2) Key Laboratory of Marine Bioactive Substances, First Institute of Oceanography, State Oceanic Administration, Qingdao 266061, China;
3) Department of Bioengineering, College of Marine Sciences and Biological Engineering, Qingdao University of Science & Technology, Qingdao 266042, China;
4) School of Basic Medicine, Qingdao University, Qingdao 266071, China;
5) Key Laboratory of Marine Science and Numerical Modeling, State Oceanic Administration, Qingdao 266061, China;
6) Center for Fundamental and Applied Microbiomics, Biodesign Institute, Arizona State University, Tempe, AZ 85287, USA
Antarctica is unique due to its isolation from other continents, extreme climate, minimum of human activity, and indigenous organisms (Hughes et al., 2014). Therefore, local habitats, home to microbes, are excellent ecological systems to study the interactions between these organisms. Surface temperature trends show significant warming across the western side of the Antarctic Peninsula since the early 1950s (Turner et al., 2005), and global warming also affects the Antarctic ecosystem (Steig et al., 2009). Under global warming, not only has the glaciers in the Antarctica melted more rapidly (Pritchard et al., 2012), but also the areas of bare soils in the Antarctic coastal area has increased (Nicolas et al., 2017). Moreover, due to the undulating surface of the Antarctic nearshore area, snow melts to form ponds and even lakes. Lakes and ponds are good sentinels of global climate change (Adrian et al., 2009). Therefore, global warming has significant effects on Antarctic landscape. Changes in microbes in the lake district can also indirectly reflect changes in climate in the Antarctic region.
Soil microbial diversity is important for long-term ecosystem sustainability (Staddon et al., 1998), as soil microorganisms fulfil essential ecosystem functions, such as biogeochemical cycling, decomposition of plants, animal and microbial wastes, and pollutants (Staddon et al., 1997; Marilley et al., 1998), including those in polar ecosystems (Hogg et al., 2006). Soil microbial community and diversity are affected by a series of factors in Antarctica, namely biological and abiotic factors, includeing organic carbon (Tytgat et al., 2016), moisture (Lavian et al., 2001), phosphorus (Kuramae et al., 2015), soil type (Berg and Smalla, 2009), and the biotic interactions (Hogg et al., 2006; Caruso et al., 2013). Therefore, soil bacterial communities are a sensitive indicator of climate change and an integral part of biogeochemistry (Oyugi et al., 2007; Santamans et al., 2017), particularly the biogeochemistry of carbon (Schaefer et al., 2014).
It is well established that the availability of organic carbon regulates the turnover and activities of microbial communities in soils (You et al., 2014), especially heterotrophic microorganisms. These heterotrophic bacteria constitute a significant proportion of the heterotrophic microbial biomass in soils (Ladd et al., 1996; Chotte et al., 1998). Furthermore, soils with higher amounts of organic carbon show higher microbial activity and catabolic activity (Lagomarsino et al., 2012). In this study, we selected the Kitezh Lake as a study site with typical slope features and collected samples to analyze bacterial composition using the 16S rRNA gene. The aim of this study is mainly to study the changes in OrC content in the soil and the effects of these changes on soil bacterium. The study is necessary to understand how ecosystem functions and community composition respond to the environment.
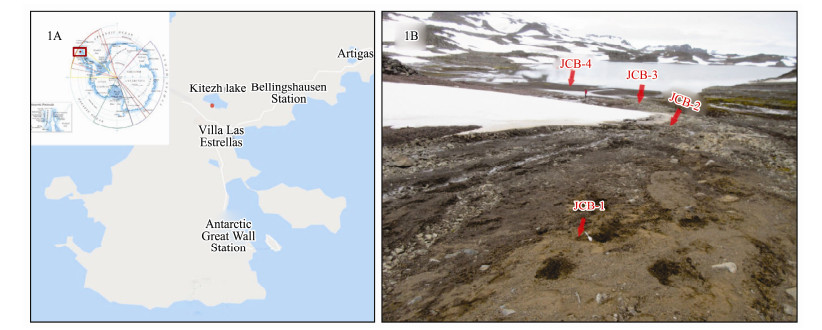
2 Materials and Methods 2.1 Sampling Site Description and Sample CollectionThe study area is located in the Kitezh Lake of the Antarctic Fildes Peninsula. Since tertiary lava, pyroclastic rock and volcanic sedimentary rock structured the main body of Fildes Peninsula, volcanic rock erosion and weathering residues have generated very pristine soils there (Wang et al., 2015). It is believed that the organic matter (e.g., carbon, nitrogen, phosphates) is transferred into soils by vegetation and animal activities (Bölter, 2011). Samples were collected in February, 2017 (Fig. 1A) at four sites (Fig. 1B). Among them, JCB1 (62.19342˚S, 58.97331˚W), JCB2 (62.19267˚S, 58.97248˚W) and JCB3 (62.19303˚S, 58.97162˚W) were located at the top, middle and bottom of the hill, respectively. JCB4 (62.19333˚S, 58.97237˚W) was located on the shoreline of Kitezh Lake. 50 grams of surface soil (0-5 cm) was collected into TWIRL'EM sterile bags (Labplas Inc., Sainte-Julie, QC, Canada) using a sterile shovel, each site with three replicates. Once collected, samples were divided into three parts and stored at −20℃ in the Great Wall Station (China) for 10 days before transport in a cooler to the home laboratory and stored at −80℃ until DNA extraction and nutrient analysis.
|
Fig. 1 Locations (red dot; A) and image (B) of the sampling sites from an Kitezh Lake area in Antarctic Fildes Peninsula. |
A total of seven soil geochemical properties were measured, including pH, organic carbon (OrC), and five soluble nutrients (NH4+-N, SiO42−-Si, NO3−-N, NO2−-N and PO43−-P) (Table 1). 10 g of soil samples were weighed, and freeze-dried with a freeze dryer, ground into powder, then stones and roots were removed. Soil pH was measured by adding 10 mL of distilled water to four grams of soil for pH measurement using a pH meter (PHS-3C, Shanghai REX Instrument Factory, Shanghai, China). OrC was measured according to the procedure described by Hu et al. (2012). The soils were treated with 10% HCl and dried to be analyzed on an element analyzer (EA3000, Euro Vector SpA, Milan, Italy). The soils used to determine nutrients were also freeze-dried and grounded, and then water was added at a ratio of 1:10 (g mL−1). After shaking once every 4 h for 48 h, a nutrient auto-analyzer (QuAAtro, SEAL, Germany) was used to determine the relative standard deviation < 5% for other physical and chemical properties (Liu et al., 2016).
|
|
Table 1 Geochemical properties of 12 samples investigated in the present study |
Genomic DNA was extracted from 0.25 g soil samples using MO BIO PowerSoil DNA Isolation Kit following manufacturer's instructions. The purity and concentration of DNA extracts were detected on an agarose gel, and the qualified samples were selected for subsequent experiments (Magoä and Salzberg, 2011). The average concentration of DNA obtained from these soils is 10-30 ng μL−1. The v3-v4 region of 16S rRNA gene was amplified using primers 806R ('5-GGACTACNNGGGTATCTAAT-3') (Michelsen et al., 2014) and 341F (5'-CCTAYGGGRB GCASCAG-3') (Jeanette et al., 2012). All PCR reactions were carried out in 30 μL reactions, including 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA, United States), 0.2 μmol L−1 of forward and reverse primers and 10 ng template DNA. The PCR amplification cycle was: initial denaturation at 98℃ for 1 min, followed by 30 cycles of denaturation at 98℃ for 10 s, annealing at 50℃ for 30 s, and elongation at 72℃ for 30 s, with a final extension of 72℃ for 5 min. PCR products were mixed with equal volume of 1× loading buffer (containing SYB green) and loaded onto 2% agarose gel for detection. Samples with a bright main strip between 400 and 450 bp were chosen for purification with Gene JET Gel Extraction Kit (Thermo Scientific, Waltham, MA, United States).
2.4 Sequence Analysis16S rRNA gene amplicons were sequenced on an Illumina MiSeq platform, and 250-bp paired-end reads were generated. Clean reads were obtained by removing barcode and primer sequences, trimming end bases and filtering low-quality bases, with QIIME (Version 1.7.0) (Caporaso et al., 2010b), chimeras were detected by the UCHIME algorithm (Edgar et al., 2011) and against the Gold database, and finally the chimeric sequences and lllumina adapters were removed (Haas et al., 2011). The resulting effective reads of all samples were clustered using the Uparse software (Version 7.0) (Edgar, 2013), and the sequences were clustered into an OTU at a 97% identity. Meanwhile, the most frequent sequence for an OTU was selected as the representative sequence of the OTU. The species were annotated and analyzed on the representative sequence of the OTUs using Qiime and the SSU rRNA database (Quast et al., 2013) of SILVA (Version 128) (Wang et al., 2007) to obtain the taxonomic information and calculate abundance at each classification level in all the samples. Finally, all samples were normalized at the same sequence depth (51249 reads), for the subsequent alpha and beta diversity analysis. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (accession number: SRP127779).
2.5 Statistical AnalysisStatistical analyses of the alpha diversity of soil samples, Chao1, Good's coverage, ACE, and Shannon's index (H'), were calculated using Qiime 1.7.0 (Caporaso et al., 2010a). Kruskal-Wallis test was performed for the geochemical properties and the diversity parameters of samples to determine the level of significance in R software (Version 3.2.4) (Tierney, 2012). The species accumulation box-plot was plotted using the R software to check if the number of samples was sufficient. A linear discriminant analysis effect size (LEfSe) method was used to identify the significantly different bacterial taxa between sampling sites (Segata et al., 2011). The relevance of geochemical parameters in explaining distribution patterns of bacterial communities by canonical correspondence analysis (CCA), and identifying sample-unique species using Adonis were analyzed using R package vegan (Oksanen et al., 2016).
3 Results 3.1 Geochemical Properties of SoilsOur results showed that geochemical properties were different between the four groups of samples in the Kitezh Lake of the Antarctic Fildes Peninsula. First, the site JCB4 has the highest values of six of the seven geochemical factors: OrC (0.5%), NH4+-N (0.83 μg g−1), SiO42−-Si (0.5 μg g−1), NO3−-N (0.32 μg g−1), NO2−-N (0.15 μg g−1), PO43−-P (0.14 μg g−1). Interestingly, the lowest values of OrC (0.15%), NH4+-N (0.33 μg g−1), SiO42−-Si (0.2 μg g−1), NO3−-N (0.13 μg g−1) NO2−-N (0.06 μg g−1), PO43−-P (0.05 μg g−1) were detected at JCB2 sample. We also found that the contents of OrC, NH4+-N, SiO42−-Si, NO3−-N, NO2−-N and PO43−-P were gradually increasing from JCB2 to JCB4.
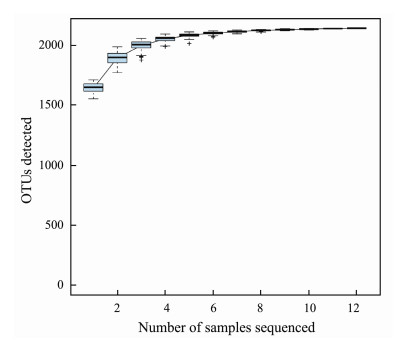
3.2 Bacterial Diversity and Community CompositionIn 12 samples, a total of 1070483 raw sequences and 803156 clean reads were retained after a series of quality control. There were more than 2000 OTUs in each sample, JCB2.2 contains the fewest OTUs (2363) and JCB1.2 contains the most OTUs (2967). The Good's coverage estimator of the OTUs in the samples ranged from 0.983 to 0.991 (Table 2), indicating that the sequences sufficeently covered most of the bacterial populations in all samples. The Shannon (8.788), Chao1 (2467.564) and AEC values (2726.765) of the JCB4.1 were the lowest among the 12 samples. The OTUs accumulation box-plot (Fig. 2) saturates with all 12 samples, indicating the OTUs of the study sites should be well represented by these samples.
|
|
Table 2 Summary data for Miseq sequencing data from the 12 samples in the present study |
|
Fig. 2 The OTUS accumulation box-plot of the 12 samples. |
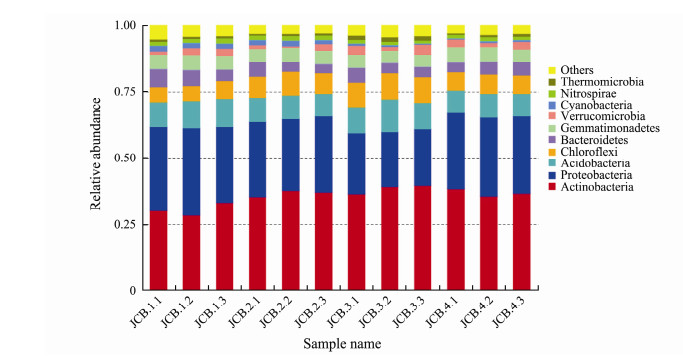
To examine how OrC and nutrients affected soil microbiomes. we first looked at the general microbiome structure. Among the 12 samples, all OTUs were mapped to 39 phyla. OTUs of Actinobacteria (28%-40%), Proteobacteria (20%-31%), Acidobacteri (8%-10%) and Chloroflexi (5%-10%) were common in all the four sites (Fig. 3). Actinobacteria and Proteobacteria were abundant phyla among the 12 soil samples. These four phyla accounted for about 75%-82% sequences. Among them, the abundance of Actinobacteria was the most abundant phylum in the 12 soil samples with an average 35.74%.
|
Fig. 3 The top10 abundance of different phyla in the 12 soil samples in the present study. |
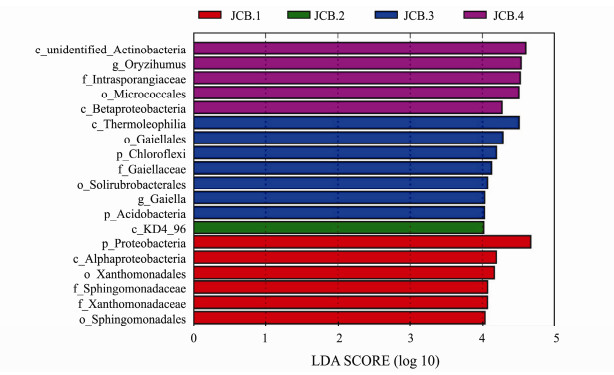
Besides the overall community composition, we also focused on bacteria with large differences in bacterial abundance. Based on the LEfSe results, 19 taxa showed LDA score (Linear Discriminant Analysis) greater than 4 (the cutoff for significance test) in the 12 samples (Fig. 4), such as, Oryzihumus, Gaiella, Micrococcales, Alphaproteobacteria, and Proteobacteria. We found that the Proteobacteria had the highest LDA (LDA score 4.72) score in all samples, followed by unidentified Actinobacteria (LDA score 4.65). In these 12 samples, there were three phyla with significant abundance differences, including Proteobacteria, Acidobacteri (LDA score 4.28), and Chioroflexi (LDA score 4.02). The abundance of Oryzihumus in JCB4 samples was higher than that of other samples (LDA score 4.5). JCB2 had most taxa, 7 of 19 (37%), more abundant than at other sites.
|
Fig. 4 The taxa showing different relative abundance among the sites using the LDA score. |
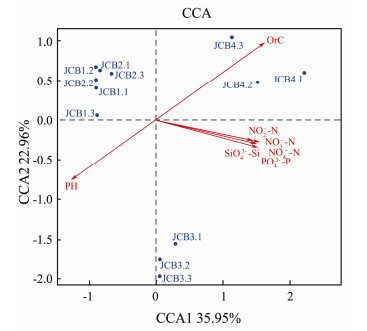
In order to explore the correlation between bacterial communities and geophysical factors, we first performed CCA analysis. The JCB4 samples were positively correlated with all of the environmental factors except pH. JCB1 and JCB2 samples were positively correlated with NH4+-N, SiO42−-Si, NO3−-N, NO2−-N and PO43−-P (Fig. 5 and Table 3). Among all of the nine geochemical properties, OrC (r2 = 0.68, P < 0.05) was one of the most important factors associated with bacterial community composition in the area. High correlation between community composition and geochemical factors was also found (Table 4). Proteobacteria was significantly related to OrC, Actinobacteria was significantly related to SiO42−-Si, NO3−-N and PO43−-P (Table 4). Hungateii was significantly associated with OrC and nutrient content (NH4+-N, SiO42−-Si, NO3−-N, NO2−-N and PO43−-P). Algae and Quitensis were affected by OrC (Table 4). A closer examination revealed that the abundance of bacteria was different at the level of phylum, class, order, family, genus, and species (Table 5). At the same time, we also found that Algae and Quitensis showed an increasing trend from JCB2 to JCB4.
|
Fig. 5 Canonical correspondence analysis to show correlations between the bacterial communities and environmental factors of the 12 samples from the four sampling sites. |
|
|
Table 3 A Monte Carlo permutation test of relationship between environmental factors and bacterial community composition |
|
|
Table 4 Correlations between bacterial and geochemical factors |
|
|
Table 5 Different taxon content in pairwise soil sample comparison |
Our research showed that the communities and diversity of soil bacteria in a lake area of the Fildes Peninsula changed with geochemical factors. In this study, the high level of Shannon diversity indices (H'= 8.92– 9.21) and the identification of 2363-2967 OTUs suggest that the presence of a surprisingly high diversity of soil bacterial communities on the lakeside. From the 16S rDNA data, we found that most of the bacterial sequences belong to the common phyla, including Actinobacteria, Proteobacteria, Acidobacteria, Chloroflexi, Bacteroidetes, Gemmatimonadetes, and Verrucomicrobia. Although the top 10 phyla of all samples are shared among the sites, the abundance was different. For example, compared with other samples, Proteobacteria (21%) in JCB3 had the lowest abundance, but Actinobacteria (38%) and Chloroflexi (9%) had the highest abundance.
In the Antarctic region, geochemical properties played an important role in the change of bacterial community diversity in soil. There was a correlation between soil bacterial community and 78 geochemical characteristics of soil. OrC (R2 = 0.68, P < 0.05) showed the highest correlation with bacterial community composition in all soil samples. Bacteria might be the main organisms responsible for the turnover of organic matter in Antarctic soils.
Proteobacteria was all significantly associated with OrC content (Table 4). OrC was a significant factor affecting the soil bacterial community in this study. The concentrations of organic carbon in JCB1 and JCB4 samples were higher than those of JCB2 and JCB3, and the abundance of Proteobacteria in JCB1 and JCB4 was also higher than that of JCB2 and JCB3 in this study, because Proteobacteria had many bacteria for heterotrophic life. As reported by the institute of organic matter addition (Parsons et al., 2004; Goldfarb et al., 2011), the organic matter related increase in Proteobacteria, specifically, the Gammaproteobacteria (Horn et al., 2014). In our study, samples with high organic carbon content were abundant in Gammaproteobacteria.
OrC also had a significant effect on heterotrophic bacteria. Through OTU analyses, we found abundant and unique species. They showed a degree of consistency with the content of OrC in soil samples, including Hungatei. Hungatei belongs to the bacteria of heterotrophic life. Because Hungatei could use OrC to decompose and exhibit chemotactic behavior (Monserrate et al., 2001), so the content of Hungatei was also high in the samples with high OrC content and has a significant correlation with organic carbon concentration. These bacteria were so closely related to OrC that changes in OrC content in the soil will have a very direct impact on the content of these bacteria.
Inorganic nitrogen (NH4+-N, NO3−-N and NO2−-N) had less effect on bacterial communities in soil than OrC (Fig. 5, Table 4). NH4+-N, NO3−-N and NO2−-N may affect the nitrogen-related bacterial population in soil (Zhang et al., 2017). We also knew that Actinobacteria was significantly correlated with PO43−-P and NO3−-N (Table 4). In Table 4, the abundance of Hungatei and Quitensis increases from JCB2 to JCB4, indicating that nutrient content changed the abundance of some species.
We also found that the OrC and nutrient concentration (NH4+-N, SiO42−-Si, NO3−-N, NO2−-N, PO43−-P) gradually increased from JCB2 to JCB4, while the pH gradually decreased. The physicochemical factors of different concentrations affected the community composition of bacteria. Through consulting the literature, we speculate that the main factors of the concentration gradient were caused by the erosive effect of snowmelt on the soil, because the samples JCB1-4 shows significant height difference from hillside to lakeside. When snowmelt starts, elution of soil solutes occurs, and nutrients also begin to elute, including NH4+-N, SiO42−-Si, NO3−-N, NO2−-N and PO43−-P. The elution of chemical solutes affects both the microbial habitats within the snow and soils (Jones, 1991; Schmidt et al., 1999; Edwards et al., 2007; Jones, 2010), and also affects the diversity of bacteria in the soil. Thus the nutrient concentration of the JCB4 sample was the highest. The concentration of organic carbon and nutrient salt in JCB1 sample is higher than that in JCB2 and JCB3, which is also due to the presence of saprophytic moss near the sampling point of JCB1 sample.
In an Kitezh Lake area, OrC plays a very important role in soil as the most important factor affecting bacterial community and diversity in soil. Changes in organic carbon content have an effect on heterotrophic bacteria in the soil. This also provides some reliable data for lak ecaused by melting snow and Antarctic climate change, and plays an important role in the carbon cycle and the Earth's biochemical cycle. It also has broad implications for predicting climate change in the Antarctic and global climate change.
Near the Kitezh Lake in Felder Peninsula, Antarctica, there are significant correlations between soil bacterial communities and geochemical properties, especially OrC. Changes in OrC and nutrient concentrations affect the bacterial community and composition in the soil, especially Hungatei.
AcknowledgementsThis research was supported by the National Natural Science Foundation of China (No. 41776198), the Basic Scientific Fund for National Public Research Institutes of China (No. GY0219Q10), and the Development Fund of Marine Bioactive Substances, SOA (No. MBSMAT-2017-01).
Adrian, R., O'Reilly, C. M., Zagarese, H., Baines, S. B., Hessen, D. O., Keller, W., Livingstone, D. M., Sommaruga, R., Straile, D. and Donk, E. V., 2009. Lakes as sentinels of climate change. Limnology & Oceanography, 54(6 part 2): 2283-2297. (  0) 0) |
Berg, G. and Smalla, K., 2009. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiology Ecology, 68(1): 1-13. (  0) 0) |
Bölter, M., 2011. Soil development and soil biology on King George Island, Maritime Antarctic. Polish Polar Research, 32(2): 105-116. DOI:10.2478/v10183-011-0002-z (  0) 0) |
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., Fierer, N., Peña, A. G., Goodrich, J. K., Gordon, J. I., Huttley, G. A., Kelley, S. T., Knights, D., Koenig, J. E., Ley, R. E., Lozupone, C. A., McDonald, D., Muegge, B. D., Pirrung, M., Reeder, J., Sevinsky, J. R., Turnbaugh, P. J., Walters, W. A., Widmann, J., Yatsunenko, T., Zaneveld, J. and Knight, R., 2010a. QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 7(5): 335-336. DOI:10.1038/nmeth.f.303 (  0) 0) |
Caruso, T., Trokhymets, V., Bargagli, R. and Convey, P., 2013. Biotic interactions as a structuring force in soil communities: Evidence from the micro-arthropods of an Antarctic moss model system. Oecologia, 172(2): 495-503. DOI:10.1007/s00442-012-2503-9 (  0) 0) |
Chotte, J. L., Ladd, J. N. and Amato, M., 1998. Sites of microbial assimilation, and turnover of soluble and particulate 14C-labelled substrates decomposing in a clay soil. Soil Biology & Biochemistry, 30(2): 205-218. (  0) 0) |
Edgar, R. C., 2013. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 10: 996-998. DOI:10.1038/nmeth.2604 (  0) 0) |
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. and Knight, R., 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27(16): 2194-2200. DOI:10.1093/bioinformatics/btr381 (  0) 0) |
Edwards, A. C., Scalenghe, R. and Freppaz, M., 2007. Changes in the seasonal snow cover of alpine regions and its effect on soil processes: A review. Quaternary International, 162(1): 172-181. (  0) 0) |
Goldfarb, K. C., Karaoz, U., Hanson, C. A., Santee, C. A., Bradford, M. A., Treseder, K. K., Wallenstein, M. D. and Brodie, E. L., 2011. Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Frontiers in Microbiology, 2(1): 94. (  0) 0) |
Haas, B. J., Gevers, D., Earl, A. M., Feldgarden, M., Ward, D. V., Giannoukos, G., Ciulla, D., Tabbaa, D., Highlander, S. K., Sodergren, E., Methé, B., DeSantis, T. Z., Petrosino, J. F., Knight, R. and Birren, B. W., 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Research, 21(3): 494-504. DOI:10.1101/gr.112730.110 (  0) 0) |
Hogg, I. D., Cary, S. C., Convey, P., Newsham, K. K., O'Donnell, A. G., Adams, B. J., Aislabie, J., Frati, F., Stevens, M. I. and Wall, D. H., 2006. Biotic interactions in Antarctic terrestrial ecosystems: Are they a factor. Soil Biology & Biochemistry, 38(10): 3035-3040. (  0) 0) |
Hu, L., Shi, X., Yu, Z., Lin, T., Wang, H., Ma, D., Guo, Z. and Yang, Z., 2012. Distribution of sedimentary organic matter in estuarine-inner shelf regions of the East China Sea: Implications for hydrodynamic forces and anthropogenic impact. Marine Chemistry, 142-144(11): 29-40. (  0) 0) |
Hughes, K. A., Convey, P., Ziska, L. H., and Dukes, J. S., 2014. Non-native species in Antarctic terrestrial environments: The impacts of climate change and human activity. In: Invasive Species & Global Climate Change, Ziska, L., ed., 81-100, DOI: 10.1079/9781780641645.0081.
(  0) 0) |
Jeanette, B., Brandt, K. K., Al-Soud, W. A., Holm, P. E., Hansen, L. H., Srensen, S. R. J. and Ole, N., 2012. Selection for Cutolerant bacterial communities with altered composition, but unaltered richness, via long-term Cu exposure. Applied & Environmental Microbiology, 78(20): 7438-7446. (  0) 0) |
Jones, H. G., 1991. Snow chemistry and biological activity: A particular perspective on nutrient cycling. NATO ASI Series, 28: 173-228. (  0) 0) |
Jones, H. G., 2010. The ecology of snow‐covered systems: A brief overview of nutrient cycling and life in the cold. Hydrological Processes, 13(14-15): 2135-2147. (  0) 0) |
Kuramae, E. E., Yergeau, E., Wong, L. C., Pijl, A. S., van Veen, J. A. and Kowalchuk, G. A., 2015. Soil characteristics more strongly influence soil bacterial communities than land-use type. Fems Microbiology Ecology, 79(1): 12-24. (  0) 0) |
Ladd, J. N., Gestel, M. V., Monrozier, L. J. and Amato, M., 1996. Distribution of organic 14C and 15N in particle-size fractions of soils incubated with 14C, 15N-labelled glucose/ NH4, and legume and wheat straw residues. Soil Biology & Biochemistry, 28(7): 893-905. (  0) 0) |
Lagomarsino, A., Grego, S. and Kandeler, E., 2012. Soil organic carbon distribution drives microbial activity and functional diversity in particle and aggregate-size fractions. Pedobiologia, 55(2): 101-110. DOI:10.1016/j.pedobi.2011.12.002 (  0) 0) |
Lavian, I. L., Vishnevetsky, S., Barness, G. and Steinberger, Y., 2001. Soil microbial community and bacterial functional diversity at Machu Picchu, King George Island, Antarctica. Polar Biology, 24(6): 411-416. DOI:10.1007/s003000100230 (  0) 0) |
Liu, J., Zang, J., Zhao, C., Yu, Z., Xu, B., Li, J. and Ran, X., 2016. Phosphorus speciation, transformation, and preservation in the coastal area of Rushan Bay. Science of the Total Environment, 565: 258-270. DOI:10.1016/j.scitotenv.2016.04.177 (  0) 0) |
Magoä, T. and Salzberg, S. L., 2011. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics, 27(21): 2957-2963. DOI:10.1093/bioinformatics/btr507 (  0) 0) |
Marilley, L., Vogt, G., Blanc, M. and Aragno, M., 1998. Bacterial diversity in the bulk soil and rhizosphere fractions of Lolium perenne and Trifolium repens as revealed by PCR restriction analysis of 16S rDNA. Plant & Soil, 198(2): 219-224. (  0) 0) |
Michelsen, C. F., Pedas, P., Glaring, M. A., Schjoerring, J. K. and Stougaard, P., 2014. Bacterial diversity in Greenlandic soils as affected by potato cropping and inorganic versus organic fertilization. Polar Biology, 37(1): 61-71. DOI:10.1007/s00300-013-1410-9 (  0) 0) |
Monserrate, E., Leschine, S. B. and Canale-Parola, E., 2001. Clostridium hungatei sp. nov., a mesophilic, N2-fixing cellulolytic bacterium isolated from soil. International Journal of Systematic & Evolutionary Microbiology, 51(1): 123-132. (  0) 0) |
Nicolas, J. P., Vogelmann, A. M., Scott, R. C., Wilson, A. B., Cadeddu, M. P., Bromwich, D. H., Verlinde, J., Lubin, D., Russell, L. M., Jenkinson, C., Powers, H. H., Ryczek, M., Stone, G., and Wille, J. D., 2017. January 2016 extensive summer melt in West Antarctica favoured by strong El Niño. 8: 15799.
(  0) 0) |
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O'Hara, R. B., and Wagner, H., 2016. Vegan: Community Ecology Package [Software].
(  0) 0) |
Oyugi, J. O., Qiu, H. and Safronetz, D., 2007. Global warming and the emergence of ancient pathogens in Canada's Arctic regions. Medical Hypotheses, 68(3): 709. DOI:10.1016/j.mehy.2006.09.006 (  0) 0) |
Parsons, A. N., Barrett, J. E., Wall, D. H. and Virginia, R. A., 2004. Soil carbon dioxide flux in antarctic dry valley ecosystems. Ecosystems, 7(3): 286-295. (  0) 0) |
Pritchard, H. D., Ligtenberg, S. R. M., Fricker, H. A., Vaughan, D. G., van den Broeke, M. R. and Padman, L., 2012. Antarctic ice-sheet loss driven by basal melting of ice shelves. Nature, 484(7395): 502-505. DOI:10.1038/nature10968 (  0) 0) |
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., Peplies, J. and Glöckner, F. O., 2013. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Research, 41(Database issue): 590-596. (  0) 0) |
Santamans, A. C., Boluda, R., Picazo, A., Gil, C., Ramosmiras, J., Tejedo, P., Pertierra, L. R., Benayas, J. and Camacho, A., 2017. Soil features in rookeries of Antarctic penguins reveal sea to land biotransport of chemical pollutants. PLoS One, 12(8): e0181901. DOI:10.1371/journal.pone.0181901 (  0) 0) |
Schaefer, K., Lantuit, H., Romanovsky, V. E., Schuur, E. A. G. and Witt, R., 2014. The impact of the permafrost carbon feedback on global climate. Environmental Research Letters, 9(8): 085003. DOI:10.1088/1748-9326/9/8/085003 (  0) 0) |
Schmidt, I. K., Jonasson, S. and Michelsen, A., 1999. Mineralization and microbial immobilization of N and P in arctic soils in relation to season, temperature and nutrient amendment. Applied Soil Ecology, 11(2-3): 147-160. DOI:10.1016/S0929-1393(98)00147-4 (  0) 0) |
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S. and Huttenhower, C., 2011. Metagenomic biomarker discovery and explanation. Genome Biology, 12(6): R60. DOI:10.1186/gb-2011-12-6-r60 (  0) 0) |
Staddon, W. J., Duchesne, L. C. and Trevors, J. T., 1997. Microbial diversity and community structure of postdisturbance forest soils as determined by sole-carbon-source utilization patterns. Microbial Ecology, 34(2): 125-130. (  0) 0) |
Staddon, W. J., Trevors, J. T., Duchesne, L. C. and Colombo, C. A., 1998. Soil microbial diversity and community structure across a climatic gradient in western Canada. Biodiversity & Conservation, 7(8): 1081-1092. (  0) 0) |
Steig, E. J., Schneider, D. P., Rutherford, S. D., Mann, M. E., Comiso, J. C. and Shindell, D. T., 2009. Warming of the Antarctic ice-sheet surface since the 1957 International Geophysical Year. Nature, 457(7228): 459-462. (  0) 0) |
Tierney, L., 2012. The R Statistical Computing Environment. Springer, New York, 1-21.
(  0) 0) |
Turner, J., Colwell, S. R., Marshall, G. J., Lachlan‐Cope, T. A., Carleton, A. M., Jones, P. D., Lagun, V., Reid, P. A. and Iagovkina, S., 2005. Antarctic climate change during the last 50 years. International Journal of Climatology, 25(3): 279-294. DOI:10.1002/joc.1130 (  0) 0) |
Tytgat, B., Verleyen, E., Sweetlove, M., D'Hondt, S., Clercx, P., Ranst, E. V., Peeters, K., Roberts, S., Namsaraev, Z. and Wilmotte, A., 2016. Bacterial community composition in relation to bedrock type and macrobiota in soils from the Sør Rondane Mountains, East Antarctica. FEMS Microbiology Ecology, 92(9): fiw126. DOI:10.1093/femsec/fiw126 (  0) 0) |
Van Horn, D. J., Okie, J. G., Buelow, H. N., Gooseff, M. N., Barrett, J. E. and Takacs-Vesbach, C. D., 2014. Soil microbial responses to increased moisture and organic resources along a salinity gradient in a polar desert. Applied Environmental Microbiology, 80(10): 3034-3043. DOI:10.1128/AEM.03414-13 (  0) 0) |
Wang, N. F., Zhang, T., Zhang, F., Wang, E. T., He, J. F., Ding, H., Zhang, B. T., Liu, J., Ran, X. B. and Zang, J. Y., 2015. Diversity and structure of soil bacterial communities in the fildes region (maritime Antarctica) as revealed by 454 pyrosequencing. Frontiers in Microbiology, 6: 1188. (  0) 0) |
Wang, Q., Garrity, G. M., Tiedje, J. M. and Cole, J. R., 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 73(16): 5261-5267. (  0) 0) |
You, Y., Wang, J., Huang, X., Tang, Z., Liu, S. and Sun, O. J., 2014. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecology & Evolution, 4(5): 633-647. (  0) 0) |
Zhang, C., Zhang, X. Y., Zou, H. T., Kou, L., Yang, Y., Wen, X. F., Li, S. G., Wang, H. M. and Sun, X. M., 2017. Contrasting effects of ammonium and nitrate additions on the biomass of soil microbial communities and enzyme activities in subtropical China. Biogeosciences, 14(20): 4815-4827. DOI:10.5194/bg-14-4815-2017 (  0) 0) |
 2019, Vol. 18
2019, Vol. 18


