2) Qingdao Ruizi Group Co., Ltd., Qingdao 266408, China;
3) Key Laboratory of Mariculture, Ministry of Education, Ocean University of China, Qingdao 266100, China
Atlantic salmon (Salmo salar) represents a pivotal species within the salmonid family, causing a significant consumer market because of its high-quality protein content. The continuous growth of global demand and the reduction of wild salmon resources highlight the necessity of aquaculture, providing a crucial strategy to alleviate the pressure on existing fish population.
The gut microbiota is recognized for its pivotal role in regulating host growth (Gholizadeh et al., 2019), development, and immune function (Ma et al., 2019), exerting effects through various mechanisms such as modulation of immune factors, enhancement of the intestinal barrier, and maintenance of intestinal homeostasis (Mancuso and Santangelo, 2018). Their composition is regulated by both intrinsic and extrinsic factors (Kohl et al., 2014), including genetics (Kohl et al., 2014; Li et al., 2015), nutritional status, environmental conditions (Li et al., 2014), and dietary habits (Liu et al., 2016). Beneficial bacteria residing in the intestinal tract play a crucial role in nutrient assimilation, synthesis of vital metabolites including vitamins, and the inhibition of pathogen colonization (Donaldson et al., 2018). At the same time, the fish intestinal tract is a major site of pathogen transmission (Zhao et al., 2023).
Notably, the composition of gut microbiota exhibits a significant correlation with the developmental stage of the host (Guttman et al., 2011). Studies indicate that adult rainbow trout (Oncorhynchus mykiss) possess a more diverse gut microbiota compared to juveniles, providing enhanced resistance against pathogen invasion (Hai et al., 2023). Similarly, distinct variations in gut microbiota composition and diversity have been observed among Japanese eels (Anguilla japonica) at different developmental stages (Zhang, 2023). However, a comprehensive understanding of the structural and functional differences in the gut microbiota of Atlantic salmon at different growth stages remains unclear, limiting the application of microbiota intervention techniques in improving fish health and quality.
The interactions between the gut microbiota and the host are primarily mediated through the production of natural products derived from the microbiome (Holmes et al., 2011). These small molecules, resulting from the biosynthesis and modification of the gut microbiome, activate specific signaling pathways through the gut-x axis, such as the gut-brain axis and gut-liver axis, exert a significant impact on the health of mammals (Dai et al., 2023). Studies indicate that Lactobacillus sakei rMA-2 supplementation may have contributed to the amino acid biosynthesis pathway to affect host gut health by increasing the production of hydroxyproline, N-acetylneuraminic acid and N-acetyl-L-phenylalanine (Zhao et al., 2023). Synchronized with gut microbiota, the derived metabolites may change with the growth and development of fish (Li et al., 2021). Therefore, identifying the bioactive molecules and their biogenesis within gut microbiota at different growth stages can enhance our understanding of the interactions between microbiota and hosts (Dai et al., 2023).
In this study, we utilized 16S rRNA sequencing and liquid chromatography-mass spectrometry (LC-MS) technology to explore the gut microbiota and associated metabolites in Atlantic salmon at different growth stages. By illustrating the differences in gut microbiota and function, and analyzing the correlation between microbiome and metabolomics, potential core microbial communities and key metabolic pathways related to quality and growth were identified.
2 Materials and Methods 2.1 Experimental Design and Fish CultureAtlantic salmon were collected from Yantai Dongfang Marine Aquaculture Company. They were introduced to a recirculating aquaculture system characterized by a pool height of 1.0 m and a diameter of 5.0 m. This system incorporated a daily water renewal rate about 20% of its total volume. The same batch of hatched salmon was raised in three parallel pools. Sampling occurred at distinct body sizes i.e., 1000 g (group S1), 2000 g (group S2), 4000 g (group S3), and 6000 g (group S4), respectively.
Throughout the experiment, the water conditions for fish culture remained consistent. The primary constituents of the basic commodity feed included fish meal (55.0%), soybean meal (25.0%), wheat meal (15.0%), refined fish oil (2.5%), lecithin (1.5%), minerals (1.0%) and vitamins. The feed components could meet the requirement of fish growth, and the particle size of the feed could be normally ingested by the fish. The water quality parameters of the culture system were monitored and the temperature was maintained at 12 – 15℃; dissolved oxygen content > 6.0 mg L−1; the pH was 6.63 – 7.06, and the photoperiod was 14 h: 10 h (light: dark).
A total of fifteen individuals of each fish size were selected for sampling and anesthetized with 200 g L−1 MS-222 (3-aminobenzoate methanesulfonate), and subsequently dissected on an ice pack. The anterior and posterior intestines were sampled, and the intestinal contents were gently washed using normal saline. The samples were mixed into steriled tubes, and promptly frozen in liquid nitrogen for subsequent analysis.
2.2 Intestinal Microbiota AnalysisTotal genomic DNA of the samples was extracted utilizing the QiAamp DNA Stool Mini kit (Qiagen, Germany). Subsequently, the quality of extracted DNA was evaluated using a spectrophotometer (ND-2000, Nanodrop, USA) and 1.0% agarose gel electrophoresis. Amplification of the 16S rRNA gene in the bacterial V3 – V4 region was facilitated by 338F (5'-ACTCCTACGGGAGGCAGCAGG-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3') primers. The amplified products were purified using PCR fragment purification kit (Takara, Japan) and subjected to detection through 1.8% agarose gel electrophoresis. Gut microbiota diversity analysis was conducted by barcoding the V3 – V4 variable region of the 16S rRNA gene, followed by sequencing the amplified products on an Illumina HiSeq platform (PE250, Illumina, USA). Qualitative filtering of the original labels for each sample were performed using Trimmomatic software (v.0.33). Furthermore, UCHIME (v.4.2) was employed for the detection and elimination of chimeric sequences. Sequence clustering was executed using UCLUST in QIIME (v.1.8.0) with 97.0% similarity cutoff. Subsequently, these tags were validated against the SILVA classification database and clustered into operational classification units (OTUs).
A Venn diagram was employed to illustrate the amount of OUT among the samples. The quantification of the Simpson's and Shannon's diversity, Chao1 richness index and ACE (Abundance-based Coverage Estimator) was performed using MOTHUR software. To depict the similarities and differences in gut microbiota composition, β-diversity was assessed. QIIME (v.1.8.0) was utilized to generate species richness at various taxonomic levels based on OTU taxonomic information, while R software (v.3.5.1) was employed for mapping the community structure.
Principal coordinate analysis (PCoA) and cluster analysis based on Bray-Curtis dissimilarity were performed on vegan packaging in R (v.3.5.1). Linear discriminant analysis effect size (LEfSe) was performed to detect potential biomarkers. The parameter was set as α = 0.05 with a linear discriminant analysis (LDA) score > 3.5.
2.3 Metabonomics AnalysisIntestinal contents were pulverized in liquid nitrogen. The homogenized samples were mixed with 500 mL (80%) of methanol in an eppendorf tube in ice bath for 5 min and centrifuged at 15000 g for 20 min at 4.0℃ to obtain the supernatant. The supernatant was subsequently diluted with mass spectrometric grade water to achieve a methanol concentration of 53%. After an additional centrifugation step at 15000 g, the samples were incubated at 4.0℃ for 20 min. The final supernatant was utilized for liquid chromatography-mass spectrometry (LC-MS) analysis. Each experimental group was analyzed with six replicates. For quality control (QC), supernatants from all samples were pooled in equal volumes with blank samples in 53% methanol to prepare QC samples.
Vanquish ultra-high performance liquid chromatography (UHPLC) was used for LC-MS analysis. Both positive and negative ion modes of electrospray ionization (ESI) were conducted on a Q Exactive HF spectrometer (Thermo Fisher, Germany) using a Hypersil Gold column (Thermo Fisher, USA). The temperature of the column was set at 40℃with a flow rate maintained at 0.2 mL min−1. The binary gradient elution system consisted of a positive elution mode containing 0.1% formic acid (A) and methanol (B) and a negative elution mode containing 5 mmol L−1 ammonium acetate (pH 9.0) (A) and methanol (B). The gradient program for separation began with 98% A and 2% B at the beginning, transitioning to 0 A and 100% B over 12 to 14 min, then returning to 98% A and 2% B from 14.1 to 17 min, covering a full scan m/z range of 100 to 1500. The mass spectrometry settings included a spray voltage of 3.2 kV, sheath gas flow rate of 40 arb (arbitrary unit), auxiliary gas flow rate of 10 arb, and a capillary temperature of 320℃. Correlation scanning was applied in the mass spectrometry analysis.
The raw data analysis and processing were conducted using Compound Discoverer v3.1 (CD3.1, Thermo Fisher) for peak detection, extraction, alignment, and integration. The mzCloud (https://www.mzcloud.org/), mzVault, and Masslist databases were used to align ion peak predictions for quantitative results. The blanks were stripped of background ions. Partial Least Squares Discriminant Analysis (PLS-DA) was used for statistical analysis. To build a PLS-DA model, the R software package was used to analyze the significant metabolites between different groups. The validity of the model was verified by 200 permutation tests. Differential metabolites were screened by combining statistically significant variable importance in projection (VIP) values (> 1.0) and fold change (FC) values (> 1.2 or < 0.833) from the PLSDA model, along with P values (< 0.05) from a two-tailed Student's t-test. Metabolites were identified using the HMDB Database (https://hmdb.ca/metabolites), KEGG database (https://www.genome.jp/kegg/pathway.html), and LIPIDMAPS database (http://www.lipidmaps.org/).
2.4 Statistical AnalysisPearson correlation analysis was utilized to investigate the association between microbiota and significantly different metabolites (SDMs). The analysis was carried out using SPSS software (version 16.0) to perform one-way analysis of variance (ANOVA), followed by Duncan's multiple comparison test for post-hoc analysis. The Kolmogorov-Smirnov test (KS test) and Levene's test were employed to check the normal distribution and variance. Statistical significance was considered at P < 0.05.
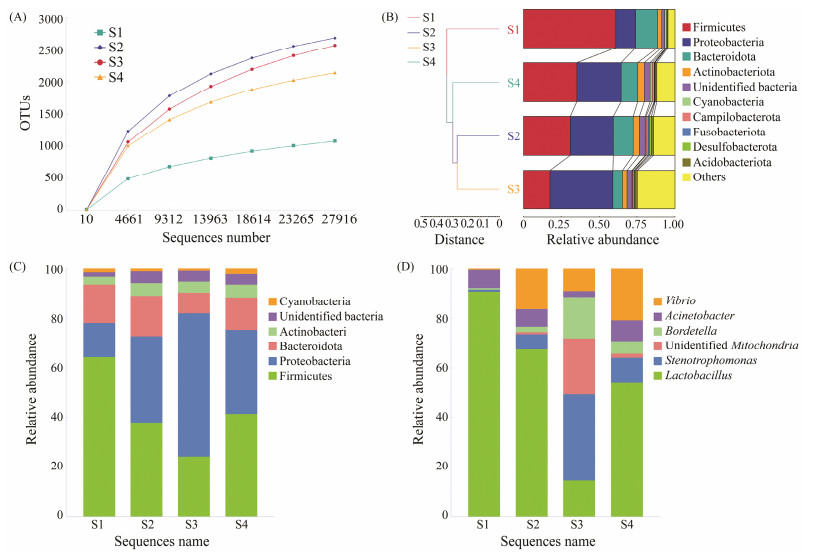
3 Results 3.1 Diversity, Composition and Cluster of Gut MicrobiotaAnalysis via Venn diagram revealed a total of 1281 operational taxonomic units (OTUs) across all groups, with unique OTUs 348, 847, 1122, and 1001 for the S1, S2, S3, and S4 groups, respectively. The S1 group exhibited the lowest OTU count. Saturation of sequencing data was confirmed by sparse curves, indicating comprehensive coverage (Fig. 1A). Both Shannon and Simpson diversity indices were the minimum in the S1 group, whereas the Chao1 and ACE indices were significantly reduced in the S1 and S4 groups compared to the S2 and S3 groups (P < 0.05) (Table 1).
|
Fig. 1 Atlantic salmon gut microbiota composition and abundance. (A), dilution curve based on sequence data; (B), UPGMA clustering tree based on unweighted UniFrac distance. (C), bar chart of phyla-level accumulation of gut microbiota; (D), bar graph of genus-level accumulation of gut microbiota. Each stacked column represents the average relative abundance of three replicates per group. S1, Atlantic salmon with 1 kg; S2, Atlantic salmon with 2 kg; S3, Atlantic salmon with 4 kg; S4, Atlantic salmon with 6 kg. |
|
|
Table 1 The α-diversity of gut microbiota in Atlantic salmon |
The UPGMA clustering tree, based on unweighted UniFrac distances, depicted the gut microbiota composition similarities and differences across groups, highlighting minor distances between the S2 and S3 groups but significant distances from the S1 group (Fig.1B).
Dominant phyla included Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteriota. The abundance of Firmicutes decreased, while the abundance of Proteobacteria showed an increased trend with the growth of fish. Notably, the S1 group showed the highest relative abundance with Firmicutes (60.97%) and the lowest one with Proteobacteria (13.19%). Differently, the S3 group exhibited the lowest relative abundance with Firmicutes (17.57%) and the highest one with Proteobacteria (41.69%) (Fig.1C).
At genus level, the top six dominant genera were Lactobacillus, Stenotrophomonas, unidentified Mitochondria, Bordetella, Acinetobacter, and Vibrio. Lactobacillus was the most dominant genus in the S1 (45.9%), S2 (8.68%), and S4 (7.37%) groups, but it showed the lowest abundance in the S3 group (3.71%). Stenotrophomonas was the most dominant genus in the S3 group (8.76%), while it showed the lowest abundance in the S1 group (0.42%). Vibrio showed the lowest abundance in the S1 group (0.06%), while its abundance increased in the S2 (2.07%), S3 (2.23%), and S4 groups (2.78%) (Fig.1D).
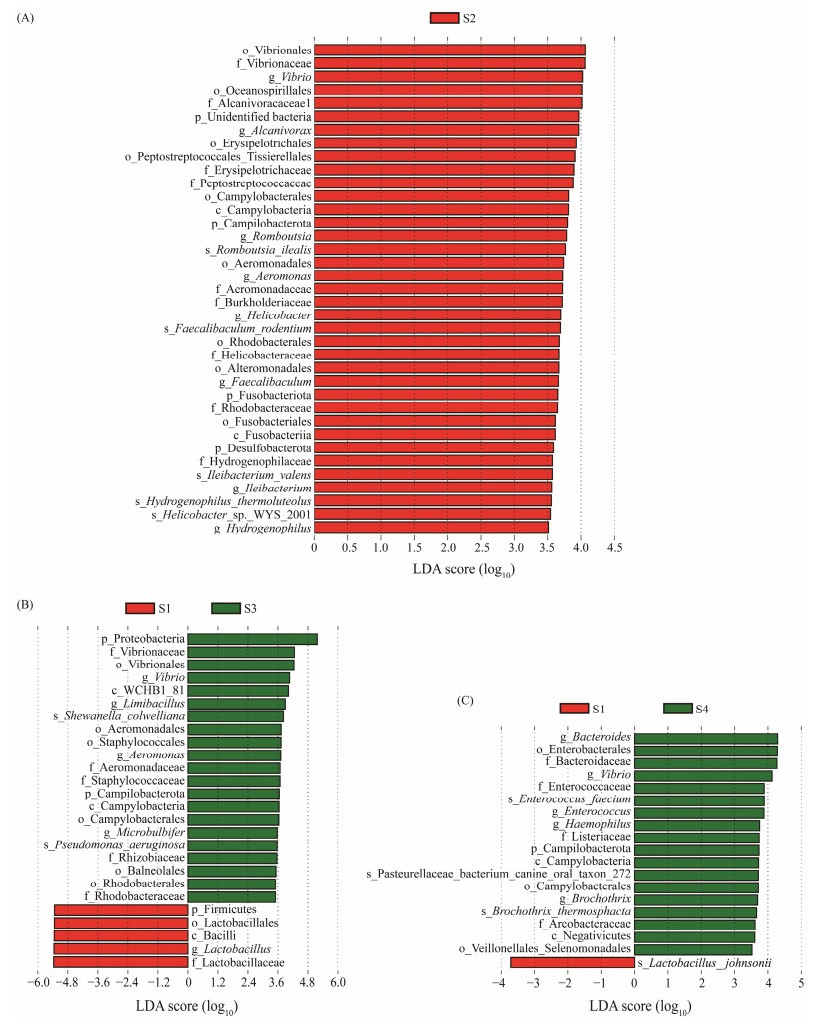
3.2 Identification of Differential BacteriaLEfSe analysis (LDA > 3.5, P < 0.05) identified distinct bacterial populations with significant differences. Compared to the S1 group, notable increases in gut microbiota such as Aeromonas, Ileibacterium, Romboutsia, Helicobacter, Alcanivorax, Hydrogenophilus, Rhodobacteraceae, and Faecalibaculum were observed in the S2 group (Fig.2A). In the S3 group, significant rises were observed in Rhodobacteraceae, Staphylococcaceae, WCHB1-81, Campylobacteria, Aeromonadales, Vibrionales, and Balneolales (Fig.2B). Noteworthy, opportunistic pathogens including Enterobacteriaceae, Enterococcaceae, Bacteroidaceae, Arcobacteraceae, Campylobacteria, Listeriaceae, Veillonellales-selenomon, and Negativicutes increased in the S4 group (Fig.2C).
|
Fig. 2 Abundance of microbiota analyzed by LEfSe (LDA > 3.5). S1, Atlantic salmon with 1 kg; S2, Atlantic salmon with 2 kg; S3, Atlantic salmon with 4 kg; S4, Atlantic salmon with 6 kg. |
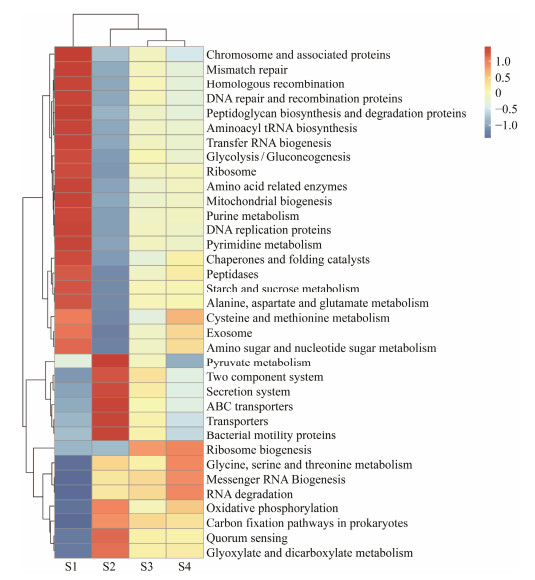
Amino acid-, nucleotide-, and carbohydrate-related metabolic pathways of the gut microbiota were predicted using Tax4Fun (Fig.3). At KEGG level 3, the predicated metabolic pathways in the S1 group including nucleotide-related metabolic pathways (e.g., purine metabolism and pyrimidine metabolism), amino acid-related metabolic pathways (e.g., alanine, aspartate and glutamate metabolism, cysteine and methionine metabolism), chromosome and associated proteins, homologous recombination, mismatch repair, DNA repair and recombination proteins, peptidoglycan biosynthesis and degradation proteins, amino acid related enzymes, transfer RNA biogenesis, glycolysis/gluconeogenesis, ribosome, mitochondrial biogenesis, DNA replication proteins, chaperones and folding catalysts, peptidases, and starch and sucrose were up-regulated. The ribosome biosynthesis pathway was significantly up-regulated in the S2 group. In the S3 group, the up-regulated metabolic pathways included carbohydrate metabolism-related pathways (pyruvate metabolism, glyoxylate and dicarboxylate metabolism), two component system and secretion system, ABC transporters, transporters, bacterial motility proteins, oxidative phosphorylation, carbon fixation pathways in prokaryotes, and quorum sensing. In the S4 group, the up-regulated metabolic pathways included amino acid metabolism-related pathways (e.g., glycine, serine and threonine metabolism), messenger RNA biogenesis, RNA degradation. Notably, pathways upregulated in the S1 group were downregulated in the S3 group, indicating a shift in functional pathway dominance at different growth stages.
|
Fig. 3 Heat map of relative abundance of different pathways. Different color means the abundance level of high and low. S1, Atlantic salmon with 1 kg; S2, Atlantic salmon with 2 kg; S3, Atlantic salmon with 4 kg; S4, Atlantic salmon with 6 kg. |
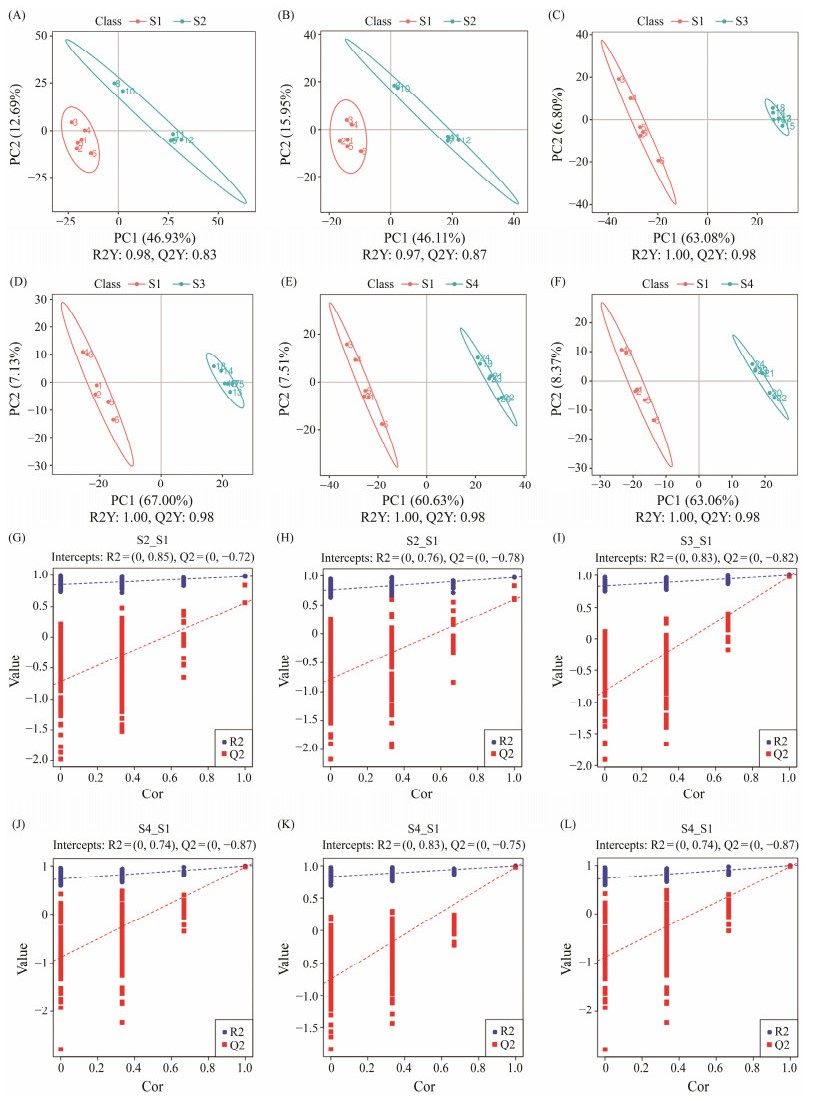
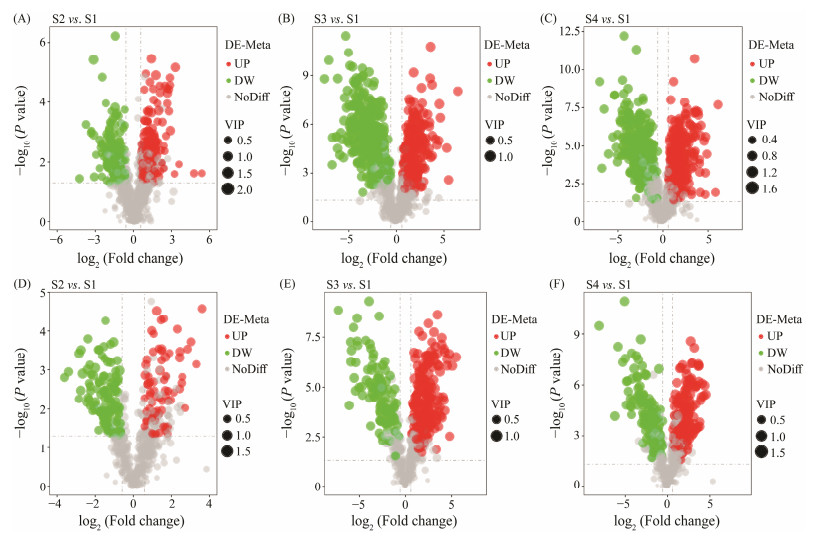
The correlation of positive and negative ion QC samples was above 0.988, indicating that the quality of the experimental data was reliable. Partial least squares discriminant analysis (PLS-DA) was used to identify important differentially enriched metabolites. There were significant changes in metabolites of gut microbiota during fish growth (Fig.4). Compared with the S1 group, a total of 568 differential metabolites were identified in the S2 group, of which 230 were up-regulated and 338 were down-regulated (Figs.5A, D). A total of 919 different metabolites were identified in the S3 group, including 500 up-regulated metabolites and 419 downregulated metabolites (Figs.5B, E). A total of 901 differential metabolites were identified in the S4 group, of which 490 were up-regulated and 411 were down-regulated (Figs. 5C, F).
|
Fig. 4 PLS-DA score scatter diagram and sorting verification diagram. (A), (C), (E), (G), (I), and (K) are positive ion mo-des, while (B), (D), (F), (H), (J), and (L) are the negative ion modes. (A) – (F), scatter plot obtained by PLS-DA; (G) – (L), validation plots of the PLS-DA model. PLS-DA was used to obtain scatter plots. R2Y represents the interpretation rate of the model, and Q2Y is used to evaluate the predictive ability of the PLS-DA model. S1, Atlantic salmon with 1 kg; S2, Atlantic salmon with 2 kg; S3, Atlantic salmon with 4 kg; S4, Atlantic salmon with 6 kg. |
|
Fig. 5 Volcanic results of different metabolites (DE-Meta) in Atlantic salmon gut microbiota. (A) – (C) are the positive ion modes. (D) – (F) are the negative ion modes. S1, Atlantic salmon with 1 kg; S2, Atlantic salmon with 2 kg; S3, Atlantic salmon with 4 kg; S4, Atlantic salmon with 6 kg. UP and DW are upand down-regulated metabolites, respectively. VIP, variable importance in projection; NoDiff, no difference. |
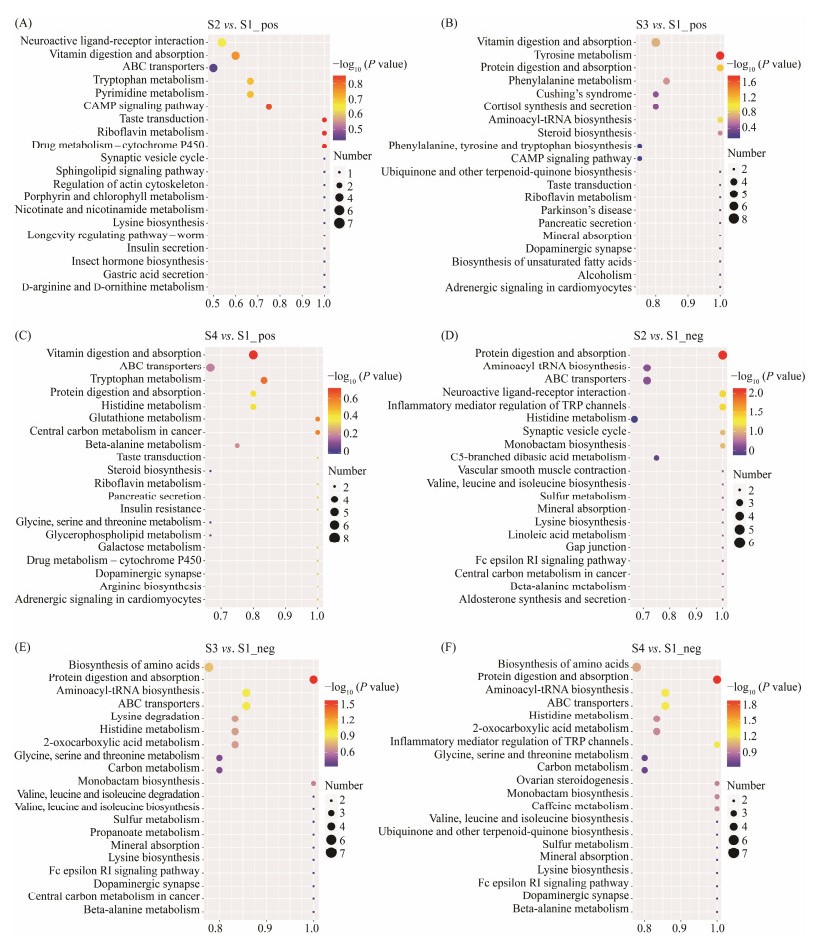
The KEGG database was used to classify and annotate differential metabolites to identify the main biochemical metabolic pathways. At KEGG level 3, in the S2 group, the corresponding up-regulated metabolites, taurine, ARA and 15(S)-HpETE were consistent with the most enriched biochemical metabolic pathways, such as protein digestion and absorption, neuroactive ligand-receptor interaction, and inflammatory mediator regulation of TRP channels (Figs.6A, D). In the S3 group, the corresponding up-regulated metabolites, 4-Hydroxyphenylacetic acid, hydroquinone and LDopa were consistent with the most enriched biochemical metabolic pathways, such as tyrosine metabolism (Figs.6B, E). The most enriched biochemical metabolic pathways in the S4 group were vitamin digestion and absorption, tryptophan metabolism, protein digestion and absorption, and biosynthesis of amino acids, etc. (Figs.6C, F). The corresponding up-regulated metabolites, including 15(S)-HpETE, citric acid, cholecalciferol, nicotinamide, pantothenic acid, riboflavin, were consistent with the most enriched biochemical metabolic pathways.
|
Fig. 6 Atlantic salmon enrichment of gut microbiota metabolism group KEGG. (A) – (C) are the positive ion modes; (D) – (F) are the negative ion modes. S1, Atlantic salmon with 1 kg; S2, Atlantic salmon with 2 kg; S3, Atlantic salmon with 4 kg; S4, Atlantic salmon with 6 kg. |
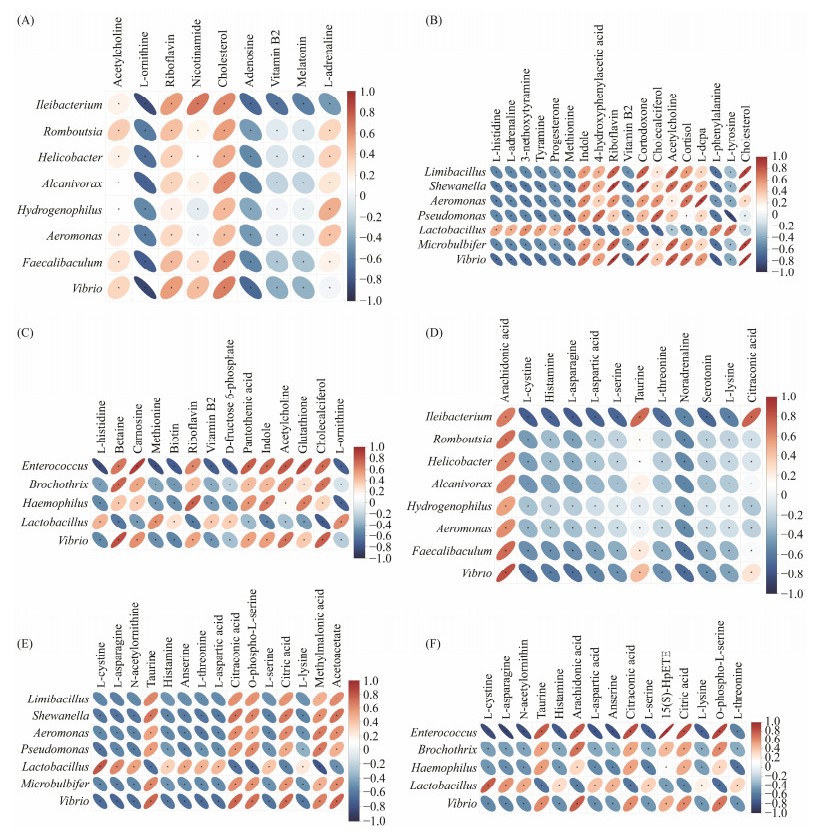
The relationships between different bacteria and different metabolites were assessed using Pearson correlation analysis (Fig.7). Among all groups, the most abundant metabolites of the gut microbiota were riboflavin and cholesterol. Compared with S1 group, the S2 group had higher levels of nicotinamide, taurine, citraconic acid, and ARA; the S3 group had more abundance of cortodoxone and acetylcholine; the S4 group had higher level of carnosine, pantothenic acid, indole, acetylcholine, cholecalciferol, glutathione, taurine, ARA, citraconic acid, and citric acid. These up-regulated metabolites were significantly and positively correlated with the abundance of microbiota, such as Ileibacterium in the S2 group (Figs.7A, D), Limibacillus, Shewanella, Microbulbifer, Vibrio, Pseudomonas, Aeromonas, and Lactobacillus in the S3 group (Figs.7B, E), as well as Brochothrix, Lactobacillus, Haemophilus, and Vibrio in the S4 group (Figs.7C, F).
|
Fig. 7 Atlantic salmon intestinal bacteria and different metabolites conjoint analysis. (A) – (C) are the positive ion modes; (D) – (F) are negative ion modes. (A) and (D) are the joint analysis cluster heatmaps of S2 vs. S1. (B) and (E) are the joint analysis cluster heatmaps of S3 vs. S1. (C) and (F) are the joint analysis cluster heatmaps of S4 vs. S1. S1, Atlantic salmon with 1 kg; S2, Atlantic salmon with 2 kg; S3, Atlantic salmon with 4 kg; S4, Atlantic salmon with 6 kg. |
Symbiotic microbes in fish gut play crucial roles in host functions (Ganguly and Prasad, 2011), encompassing the production of digestion related enzymes (Ray et al., 2012), synthesis of vitamins (Tran et al., 2017), and facilitation of gut-associated immune systems development (Nayak, 2010). In this study, the composition and abundance of the gut microbiota exhibited significant changes during the fish's growth, particularly a gradual increase in microbiota diversity. These alterations in microbiota composition may be linked to the growth rate and can influence the physiological and immune status of fish at various development stages (Nie et al., 2019).
Throughout the growth processes, Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteriota, and Cyanobacteria consistently dominated the phyla. Notably, the abundances of Firmicutes and Bacteroidetes decreased with fish growth, while the abundance of Proteobacteria gradually increased. Intriguingly, the diversities of specification microbiota in the S2 and S3 groups were significantly higher than these in the S1 and S4 groups, potentially caused by more nutrient demand during rapid fish growth. At this stage, the metabolism of fish becomes more vigorous, and the host requires the microbial communities to improve their ability to process nutrients to meet growth needs. The observed diversity spike during the S2 and S3 stages implies a metabolic intensification to fulfill escalating growth demands, marking a shift in dominant microbiota from Firmicutes to Proteobacteria.
This transition mirrors the findings in metabolic disorder studies, where a surge in Proteobacteria signifies dysbiosis (De Filippo et al., 2010) and potential disease markers (Shin et al., 2015). Unregulated expansion of Proteobacteria could exacerbate inflammation or pathogen susceptibility (Shin et al., 2015), highlighting the critical role of microbiota management for optimal fish health and immunity during crucial growth phases.
The Firmicutes to Bacteroidetes (F/B ratio) ratio is considered positively related to weight gain and intestinal homeostasis (Ley, 2006), with an imbalance potentially leading to metabolic syndrome in higher animals (Mariat et al., 2009). Studies in zebrafish have linked the Firmicutes and Bacteroidetes imbalance to energy, glucose and fat metabolism (Wan et al., 2019). In this study, the F/B ratio in the gut microbiota decreased in the S2 and S3 groups compared to that in the S1 group, indicating the weaken energy, glucose and fat metabolism capacity in these stages of Atlantic salmon.
Notably, Lactobacillus, a common probiotic belonging to Firmicutes, dominated the gut microflora in the S1 group. Lactobacillus is known for producing acetate (another health-promoting short-chain fatty acid), lactate, and antimicrobial substances that hinder pathogenic interference in health (Chomwong et al., 2018). For instance, Leuconostoc mesenteroides HY2 strain has shown to enhance the growth performance and regulate the immune function of turbot (Guo et al., 2020). In contrast, the abundance of opportunistic pathogens such as Stenotrophomonas and Vibrio increased as Atlantic salmon grew, potentially posing susceptibility risks under stress conditions.
Probiotics are commonly recommended to regulate the composition and abundance of fish gut microbiota. Therefore, the use of probiotics or their derivatives to effectively intervene in the gut microbiota may improve the ability of fish to cope with complex environments and disease resistance (Xia et al., 2020).
The function of the gut microbiota is generally adjusted according with the changes in microbial structure. In this study, most of the KEGG pathways were up-regulated in the S1 group while they were basically down-regulated in the S3 group. The gut microbiota in the S1 group was found to be enriched in cysteine and methionine metabolism, alanine, aspartate and glutamic acid metabolism, as well as nucleotide-related pathways, such as purine metabolism and pyrimidine metabolism. Purine metabolic pathway can increase the deposition of aromatic substances in fish and enhance their flavor (Cai et al., 2022). Pyruvate metabolism pathway was found to be enriched in the S3 group. Pyruvate metabolic pathway involves glycolysis, tricarboxylic acid cycle, fatty acid metabolism and other metabolic activities (Wang et al., 2011), which can lead to the production of short chain fatty acids (SCFAs) (Martin et al., 2010).
Bacterial metabolites have shown to provide an important link between gut microbiota and host physiological homeostasis (Nicholson et al., 2012). In this study, metabolites of ARA and taurine in the gut microbiota were significantly up-regulated in the S2 and S3 groups. ARA supplemented in diet is shown to promote the growth and survival of turbot Scophthalmus maximus and tonguefish Cynoglossus semilaevis by regulating the antioxidant capacity and immune function (Xu et al., 2017), as well as affecting fat deposition and fatty acid composition (Tian et al., 2014). Taurine is proved to accelerate glycolysis pathway which is beneficial for fish growth and feed conversion (Gobi et al., 2016), and it also plays a role in regulating the osmotic pressure of fish. In this study, the metabolites citric acid, riboflavin and pantothenic acid were significantly up-regulated in the S4 group. Citric acid, an intermediate product of the tricarboxylic acid cycle, may play an important role in providing energy to the continuous growth and development of Atlantic salmon (Dai et al., 2018). Riboflavin catalyzes a variety of enzymatic reactions in fish, such as dehydrogenation, hydroxylation, oxidative dehydroxylation and dioxygenation, and affects the metabolism of protein, fat and carbohydrate in fish. Riboflavin deficiency in Salmo fish was shown to cause a variety of symptoms such as growth retardation, photophobia, cataract, hemorrhage and corneal neovascularization (Yang et al., 2020). Pantothenic acid, as a precursor for coenzyme A synthesis, participates in processes such as carbohydrate metabolism, amino acid breakdown metabolism, and tricarboxylic acid cycle, which can promote fish growth, improve weight gain rate and feed utilization efficiency (Islam et al., 2022).
In this study, the amino acid content in the muscle tissues of Atlantic salmon undergoes significant changes across different growth stages (Table 2). Notably, levels of essential amino acids such as threonine and lysine, alongside others, diminish in the body tissues when the salmon grows. Both threonine and lysine are crucial for the growth of fish, with threonine playing a key role in a range of physiological and biochemical functions including growth promotion, feed efficiency, and the enhancement of absorption and immune responses (Ma et al., 2021). Lysine, on the other hand, is vital for the normal growth and development of aquatic animals, contributing significantly to the functions of the nervous system (Cai et al., 2018). Concurrently, we discovered a significant downregulation in the levels of these essential amino acids, including threonine and lysine, within the metabolites of the gut microbiota. Serine, a non-essential amino acid in fish, contributes to fat and fatty acid metabolism as well as muscle growth. Our findings showed a decrease in serine levels within body tissues, paralleled by a reduction in serine metabolites within the gut microbiota. As the fish continue to grow, we noted a gradual decrease in the taurine content within body tissues. Interestingly, the levels of taurine within the gut microbiota metabolites saw a significant increase, indicating a complex interplay between fish growth stages and amino acid availability both in the fish tissues and their gut microbiota. Further analysis revealed a relationship between gut microbiota metabolites, specifically amino acids like threonine, lysine, serine, and the abundance of various bacteria across different growth stages. These amino acids showed negative correlations with potential harmful bacteria, including Helicobacter, Alcanivorax, Faecalibaculum, Aeromonas, and Vibrio in the S2 stage. Conversely, they positively correlated with Lactobacillus, a beneficial probiotic, across all stages, while showing negative associations with Shewanella, Aeromonas, Pseudomonas, Microbulbifer, and Vibrio in the S3 stage, and with Enterococcus, Haemophilus, and Vibrio in the S4 stage. The increase of some pathogenic or potential pathogenic bacteria, such as Helicobacterium, Vibrio, Aeromonas, Pseudomonas, might have potential effects on the reduction of threonine, lysine and serine in muscle and gut microbiota. Notably, Lactobacillus showed a significant positive correlation with threonine, lysine and serine. As a probiotic Lactobacillus not only plays an important role in maintaining the normal gut microbial balance, but also can promote the production of amino acids. However, a decline in Lactobacillus abundance concurrent with fish growth was noted, potentially explaining the reduction in threonine, lysine, and serine levels. This suggests that effective gut interventions at different growth stages of Atlantic salmon may modulate the fish's nutritional profile. As shown in previous studies, supplementation of L. mesenteroides HY2 in diet increased the content of amino acids in turbot Scophthalmus maximus (Guo et al., 2020), the use of Lactobacillus sakei rMA-2 contributed to the nutritional composition of rainbow trout (Zhao et al., 2023). This study identified changes in gut microbiota and related metabolites at different growth stage. Obviously, the strategy of using probiotics to regulate the gut function needs to consider the effects of probiotics at different growth stages. Further research is needed to investigate the effects of gut microbiota derived metabolites of Atlantic salmon at different growth stages on fish growth, immunity, and gut microbiota.
|
|
Table 2 Composition and content of four nonvolatile flavor components in dorsal muscle of Atlantic salmon |
The gut microbiota and its associated metabolites exhibit notable changes throughout the growth of Atlantic salmon, marked by abundance of Proteobacteria coupled with a diminished Firmicutes/Bacteroidetes ratio. Metabolites produced by the gut microbiota, including ARA, taurine, citric acid, riboflavin, biotin, pantothenic acid, threonine, lysine and serine, may play a distinct role at various growth stages. Especially, the S1 group exhibited a more active microbial communities and metabolites related to amino acid metabolism. In addition, threonine, lysine, and serine produced by the gut microbiota are positively correlated with their contents in the muscles of Atlantic salmon. Among them, the abundance of Lactobacillus shows a positive correlation with threonine, lysine, and serine. This finding provides valuable insights for the development of targeted strategies aimed at manipulating the gut microbiota to optimize the nutritional status and overall health of Atlantic salmon.
AcknowledgementsThis research was supported by the Key R & D Program of Shandong Province (No. 2021LZGC027), the Shandong Provincial Natural Science Foundation (No. ZR20210225 0235), the Major Agricultural Application Technology Innovation Projects in Shandong Province (No. SD2019YY 006), and the 'First Class Fishery Discipline' Program in Shandong Province, China. Miss Qianqian Shan was appreciated for providing data on the amino acid content of Atlantic salmon muscle.
Author Contributions
Material preparation, data collection, analysis and writing-original draft were performed by Bowen Wang. Material preparation and formal analysis were performed by Xiyu Cao. Data curation was performed by Wenhao Ren. Methodology and resources were performed by Chunyan Zhao. Investigation was performed by Qing Li. Resources was performed by Ruiyong Fan. Methodology was performed by Xianhui Men. Project administration and resources were performed by Yangen Zhou. Conceptualization, methodology, funding acquisition, supervision and writingreview and editing were performed by Yichao Ren.
Data Availability
All data generated and analyzed during this study are included in this published article and its additional files.
Declarations
Ethics Approval and Consent to Participate
The experiments were conducted according to the gui-delines approved by the ethics committee of Qingdao Agricultural University (No. 2023002) and the Academy of Experimental Animal Center of Qingdao Agricultural University.
Consent for Publication
Informed consent for publication was obtained from all participants.
Conflict of Interests
The authors declare that they have no conflict of interests.
Cai, W. C., Liu, W. B., Jiang, G. Z., Wang, K. Z., Sun, C. X., and Li, X. F., 2018. Lysine supplement benefits the growth performance, protein synthesis, and muscle development of Megalobrama amblycephala fed diets with fish meal replaced by rice protein concentrate. Fish Physiology and Biochemistry, 44(4): 1159-1174. DOI:10.1007/s10695-018-0503-3 (  0) 0) |
Cai, W. J., Fu, L. L., Liu, C., He, L. Y., Liu, H. K., Han, D., et al., 2022. Dietary ribose supplementation improves flesh quality through purine metabolism in gibel carp (Carassius auratus gibelio). Animal Nutrition Journal, 13: 50-63. DOI:10.1016/j.aninu.2022.12.006 (  0) 0) |
Chomwong, S., Charoensapsri, W., Amparyup, P., and Tassanakajon, A., 2018. Two host gut-derived lactic acid bacteria activate the proPO system and increase resistance to an AHPND-causing strain of Vibrio parahaemolyticus in the shrimp Litopenaeus vannamei. Developmental and Comparative Immunology, 89: 54-65. DOI:10.1016/j.dci.2018.08.002 (  0) 0) |
Dai, H. Q., Han, J. J., Wang, T., Yin, W. B., Chen, Y. H., and Liu, H. W., 2023. Recent advances in gut microbiota-associated natural products: Structures, bioactivities, and mechanisms. Natural Product Reports, 40(6): 1078-1093. DOI:10.1039/d2np00075j (  0) 0) |
Dai, J. H., Li, Y. X., Yang, P., Liu, Y., Chen, Z. C., Ou, W. H., et al., 2018. Citric acid as a functional supplement in diets for juvenile turbot, Scophthalmus maximus L. : Effects on phosphorus discharge, growth performance, and intestinal health. Aquaculture, 495: 643-653. DOI:10.1016/j.aquaculture.2018.04.004 (  0) 0) |
De Filippo, C., Cavalieri, D., Di Paola, M., Ramazzotti, M., Poullet, J. B., Massart, S., et al., 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences, 107(33): 14691-14696. DOI:10.1073/pnas.1005963107 (  0) 0) |
Donaldson, G. P., Ladinsky, M. S., Yu, K. B., Sanders, J. G., Yoo, B. B., Chou, W. C., et al., 2018. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science, 360(6390): 795. DOI:10.1126/science.aaq0926 (  0) 0) |
Ganguly, S., and Prasad, A., 2011. Microflora in fish digestive tract plays significant role in digestion and metabolism. Reviews in Fish Biology and Fisheries, 22(1): 11-16. DOI:10.1007/s11160-011-9214-x (  0) 0) |
Gholizadeh, P., Mahallei, M., Pormohammad, A., Varshochi, M., Ganbarov, K., Zeinalzadeh, E., et al., 2019. Microbial balance in the intestinal microbiota and its association with diabetes, obesity and allergic disease. Microbial Pathogenesis, 127: 48-55. DOI:10.1016/j.micpath.2018.11.031 (  0) 0) |
Gobi, N., Malaikozhundan, B., Sekar, V., Shanthi, S., Vaseeharan, B., Jayakumar, R., et al., 2016. GFP tagged Vibrio parahaemolyticus Dahv2 infection and the protective effects of the probiotic Bacillus licheniformis Dahb1 on the growth, immune and antioxidant responses in Pangasius hypophthalmus. Fish and Shellfish Immunology, 52: 230-238. DOI:10.1016/j.fsi.2016.03.006 (  0) 0) |
Guo, G. X., Li, C., Xia, B., Jiang, S. H., Zhou, S., Men, X. H., et al., 2020. The efficacy of lactic acid bacteria usage in turbot Scophthalmus maximus on intestinal microbiota and expression of the immune related genes. Fish and Shellfish Immunology, 100: 90-97. DOI:10.1016/j.fsi.2020.03.003 (  0) 0) |
Guttman, D. S., Frese, S. A., Benson, A. K., Tannock, G. W., Loach, D. M., Kim, J., et al., 2011. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genetics, 7(2): e1001314. DOI:10.1371/journal.pgen.1001314 (  0) 0) |
Hai, Q., Wang, J. F., Kang, W. G., Lv, N. N., Liu, Y., Liu, Z., et al., 2023. Analysis of immune-related gene expression and differences in gut microflora between two sizes of rainbow trout (Oncorhynchus mykiss). Journal of Agricultural Biotechnology, 31(5): 1043-1052. DOI:10.3969/j.issn.1674-7968.2023.05.014 (  0) 0) |
Holmes, E., Li, J. V., Athanasiou, T., Ashrafian, H., and Nicholson, J. K., 2011. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends in Microbiology, 19(7): 349-359. DOI:10.1016/j.tim.2011.05.006 (  0) 0) |
Islam, M. A., Park, E., Jeong, B., Gwak, Y. J., Kim, J., Hong, W. H., et al., 2022. Validation of vitamin B-5 (pantothenic acid) and B-6 (pyridoxine, pyridoxal, and pyridoxamine) analyses in seafood. Journal of Food Composition and Analysis: 109. DOI:10.1016/j.jfca.2022.104518 (  0) 0) |
Kohl, K. D., Amaya, J., Passement, C. A., Dearing, M. D., and McCue, M. D., 2014. Unique and shared responses of the gut microbiota to prolonged fasting: A comparative study across five classes of vertebrate hosts. FEMS Microbiology Ecology, 90(3): 883-894. DOI:10.1111/1574-6941.12442 (  0) 0) |
Li, T. T., Long, M., Gatesoupe, F. J., Zhang, Q. Q., Li, A., and Gong, X. N., 2015. Comparative analysis of the intestinal bacterial communities in different species of carp by pyrosequencing. Microbial Ecology, 69(1): 25-36. DOI:10.1007/s00248-014-0480-8 (  0) 0) |
Li, X. M., Zhu, Y. J., Yan, Q. Y., Ringo, E., and Yang, D. G., 2014. Do the intestinal microbiotas differ between paddlefish (Polyodon spathala) and bighead carp (Aristichthys nobilis) reared in the same pond?. Journal of Applied Microbiology, 117(5): 1245-1252. DOI:10.1111/jam.12626 (  0) 0) |
Li, Y. J., Wen, Z. X., Meng, N., Li, X. J., Mi, R., and Du, X. F., 2021. Metabolomic analyses of tussah pupa-cultivated Cordyceps militaris at different growth stages. Mycosystema, 40(5): 1023-1038. DOI:10.13346/j.mycosystema.200243 (  0) 0) |
Liu, H., Guo, X. W., Gooneratne, R., Lai, R., Zeng, C., Zhan, F. B., et al., 2016. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Scientific Reports, 6: 24340. DOI:10.1038/srep24340 (  0) 0) |
Ma, L., Wu, X. Y., Ye, B., Geng, L. N., Zhou, Z. Y., Wang, X., et al., 2021. The optimum threonine requirement in diets of juvenile hybrid grouper (Epinephelus fuscoguttatus female × Epinephelus lanceolatus male). Aquaculture Nutrition, 27(3): 829-840. DOI:10.1111/anu.13227 (  0) 0) |
Ma, Q. T., Li, Y. Q., Li, P. F., Wang, M., Wang, J. K., Tang, Z. Y., et al., 2019. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomedicine & Pharmacotherapy, 117: 109138. DOI:10.1016/j.biopha.2019.109138 (  0) 0) |
Mancuso, C., and Santangelo, R., 2018. Alzheimer's disease and gut microbiota modifications: The long way between preclinical studies and clinical evidence. Pharmacological Research, 129: 329-336. DOI:10.1016/j.phrs.2017.12.009 (  0) 0) |
Mariat, D., Firmesse, O., Levenez, F., Guimarăes, V. D., Sokol, H., Doré, J., et al., 2009. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiology, 9: 123. DOI:10.1186/1471-2180-9-123 (  0) 0) |
Martin, S. A. M., Douglas, A., Houlihan, D. F., and Secombes, C. J., 2010. Starvation alters the liver transcriptome of the innate immune response in Atlantic salmon (Salmo salar). BMC Genomics, 11: 418. DOI:10.1186/1471-2164-11-418 (  0) 0) |
Nayak, S. K., 2010. Role of gastrointestinal microbiota in fish. Aquaculture Research, 41(11): 1553-1573. DOI:10.1111/j.1365-2109.2010.02546.x (  0) 0) |
Nicholson, J. K., Holmes, E., Kinross, J., Burcelin, R., Gibson, G., Jia, W., et al., 2012. Host-gut microbiota metabolic interactions. Science, 336(6086): 1262-1267. DOI:10.1126/science.1223813 (  0) 0) |
Nie, P. Q., Li, Z. Q., Wang, Y. M., Zhang, Y. B., Zhao, M. N., Luo, J., et al., 2019. Gut microbiome interventions in human health and diseases. Medicinal Research Reviews, 39(6): 2286-2313. DOI:10.1002/med.21584 (  0) 0) |
Ray, A. K., Ghosh, K., and Ringø, E., 2012. Enzyme-producing bacteria isolated from fish gut: A review. Aquaculture Nutrition, 18(5): 465-492. DOI:10.1111/j.1365-2095.2012.00943.x (  0) 0) |
Ley, R. E., Turnbaugh, P. J., Klein, S., and Gordon, J. I., 2006. Human gut microbes associated with obesity. Microbial Ecology, 444(7122): 1022-1023. DOI:10.1038/nature4441022a (  0) 0) |
Shin, N. R., Whon, T. W., and Bae, J. W., 2015. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends in Biotechnology, 33(9): 496-503. DOI:10.1016/j.tibtech.2015.06.011 (  0) 0) |
Tian, J., Ji, H., Oku, H., and Zhou, J., 2014. Effects of dietary arachidonic acid (ARA) on lipid metabolism and health status of juvenile grass carp, Ctenopharyngodon idellus. Aquaculture, 430: 57-65. DOI:10.1016/j.aquaculture.2014.03.020 (  0) 0) |
Tran, N. T., Wang, G. T., and Wu, S. G., 2017. A review of intestinal microbes in grass carp Ctenopharyngodon idellus (Valenciennes). Aquaculture Research, 48(7): 3287-3297. DOI:10.1111/are.13367 (  0) 0) |
Wan, Z. Q., Wang, C. Y., Zhou, J. J., Shen, M. L., Wang, X. Y., Fu, Z. W., et al., 2019. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere, 217: 646-658. DOI:10.1016/j.chemosphere.2018.11.070 (  0) 0) |
Wang, D. L., Ye, W. W., Wang, J. J., Song, L. Y., Fan, W. L., and Cui, Y. P., 2011. Construction of SSH library and its analyses of cotton drought associated genes under drought stress. Acta Agronomica Sinica, 36(12): 2035-2044. DOI:10.3724/sp.J.1006.2010.02035 (  0) 0) |
Xia, Y., Yu, E. M., Lu, M. X., and Xie, J., 2020. Effects of probiotic supplementation on gut microbiota as well as metabolite profiles within Nile tilapia, Oreochromis niloticus. Aquaculture, 527: 735428. DOI:10.1016/j.aquaculture.2020.735428 (  0) 0) |
Xu, H. G., Cao, L., Zhang, Y. Q., Johnson, R. B., Wei, Y. L., Zheng, K. K., et al., 2017. Dietary arachidonic acid differentially regulates the gonadal steroidogenesis in the marine teleost, tongue sole (Cynoglossus semilaevis), depending on fish gender and maturation stage. Aquaculture, 468: 378-385. DOI:10.1016/j.aquaculture.2016.11.002 (  0) 0) |
Yang, L. X., Xiang, X., Zhou, X. H., Chen, J., and Luo, L., 2020. Effects of riboflavin on growth performance, body composition, immunity and antioxidant capacity of Schizothorax prenanti. Journal of fisheries of China, 44(5): 836-844. DOI:10.11964/jfc.20190511780 (  0) 0) |
Zhang, J. L., 2023. Analysis of the structure and functional differences of intestinal flora in Anguilla japonica of different sizes. Journal of Wuhan Polytechnic University, 42(2): 16-23. DOI:10.3969/j.issn.2095-7386.2023.02.003 (  0) 0) |
Zhao, C. Y., Men, X. H., Dang, Y. J., Zhou, Y. G., and Ren, Y. C., 2023. Probiotics mediate intestinal microbiome and microbiotaderived metabolites regulating the growth and immunity of rainbow trout (Oncorhynchus mykiss). Microbiology Spectrum, 11(2): e03980-22. DOI:10.1128/spectrum.03980-22 (  0) 0) |
 2025, Vol. 24
2025, Vol. 24


