2) Department of Biological Sciences, University of Bergen, High Technology Center, 5020 Bergen, Norway;
3) Akvaplan-niva, Trondheim Office, Havnegata 9, 7010 Trondheim, Norway;
4) Akvaplan-niva, Framsenteret, 9296 Tromsø, Norway;
5) Nordlaks Oppdrett AS, Post Box 224, 8455 Stokmarknes, Norway;
6) Cermaq Finnmark AS, Markedsgata 3, 9510 Alta, Norway;
7) GIFAS AS, Gildeskål, 8140 Inndyr, Norway
The sea lice, Lepeophtheirus salmonis and various Caligus species, are ectoparasites of marine finfish (Copepoda: Caligidae). They have a major impact on salmonid aquaculture worldwide (Igboeli et al., 2012, 2014); they cause a loss of over €440 million in Norway annually (Abolofia et al., 2017). The lice live on the mucus and skin and in the blood of fish, resulting in wounds if not removed. The lice occur naturally on salmon in sea water and were described as early as in the middle of the 18th century (Torrissen et al., 2013). However, the problem has escalated with the commercial production of Atlantic salmon (Salmo salar L) and rainbow trout (Oncorhynchus mykiss Walbaum) in sea cages. The effectiveness of medicinal treatments by either bathing or oral administration may be affected by the development of reduced sensitivity, leading to reducing treatment efficacy. Therefore, more emphasis has being giving to mechanical treatments such as thermolicing and high pressure washing. Biological control using cleaner fish that pick the sea lice from salmonids is effective in reducing lice density and is adopted widely by salmon farming industry. As an alternative of cold-water cleaner fish, the common lumpfish, Cyclopterus lum-pus L., is currently used to control sea lice infestation (Imsland et al., 2014a, b, c, 2015a, b).
The parasitic copepod family Caligidae comprises more than 30 genera (Kabata, 1979; Hemmingsen et al., 2020) and more than 450 species (Dojiri and Ho, 2013). Mem-bers of two of these genera, Lepeophtheirus and Caligus, have received notoriety; they have the greatest economic impact among parasite genera in salmonid fish maricul-ture (Costello, 2006) and have evolved collectively as so called 'sea lice'. Although this notoriety is mainly due to the serious impact of L. salmonis, the members of genus Caligus are also implicated. Johnson et al. (2004) estimated that 61% of copepod infestations in marine and brackish water fish culture are caused by the members of family Caligidae, 40% by the species of Caligus and 14% by the species of Lepeophtheirus. A major difference be-tween L. salmonis and Caligus sp. is their host specificity. L. salmonis is an obligate parasite of salmonid fish (Kabata, 1979) whereas many Caligus sp. tend to be facultative (Kabata, 1979; Pike and Wadsworth, 1999) and have been found on > 80 fish species (Kabata, 1979). In the central and northern parts of Norway, a high C. elongatus Nordmann abundance on farmed fish frequently occurs in autumn (Øines et al., 2006). Infections have been assum-ed to connect to passing schools of pollock (Pollachius pollachius L.), saithe (Pollachius virens L.) and herring (Clupea harengus L.) (á Norði et al., 2015).
Mature C. elongatus is smaller than mature L. salmonis (Piasecki, 1996), and its two sexes are at an equal size (around 6 mm). C. elongatus is a much better swimmer than L. salmonis and can re-infect other fish species if being removed from its original host (Øines et al., 2006; Hemmingsen et al., 2020). Hence, determining if mature C. elongatus infects species like lumpfish and saithe should help to explain the rapid increase of C. elongatus in sea pens during certain periods of year (Heuch et al., 2007) in northern Norway. Lumpfish are now extensively used as cleaner fish in northern Norway (Imsland et al., 2018), Ireland (Bolton-Warberg, 2018), Scotland (Treasurer et al., 2018), Iceland (Steinarson and Árnason, 2018) and the Faroese Island (Eliasen et al., 2018). However, to this date, there exists no systematic knowledge and guiding line of the effect of lumpfish on C. elongatus. Earlier researches have clearly indicated that lumpfish prefer to graze the adult female L. salmonis (Imsland et al., 2014a, c, 2016, 2018). However, lumpfish in sea pens can be classified as the strongly opportunistic (Imsland et al., 2014c) and they do not restrict themselves to graze a single food source if others exist. They may readily graze on mature sea lice males as well as C. elongatus.
In this mini review, we summarized the findings from both small and large scale trials with lumpfish where grazing on C. elongatus has been reported in order to give recommendations on the possible use of lumpfish to com-bat C. elongatus on Atlantic salmon in sea pens.
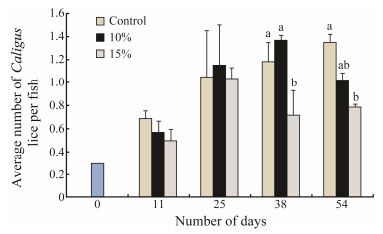
2 Different Densities of Lumpfish: Effect on the Occurrence of C. elongatus on Atlantic Salmon in Small Scale StudiesImsland et al. (2014a) investigated the efficacy of lump-fish grazing on attached C. elongatus on Atlantic salmon at two different lumpfish densities, 10% and 15%. C. elongatus were counted every two weeks during the trial (54 days). To investigate the stomach content of lumpfish, a gastric lavage was performed. The results showed that on day 38, 15% stocked cages had a significantly lower average number (0.72) of C. elongatus per salmon compared to that of control (1.18) and 10% stocked cages (1.37) (Tukey's multiple test, P < 0.05, Fig. 1). Similarly, on day 54, 15% stocked cages had a significantly lower average number per fish (0.78) compared to that of control cages (1.35) and 10% stocked cages (1.02) (Tukey's multiple test, P < 0.05).
|
Fig. 1 Total average number of C. elongatus per fish recorded for each duplicate treatment during each of the sampling dates in the trial of Imsland et al. (2014a). Values are presented as means ± S.D. Mean values which do not share a letter are significantly different (ANOVA, Tukey's multiple range test, P < 0.05). The average number refers to the total number of fish individuals sampled from two cages at each sampling time. |
Both visual inspection and gastric lavage indicated the consumption of C. elongatus in the trial of Imsland et al. (2014a). The average number per fish varied throughout the trial although both 10% and 15% stocked cages had 25% and 42% fewer C. elongatus lice than controls on day 54. This finding strongly indicated that the presence of lumpfish has lowered the infestations of C. elongatus among Atlantic salmon. The results from the gastric lavage used to assess food choices in lumpfish displayed the presence of C. elongatus in the stomachs of several fish throughout the study. The proportion of lumpfish with sea lice (L. salmonis and C. elongatus) increased from 10% on day 11 to 28% on day 54. The number of lumpfish eating lice may in fact be much higher as these values were only determined from lavage fish every 14 days throughout the trial. The number of days between sample points allowed for lumpfish to consume sea lice and fully digest them, thus only giving a snapshot on lice eating. However, the relatively large increase in numbers of lumpfish found with ingested sea lice in their stomachs suggest that the level of grazing intensified throughout the study period. This may be an indicator of some forms of learning or habituation of lumpfish, which was investigated in a follow-up trial (see below).
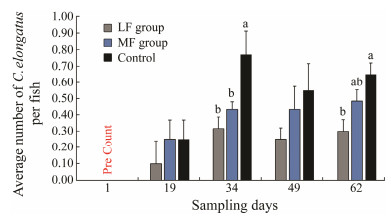
3 Habituation of Lumpfish by Feeding Live Feeds Prior to Transfer to Atlan-tic Salmon Net Pens: Effect on the Occurrence of C. elongatusImsland et al. (2019) established two groups of individually tagged lumpfish in land-based tanks. One group received marine pelleted feed (MF group) whilst the other received a mix of pelleted feed, live adult Artemia and frozen sea lice (LF group). Sixty lumpfish each group were tagged and transferred to small scale sea pens with 300 Atlantic salmon each, and the occurrence of C. elongatus on the salmon was investigated for 62 days. They found that there were significantly less C. elongatus stage on salmon from both LF and MF groups on day 34 compared to the control (SNK post hoc test, P < 0.05, Fig. 2). On day 62, there was significantly less C. elongatus found on salmon from LF group compared to the control (SNK post hoc test, P < 0.05) as there was 38% less C. elongatus found on salmon reared with LF lumpfish compared to MF lumpfish.
|
Fig. 2 Total average number of C. elongatus per Atlantic salmon recorded for each duplicate treatment during each of the sampling dates of the sea pen study carried out by Imsland et al. (2019). Values are presented as means ± S.D. Mean values which do not share a letter are significantly different (ANOVA, SNK post hoc test, P < 0.05). The average number refers to the total number of fish individuals sampled from two cages (n = 60) each group each sampling time. |
In the study of Imsland et al. (2019), the level of C. e-longatus was significantly different between control and LF groups, indicating that the dietary treatment influenced the ability of lumpfish to effectively forage on C. elongatus as lumpfish conditioned prior to sea pen rearing were nearly 40% more efficient in grazing C. elongatus compared to controls. These results provided further supports to previous studies which reported that lumpfish do graze on C. elongatus (Imsland et al., 2014a). C. elongatus is not included in Norwegian legislation, and there is therefore no legal limit to the level of infestation of C. elongatus at which a treatment should be initiated. However, the spe-cies has an economic impact in the production cycle of salmon (Boxaspen, 2006). There have been some concerns on using lumpfish as a cleaner fish; the fish is considered to be a preferred host of C. elongatus (Heuch et al., 2007; Mitamura et al., 2012), and lumpfish has the potential to act as a vector of C. elongatus that can infect salmon (Powell et al., 2017). These concerns can be reduced if lumpfish graze indiscriminately on both species of lice and domesticated lumpfish free of C. elongatus were introduced into sea cages.
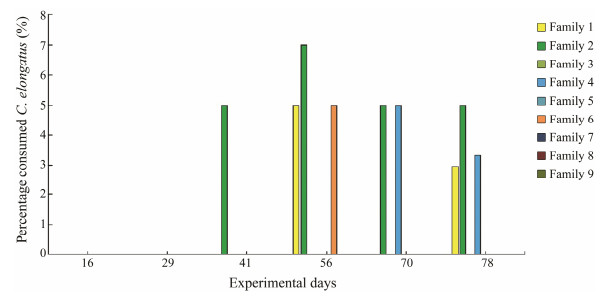
4 Lumpfish Grazing on C. elongatus: Possible Parental ControlPossible heritable component of C. elongatus grazing was investigated in two recent trials. Imsland et al. (2016) investigated possible parental control in grazing of C. e-longatus in nine families of lumpfish distributed in dup-licates among nine small sea cages stocked with 400 Atlantic salmon each. During the trial (78 d), gastric lavage was performed every two weeks to assess the feeding pre-ference of individual lumpfish. Although C. elongatus in-festation rate was found to be very low in the study (Fig. 3), the percentage of lumpfish found to have consumed C. elongatus varied significantly between families, indicating a possible parental control of C. elongatus grazing.
|
Fig. 3 Mean percentage values of C. elongatus found in nine lumpfish families sampled at each sampling time point. Data are from Imsland et al. (2016). |
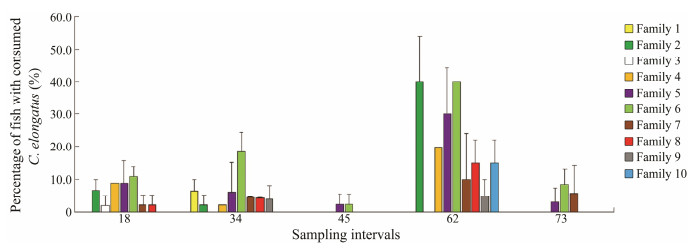
In a study carried out by Imsland et al. (unpublished), 10 families of lumpfish, 480 individuals each, 46.5 ± 4.3 g in mean weight, were distributed into ten sea cages (5 m × 5 m × 5 m) stocked with 400 Atlantic salmon each, 387.3 ± 10.3 g in mean weight. From each family, 20 lumpfish were stocked into one of 10 sea cages and 20 into another cage, thus establishing duplicate treatments each family, two fa-milies each cage. During the trial (73 d), gastric lavage was performed every two weeks to assess the feeding pre-ference of lumpfish individuals.
Consumption of C. elongatus varied between families (Fig. 4). Seven of the ten families were found to consume C. elongatus on day 18. Percentage of lumpfish that had consumed C. elongatus varied between 2% and 11% on day 18. On day 62, between 5% and 40% of lumpfish were found with C. elongatus in their stomach. Families 5 and 6 (half-siblings, same father) had the highest consumption of C. elongatus throughout the study (Fig. 4).
|
Fig. 4 Percentage values of C. elongatus found in stomach of lumpfish of ten families sampled at each sampling time point. Values are presented as means ± S.D. Data are from Imsland et al. (unpublished). |
Given the difference in consumption of C. elongatus in two family trials and other natural source (see Imsland et al., 2016 for details), it seems that some lumpfish may be more predisposed in actively seeking natural food sources, including that C. elongatus should have a genetic basis to underline such a behaviour. It is well known that behavioural traits respond to both natural and sexual selection. Fish from families 5 and 6 in the second trial where consumption of C. elongatus was much more pronounced shared the same father but had different mothers. Given that these differences had a degree of genetic influence, then it would appear more likely that this difference was passed through paternal rather than maternal lines. Recent studies have revealed both maternal (Royle et al., 2012) and paternal (McGhee and Bell, 2014) effects on offspring behaviour via epigenetic alterations of genome.
Results from two family trials indicated that consumption of C. elongatus varies among families. The families with the highest consumption of C. elongatus (trial 1, fa-mily 2; trial 2, families 5 and 6) were also those with the highest consumption of L. salmonis. Although energy rich salmon pellets were available, family 2 in trial 1 and fami-lies 5 and 6 in trial 2 preferred natural pray to a larger ex-tent than other families did. This confirmed that the gene-tic influence of sea lice consumption can be strong (Imsland et al., 2016). Given the difference in the consumption of natural C. elongatus among families, we may spe-culate that these fish are more disposed to seek out natural food sources. If this behaviour has a genetic basis, it may be further enhanced through selection and targeted breeding.
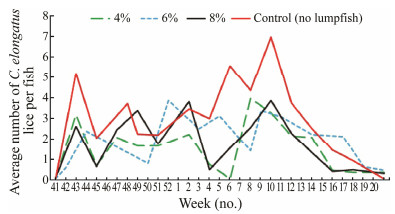
5 The Effect of Lumpfish on C. elongatus Incidence on Atlantic Salmon: Large-Scale Observations 5.1 Large-Scale Trial at Lerøy Aurora, Troms, NorwayImsland et al. (2018) performed a large-scale trial at a commercial Atlantic salmon sea farm (69.80°N, 19.41°E) (Lerøy Aurora, Troms County, Norway) from October 6, 2015 to May 17, 2016. The experiment was conducted in eight large sea cages (130 m in circumference, 37688 m3 in volume) holding 193304 ± 2089 smolts (Atlantic salmon) each, initial mean body weight 198 g ± 20 g. Lumpfish were stocked at densities 4%, 6% and 8%, respectively, in duplicate sea cages. During the trial, C. elongatus on 240 sal-mon were counted every two weeks.
The level of C. elongatus rose in all groups in autumn (Fig. 5). Significantly, a lower level of C. elongatus was seen in lumpfish groups from late February to early April (Student-Newman-Keuls post hoc test, P < 0.05, Fig. 5). In April, C. elongatus level decreased in all experimental groups, and the final level in May was similar to the initial ones in October last year.
|
Fig. 5 Occurrence of C. elongatus per salmon (n = 60) each group each sampling point in large scale sea cages at Lerøy Aurora, northern Norway, with 0 (control), 4%, 6% and 8% of lumpfish recorded each duplicate treatment each sampling dates (bi-weekly). |
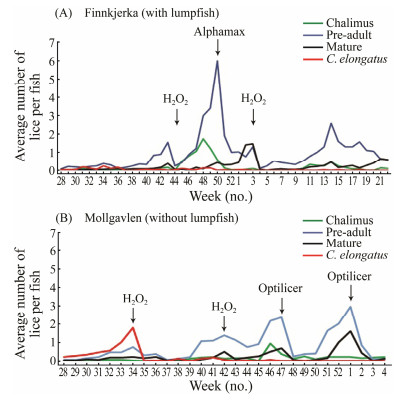
A large-scale observation was performed at a commercial Atlantic salmon sea farm (68.40°N, 15.11°E) (Nordlaks, Nordland county, Norway) from July 1, 2017 to February 2, 2018. The observation was conducted in 12 large sea cages (160 m in circumference, 35000 m3 in volume) holding smolts (Atlantic salmon). Two nearby locations, Finnkjerka and Mollgavlen, in the same seawater basin (10 km between them) were monitored. At Finnkjerka, there were six sea pens holding 198250 ± 3200 salmon smolts each, initial mean body weight, 75 g ± 9 g, in October, 2016. In July 2017, about 12000 lumpfish each pen, mean weight, 32 g ± 3 g, were released to all these sea pens. In the nearby location of Mollgavlen, there were six sea pens holding 164724 ± 8632 salmon smolts each, initial mean body weight, 76 g ± 12 g in October, 2016. Every other week during observation, thirty salmon each sea pen were sedated and weighted individually with lice on them recorded. Af-ter counting, the lice remaining in container if any were also recorded. Lice were registered in 4 categories: 1) Le-peophtheirus salmonis, adult female; 2) L. salmonis, pre-adult; 3) L. salmonis, Chalimus; 4) Caligus elongatus.
Overall, less C. elongatus were found on the salmon in pens with lumpfish (Finnkjerka location) compared with those without lumpfish (Fig. 6). This effect was most evi-dent in winter and spring and also in summer; C. elonga-tus increased at Mollgavlen (without lumpfish) from July whereas such increase was not seen at nearby Finnkjerka (with lumpfish in sea pens). Overall, there were more sea lice challenges at Mollgavlen, resulting in approximately 600 g loss of final slaughtering weight of salmon.
|
Fig. 6 Sea lice development at two production sites of Nord-laks in northern Norway 2017–2018. Arrows indicate me-chanical and chemotherapeutical delouse operations during the observation period. |
The relatively high number of C. elongatus observed in two large-scale studies was the indicative of all production sites, as these lice are known to transfer easily between fish (Heuch et al., 2007). Despite the presence of lumpfish, there were a sufficiently high number of lice elsewhere at the site to allow continual re-colonization in the cages stocked with lumpfish. Nevertheless, the mean num-ber of C. elongatus was lower in groups with lumpfish at both salmon farms. The added positive effect of lumpfish at the Nordlaks production sites was around 600 g gain of slaughter weight (3.82 kg vs. 3.18 kg) at the location with lumpfish and this is almost surely linked to less problems with sea lice (both L. salmonis and C. elongatus) at this lo-cation.
6 Summary 6.1 Lumpfish Efficacy for C. elongatus RemovalTo summarize the relationship between the use of lump-fish and the occurrence of C. elongatus on Atlantic sal-mon, we have compiled current knowledge from the published literatures and reports and by interviewing fish health care persons in Atlantic salmon farming industry in Table 1. Available data clearly indicates that lumpfish grazes on C. elongatus and it is possible to enhance this grazing with the assistance of live-feed conditioning prior to sea-pen transfer and selective breeding. Grazing is observed in va-rious size classes (25 g to 550 g), at temperatures ranging from 4 to 13℃ and in all seasons. Majority of published data come from northern Norway. There are also indications from the Faroe Islands, Scotland and Iceland that lumpfish grazes on C. elongatus. In the Faroe Islands, an investigation into 5511 lumpfish stomach (Eliasen et al., 2018) showed that L. salmonis was found in 13.5% of 743 individuals, of them, around 80% had also C. elongatus in their stomach (Kirsten Eliasen, Fiskaaling, Faroe Islands, pers. comm.). The consensus in the salmon farming indus-try in Faroe Islands is that lumpfish is effective in reducing the number of C. elongatus, but the infestation pattern is different from that of L. salmonis. Lumpfish is not systematically used as a biological delouser for C. elongatus (Kirsten Eliasen, Fiskaaling, Faroe Islands, pers. comm.). In Scotland, the C. elongatus number can be seasonally im-portant, but the efficacy of cleaner fish to C. elongatus can be difficult to assess in summer as C. elongatus continue to re-infect from a range of wild fish species. Even after bath treatments, re-infestation of C. elongatus can be rapid (Jim Treasurer, FAI Aquaculture, Scotland, pers. comm.). In the Westfjords area of Iceland, C. elongatus infestations are presently considered a more severe problem than the L. salmonis and the number of C. elongatus on salmon can be high (> 10) in late autumn (October – November) (Eva D. Jóhannesdóttir, Arctic Sea Farm Ltd., pers. comm., Hjörtur Methúsalemsson, Arnarlax Ltd., pers. comm.). In this area, large scale trials have clearly shown that lumpfish is very effective in lowering the number of C. elongatus.
|
|
Table 1 A summary of the current literature (peer-reviewed journal articles and scientific reports) and observations (including pers. comm.) on experiments with lumpfish and its effect on C. elongatus infestations on farmed Atlantic salmon |
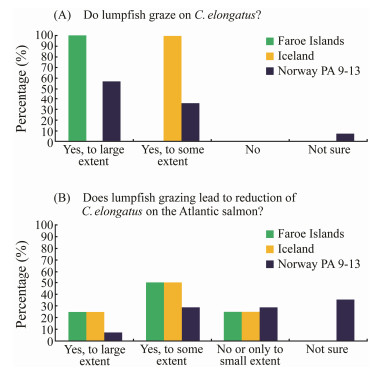
To investigate in more details the possible effect of lump-fish on C. elongatus on Atlantic salmon, we conducted a survey by interviewing fish health personnel and biological controllers working in salmon farming industry in Nor-way (n = 18), Faroe Islands (n = 5) and Iceland (n = 2) (https://www.fhf.no/prosjekter/prosjektbasen/901539/). In Norway, we interviewed persons working in companies in northern Norway (i.e., from Production Areas (PA) 9-13, see Fig. 1 in Overton et al., 2018). The survey findings showed that almost all participants agreed that lumpfish grazes on C. elongatus (Fig. 7A), but the extent of grazing is unclear. On the Faroes Islands, all participants agreed that lumpfish grazes to a large extent on C. elongatus whereas in northern Norway the views can be divided into large extent and some extent (Fig. 7A). The survey on whether the grazing of lumpfish leads to reduction of C. elongatus on Atlantic salmon showed different views on the extent of C. elongatus reduction on salmon (Fig. 7B). The majority in all three countries think that the grazing reduces C. elongatus on salmon to a large or some extent. In all three countries, it was commented that the lumpfish influences the number of C. elongatus if the number of C. elongatus on salmon is moderate or low.
|
Fig. 7 Results from interview survey of fish health care per-son and biological controllers working in the salmon farm-ing industry in Norway (n = 18), Faroe Islands (n = 4) and Iceland (n = 2). |
Financial support was given by the Norwegian Seafood Research Found (Nos. KEKS901539 and 901652EFFEK TIV).
Abolofia, J., Asche, F. and Wilen, J. E., 2017. The cost of lice: Quantifying the impacts of parasitic sea lice on farmed sal-mon. Marine Resource Economics, 32: 329-349. DOI:10.1086/691981 (  0) 0) |
á Norði, G., Simonsen, K., Danielsen, E., Eliasen, K., Mols-Mor-tensen, A., Christiansen, D. H., Steingrund, P., Galbraith, M. and Patursson, Ø., 2015. Abundance and distribution of plank-tonic Lepeophtheirus salmonis and Caligus elongatus in a fish farming region in the Faroe Islands. Aquaculture Environment Interactions, 7: 15-27. DOI:10.3354/aei00134 (  0) 0) |
Bolton-Warberg, M., 2018. An overview of cleaner fish use in Ireland. Journal of Fish Diseases, 41: 935-939. DOI:10.1111/jfd.12731 (  0) 0) |
Boxaspen, K., 2006. A review of the biology and genetics of sea lice. ICES Journal of Sea Research, 63: 1304-1316. DOI:10.1016/j.icesjms.2006.04.017 (  0) 0) |
Costello, M. J., 2006. Ecology of sea lice parasitic on farmed and wild fish. Trends in Parasitology, 22: 475-483. DOI:10.1016/j.pt.2006.08.006 (  0) 0) |
Costello, M. J., 2009. The global economic cost of sea lice to the salmonid farming industry. Journal of Fish Diseases, 32: 115-118. DOI:10.1111/j.1365-2761.2008.01011.x (  0) 0) |
Denholm, I., Devine, G. J., Horsberg, T. E., Sevatdal, S., Fallang, A., Nolan, D. V. and Powell, R., 2002. Analysis and management of resistance to chemotherapeutants in salmon lice Lepeophtheirus salmonis (Krøyer) (Copepoda: Caligidae). Pest Management Science, 58: 528-536. DOI:10.1002/ps.482 (  0) 0) |
Dojiri, M. and Ho, J. S., 2013. Systematics of the Caligidae, Co-pepods Parasitic on Marine Fishes. Crustaceana Monographs Vol. 18. Brill Publishers, Netherlands, 448pp.
(  0) 0) |
Eliasen, K., Danielsen, E., Johannesen, Á., Joensen, L. L. and Patursson, E. J., 2018. The cleaning efficacy of lumpfish (Cy-clopterus lumpus L. ) in Faroese salmon (Salmo salar L.) farm-ing pens in relation to lumpfish size and season. Aquaculture, 488: 61-65. (  0) 0) |
Hemmingsen, W., Sagerup, K., Remen, M., Bloch-Hansen, K. and Imsland, A. K. D., 2020. Caligus elongatus and other sea lice of the genus Caligus as parasites of farmed salmonids: A review. Aquaculture, 522: 735160. DOI:10.1016/j.aquaculture.2020.735160 (  0) 0) |
Heuch, P. A., Oines, O., Knutsen, J. A. and Schram, T. A., 2007. Infection of wild fishes by the parasitic copepod Caligus elon-gatus on the south east coast of Norway. Diseases of Aquatic Organisms, 77: 149-158. DOI:10.3354/dao01833 (  0) 0) |
Igboeli, O. O., Fast, M. D., Heumann, J. and Burka, J. F., 2012. Role of P-glycoprotein in emamectin benzoate (SLICE (R)) re-sistance in sea lice, Lepeophtheirus salmonis. Aquaculture, 344: 40-47. (  0) 0) |
Igboeli, O. O., Burka, J. F. and Fast, M. D., 2014. Lepeoph-theirus salmonis: A persisting challenge for salmon aquaculture. Animal Frontiers, 4: 22-32. DOI:10.2527/af.2014-0004 (  0) 0) |
Imsland, A. K., Reynolds, P., Eliassen, G., Hangstad, T. A., Foss, A., Vikingstad, E. and Elvegård, T. A., 2014a. The use of lump-fish (Cyclopterus lumpus L.) to control sea lice (Lepeoph-theirus salmonis Krøyer) infestations in intensively farmed Atlantic salmon (Salmo salar L.). Aquaculture, 424-425: 18-23. DOI:10.1016/j.aquaculture.2013.12.033 (  0) 0) |
Imsland, A. K., Reynolds, P., Eliassen, G., Hangstad, T. A., Foss, A., Vikingstad, E. and Elvegård, T. A., 2014b. Notes on the behaviour of lumfish with and without Atlantic salmon present. Journal of Ethology, 32: 117-122. DOI:10.1007/s10164-014-0397-1 (  0) 0) |
Imsland, A. K., Reynolds, P., Eliassen, G., Hangstad, T. A., Nytrø, A. V., Foss, A., Vikingstad, E. and Elvegård, T. A., 2014c. Assessment of growth and sea lice infection levels in Atlantic salmon stocked in small-scale cages with lumpfish. Aquaculture, 433: 137-142. DOI:10.1016/j.aquaculture.2014.06.008 (  0) 0) |
Imsland, A. K., Reynolds, P., Eliassen, G., Hangstad, T. A., Nytrø, A. V., Foss, A., Vikingstad, E. and Elvegård, T. A., 2015a. Feeding preferences of lumpfish (Cyclopterus lumpus L.) main-tained in open net-pens with Atlantic salmon (Salmo salar L.). Aquaculture, 436: 47-51. DOI:10.1016/j.aquaculture.2014.10.048 (  0) 0) |
Imsland, A. K., Reynolds, P., Eliassen, G., Hangstad, T. A., Nytrø, A. V., Foss, A., Vikingstad, E. and Elvegård, T. A., 2015b. Assessment of suitable substrates for lumpfish in sea pens. Aquaculture International, 23: 639-645. DOI:10.1007/s10499-014-9840-0 (  0) 0) |
Imsland, A. K., Reynolds, P., Eliassen, G., Mortensen, A., Hansen, Ø. J., Puvanendran, V., Hangstad, T. A., Jónsdóttir, Ó. D. B., Emaus, P. A., Elvegård, T. A., Lemmens, S. C. A., Ryd-land, R., Nytrø, A. V. and Jonassen, T. M., 2016. Is cleaning behaviour in lumpfish (Cyclopterus lumpus) parentally con-trolled?. Aquaculture, 459: 156-165. DOI:10.1016/j.aquaculture.2016.03.047 (  0) 0) |
Imsland, A. K., Hanssen, A., Reynolds, P., Nytrø, A. V., Jonassen, T. M., Hangstad, T. A., Elvegård, T. A., Urskog, T. C. and Mikalsen, B., 2018. It works! Lumpfish can significantly lower sea lice infections in large scale salmon farming. Biology Open, 7(8): bio036301. DOI:10.1242/bio.036301 (  0) 0) |
Imsland, A. K. D., Frogg, N. E., Stefansson, S. O. and Reynolds, P., 2019. Improving sea lice grazing of lumpfish (Cyclopterus lumpus L.) by feeding live feeds prior to transfer to Atlantic salmon (Salmo salar L.) net-pens. Aquaculture, 511: 734224. DOI:10.1016/j.aquaculture.2019.734224 (  0) 0) |
Johnson, S. C., Treasurer, J. W., Bravo, S., Nagasawa, K. and Kabata, Z., 2004. A review of the impact of parasitic copepods on marine aquaculture. Zoological Studies, 43: 229-243. (  0) 0) |
Kabata, Z., 1979. Parasitic Copepoda of British Fishes. The Ray Society, London, 468pp.
(  0) 0) |
McGhee, K. E. and Bell, A. M., 2014. Paternal care in a fish: Epigenetics and fitness enhancing effects on offspring anxiety. Proceedings of the Royal Society B Biological Sciences, 281: 1146-1151. (  0) 0) |
Mitamura, H., Thorstad, E. B., Uglem, I., Bjorn, P. A., Okland, F., Naesje, T. F., Dempster, T. and Arai, N., 2012. Movements of lumpsucker females in a northern Norwegian fjord during the spawning season. Environmental Biology of Fishes, 93: 475-481. DOI:10.1007/s10641-011-9942-8 (  0) 0) |
Overton, K., Dempster, T., Oppedal, F., Kristiansen, T. S. and Gismervik, K., 2018. Salmon lice treatments and salmon mor-tality in Norwegian aquaculture: A review. Reviews in Aqua-culture, 11: 1398-1417. (  0) 0) |
Piasecki, W., 1996. The developmental stages of Caligus elongatus von Nordmann, 1832 (Copepoda: Caligidae). Canadian Jour-nal of Zoology, 74: 1459-1478. DOI:10.1139/z96-161 (  0) 0) |
Pike, A. W. and Wadsworth, S. L., 1999. Sealice on salmonids: Their biology and control. Advances in Parasitology, 44: 233-337. DOI:10.1016/S0065-308X(08)60233-X (  0) 0) |
Powell, A., Treasurer, J. W., Pooley, C. L., Keay, A. J., Lloyd, R., Imsland, A. K. and Garcia de Leaniz, C., 2018. Cleaner fish for sea-lice control in salmon farming: Challenges and op-portunities using lumpfish. Reviews in Aquaculture, 10: 683-702. DOI:10.1111/raq.12194 (  0) 0) |
Royle, N. J., Smiseth, P. T., Kolliker, M., Champagne, F. A. and Curley, J. M., 2012. The Evolution of Parental Care. Oxford University Press, Oxford, 304-324.
(  0) 0) |
Steinarson, A., and Árnason, T., 2018. Rearing of cleaner fish in Iceland. In: Cleaner Fish Biology and Aquaculture Applications. Treasurer, J. W., ed., 5M Publishing Ltd., Sheffield, 420-435.
(  0) 0) |
Torrissen, O., Jones, S., Asche, F., Guttormsen, A., Skilbrei, O. T., Nilsen, F., Horsberg, T. E. and Jackson, D., 2013. Salmon lice -Impact on wild salmonids and salmon aquaculture. Jour-nal of Fish Diseases, 36: 171-194. DOI:10.1111/jfd.12061 (  0) 0) |
Treasurer, J. W., 2002. A review of potential pathogens of sea lice and the application of cleaner fish in biological control. Pest Management Science, 58: 546-558. DOI:10.1002/ps.509 (  0) 0) |
Treasurer, J., Prickett, R., Zietz, M., Hempleman, C., and Garcia de Leaniz, C., 2018. Cleaner fish rearing and deployment in the UK. In: Cleaner Fish Biology and Aquaculture Applications. Treasurer, J. W., ed., 5M Publishing Ltd., Sheffield, 376-391.
(  0) 0) |
Øines, Ø., Simonsen, J., Knutsen, J. and Heuch, P., 2006. Host preference of adult Caligus elongatus Nordmann in the laboratory and its implications for Atlantic cod aquaculture. Jour-nal of Fish Diseases, 29: 167-174. DOI:10.1111/j.1365-2761.2006.00702.x (  0) 0) |
 2020, Vol. 19
2020, Vol. 19


