2) Guangdong Laboratory of Marine Ecology Environment Monitoring and Warning, Zhanjiang 524088, China
Harmful algal blooms (HABs), a natural ecological phenomenon, usually refers to the phenomenon that some marine microalgae and macroalgae overproliferate or accumulate in the water, causing discoloration (Glibert et al., 2001). Excess algae depletes oxygen from the water, which can kill aquatic animals and then rot and release toxins (Sellner et al., 2003; Yussof et al., 2021). Phycotoxins, primarily produced by certain dinoflagellates and cyanobacteria, have toxic effects on aquatic animals and can pose a threat to human health when the people consume contaminated water or aquatic animals (Indeck et al., 2015). When exposed to dinoflagellates, the fish exhibited gasping for breath, loss of balance, and convulsions. The proteomic analysis results suggest that this could be caused by oxidative stress mediated muscle damage (Kwok et al., 2021). Dinoflagellate can also lead to a significant inhibition of the opercular respiratory rate in fish, induce GSH-mediated antioxidant defense responses, and modulate DNA stability in gill tissues at sublethal concentrations (Shin et al., 2019). Exposure to the harmful dinoflagellate Prorocentrum minimum caused temporary oxidative damage and histological injury in ridgetail white prawn, Exopalaemon carinicauda (Mu et al., 2017).
Dinoflagellate is a typical group of HABs causative species, and some of them can produce dinoflagellate toxins. Most of Alexandrium species produce paralytic shellfish poison (PSP). The main components of the toxin spectrum are marine dinoflagellate-sulfocarbamoyl derivatives (C1/C2, B1), gonyautoxins (GTX1/GTX4), and neo-saxitoxin (NEO) (Krock et al., 2007; Zou et al., 2014), while the toxin content of different algal strains varies greatly. Alexandrium is widely distributed in the Arctic, temperate, and tropical regions (Taylor et al., 2003), with the highest frequency in the temperate waters of the northern hemisphere. Once the bloom occurs, it will cause serious environmental problems and economic losses to the aquaculture industry (Tillmann et al., 2020). Among them, Alexandrium pacificum, formerly a member of the Alexandrium tamarense species complex, was isolated and renamed in 2014, and can often be found in the Yellow Sea and the Bohai Sea in China (John et al., 2014). A. pacificum can produce PSP, which can accumulate in fish, shrimp, shellfish, and other organisms through the food chain, threatening human health (Robineau et al., 1991; Linares et al., 2009). High concentrations of A. pacificum can affect the survival rate of L. vannamei at nauplii and mysis stages (Wang et al., 2007), and is lethal to Penaeus monodon (Su et al., 1993). What's more, low concentrations of A. pacificum affected the reproduction of Neomysis awatschensis and reduce the survival rate of juvenile shrimp (Yan et al., 2004). The PSP toxin extracted from dinoflagellates is lethal to L. vannamei (Pérez et al., 2008). PSP will damage the ganglia of shrimp, causing ganglionic medullary division and cell lysis, resulting in paralysis, spasms, abnormal behavior, or death (Linares et al., 2009; Liang et al., 2014). If people accidentally ingest shellfish products enriched with PSP due to ingesting toxic algae, they will have poisoning reactions with neurological symptoms and, in severe cases, die due to respiratory muscle paralysis (Yang et al., 2000).
The PSP produced by A. pacificum can cause physiological changes of L. vannamei in various ways (Linares et al., 2009; Yeganeh et al., 2020). The mechanisms of the toxic effects of red tide poisonous algae on aquatic animals has always been a topic of concern. The nerves system is an important target organ for paralytic toxins (Pérez et al., 2008). PSP can selectively act on voltage-sensitive Na+ channels of nerve cells, block Na+ influx, and cause signal transmission between neurons to be blocked, thereby causing neurological paralysis (Bricelj et al., 2005; Tillmann et al., 2020). Previous studies have mainly focused on the response of immune organs to PSP and the presence of toxin residues, while the research on the specific toxicity of PSP on neural tissues and their response is limited (Mu et al., 2019; Mu et al., 2023). In this study, high-throughput sequencing techniques were used to explore the changes in the neural transcriptional levels in L. vannamei after acute exposure to high concentrations of A. pacificum. The response mechanism of shrimp to dinoflagellate toxins provides a reference for healthy shrimp farming and disease control (Landsberg, 2002).
2 Materials and Methods 2.1 Experimental MaterialsA. pacificum was obtained from Shanghai Guangyu Biotechnology Co., Ltd., China. A. pacificum were cultured in f/2 medium with salinity of 25 (Guillard, 1975) under conditions of 22℃, 50 μmol m−2 s−1, and a photo period of 14 h: 10 h (light-dark ratio). The natural seawater was collected from the Donghai Island Marine Biological Research Base in Zhanjiang, China (110˚32΄22.07΄΄E, 21˚01΄33.12΄΄N). Cultures were shaken three times a day at regular intervals.
L. vannamei was provided by the Zhanjiang Donghai Island Marine Biology Research Base, Guangdong Province, with an initial body length of 5.3 cm ± 0.3 cm and an initial body weight of 2.5 g ± 0.5 g. Before experiment, L. vannamei were domesticated for 2 weeks in the indoor laboratory. The shrimps were maintained in 200 L containers containing sterilized seawater (temperature 28℃ ± 2℃, DO (6.18 ± 0.08) mg L−1, pH 8.0 ± 0.2).
2.2 Preparation of A. pacificum Fragmentation SolutionThe A. pacificum algal lysate includes cell fragments, cell contents, and extracellular filtrate, all of which contain PSP toxins. Hence, we chose to add this lysate to the experimental water to simulate the natural environment (Landsberg, 2002; Pérez et al., 2008; EFSA, 2009).
A. pacificum cultures were centrifuged at 4℃ with 4000 r min−1 for 2 min. The supernatant and algal cells were collected, respectively, and the algal cells were concentrated to 5×105 cells mL−1. Then, the algal cells were mixed with 0.5 mm zirconium beads and crushed with a tissue crusher at 70 Hz for 2 min.
2.3 A. pacificum Exposure ExperimentCombined with our preliminary experiments, the concentration of 1.0×104 cells mL−1 was chosen as the acute study.
The crushed A. pacificum was diluted to 1×104 cells mL−1 in seawater, and the L. vannamei was exposed to the dilution solution for the experiment (treatment group). The control was normal, sterilized seawater without algal fluid. Three replicates were set up in each group, and 30 prawns were randomly placed in each replicate, with 50 L experimental water volume. During the experiment, the oxygen pump was continuously inflated. The prawn was fed with the formulated pellet feed (No. 2 white prawn feed produced by Yuehai Feed Group of Zhanjiang, China), 3 times a day. The feces were regularly removed to ensure the cleanliness of aquatic environment.
At 72 h, 16 shrimps were randomly selected from each parallel of the control and experimental groups. Their ventral nerves were quick-frozen in liquid nitrogen and stored at −80℃ for further analyses.
2.4 RNA Extraction, cDNA Library Construction, and SequencingThe TRIzol kit (CWBIO) was used to extract the neural RNA of L. vannamei, and the operation process was carried out strictly following the instructions. Three replicates were conducted in each group, and sixteen nerves per replicate were mixed together as one sample to decrease interindividual variation. Nanodrop was used to measure the quality of the extracted samples, and RNA-specific agarose electrophoresis was used to test the integrity of the samples. Total RNA was extracted from the samples using RNA-prep Pure Tissue Kit (TIANGEN BIOTECH, Beijing, China). After detection of RNA concentration, purity and integrity, rRNA in total RNA was removed. The product joint was connected, the joint was purified, the enrichment fragment library was amplified by PCR, and the sequencing library was constructed. The high-throughput sequencing part of this experiment was conducted by Biomarker Technologies Co., Ltd. using Illumina NovaSeq 6000 platform for transcriptome sequencing.
2.5 Transcriptome Data AnalysisHigh-quality data filtered from the raw data were compared and annotated with the reference genome of L. vannamei (https://www.ncbi.nlm.nih.gov/assembly/GCA_003789085.1, accessed on 30 December 2022). HISAT2 was used to align reads from RNA sequencing experiments. The reads were assembled by StringTie and the expression was evaluated according to the maximum flow algorithm. Their expressions were standardized using FPKM. DESeq2 was used to analyze the differential expression between the samples, based on negative binomial distribution. |Fold change| > 1.5, P-value < 0.05 was used as the screening standard during the process of differentially expressed genes (DEGs) detection. The number of common DEGs in three replicates between each comparison group was counted, and the gene expression was averaged over each group sample. GO (http://geneontology.org/, accessed on 30 December 2022) and KEGG databases (https://www.kegg.jp/, accessed on 30 December 2022) were used to annotate the differential genes. GO enrichment analysis of the DEGs was implemented by the GOseq R packages based Wallenius non-central hyper-geometric distribution (Young et al., 2010), which can adjust gene length bias in DEGs. KOBAS software was used to test the statistical enrichment of DEGs in KEGG pathways (Mao et al., 2005).
2.6 Quantitative PCR (qPCR) VerificationSeven DEGs were selected for real-time (quantitative) PCR (qPCR) to verify the reliability and authenticity of transcriptomic data. The primers were designed according to the whole genome sequence of L. vannamei (Table 1) and synthesized by Jinweizhi Biotechnology Co., Ltd. (Suzhou, China). HiScriptR Ⅲ RT SuperMix for qPCR (+gDNA wiper) was used to reversely transcrib RNA into cDNA. ChamQ Universal SYBR qPCR Master Mix was used for qPCR. The experiments in each group were repeated 3 times, and the expression level of differential genes was calculated by 2−∆∆CT method with β-actin gene as the reference gene.
|
|
Table 1 Primer sequences used for qPCR |
The original sequence quality of the transcriptome samples after library construction and sequencing is shown in Table 2. After filtering, an average of 21197098 bp of clean reads were screened for each sample. The analysis showed that the Q20 (the proportion of bases with a base recognition accuracy of more than 99%) in both the experimental group and the control group was higher than 97%, and the Q30 (the proportion of bases with a base recognition accuracy of more than 99.9%) was higher than 92%.
|
|
Table 2 Quality analysis of sequence after filtration |
Compared with the control group, 300 differentially expressed genes were identified in the A. pacificum treatment group, of which 194 were up-regulated, and 106 were down-regulated. Except for hypothetical proteins with unknown function, the most significantly up-regulated genes were functionally classified as serine protease inhibitors, while the most significantly down-regulated genes were ribosomal proteins. Among the top 10 genes with the most significant differential expression, transferrin, cytoskeleton, and ribosomal protein-related functional genes accounted for a relatively high proportion.
3.3 GO Enrichment AnalysisGO classification and enrichment analysis were performed on the differential genes in two groups (Fig.1). There were 179 genes with significant differential expression in the neural sample group. GO pathway analysis results of the DEGs were classified according to biological processes (BP), cellular components (CC), and molecular functions (MF). Cortical cytoskeleton organization, troponin complex, glycogen catabolic process and pyrimidine nucleobase metabolic process were enriched in cell components. Amyloalpha-1, 6-glucosidase and thymidine phosphorylase were both significantly enriched in molecular functions. Troponin complex and cell cortex were significantly enriched in biological processes.
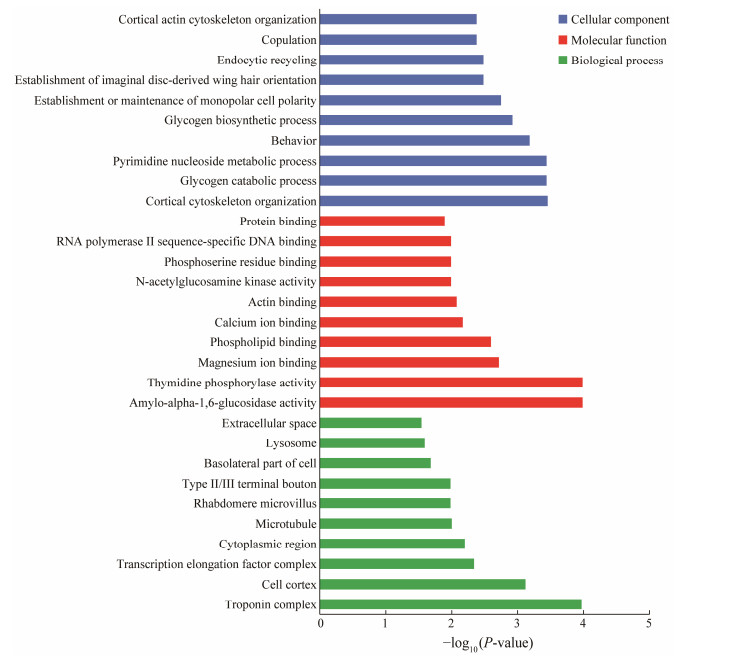
|
Fig. 1 Gene ontology (GO) enrichment analysis of the differently expressed genes. |
KEGG classification and enrichment analysis (Fig.2) showed that DEGs were mainly enriched in oxidative phosphorylation, intercellular tight junctions, mitophagy, drug metabolism enzymes, leukocyte transendothelial migration, etc. The significant enriched pathways are shown in Table 3. The results showed that after exposure to A. pacificum, the proteoglycans, signaling pathways, and various metabolic processes that regulate cell proliferation, differentiation, and apoptosis all played an essential role in the response of L. vannamei to algal toxins.
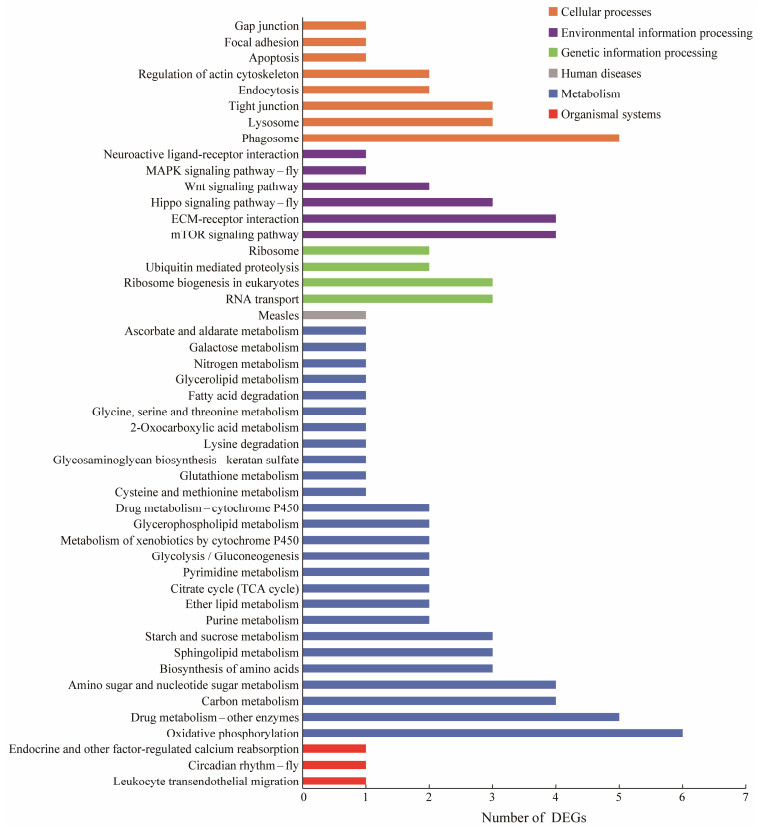
|
Fig. 2 KEGG pathway classification of the differently expressed genes. |
|
|
Table 3 Statistic of significant enriched KEGG pathways in DEGs (P-value < 0.05) |
Combined with the KEGG pathway analysis and transcriptome sequencing results, the genes related to the oxidative phosphorylation pathway and significantly changed in the nerves of L. vannamei after exposed in A. pacificum were identified. Some of the genes were significantly up-regulated, such as SDHA and SDH1, while NDUFB5, NDUFS6, UQCRFS1, RIP1, petA, ATPeV1C, ATP6C, ATPeV1B, and ATP6B were all significantly down-regulated (Fig.3 and Table 4). In PI3K-Akt signaling pathway, the PHLPP gene was significantly up-regulated. Comparing the differential expressions of the genes in the hippo signaling pathway, it was found that the SCRIB gene was significantly up-regulated, and YWHAE gene was significantly down-regulated (Table 3).
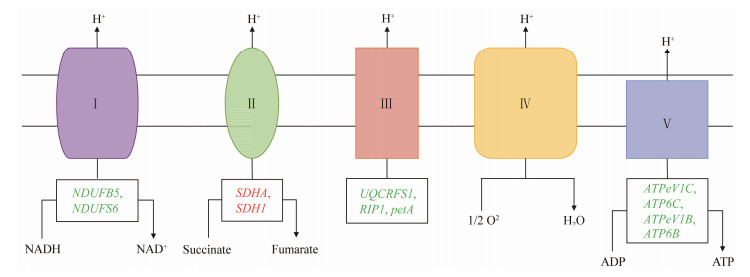
|
Fig. 3 Schematic diagram of oxidative phosphorylation pathway of shrimp exposed to A. pacificum. The red and green letters indicate up-regulated and down-regulated genes, respectively. Complex Ⅰ, NADH dehydrogenase; complex Ⅱ, succinate-Q oxidoreductase; complex Ⅲ, cytochrome c oxidoreductase; complex Ⅳ, cytochrome oxidase; complex Ⅴ, ATPase. |
|
|
Table 4 Gene screens related to detoxification and metabolic functions |
Seven genes were selected for validating RNA-seq results using a qPCR assay (Fig.4). The log2 (Fold change) of each gene values obtained from RNA-seq and qPCR were as follows: optB −2.32 vs. −4.29, RpL9 −2.96 vs. −3.86, TCHHL −2.06 vs. −1.07, Mo25 −1.92 vs. −1.06, RNase K −1.58 vs. −1.33, GABRG1 1.95 vs. 3.26, LeiL 2.06 vs. 6.92. Although the fold changes were not identical, the up- and down-regulation patterns of the seven genes were similar in RNA-seq and qPCR results, indicating that the transcriptome results were reliable.
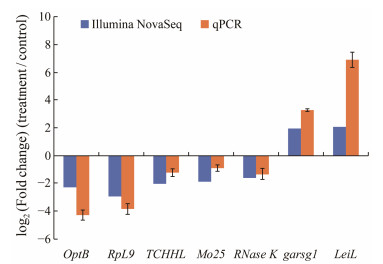
|
Fig. 4 Comparison of the expressions of nine differentially expressed genes determined by Illumina sequencing and qPCR. |
Worldwide, the species responsible for most of harmful algal bloom events are dinoflagellates, especially Alexandrium. The PSP toxin are a threat to humans, among which saxitoxin (STX) and gonyautoxin (GTX) are the most important (Anderson et al., 2002). Alexandrium and the toxins it produces, such as PSP, hemolysin, etc., can have acute toxic effects on aquatic animals, directly causing the death of marine organisms, but also cause behavior changes, reproduction interruptions, growth retardation, and enzymatic activity decrease in aquatic animals (Eschbach et al., 2001; Emura et al., 2004). In addition, A. pacificum can produce non-paralytic shellfish toxicity that reduces the fertilization rate of scallops (Yan et al., 2003), as well as produce active oxygen such as hydrogen peroxide (Kim et al., 1999). Moreover, bioactive extracellular compounds secreted by Alexandrium strongly modify the valve-activity behavior of Crassostrea gigas, inducing hemocyte mobilization within the gills (Castrec et al., 2018). Alterations and subacute toxicity, such as apoptosis or necrosis, result in decreased adaptation of aquatic animals to the environment. In Chinese shrimp culture ponds, blooms caused by Alexandrium have occurred frequently, which threatens the shrimp farming. Therefore, the potential harmful mechanism of Alexandrium to shrimp, especially the immune function of shrimp, is necessary to be studied to increase the disease resistance (Su et al., 1993). Previous studies have mainly focused on the effects of toxins on immune organs, like hepatopancreas, which play a crucial role in detoxification (Mu et al., 2019; Mu et al., 2023). Additionally, researchers have also concerned about the presence of toxin residues in various tissues due to the potential adverse impacts they can have on human health (Yu et al., 2021). Our study aimed to investigate the direct effects of this neurotoxin on the nervous system, which is another target organ of the toxin. We specifically examined the molecular transcription level response of neural genes, indicating that the nervous system also actively responds to the toxin.
Toxins can accumulate in zooplankton, shellfish, fish, and crustacea (Blanco et al., 2003; Kwong et al., 2006; Sephton et al., 2006). It's found that the mortality of Penaeus monodon reached as high as 90% after 12 hours exposure to a concentration of 1.0 × 104 cells mL−1 of A. pacificum (Su et al., 1993). The semi-lethal concentration (LC50) of A. pacificum to Penaeus chinensis through bath exposure was 1.0 × 104 cells mL−1 with a PSP concentration of 3.9 × 10–5 MU cell−1 determined by Liang et al. (2014). According to the toxin content of 1 MU to Kunming mice is equivalent to 0.189 g of saxitoxin (STX), the toxicity of PSP of A. pacificum is 7.3 pg STX Equal cell−1 (Lin et al., 2001; Liang et al., 2014). Although the amount of toxin varies from different strains, the toxicity of A. pacificum measured by Yan et al. (2002) and Tan et al. (2007) is basically the same. It is worth considering that both the intracellular and extracellular secretions, as well as cell fragments of A. pacificum, may potentially be toxic to L. vannamei. Combined with our preliminary experiments, the concentration of 1.0 × 104 cells mL−1 was chosen as the acute study, and the experimental group was finally added with algal cell fragments, intracellular secretions, and algal culture medium. The nerve is one of the target organs of PSP toxin (Linares et al., 2009). In this experiment, prawn nerves were taken for mRNA transcriptome sequencing to screen different expressions of immunity- and detoxification-related genes.
Oxidative phosphorylation is an essential metabolic pathway. Adenosine triphosphate (ATP) is produced by mitochondria via oxidative phosphorylation. Five major protein complexes make up the respiratory chain, including NADHQ oxidoreductase, Q-cytochrome c oxidoreductase, cytochrome c reductase, cytochrome-c-oxidase, and ATP synthase (complexes Ⅰ, Ⅱ, Ⅲ, Ⅳ, and Ⅴ). These complexes are located in mitochondrial membranes. By oxidizing NADH or FADH2, complex Ⅰ or Ⅱ triggers the transfer of electrons from complex Ⅰ to complex Ⅳ. It transfers electrons from ubiquinol to water-soluble cytochrome c through complex Ⅲ. In cytochrome c oxidase (COX), electrons are transferred from cytochrome c to oxygen. By phosphorylating ADP into ATP as protons return to the mitochondrial matrix through the complex Ⅴ, the energy stored in the proton gradient is used to fuel respiration (electron transport) (Boekema et al., 2007). In this study, the pathway enriched for metabolic function accounted for the highest proportion, of which oxidative phosphorylation was the most significant. Significant up-regulation of genes in the oxidative phosphorylation pathway, such as the genes related to electrons passing through complex Ⅱ, suggests that exposure to A. pacificum fragment solution activates this pathway, increasing intracellular energy production to defend against algal toxins.
When algal toxins stimulate the lymphocyte membrane receptor, it will cause a series of metabolic reactions, such as oxidative phosphorylation. The oxidative phosphorylation process will generate reactive oxygen species (ROS), a positive signal for lymphocyte activation (Fuentes et al., 2011; Belt et al., 2017). However, reactive oxygen species can cause free radicals to proliferate, damage cells, and lead to aging and disease (Kuno et al., 1985). In addition, the function of lymphocyte membrane receptors is regulated by the cytoskeleton (microtubules and microfilaments), which plays a regulatory role in the generation of ROS (Rinnerthaler et al., 2012). Oxidative phosphorylation is the coupling reaction of the energy released by the oxidation of substances in the body to supply ADP and inorganic phosphorus to synthesize ATP through the respiratory chain (Walker, 2013; Mühleip et al., 2016). Among the genes enriched in oxidative phosphorylation, two genes were up-regulated, nine were down-regulated. Among them, genes related to ATP synthesis, a critical step in the production of ATP (Senior, 1990), were significantly down-regulated when the electron acceptor was nicotinamide adenine dinucleotide (NADH). Additionally, the genes related to electrons passing through complexes Ⅰ, Ⅲ, and Ⅴ in the respiratory chain were also significantly down-regulated, indicating that the L. vannamei had suffered oxidative damage under the stress of A. pacificum. However, when the electron acceptor was flavin adenine dinucleotide (FAD), genes related to electrons passing through complex Ⅱ in the respiratory chain were significantly up-regulated, indicating that the oxidative phosphorylation function of L. vannamei may also be enhanced under stress, improving the efficiency of ATP synthesis and obtain energy, which can more effectively ensure normal life activities.
These leucine rich repeat protein phosphatases (PHLPPs) are comprised of two genes: PHLPP1 and PHLPP2 (Liu et al., 2011). PHLPP dephosphorylates Akt on its hydrophobic motif, terminates Akt signaling, playing an inhibitory role in this pathway (Gao et al., 2005). The PI3K-Akt signaling pathway is activated by A. pacificum to regulate essential functions, including cell growth, survival, transcription, translation, and proliferation. After binding growth factors to receptor tyrosine kinases (RTKs) or G protein-coupled receptors (GPCRs), the class Ia and class Ib PI3K subtypes are activated, respectively. The effector phosphatidylinositol-3, 4, 5-triphosphate (PIP3) is a major product of PI3K activation, which is the second messenger of Akt activation (Vogt et al., 2011). Once activated, Akt controls vital cellular processes by phosphorylating substrates involved in apoptosis, protein synthesis, metabolism, and the cell cycle. The significant up-regulation of PHLPP in this study indicates that under the stress of A. pacificum, the cell survival and proliferation of L. vannamei were inhibited, while the apoptosis rate increased.
As an essential component of a wide variety of cellular processes, protein lap4-like SCRIB has been implicated in polarity, migration, proliferation, differentiation, apoptosis, stem cell maintenance, and vesicle trafficking (Humbert et al., 2008). SCRIB plays an inhibitory role in hippo signaling pathway (Pearson et al., 2011). The pathway can regulate cell proliferation and apoptosis and plays a vital role in cancer occurrence, tissue regeneration, and the regulation of stem cell function (Boggiano et al., 2012). A. pacificum induced physiological injury of shrimp cells, and apoptosis was activated to induce programmed cell death. The significant up-regulation of SCRIB in this study indicates that under the stress of A. pacificum, the functions of cell proliferation and tissue regeneration of L. vannamei are inhibited. At the same time the rate of apoptosis is increased.
Apoptosis is a genetically programmed process that eliminates no longer useful cells (Opferman et al., 2003). PI3KAkt signaling pathway and the Hippo signaling pathway play an essential role in the regulation of apoptosis and are two critical pathways to control the initiation and execution of apoptosis (Huang et al., 2005; Franke, 2008; Nguyen et al., 2020). Up-regulation of PHLPP and SCRIB indicates the accelerated apoptosis in L. vannamei under A. pacificum stress.
In this experiment, the significant differential expression of pathways closely related to apoptosis in the KEGG significance analysis indicated that apoptosis played a vital role in the process of L. vannamei against A. pacificum.
5 ConclusionsIn summary, we investigated the immune defense mechanisms of neural tissue in L. vannamei acutely exposed to A. pacificum through transcriptional level analysis. Compared with the control group, 300 DEGs were identified in the experimental group. Transcriptome analysis showed that A. pacificum caused oxidative damage to shrimp by inhibiting ATP synthesis, and regulated cell proliferation, apoptosis, and other processes by inhibiting protein synthesis. During the stress-resistance process, L. vannamei improved the function of oxidative phosphorylation and the efficiency of ATP synthesis, while various metabolic processes, apoptosis, and cell cortex defense mechanisms also played an essential role in immune defense. The results are helpful to understand the neurotoxic effects of PSP on shrimp. In shrimp aquaculture, targeting genes associated with neurotoxicity allows the implementation of relevant disease-resistant breeding strategies, which may be effective in combating certain outbreaks of dinoflagellate red tides.
AcknowledgementsThis research was supported by the Modern Seed Industry Park for Whiteleg Shrimp of Guangdong Province (No. K22226), the National Natural Science Foundation of China (No. 32102796), the Natural Science Foundation of Guangdong Province (No. 2020A1515110086), the Program for Scientific Research Start-up Funds of Guangdong Ocean University (Nos. 060302022102, 060302022201), the Program of Shrimp Aquaculture Talent Development (No. B2 2424), and the Undergraduate Innovation Team of Guangdong Ocean University (No. CXTD2023002).
Anderson, D. M., and Burkholder, G. J. M., 2002. Harmful algal blooms and eutrophication: Nutrient sources, composition and consequences. Estuaries, 25(4): 704-726. DOI:10.1007/BF02804901 (  0) 0) |
Belt, K., Huang, S., Thatcher, L. F., Casarotto, H., Singh, K. B., Aken, O. V., et al., 2017. Salicylic acid-dependent plant stress signaling via mitochondrial succinate dehydrogenase. Plant Physiology, 173(4): 2029-2040. DOI:10.1104/pp.16.00060 (  0) 0) |
Blanco, J., Reyero, M. I., and Franco, J., 2003. Kinetics of accumulation and transformation of paralytic shellfish toxins in the blue mussel Mytilus galloprovincialis. Toxicon, 42(7): 777-784. DOI:10.1016/j.toxicon.2003.10.007 (  0) 0) |
Boekema, E. J., and Braun, H. P., 2007. Supramolecular structure of the mitochondrial oxidative phosphorylation system. Journal of Biological Chemistry, 282(1): 1-4. DOI:10.1074/jbc.R600031200 (  0) 0) |
Boggiano, J. C., and Fehon, R. G., 2012. Growth control by committee: Intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Developmental Cell, 22(4): 695-702. DOI:10.1016/j.devcel.2012.03.013 (  0) 0) |
Bricelj, V. M., Connell, L., Konoki, K., Macquarrie, S., Scheuer, T., Catterall, W., et al., 2005. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature, 434(7034): 763-767. DOI:10.1038/nature03415 (  0) 0) |
Castrec, J., Soudant, P., Payton, L., Tran, D., Miner, P., Lambert, C., et al., 2018. Bioactive extracellular compounds produced by the dinoflagellate Alexandrium minutum are highly detrimental for oysters. Aquatic Toxicology, 199: 188-198. DOI:10.1016/j.aquatox.2018.03.034 (  0) 0) |
Chain, E. P. O. C. I., 2009. Marine biotoxins in shellfish-saxitoxin group. EFSA Journal, 7(4): 1019. (  0) 0) |
Emura, A., Matsuyama, Y., and Oda, T., 2004. Evidence for the production of a novel proteinaceous hemolytic exotoxin by dinoflagellate Alexandrium taylori. Harmful Algae, 3(1): 29-37. DOI:10.1016/j.hal.2003.08.004 (  0) 0) |
Eschbach, E., Scharsack, J. P., John, U., and Medlin, L. K., 2001. Improved erythrocyte lysis assay in microtitre plates for sensitive detection and efficient measurement of haemolytic compounds from ichthyotoxic algae. Journal of Applied Toxicology, 21(6): 513-519. DOI:10.1002/jat.797 (  0) 0) |
Franke, T. F., 2008. PI3K/Akt: Getting it right matters. Oncogene, 27(50): 6473-6488. DOI:10.1038/onc.2008.313 (  0) 0) |
Fuentes, D., Meneses, M., Nunes-Nesi, A., Araújo, W. L., Tapia, R., Gómez, I., et al., 2011. A deficiency in the flavoprotein of Arabidopsis mitochondrial complex Ⅱ results in elevated photosynthesis and better growth in nitrogen-limiting conditions. Plant Physiology, 157(3): 1114-1127. DOI:10.1104/pp.111.183939 (  0) 0) |
Gao, T., Furnari, F., and Newton, A. C., 2005. PHLPP: A phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Molecular Cell, 18(1): 13-24. DOI:10.1016/j.molcel.2005.03.008 (  0) 0) |
Glibert, P., and Pitcher, G., 2001. Global Ecology and Oceanography of Harmful Algal Blooms: Science Plan. SCOR and IOC, Baltimore and Paris, 87pp.
(  0) 0) |
Guillard, R. R. L., 1975. Culture of phytoplankton for feeding marine invertebrates. In: Culture of Marine Invertebrate Animals: Proceedings – 1st Conference on Culture of Marine Invertebrate Animals Greenport. Springer US, Boston, MA, 29-60.
(  0) 0) |
Huang, J., Wu, S., Barrera, J., Matthews, K., and Pan, D., 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the drosophila homolog of YAP. Cell, 122(3): 421-434. DOI:10.1016/j.cell.2005.06.007 (  0) 0) |
Humbert, P. O., Grzeschik, N. A., Brumby, A. M., Galea, R., Elsum, I., and Richardson, H. E., 2008. Control of tumourigenesis by the scribble/dlg/lgl polarity mod-ule. Oncogene, 27(55): 6888-6907. DOI:10.1038/onc.2008.341 (  0) 0) |
Indeck, K. L., Simard, P., Gowans, S., Lowerre-Barbieri, S., and Mann, D. A., 2015. A severe red tide (Tampa Bay, 2005) causes an anomalous decrease in biological sound. Royal Society Open Science, 2(9): 150337. DOI:10.1098/rsos.150337 (  0) 0) |
John, U., Litaker, R. W., Montresor, M., Murray, S., Brosnahan, M. L., and Anderson, D. M., 2014. Formal revision of the Alexandrium tamarense species complex (Dinophyceae) taxonomy: The introduction of five species with emphasis on molecular-based (rDNA) classification. Protist, 165(6): 779-804. DOI:10.1016/j.protis.2014.10.001 (  0) 0) |
Kim, C. S., Lee, S. G., Lee, C. K., Kyu, L. C., Gyoon, K. H., Jin, J., et al., 1999. Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. Journal of Plankton Research, 21(11): 2105-2115. DOI:10.1093/plankt/21.11.2105 (  0) 0) |
Krock, B., Seguel, C. G., and Cembella, A. D., 2007. Toxin profile of Alexandrium catenella from the Chilean coast as determined by liquid chromatography with fluorescence detection and liquid chromatography coupled with tandem mass spectrometry. Harmful Algae, 6(5): 734-744. DOI:10.1016/j.hal.2007.02.005 (  0) 0) |
Kuno, S., Bacher, A., and Simon, H., 1985. Structure of enoate reductase from a Clostridium tyrobutyricum (C. spec. La1). Biological Chemistry, 366(1): 463-472. (  0) 0) |
Kwok, C. S., Lai, K. K., Lam, W., Xu, S. J., Lam, S. W., and Lee, F. W., 2021. Proteome analysis of whole-body responses in medaka experimentally exposed to fish-killing dinoflagellate Karenia mikimotoi. International journal of Molecular Sciences, 22(21): 11625. DOI:10.3390/ijms222111625 (  0) 0) |
Kwong, R. W. M., Wang, W. X., and Lam, P. K. S., 2006. The up-take, distribution and elimination of paralytic shellfish toxins in mussels and fish exposed to toxic dinoflagellates. Aquatic Toxicology, 80(1): 82-91. DOI:10.1016/j.aquatox.2006.07.016 (  0) 0) |
Landsberg, J. H., 2002. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science, 10(2): 113-390. DOI:10.1080/20026491051695 (  0) 0) |
Liang, Z., Li, J., Li, J., Tan, Z., Ren, H., and Zhao, F., 2014. Toxic dinoflagellate Alexandrium tamarense induces oxidative stress and apoptosis in hepatopancreas of shrimp (Fenneropenaeus chinensis). Journal of Ocean University of China, 13(6): 1005-1011. DOI:10.1007/s11802-014-2397-8 (  0) 0) |
Lin, Y., Jia, X., Yang, M., and Quan, Q., 2001. Primary study on detected method of paralytic shellfish poison used Kunming strain mouse. Journal of Tropical Oceanography, 20(2): 88-91. (  0) 0) |
Linares, J. P., Ochoa, J. L., and Martínez, A. G., 2009. Retention and tissue damage of PSP and NSP toxins in shrimp: Is cultured shrimp a potential vector of toxins to human population. Toxicon, 53(2): 185-195. DOI:10.1016/j.toxicon.2008.10.022 (  0) 0) |
Liu, J., Stevens, P. D., Li, X., Schmidt, M. D., and Gao, T., 2011. PHLPP-mediated dephosphorylation of S6K1 inhibits protein translation and cell growth. Molecular and Cellular Biology, 31(24): 4917-4927. DOI:10.1128/MCB.05799-11 (  0) 0) |
Mao, X., Cai, T., Olyarchuk, J. G., and Wei, L., 2005. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics, 21: 3787-3793. DOI:10.1093/bioinformatics/bti430 (  0) 0) |
Mu, C., Ge, Q., and Li, J., 2019. Exposure to Prorocentrum minimum induces oxidative stress and apoptosis in the ridgetail white prawn, Exopalaemon carinicauda. Journal of Ocean University of China, 18(3): 727-734. DOI:10.1007/s11802-019-3846-1 (  0) 0) |
Mu, C., Ren, X., and Li, J., 2023. Immune response of the ridgetail white prawn Exopalaemon carinicauda after exposure to the dinoflagellate Prorocentrum minimum. Journal of Ocean University of China, 22(3): 821-830. DOI:10.1007/s11802-023-5336-8 (  0) 0) |
Mu, C., Ren, X., Ge, Q., Wang, J., and Li, J., 2017. Antioxidant response of ridgetail white prawn Exopalaemon carinicauda to harmful dinoflagellate Prorocentrum minimum exposure and its histological change. Journal of Ocean University of China, 16: 285-293. DOI:10.1007/s11802-017-3170-6 (  0) 0) |
Mühleip, A. W., Joos, F., Wigge, C., Frangakis, A. S., Kühlbrandt, W., and Davies, K. M., 2016. Helical arrays of U-shaped ATP synthase dimers form tubular cristae in ciliate mitochondria. Proceedings of the National Academy of Sciences, 113(30): 8442-8447. DOI:10.1073/pnas.1525430113 (  0) 0) |
Nguyen, T. H., Ralbovska, A., and Kugler, J. M., 2020. RhoBTB proteins regulate the Hippo pathway by antagonizing ubiquitination of LKB1. G3: Genes, Genomes, Genetics, 10(4): 1319-1325. DOI:10.1534/g3.120.401038 (  0) 0) |
Opferman, J. T., and Korsmeyer, S. J., 2003. Apoptosis in the development and maintenance of the immune system. Nature Immunology, 4(5): 410-415. DOI:10.1038/ni0503-410 (  0) 0) |
Pearson, H. B., Perez-Mancera, P. A., Dow, L. E., Ryan, A., Tennstedt, P., Bogani, D., et al., 2011. SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. The Journal of Clinical Investigation, 121(11): 4257-4267. DOI:10.1172/JCI58509 (  0) 0) |
Pérez-Linares, J., Ochoa, J. L., and Gago-Martínez, A., 2008. Effect of PSP toxins in white leg shrimp Litopenaeus vannamei boone. Food Science, 73(4): 233-237. (  0) 0) |
Rinnerthaler, M., Büttner, S., Laun, P., Heeren, G., Felder, T. K., Klinger, H., et al., 2012. Yno1p/Aim14p, a NADPH-oxidase ortholog, controls extramitochondrial reactive oxygen species generation, apoptosis, and actin cable formation in yeast. Proceedings of the National Academy of Sciences, 109(22): 8658-8663. DOI:10.1073/pnas.1201629109 (  0) 0) |
Robineau, B., Gagné, J. A., Fortier, L., and Cembella, A. D., 1991. Potential impact of a toxic dinoflagellate (Alexandrium excavatum) bloom on survival of fish and crustacean larvae. Marine Biology, 108(2): 293-301. DOI:10.1007/BF01344344 (  0) 0) |
Sellner, K. G., Doucette, G. J., and Kirkpatrick, G. J., 2003. Harmful algal blooms: Causes, impacts and detection. Journal of Industrial Microbiology & Biotechnology, 30: 383-406. (  0) 0) |
Senior, A. E., 1990. The proton-translocating ATPase of Escherichia coli. Annual Review of Biophysics and Biophysical Chemistry, 19(1): 7-41. DOI:10.1146/annurev.bb.19.060190.000255 (  0) 0) |
Sephton, D. H., Haya, K., and Martin, J. L., 2007. Paralytic shellfish toxins in zooplankton, mussels, lobsters and caged Atlantic salmon, Salmo salar, during a bloom of Alexandrium fundyense off Grand Manan Island, in the Bay of Fundy. Harmful Algae, 6(5): 745-758. DOI:10.1016/j.hal.2007.03.002 (  0) 0) |
Shin, Y. K., Nam, S. E., Kim, W. J., Seo, D. Y., Kim, Y. J., and Rhee, J. S., 2019. Red tide dinoflagellate Cochlodinium polykrikoides induces significant oxidative stress and DNA damage in the gill tissue of the red seabream Pagrus major. Harmful Algae, 86: 37-45. DOI:10.1016/j.hal.2019.04.008 (  0) 0) |
Su, H. M., Liao, I. C., and Chiang, Y. M., 1993. Mass mortality of prawn caused by Alexandrium pacificum blooming in a culture pond in southern Taiwan. In: Toxic Phytoplankton Blooms in the Sea. Tomas, C. R., and Baden, D. G., eds., Elsevier Science Publishers, New York, 329-333.
(  0) 0) |
Tan, Z. J., Yan, T., and Yu, R. C., 2007. Transfer of paralytic shellfish toxins via marine food chains: A simulated experiment. Biomedical and Environmental Sciences, 20(3): 235. (  0) 0) |
Taylor, F. J. R., Fukuyo, Y., Larsen, J., and Hallegrae, F. F., 2003. Taxonomy of harmful dinoflagellates. Manual on Harmful Marine Microalgae, 33: 389-432. (  0) 0) |
Tillmann, U., Krock, B., and Wietkamp, S., 2020. A mediterranean Alexandrium taylorii (Dinophyceae) strain produces gonio-domin A and lytic compounds but not paralytic shellfish toxins. Toxins, 12(9): 564. DOI:10.3390/toxins12090564 (  0) 0) |
Vogt, P. K., Hart, J. R., Gymnopoulos, M., Gymnopoulos, M., Jiang, H., Kang, S., et al., 2011. Phosphatidylinositol 3-kinase: The oncoprotein. Current Topics in Microbiology & Immunology, 2: 79-104. (  0) 0) |
Walker, J., 2013. The ATP synthase: The understood, the uncertain and the unknown. Biochemical Society Transactions, 41(1): 1-16. DOI:10.1042/BST20110773 (  0) 0) |
Wang, X. H., and Ma, S., 2007. The research on toxicity effects of Alexadrium tamarense ATDH01 on nauplii of Penaeus vannamei. Journal of Fujian Normal University, 23(3): 58-62. (  0) 0) |
Yan, T., Tan, Z. J., and Yu, R. C., 2002. The effect of dinoflagellate Alexandrium tamarense on juvenile perch Lateolabrax japonicus. Acta Scientiae Circumstantiae, 22(6): 749-753. (  0) 0) |
Yan, T., Tan, Z. J., Li, J., Yu, R. C., Wang, Y. F., and Zhou, M. J., 2004. The toxicity study of Alexandrium tamarense and Heterosigma akashiwo to two crustacean species Neomysis awatschensis and Artemia salina. Acta Oceanologica Sinica, 26(1): 76-81. (  0) 0) |
Yan, T., Zhou, M., and Fu, M., 2003. Effects of the dinoflagellate Alexandrium tamarense on early development of the scallop Argopecten irradians concentricus. Aquaculture, 217(1-4): 167-178. DOI:10.1016/S0044-8486(02)00117-5 (  0) 0) |
Yang, M. L., Lin, Y. T., and Quan, G. Y., 2000. Characteristics of paralytic shellfish toxins content in Shenzhen sea area. Journal of Zhanjiang Ocean University, 20(4): 13-17. (  0) 0) |
Yeganeh, V., Sharifinia, M., Mobaraki, S., Dashtiannasab, A., Aeinjamshid, K., Borazjani, J. M., et al., 2020. Survey of survival rate and histological alterations of gills and hepatopancreas of the Litopenaeus vannamei juveniles caused by exposure of Margalefidinium /Cochlodinium polykrikoides isolated from the Persian Gulf. Harmful Algae, 97: 101856. DOI:10.1016/j.hal.2020.101856 (  0) 0) |
Young, M. D., Wakefield, M. J., and Smyth, G. K., 2010. Gene ontology analysis for RNA-Seq: Accounting for selection bias. Genome Biology, 11(2): 14. DOI:10.1186/gb-2010-11-2-r14 (  0) 0) |
Yu, R. C., Zhang, Q. C., and Liu, Y., 2021. The dinoflagellate Alexandrium catenella producing only carbamate toxins may account for the seafood poisonings in Qinhuangdao, China. Harmful Algae, 103(1): 101980. (  0) 0) |
Yussof, F. N., Maan, N., and Reba, M. N., 2021. Lstm networks to improve the prediction of harmful algal blooms in the west coast of Sabah. International Journal of Environmental Research and Public Health, 18: 7650. DOI:10.3390/ijerph18147650 (  0) 0) |
Zou, C., Ye, R. M., Zheng, J. W., Luo, Z. H., Gu, H. F., Yang, W. D., et al., 2014. Molecular phylogeny and PSP toxin profile of the Alexandrium pacificum species complex along the coast of China. Marine Pollution Bulletin, 89(1-2): 209-219. DOI:10.1016/j.marpolbul.2014.09.056 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


