2) UMT-OUC Joint Center for Marine Studies, Qingdao 266003, China;
3) Institute of Marine Biotechnology, Universiti Malaysia Terengganu, 21030 Kuala Terengganu, Malaysia;
4) Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, TAS 7001, Australia;
5) Haide College, Ocean University of China, Qingdao 266100, China;
6) The Affiliated Hospital of Qingdao University, Qingdao 266001, China
Viruses rely on living cells to multiply and survive in cells of animals, plants, and microbes (Taylor and Leiman, 2020). They are thought to have a key impact on global ecosystems. Bacteriophages (phages), viruses that infect bacteria, are the most abundant 'biological entities' on Earth, with an estimated total number of 1031 particles (Hendrix et al., 1999), which corresponds to about 200 million tons of biomass (Suttle, 2013). Phages and their host cells are integral components of the marine environment, and play important roles in marine food webs (Rohwer and Thurber, 2009). Given their dominance, exploring the interactions between phages and their hosts, rather than studying phages individually, may be more beneficial in predicting the effect of viruses on microbial communities (Weitz et al., 2013).
Pseudomonas is a genus of gram-negative aerobic microorganisms in the family Pseudomonadaceae. Its athletic ability is achieved through a single polarity flagella (Iglewski, 1996). Members of the Pseudomonas genus inhabit in a wide variety of environment, including soil, water, plants, and the intestinal tract of animals (Palleroni, 1981). It is closely related to its remarkable metabolic and physiological diversity (Ben Haj Khalifa et al., 2011). Since Migula first described the Pseudomonas genus in 1984 (Schroeter, 1872), more Pseudomonas species have been discovered and studied for their functions. For example, Pseudomonas aeruginosa is one of the pathogens with the highest mortality rate in the global medical system, and is capable of causing various chronic or acute diseases (Diggle and Whiteley, 2020). Its pathogenicity includes ordinary wound infections to sepsis, pneumonia, and even cancer (Qin et al., 2022). In addition to its close connection with humans, another species of Pseudomonas, Pseudomonas stutzeri, has important ecological functions.
Recently, the Pseudomonas genus has been reclassified. Strains that previously belonged to the phylogenetic population of Pseudomonas stutzeri were classified as a new genus, Stutzerimonas (Gomila et al., 2022; Lalucat et al., 2022). Stutzerimonas stutzeri, a fluorescent denitrifying bacterium, is mainly found in soil and marine environments (Lalucat et al., 2006; Yang et al., 2012). Some strains have important ecological functions and can perform various chemical metabolisms (Rossello et al., 1991), such as nitrogen fixation (Yan et al., 2008), denitrification (Peña et al., 2012), and participation in the degradation of environmental pollutants, including high concentrations of metals (Waite et al., 2020). In addition to its important ecological function, members of Stutzerimonas have also gradually been recognized as important causes of human infection (Halabi et al., 2019) and are considered opportunistic pathogens of humans. While S. stutzeri has been extensively studied due to its importance, little research has been conducted on its corresponding phages. Till April 2023, only two phages with complete sequences isolated from S. stutzeri have been recorded in the GenBank database. Phage 8P (Liu et al., 2021a) (GenBank: MT152150.1) was isolated from S. stutzeri surviving in an oil reservoir, and another novel phage, named vB_PstS-pAN (GenBank: MW6518 59.1), was induced from S. stutzeri AN10 by mitomycin (Feng et al., 2021).
In this study, we described a phage called vB_SstM-PG1, which was isolated from S. stutzeri G1 in the ocean. Analysis of its physiology and genome showed that vB_SstM-PG1 differs from previously isolated phages and represents a new genus along with six uncultured viruses from the IMG/VR v4 database. The discovery of vB_SstM-PG1 broadens our understanding of S. stutzeri phages, makes a remarkable contribution to the expansion of Stutzerimonas phage taxa, and provides new insights into the interactions between phages and marine microbial populations with important ecological functions.
2 Methods 2.1 Sample Collection, Isolation, and Purification of Phage and Its Host StrainBoth Stutzerimonas stutzeri G1 and its phage were isolated from the coastal waters of Xiaogang in Qingdao, China (36˚04΄20΄΄N, 120˚18΄30΄΄E) in October 2020.
Coarsely sieved seawater samples were diluted to 10−5 with 2216E (peptone 5 wt.%, yeast extract 1 wt.%) seawater culture solution in a gradient. Totally 200 μL of samples were taken from each gradient and inoculated onto 2216E solid medium using a plate coating method. Three sets of parallel experiments were performed for each gradient and incubated at 28℃ for 12 h. After resting incubation, single colonies with different morphological characteristics were selected, and the final purified single colonies were obtained through three-zone delineations. The activated pure culture bacteria were placed in a 50 mL sterile centrifuge tube and centrifuged at 3000 r min−1 and 28 ℃ for 10 min. After centrifugation, the supernatant was discarded, and the pellet was sent to Sangon Biotech (Shanghai) for 16S rRNA gene sequencing.
Another portion of the collected seawater sample was filtered through 0.22 µm pore-size membranes (Millipore) and concentrated to obtain viral water. The phage was isolated using the double-layer plate method. Briefly, 200 µL sea water with bacteria was mixed homogeneously with 200 µL sea water with virus, and the mixture was left to stand for 20 min. Then, 3.5 mL of a semi-solid culture at 45℃ was added to the bacteria-viruses mixture. After vortex oscillation, it was evenly spread on the 2216E solid medium to form a double-layer plate culture mode. Incubation at 28℃ for 12 h was used to indicate the presence of phage based on the formation of plaques. Individual plaques were picked out and placed in 1 mL of SM buffer (100 mmol L−1 NaCl, 8 mmol L−1 MgSO4·7H2O, 50 mmol L−1 Tris-Cl, pH = 7.5) (Liu et al., 2021b), filtered through a 0.22 µm PES millipore to obtain viral water (Dong et al., 2022), and its host was infected again by the double-layer plate method (Kropinski et al., 2009a). These steps were repeated 3 to 5 times until a purified phage solution was obtained.
2.2 Identification of Phage MorphologyThe morphology of vB_SstM-PG1 was observed using transmission electron microscopy (TEM) (Deveau et al., 2006). Purified phage samples were added to dry copper grids and left to stand until completely dry. The grids were then negatively stained with 2% (w/v) potassium phosphotungstate and placed under a transmission electron microscope for observation (Huang et al., 2013).
2.3 Determination of the One-Step Growth CurveTo analyze the infectivity and replication ability of phages, a one-step growth curve test was performed. Totally 0.5 mL bacterial culture was mixed with 0.5 mL virus solution and incubated for 15 min at a multiplicity of infection (MOI) of 0.1. The mixture was then centrifuged, the supernatant was discarded, and the pellet was resuspended three times with 2216E liquid medium before being transferred into 50 mL of 2216E liquid medium. Samples were taken every 5 min for the first half hour and diluted in gradients of -2, -4, -6; every 10 min for the middle hour to -6, -8, -10; and every 30 min for the last half hour to -8, -10, -12. The virus titer was determined using the double-layer plate, and three replicate parallel experiments were performed to calculate the number of plaques at different time points to plot the growth curve.
2.4 Determination of Temperature StabilityOne milloliter (1 mL) of phage samples with a titer of 108 PFU mL−1 were processed at temperatures of −20℃, 4℃, 25℃, 35℃, 45℃, 55℃, 65℃, 75℃, 85℃, and 100℃ for 2 h. The treated phage samples were diluted in gradients of -4, -6, and -8, and the activity of the phage was determined using the double-layer plate method. Three parallel samples were set up in each group.
2.5 Determination of pH SensitivitySM buffers with pH values of 2, 3, 4, 5, 6, 7, 8, 9, 10, and 11 were prepared. vB_SstM-PG1 samples with the same properties described above were diluted tenfold with SM buffer at different pH values, mixed well, and left to stand for 2 h at room temperature. The treated phage samples were further diluted in three gradients, and the phage activity was determined using the double-layer plate method. Three parallel samples were set up in each group.
2.6 DNA Extraction, Sequencing, and AnnotationViral nucleic acids were extracted according to the instructions of the OMEGA Viral DNA Kit. Purified phage genomic DNA was sequenced using the Illumina HiSeq 4000 platform with a 150-bp paired-end DNA library. Trimmomatic was used to trim and filter the raw paired-end reads (parameters: SLIDING WINDOW: 4:15 MINLEN: 75) (Bolger et al., 2014). The filtered reads were then assembled with ABySS using multiple-Kmer parameters (Simpson et al., 2009) and further processed using GapCloser (Xu et al., 2020) to fill in local inner gaps, correct single base polymorphisms in the assembly, and for subsequent analyses.
Phage open reading frames (ORFs) were predicted using the comprehensive results from GeneMarkS (Besemer et al., 2001), GeneMark.hmm (Lukashin and Borodovsky, 1998), RAST (https://rast.nmpdr.org/rast.cgi), and ORF-finder (https://www.ncbi.nlm.nih.gov/orffinder/). Gene functions were further annotated using Pfam (http://pfam-legacy.xfam.org/search) with E-value < 1e − 5 and the online servers HHpred (https://toolkit.tuebingen.mpg.de/tools/hhpred). Inconsistent results produced by the various prediction and annotation softwares were manually checked.
2.7 Structural Analysis of ORF41 and ORF11The protein structures encoded by ORF41 and ORF11 were predicted and visualized using the AlphaFold2 tool in COSMIC2 (https://cosmic2.sdsc.edu:8443/gateway/home.action) (Cianfrocco et al., 2017). Protein structure-based searches were performed using the Dali server (http://ekhidna.biocenter.helsinki.fi/dali/) (Holm and Sander, 1995).
2.8 Phylogenetic Analysis of Stutzerimonas stutzeri G1Using the online website List of Prokaryotic names with Standing in Nomenclature (LPSN) (https://lpsn.dsmz.de/) (Parte et al., 2020), a search was conducted for reference sequences, resulting in the selection of 16 Stutzerimonas and 34 Pseudomonas sequences. The LPSN Application Programming Interface (API) was then used to construct a 16S rRNA gene phylogenetic tree (Meier-Kolthoff et al., 2021) for Stutzerimonas species G1 and the reference sequences, and to calculate the exact pairwise similarities between the gene sequences.
2.9 Phylogenetic and Comparative Genomic Analysis of vB_SstM-PG1Using the full-genome amino acid sequences of vB_SstM-PG1 and S. stutzeri phage, a proteomic tree was generated using ViPTree v3.1 (http://www.genome.jp/viptree) (Nishimura et al., 2017). Genome comparison between phage PG1 and its relatives was then conducted using tblastx and ViPTree. The relationship between vB_SstM-PG1 and its close relatives was subsequently described.
2.10 Whole Genomic Network Analysis and Protein ClusteringTo further investigate the classification status of vB_SstM-PG1, blastp was used to expand the phage population by querying each coding sequence of PG1 against the Integrated Microbial Genomes/Virus (IMG/VR v4) database with an E-value cutoff of < 1e − 10, identity cutoff of > 30%, and alignment coverage cutoff of > 50% (Teeling et al., 2004; Bolduc et al., 2017; Camargo et al., 2023). In vConTACT analysis, ClusterONE was used to identify viral clusters (VCs) with default parameters defined in vCon-TACT 2.0 (Bin Jang et al., 2019). Gephi was used for network visualization and modular analysis based on vCon-TACT results (Bastian et al., 2009). Protein clustering analysis was based on the ClusterONE algorithm and the generated vConTACT protein file. The VC containing vB_SstM-PG1 and other related VCs' viral proteins were extracted, corresponding to different protein cluster (PC) numbers. A protein clustering matrix was generated based on the obtained data, and a protein co-expression network heatmap was plotted using R. The average nucleotide identity (ANI) value of vB_SstM-PG1 was calculated using VIRIDIC (Moraru et al., 2020) and visualized using R.
2.11 Ecological Distribution of vB_SstM-PG1 in the OceanThe relative abundance of viral genomes in the Global Ocean Viromes 2.0 (GOV 2.0) database was calculated using minimap2 (parameters: -min-read-percent-identity 0.95, -min-read-aligned-percent 0.75, -m rpkm), a metagenomic tool (Li, 2018). The reference sequence mainly included isolated and uncultured viruses associated with vB_SstM-PG1, as well as two strains of Stutzerimonas phages, one being virulent and the other temperate. Additionally, it also included representative Pelagibacter phages from the ocean (HTVC010P, HTVC019P, HTVC011P) (Zhao et al., 2013), cyanophages (P-SSB7, P-SSM7), and the SIO1 phage (Angly et al., 2009). The relative abundance of viruses from five oceanic viral ecological zones (VEZs) defined by the GOV 2.0 dataset was aggregated, including the Arctic (ARC), Antarctic (ANT), epipelagic of the tropical and subtropical oceans (EPI), mesopelagic of the tropical and subtropical oceans (MES), and bathypelagic zone (BATHY) (Gregory et al., 2019). The relative abundance of vB_SstM-PG1 was expressed in transcripts per million (TPM), and the final result was visualized using heatmaps and histograms generated in R.
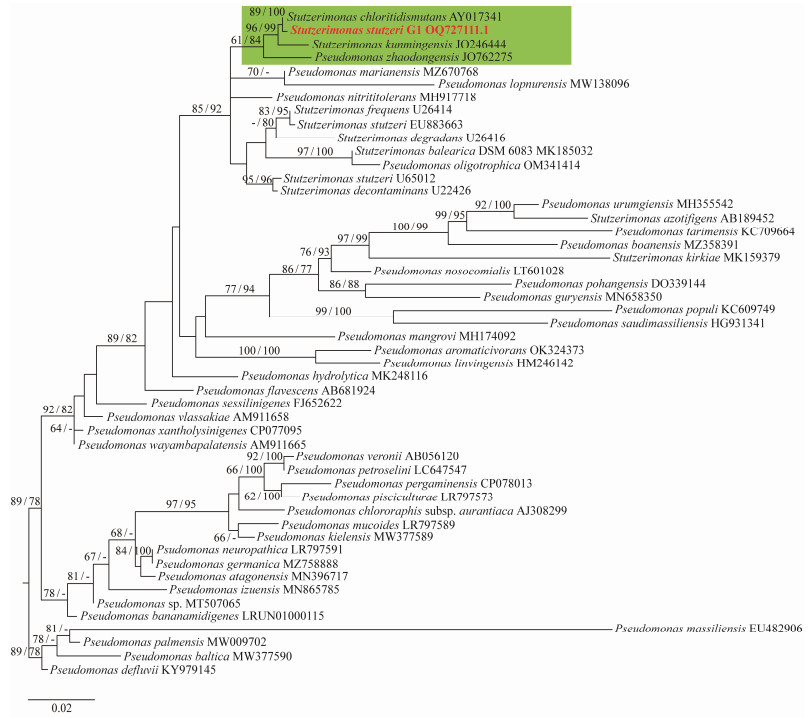
3 Results and Discussion 3.1 Phylogeny of Host Strain Stutzerimonas stutzeri G1From the maximum likelihood phylogenetic tree, G1 forms a separate clade with members of the Stutzerimonas genus, and is most closely related to Stutzerimonas chloritidismutans (Fig.1). The results indicate that G1 is distinct from other members of S. stutzeri and represents a novel species of this genus.
|
Fig. 1 16S rRNA phylogenetic tree of S. stutzeri G1. The green parts are members of Stutzerimonas. Bold red is the host S. stutzeri G1. The reference sequences are the 50 of 16S rRNA sequences from NCBI (16 from Stutzerimonas and 34 from Pseudomonas). |
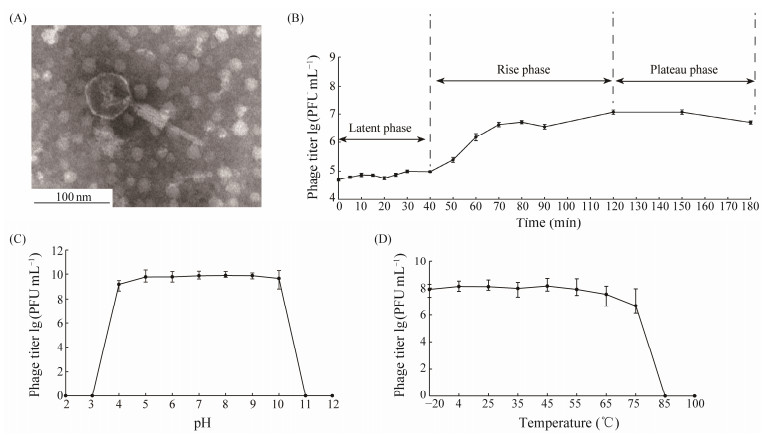
Based on the TEM result, the phage has an icosahedral head with an average diameter of 59 nm and a contractile tail with an average length of 116 nm containing a baseplate complex, shown in Fig.2A (Ackermann, 2007). Only long-tail and tailless S. stutzeri phages have been reported, so this phage is a S. stutzeri phage characterized by a novel structural feature. According to commonly accepted phage nomenclature (Kropinski et al., 2009b), we named this phage vB_SstM-PG1 (PG1 for short).
|
Fig. 2 Morphological and physiological properties of vB_SstM-PG1. (A) vB_SstM-PG1 under transmission electron microscopy. (B) one-step growth curve of vB_SstM-PG1. (C) pH sensitivity curve of vB_SstM-PG1. (D) temperature stability curve of vB_SstM-PG1. |
The one-step growth curve experiment shows that the first 40 min is the latent phase of the phage PG1 (Fig.2B). During the rising phase, the time interval between the latent and plateau phases is from 40 to 120 min, lasting a total of 80 min before entering the plateau phase after 120 min.
In addition to its one-step growth, the viability of phages is also influenced by external unfavorable conditions (Jończyk et al., 2011). Therefore, the pH sensitivity and temperature stability of PG1 were determined. PG1 within a pH range from 3 to 10, and is active within the temperature range from −20℃ to 65℃, with an optimum temperature of about 45℃ (Figs.2C, 2D). These results indicate that an environment ranging from weakly acidic to alkaline is suitable for the survival of phage PG1.
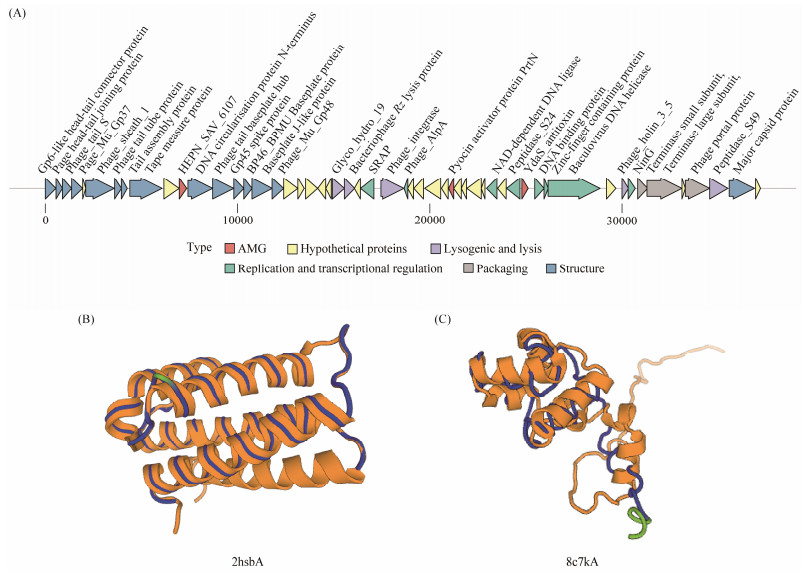
3.3 Genomic Features of Phage PG1The assembled PG1 genome had a complete linear double-stranded DNA sequence with a length of 37204 bp and a GC content of 64.14%. The minimum and maximum values of GC-skew can be used to predict the origin and termination points of replication (Zhang et al., 2021). The results of GC-skew analysis indicate that the replication origin of PG1 phage is located at position 0 nt, while the termination point may locate in the region around 33300 nt. A total of 54 open reading frames (ORFs) were predicted. After Pfam and HHpred analyses, 34 ORFs were identified with clear function (Table 1), which can be broadly divided into five functional modules: phage structure (ORFs 1, 2, 3, 4, 6, 7, 8, 9, 12, 13, 14, 15, 16, 17 and 53), phage packaging (ORFs 48, 49, and 51), DNA replication and transcriptional regulation (ORFs 26, 28, 38, 40, 42, 43, 44, and 47), lysogenic and lysis genes (ORFs 27, 23, 24, 46 and 52) and auxiliary metabolic genes (ORFs 11, 33, and 41). The remaining 20 ORFs were not annotated with any function and were identified as hypothetical proteins (Fig. 3A).
|
|
Table 1 Genomic annotation of the open reading frames (ORFs) of vB_SstM-PG1 |
|
Fig. 3 (A) Genome map of vB_SstM-PG1. The predicted proteins of vB_SstM-PG1 were divided into six modules according to their functions, and were represented with different colors. (B) Structures of ORF11 and 2hsbA are rendered as cartoons by the DALI server. The blue part represents the conserved structure, and the yellow part represents the structure with the highest Z value that ORF11 is aligned to. (C) Structures of ORF41 and 8c7kA are rendered as cartoons by the DALI server. The blue part represents the conserved structure, and the yellow part represents the structure with the highest Z value that ORF41 is aligned to. |
A total of eight ORFs were annotated in the DNA replication and transcription regulation module of the phage PG1 genome. ORF26 encodes an SOS response-associated peptidase (SRAP), which may be involved in DNA repair processes (Aravind et al., 2013). ORF40 in the PG1 phage genome encodes Peptidase_S24 LexA-like proteins. LexA, as a gene repressor, plays an important role in the SOS response by controlling the expressions of a range of genes (Luo et al., 2001). ORF28 encodes a DNA-binding transcription regulator, AlpA, formerly known as Alp protease in Escherichia coli. This gene is widely found in various bacteria, and its regulation mechanism involves binding to DNA and regulating the transcription level of downstream genes. In Pseudomonas aeruginosa, the AlpA system encodes a programmed cell death pathway involved in the expression of virulence in the organism (Peña et al., 2021). Hence, PG1 may have the ability to control the expression of host virulence genes. ORFs 38 and 44 encode enzymes involved in DNA replication, namely NAD-dependent DNA ligase and rod-shaped virus helicase, respectively. ORF43 was annotated as a Zinc-finger containing protein, which has 100% coverage and 83.56% similarity with ORF46 (QSH74620.1) of Stutzerimonas phage vB_PstS-pAN. The phage regulatory protein CII, encoded by ORF42, is thought to be a DNA-binding protein that can control lysogenic development of phages by regulating promoters (Kalionis et al., 1986). The last member of this module is from the ORF47-encoded NinG protein. The ninG gene, also known as the rap gene, is associated with recombination (Tarkowski et al., 2002).
3.5 Lysogenic and Lysis Module of Phage PG1ORF27 in the PG1 genome encodes an integrase family protein, and the presence of integrase genes is a typical feature of temperate phages (Omata et al., 2021). No tRNA genes were identified in PG1, and temperate phages usually contain fewer tRNAs than virulent phages (Bailly-Bechet et al., 2007). Therefore, we infer that PG1 is a temperate phage with a lysogenic life cycle. ORFs 23, 24, 46, and 52 were found to encode four bacterial lysis-associated genes, including Chitinase class Ⅰ of the Glyco_hydro_19 family, phage Rz lysis protein, phage_holin_3_5 protein, and peptidase_S49, respectively. The glycoside hydrolase family 19 is a family with both chitinase and endolysin. Chitinases class Ⅰ are enzymes capable of hydrolyzing the glycosidic bonds present in chitin (Henrissat, 1991). They have the ability to hydrolyze the bacterial cell wall, resulting in host cell lysis and phage release. Thus, PG1 with ORF23 can be considered to have lysozyme activity. Consistent with previous reports, the holin gene is generally found to coexist with endolysin belonging to the lysis module, which is jointly responsible for bacterial lysis and release of phage progeny (Daniel et al., 2007). The holin gene attacks the cytoplasmic membrane, forming non-specific channels or pores in the membrane that allow endolysins to escape and attack peptidoglycans. This is often achieved by a third functional class of lysis proteins, the spanins (Berry et al., 2012). The first spanins identified were lambda Rz and Rz1 (Zhang and Young, 1999), which worked by forming complexes. In this study, only the Rz lysis protein was found to be encoded by ORF24, suggesting that PG1 may contain proteins that have not been annotated for Rz1 function or can play a role in host lysis by the Rz gene alone. In addition to the genes associated with lysing the host described above, some proteins exhibit hydrolysis ability during phage packaging. For example, ORF52 encodes the peptidase_S49 protein of the serine protease family, one of the four major families of prohead proteases. According to previous reports, the prohead protease gene is located between the portal protein gene and the major capsid protein gene and plays a crucial role in capsid morphogenesis (Liu and Mushegian, 2004). It is shown that PG1 phage needs to undergo proteolytic cleavage by peptidase_S49 during assembly to achieve capsid maturation.
3.6 Temperate Phages' Genomic Elements Encoded by PG1In the structure module, PG1 annotation reveals a significantly larger number of structurally relevant proteins than previously reported Stutzerimonas phages, including a total of 15 ORFs. ORF1, ORF2, and ORF3 were all annotated as phage head-tail connector proteins. ORF1 is similar to the Gp6 protein, which is involved in the assembly of phage HK97 connectors (Cardarelli et al., 2010), and is a key component in the junction between the head and tail. The S protein encoded by ORF3 is thought to act in tail completion and stable head. ORF6 encodes a tail sheath protein, while ORF7 is a phage tail tube protein that helps the phage inject its genome into the host bacteria's cytoplasm without disrupting the cell's integrity (Cuervo et al., 2013). ORF8 was annotated as a tail assembly protein. ORF4 performs the same function as phage Mu Gp37, encoding a tail terminator protein related to the polymerization of the tail tube (Pell et al., 2009), while the basis of tail tube polymerization is tape measure protein, encoded by ORF9. The phage tail tape measure protein (TMP) is also involved in the genomic injection process (Cumby et al., 2015). Additionally, there are other ORFs encoding proteins of the phage Mu tail structure. For example, the phage tail baseplate hub (GPD) protein (ORF13) is homologous to phage Mu P proteins, which form the phage central baseplate hub (Gloor and Chaconas, 1988). The phage baseplate puncturing device (ORF14) is functionally similar to phage Mu Gp45, while BP46_BPMU Baseplate protein (ORF15) and Phage_ Mu_Gp48 (ORF17) are homologs of phages Mu Gp46 and Gp48 (Morgan et al., 2002), respectively. The DNA circularization protein N-terminus (DCN) (ORF12) is a protein found in phages that plays a crucial role during host cell infection, helping phages complete genome circulation and packaging. Upon entering the host, the phage's linear DNA is converted into a circular form, facilitating replication and packaging within the host cell. A similar situation occurs in the N-terminal region of Escherichia phage Mu DNA circularization protein N (Puspurs et al., 1983; Gloor and Chaconas, 1986). It is evident that PG1's genome is highly similar to that of the phage Mu tail plate protein in terms of structural modules, suggesting that PG1 may have a tail structure assembly mechanism similar to that of Mu phage. At the same time, it is well known that Mu phages are temperate phages, further validating PG1's classification as a temperate phage based on its tail structural features. Another structural protein located at the edge of the baseplate that is not similar to Mu baseplate protein is encoded by ORF16. The major capsid protein appears to be encoded by ORF53, which assembles like Enterobacter phage HK97 to form icosahedral capsids (Wikoff et al., 2000). In a summary, most structure-related proteins are primarily distributed at the upstream of PG1's genome.
3.7 DNA Packaging Gene RegionThree gene products involved in DNA packaging were identified in the packaging module of the phage PG1 genome, including the phage terminase small subunit encoded by ORF48, the terminase large subunit encoded by ORF49, and the phage portal protein encoded by ORF51. These genes are located at the downstream portion of the PG1 genome, with the terminase small subunit adjacent to the terminase large subunit in the genome location. Terminating enzymes are essential functional proteins in the genome packaging process of dsDNA virus heads (Petrovski et al., 2011). The portal protein serves to link genome packaging to the maturation of the icosahedral shell.
3.8 Auxiliary Metabolic GenesThe phage PG1 genome contains three AMGs, including the pyocin activator PrtN family protein (ORF33), YdaS_ antitoxin (ORF41), and HEPN_SAV_6107 (ORF11). PrtN is associated with pyocin, a bacterial toxin secreted by Pseudomonas aeruginosa that promotes intraspecific competition by killing competing strains (Matsui et al., 1993). PrtN is a transcriptional activator of the pyocyanin synthesis gene, activating the expression of various pyocyanin genes by interacting with conserved DNA sequences in the 5' noncoding region of the pyocyanin gene. YdaS_antitoxin is a family of putative bacterial antitoxins that form a stable complex by neutralizing the homologous toxin YdaT family due to its poor intracellular stability (Yamaguchi and Inouye, 2011). This toxin found in PG1 may potentially modulate the proliferation and apoptosis of host cells. ORF11 represents the HEPN domain found in SAV_6107. The HEPN domain is an important component of many toxin-antitoxin (TA) and abortive infection (Abi) systems in prokaryotes (Songailiene et al., 2020). It acts as a toxic RNase and is widely used in various intragenomic, intergenomic, and inter-organism conflicts. Host cells often secrete toxin RNases to lyse viral RNA or target their RNA (Anantharaman et al., 2013). It can therefore be speculated that PG1 can cleave other viral RNAs by carrying toxin RNases, thereby resisting other viruses competing for hosts.
To further ensure the accuracy of the results, we performed structural analysis of ORF41 and ORF11 using the Dali server. As DALI supports pairwise structure alignment and database searches, we searched the PDB files of the two proteins predicted by Alphafold2 in the DALI database. The structural similarity between proteins is evaluated based on the DALI Z score, which is a standard for measuring the quality of structural arrangement. When the Z score is greater than or equal to 0.2, the result is considered meaningful (Holm, 2020). Fig.3B shows a comparison of the structure of 2hsbA (Z = 15.4) and the ORF11 structure. We found that ORF11 and UPF0332 family containing HEPN domain (2hsbA) are highly homologous (the structurally conserved region is shown in blue in Fig.3B). Fig.3C shows a comparison of ORF41 with the structure of 8c7kA (Z = 8.8). We found that ORF41 has significant similarity with YdaS structure (8c7kA), which may indicate that they have a common origin. In a summery, ORF11 and ORF41 code for proteins that have the functions of HEPN_SAV_6107 and YdaS_antitoxin, respectively.
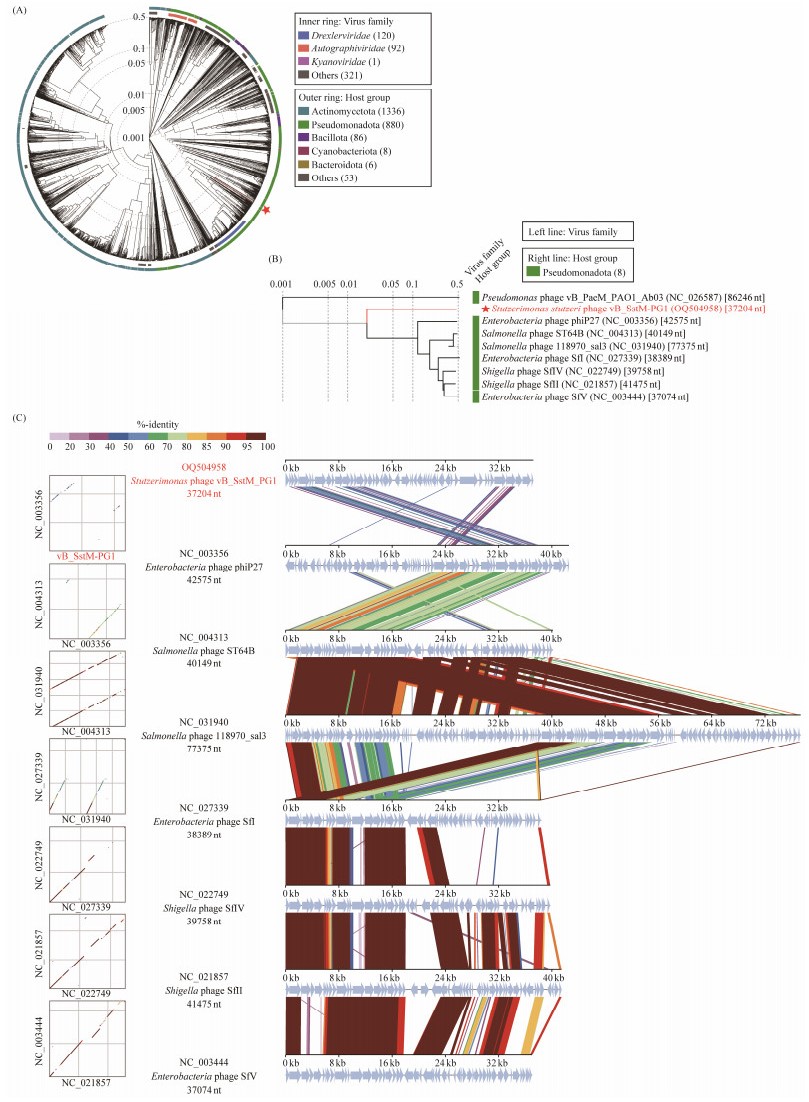
3.9 Phylogenetic Analysis Revealed That PG1 Represents a Novel Viral ClusterTo further determine the taxonomic status of PG1, a whole-genome amino acid sequence-based phylogenetic tree was constructed using ViPTree. This tree included PG1 and other reference phages (Fig.4A). PG1 formed a separate clade, from which seven phage genomes similar to PG1 were extracted to construct a rectangular phylogenetic tree (Fig.4B). Evidently, PG1 is not similar to any isolated phage of the genus Stutzerimonas, suggesting that it may have a different genome composition from other isolated phages. To further validate this hypothesis, PG1 was compared with these seven isolated phages for comparative genomic analysis and nucleotide sequence identification. In the comparative genome collinearity analysis (Fig.4C), it is clear that the similarity between phage PG1 and isolated phage homologous genes is not high. Meanwhile, the nucleotide identity analysis revealed that although the genome length of PG1 and the seven separated phages were roughly similar, the ANI between PG1 and the separated phages was even lower than 10%. This double verification confirmed our previous speculation that PG1 is not closely related to the separated phages, suggesting that it represents a novel cluster.
|
Fig. 4 Phylogenetic and comparative genomic analyses of vB_SstM-PG1. (A) Whole-genome amino acid sequences constructed by ViPTree based on vB_SstM-PG1 and other reference phages. vB_SstM-PG1 is marked with a red pentagram. (B) Phylogenetic tree of vB_SstM-PG1 and the seven closest virus genomes. (C) Comparative genomic analysis of vB_SstM-PG1 and the seven most closely related phages. The shading parts beneath each genome represent sequence similarities between the genomes, and different colors indicate different levels of similarity. |
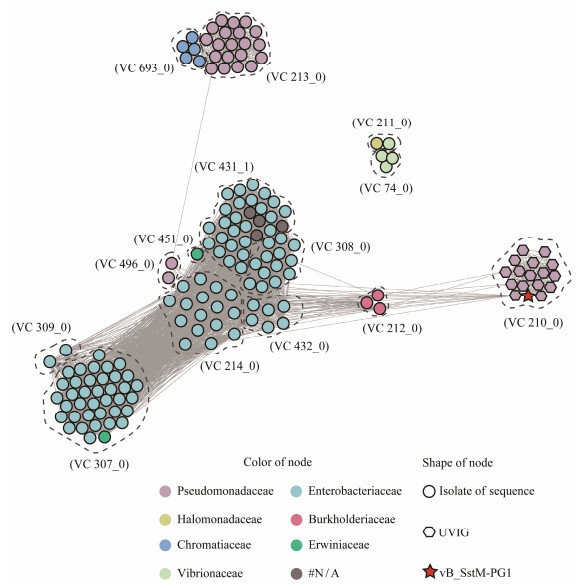
Despite representing a new viral group, it is difficult to determine whether PG1 represents a new genus or family based solely on its relationship with isolated viruses. Therefore, genomic analysis was conducted to compare PG1 with uncultivated viruses from IMG/VR v4 to explore the relationship between PG1 and metagenomic-assembled viruses. Surprisingly, using an all-vs-all blastp search in the IMG/VR v4 dataset, 17 high-quality uncultured viral genomes (UViGs) were found to be similar to PG1, with over 50% of ORF coverage. Following this result, PG1, UViGs similar to PG1, and reference sequences from the International Committee on the Taxonomy of Viruses (ICTV) were partitioned into different VCs using vConTACT2.0. Finally, the clustering results were visualized as a network graph using Gephi (Fig.5), showing that PG1 and 16 UViGs clustered together as members of VC_210, while the remaining 140 members from ICTV were divided into 13 different VC groups. According to the host information, it can be observed that PG1 is clustered together with uncultured viruses that are predicted to infect members of the Pseudomonadaceae family in the metagenomic library, rather than being clustered to previous isolated viruses.
|
Fig. 5 The network of the whole genome of vB_SstM-PG1 from NCBI RefSeq database and other related viral sequences from IMG/VR v4. The nodes represent the viral genomic sequences. The edges represent the similarity score between genomes based on shared gene content. In each VC, viral genomes that belong to different host clusters are indicated by different colors. VCs are generated using vConTACT2, and the network is visualized using Gephi. |
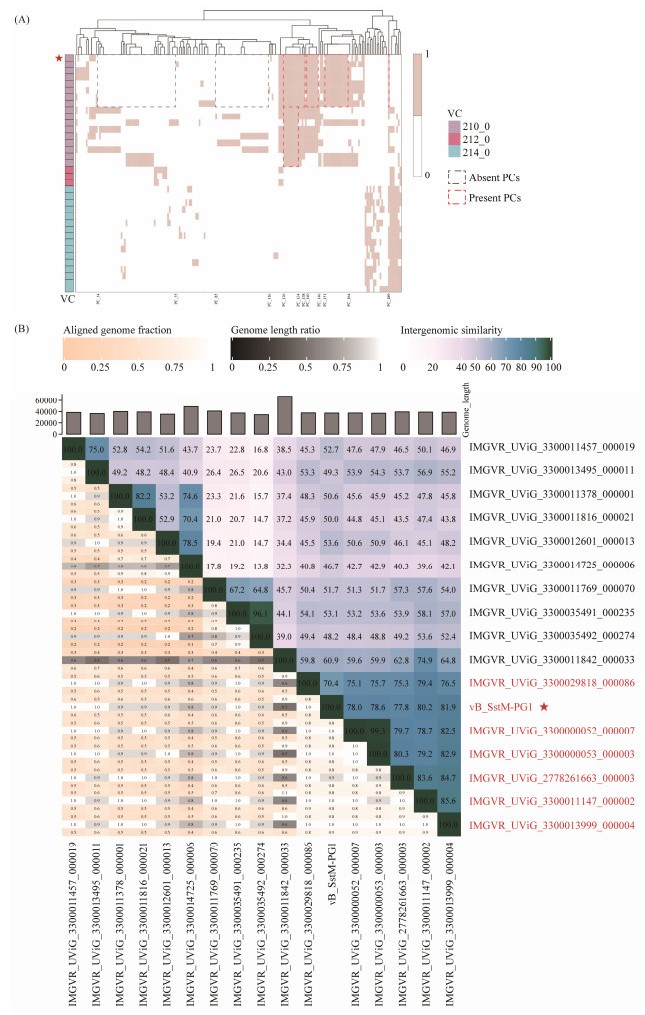
The presence or absence of key proteins plays an important role in the classification of phages. To further explore the relationship between PG1 and other members of VC_210, protein numbers for PG1 and related viruses in the same viral cluster were extracted based on the vConTACT results and represented by different PC values. A heat map was generated using an R package to perform protein clustering network analysis as shown in Fig.6A. The results show that there are three related VCs, and except for VC_210, the components of the other VCs are already isolated viruses. A total of 196 PC members are involved, and it can be seen that the two VCs below have very few shared proteins with VC_210. The members of VC_210 collectively possess nine PCs, namely PC126 – PC134. In addition, there are four distinctive PC modules in PG1, with the first three modules consisting of consecutive PC numbers, including PC126 – PC138, PC140 – PC146, and PC151 – PC164. The last one is a standalone PC, which is PC189. These four types of PCs were also identified in seven uncultured viruses below and have been annotated in Fig.6A. Among the uncultured viruses, seven strains (PC14 – PC55 and PC85 – PC116) lack PC types as observed in PG1. These two groups of PCs are commonly absent in these strains. Therefore, it is inferred that PG1 has a closer relationship with these seven viruses in VC_210. Further nucleotide identity analysis (Fig.6B) confirmed that PG1 exhibited an ANI greater than 70% with the six uncultured viruses mentioned earlier. As stipulated by ICTV, they can be considered as a new genus. PG1 is the only isolated member of this new genus, named Metabovirus.
|
Fig. 6 (A) Protein clustering network heatmap. vConTACT2 classifies PG1 and uncultured viral genomes into VC210, and the other three related VCs are composed of viruses from the NCBI RefSeq database, with a total of 196 related PCs. (B) Nucleotide identity heatmap between vB_SstM-PG1 and seven uncultured viruses. The Bacterial and Archaeal Viruses Subcommittee (BAVS) of the ICTV considers phages that share 70% ANI as members of the same genus. In the right half of the diagram, color coding is used to quickly visualize phage genome clustering based on intergenic similarity. The closer the relationship between genomes, the darker is the color. The numbers represent the similarity between each pair of genomes, i.e., nucleotide identity. The left half of the diagram can be used to analyze three indicators between each pair of genomes. From top to bottom, the figure indicates aligned partial genome 1 (for the genome found in this row), genome length ratio (for two genomes in this pair), and aligned partial genome 2 (for the genome found in this column), respectively. |
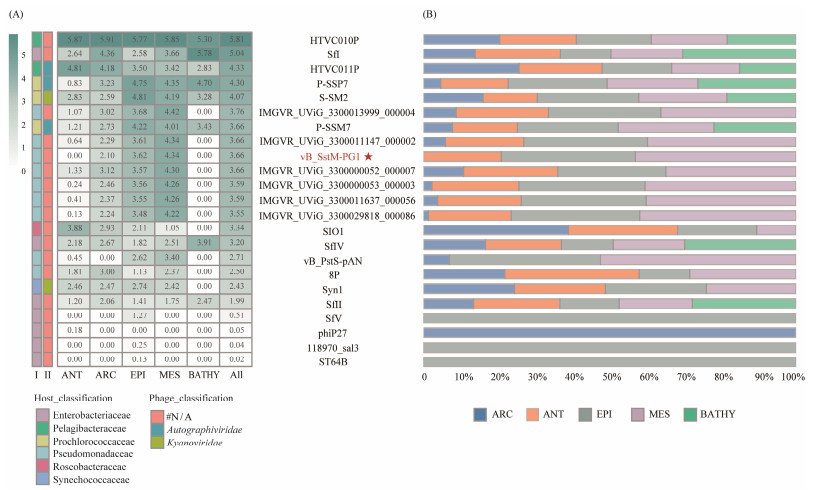
To investigate the ecological distribution of PG1 in the ocean, we described the biogeographical distribution characteristics of Pseudomonas aeruginosa phage PG1 in 154 viral metagenomes from five VEZs in the sea dataset (GOV2.0). The results (Fig.7) show that the distribution of phages belonging to the Enterobacteriaceae family, which were on the same branch as PG1 in the phylogenetic tree, did not exhibit significant regularity and did not show ecological similarity with PG1. Some members of Enterobacteriaceae such as Sfl had high abundance, even higher than Pelagibacter phage HTVC011P, while others belonging to the same family had very low abundance or were not detected, such as phages phiP27, 118970_sal3, and ST64B. The presence of PG1 has been detected in three ecotypes, including ARC, EPI, and MES, but not in ANT and BATHY. Although PG1 has demonstrated activity across a wide temperature range in physiological experiments, the temperatures in ANT and BATHY are significantly below PG1's optimal temperature of 45℃. This temperature discrepancy could potentially inhibit PG1 from achieving its maximum activity, thereby affecting its distribution within these environments. Related viruses within the same genus also exhibit a similar distribution pattern to PG1, and all members of this genus have higher abundance than the representative marine Roseophage SIO1. The above results indicate that the abundance of S. stutzeri phage PG1 in the ocean is relatively high. The abundance of the two other known phages of S. stutzeri was not as high as that of PG1, while no S. stutzeri phages were detected in the deep sea. Therefore, it is speculated that the members of the genus containing phage PG1 may be the most abundant Stutzerimonas phages in the ocean.
|
Fig. 7 Relative abundance of vB_SstM-PG1 in 154 viromes that are chosen from the Global Ocean Viromes (GOV 2.0) data set. The relative abundance was expressed as TPM (B), and the values are calculated by CoverM (v0.3.1) (A). Values were normalized by the number of databases of each VEZ for the right bar chart, and the result was log10 transformed for the left heatmap. The reference sequence mainly included isolated and uncultured viruses associated with vB_SstM-PG1, two strains of Stutzerimonas phages, representative Pelagibacter phages from the ocean (HTVC010P, HTVC019P, HTVC011P), cyanophages (P-SSB7, P-SSM7), and the SIO1 phage. Five marine VEZs are ARC, ANT, EPI, MES, and BATHY. |
This study isolated phage PG1 infecting S. stutzeri G1 and performed a comprehensive characterization including physiological feature analysis, genomic, phylogenetic, comparative genomic, and biogeographic analysis. The majority of PG1 genes encode structural proteins with a tail structure of baseplate complex, which is an important feature differentiating it from known Stutzerimonas phages. An integrase was identified in PG1, which lacks tRNA and exhibits structural protein characteristics similar to those of temperate phages. In addition, three AMGs have been found in the PG1 genome, including pyocin activator PrtN family protein, YdaS_antitoxin, and HEPN_SAV_6107, which play important roles in regulating host growth and metabolism. Genomic and phylogenetic analyses suggest that PG1 is novel, and represents a new viral genus together with six UViGs. Biogeographic analysis reveals that PG1 is the most abundant phage infecting S. stutzeri, highlighting the diversity, evolution, and ecology of phage-microbe interactions in the marine environment. In general, the comprehensive study on the lytic phage PG1 has expanded the taxonomic group of Stutzerimonas phages, enriched the existing phage library, and will provide an important reference for future studies on implementing S. stutzeri phages.
5 Data AvailabilityThe genome sequence of phage vB_SstM-PG1 has been deposited in GenBank under accession number OQ504958. The 16S rRNA sequence of the host also has been deposited in NCBI under accession number OQ727111.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (Nos. 42188102, 42120104006, 41976117, 42176111 and 42306111), and the Fundamental Research Funds for the Central Universities (No. 2018 12002 and Andrew McMinn). We thank the Center for High Performance Computing and System Simulation, Laoshan Laboratory (Qingdao) for the support of the high-performance servers; the Institute of Evolution and Marine Biodiversity for operating the IEMB-1, a high-performance computing cluster; and the Marine Big Data Center of Institute for Advanced Ocean Study of Ocean University of China.
Ackermann, H. W., 2007. 5500 phages examined in the electron microscope. Archives of Virology, 152(2): 227-243. DOI:10.1007/s00705-006-0849-1 (  0) 0) |
Anantharaman, V., Makarova, K. S., Burroughs, A. M., Koonin, E. V., and Aravind, L., 2013. Comprehensive analysis of the HEPN superfamily: Identification of novel roles in intra-genomic conflicts, defense, pathogenesis and RNA processing. Biology Direct, 8: 15. DOI:10.1186/1745-6150-8-15 (  0) 0) |
Angly, F., Youle, M., Nosrat, B., Srinagesh, S., Rodriguez-Brito, B., McNairnie, P., et al., 2009. Genomic analysis of multiple Roseophage SIO1 strains. Environmental Microbiology, 11(11): 2863-2873. DOI:10.1111/j.1462-2920.2009.02021.x (  0) 0) |
Aravind, L., Anand, S., and Iyer, L. M., 2013. Novel autoproteolytic and DNA-damage sensing components in the bacterial SOS response and oxidized methylcytosine-induced eukaryotic DNA demethylation systems. Biology Direct, 8: 20. DOI:10.1186/1745-6150-8-20 (  0) 0) |
Bailly-Bechet, M., Vergassola, M., and Rocha, E., 2007. Causes for the intriguing presence of tRNAs in phages. Genome Research, 17: 1486-1495. DOI:10.1101/gr.6649807 (  0) 0) |
Bastian, M., Heymann, S., and Jacomy, M., 2009. Gephi: An open source software for exploring and manipulating networks. The Proceedings of the International AAAI Conference on Web and Social Media, 3(1): 361-362. (  0) 0) |
Ben Haj Khalifa, A., Moissenet, D., Vu Thien, H., and Khedher, M., 2011. Virulence factors in Pseudomonas aeruginosa: Mechanisms and modes of regulation. Annales de Biologie Clinique, 69(4): 393-403. DOI:10.1684/abc.2011.0589 (  0) 0) |
Berry, J., Rajaure, M., Pang, T., and Young, R., 2012. The spanin complex is essential for lambda lysis. Journal of Bacteriology, 194(20): 5667-5674. DOI:10.1128/jb.01245-12 (  0) 0) |
Besemer, J., Lomsadze, A., and Borodovsky, M., 2001. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Research, 29(12): 2607-2618. DOI:10.1093/nar/29.12.2607 (  0) 0) |
Bin Jang, H., Bolduc, B., Zablocki, O., Kuhn, J. H., Roux, S., Adriaenssens, E. M., et al., 2019. Taxonomic assignment of uncultivated prokaryotic virus genomes is enabled by genesharing networks. Nature Biotechnology, 37: 632-639. DOI:10.1038/s41587-019-0100-8 (  0) 0) |
Bolduc, B., Jang, H. B., Doulcier, G., You, Z. Q., Roux, S., and Sullivan, M. B., 2017. vConTACT: An iVirus tool to classify double-stranded DNA viruses that infect Archaea and Bacteria. PeerJ, 5: e3243. DOI:10.7717/peerj.3243 (  0) 0) |
Bolger, A. M., Lohse, M., and Usadel, B., 2014. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30(15): 2114-2120. DOI:10.1093/bioinformatics/btu170 (  0) 0) |
Camargo, A. P., Nayfach, S., Chen, I. A., Palaniappan, K., Ratner, A., Chu, K., et al., 2023. IMG/VR v4: An expanded database of uncultivated virus genomes within a framework of extensive functional, taxonomic, and ecological metadata. Nucleic Acids Research, 51(D1): D733-D743. DOI:10.1093/nar/gkac1037 (  0) 0) |
Cardarelli, L., Lam, R., Tuite, A., Baker, L. A., Sadowski, P. D., Radford, D. R., et al., 2010. The crystal structure of bacteriophage HK97 gp6: Defining a large family of head-tail connector proteins. Journal of Molecular Biology, 395(4): 754-768. DOI:10.1016/j.jmb.2009.10.067 (  0) 0) |
Cianfrocco, M. A., Wong-Barnum, M., Youn, C., Wagner, R., and Leschziner, A., 2017. COSMIC2: A science gateway for cryoelectron microscopy structure determination. The Proceedings of the Practice and Experience in Advanced Research Computing 2017 on Sustainability, Success and Impact. New Orleans, LA, USA, Association for Computing Machinery.
(  0) 0) |
Cuervo, A., Pulido-Cid, M., Chagoyen, M., Arranz, R., González-García, V. A., Garcia-Doval, C., et al., 2013. Structural characterization of the bacteriophage T7 tail machinery. Journal of Biological Chemistry, 288(36): 26290-26299. DOI:10.1074/jbc.M113.491209 (  0) 0) |
Cumby, N., Reimer, K., Mengin-Lecreulx, D., Davidson, A. R., and Maxwell, K. L., 2015. The phage tail tape measure protein, an inner membrane protein and a periplasmic chaperone play connected roles in the genome injection process of E. coli phage HK97. Molecular Microbiology, 96(3): 437-447. DOI:10.1111/mmi.12918 (  0) 0) |
Daniel, A., Bonnen, P. E., and Fischetti, V. A., 2007. First complete genome sequence of two Staphylococcus epidermidis bacteriophages. Journal of Bacteriology, 189(5): 2086-2100. DOI:10.1128/jb.01637-06 (  0) 0) |
Deveau, H., Labrie, S. J., Chopin, M. C., and Moineau, S., 2006. Biodiversity and classification of lactococcal phages. Applied and Environmental Microbiology, 72(6): 4338-4346. DOI:10.1128/AEM.02517-05 (  0) 0) |
Diggle, S. P., and Whiteley, M., 2020. Microbe profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiology, 166(1): 30-33. DOI:10.1099/mic.0.000860 (  0) 0) |
Dong, Y., Zheng, K., Zou, X., Liang, Y., Liu, Y., Li, X., et al., 2022. Characterization and genomic analysis of the first podophage infecting Shewanella, representing a novel viral cluster. Frontiers in Microbiology, 13: 853973. DOI:10.3389/fmicb.2022.853973 (  0) 0) |
Feng, Z., Liu, X., Wang, M., Nie, Y., and Wu, X. L., 2021. A novel temperate phage, vB_PstS-pAN, induced from the naphthalene-degrading bacterium Pseudomonas stutzeri AN10. Archives of Virology, 166(8): 2267-2272. DOI:10.1007/s00705-021-05098-8 (  0) 0) |
Gloor, G., and Chaconas, G., 1986. The bacteriophage Mu N gene encodes the 64-kDa virion protein which is injected with, and circularizes, infecting Mu DNA. Journal of Biological Chemistry, 261(35): 16682-16688. DOI:10.1016/S0021-9258(18)66619-0 (  0) 0) |
Gloor, G., and Chaconas, G., 1988. Sequence of bacteriophage Mu N and P genes. Nucleic Acids Research, 16(11): 5211-5212. DOI:10.1093/nar/16.11.5211 (  0) 0) |
Gomila, M., Mulet, M., García-Valdés, E., and Lalucat, J., 2022. Genome-based taxonomy of the genus Stutzerimonas and proposal of S. frequens sp. nov. and S. degradans sp. nov. and emended descriptions of S. perfectomarina and S. chloritidismutans. Microorganisms, 10(7): 1363. (  0) 0) |
Gregory, A. C., Zayed, A. A., Conceição-Neto, N., Temperton, B., Bolduc, B., Alberti, A., et al., 2019. Marine DNA viral macro- and microdiversity from pole to pole. Cell, 177(5): 1109-1123.e1114. DOI:10.1016/j.cell.2019.03.040 (  0) 0) |
Halabi, Z., Mocadie, M., El Zein, S., and Kanj, S. S., 2019. Pseudomonas stutzeri prosthetic valve endocarditis: A case report and review of the literature. Journal of Infection and Public Health, 12(3): 434-437. DOI:10.1016/j.jiph.2018.07.004 (  0) 0) |
Hendrix, R. W., Smith, M. C., Burns, R. N., Ford, M. E., and Hatfull, G. F., 1999. Evolutionary relationships among diverse bacteriophages and prophages: All the world's a phage. The Proceedings of the National Academy of Sciences of the United States of America, 96(5): 2192-2197. DOI:10.1073/pnas.96.5.2192 (  0) 0) |
Henrissat, B., 1991. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochemical Journal, 280(Pt 2): 309-316. DOI:10.1042/bj2800309 (  0) 0) |
Holm, L., 2020. DALI and the persistence of protein shape. Protein Science, 29(1): 128-140. DOI:10.1002/pro.3749 (  0) 0) |
Holm, L., and Sander, C., 1995. Dali: A network tool for protein structure comparison. Trends in Biochemical Sciences, 20(11): 478-480. DOI:10.1016/s0968-0004(00)89105-7 (  0) 0) |
Huang, G., Le, S., Peng, Y., Zhao, Y., Yin, S., Zhang, L., et al., 2013. Characterization and genome sequencing of phage Abp1, a new phiKMV-like virus infecting multidrug-resistant Acinetobacter baumannii. Current Microbiology, 66(6): 535-543. DOI:10.1007/s00284-013-0308-7 (  0) 0) |
Iglewski, B. H., 1996. Pseudomonas. In: Medical Microbiology. Baron, S., ed., University of Texas Medical Branch at Galveston, 1340pp.
(  0) 0) |
Jończyk, E., Kłak, M., Międzybrodzki, R., and Górski, A., 2011. The influence of external factors on bacteriophages – Review. Folia Microbiologica, 56(3): 191-200. DOI:10.1007/s12223-011-0039-8 (  0) 0) |
Kalionis, B., Dodd, I. B., and Egan, J. B., 1986. Control of gene expression in the P2-related template coliphages. Ⅲ. DNA sequence of the major control region of phage 186. Journal of Molecular Biology, 191(2): 199-209. DOI:10.1016/0022-2836(86)90257-3 (  0) 0) |
Kropinski, A. M., Mazzocco, A., Waddell, T. E., Lingohr, E., and Johnson, R. P., 2009a. Enumeration of bacteriophages by double agar overlay plaque assay. Methods in Molecular Biology, 501: 69-76. DOI:10.1007/978-1-60327-164-6_7 (  0) 0) |
Kropinski, A. M., Prangishvili, D., and Lavigne, R., 2009b. Position paper: The creation of a rational scheme for the nomenclature of viruses of Bacteria and Archaea. Environmental Microbiology, 11(11): 2775-2777. DOI:10.1111/j.1462-2920.2009.01970.x (  0) 0) |
Lalucat, J., Bennasar, A., Bosch, R., García-Valdés, E., and Palleroni, N. J., 2006. Biology of Pseudomonas stutzeri. Microbiology and Molecular Biology Reviews, 70(2): 510-547. DOI:10.1128/mmbr.00047-05 (  0) 0) |
Lalucat, J., Gomila, M., Mulet, M., Zaruma, A., and Garcia-Valdes, E., 2022. Past, present and future of the boundaries of the Pseudomonas genus: Proposal of Stutzerimonas gen. nov. Systematic and Applied Microbiology, 45(1): 126289. DOI:10.1016/j.syapm.2021.126289 (  0) 0) |
Li, H., 2018. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics, 34(18): 3094-3100. DOI:10.1093/bioinformatics/bty191 (  0) 0) |
Liu, J., and Mushegian, A., 2004. Displacements of prohead protease genes in the late operons of double-stranded-DNA bacteriophages. Journal of Bacteriology, 186(13): 4369-4375. DOI:10.1128/jb.186.13.4369-4375.2004 (  0) 0) |
Liu, X., Feng, Z., Fan, X., Nie, Y., and Wu, X. L., 2021a. Isolation and characterization of the novel Pseudomonas stutzeri bacteriophage 8P. Archives of Virology, 166(2): 601-606. DOI:10.1007/s00705-020-04912-z (  0) 0) |
Liu, Y., Zheng, K., Liu, B., Liang, Y., You, S., Zhang, W., et al., 2021b. Characterization and genomic analysis of marinobacter phage vB_MalS-PS3, representing a new lambda-like temperate siphoviral genus infecting algae-associated bacteria. Frontiers in Microbiology, 12: 726074. DOI:10.3389/fmicb.2021.726074 (  0) 0) |
Lukashin, A. V., and Borodovsky, M., 1998. GeneMark. hmm: New solutions for gene finding. Nucleic Acids Research, 26(4): 1107-1115. DOI:10.1093/nar/26.4.1107 (  0) 0) |
Luo, Y., Pfuetzner, R. A., Mosimann, S., Paetzel, M., Frey, E. A., Cherney, M., et al., 2001. Crystal structure of LexA: A conformational switch for regulation of self-cleavage. Cell, 106(5): 585-594. DOI:10.1016/s0092-8674(01)00479-2 (  0) 0) |
Matsui, H., Sano, Y., Ishihara, H., and Shinomiya, T., 1993. Regulation of pyocin genes in Pseudomonas aeruginosa by positive (prtN) and negative (prtR) regulatory genes. Journal of Bacteriology, 175(5): 1257-1263. DOI:10.1128/jb.175.5.1257-1263.1993 (  0) 0) |
Meier-Kolthoff, J. P., Carbasse, J. S., Peinado-Olarte, R. L., and Göker, M., 2021. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Research, 50(D1): D801-D807. DOI:10.1093/nar/gkab902 (  0) 0) |
Moraru, C., Varsani, A., and Kropinski, A. M., 2020. VIRIDIC – A novel tool to calculate the intergenomic similarities of prokaryote-infecting viruses. Viruses, 12(11): 1258. DOI:10.3390/v12111268 (  0) 0) |
Morgan, G. J., Hatfull, G. F., Casjens, S., and Hendrix, R. W., 2002. Bacteriophage Mu genome sequence: Analysis and comparison with Mu-like prophages in Haemophilus, Neisseria and Deinococcus. Journal of Molecular Biology, 317(3): 337-359. DOI:10.1006/jmbi.2002.5437 (  0) 0) |
Nishimura, Y., Yoshida, T., Kuronishi, M., Uehara, H., Ogata, H., and Goto, S., 2017. ViPTree: The viral proteomic tree server. Bioinformatics, 33(15): 2379-2380. DOI:10.1093/bioinformatics/btx157 (  0) 0) |
Omata, K., Hibi, N., Nakano, S., Komoto, S., Sato, K., Nunokawa, K., et al., 2021. Distribution and genome structures of temperate phages in acetic acid bacteria. Scientific Reports, 11(1): 21567. DOI:10.1038/s41598-021-00998-w (  0) 0) |
Palleroni, N. J., 1981. Introduction to the family Pseudomonadaceae. In: The Prokaryotes: A Handbook on Habitats, Isolation, and Identification of Bacteria. Starr, M. P., et al., eds., Berlin, Heidelberg, Springer Berlin Heidelberg, 655-665.
(  0) 0) |
Parte, A. C., Sardà Carbasse, J., Meier-Kolthoff, J. P., Reimer, L. C., and Göker, M., 2020. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. International Journal of Systematic and Evolutionary Microbiology, 70(11): 5607-5612. DOI:10.1099/ijsem.0.004332 (  0) 0) |
Pell, L. G., Liu, A., Edmonds, L., Donaldson, L. W., Howell, P. L., and Davidson, A. R., 2009. The X-ray crystal structure of the phage lambda tail terminator protein reveals the biologically relevant hexameric ring structure and demonstrates a conserved mechanism of tail termination among diverse long-tailed phages. Journal of Molecular Biology, 389(5): 938-951. DOI:10.1016/j.jmb.2009.04.072 (  0) 0) |
Peña, A., Busquets, A., Gomila, M., Bosch, R., Nogales, B., García-Valdés, E., et al., 2012. Draft genome of Pseudomonas stutzeri strain ZoBell (CCUG 16156), a marine isolate and model organism for denitrification studies. Journal of Bacteriology, 194(5): 1277-1278. DOI:10.1128/jb.06648-11 (  0) 0) |
Peña, J. M., Prezioso, S. M., McFarland, K. A., Kambara, T. K., Ramsey, K. M., Deighan, P., et al., 2021. Control of a programmed cell death pathway in Pseudomonas aeruginosa by an antiterminator. Nature Communications, 12(1): 1702. DOI:10.1038/s41467-021-21941-7 (  0) 0) |
Petrovski, S., Seviour, R. J., and Tillett, D., 2011. Genome sequence and characterization of the Tsukamurella bacteriophage TPA2. Applied and Environmental Microbiology, 77(4): 1389-1398. DOI:10.1128/aem.01938-10 (  0) 0) |
Puspurs, A. H., Trun, N. J., and Reeve, J. N., 1983. Bacteriophage Mu DNA circularizes following infection of Escherichia coli. EMBO Journal, 2(3): 345-352. DOI:10.1002/j.1460-2075.1983.tb01429.x (  0) 0) |
Qin, S., Xiao, W., Zhou, C., Pu, Q., Deng, X., Lan, L., et al., 2022. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduction and Targeted Therapy, 7(1): 199. DOI:10.1038/s41392-022-01056-1 (  0) 0) |
Rohwer, F. L., and Thurber, R. V., 2009. Viruses manipulate the marine environment. Nature, 459: 207-212. (  0) 0) |
Rossello, R., García-Valdés, E., Lalucat, J., and Ursing, J., 1991. Genotypic and phenotypic diversity of Pseudomonas stutzeri. Systematic and Applied Microbiology, 14: 150-157. (  0) 0) |
Schroeter, J., 1872. Über einige durch Bacterien gebildete Pigmente. Beiträge zur Biologie der Pflanzen, Max Müller, Breslau, 109-126.
(  0) 0) |
Simpson, J. T., Wong, K., Jackman, S. D., Schein, J. E., Jones, S. J., and Birol, I., 2009. ABySS: Aparallel assembler for short read sequence data. Genome Research, 19(6): 1117-1123. DOI:10.1101/gr.089532.108 (  0) 0) |
Songailiene, I., Juozapaitis, J., Tamulaitiene, G., Ruksenaite, A., Šulčius, S., Sasnauskas, G., et al., 2020. HEPN-MNT toxinantitoxin system: The HEPN ribonuclease is neutralized by OligoAMPylation. Molecular Cell, 80(6): 955-970.e957. DOI:10.1016/j.molcel.2020.11.034 (  0) 0) |
Suttle, C. A., 2013. Viruses: Unlocking the greatest biodiversity on Earth. Genome, 56(10): 542-544. DOI:10.1139/gen-2013-0152 (  0) 0) |
Tarkowski, T. A., Mooney, D., Thomason, L. C., and Stahl, F. W., 2002. Gene products encoded in the ninR region of phage lambda participate in Red-mediated recombination. Genes to Cells, 7(4): 351-363. DOI:10.1046/j.1365-2443.2002.00531.x (  0) 0) |
Taylor, N. M. I., and Leiman, P. G., 2020. Editorial overview: Virus structure and expression. Current Opinion in Virology, 45: ⅲ-ⅴ. DOI:10.1016/j.coviro.2020.11.005 (  0) 0) |
Teeling, H., Meyerdierks, A., Bauer, M., Amann, R., and Glöckner, F. O., 2004. Application of tetranucleotide frequencies for the assignment of genomic fragments. Environmental Microbiology, 6(9): 938-947. DOI:10.1111/j.1462-2920.2004.00624.x (  0) 0) |
Waite, C. C. D. C, Silva, G. O. A. D., Bitencourt, J. A. P., Chequer, L. P. T., Pennafirme, S., Jurelevicius, D. D. A., et al., 2020. Potential application of Pseudomonas stutzeri W228 for removal of copper and lead from marine environments. PLoS One, 15(10): e0240486. DOI:10.1371/journal.pone.0240486 (  0) 0) |
Weitz, J. S., Poisot, T., Meyer, J. R., Flores, C. O., Valverde, S., Sullivan, M. B., et al., 2013. Phage-bacteria infection networks. Trends in Microbiology, 21(2): 82-91. DOI:10.1016/j.tim.2012.11.003 (  0) 0) |
Wikoff, W. R., Liljas, L., Duda, R. L., Tsuruta, H., Hendrix, R. W., and Johnson, J. E., 2000. Topologically linked protein rings in the bacteriophage HK97 capsid. Science, 289(5487): 2129-2133. DOI:10.1126/science.289.5487.2129 (  0) 0) |
Xu, M., Guo, L., Gu, S., Wang, O., Zhang, R., Peters, B. A., et al., 2020. TGS-GapCloser: A fast and accurate gap closer for large genomes with low coverage of error-prone long reads. GigaScience, 9(9): giaa094. DOI:10.1093/gigascience/giaa094 (  0) 0) |
Yamaguchi, Y., and Inouye, M., 2011. Regulation of growth and death in Escherichia coli by toxin-antitoxin systems. Nature Reviews: Microbiology, 9(11): 779-790. DOI:10.1038/nrmicro2651 (  0) 0) |
Yan, Y., Yang, J., Dou, Y., Chen, M., Ping, S., Peng, J., et al., 2008. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. The Proceedings of the National Academy of Sciences, 105(21): 7564-7569. DOI:10.1073/pnas.0801093105 (  0) 0) |
Yang, X., Wang, S., and Zhou, L., 2012. Effect of carbon source, C/N ratio, nitrate and dissolved oxygen concentration on nitrite and ammonium production from denitrification process by Pseudomonas stutzeri D6. Bioresource Technology, 104: 65-72. DOI:10.1016/j.biortech.2011.10.026 (  0) 0) |
Zhang, N., and Young, R., 1999. Complementation and characterization of the nested Rz and Rz1 reading frames in the genome of bacteriophage lambda. Molecular and General Genetics, 262(4-5): 659-667. DOI:10.1007/s004380051128 (  0) 0) |
Zhang, W., Liang, Y., Zheng, K., Gu, C., Liu, Y., Wang, Z., et al., 2021. Characterization and genomic analysis of the first Oceanospirillum phage, vB_OliS_GJ44, representing a novel siphoviral cluster. BMC Genomics, 22(1): 675. DOI:10.1186/s12864-021-07978-4 (  0) 0) |
Zhao, Y., Temperton, B., Thrash, J. C., Schwalbach, M. S., Vergin, K. L., Landry, Z. C., et al., 2013. Abundant SAR11 viruses in the ocean. Nature, 494(7437): 357-360. DOI:10.1038/nature11921 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


