022. Effects of salinity and tempera-ture on growth and survival of diploid and tetraploid larvae of the Pacific oyster, Crassostrea gigas. Aquaculture, 550: 737809. DOI:10.1016/j.aquaculture.2021.737809 (  0) 0) |
Liu, X., Chang, Y., Xiang, J., and Cao, X., 2005. Estimates of genetic parameters for growth traits of the sea urchin, Strongylocentrotus intermedius. Aquaculture, 243: 27-32. DOI:10.1016/j.aquaculture.2004.10.014 (  0) 0) |
Matt, J. L., and Allen, S. K., 2014. Heteroploid mosaic tetra-ploids of Crassostrea virginica produce normal triploid larvae and juveniles as revealed by flow cytometry. Aquaculture, 432: 336-345. DOI:10.1016/j.aquaculture.2014.05.015 (  0) 0) |
McCombie, H., Lapѐgue, S., Cornette, F., Ledu, C., and Boudry, P., 2005. Chromosome loss in bi-parental progenies of tetraploid Pacific oyster Crassostrea gigas. Aquaculture, 247: 97-105. DOI:10.1016/j.aquaculture.2005.02.003 (  0) 0) |
Newkirk, G. F., and Haley, L. E., 1982. Phenotypic analysis of the European oyster Ostrea edulis L. : Relationship between length of larval period and postsetting growth rate. Journal of Experimental Marine Biology and Ecology, 59: 177-184. DOI:10.1016/0022-0981(82)90114-9 (  0) 0) |
Newkirk, G. F., Haley, L. E., Waugh, D. L., and Doyle, R., 1977. Genetics of larvae and spat growth rate in the oyster Crassostrea virginica. Marine Biology, 41: 49-52. DOI:10.1007/BF00390580 (  0) 0) |
Nguyen, T. T. T., Hayes, B. J., and Ingram, B. A., 2014. Genetic parameters and response to selection in blue mussel (Mytilus galloprovincialis) using a SNP-based pedigree. Aquaculture, 420-421: 295-301. DOI:10.1016/j.aquaculture.2013.11.021 (  0) 0) |
Qin, Y., Zhang, Y., and Yu, Z., 2022. Aquaculture performance comparison of reciprocal triploid C. gigas produced by mating tetraploids and diploids in China. Aquaculture, 552: 738044. DOI:10.1016/j.aquaculture.2022.738044 (  0) 0) |
Rauw, W. M., Kanis, E., Noordhuizen-Stassen, E. N., and Grommers, F. J., 1998. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livestock Production Science, 56: 15-33. DOI:10.1016/S0301-6226(98)00147-X (  0) 0) |
Toro, J. E., Alcapán, A. C., Vergara, A. M., and Ojeda, J. A., 2004. Heritability estimates of larval and spat shell height in the Chilean blue mussel (Mytilus chilensis Hupe 1854) produced under controlled laboratory conditions. Aquaculture Research, 35: 56-61. DOI:10.1111/j.1365-2109.2004.00985.x (  0) 0) |
Wan, S., Yu, H., Liu, S., and Kong, L., 2019. Estimating heritability for meat composition traits in the golden shell strain of Pacific oyster (Crassostrea gigas). Aquaculture, 516: 734532. DOI:10.1016/j.aquaculture.2019.734532 (  0) 0) |
Wan, W., Qin, Y., Shi, G., Li, S., Liao, Q., Ma, H., et al., 2023. Genetic improvement of aquaculture performance for tetraploid Pacific oysters, Crassostrea gigas: A case study of four consecutive generations of selective breeding. Aquaculture, 563: 738910. DOI:10.1016/j.aquaculture.2022.738910 (  0) 0) |
Wu, F., Liu, C., Zhang, J., and Zhang, G., 2022. Genetic evalua-tion of growth and survival-related traits in Yesso scallop Patinopecten yessoensis in sea-based culture system. Frontiers in Marine Science, 9: 865736. DOI:10.3389/fmars.2022.865736 (  0) 0) |
Xu, C., Li, Q., Chong, J., Liu, S., and Kong, L., 2019. Mass se-lection for growth improvement in black shell line of Pacific oyster Crassostrea gigas. Journal of Ocean University of China, 18: 1411-1416. DOI:10.1007/s11802-019-4041-0 (  0) 0) |
Yue, C., Qin, Y., Wan, W., Shi, G., Li, S., Li, J., et al., 2024. Phe-notypic traits of reciprocal tetraploid hybrids derived from tetraploid Crassostrea gigas and tetraploid Crassostrea angulata. Aquaculture, 582: 740495. DOI:10.1016/j.aquaculture.2023.740495 (  0) 0) |
Zhai, S., Yang, B., Zhang, F., Li, Q., and Liu, S., 2021. Estimation of genetic parameters for resistance to Vibrio alginolyticus infection in the Pacific oyster (Crassostrea gigas). Aquaculture, 538: 736545. DOI:10.1016/j.aquaculture.2021.736545 (  0) 0) |
Zhang, Y., Li, J., Qin, Y., Zhou, Y., Zhang, Y., and Yu, Z., 2016. A comparative study of the survival, growth and gonad development of the diploid and triploid Hong Kong oyster, Crassostrea hongkongensis (Lam & Morton 2003). Aquaculture Research, 48: 2453-2462. DOI:10.1111/are.13081 (  0) 0) |
Zhang, Y., Qin, Y., and Yu, Z., 2022. Comparative study of tetra-ploid-based reciprocal triploid Portuguese oysters, Crassostrea angulata, from seed to marketsize. Aquaculture, 547: 737523. DOI:10.1016/j.aquaculture.2021.737523 (  0) 0) |
Zhao, L., Yan, X., Huo, Z., Yang, F., and Zhang, G., 2012. Divergent selection for shell length in the Manila clam, Ruditapes philippinarum. Journal of the World Aquaculture Society, 43: 878-884. DOI:10.1111/j.1749-7345.2012.00612.x (  0) 0) |
Zheng, H., Li, L., and Zhang, G., 2012. Inbreeding depression for fitness-related traits and purging the genetic load in the hermaphroditic bay scallop Argopecten irradians irradians (Mollusca: Bivalvia). Aquaculture, 366-367: 27-33. DOI:10.1016/j.aquaculture.2012.08.029 (  0) 0) |
Zheng, H., Zhang, G., Liu, X., Zhang, F., and Guo, X., 2004. Different responses to selection in two stocks of the bay scallop, Argopecten irradians irradians Lamarck (1819). Journal of Experimental Marine Biology and Ecology, 313: 213-223. DOI:10.1016/j.jembe.2004.04.015 (  0) 0) |
Zhou, J., Jiang, G., Xu, C., Bai, X., and Li, Q., 2023. Growth, survival and gonad development of diploids, triploids and tetraploids of 'Haida No. 3' line of the Pacific oyster Crassostrea gigas. Aquaculture, 571: 739472. DOI:10.1016/j.aquaculture.2023.739472 (  0) 0) |
Zhou, X., Abbas, K., Li, M., Fang, L., Li, S., and Wang, W., 2010. Comparative studies on survival and growth performance among diploid, triploid and tetraploid dojo loach Misgurnus anguillicaudatus. Aquaculture International, 18: 349-359. DOI:10.1007/s10499-009-9248-4 (  0) 0) |
 2025, Vol. 24
2025, Vol. 24


