The diversity and complexity of pigmentation patterns on mollusk shells have been an active research field. The color patterns on their shells make the mollusca fascinating models to study morphogenesis. The generation of a mollusk shell pattern is, in fundamental terms, a symmetry-breaking process. Such a process can be efficiently modeled by one-dimensional reaction diffusion (Kondo and Miura, 2010). It requires an autonomous line of secretory cells experiencing local and lateral interactions along the mantle edge to control pigmentation (Budd et al., 2014). Mollusk shell formation is controlled by an evolutionarily homologous organ, i.e., mantle, which covers the visceral mass and directly contact with shell (Jabbour-Zahab et al., 1992; Budd et al., 2014). The mantle is divided into distal, central, and proximal zones, and they are responsible for the secretion of structurally different shell layers (Jolly et al., 2004; Marin and Luquet, 2004; McDougall et al., 2011). Shells grow in a linear way by the addition of new material to the growing edge that contacts with the mantle (Williams, 2017; Feng et al., 2019). It is here that new pigments produced by the mantle are established in the periostracum (Gantsevich et al., 2005; Li et al., 2014; Williams, 2017).
Melanin of Mollusca is the most widespread pigment in nature (Williams, 2017). In vertebrates, melanocytes are the most common type of chromatophores and are responsible for most of the dorsal pigmentation. Each melanocyte contains thousands of melanin-containing melanosomes. Melanosomes are morphologically and functionally unique organelles within which melanin pigments are synthesized and stored (Marks and Seabra, 2001). The size and maturation of melanosomes can cause different skin colors (Miyamura et al., 2007). Several studies have focused on the distribution and formation of melanin or melanocytes in invertebrates. It has been reported that the number of melanosomes in the melanocytes of albino Apostichopus japonicus is less than it in the normal ones (Zhao et al., 2012). Moreover, in Cephalopods, the biochemical steps in melanin production have been linked to melanosomes in the melanin-producing cells in the ink gland (Derby, 2014). In Pinctada persica, the mantle edge stained with haematoxylin and eosin is lined by cuboidal and columnar epithelia, and some cells containing brown granules were distributed among these epidermal cells (Parvizi et al., 2018). In addition, brown pigmented cells were sparsely distributed in the outer fold and middle fold in Pinctada margaritifera (Jabbour-Zahab et al., 1992). The presence of pigment in mantle epithelia showed their possible role in black calcitic prism deposition.
The Pacific oyster is a shellfish with high commercial value and possesses multiple traits, such as fast growth rate, high reproductive performance, and rapid adaptation, making it desirable for aquaculture (Ge et al., 2014). The complex pigment patterns (black, golden, orange and white) on C. gigas shells makes it a fascinating model to study morphogenesis of pigmentation. The shell colors have been considered as a quantitative trait which lays a foundation for future marker-assisted breeding (Ge et al., 2014; Xu et al., 2019). Recently, genome-wide expression profiling compared C. gigas with different shell colors, and the results indicated that several candidate genes, such as Rab7 and Tyrp1, might be involved in shell pigmentation (Feng et al., 2015). Among these candidates, tyrosinase and peroxidase have been identified to play key roles in shell matrices and melanin pigmentation in C. gigas shells (Feng et al., 2019; Zhu et al., 2021). Previous morphological studies on the mantle histology and ultrastructure of various oyster species have reported the presence of pigments found in mantle edges. (Jabbour-Zahab et al., 1992; Fang et al., 2008; Parvizi et al., 2018). Additionally, Han et al. (2022) confirmed the presence of melanin in oysters using three melanin staining methods, including DOPA, Masson-Fontana and ferrous sulfate staining methods. Besides, melanocytes containing melanosomes with electron-density components were found below the membrane, indicating the melanosomes exist in the mantle tissues of the Pacific oyster (Han et al., 2022). It is believed that the mantle edges have three distinct folds, including outer secretory, middle sensory, and inner muscular folds in most advanced mollusks. Moreover, the epithelia of the mantle edges that secrete different components of the shell show several distinctive features. However, little is known about patterning mechanisms of pigmentation among the three distinct lobes in the mollusks.
In this study, the fine structures of three distinct lobes were investigated to identify the cellular basis of the pigmentation patterns in C. gigas. Data from this study will provide functional morphology of the mollusks integument information to understand the mechanisms of the shell coloration, which will bring new insights into the genetic basis for the selective breeding the C. gigas with different shell colors.
2 Materials and Methods 2.1 Pigmentation MappingWild populations of juvenile C. gigas (shell height: 48.13 mm ± 4.69 mm; shell length: 31.08 mm ± 4.07 mm; shell width: 11.13 mm ± 1.86 mm) were collected from the sea along Weihai City of China in November 2021. The C. gigas were cultured in filtered seawater with in 1 mol L−1 MgCl2 for 8 h. Then the mantles were dissected and mounted on slides. Image acquisitions were performed with Olympus DP80 microscope.
2.2 Electron MicroscopyThe oysters were anesthetized in 1 mol L−1 MgCl2 in 50% seawater and 50% fresh water for 8 h. Mantle samples were fixed in 2.5% glutaraldehyde for 2 h. They were postfixed in osmium tetroxide, dehydrated in acetone, and embedded in EPON 812 resin. Then the sections were cut at 5 μm and then stained with toluidine blue to identify different mantle regions under the light microscope. Ultrathin sections of 60 nm were stained with uranyl acetate and lead citrate and observed with a transmission electron microscope (JEM-1200EX) at 80.0 kV.
2.3 Histological Observation of the MantlesMantle tissues were fixed in buffered formalin following the method of Carson et al. (1973). All samples were dehydrated through a graded series of EtOH (70%, 80%, 90%, and 100%), cleared in xylene, and then embedded in paraffin. The sections were cut at 5 μm using a Leica RM 2016 microtome (Leica). The paraffin-embedded microsections were stained for morphological studies with hematoxylin and eosin (H & E).
The chelation of ferrous ions by the melanin pigment in the mantles was estimated by the method of Jabbour-Zahab et al. (1992). Briefly, the sections were dewaxed with xylene and then dehydrated in gradient alcohol. After the sections were stained in ferrous solution for 1 h and then washed for 12 min four times in ultrapure water. Sections were immersed in acid potassium ferricyanide solution for 30 min. After washed for four times, the sections were then placed in nuclear fast red solution for 10 min. Images of stained mantle tissues were captured using an Olympus DP80 microscope.
3 Results 3.1 Histological Observation of the MantleThe mantle tissue of the C. gigas is divided into two zones, namely the marginal mantle and central mantle. The mantle with pigmented granules that can be clearly viewed in the light microscopy (Fig. 1). The degree of pigment deposition within the marginal and central mantles is different, which indicates the pigmentation and scattering properties vary among mantle areas.
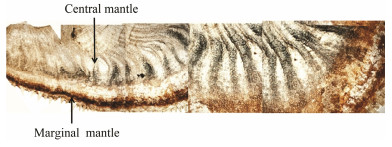
|
Fig. 1 Mantle tissue of C. gigas. |
To further study the structures of the mantles in the oyster, we performed H & E stain. The C. gigas marginal mantle is a thick and muscular membrane, and is characterized by three different folds, including the outer secretory, middle sensory, and inner muscular folds, as shown in Fig. 2A. These folds are composed of the outer epithelium, inner epithelium, and connective tissues. Close to the shell is the outer mantle fold, and the periostracum is formed within the periostracal groove between the outer fold and the middle fold. It is clear that the middle fold has finger-like ciliated tentacles on its outer surface (Fig. 2B). Moreover, a large rounded ganglion is located at the convergent boundaries of the three folds (shown in Fig. 2C). Central mantle is a transparent and epithelial membrane, which consists of the epithelial tissue that is attached to irregular connective tissues. Hemocytes and muscle fibers are observed within the central mantle (Fig. 2D). Ferrous ion chelating assays against the tyrosine hydroxylase showed that a large amount of melanin was present in the epithelium of marginal mantle (Fig. 2E), particularly in the inner fold and the inner surface of the middle fold, as shown in Fig. 2F. However, melanin granules are more abundant in the inner surface of the middle fold than those of the inner fold and outer fold.
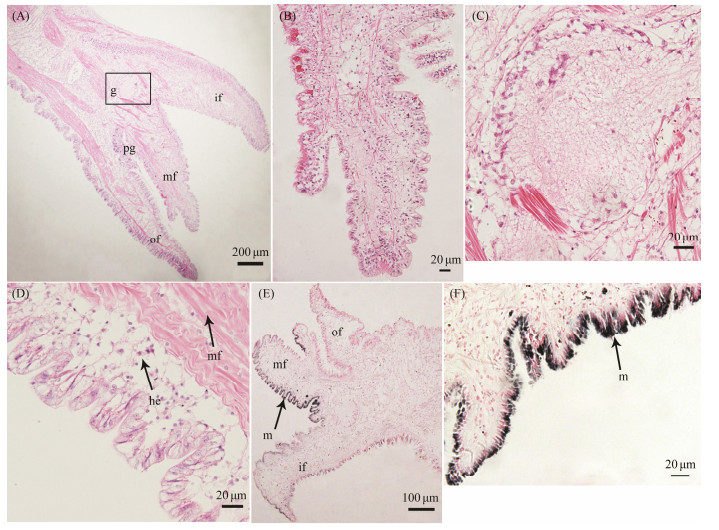
|
Fig. 2 Histological sections of the C. gigas mantle. A, marginal mantle and central mantle. B, middle fold of the marginal mantle. C, ganglion in the marginal mantle. D, epithelium of the central mantle. E, distribution of melanin of the mantle. F, distribution of melanin in the middle fold. of, outer fold; mf, middle fold; if, inner fold; pg, periostracal groove; g, ganglion; mf, muscle fiber; he, hemocytes; m, melanin. |
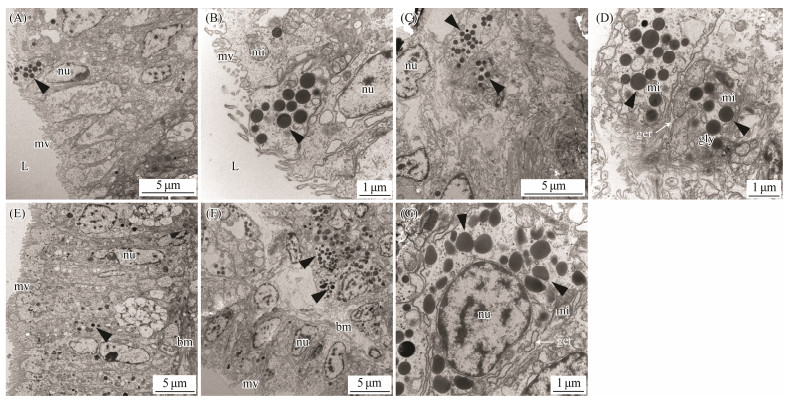
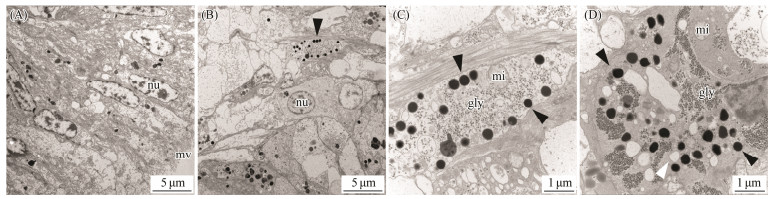
By taking advantage of the high spatial resolution of a transmission electron microscope, we compared the difference of the ultrastructure among the marginal and central mantle of C. gigas. The apical plasma membrane of the epithelia is arranged in an array of microvilli in all cases (Figs. 3A, 3E, 4A, 4E, 5A, 5E, 6A). Most of the cells in the epidermis are cuboidal epidermal cells, lying on a membrane. The epithelium is separated with its neighbour by an intercellular junction. Apparently, melanocytes are distributed in the marginal and central mantle, which is a characteristic of melanin-containing melanosomes, varying from 0.3 to 0.6 μm in size (Figs. 3D, 3H, 4C, 4D, 4G, 5D, 5G, 6C, 6D). In the inner (Figs. 3A – D) and outer (Figs. 3E – H) surfaces of the inner fold, cells located below the membrane possess more melanosomes than those in the epidermis (Figs. 3A, B, E, F). Only several melanosomes are found within the inner epithelium of the inner fold (black arrow-head) (Fig. 3C). Interestingly, non-pigmented vesicles also appear in the basal portion of the inner epithelium of the inner fold (white arrowheads) (Fig. 3D). However, in the outer surface, the number of melanosome is higher than that in the inner surface. Some single dispersion, or aggregation of melanosomes with different degrees of melanization are found in this region (Fig. 3H). In the middle fold, it is evident that the melanosome within the outer surface (Figs. 4E, F) are concentrated in the apical region compared with inner surface (Figs. 4A, B). Notably, the number of melanosome within the inner surface (Figs. 4A – D) is significantly higher than that in the outer surface (Figs. 4E – H). Additionally, some melanosomes with partially pigmented granules were also observed on the outer surface (white arrowheads) (Figs. 4G, H). In the outer fold, there are numerous single and large melanosomes within the inner (Figs. 5A – D) and outer (Figs. 5E – H) surfaces, characterized by ellipsoidal and intensely melanotic. Overall, the melanosome count in the outer fold is higher than that in the inner fold and middle fold. The vesicles loaded with multiple melanosomes (black arrowhead) (Fig. 5B) were concentrated towards the apical microvillar surface near the lumen (shell-abutting). The majority of melanosomes reside along the membrane within the inner epithelium and there are usually aggregations of glycogen rosettes in this region (Fig. 5D). Typically, granular endoplasmic reticulum are present in the peripheral region of melanosomes (Figs. 5D, G). Surprisingly, melanosomes are occasionally observed in the central mantle, but they are relatively less (Fig. 6). There appear to be an increase in the amount of glycogen when compared with those in the marginal mantle.
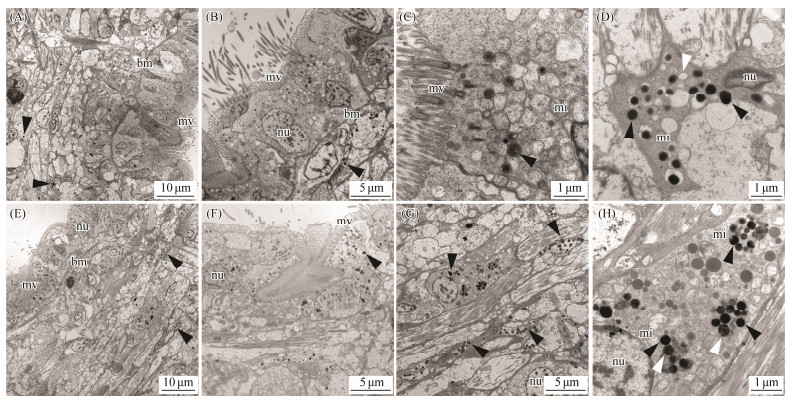
|
Fig. 3 Transmission electron microphotographs of the inner fold of mantle epithelia. A, B, C and D: Ultrastructure of the inner surface of the inner fold. Black arrowheads indicate melanosomes. White arrowheads indicate non-pigmented vesicles. E, F, G and H: Ultrastructure of the outer surface of the inner fold. Black arrowheads indicate melanosomes. White arrowheads indicate melanosomes with partially pigmented granules. mv, microvilli; bm, basal membrane; nu, nuclei; mi, mitochondria. |
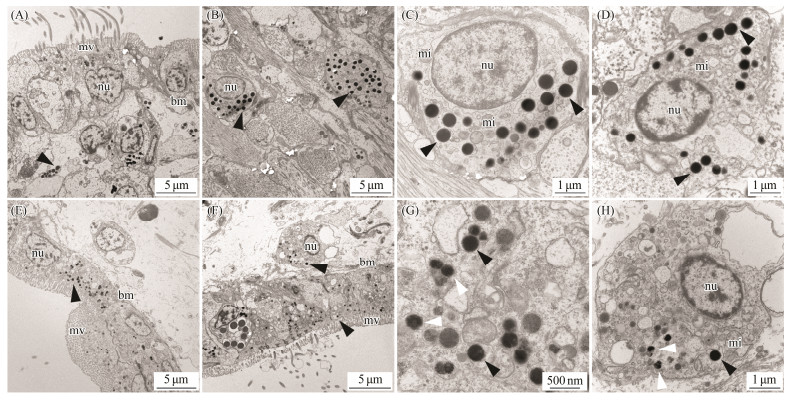
|
Fig. 4 Transmission electron microphotographs of the middle fold of mantle epithelia. A, B, C and D: Ultrastructure of the inner surface of the middle fold. E, F, G and H: Ultrastructure of the outer surface of the middle fold. Black arrowheads indicate melanosomes. White arrowheads indicate melanosomes with partially pigmented granules. mv, microvilli; bm, basal membrane; nu, nuclei; mi, mitochondria. |
|
Fig. 5 Transmission electron microphotographs of the outer fold of mantle epithelia. A, B, C and D: Ultrastructure of the inner surface of the outer fold. E, F, G and H: Ultrastructure of the outer surface of the outer fold. Black arrowheads indicate melanosomes. mv, microvilli; bm, basal membrane; nu, nuclei; mi, mitochondria; L, luman; ger, granular endoplasmie retieulum; gly, glycogen. |
|
Fig. 6 Transmission electron microphotographs of central mantle epithelia. Black arrowheads indicate melanosomes. White arrowheads indicate non-pigmented vesicles. mv, microvilli; nu, nuclei; mi, mitochondria; gly, glycogen. |
The various mantle edge morphology may affect shell characteristics, including shape, colour, size and thickness (Parvizi et al., 2018). In the Yesso Scallop, the middle fold of mantle was composed of several protuberances with abundant nerve fibers, whereas the inner fold was highly muscular and was much larger than the other folds (Mao et al., 2019). It is conceived that the muscular curtain of the inner fold is used to regulate water flow into and out of the mantle cavity (Audino et al., 2015). Meanwhile, in contrast to other folds, the inner fold was taller and bigger, and contained pleated epithelium in the pearl oysters (Parvizi et al., 2018). However, the outer fold was longer than the inner fold in Velesunio ambiguus and Hyridella depressa, and it was divided into two or more lobes (Richardson et al., 1981; Colville and Lim, 2003). In some species, such as Pulvinites exempla, the mantle edge was composed of four folds, including two inner folds and two outer folds (Tëmkin, 2006). In C.gigas, the mantle edge was divided into three folds, they were similarly unfused along the mantle edge and free from attachment to the inner surface of mantles to allow deep movement by pallial retractor muscles either three or four folds (Yonge, 1977; Tëmkin, 2006). Altogether, the mantle has specific and different morphology in each species of bivalves. It is believed that the mantle edges have three distinct folds, including outer secretory, middle sensory, and inner muscular folds in most advanced mollusks. Moreover, the epithelia of the mantle edges concerned with the secretion of the different components of the shell show several distinctive features.
Indeed, outer fold of P. exempla contains amounts of pigmented granules, while a very low concentration of pigmented cells was present in the inner fold and the adjacent portion of the inner mantle surface, suggesting their role in secretion (Tëmkin, 2006). A recent study conducted in specimens of C. gigas through staining with Masson-Fontana and ferrous ion showed a distribution of melanin in the mantle tissues, suggesting the presence of melanin granules (Han et al., 2022). In this study, the melanin was mainly found in the inner fold and the inner surface of the middle fold using the ferrous ion staining. Fibres of the muscles run along the inner and outer surfaces of mantle tissue, penetrating into inner and outer mantle folds, suggesting the possibility of gradual transfer of melanin from the inner and middle folds to the outer fold along actin filaments directly, as reported in the fish melanophores previously (Aspengren et al., 2008). In addition, the cells of the outer fold mantle secrete organic matrix into the extrapallial space, the matrix can combine with inorganic ions to form calcium carbonate, and then deposit on the shell (Ballarini and Heuer, 2007). These observations led to the conclusion that the melanin granules could be involved in the shell coloration. Similar results were found in the Pinctada maritlza, in that species melanins were observed in the middle and inner fold of the mantle epithelia (JabbourZahab et al., 1992).
Melanin, a black or brown biological polymer formed from tyrosine, was founded in the body wall of sea cucumbers (Zhao et al., 2012). Meanwhile, melanin was also histologically distributed in the epithelium of Haliotis tuberculata side foot (Bravo Portela et al., 2012). In these species, the pigment was in the form of melanosomes. Pigmented granules were commonly observed in bivalve's mantles, and most appeared as secretory vesicles. Secretory vesicles associate with the Golgi complex and will change size and electron density when they move to the cell apex region (Álvarez Nogal and Molist García, 2015). These vesicles can contact with the plasma membrane (Colville and Lim, 2003), and then they can take up or release materials. The epidermal cells of the mantle were associated with pigmentation of the shell and soft tissues. Recently, melanocytes containing melanosomes were identified below the mantle epidermis in C. gigas (Han et al., 2022). In this study, ultrastructures of the marginal and central mantle of C. gigas were observed. A large number of melanocytes which contained melanosomes were distributed on the mantle surface. Melanosomes with many electron dense particles known as the melanin granules become more and larger from the epidermis of the inner fold to the outer fold. The increase of number and size of melanosome may be ascribed to function of specific mantle region. In general, pigment granules of epidermal cells of the outer mantle contribute to the formation and structure of the shell through chitin (Álvarez Nogal and Molist García, 2015). The growth process of a bivalve shell was controlled by the material that was secreted by the epithelium of the outer mantle fold (Checa, 2000). It was indicated that the difference of human skin color was mainly due to the melanocytic activity in each melanocyte, as well as the size and maturation of melanosomes (Aspengren et al., 2008). Thus, it can be hypothesized that melanosomes within the outer mantle may release the melanin to control shell color as these cells secrete materials to form the shell.
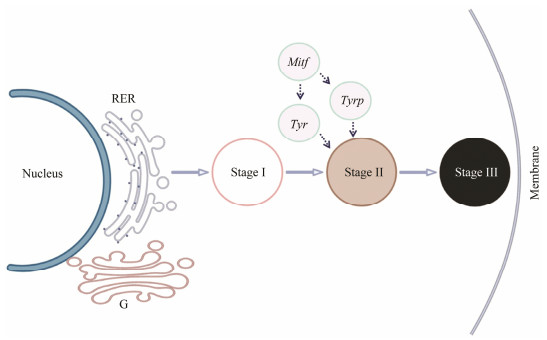
Regarding the variations in the distribution of melanosomes within epithelium in mantle, three distinct stages were defined. Stage Ⅰ represents vesicles with non-pigmented, stage Ⅱ represents melanosomes with partially pigmented granules, stage Ⅲ represents melanosomes with completely pigmented granules. According to Derby (2014), nonpigmented melanosome vesicles represent premelanosomes in which melanins were not yet deposited. The change from stage Ⅱ to stage Ⅲ was a progress from early stage melanosomes to mature melanosomes. Similar studies were reported in H. tuberculata, pigmented cells on a groove of foot containing mature and immature melanosomes (Bravo Portela et al., 2012). Formation of the periostracum takes place over the basal third of the inner face of the outer fold epithelium (Bubel, 1973). Our observations indicate that the inner surface of the outer fold epithelium is with one cell type, characterized by the presence of numerous melanosomes. It is likely that these melanosomes take part in the formation of periostracum via extrapallial space (luman). Furthermore, in the inner surface of the outer fold, there are a large number of mitochondria within the melanocyte and a highly developed rough endoplasmic reticulum. The presence of mitochondria suggests high metabolic activity in this area. Moreover, it is generally accepted that rough endoplasmic reticulum can form early premelanosomes (Palumbo, 2003). However, the process of melanin synthesis in C. gigas is complex and is involved in various melanogenic enzymes. Whether the melanogenic enzymes co-localize and are functionally interactive in the melanogenic compartments of mantle cells is not clear. With this issue, large amounts of active tyrosinase were found in the mantle edge in our previous studies, and the localization of tyrosinase gene (Tyr) was located in the inner surface of the outer fold by ISH analysis (Zhu et al., 2021). These evidences suggest that the tyrosinase can take part in the melanization. Another important factor related to the melanin formation is microphthalmia-associated transcription factor (Mitf), which not only regulates the survival and proliferation of melanocytes, but also promotes the transcription of tyrosinase gene (Noguchi et al., 2014). It is probable that the rough endoplasmic reticulum formed vesicles, then, melanization occurred at the outside of the vesicles with the catalytic action of melanogenic enzymes, forming particulate melanosomes. Finally, melanosomes fuse with the cell membrane at the apical pole, releasing the melanin into the extrapallial space (Fig. 7). Similar study can be found in melanin-producing systems of ink gland of Sepia officinalis (Derby, 2014).
|
Fig. 7 Overview of melanin formation in the mantle of C. gigas. Stage Ⅰ represent non-pigmented vesicles. Stage Ⅱ represent melanosomes with partially pigmented granules. Stage Ⅲ represent melanosomes with completely pigmented granules. Tyr, tyrosinase gene; Tyrp, tyrosinase-like protein gene; Mitf, microphthalmia-associated transcription factor; RER, rough endoplasmic reticulum; G, Golgi. |
What's more, there is a tendency of reduction in the protein synthetic apparatus of the cells from the edge mantle to the central mantle, whereas the quantity of glycogen increased. Early studies showed that this may be associated with a change of secretory activity from periostracum formation to calcium secretion (Kniprath, 1972). In addition, it may be related to shell calcification, since carbon dioxide generated as a result of glycogen metabolism can provide a carbonate source for the calcification process (Bubel, 1973).
In conclusion, the pigmented granules of the mantles vary among the three lobes and melanosomes at different stages, and are enriched in distinct cargo molecules. It causes a remarkable difference between the marginal mantle and central mantle. Most importantly, melanosomes within the epidermal cells of the outer mantle might be associated with shell pigmentation. In particular, the maturation of melanosomes probably affects the growth and proliferation of melanosomes. This study sheds new light on the cellular basis for mantle melanin formation, which can contribute to understand better the shell pigmentation in the C. gigas.
AcknowledgementsThis research was supported by grants from the National Natural Science Foundation of China (Nos. 31772843 and 31972789), the National Key R & D Program of China (No. 2018YFD0900200), the Earmarked Fund for Agriculture Seed Improvement Project of Shandong Province (No. 2017LZGC009), and the Ocean University of China-Auburn University Joint Research Center for Aquaculture and Environmental Science.
Álvarez Nogal, R., and Molist García, P., 2015. The outer mantle epithelium of Haliotis tuberculata (Gastropoda Haliotidae): An ultrastructural and histochemical study using lectins. Acta Zoologica, 96: 452-459. DOI:10.1111/azo.12090 (  0) 0) |
Aspengren, S., Hedberg, D., Sköld, H, N., and Wallin, M., 2008. Chapter 6 new insights in melanosome transport in vertebrate pigment cells. International Review of Cell and Molecular Biology, 272: 245-302. (  0) 0) |
Audino, J. A., Marian, J. E. A. R., Wanninger, A., and Lopes, S. G. B. C., 2015. Mantle margin morphogenesis in Nodipecten nodosus (Mollusca: Bivalvia): New insights into the development and the roles of bivalve pallial folds. BMC Developmental Biology, 15(1): 22. DOI:10.1186/s12861-015-0074-9 (  0) 0) |
Ballarini, R., and Heuer, A. H., 2007. Secrets in the shell. American Scientist, 95: 422-429. DOI:10.1511/2007.67.3726 (  0) 0) |
Bravo Portela, I., Martinez Zorzano, V. S., Molist-Perez, I., and Molist Garcia, P., 2012. Ultrastructure and glycoconjugate pattern of the foot epithelium of the abalone Haliotis tuberculata (Linnaeus, 1758) (Gastropoda, Haliotidae). Scientific World Journal, 2012: 1-12. (  0) 0) |
Bubel, A., 1973. An electron-microscope study of periostracum formation in some marine bivalves. Ⅱ. The cells lining the periostracal groove. The cells lining the periostracal groove. Marine Biology, 20: 222-234. (  0) 0) |
Budd, A., McDougall, C., Green, K., and Degnan, B. M., 2014. Control of shell pigmentation by secretory tubules in the abalone mantle. Frontiers in Zoology, 11: 62. DOI:10.1186/s12983-014-0062-0 (  0) 0) |
Carson, F. L., Martin, J. H., and Lynn, J. A., 1973. Formalin fixation for electron microscopy: A re-evaluation. American Journal of Clinical Pathology, 59: 365-373. DOI:10.1093/ajcp/59.3.365 (  0) 0) |
Checa, A., 2000. A new model for periostracum and shell formation in Unionidae (Bivalvia, Mollusca). Tissue and Cell, 32: 405-416. DOI:10.1054/tice.2000.0129 (  0) 0) |
Colville, A. E., and Lim, R. P., 2003. Microscopic structure of the mantle and papls in the freashwater mussles Velesunio ambiguus and Hyridella depressa. Molluscan Research, 23(1): 1-20. DOI:10.1071/MR02014 (  0) 0) |
Derby, C. D., 2014. Cephalopod ink: Production, chemistry, functions and applications. Marine Drugs, 12: 2700-2730. DOI:10.3390/md12052700 (  0) 0) |
Fang, Z., Feng, Q., Chi, Y., Xie, L., and Zhang, R., 2008. Investigation of cell proliferation and differentiation in the mantle of Pinctada fucata (Bivalve, Mollusca). Marine Biology, 153(4): 745-754. DOI:10.1007/s00227-007-0851-5 (  0) 0) |
Feng, D., Li, Q., and Yu, H., 2019. RNA interference by ingested dsRNA-expressing bacteria to study shell biosynthesis and pigmentation in Crassostrea gigas. Marine Biotechnology, 21(4): 526-536. DOI:10.1007/s10126-019-09900-2 (  0) 0) |
Feng, D., Li, Q., Yu, H., Zhao, X., and Kong, L., 2015. Comparative transcriptome analysis of the Pacific oyster Crassostrea gigas characterized by shell colors: Identification of genetic bases potentially involved in pigmentation. PLoS One, 10: e0145257. DOI:10.1371/journal.pone.0145257 (  0) 0) |
Gantsevich, M., Tyunnikova, A., and Malakhov, V., 2005. The genetics of shell pigmentation of the Mediterranean mussel Mytilus galloprovincialis Lamarck, 1819 (Bivalvia, Mytilida). Doklady Biological Sciences, 404: 370-371. DOI:10.1007/s10630-005-0139-1 (  0) 0) |
Ge, J., Li, Q., Yu, H., and Kong, L., 2014. Identification and mapping of a SCAR marker linked to a locus involved in shell pigmentation of the Pacific oyster (Crassostrea gigas). Aquaculture, 434: 249-253. DOI:10.1016/j.aquaculture.2014.08.027 (  0) 0) |
Han, Y., Xie, C., Fan, N., Song, H., Wang, X., Zheng, Y., et al., 2022. Identification of melanin in the mantle of the Pacific oyster Crassostrea gigas. Frontiers in Marine Science, 9: 880337. DOI:10.3389/fmars.2022.880337 (  0) 0) |
Jabbour-Zahab, R., Chagot, D., Blanc, F., and Grizel, H., 1992. Mantle histology, histochemistry and ultrastructure of the pearl oyster Pinctada margaritifera (L.). Aquatic Living Resources, 5: 287-298. DOI:10.1051/alr:1992027 (  0) 0) |
Jolly, C., Berland, S., Milet, C., Borzeix, S., Lopez, E., and Doumenc, D., 2004. Zonal localization of shell matrix proteins in mantle of Haliotis tuberculata (Mollusca, Gastropoda). Marine Biotechnology, 6: 541-551. DOI:10.1007/s10126-004-3129-7 (  0) 0) |
Kniprath, E., 1972. Formation and structure of the periostraeum in Lymnaea stagnalis. Tissue Research, 9: 260-271. DOI:10.1007/BF02061966 (  0) 0) |
Kondo, S., and Miura, T., 2010. Reaction-diffusion model as a framework for understanding biological pattern formation. Science, 329(5999): 1616-1620. DOI:10.1126/science.1179047 (  0) 0) |
Li, X., Bai, Z., Luo, H., Liu, Y., Wang, G., and Li, J., 2014. Cloning, differential tissue expression of a novel hcApo gene, and its correlation with total carotenoid content in purple and white inner-shell color pearl mussel Hyriopsis cumingii. Gene, 538(2): 258-265. DOI:10.1016/j.gene.2014.01.046 (  0) 0) |
Mao, J., Zhang, W., Wang, X., Song, J., Yin, D., Tian, Y., et al., 2019. Histological and expression differences among different mantle regions of the Yesso scallop (Patinopecten yessoensis) provide insights into the molecular mechanisms of biomineralization and pigmentation. Marine Biotechnology, 21(5): 683-696. DOI:10.1007/s10126-019-09913-x (  0) 0) |
Marin, F., and Luquet, G., 2004. Molluscan shell proteins. Comptes Rendus Palevol, 3: 469-492. DOI:10.1016/j.crpv.2004.07.009 (  0) 0) |
Marks, M. S., and Seabra, M. C., 2001. The melanosome: Membrane dynamics in black and white. Nature Reviews Molecular Cell Biology, 2: 738-748. DOI:10.1038/35096009 (  0) 0) |
Mcdougall, C., Green, K., Jackson, D. J., and Degnan, B. M., 2011. Ultrastructure of the mantle of the gastropod Haliotis asinina and mechanisms of shell regionalization. Cells Tissues Organs, 194(2-4): 103-107. DOI:10.1159/000324213 (  0) 0) |
Miyamura, Y., Coelho, S. G., Wolber, R., Miller, S. A., Wakamatsu, K., Zmudzka, B. Z., et al., 2007. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Research, 20: 2-13. DOI:10.1111/j.1600-0749.2006.00358.x (  0) 0) |
Noguchi, S., Kumazaki, M., Yasui, Y., Mori, T., Yamada, N., and Akao, Y., 2014. MicroRNA-203 regulates melanosome transport and tyrosinase expression in melanoma cells by targeting kinesin superfamily protein 5b. Journal of Investigative Dermatology, 134: 461-469. DOI:10.1038/jid.2013.310 (  0) 0) |
Palumbo, A., 2003. Melanogenesis in the ink gland of Sepia officinalis. Pigment Cell Research, 16(5): 575-522. (  0) 0) |
Parvizi, F., Monsefi, M., Noori, A., and Ranjbar, M. S., 2018. Mantle histology and histochemistry of three pearl oysters: Pinctada persica, Pinctada radiata and Pteria penguin. Molluscan Research, 38(1): 11-20. DOI:10.1080/13235818.2017.1387039 (  0) 0) |
Richardson, C. A., Runham, N. W., and Crisp, D. J., 1981. A histological and ultrastructural study of the cells of the mantle edge of a marine bivalve, Cerastoderma edule. Tissue Cell, 13(4): 715-730. DOI:10.1016/S0040-8166(81)80008-0 (  0) 0) |
Tëmkin, I., 2006. Anatomy, shell morphology, and microstructure of the living fossil Pulvinites exempla (Hedley, 1914) (Mollusca: Bivalvia: Pulvinitidae). Zoological Journal of the Linnean Society, 148(3): 523-552. DOI:10.1111/j.1096-3642.2006.00263.x (  0) 0) |
Williams, S. T., 2017. Molluscan shell colour. Biological Reviews, 92(2): 1039-1058. DOI:10.1111/brv.12268 (  0) 0) |
Xu, C., Li, Q., Yu, H., Liu, S., Kong, L., and Chong, J., 2019. Inheritance of shell pigmentation in Pacific oyster Crassostrea gigas. Aquaculture, 512: 734249. DOI:10.1016/j.aquaculture.2019.734249 (  0) 0) |
Yonge, C. M., 1977. Form and evolution in the Anomiacea (Mollusca: Bivalvia) – Pododesmus, Anomia, Patro, Enigmonia (Anomiidae); Placunanomia, Placuna (Placunidae Fam. Nov). Philosophical Transactions of the Royal Society of London Series B, 276(950): 453-523. DOI:10.1098/rstb.1977.0005 (  0) 0) |
Zhao, H., Yang, H., Zhao, H., Liu, S., and Wang, T., 2012. Differences in MITF gene expression and histology between albino and normal sea cucumbers (Apostichopus japonicus Selenka). Chinese Journal of Oceanology and Limnology, 30(1): 80-91. DOI:10.1007/s00343-012-1043-9 (  0) 0) |
Zhu, Y., Li, Q., Yu, H., Liu, S., and Kong, L., 2021. Shell biosynthesis and pigmentation as revealed by the expression of tyrosinase and tyrosinase-like protein genes in Pacific oyster (Crassostrea gigas) with different shell colors. Marine Biotechnology, 23: 777-789. DOI:10.1007/s10126-021-10063-2 (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


