2) Department of Animal Production, Faculty of Agriculture, Al-Azhar University, Nasr City, Cairo 11884, Egypt;
3) CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266000, China;
4) East China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shanghai 20090, China
Because of the extensive use of plastics and their resistance to degradation, plastic contaminants have become a global environmental issue. This contamination poses ecological risks and may have negative effects on human health. Microplastics (MPs) are plastic particles with a particle size of < 5 mm (Arthur et al., 2009), and nanoplastics (NPs) are usually defined as tiny particles < 100 nm or 1 µm in size (Koelmans et al., 2015; Hartmann et al., 2019). Microplastics are widespread in aquatic eco-systems, and they can be ingested by aquatic organisms and transferred along food webs (Rebelein et al., 2021). In recent years, many field investigations have shown that microplastics have been widely detected in commercial aquatic products (Jabeen et al., 2017; Azevedo-Santos et al., 2019; Ding et al., 2021; Wu et al., 2022).
Bivalves, such as mussels and clams, are crucial members of the benthic community assemblage, and they have been used to monitor MP pollution in the marine environment because of their sessile life, widespread distribution and ability to filter large volumes of water (Browne et al., 2008; Li et al., 2020). Ding et al. (2021) suggested that bivalves play a crucial role as environmental bioindicators, and attention should be given to the pollution of microplastics in bivalves. However, currently there is a limited body of research on the assessment of oxidative stress in entrenched shellfish inhabiting mudflats, while more studies have been conducted on attached shellfish (Magara et al., 2018; Cole et al., 2020). In fact, as hydrophobic materials, MPs generally tend to deposit in the sediment, which becomes a source and sink, causing accumulation in the benthos (Li et al., 2020; Vasanthi et al., 2021). And Abdelsaleheen et al. (2021) have shown that beyond a certain time, the amount of nanoplastics in the sediment is significantly higher than the amount of nanoplastics in the water. The biological effects of MPs on bivalves have been reported on various aspects, including disturbed amino acid metabolism, oxidative stress, and immunotoxicity (Huang et al., 2021). They have also been found to induce structural alterations in the gills and digestive diverticula, leading to oxidative stress and impaired digestive performance (Wang et al., 2020). Compared to MPs, there is limited information about the accumulation and toxicity of NPs in bivalves because of methodological challenges to detection. Multiple studies have reported that smaller plastic particles, including NPs, generally exhibit higher toxicity to invertebrates compared to larger particles (Provenza et al., 2020). This increased toxicity can be attributed to the smaller particle size of NPs, resulting in a larger surface area to volume ratio and a greater tendency to aggregate. Moreover, NPs have the capability to easily traverse biological barriers, further contributing to their potential harmful effects on organisms (Lee et al., 2013; Rist et al., 2017; Li et al., 2020; Liu et al., 2021; Muhammad et al., 2021).
Meretrix meretrix and Sinonovacula constricta are economically significant marine bivalves in Asia, inhabiting the sediments of tidal flats. They are representative examples of sessile filter-feeding and sediment-dwelling bivalves. A previous study reported that the habitat environment greatly influenced the ingestion of microplastics in bivalves (Ding et al., 2021), moreover they suggested that the clam (R. philippinarum) can serve as a bioindicator to monitor the spatial distribution pattern of microplastic polymer types in the sediment. However, previous studies emphasized the toxicity of MP/NP on water-dwelling bivalves such as Mytilus galloprovincialis, Mytilus coruscus, and Crassostrea gigas (Brandts et al., 2018; Huang et al., 2021; Choi et al., 2022), but there is limited information available regarding the accumulation and toxicity of NPs on sediment-dwelling bivalve. Li et al. (2020) conducted a study using fluorescence tags to trace organ accumulation of NPs (80 nm diameter) in C. fluminea. They discovered that NPs could accumulate in the mantle, visceral mass, and gills. Furthermore, the study revealed that clams had different responses to oxidative stress after exposure to NPs. However, the mechanisms responsible for these negative responses of NP remain unclear. Liu et al., (2021) demonstrated that functionalized polystyrene nanoplastics (PS-NH2 and PS-COOH) inhibited the growth of M. meretrix. The mechanism involved included an imbalance in energy homeostasis, decreased phagocytosis and disturbing the stabilization of the lysosomal membrane. Moreover, the toxic mechanism of NPs on lysosomal membrane stabilization was mainly attributed to oxidative damage.
In this study, we explored the effect of NPs on oxidative stress responses and oxidative damage in the mantle, digestive gland, and gills of M. meretrix and S. constricta. The clams were independently exposed to different concentrations of fluorescently labeled NP particles in seawater for 7 d and then returned to clean conditions for 3 d. Catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) activities as well as malondialdehyde (MDA) content were measured at different time points during the experiment.
2 Materials and Methods 2.1 Experimental MusselsHealthy M. meretrix (average length of 45 ± 1.2 mm) and S. constricta (62.5 ± 2.1 mm) were collected from Yancheng (Jiangsu Province, China). Before the experiment, they were kept in an aquarium for 2 weeks to acclimatize to the experimental conditions. During the acclimatization period, the physical and chemical conditions of the water were as follows: temperature, 19 ± 1℃; pH, 8.0 ± 0.05; salinity, 20; dissolved oxygen concentration, > 6 mg L−1. The mussels were fed with the alga Chlorella vulgaris (1 × 105 ind mL−1) every day.
2.2 Experimental DesignThree concentrations (0, 0.1, and 1 mg L−1) of fluorescently labeled PS-NPs (80 nm diameter, excitation wavelength of 470 nm, and emission wavelength of 526 nm) were purchased for use in this experiment (Tianjin BaseLine ChromTech Research Center, Tianjin, China). These concentrations were chosen according to the previously reported that NPs concentration in natural waters is estimated to be less than 1 mg L−1 (Lenz et al., 2016). The surface morphology and particle size of the PS-NPs particles were analyzed by scanning electron microscope (Nova Nano SEM 450, FEI company, USA) (Fig.1). After the acclimatization, both M. meretrix and S. constricta were randomly assigned to the three treatments. Each treatment consisted of three glass aquariums (20 cm × 15 cm × 10 cm). C. vulgaris (1 × 105 ind mL−1) was fed to the clams daily. After the 7 d exposure experiment, the clams were transferred to clean seawater (no PS-NPs) and subjected to a 3 d recovery period under normal conditions. The seawater was renewed daily and clams were fed C. vulgaris (1 × 105 ind mL−1) during recovery. Sampling was performed on exposure days 1, 3, and 7 and on recovery days 1 and 3. At each sampling point, 18 clams were randomly selected from each glass aquarium and dissected to extract the mantle, digestive gland, and gills. All samples were stored at − 80℃ for subsequent measurement of enzyme activities. The experimental design for S. constricta was identical. All mandatory animal welfare and laboratory health and safety procedures were followed during the course the experiments.
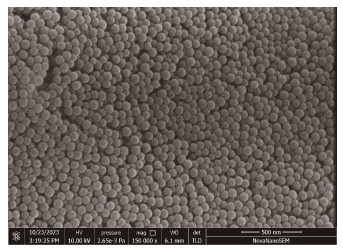
|
Fig. 1 SEM images of polystyrene nanoplastics (80 nm). |
Each sample of tissue was mixed with a saline solution, and the saline solution had a 1:9 ratio of normal saline to homogenate, and the mixtures were centrifuged at 960 g (3000 r min−1) for 10 min at 4℃ (Centrifuge 5810 R, Eppendorf, Germany). Normal saline is an aqueous solution of sodium chloride with a pH value of 7. The supernatant was collected for subsequent enzyme activity analyses. The activities of SOD, CAT, and GPx and the MDA content were measured using commercially available kits (Jiancheng Bioengineering Institute, Nanjing, China). The protein concentrations were determined using a Coomassie Brilliant Blue protein assay kit (Jiancheng Bioengineering Institute). The methods used followed the instructions provided by the manufacturer.
2.4 Integrated Biomarker Response Index'Integrated Biomarker Response Index version 2' (IBRv2) was described by Beliaeff and Burgeot (2002) with a modification (Sanchez et al., 2013). Briefly, for each individual biomarker, the ratio between the mean value obtained for each experimental treatment at each time point and concentration and the respective mean baseline value (T0) was log-transformed (Yi). In the next step, a general mean (μ) and standard deviation (s) were calculated considering all Yi values. The Yi values then were standardized using the formula Zi = (Yi − μ)/s, and the difference between Zi and Z0 (T0) was used to define the biomarker deviation index (A). To obtain a value for the integrated multiple biomarker responses, the value of A for each biomarker was calculated for every organ and every time point, and the IBRv2 was calculated for each organ as the sum of the absolute values of A.
2.5 Statistical AnalysesStatistical analyses were performed using SPSS 19 (SPSS Inc., Chicago, IL, USA). The effect of PS-NPs on the antioxidant enzyme activities and MDA content of clams in the different treatments at each sampling date was evaluated using one-way analysis of variance, followed by least significant difference multiple comparisons. The results are expressed as the mean ± standard error, and results were considered to be significantly different at P < 0.05.
3 Results 3.1 Antioxidant Enzyme Activities and MDA Content of M. meretrixThere was no significant difference in SOD activity in the digestive gland of M. meretrix among all treatments after the PS-NPs exposure period or recovery period (P > 0.05) (Fig.2A). Both the low and high concentrations of PS-NPs significantly increased the SOD activity in the gills on exposure day 1 (P < 0.05), but it then decreased on exposure day 3 compared with the control (P < 0.05) (Fig.2B). Both PS-NPs treatments showed a significantly decrease in the mantle's SOD activity on exposure day 1 (P < 0.05). However, SOD activity was not significantly different between the control and PS-NPs treatments on exposure day 3 and day 7 (P > 0.05) (Fig.2C). During the recovery period, the SOD activities in the gills and mantle were not significantly different in all treatments (P > 0.05).
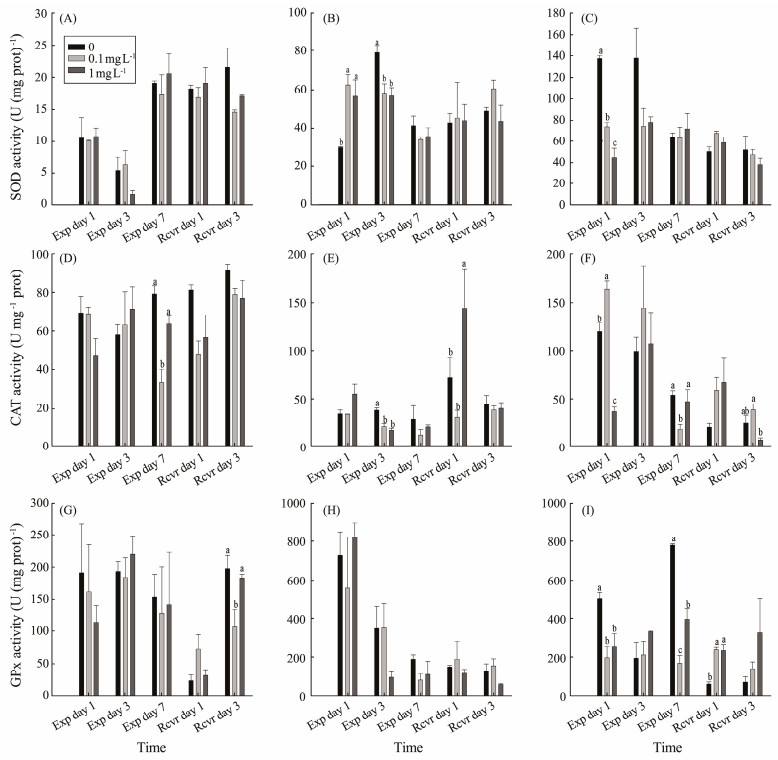
|
Fig. 2 Effect of polystyrene nanoplastic exposure on antioxidant enzyme activities in M. meretrix. |
Compared to the control and high-concentration treatments, the CAT activity of the digestive gland in the 0.1 mg L−1 PS-NPs treatment was significantly lower on exposure day 7 (P < 0.05). However, no significant effects among treatments were detected during the recovery period (P > 0.05) (Fig.2D). The CAT activity of the gills in both PS-NPs treatments was lower than that of the control on exposure day 3. The PS-NPs treatment of 1 mg L−1 was significantly higher than those of the 0.1 mg L−1 PS-NPs and control treatments on recovery day 1 (P < 0.05), but the activity did not differ significantly among treatments on recovery day 3 (P > 0.05) (Fig.2E). In the mantle, the CAT activity in the 0.1 mg L−1 PS-NPs treatment was significantly higher than that of the control on exposure day 1, and it significantly decreased on exposure day 7 (P < 0.05). The PS-NPs treatment of 1 mg L−1 was significantly lower than that of the control on exposure day 1 (P < 0.05). However, the value was not significantly different from that of the control (P > 0.05). The CAT activity of the mantle in the 1 mg L−1 PS-NPs treatment was significantly lower than that of 0.1 mg L−1 PS-NPs treatment on recovery day 3 (P < 0.05) (Fig.2F).
No significant difference in GPx activity was detected in the digestive gland of M. meretrix after PS-NPs exposure (P > 0.05), but the value was significantly lower in 0.1 mg L−1 PS-NPs treatment on recovery day 3 (P < 0.05) (Fig.2G). There was no significant difference in GPx activity in the gills (P > 0.05) (Fig.2H). Compared with the control, the GPx activity in the mantle was significantly lower in the 0.1 mg L−1 and 1 mg L−1 PS-NPs treatments on exposure days 1 and 7, respectively (P < 0.05). However, the value in both treatments was significantly higher compared with the control on recovery day 1 (P < 0.05) (Fig.2I).
The histogram data are presented as the mean ± SEM. Different letters above bars in the same series indicate significant differences (P < 0.05) among treatments. SOD in the digestive gland (A), SOD in the gills (B), SOD in the mantle (C), CAT in the digestive gland (D), CAT in the gills (E), CAT in the mantle (F), GPx in the digestive gland (G), GPx in the gills (H), GPx in the mantle (I) are showed in Fig.2.
The digestive gland of M. meretrix had significantly more MDA content than that of the control on exposure day 7 in low and high PS-NPs concentrations (P < 0.05). But it significantly decreased compared to the control on recovery day 3 (P < 0.05) (Fig.3A). No significant difference in MDA content in the gills was detected (P > 0.05) (Fig.3B). The MDA content in the mantle in the 1 mg L−1 PS-NPs treatment was significantly lower than those of the control and 0.1 mg L−1 treatments on exposure day 1 (P < 0.05), but there was no significant difference on exposure day 3 and day 7 (P > 0.05) (Fig.3C).
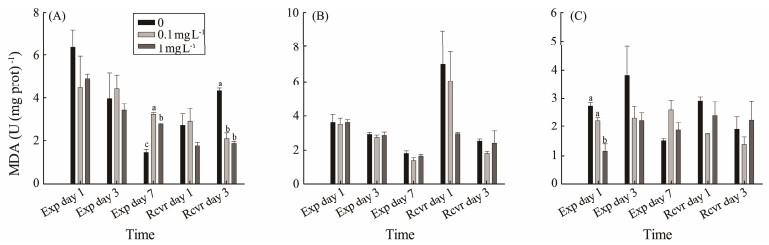
|
Fig. 3 Effect of polystyrene nanoplastic exposure on MDA content in M. meretrix. |
The histogram data are presented as the mean ± SEM. Different letters above bars in the same series indicate significant differences (P < 0.05) among treatments. MDA in the digestive gland (A), MDA in the gills (B), MDA in the mantle (C) are showed in Fig.3.
3.2 Antioxidant Enzyme Activities and MDA Content of S. constrictaCompared with the control, the SOD activity in the digestive gland of S. constricta in the 0.1 mg L−1 PS-NPs treatment was significantly higher on exposure day 7 (P < 0.05), whereas that of the 1 mg L−1 PS-NPs treatment was significantly lower (P < 0.05). During the recovery period, there was no significant difference in SOD activity among all treatments (P > 0.05) (Fig.4A). The SOD activity in the gills in the high-concentration treatment was significantly higher compared with the control and low-concentration treatments on exposure day 1, and the activity of the low-concentration treatment was significantly lower than that of the control (P < 0.05). The SOD activity in the 0.1 mg L−1 PS-NPs treatment was significantly lower than that of the control on recovery day 1 (P < 0.05), and the activity in both PS-NPs treatments was significantly higher than that of the control on recovery day 3 (P < 0.05) (Fig.4B). No significant difference in SOD activity in the mantle was detected at any PS-NPs concentration or time point (P > 0.05) (Fig.4C).
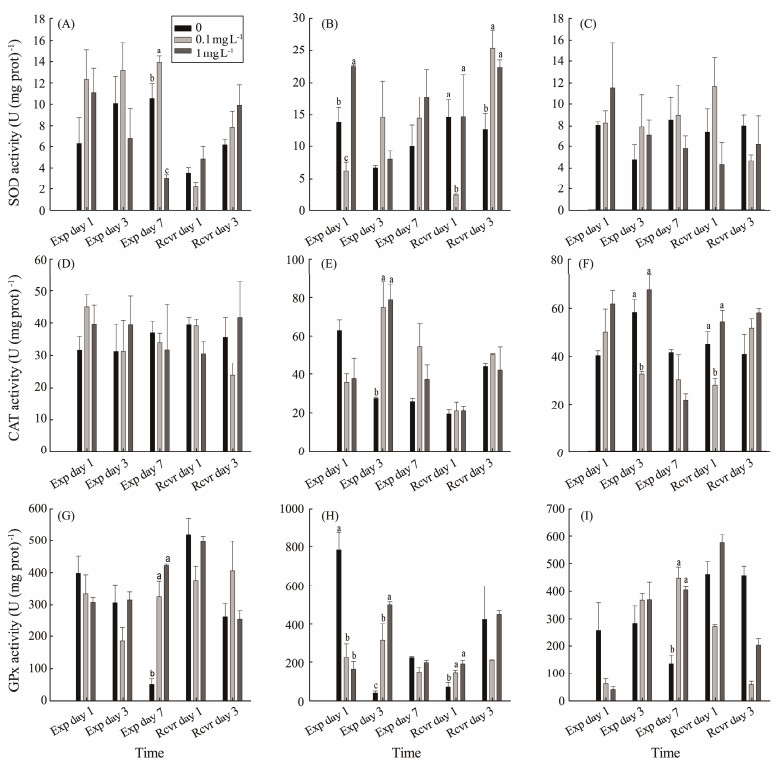
|
Fig. 4 Effect of polystyrene nanoplastic exposure on antioxidant enzyme activities in S. constricta. |
There was no significant difference in CAT activity in the digestive gland (P > 0.05) (Fig.4D). The CAT activity in the gills at both PS-NPs concentrations was significantly higher than that of the control on exposure day 3 (P < 0.05), but no significant difference was revealed on day 1 and day 7 (P > 0.05) (Fig.4E). The CAT activity of the mantle in the low concentration treatment was significantly lower than that of the control and high concentration treatments on exposure day 3 and recovery day 1 (P < 0.05). However, there was no significant difference observed on exposure day 1, day 7 and recovery day 3 (P > 0.05) (Fig.4F).
The GPx activity in the digestive gland at low and high PS-NPs concentrations was significantly higher than that of the control on exposure day 7 (P < 0.05), but no significant differences were detected on exposure day 1 and day 3 (P > 0.05) (Fig.4G). The GPx activity in the gills at both PS-NPs concentrations was significantly lower than that of the control on exposure day 1 (P < 0.05), but it was significantly higher on exposure day 3 and recovery day 1 (P < 0.05) (Fig.4H). The GPx activity in the mantle in both PS-NPs concentrations were significantly higher than that of the control on exposure day 7 (P < 0.05), but there was no significant difference observed on exposure day 1 and day 3 (P > 0.05) (Fig.4I).
The histogram data are presented as the mean ± SEM. Different letters above bars in the same series indicate significant differences (P < 0.05) among treatments. SOD in digestive gland (A), SOD in the gills (B), SOD in the mantle (C), CAT in the digestive gland (D), CAT in gills (E), CAT in the mantle (F), GPx in the digestive gland (G), GPx in the gills (H), GPx in the mantle (I) are showed in Fig.5.

|
Fig. 5 Effect of polystyrene nanoplastic exposure on MDA content in S. constricta. |
There was no significant difference in MDA content in the digestive gland (P > 0.05) (Fig.5A). The MDA content in the gills in both PS-NPs treatments were significantly higher than that of the control on exposure day 3 (P < 0.05). The MDA content in the high concentration PS-NPs treatment was also higher than that of the low concentration treatment on recovery day 3 (Fig.5B). The MDA content in the mantle in the high concentration treatment was significantly higher than that of the control and low concentration treatments on exposure day 7 (P < 0.05), but no significant difference was detected on exposure day 1 and day 3 (P > 0.05) (Fig.5C).
The histogram data are presented as the mean ± SEM. Different letters above bars in the same series indicate significant differences (P < 0.05) among treatments, MDA in the digestive gland (A), MDA in the gills (B), MDA in the mantle (C).
3.3 Evaluation of Antioxidant Stress Capacity of OrgansIBRv2 values were calculated using the following biomarkers: SOD, CAT, GPx and MDA. We detected differences between the M. meretrix and S. constricta responses for PS-NPs. For M. meretrix, the IBRv2 values for digestive gland, gills, and mantle decreased in the following order on day 1: gills (2.40) > mantle (1.70) > digestive gland (0.30) in the low concentration treatment, and mantle (4.80) > gills (3.02) > digestive gland (0.13) in the high concentration treatment. SOD and CAT activities of the gills contributed the most to the value of IBRv2, respectively. Additionally, the IBRv2 value for digestive gland was the highest among the organs in the high concentration treatment on day 7 (Fig.6). For S. constricta, the IBRv2 values decreased in the order on day 1: gills (6.87) > mantle (3.72) > digestive gland (0.36) in the low concentration treatment, and gills (4.27) > mantle (1.29) > digestive gland (0.85) in the high concentration treatment. Additionally, SOD activities of the digestive gland contributed the most to the value of IBRv2. The IBRv2 value for the digestive gland was the highest among the organs for both PS-NPs concentrations on day 7 (Fig.7).
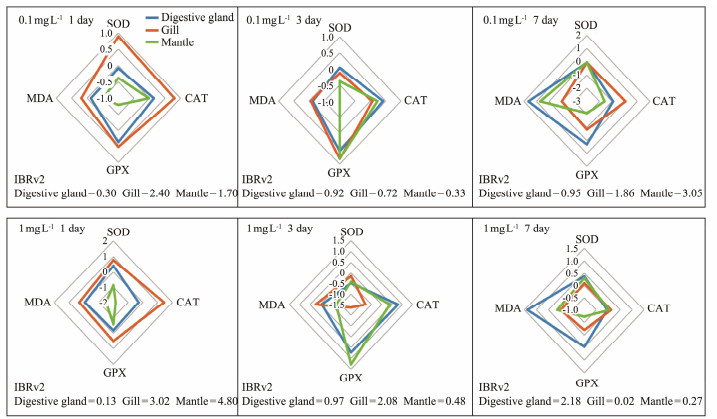
|
Fig. 6 Integrated biomarker response index version 2 (IBRv2) value in M. meretrix based on the following biomarker: SOD, CAT, GPx and MDA. |
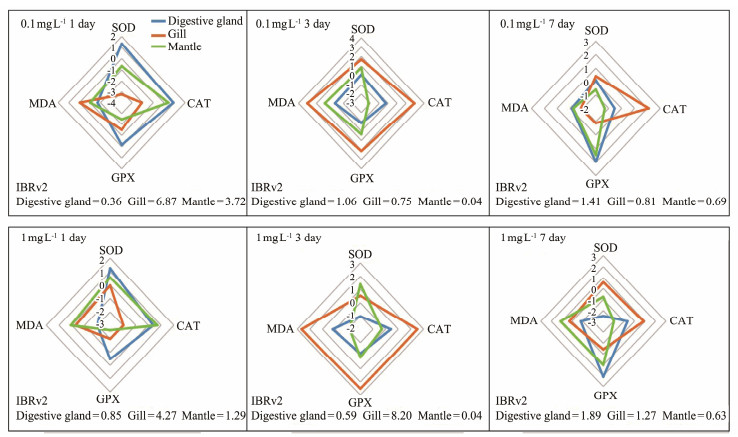
|
Fig. 7 Integrated biomarker response index version 2 (IBRv2) value in S. constricta based on the following biomarker: SOD, CAT, GPx and MDA. |
Microplastics from seawater can accumulate in different tissues of bivalves, such as the gills, muscles, and digestive gland (Ribeiro et al., 2017; Al-Sid-Cheikh et al., 2018; González-Soto et al., 2019; Sendra et al., 2020; Liu et al., 2021). The main fate of NPs is that they converge upon the lysosomes in the cells by endocytic routes (Sendra et al., 2020). NPs can affect lysosomal function, stimulate the production of reactive oxygen species, and decrease the phagocytic activity in hemocytes, which are immune cells in Mytilus (Canesi and Procházková, 2014). Canesi et al. (2015) reported PS-NH2 NPs stimulated an increase in extracellular production of reactive oxygen species (ROS) and nitric oxide (NO) in haemocytes of M. galloprovincialis. These results confirmed that the toxic mechanism of NPs on lysosomal membrane stabilization was mainly associated with oxidative damage. Antioxidant enzymes such as SOD, CAT, and GPx can effectively inhibit oxidation and play important roles in maintaining body homeostasis. Extensive studies have reported the observation of oxidative stress response in certain parts of mussels after nanoplastic ingestion, for instance, in the digestive gland and gills of M. edulis (Magara et al., 2018), the gills and digestive gland of S. plana (Ribeiro et al., 2017), as well as visceral mass, gills, and mantle of C. fluminea (Li et al., 2020). The results of the present study indicate that the oxidative stress response is dependent on tissue type, exposure time, and species. SOD catalysed the dismutation of the superoxide anion into oxygen and hydrogen peroxide, serving as the first defence line of defense to protect these tissues against oxidative stress probably caused by injures of PS-NPs. And the next defense is CAT. CAT can effectively catalyze the decomposition of hydrogen peroxide (H2O2), thereby maintaining a balance between the generation of de novo H2O2 and its efficient elimination (Wang et al., 2012). For M. meretrix, SOD activity in the gills increased at 0.1 mg L−1 and 1 mg L−1 groups after 1 d of exposure (P < 0.05). For S. constricta, the SOD activity in the gills at 1 mg L−1 group increased after 1 d exposure (P < 0.05). Additionally, the CAT activity in the gills at both PS-NPs concentrations increased after 3 d of exposure (P < 0.05). CAT and GPx are both involved in the removal of H2O2. The GPx activity in the gills of S. constricta increased after 3 d of exposure, and it also increased in digestive gland and mantle after 7 d of exposure (P < 0.05). Therefore, GPx could play an important role in defending against oxidative damage in S. constricta. Similar responses were occurred in the visceral mass, gills, and mantle of clam C. fluminea exposed to 5 mg L−1 PS-NPs (80 nm) after 4 d of exposure, and concentration dependence of SOD was exhibited in these organs (Li et al., 2020).
After recovery day 1, the GPx activity in the gills of S. constricta continued to increase at both PS-NPs concentrations (P < 0.05), without any significant difference observed on recovery day 3 (P > 0.05). However, SOD activity in the gills of S. constricta continued to increase in both PS-NPs concentrations after recovery day 3 (P < 0.05). Ribeiro et al. (2017) reported that the SOD activity continued to increase in the gills and digestive glands of the clam Scrobicularia plana after 7 d of depuration, while microplastics were still present in both tissues even after a week of depuration. So, the increase in enzymatic activity observed at the end of the depuration period in this study should be attributed to the presence of remaining PS-NPs in those tissues of S. constricta, that still induce an enzymatic response. There were also different responses between the S. constricta and M. meretrix in the depuration period. The GPx activity of the mantle of M. meretrix increased at both PS-NPs concentrations after recovery day 1 (P < 0.05). The different response patterns observed between S. constricta and M. meretrix could potentially be explained by their different type and time of accumulation and elimination concerning PS-NPs. In addition, Sendra et al. (2020) indicated that NPs (50 nm, 100 nm and 1 μm) were found in the hemolymph of M. galloprovincialis after a very short time, and due to the open circulatory system in mussels and the higher translocation of 50 nm PS-NPs, these particles could be the primary candidate to reach the gonads and the hepatopancreas. Meanwhile, Li et al. (2020) indicated that PS-NPs (80 nm) are more likely to accumulate in the gill, intestine and stomach of C. fluminea than in other tissues. Alsid-Cheikh et al. (2018) also observed a higher concentration of C14 PS-NPs in the digestive gland of the scallop Pecten maximus.
4.2 Evaluation of the Antioxidant Stress Capacity of OrgansIBRv2 values integrate all responses to identify the health status of organisms exposed to environmental stress. It is an effective tool for assessing the responses of individual biomarkers to stress and is widely used in toxicological studies (Sanchez and Porcher, 2013; DeAndrade et al., 2019; Li et al., 2020). In this study, the gills exhibited a more sensitive response to oxidative stress related to PS-NPs exposure compared to the mantle and digestive gland of M. meretrix and S. constricta at low concentration on day 1. Ribeiro et al. (2017) also reported that the gills of bivalves demonstrate a more effective response to oxidative stress related to MPs compared to the digestive gland. However, with longer exposure (day 7), both M. meretrix and S. constricta exhibited a more effective response to oxidative stress in the digestive gland than in the gills and mantle, particularly at high PS-NPs concentration. Li et al. (2020) also found that the digestive gland of C. fluminea exhibited a more effective response to oxidative stress than the gills and mantle after 96 h of exposure to NPs. These results suggest that the impact of PS-NPs on the antioxidant capacity of different organs could be based on exposure time.
4.3 Oxidative DamageMDA is a product of lipid oxidation, and it is a biomarker that reflects the degree of oxidative damage. Lin et al. (2006) reported that SiO2 nanoparticles increased ROS levels in cultured human bronchoalveolar carcinoma-derived cells, resulting in a significant increase in cellular MDA levels as well. Li et al. (2020) also reported significantly elevated MDA content in the visceral mass of C. fluminea treated with low (0.1 mg L−1) and high (5 mg L−1) concentrations of NPs. In the current study, the content of MDA in both PS-NPs treatments was significantly induced in the digestive gland of M. meretrix on day 7, but the level significantly decreased compared with that of the control after recovery day 3. At the same time, Liu et al. (2021) reported that both PS-NH2 and PS-COOH were observed in the digestive glands of the exposed M. meretrix, which induced atrophy and necrosis in clam digestive tubules, thus leading to digestion dysfunction. In the case of S. constricta, the content of MDA in both treatments was significantly induced in the gills on day 3 and in the mantle of the 1 mg L−1 PS-NPs treatment on day 7. These results suggest that the oxidative damage was most pronounced in the digestive gland in of M. meretrix and in the gills of S. constricta. This finding may be attributable to their different characteristics. Both M. meretrix and S. constricta are filter-feeding organisms, M. meretrix is a fully closed species, whereas S. constricta is not. Therefore, Kolandhasamy et al. (2018) proposed that both ingestion and adherence are involved in the uptake of MPs in mussels, and adherence to soft tissue leads to a higher MP accumulation than ingestion alone. Thus, PS-NPs might accumulate more easily in the gills of nonfully closed S. constricta by adherence than in the tissues of fully closed M. meretrix. This could explain why oxidative damage may occur earlier in the gills of S. constricta.
5 ConclusionsThe oxidative enzyme responses to PS-NPs varied depending on exposure time, dose, organ, and species. GPx play an important role in defending against oxidative damage in S. constricta. The gills were most effective at responding to oxidative stress during early exposure, while the digestive gland was most effective during the long exposure. The molecular pathways responsible for the activation and inhibition of antioxidant enzymes should be researched in the future.
AcknowledgementThis work was supported by the Natural Natural Science Foundation of China (No. 41706142), and the Earmarked Fund for CARS-49 (No. CARS-49).
Author Contributions
Linlan Lv, raw material preparation, writing-original draft, writing-review and editing, data curation. Wanjun Feng, raw material preparation, writing-original draft, writing-review and editing, data curation. Ke Sun, raw material preparation and data analysis. Weihong Zhao, administration and supervision. Fengjuan Jiang, supervision and data curation. Mohsen Mohamed, writing-review and editing. Lei Li, supervision, project administration. Yue Yang, conceptualization and investigation. Yingying Zhang, raw material preparation and data analysis. Yanming Sui, funding acquisition and supervision. Xuexing Dong, writing-original draft and data curation.
Data Availability
The data and references presented in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
Informed consent for publication was obtained from all participants.
Conflict of Interests
The authors declare that they have no conflict of interests.
Abdelsaleheen, O., Monikh, F. A., Monikh, S., and Keski-Saari, J., 2021. The joint adverse effects of aged nanoscale plastic debris and their co-occurring benzo[α]pyrene in freshwater mussel (Anodonta anatina). Science of the Total Environment, 798: 149196. DOI:10.1016/j.scitotenv.2021.149196 (  0) 0) |
Al-Sid-Cheikh, M., Rowland, S. J., Stevenson, K., Rouleau, C., Henry, T. B., and Thompson, R. C., 2018. Uptake, wholebody distribution, and depuration of nanoplastics by the scallop pecten maximus at environmentally realistic concentrations. Environmental Science & Technology, 52: 14480-14486. DOI:10.1021/acs.est.8b05266 (  0) 0) |
Arthur, C., Baker, J., and Bamford, H., 2009. Proceeding of the international research work-shop on the occurrence, effects, and fate of microplastic marine debris. NOAA Marine Debris Division, Washington, D. C., American.
(  0) 0) |
Azevedo-Santos, V. M. D., Gonalves, G. R. L., Manoel, P. S., Andrade, M. C., and Pelicice, F. M., 2019. Plastic ingestion by fish: A global assessment. Environmental Pollution, 255: 112994. DOI:10.1016/j.envpol.2019.112994 (  0) 0) |
Beliaeff, B., and Burgeot, T., 2002. Integrated biomarker response: A useful tool for ecological risk assessment. Environmental Toxicology and Chemistry, 21(6): 1316-1322. (  0) 0) |
Brandts, I., Teles, M., Goncalves, A. P., Barreto, A., Franco-Martinez, L., Tvarijonaviciute, A., et al., 2018. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Science of the Total Environment, 643: 775-784. DOI:10.1016/j.scitotenv.2018.06.257 (  0) 0) |
Browne, M. A., Dissanayake, A., Galloway, T. S., Lowe, D. M., and Thompson, R. C., 2008. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L). Environmental Science & Technology, 42(13): 5026-5031. DOI:10.1021/es800249a (  0) 0) |
Canesi, L., and Procházková, P., 2014. The invertebrate immune system as a model for investigating the environmental impact of nanoparticles. Nanoparticles and the Immune System, 7: 91-112. DOI:10.1016/B978-0-12-408085-0.00007-8 (  0) 0) |
Canesi, L., Ciacci, C., Bergami, E., Monopoli, M. P., Dawson, K. A., Papa, S., et al., 2015. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Marine Environmental Research, 111: 34-40. DOI:10.1016/j.marenvres.2015.06.008 (  0) 0) |
Choi, H., Im, D. H., Park, Y. H., Lee, J. W., and Yoon, S. J., 2022. Ingestion and egestion of polystyrene microplastic fragments by the Pacific oyster, Crassostrea gigas. Environmental Pollution, 307: 119217. DOI:10.1016/j.envpol.2022.119217 (  0) 0) |
Cole, M., Liddle, C., Consolandi, G., Drago, C., Hird, C., Lindeque, P. K., et al., 2020. Microplastics, microfibres and nanoplastics cause variable sublethal responses inmussels (Mytilus spp.). Marine Pollution Bulletin, 160: 111552. DOI:10.1016/j.marpolbul.2020.111552 (  0) 0) |
De Andrade, L. L., Santo Pereira, A. D. E., Fraceto, L. F., and Bueno, D. R. M. C., 2019. Can atrazine loaded nanocapsules reduce the toxic effects of this herbicide on the fish Prochilodus lineatus? A multibiomarker approach. Science of the Total Environment, 663: 548-559. DOI:10.1016/j.scitotenv.2019.01.380 (  0) 0) |
Ding, J., Sun, C., He, C., Li, J., Ju, P., and Li, F., 2021. Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Science of the Total Environment, 782: 146830. DOI:10.1016/j.scitotenv.2021.146830 (  0) 0) |
González-Soto, N., Hatfifield, J., Katsumiti, A., Duroudier, N., Lacave, J. M., Bilbao, E., et al., 2019. Impacts of dietary exposure to different sized polystyrene microplastics alone and with sorbed benzo[a]pyrene on biomarkers and whole organism responses in mussels Mytilus galloprovincialis. Science of the Total Environment, 684: 548-566. DOI:10.1016/j.sci-totenv.2019.05.161 (  0) 0) |
Hartmann, N. B., Hüffer, T., Thompson, R. C., Hassellöv, M., Verschoor, A., Daugaard, A. E., et al., 2019. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environmental Science & Technology, 53: 1039-1047. DOI:10.1021/acs.est.8b05297 (  0) 0) |
Huang, W., Wang, X., Chen, D., Xu, E. G., Luo, X., Zeng, J., et al., 2021. Toxicity mechanisms of polystyrene microplastics in marine mussels revealed by high-coverage quantitative metabolomics using chemical isotope labeling liquid chromatography mass spectrometry. Journal of Hazardous Materials, 417: 126003. DOI:10.1016/j.jhazmat.2021.126003 (  0) 0) |
Jabeen, K., Su, L., Li, J., Yang, D., Tong, C., Mu, J., et al., 2016. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environmental Pollution, 221: 141-149. DOI:10.1016/j.envpol.2016.11.055 (  0) 0) |
Koelmans, A. A., Besseling, E., and Shim, W. J., 2015. Nanoplastics in the aquatic environment. In: Marine Anthropogene Litter. Bergmann, M., et al., eds., 325-340, DOI: 10.1007/978-3-319-16510-3_12.
(  0) 0) |
Kolandhasamy, P., Su, L., Li, J. N., Qu, X. Y., Jabeen, K., and Shi, H. H., 2018. Adherence of microplastics to soft tissue of mussels: A novel way to uptake microplastics beyond ingestion. Science of the Total Environment, 610-611: 635. DOI:10.1016/j.scitotenv.2017.08.053 (  0) 0) |
Lee, K. W., Shim, W. J., Kwon, O. Y., and Kang, J. H., 2013. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environmental Science & Technology, 47: 11278-11283. DOI:10.1021/es401932b (  0) 0) |
Lenz, R., Enders, K., and Nielsen, T. G., 2016. Microplastic exposure studies should be environmentally realistic. Proceedings of the National Academy of Sciences of the United States of America, 113: 4121-4122. DOI:10.1073/pnas.1606615113 (  0) 0) |
Li, Z., Feng, C., Wu, Y., and Guo, X., 2020. Impacts of nanoplastics on bivalve: Fluorescence tracing of organ accumulation, oxidative stress and damage. Journal of Hazardous Materials, 392: 122418. DOI:10.1016/j.jhazmat.2020.122418 (  0) 0) |
Lin, W. S., Huang, Y. W., Zhou, X. D., and Ma, Y. F., 2006. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicology and Applied Pharmacology, 217: 252-259. DOI:10.1016/j.taap.2006.10.0 (  0) 0) |
Liu, L., Zheng, H., Luan, L. P., Luo, X. X., Wang, X., Lu, H., et al., 2021. Functionalized polystyrene nanoplastic-induced energy homeostasis imbalance and the immunomodulation dysfunction of marine clams (Meretrix meretrix) at environmentally relevant concentrations. Environmental Science-Nano, 8: 2030-2048. DOI:10.1039/D1EN00212K (  0) 0) |
Magara, G., Elia, A. C., Syberg, K., and Khan, F. R., 2018. Single contaminant and combined exposures of polyethylene microplastics and fluoranthene: Accumulation and oxidative stress response in the blue mussel, Mytilus edulis. Journal of Toxicology and Environmental Health, Part A, 81: 761-773, DOI: 10.1080/15287394.2018.1488639.
(  0) 0) |
Muhammad, A., Zhou, X., He, T., Zhang, N., Shen, X., Sun, C., et al., 2021. Toxic effects of acute exposure to polystyrene microplastics and nanoplastics on the model insect, silkworm Bombyx mori. Environmental Pollution, 285: 117255. DOI:10.1016/j.envpol.2021.117255 (  0) 0) |
Provenza, F., Piccardo, M., Terlizzi, A., and Renzi, M., 2020. Exposure to pet-made microplastics: Particle size and pH effects on biomolecular responses in mussels. Marine Pollution Bulletin, 156: 111228. DOI:10.1016/j.marpolbul.2020.111228 (  0) 0) |
Rebelein, A., Int-Veen, I., Kammann, U., and Scharsack, J. P., 2021. Microplastic fibers – Underestimated threat to aquatic organisms? Science of the Total Environment, 777: 146045, DOI: 10.1016/j.scitotenv.2021.146045.
(  0) 0) |
Ribeiro, F., Garcia, A. R., Pereira, B. P., Fonseca, M., Mestre, N. C., Fonseca, T., et al., 2017. Microplastics effects in Scrobicularia plana. Marine Pollution Bulletin, 122(1-2): 379-391. DOI:10.1016/j.marpolbul.2017.06.078 (  0) 0) |
Rist, S., Baun, A., and Hartmann, N. B., 2017. Ingestion of micro and nanoplastics in Daphnia magna – Quantification of body burdens and assessment of feeding rates and reproduction. Environmental Pollution, 228: 398-407. DOI:10.1016/j.envpol.2017.05.048 (  0) 0) |
Sanchez, W., Burgeot, T., and Porcher, J. M., 2013. A novel 'Integrated Biomarker Response' calculation based on reference deviation concept. Environmental Science & Technology, 20(5): 2721-2725. DOI:10.1007/s11356-012-1359-1 (  0) 0) |
Sendra, M., Saco, A., Yeste, M. P., Romero, A., Novoa, B., and Figueras, A., 2020. Nanoplastics: From tissue accumulation to cell translocation into Mytilus galloprovincialis hemocytes. resilience of immune cells exposed to nanoplastics and nanoplastics plus Vibrio splendidus combination. Journal of Hazardous Materials, 388: 121788. DOI:10.1016/j.jhazmat.2019.121788 (  0) 0) |
Vasanthi, R. L., Arulvasu, C., Kumar, P., and Srinivasan, P., 2021. Ingestion of microplastics and its potential for causing structural alterations and oxidative stress in Indian green mussel Perna viridis – A multiple biomarker approach. Chemosphere, 283: 130979. DOI:10.1016/j.chemosphere.2021.130979 (  0) 0) |
Wang, C., Yue, X., Lu, X., and Liu, B. Z., 2012. The role of catalase in the immune response to oxidative stress and pathogen challenge in the clam Meretrix meretrix. Fish & Shellfish Immunology, 34(1): 91-99. DOI:10.1016/j.fsi.2012.10.013 (  0) 0) |
Wang, X., Huang, W., Wei, S., Shang, Y., Gu, H., Wu, F., et al., 2019. Microplastics impair digestive performance but show little effects on antioxidant activity in mussels under low pH conditions. Environmental Pollution, 258: 113691. DOI:10.1016/j.envpol.2019.113691 (  0) 0) |
Wu, Y., Yang, J., Li, Z., He, H., Wang, Y., Wu, H., et al., 2022. How does bivalve size influence microplastics accumulation? Environmental Research, 214 (1): 113847, https://doi.org/10.1016/j.envres.2022.113847.
(  0) 0) |
 2025, Vol. 24
2025, Vol. 24


