2) Key Laboratory of Marine Biotechnology of Zhejiang Province, Ningbo University, Ningbo 315832, China;
3) College of Marine Sciences, Ningbo University, Ningbo 315832, China
In recent years, lots of researches and reviews have been conducted on the intestinal microbiota of fish species (Ruiz et al., 2024; Zhang et al., 2024a). This is due to the fact that the intestinal tract of fish harbors a diverse array of microbiota, which play vital roles in producing digestive enzymes (Stanley et al., 2018; Peter et al., 2020), regulating energy metabolism (Zhang et al., 2020a), and enhancing host immunity (Meng et al., 2019). Nonetheless, the gut microbiota is closely associated with the development of diseases. Specific microbiota have the ability to breach the gut epithelium or induce tissue inflammation, leading to the onset of various diseases (Xiong et al., 2019). For instance, Aeromonas punctata f. intestinalis and Aeromonas hydrophila have shown to cause intestinal inflammation and structural changes in the mucosa of farmed grass carp (Ctenopharyngodon idella) (Sun et al., 2007). In addition to the pathogenic bacteria, the structure and function of the gut microbiota also have a significant impact on the overall health of the hosts (Zheng et al., 2019). Studies have indicated that grass carp with intestinal inflammation exhibited notable differences in the diversity, structure, and function of the gut microbiota, particularly in terms of distinct alterations in Dechloromonas, Methylocaldum, Planctomyces, Rhodobacter, Caulobacter, Flavobacterium, and Pseudomona (Tran et al., 2018). Furthermore, the occurrence of intestinal diseases has been primarily associated with specific microorganisms and their metabolic byproducts (Carding et al., 2015). Overall, these investigations indicated that variations in the diversity, composition, and function of the intestinal microbiota are closely linked to the overall health of the host.
Pampus argenteus is a valuable piscine species and is distributed along the coastal regions of China, spanning from the Bohai Sea to the South China Sea (Peng et al., 2012). In recent decades, the wild population of P. argenteus has experienced substantial depletion as a result of overfishing and environmental changes (Sun et al., 2019). However, with the success of artificial propagation, P. argenteus has been deemed as a potential candidate for aquaculture (James and Almatar, 2008). During the breeding process, intestinal flatulence has been observed to lead to increased mortality of larvae and losses of fry production. Intestinal flatulence results in visible abdominal gas accumulation, causing the fish's body to turn over to one side, hampering their swimming and eating abilities. Researchers have characterized this phenomenon as 'intestinal gas bubble accumulation', which leads to alterations in antioxidant enzymes and metabolic disturbances (Zheng, 2020). In our previous study, we observed a disparity in the relative abundance of dominant bacteria between affected individuals and healthy individuals. Specifically, we noted an increase of aerogenic bacteria, which may contribute to excessive gas production and consequently lead to flatulence (Zhang et al., 2020). Similar findings were reported in another study, which revealed a decrease in the abundance and evenness of the gut microbiota in P. argenteus affected by gas bubble accumulation. Furthermore, the relative abundance of Acinetobacter and Ilumatobacter was significantly reduced in these individuals (Zheng et al., 2020). These studies provided compelling evidence for the close relationship between intestinal flatulence and disruptions in the gut microbiota.
Our observations have indicated that alterations in the type or quantity of feed can sometimes contribute to intestinal flatulence. However, it remains uncertain whether these observations are associated with changes in the gut microbiota. The objective of this study was to examine the disparities in intestinal microbiota composition between flatulent and healthy P. argenteus. To achieve this aim, 16S amplicons were analyzed with high-throughput sequencing. The findings of this study could establish a theoretical framework for early detection and play a crucial role in the prevention and treatment of flatulence through the modu-
lation of the intestinal microbiota.
2 Materials and Methods 2.1 Experimental Design and SamplingThe experimental P. argenteus with two months old were obtained from Xiangshan Harbor Aquaculture and Larva Company Limited, Ningbo, China. The fish were reared in 2500 L ponds maintained with a temperature of 25℃± 0.1℃, pH of 8.22 ± 0.3, dissolved oxygen level of (7.22 ± 0.22) mg L−1, and salinity of 22 ± 0.4 (121˚95΄E, 29˚77΄N). With a recirculating water system, daily water exchange was conducted at a rate of 200% of the pond volume. The fish were divided into intestinal flatulence group (F) and healthy group (N). After an initial acclimation period of one week, sixty flatulent fish were identified based on their swimming posture and the presence of gas in the abdomen, with no additional anatomical abnormalities beyond intestinal enlargement after anatomy. Another sixty healthy fish were selected as the control group (Fig.1). Three replicates were conducted (n = 3) and each replicate consisted of 20 fish with an average weight of 4.5 g ± 1.08 g and an average body length of 5.65 cm ± 1.17 cm. Additionally, water samples were collected from the cultured environment (W). All fish were euthanized using buffered MS-222 (Finquel MS-222, Sigma Inc.), and the whole gut was carefully extracted from the anus using sterile scissors. Simultaneously, 10 L of cultured seawater was promptly filtered through a 0.22-μm polycarbonate membrane (47 mm-diameter, Millipore, USA) (n = 3), then stored at −80℃ for DNA extraction.
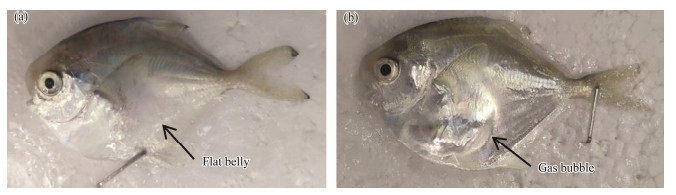
|
Fig. 1 Comparison of the healthy and flatulent P. argenteus. (a), healthy fish (N) without gas bubble in the abdomen; (b), intestinal flatulent fish (F) with visible gas bubble in the abdomen. |
The total genomic DNA was extracted with OMEGA bacterial DNA kit following the manufacturer's protocol. The quantification of DNA was executed via the NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, USA). The DNA was subsequently diluted to a concentration of 1 ng mL−1 using sterile water, based on the concentration analysis. The V4 hypervariable region of the 16S rRNA gene was amplified with barcode sequence via the universal primers 515F (5'-GTGCCAGCMGCCGCGG-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3'). The PCR solution consisted of 15 µL Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 µL forward and reverse primers, and 10 ng of template DNA. The PCR protocol included an initial denaturation at 98℃ for 1 min, 30 cycles of denaturation at 98℃ for 10 s, annealing at 50℃ for 30 s, and elongation at 72℃ for 30 s, with a final elongation step at 72℃ for 5 min. The Agilent 5400 fragment analyzer was utilized to verify the quality and concentration of PCR products.
The TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA) was employed to establish sequencing libraries and supplemented with index codes. Evaluation of library quality was executed through Qubit@ 2.0 Fluorometer (Thermo Scientific) and the Agilent Bioanalyzer 2100 system. The qualified libraries were subjected to sequencing on an Illumina NovaSeq PE 250 platform.
2.3 Data AnalysisEqual amount of amplicons were selected from each sample and were pooled together, then they were sequenced using an Illumina MiSeq platform (Illumina, San Diego, USA). The 16S rRNA gene sequences were processed using QIIME2 (Bolyen et al., 2019). In short, primers were trimmed utilizing the Cutadapt plugin (Martin, 2011). Based on the high-confidence representative sequences, amplicon sequence variants (ASVs) table was denoised and generated by DADA2 (Callahan et al., 2016). The taxonomy of the representative sequences was classified with the silva database (v. 138). Nonbacterial ASVs, chloroplast, mitochondria, archaea, and rare bacteria (< 20 reads) were removed. Finally, to adjust unequal sequencing depth, all samples were rarefied to the same sequencing depth in downstream analysis.
2.4 Statistical AnalysisThe vegan package (Oksanen et al., 2022) in R 4.3.1 was utilized for subsequent analyses unless otherwise stated. The estimateR function from the vegan package, was utilized to calculate observed species and Shannon index was calculated by diversity. To determine significant differences between samples (groups), Tukey's HSD test were carried out with a significance level of 0.05 (Liu et al., 2023). Permutational multivariate analysis of variance (PERMANOVA) was further conducted to quantify the strength and significance of different conditions in determining the variations in microbiota (Anderson, 2001). Microbial beta diversity was evaluated by calculating Bray-Curtis distance matrices and was depicted by Constrained principal coordinates analysis (CPCoA). The permutational analysis of multivariate dispersions (PERMDISP) was adopted to examine the significance of the differences in microbial communities by betadisper. VennDiagram was used to detect the different ASVs between flatulence and healthy fish. The package of edgeR was utilized to screen the differential ASVs among flatulence and healthy fish. Only ASVs with FDR-adjusted P < 0.05 and log2 (Fold change) > 1 or < −1 were regarded as significantly 'enriched' or 'depleted', respectively (Gao et al., 2020). SourceTracker was used to analyze the origin of gut microbes (Knights et al., 2011). Similarity was analyzed using Jaccard distance and Cohesion algorithm (Herren and McMahon, 2017). Microbiome co-occurrence networks and correlation were analyzed by ggClusterNet (Wen et al., 2022). Additionally, PICRUSt2 was utilized to predict functional content of the metagenome from 16S rRNA (Douglas et al., 2020). The difference of PICRSUt2 results between flatulence and healthy fish was calculated by wilcox text. Metabolic pathways were visualized by iPath (Interactive Pathways Explorer) (https://pathways.embl.de/) (Darzi et al., 2018).
3 Results 3.1 The α Diversity 16S rRNA Sequencing ResultsA total of 227571 high-quality and classifiable reads were obtained in this study, ranging from 3166 to 5646 reads per sample. These reads corresponded to 312 Amplicon Sequence Variants (ASVs) across all samples. The rarefaction curves demonstrated that most of the bacterial species were identified, as the curves approached a plateau (Fig.2a). The Good's coverage analysis indicated that the identified bacterial species were representative, with coverage exceeding 99.2%. Our amplicon sequencing analysis revealed notable differences in the diversity of the water, flatulent, and healthy groups (Table 1). The healthy group exhibited the lowest observed species index and Shannon diversity, and there were significant differences between the flatulent and healthy groups (Figs.2b, c). The observed species and Shannon indices in water samples were significantly higher than those in the gut microbiota collected from the fish. The Venn diagram illustrated that five ASVs were present in both the flatulent and healthy fish, and in water samples (Fig.2d), while six ASVs were exclusively found in the gut of healthy fish. Twenty-five ASVs were unique in the flatulent samples, and 95 ASVs were exclusive in the water samples.
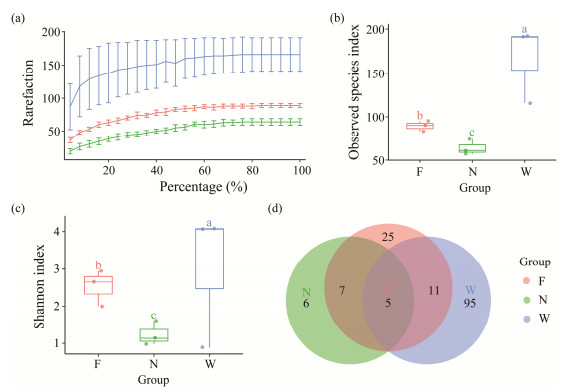
|
Fig. 2 Sequencing results and alpha diversity analysis. (a), the rarefaction curves of all samples; (b), the boxplot of observed species index; (c), the boxplot of index of Shannon; (d), Venn diagrams depicted the different kinds of ASVs among tween three groups. Different lowercase letters in (b) and (c) indicate significant difference among groups (P < 0.05). |
|
|
Table 1 P value of analyzing α-diversity in flatulence and control groups, and in environmental waters using Tukey's HSD test |
CPCoA based on Bray-Curtis distances revealed distinct separation of bacterial communities (P < 0.05, PERMANOVA) among the flatulent fish, healthy fish, and water sample (Fig.3a). The flatulent and healthy groups were separated along the second axis, explaining 30.87% of the variation, while the water samples were separated from both the flatulent and healthy groups along the first axis (69.13%). These results indicate that the condition of the fish was the main factor influencing the composition of the bacterial communities (Fig.4). In terms of taxonomic classification, the most dominant phylum in all samples was Proteobacteria, with the lowest relative abundance observed in the flatulent group (85.3%) (Fig.3b). Comparatively, the relative abundance of Proteobacteria in healthy samples (N) was 97.2%, and in water samples (W) was 97.8% during the same period. However, Firmicutes accounted for 11.28% in the flatulent group, which was significantly higher than in both the healthy fish (1.74%) and water (0.12%) (Fig.3b). Additionally, Fusobacteriota was present at 1% in the healthy group, while its abundance in the flatulent group (F) was less than 0.1%, and in the water samples (W) it was less than 0.01%. Gracilibacteria and Thaumarchaeota were exclusively found in water samples, while Spirochaetae was not detected.
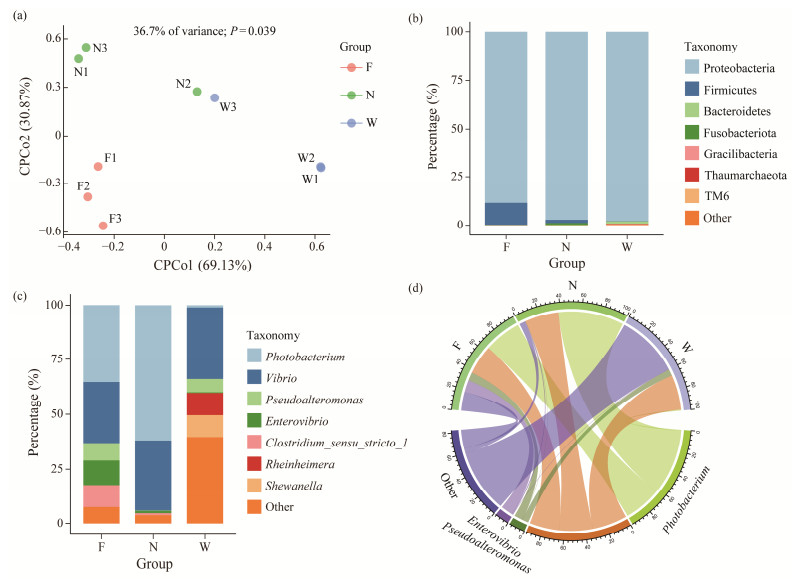
|
Fig. 3 Composition of gut microbial communities between different samples. (a), CPCoA analysis based on Bray-Curtis distance matrices of bacterial communities; (b), composition of taxa at phylum level; (c), composition of taxa at genus level; (d), composition of the entire aquaculture environment. |
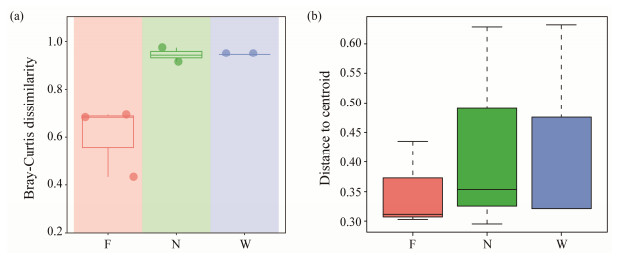
|
Fig. 4 Comparison of the dispersion among healthy, flatulent P. argenteus and water environment. (a), community dissimilarity calculated by Bray-Curtis distance; (b), comparison of community β-diversity using type as centroid in PERMDISP. |
Microbial composition at the genus level revealed distinct differences (Fig.3c). The flatulent group demonstrated significantly higher levels of Pseudoalteromonas (7.86%), Enterovibrio (11.20%), and Clostridium_sensu_stricto_1 (10.12%) when compared to the healthy group (0.17%, 1.07%, and 0.93%, respectively). Conversely, Photobacterium was notably less abundant in the flatulent group (35.67%) than in the healthy group (62.3%). The water contained a large number of Rheinheimera and Shewanella, which were hardly observed in the gut of both flatulent and healthy fish, while Clostridium_sensu_stricto_1 was absent. The bacterial communities dominated by Photobacterium, Vibrio, Pseudoalteromonas, and Enterovibrio were representative of the majority of microbes in the aquaculture environment as a whole (Fig.3d).
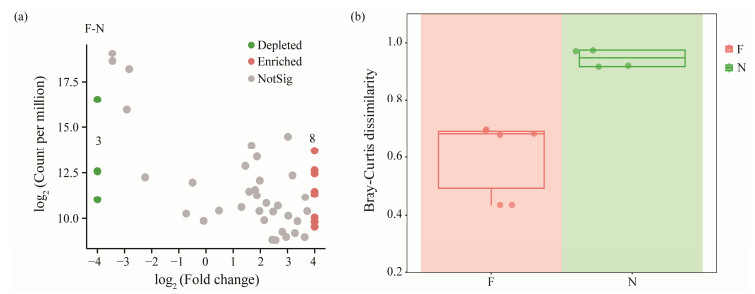
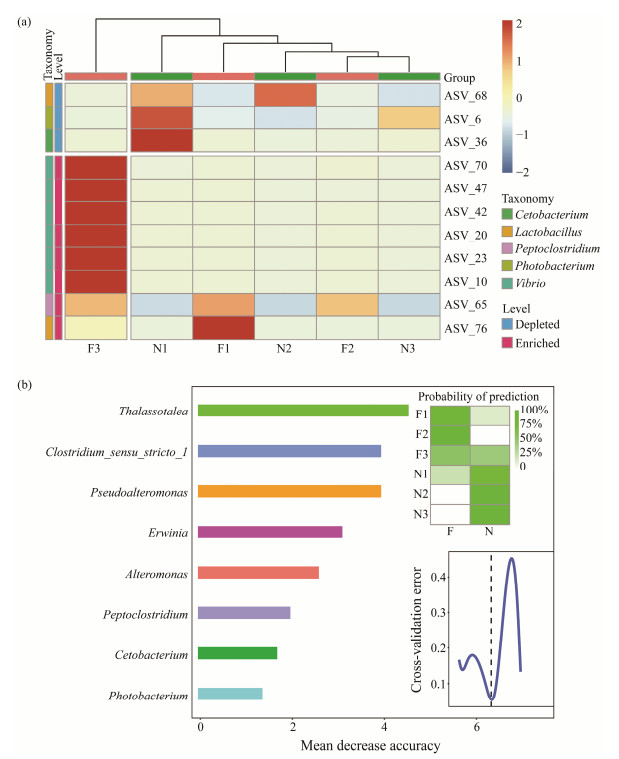
3.3 Differences of Gut Microbiota Between Flatulent and Healthy P. argenteusTo identify the ASVs that contributed to the divergence in microbial community composition between the flatulent and healthy groups, differential abundance analyses were conducted using a negative binomial distribution with ASV counts. The flatulent group was set as the control, and an adjusted P value cutoff of 0.05 was applied (Fig.5a). The dissimilarities of microbial community composition between the healthy and flatulent groups were also compared. Higher dissimilarities were observed in the healthy group (Fig.5b), indicating greater diversity bacterial communities in the gut of healthy fish. The ASVs were annotated at the genus level (Fig.6a), and eight ASVs were found to be enriched in the flatulent group (P < 0.05), primarily belonging to Vibrio, Peptoclostridium, and Lactobacillus. Three ASVs (ASV6, ASV36, ASV68) were significantly depleted in the flatulent group (P < 0.05), corresponding to Photobacterium, Cetobacterium, and Lactobacillus, respectively.
|
Fig. 5 Difference between flatulent and healthy groups. (a), volcano plots represent the proportion of ASVs enriched or depleted by microbiomes in the flatulence relative to healthy samples; (b), community dissimilarity calculated by Bray-Curtis distance. |
|
Fig. 6 Significantly different microbes in the flatulent and healthy P. argenteus. (a), significant enriched and depleted of ASVs in the flatulent over healthy fish gut microbes annotated at the genus level; (b), the top 8 taxa for distinguishing the flatulent and healthy P. argenteus in genus. The heatmap shows the predicted probabilities of planting location based on the profiles of taxa. |
To further understand the relationship between the microbial features of flatulent and healthy P. argenteus, a comparison was conducted at each taxonomic level. The best results were obtained at the genus level, which provided an appropriate number of taxa. Discriminatory taxa across the two groups were identified using the RandomForest machine learning algorithm. The top-ranking eight conditiondiscriminatory taxa were selected based on their variable importance measures (Fig.6b), which contributed an overall diagnostic accuracy of 100%. This indicated their ability to accurately predict the fish condition.
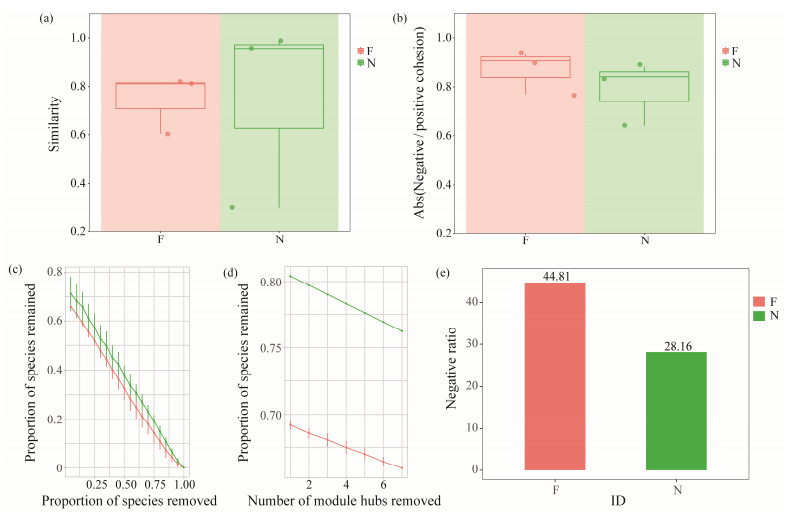
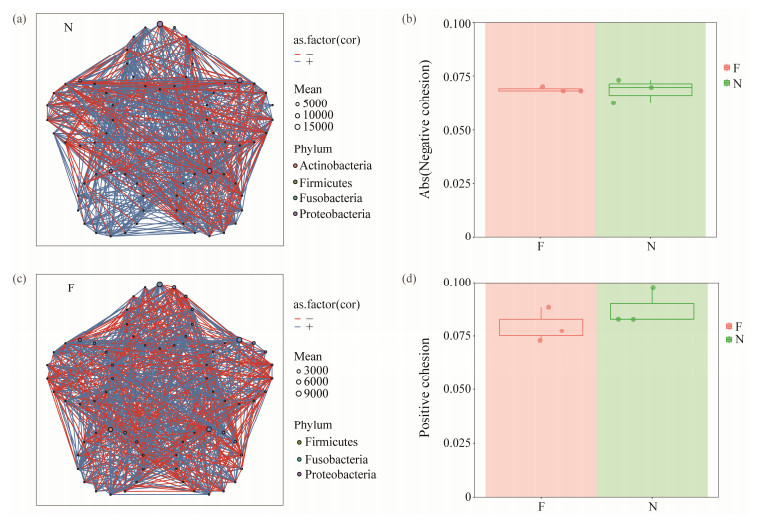
3.4 Flatulent P. argenteus Possess a Less Stable and Resistant Gut Bacterial CommunityThe flatulent group exhibited a significant enhancement in within-group similarity of the bacterial community (Fig. 7a), indicating that the flatulent fish displayed a disruption in bacterial community stability. Bacterial co-occurrence net-works were constructed using ggClusterNet to further explore microbe-microbe interactions in the flatulent and healthy groups (Figs.8a, b). The ratio of negative to positive cohesion was increased in the flatulent group, primarily due to a significant decrease in positive cohesion (Fig.7b and Figs.8c, d). The robustness of the bacterial networks, measured by removing random and targeted nodes, was consistently low when an equal number of nodes were randomly removed in the flatulent group compared to the healthy group (Figs.7c, d). This suggests that the roles of the nodes in maintaining the stability of bacterial networks were weakened in the flatulent group. Additionally, a higher proportion of negative correlations were observed in the co-occurrence networks of the flatulent group (Fig.7e). These findings indicated that the flatulent group had a higher degree of competition and instability within the gut microbial community.
|
Fig. 7 Stability parameters of gut bacteria community of the flatulence and healthy group. (a), the within-group similarities of gut bacterial community in the flatulent and healthy P. argenteus; (b), the absolute value of negative: positive cohesion ratio (N/P cohesion) of bacterial networks; (c), the robustness after removed random nodes; (d), the robustness after removed target hub nodes; (e), the radio of negative correlation in bacterial networks. |
|
Fig. 8 The bacterial co-occurrence network and cohesion characteristics of the flatulent and healthy P. argenteus. (a), the bacterial co-occurrence network characteristics of the P. argenteus with flatulence; (b), the bacterial co-occurrence net-work characteristics of the healthy P. argenteus; (c), the absolute value of negative cohesion ratio of bacterial networks; (d), the value of positive cohesion ratio of bacterial networks. |
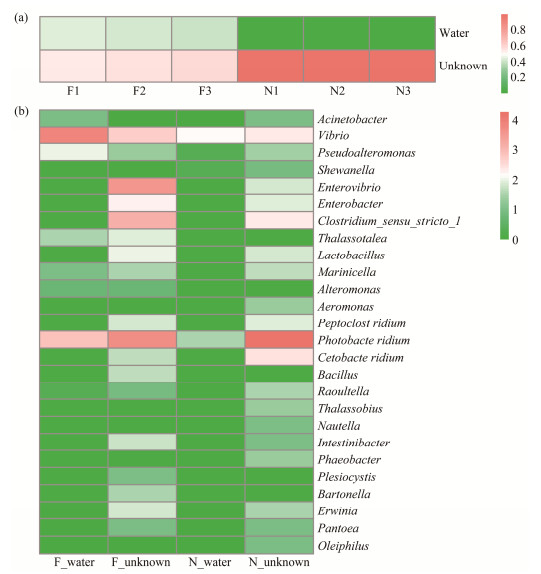
In both wild and farmed fish, the gut of the first hatchlings is initially a vacuum sterile environment. During their growth, water enters the gut. To investigate whether the bacterial community in the guts of flatulent and healthy fish selects microbes from the culture water, an analysis was performed (Fig.9a). It was found that in the gut of flatulent fish, 43.35% of the microbes originated from the culture water, while only 0.1% of microbes were from the culture water in the healthy fish gut. Furthermore, at the genus level, the abundance of certain microbes differed between the flatulent and healthy groups (Fig.9b). The flatulent fish was able to take up more Vibrio, Pseudoalteromonas, Acinetobacter, Thalassotalea, Marinicella, and Alteromonas from the culture water. On the other hand, Clostridium_sensu_ stricto_1 and Enterovibrio were found to be more abundant in the flatulent fish and were not from the water. Photobacterium, which was not from the environmental water, was more abundant in the healthy group than in the flatulent group. The study reveals that the microbiomes of flatulent and healthy fish intestine differ in their selection and acquisition of environmental microbes.
|
Fig. 9 Source analysis of gut microbes. (a), proportion of microbes from the aqueous environment in each sample; (b), intestinal flatulent and healthy fish gut microbes from the aquatic environment species at genus level. |
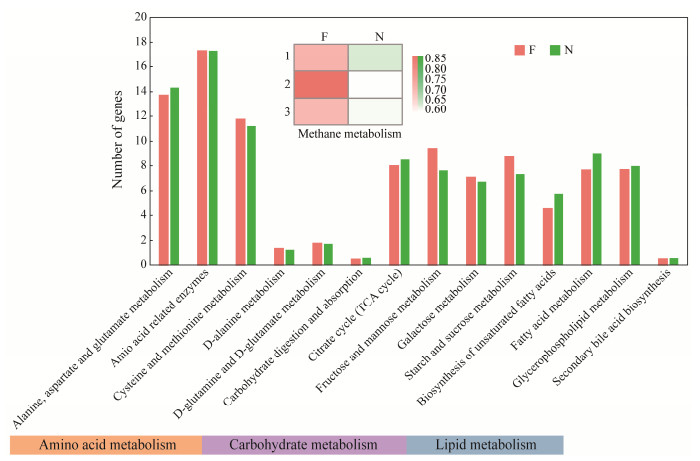
According to Fig.10, the relative abundances of key genes involved in carbohydrate metabolism, amino acid metabolism, and lipid metabolism differed between flatulent and healthy P. argenteus. In the gut microbes of healthy fish, there was a higher abundance of functional microbiota related to alanine, aspartate, and glutamate metabolism compared to the flatulent group. On the other hand, the abundance of functional microbes related to cysteine and methionine metabolism was higher in the flatulent group. In terms of carbohydrate metabolism, the healthy group exhibited a higher abundance of functional microorganisms related to carbohydrate digestion and absorption, as well as the citrate cycle (TCA cycle). Conversely, other aspects of carbohydrate metabolism showed higher abundance in the flatulent group. In lipid metabolism, the abundance of functional microbiota was generally higher in healthy fish than in the flatulent group. Notably, the methane metabolism function was stronger in the flatulent group than in the healthy group. Furthermore, metabolic pathway analysis (picture not shown) revealed that certain pathways were unique to the flatulent group. These included pantothenate and CoA biosynthesis, riboflavin metabolism, and methane metabolism, as visualized by iPath. On the other hand, some lipid metabolism pathways, such as glycerophospholipid metabolism and arachidonic acid metabolism, were lost or reduced in flatulent guts. Tyrosine metabolism and propanoate metabolism were also enriched exclusively in the healthy group. These findings highlight differences in the metabolic pathways and functional microbiota between the flatulent and healthy P. argenteus.
|
Fig. 10 Functional analysis of gut core microbiome in the flatulent and healthy P. argenteus annotated in KEGG database by PICRUSt2. |
Numerous studies have firmly established the pivotal roles of gut microbial communities in host metabolic processes, immune function, and physiology (Ji et al., 2020; Srivastava et al., 2021). Previous investigations have unveiled the effects of diet and aquatic habitat on the gut microbiota of aquatic organisms (Sado et al., 2014; Li, 2021). Hence, our analysis focused specifically on the effects of water and feed, as pellet feed lacks the presence of microflora. This allowed us to exclusively examine the microbiota of tissue and water samples. Our findings revealed a notable disparity in the number, abundance, and diversity of microbiota between water and tissue samples, while the former sample exhibited significantly higher levels (Fig.2). The CPCoA analysis (Fig.3a) demonstrated significant dissimilarities between samples obtained from the flatulence, healthy groups and water. This disparity can be attributed to the fact that water serves as an external environment that naturally harbors a diverse range of microbes. Similarly, a previous study by Kuang et al. (2020) investigated the microbiota in the gut, gill, and habitat (water and sediment) of two filtered-feeding fish species (Hypophthalmichthys molitrix and Hypophthalmichthys nobilis). The study revealed higher microbial diversity in the habitat samples compared to the fish samples. The SourceTracker analyses (Fig.9) indicated that only a minor fraction of gut microbes in healthy fish were from enviromental water. Specifically, microbes such as Vibrio and Photobacterium were identified, similar with previous reports (Takahashi et al., 2008; InaSalwany et al., 2019). However, a substantial portion of Photobacterium does not originate from the water. This observation could be attributed to the variations of water bodies and cultivation practices employed in our current study. Previous research has indicated that silver pomfret possesses a distinctive esophageal sac and pharyngeal sac structure (Jiang et al., 2023; Zheng et al., 2023). These anatomical features enable them to achieve more thorough food grinding during digestion. Additionally, their elongated digestive tract creates an environment rich with digestive enzymes, leading to the excretion of fecal matter that contributes to increased water turbidity. The turbidity of the water cause outbreaks of culture diseases (Pérez Martínez et al., 2003; Wold et al., 2014). So we change the water in the culture ponds every day. Additionally, it is important to note that the silver pomfret specimens used in our temporary culture were not obtained from the harbor for an extended duration. As a result, there was a limited correlation between the gut microorganisms and the environment of the temporary water body. While it is generally expected that external environmental factors do not have a significant impact on the structure of the gut microbiota in aquatic animals over longer periods (Zhang et al., 2020b), a previous study has acknowledged that fish in different developmental stages selectively establish distinct gut microbiota regardless of environmental factors (Yan et al., 2016).
4.2 Bacterial Community in Flatulent Gut Possessed Less StabilityThe stability of the host microbiome is crucial for maintaining host health, as it ensures the preservation of beneficial commensal bacteria and their associated functions over time (Coyte et al., 2015). Disruption of the intestinal microbiota can cause diseases through ways such as damaging epithelial layers and traversing them, or triggering inflammatory responses that harm the tissues (Pérez Sánchez et al., 2010). The progression of the disease is contingent upon the interplay between the colonic microbiota, their metabolic byproducts, and the host's immune system (Carding et al., 2015). Previous research has demonstrated that communities exhibiting greater negative cohesion tend to display higher stability (Hernandez et al., 2021). However, in our study, we observed a healthy group characterized by greater positive cohesion and enhanced stability. In the gut of P. argenteus, flatulence significantly reduced positive interactions between taxa, resulting in a more pronounced decrease in positive cohesion than in negative cohesion (Fig.8). The flatulent group exhibited a higher proportion of negative correlation in bacterial networks (Fig.7e), suggesting the occurrence of competition, ecological niche differentiation, and modular dispersion among microbial species. Compared with the normal intestinal microbiota, the intestinal microbiota of flatulence changed significantly (Fig.4). Consequently, there is a possibility that flatulence increases the susceptibility to fish diseases. Furthermore, the relative abundance analysis of the genus revealed an increase in opportunistic pathogens such as Enterovibrio and Vibrio in flatulent P. argenteus, which has been previously reported (Austin et al., 2005; Pascual et al., 2009; Wu et al., 2015).
Photobacterium damselae is the main bacteria affecting silver pomfret culture (Takahashi et al., 2008), causing massive skin ulcers and focal necrosis of the liver, spleen, kidneys and other tissues (Zhang et al., 2024b). Interestingly, we observed a lower abundance of Photobacterium damselae in the gut of the flatulence group compared to the healthy group. In the flatulence gut, there appears to be a competition between Photobacterium damselae and Vibrio, with Vibrio emerging as the dominant bacterium (Fig.3c). Numerous studies have demonstrated that Vibrio can induce dynamic changes in gut bacteria in aquatic animals (Xia et al., 2018; Shi et al., 2019; Kim et al., 2023), which raises the potential for a disease outbreak in flatulent silver pomfret. Indeed, feces harbor a significant number of bacterial taxa. Fecal microbiota transplantation has been demonstrated to alter the gut microbial community structure in shrimp, bolstering resistance to Vibrio infection (Guo et al., 2023). This technique also impacts gut microbe composition and offers therapeutic benefits in clinical treatments (Flannigan et al., 2017). Since both flatulent and healthy silver pomfrets are cultured in the same water body, there is a possibility that fecal microorganisms from the flatulent group can contaminate the gut of healthy individuals, which aligns with our observation that healthy groups can be infected by flatulence.
4.3 Differential Bacteria and Function in the Flatulent P. argenteusMethane is produced by methanogenic bacteria and is commonly expelled as a waste gas (Hackstein et al., 1996; St-Pierre et al., 2015). The production of methane can decrease the volume of intestinal gas and establish anaerobic conditions favorable for other bacteria (Roccarina et al., 2010; Mutuyemungu et al., 2023). Nevertheless, an overgrowth of methanogenic bacteria can lead to excessive methane production, which in turn can cause undesirable gastrointestinal symptoms like bloating and constipation in humans (Ge et al., 2015; Gottlieb et al., 2016; Lyu, 2021). In our previous study, we also observed an increase in methanogens in the intestinal flora of both flatulent and healthy P. argenteus, with a significant enrichment in the methane metabolic pathway (Zhang et al., 2020). Abnormal methane metabolism may be a contributing factor to the flatulence (Tran et al., 2018). Our study revealed notable differences in the functional prediction results related to methane metabolism between the groups. The enrichment analysis of the KEGG pathway indicated a substantial increase in the flatulent samples than in healthy samples. It is important to note that methane biosynthesis occurs exclusively through the anaerobic fermentation of carbohydrates, both endogenous and exogenous, by methanogenic microorganisms (Ge et al., 2015). This process includes the glycolytic pathway, tricarboxylic acid cycle, and amino acid and nucleotide metabolism. When comparing the flatulent and healthy groups, a common differential bacterium Clostridium sensu stricto 1 was identified (Fig.3c). This genus produces acid and hydrogen through fermentation (Yang et al., 2019a, b; Lu et al., 2020), and has been linked to sturgeon flatulence in previous studies (Brocca et al., 2022). Peptoclostridium in the flatulent samples (Fig.6a), is a group of Gram-positive, anaerobic, cell-producing bacteria belonging to Firmicutes. They can utilize a wide range of organic matters to produce different metabolites such as acetic acid, butyric acid, ethanol and hydrogen (Galperin et al., 2016). Additionally, another differential genus, Cetobacterium, was significantly more abundant in the gut of the healthy group than in the flatulent group. As a promising probiotic in aquaculture, Cetobacterium is also capable of producing proteases, lipases (Kim et al., 2007; Liu et al., 2016). It can use acetate, inhibits steatosis, and enhances glucose utilization (Finegold et al., 2003; Larsen et al., 2014; Wang et al., 2021; Sa et al., 2022). Lactobacillus was able to remodel the gut microbiota and enhance bile acid production, allowing normalisation of intestinal motility disorders, inhibition of Vibrio spp. growth, and improved ossification and survival (Ljubobratovic et al., 2020; Sun et al., 2021; Wang et al., 2022). We found a significant reduction of ASV68 (Lactobacillus spp.) in the flatulence gut compared to the healthy group (Fig.6), which is consistent with previous findings (Ruizhe et al., 2023; Yu et al., 2024). Our observation revealed that though cultured with the same conditions, some silver pomfret exhibited flatulence while others did not. This discrepancy can be attributed to individual differences. Each silver pomfret possesses distinct physiological and genetic characteristics that influence its capacity to digest food. These variations can be attributed to factors such as the individual's esophageal sac, pharyngeal sac, and the activity levels of digestive enzymes, which can vary among individuals (Jiang et al., 2023; Zheng et al., 2023). Some fish may possess a digestive system that is more adept at processing a specific type of feed, enabling better digestion. Conversely, other fish may have a less well-adapted digestive system, leading to flatulence or other digestive problems. Secondly, during the farming process, it is unavoidable that silver pomfret will experience artificial stress, which can impact their digestive systems and sffect their digestion of the feed (Campbell et al., 1992; Øverli et al., 2005; Jia et al., 2016).
5 ConclusionsOur research is pioneering in associating flatulence in silver pomfret with alterations in gut microbiome composition and functionality. We discovered a correlation between increased presence of Vibrio and heightened flatulence susceptibility and microbial instability. Functional analysis revealed associations between flatulence and aberrant methane and lipid metabolism. Excessive methane synthesis may utilize hydrogen and acetic acid as substrates, which are produced by specific bacteria such as Clostridium sensu stricto 1, Cetobacterium, and Peptoclostridium. Future investigations need to combine ecological and physiological analyses to further understand gut microbiota dynamics in silver pomfret.
AcknowledgementsThis research was funded by the National Key R&D Program of China (No. 2022YFD2400100), the Ningbo 2025 Major Project of Science Technology and Innovation (No. 2021Z003), the Public Welfare Program of Ningbo City (Science and Technology Special Commissioner Project) (No. 2022S204), the National Natural Science Foundation of China (Nos. 31872195, 42076118 and 42306114), the China Postdoctoral Science Foundation (No. 2022M721729), the General Scientific Research Projects of Zhejiang Provincial Department of Education (No. Y202249062), the Ningbo Public Welfare Science and Technology Project (No. 2021S061), and the Ningbo Yongjiang Talent Introduction Programme (No. 2021B-029-C).
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jiabao Hu. The draft of the manuscript was written by Wenhao Nie, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
All data generated and analyzed during this study are included in this published article and its additional files.
Declarations
Ethics Approval
This study was conducted in accordance with the recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocols were approved by the Animal Care and Use Committee of Ningbo University (NBU20220079).
Consent for Publication
Informed consent for publication was obtained from all participants.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that can influence the research.
Anderson, M., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology, 26: 32-46. (  0) 0) |
Austin, B., Austin, D., Sutherland, R., Thompson, F., and Swings, J., 2005. Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environmental Microbiology, 7: 1488-1495. DOI:10.1111/j.1462-2920.2005.00847.x (  0) 0) |
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al., 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology, 37: 1019. (  0) 0) |
Brocca, G., Zamparo, S., Pretto, T., Calore, A., Marsella, A., Xiccato, R. L., et al., 2022. Severe gastroenteropathy associated with Clostridium perfringens isolation in starving juvenile sturgeons. Journal of Fish Diseases, 45: 471-477. DOI:10.1111/jfd.13579 (  0) 0) |
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J., and Holmes, S. P., 2016. DADA2: High resolution sample inference from Illumina amplicon data. Nature Methods, 13: 581-583. DOI:10.1038/nmeth.3869 (  0) 0) |
Campbell, P. M., Pottinger, T. G., and Sumpter, J. P., 1992. Stress reduces the quality of gametes produced by rainbow trout. Biology of Reproduction, 47: 1140-1150. DOI:10.1095/biolreprod47.6.1140 (  0) 0) |
Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M., and Owen, L. J., 2015. Dysbiosis of the gut microbiota in disease. Microbial Ecology in Health and Disease, 26: 26191. (  0) 0) |
Coyte, K. Z., Schluter, J., and Foster, K. R., 2015. The ecology of the microbiome: Networks, competition, and stability. Science, 350: 663-666. DOI:10.1126/science.aad2602 (  0) 0) |
Darzi, Y., Letunic, I., Bork, P., and Yamada, T., 2018. iPath3.0: Interactive pathways explorer v3. Nucleic Acids Research, 46: W510-W513. (  0) 0) |
Douglas, G. M., Maffei, V. J., Zaneveld, J. R., Yurgel, S. N., Brown, J. R., Taylor, C. M., et al., 2020. PICRUSt2 for prediction of metagenome functions. Nature Biotechnology, 38: 685-688. DOI:10.1038/s41587-020-0548-6 (  0) 0) |
Finegold, S. M., Vaisanen, M. L., Molitoris, D. R., Tomzynski, T. J., Song, Y., Liu, C., et al., 2003. Cetobacterium somerae sp. nov. from human feces and emended description of the genus Cetobacterium. Systematic and Applied Microbiology, 26: 177-181. (  0) 0) |
Flannigan, K. L., Rajbar, T., Moffat, A., McKenzie, L. S., Dicke, F., Rioux, K., et al., 2017. Changes in composition of the gut bacterial microbiome after fecal microbiota transplantation for recurrent Clostridium difficile infection in a pediatric heart transplant patient. Frontiers in Cardiovascular Medicine, 4: 17. (  0) 0) |
Galperin, M. Y., Brover, V., Tolstoy, I., and Yutin, N., 2016. Phylogenomic analysis of the family Peptostreptococcaceae (Clostridium cluster XI) and proposal for reclassification of Clostridium litorale (Fendrich et al. 1991) and Eubacterium acidaminophilum (Zindel et al. 1989) as Peptoclostridium litorale gen. nov. comb. nov. and Peptoclostridium acidaminophilum comb. nov. International Journal of Systematic and Evolutionary Microbiology, 66: 5506-5513. DOI:10.1099/ijsem.0.001548 (  0) 0) |
Gao, M., Xiong, C., Gao, C., Tsui, C. K. M., Wang, M., Zhou, X., et al., 2020. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome, 9: 187. (  0) 0) |
Ge, T., Mu, C., and Zhu, W., 2015. Methanogen and human gut health – A review. Acta Microbiologica Sinica, 55: 661-666. (  0) 0) |
Gottlieb, K., Carter, S., Cruz, C., Porter, T., Le, C., Wacher, V., et al., 2016. Accurate identification of excessive methane gas producers is possible by a single fasting measurement of methane: Analysis of a large national database confirms prior results. American Journal of Gastroenterology, 111: S242-S243. (  0) 0) |
Guo, H. P., Fu, X. Z., He, J. K., Wang, R. Y., Yan, M. C., Wang, J., et al., 2023. Gut bacterial consortium enriched in a biofloc system protects shrimp against Vibrio parahaemolyticus infection. Microbiome, 11: 230. DOI:10.1186/s40168-023-01663-2 (  0) 0) |
Hackstein, J. H. P., Langer, P., and Rosenberg, J., 1996. Genetic and evolutionary constraints for the symbiosis between animals and methanogenic bacteria. Environmental Monitoring and Assessment, 42: 39-56. DOI:10.1007/BF00394041 (  0) 0) |
Hernandez, D. J., David, A. S., Menges, E. S., Searcy, C. A., and Afkhami, M. E., 2021. Environmental stress destabilizes microbial networks. The ISME Journal, 15: 1722-1734. DOI:10.1038/s41396-020-00882-x (  0) 0) |
Herren, C. M., and McMahon, K. D., 2017. Cohesion: A method for quantifying the connectivity of microbial communities. The ISME Iournal, 11: 2426-2438. DOI:10.1038/ismej.2017.91 (  0) 0) |
Ina-Salwany, M. Y., Al-saari, N., Mohamad, A., Mursidi, F. A., Mohd-Aris, A., Amal, M. N. A., et al., 2019. Vibriosis in fish: A review on disease development and prevention. Journal of Aquatic Animal Health, 31: 3-22. DOI:10.1002/aah.10045 (  0) 0) |
James, C. M., and Almatar, S. M., 2008. Potential of silver pomfret (Pampus argenteus) as a new candidate species for aquaculture. Aquaculture Asia Magazine, 2008: 49-50. (  0) 0) |
Ji, Y. Y., Groer, M., Dutra, S., Sarkar, A., and Mcskimming, D. I., 2020. Gut microbiota and immune system interactions. Microorganisms, 8: 1587. DOI:10.3390/microorganisms8101587 (  0) 0) |
Jia, R., Liu, B. L., Feng, W. R., Han, C., Huang, B., and Lei, J. L., 2016. Stress and immune responses in skin of turbot (Scophthalmus maximus) under different stocking densities. Fish & Shellfish Immunology, 55: 131-139. (  0) 0) |
Jiang, H., Hu, J., Xie, H., Zhang, M., Guo, C., Zhang, Y., et al., 2023. Morphological and molecular functional evidence of the pharyngeal sac in the digestive tract of silver pomfret, Pampus argenteus. International Journal of Molecular Sciences, 24: 1663. DOI:10.3390/ijms24021663 (  0) 0) |
Kim, D. G., Lee, S. J., Lee, J., Lee, E. W., and Jang, W. J., 2023. Changes in the gut microbiota composition of juvenile olive flounder (Paralichthys olivaceus) caused by pathogenic bacterial infection. Fishes, 8: 294. DOI:10.3390/fishes8060294 (  0) 0) |
Kim, D. H., Brunt, J., and Austin, B., 2007. Microbial diversity of intestinal contents and mucus in rainbow trout (Oncorhynchus mykiss). Journal of Applied Microbiology, 102: 1654-1664. DOI:10.1111/j.1365-2672.2006.03185.x (  0) 0) |
Knights, D., Kuczynski, J., Charlson, E. S., Zaneveld, J. R., Mozer, M. C., Collman, R. G., et al., 2011. Bayesian communitywide culture-independent microbial source tracking. Nature Methods, 8: 761-763. DOI:10.1038/nmeth.1650 (  0) 0) |
Kuang, T., He, A., Lin, Y., Huang, X., and Zhou, L., 2020. Comparative analysis of microbial communities associated with the gill, gut, and habitat of two filter-feeding fish. Aquaculture Reports, 18: 100501. DOI:10.1016/j.aqrep.2020.100501 (  0) 0) |
Larsen, A. M., Mohammed, H. H., and Arias, C. R., 2014. Characterization of the gut microbiota of three commercially valuable warmwater fish species. Journal of Applied Microbiology, 116: 1396-1404. DOI:10.1111/jam.12475 (  0) 0) |
Li, H., 2021. Connection between the gut microbiota of largemouth bass (Micropterus salmoides) and microbiota of the pond culture environment. Microorganisms, 9(8): 1770. DOI:10.3390/microorganisms9081770 (  0) 0) |
Liu, C., Zhao, L. P., and Shen, Y. Q., 2021. A systematic review of advances in intestinal microflora of fish. Fish Physiology and Biochemistry, 47: 2041-2053. DOI:10.1007/s10695-021-01027-3 (  0) 0) |
Liu, H., Guo, X., Gooneratne, R., Lai, R., Zeng, C., Zhan, F., et al., 2016. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Scientific Reports, 6: 24340. DOI:10.1038/srep24340 (  0) 0) |
Liu, R. Z., Wang, S., Huang, D. L., Huang, Y. L., He, T. L., and Chen, X. H., 2023. The probiotic roles of Lactiplantibacillus plantarum E2 as a dietary supplement in growth promotion and disease resistance of juvenile large yellow croaker (Larimichthys crocea). Aquaculture, 578: 740082. (  0) 0) |
Liu, Y. X., Chen, L., Ma, T., Li, X., Zheng, M., Zhou, X., et al., 2023. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. iMeta, 2: e83. DOI:10.1002/imt2.83 (  0) 0) |
Ljubobratovic, U., Kosanovic, D., Demény, F. Z., Krajcsovics, A., Vukotic, G., Stanisavljevic, N., et al., 2020. The effect of live and inert feed treatment with lactobacilli on weaning success in intensively reared pike-perch larvae. Aquaculture, 516: 734608. DOI:10.1016/j.aquaculture.2019.734608 (  0) 0) |
Lowrey, L. T., and Salinas, I., 2013. Sequencing the rainbow trout environmental and gut microbiome. Fish & Shellfish Immunology, 34: 1721. (  0) 0) |
Lu, J. H., Chen, C., Huang, C., and Lee, D. J., 2020. Glucose fermentation with biochar-amended consortium: Microbial consortium shift. Bioengineered, 11: 272-280. DOI:10.1080/21655979.2020.1735668 (  0) 0) |
Lyu, Z., 2021. Back to the source: Molecular identification of methanogenic archaea as markers of colonic methane production. Digestive Diseases and Sciences, 66: 3661-3664. DOI:10.1007/s10620-021-06839-0 (  0) 0) |
Martin, M., 2011. CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet Journal, 17(1): 10-12. DOI:10.14806/ej.17.1.200 (  0) 0) |
Meng, X., Hu, W., Wu, S., Zhu, Z., and Nie, G., 2019. Chinese yam peel enhances the immunity of the common carp (Cyprinus carpio L. ) by improving the gut defence barrier and modulating the intestinal microflora. Fish & Shellfish Immunology, 95: 528-537. (  0) 0) |
Mutuyemungu, E., Singh, M., Liu, S., and Rose, D. J., 2023. Intestinal gas production by the gut microbiota: A review. Journal of Functional Foods, 100: 105367. DOI:10.1016/j.jff.2022.105367 (  0) 0) |
Oksanen, J., Simpson, G., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P., et al., 2022. Vegan community ecology package version 2.6-2 April 2022.
(  0) 0) |
Øverli, Ø., Winberg, S., and Pottinger, T. G., 2005. Behavioral and Neuroendocrine correlates of selection for stress responsiveness in rainbow trout – A review. Integrative and Comparative Biology, 45: 463-474. DOI:10.1093/icb/45.3.463 (  0) 0) |
Pascual, J., Macian, M. C., Arahal, D. R., Garay, E., and Pujalte, M. J., 2009. Description of Enterovibrio nigricans sp. nov., reclassification of Vibrio calviensis as Enterovibrio calviensis comb. nov. and emended description of the genus Enterovibrio Thompson et al. 2002. International Journal of Systematic and Evolutionary Microbiology, 59: 698-704.
(  0) 0) |
Peng, S., Shi, Z., Fei, Y., and Peng, S., 2012. Population genetic structure and demographic history of Pampus argenteus in the Indo-West Pacific inferred from mitochondrial cytochrome b sequences. Biochemical Systematics & Ecology, 43: 54-63. (  0) 0) |
Pérez Martínez, Ó., Ross, L., Telfer, T., and Barquin, L. M., 2003. Water quality requirements for marine fish cage site selection in Tenerife (Canary Islands): Predictive modelling and analysis using GIS. Aquaculture, 224: 51-68. DOI:10.1016/S0044-8486(02)00274-0 (  0) 0) |
Pérez Sánchez, T., Balcazar, J., Ruiz-Zarzuela, I., Halaihel, N., Vendrell, D., de Blas, I., et al., 2010. Host-microbiota interactions within the fish intestinal ecosystem. Mucosal Immunology, 3: 355-360. DOI:10.1038/mi.2010.12 (  0) 0) |
Peter, M., Lv, H., Jiang, X., Liu, Y., and Chu, Z., 2020. Effects of starvation on enzyme activities and intestinal microflora composition in loach (Paramisgurnus dabryanus). Aquaculture Reports, 18: 100467. DOI:10.1016/j.aqrep.2020.100467 (  0) 0) |
Roccarina, D., Lauritano, E., Gabrielli, M., Franceschi, F., Ojetti, V., and Gasbarrini, A., 2010. The role of methane in intestinal diseases. The American Journal of Gastroenterology, 105: 1250-1256. DOI:10.1038/ajg.2009.744 (  0) 0) |
Ruiz, A., Gisbert, E., and Andree, K. B., 2024. Impact of the diet in the gut microbiota after an inter-species microbial transplantation in fish. Scientific Reports, 14: 4007. DOI:10.1038/s41598-024-54519-6 (  0) 0) |
Sa, R., Feng, C., Bai, H., Yin, X., Song, L., Hu, X., et al., 2022. Inhibitory effects of Mongolian medicine Yihe-Tang on continuous darkness induced liver steatosis in zebrafish. EvidenceBased Complementary and Alternative Medicine: eCAM, 2022: 1-12. (  0) 0) |
Sado, R. Y., Bicudo, Á. J., and Cyrino, J. E., 2014. Hematology of juvenile pacu, Piaractus mesopotamicus (Holmberg, 1887) fed graded levels of mannan oligosaccharides (MOS). Latin American Journal of Aquatic Research, 42: 30-39. DOI:10.3856/vol42-issue1-fulltext-3 (  0) 0) |
Shi, C., Xia, M., Li, R., Mu, C., Zhang, L., Liu, L., et al., 2019. Vibrio alginolyticus infection induces coupled changes of bacterial community and metabolic phenotype in the gut of swimming crab. Aquaculture, 499: 251-259. DOI:10.1016/j.aquaculture.2018.09.031 (  0) 0) |
Srivastava, A., Prabhakar, M. R., Mohanty, A., and Meena, S. S., 2021. Influence of gut microbiome on the human physiology. Systems Microbiology and Biomanufacturing, 2: 217-231. (  0) 0) |
Stanley, H., Onianwah, F., and Oyakhire, M., 2018. Microorganisms in aquaculture development. Global Advanced Research Journal of Microbiology, 7: 127-131. (  0) 0) |
St-Pierre, B., Cersosimo, L., Ishaq, S., and Wright, A. D., 2015. Toward the identification of methanogenic archaeal groups as targets of methane mitigation in livestock animals. Frontiers in Microbiology, 6: 00776. (  0) 0) |
Sun, B., Liu, M., Tang, L., Hu, C., Huang, Z., Zhou, X., et al., 2021. Probiotic supplementation mitigates the developmental toxicity of perfluorobutanesulfonate in zebrafish larvae. Science of the Total Environment, 799: 149458. DOI:10.1016/j.scitotenv.2021.149458 (  0) 0) |
Sun, D., Ge, Y., and Cheng, Q., 2019. Genetic diversity of eight wild populations of Pampus argenteus along the coast of China inferred from fifteen polymorphic microsatellite markers. Brazilian Journal of Oceanography, 67: e19251. (  0) 0) |
Sun, H. C., Geng, X. X., Zhang, F., Li, H., and Chai, J., 2007. Immunogenecity and protective effects of an oral vaccine microparticle against Aeromonas punctata f. intestinalis in grass carp (Ctenopharyngodon idella). Journal of Southwest University, 29: 115-118. (  0) 0) |
Takahashi, H., Miya, S., Kimura, B., Yamane, K., Arakawa, Y., and Fujii, T., 2008. Difference of genotypic and phenotypic characteristics and pathogenicity potential of Photobacterium damselae subsp. damselae between clinical and environmental isolates from Japan. Microbial Pathogenesis, 45: 150-158. (  0) 0) |
Tran, N. T., Zhang, J., Xiong, F., Wang, G. T., Li, W. X., and Wu, S. G., 2018. Altered gut microbiota associated with intestinal disease in grass carp (Ctenopharyngodon idellus). World Journal of Microbiology and Biotechnology, 34: 71. (  0) 0) |
Wang, A., Zhang, Z., Ding, Q., Yang, Y., Bindelle, J., Ran, C., et al., 2021. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes, 13: 1-15. (  0) 0) |
Wang, J., Yin, L., Zheng, W., Shi, S., Hao, W., Liu, C., et al., 2022. Lactobacillus rhamnosus GG normalizes gut dysmotility induced by environmental pollutants via affecting serotonin level in zebrafish larvae. World Journal of Microbiology and Biotechnology, 38: 222. (  0) 0) |
Wen, T., Xie, P., Yang, S., Niu, G., Liu, X., Ding, Z., et al., 2022. ggClusterNet: An R package for microbiome network analysis and modularity-based multiple network layouts. iMeta, 1: e32. (  0) 0) |
Wold, P. A., Holan, A., Øie, G., Attramadal, K., Bakke, I., Vadstein, O., et al., 2014. Effects of membrane filtration on bacterial number and microbial diversity in marine recirculating aquaculture system (RAS) for Atlantic cod (Gadus morhua L. ) production. Aquaculture, 422-423: 69-77. (  0) 0) |
Wu, F., Tang, K., Yuan, M., Shi, X., Shakeela, Q., and Zhang, X. H., 2015. Studies on bacterial pathogens isolated from diseased torafugu (Takifugu rubripes) cultured in marine industrial recirculation aquaculture system in Shandong Province, China. Aquaculture Research, 46: 736-744. (  0) 0) |
Xia, M. J., Feng, P., Mu, C. K., Ye, Y. F., and Wang, C. L., 2018. Disruption of bacterial balance in the gut of Portunus trituberculatus induced by Vibrio alginolyticus infection. Journal of Oceanology and Limnology, 36(5): 1891-1898. (  0) 0) |
Xiong, J. B., Nie, L., and Chen, J., 2019. Current understanding on the roles of gut microbiota in fish disease and immunity. Zoological Research, 40: 1-7. (  0) 0) |
Yan, Q., Li, J., Yu, Y., Wang, J., He, Z., Van Nostrand, J. D., et al., 2016. Environmental filtering decreases with fish development for the assembly of gut microbiota. Environmental Microbiology, 18: 4739-4754. (  0) 0) |
Yang, G., Yin, Y., and Wang, J., 2019a. Microbial community diversity during fermentative hydrogen production inoculating various pretreated cultures. International Journal of Hydrogen Energy, 44: 13147-13156. (  0) 0) |
Yang, Q., Huang, X., Zhao, S., Sun, W., Yan, Z., Wang, P., et al., 2017. Structure and function of the fecal microbiota in diarrheic neonatal piglets. Frontiers in Microbiology, 8: 00502. (  0) 0) |
Yang, W. Y., Lee, Y., Lu, H., Chou, C. H., and Wang, C., 2019b. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS One, 14: e0205784. (  0) 0) |
Yu, J. Y, Sun, J. G., Sun, M., Li, W. D., Qi, D. M., Zhang, Y. Q., et al., 2024. Protective mechanism of Coprinus comatus polysaccharide on acute alcoholic liver injury in mice, the metabolomics and gut microbiota investigation. Food Science and Human Wellness, 13: 401-413. (  0) 0) |
Zhang, B., Yang, H., Cai, G., Nie, Q., and Sun, Y., 2024a. The interactions between the host immunity and intestinal microorganisms in fish. Applied Microbiology and Biotechnology, 108: 30. (  0) 0) |
Zhang, H., Ding, Q., Wang, A., Liu, Y., and Zhou, Z., 2020a. Effects of dietary sodium acetate on food intake, weight gain, intestinal digestive enzyme activities, energy metabolism and gut microbiota in cultured fish: Zebrafish as a model. Aquaculture, 523: 735188. (  0) 0) |
Zhang, M., Hu, J. B., Wang, Y. J., Le, Q. J., Xu, S. L., Ying, X. Y., et al., 2020. Correlation between gut microbial diversity and flatulence of silver pomfret (Pampus argenteus) during ontogenesis. Aquaculture Research, 51: 3139-3153. (  0) 0) |
Zhang, Y. Y., Hu, J. B., Yan, K. H., Yuan, F., Li, Y., Zhang, M., et al., 2024b. Immune response of silver pomfret (Pampus argenteus) to Photobacterium damselae subsp. Damselae: Virulence factors might induce immune escape by damaging phagosome. Aquaculture, 578: 740014. (  0) 0) |
Zhang, Z., Yu, Y. X., Jiang, Y., Wang, Y. G., Liao, M. J., Rong, X. J., et al., 2020b. The intestine of artificially bred larval turbot (Scophthalmus maximus) contains a stable core group of microbiota. Archives of Microbiology, 202: 2619-2628. (  0) 0) |
Zheng, A., Yi, H., Li, F., Han, L., and Li, J., 2019. Changes in gut microbiome structure and function of rats with isoproterenolinduced heart failure. International Heart Journal, 60: 1176-1183. (  0) 0) |
Zheng, D., 2020. Effect of intestinal gas bubble accumulation on the metabolizing enzymes in four tissues of silver pomfret. Open Journal of Fisheries Research, 7: 77-83. (  0) 0) |
Zheng, D., Wang, Q., Wang, L., Shi, Z. H., and Peng, S. M., 2020. Effects of the intestinal gas bubble accumulation on intestinal bacterial communitis in silver pomfret. Chinese Journal of Zoology, 55: 247-255. (  0) 0) |
Zheng, X. B., Du, C., Gao, X. M., Ni, J. J., Wang, Y. J., Hou, C. C., et al., 2023. Changes in the histology and digestive enzyme activity during digestive system development of silver pomfret (Pampus argenteus). Aquaculture, 577: 739905. (  0) 0) |
 2025, Vol. 24
2025, Vol. 24


