2) Department of Environmental Science and Engineering, Xiamen University Tan Kah Kee College, Zhangzhou 363105, China;
3) Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, China;
4) South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou 510300, China;
5) Laboratory of Marine Biology and Ecology, Third Institute of Oceanography, Ministry of Natural Resources, Xiamen 361005, China
Horseshoe crabs are benthic macrofauna and are from an ancient arthropod lineage. From the late 1950s to the 1990s, the Chinese horseshoe crab, Tachypleus tridentatus, was found in the benthic macrofaunal communities in the silt intertidal zone of Xiamen Island (Zeng et al., 1996). Horseshoe crabs play an important role in the marine food chain. They are both predators and prey in the benthic ecosystem. They feed on plants, animals, and debris, but their eggs and juveniles are food sources for birds and other large marine animals (Chen et al., 2015).
In the coastal areas of China, the habitat preferences of horseshoe crabs have been noted by researchers. Horseshoe crabs prefer to live in small sheltered coves without waves and hibernate on the seafloor (Weng et al., 2012; Chen et al., 2015). There are many mud and sand beaches in the west of Haitan Island. It is the largest horseshoe crabproducing area in the history of Pingtan, but human activities have caused decline of the Chinese horseshoe crab population around Pingtan Island (Huang et al., 2003). Tachypleus tridentatus and Carcinoscorpius rotundicauda are known to occur in Hong Kong (Chiu and Morton, 2003). Elsewhere, in the intertidal zones of Xibeiling, Beihai, Guangxi region of China, the number of horseshoe crab larvae has increased in areas close to the mangrove communities (Li and Hu, 2011). Horseshoe crabs were also once widely distributed to the south of the Yangtze River Estuary and the vast Chinese sea area up to the Beibu Gulf. The sandy beach and the bay with calm wind and waves were the habitats of the horseshoe crab. These sheltered bays and islands include Zhoushan Islands, Taizhou Bay, Sanmen Bay, Wenzhou Bay in Zhejiang Coast, and Haitan Island, Xinghua Bay, Meizhou Bay, Xiamen Bay, Futou Bay, Liu'ao Peninsula, Dongshan Island in Fujian Coast, and Nan'ao Island, Red Bay, Chuanshan Islands, Leizhou Bay at Guangdong Coast, and Beihai at Guangxi Coast (Weng et al., 2012).
Intertidal zones around Xiamen Island, and along the Jimei Coast and Tong'an Bay have sandy beaches, which are very suitable habitats for the breeding of horseshoe crabs (Weng and Hong, 2001). There used to be a large number of Chinese horseshoe crabs in the seas near Xiamen. However, due to land reclamation, mariculture, and widespread hunting, as well as the deterioration of seawater quality and sediment environment, horseshoe crab populations in the seas near Xiamen have declined sharply (Chen, 2009). Due to the influence of other human activities such as oyster farming and pond fish farming, populations of Tachypleus tridentatus and its habitat in the intertidal zone of Eyu islet (Crocodile Island) in Xiamen are small (Cai et al., 2021).
Although many studies have focused on the population resources and ecological habits of horseshoe crabs in China, the relationship between horseshoe crabs and their habitats, and the differences between the benthic macrofaunal communities in intertidal zones with and without horseshoe crab presence have not been studied systematically. Therefore, we undertook this study in order to explore the main environmental factors affecting horseshoe crab populations and to determine what benthic macrofauna, including prey, often co-exist with horseshoe crabs. This study includes the following components. 1) The differences of benthic macrofaunal communities with and without horseshoe crab presence in the Crocodile Island intertidal zone, Xiamen. 2) Distribution characteristics of dominant species of benthic macrofauna in the Crocodile Island intertidal zone, Xiamen. 3) The difference in sedimentary environmental factors as ociated with and without horseshoe crab presence in the Crocodile Island intertidal zone, Xiamen.
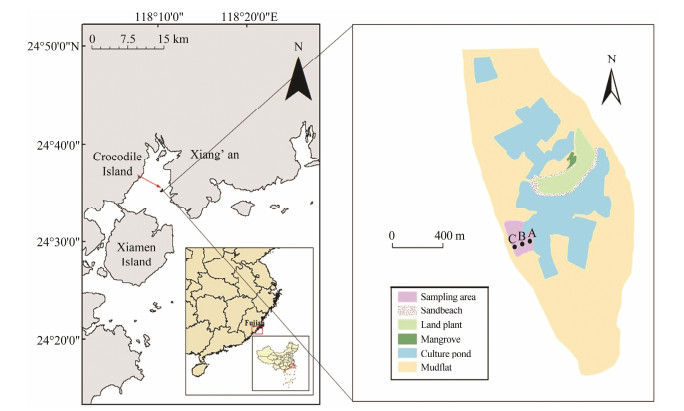
2 Materials and Methods 2.1 Study SitesCrocodile Island, also known as Eyuyu or White Island, is the only uninhabited island with fresh groundwater in Xiamen. The island is named for its shape that resembles a crocodile. Crocodile Island is 0.61 km long and 0.21 km wide, with an area of 0.13 km2. Water depths range from 0.3 m to 3.1 m around the island. The waters around Crocodile Island were once teeming with amphioxus but now are occupied by oyster farms. According to satellite telemetry obtained in 2015, the areas of sandy beach, mudflat, mangroves, and culture ponds in the Crocodile Island intertidal zone are 1.8 × 10−2, 127.4 × 10−2, 0.5 × 10−2, and 45.8 × 10−2 km2, respectively (Fig.1).
|
Fig. 1 The map of the Xiamen and Crocodile Island region. The red arrow points to the location of Crocodile Island, Xiamen. Site A, site B, and site C are located in the southwest of Crocodile Island. |
Benthic macrofauna were collected quantitatively at three sampling sites, sites A, B, and C, in the Crocodile Island intertidal zone in October 2018 (autumn), January 2019 (winter), April 2019 (spring), and July 2019 (summer). Horseshoe crabs were found at sampling site A, but no horseshoe crabs were observed at sampling sites B and C (Cai et al., 2021). Consequently, we considered site A to be a horseshoe crab area and sites B and C to be non-horseshoe crab areas. The height of tide at sites A, B, and C are 270 cm, 225 cm, and 180 cm respectively. Site A has a high content of sand, site B has a high content of silt and covering many oyster stones, while site C also has a high content of silt but more clay than site B.
For benthic macrofaunal samples, five quadrats (25 cm × 25 cm × 30 cm depth) were collected at each sampling site. Sediments including benthic macrofauna at each quadrat were washed through a 0.5 mm mesh size sieve. Benthic macrofauna and debris were packed into plastic bottles and fixed in 7% formalin. Benthic macrofauna and debris were sorted and identified to the species level under a stereoscopic microscope at the laboratory. Identified benthic macrofauna were numbered and weighed with an electronic balance (minimum sensitivity was 0.1 mg).
2.3 Sedimentary Factor DeterminationThree replicate samples for each site were assayed for sediment grain size using a Mastersizer 2000 particle size analyser with a relative error of < 3%. The four particle size ranges were gravel (> 2000 μm), sand (50 – 2000 μm), silt (2 – 50 μm), and clay (< 2 μm). The metal concentrations of Cr (chromium), Co (cobalt), and Ni (nickel) were determined by inductively coupled plasma mass spectrometry (Agilent 7700x, Agilent Technologies, USA). Quality assurance was determined with the procedure of Wang et al. (2019). Organic carbon and organic nitrogen quantification was detected using a Vario EL Ⅲ Elemental Analyzer. The samples were treated with dilute hydrochloric acid and burned in a pure oxygen environment (960 – 970℃) under static conditions. The organic carbon and organic nitrogen in the samples were oxidized to CO2 and NOX. CO2 and NOX were measured by a thermal conductivity detector with helium as a carrier gas, and the measured signal was used to calculate the content of organic carbon and organic nitrogen. Polycyclic aromatic hydrocarbons (PAHs) such as Phenanthrene, Fluoranthene, and Pyrene in sediments were detected with Gas Chromatography-Mass Spectrometry (Agilent 7890B – 7000C). Injection temperature was 280℃, no shunt, with 1 mL min−1 constant flow. The initial column temperature was kept at 80℃ for 2 min, rose to 180℃ for 5 min at 20℃ min−1, and rose to 290℃ for 5 min at 10℃ min−1. All the above metals and PAHs were determined for three replicate samples at each site.
2.4 Statistical AnalysisWe calculated the species number, density, and biomass of benthic macrofauna, Shannon-Wiener diversity index (Hˊ using log2), Pielou's evenness index (Jˊ), Margalef's richness index (d), AZTI's Marine Biotic Index (AMBI), Multivariate AZTI's Marine Biological Index (M-AMBI), and Macrozoobenthos Pollution Index (MPI) (Cai, 2003). Variance analysis (ANOVA) and correlation analysis were performed using SPSS v22 software. ANOVA was used to determine whether there were significant differences in the community parameters of benthic macrofauna in different seasons and sites. Correlation analysis was used to determine whether the community parameters of benthic macrofauna and densities of common benthic macrofauna were significantly correlated with sedimentary environmental factors. Cluster, Non-metric multidimensional scaling (NMDS), and BIOENV analyses were run in PRIMER v7 (Anderson et al. 2008). Similarities of benthic macrofauna between each pair of sites were determined using the Bray-Curtis similarity measure based on the fourth root transformed abundance data. NMDS ordination based on the Bray-Curtis similarity was performed to explore the season and site variation of the macrofaunal community. BIOENV analyses examined the major environmental factors affecting the benthic macrofaunal community.
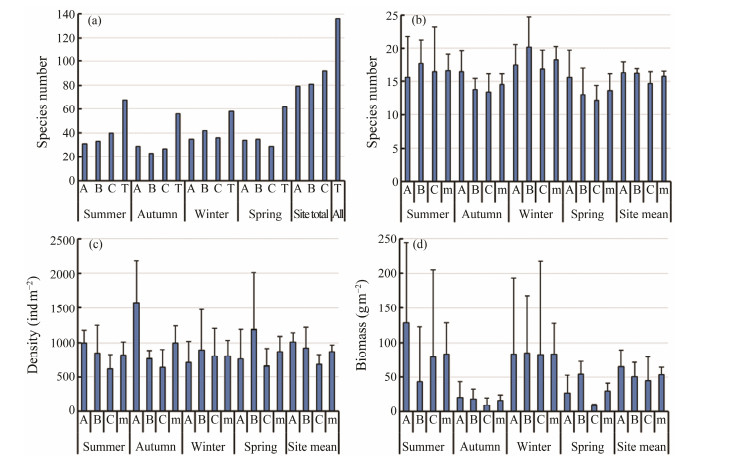
3 Results 3.1 Species Number, Density, and Biomass of Benthic Macrofauna at Three Sites in the Crocodile Island Intertidal Zone over Four SeasonsA total of 136 species of benthic macrofauna were obtained in the three sampling sites over four seasons in the Crocodile Island intertidal zone. The species number of benthic macrofauna was the highest in summer (68), followed by those in spring (62), winter (58), and autumn (52). The species number of benthic macrofauna in sampling site C was the largest (92), followed by those in sampling site B (81) and sampling site A (79). The number of benthic macrofauna species tends to increase with decreasing tide levels (Fig.2a). The average number of benthic macrofauna species among all sites was the highest in winter (18), followed by those in summer (17), autumn (15), and spring (14). The average species number of benthic macrofauna by sampling site was the highest at A (16), followed by those at sampling site B (16) and sampling site C (15). The number of benthic macrofauna species tends to decrease with decreasing tide levels (Fig.2b).
|
Fig. 2 Species number, density, and biomass of benthic macrofauna at three sites at the Crocodile Island intertidal zone over four seasons. The relevant data are presented as mean ± SD. (a), the number of benthic macrofauna species at each site over four seasons, and the total number of benthic macrofauna species in each season; (b), the average number of benthic macrofauna species by sampling site; (c), the average density of benthic macrofauna in three sampling sites over four seasons; (d), the average biomass of benthic macrofauna in three sampling sites over four seasons. m, the average value. |
The average density of benthic macrofauna in three sampling sites over four seasons in the intertidal zone is (884 ± 95) ind m−2. The average density of benthic macrofauna is the highest in autumn at (996 ± 248) ind m−2, followed by those in spring at (870 ± 219) ind m−2, in summer at (825 ± 184) ind m−2, and the lowest in winter at (805 ± 228) ind m−2. The average density of benthic macrofauna is the highest at sampling site A with (1012 ± 126) ind m−2, at site B it is the second with (927 ± 291) ind m−2, and at site C it is the lowest with (683 ± 146) ind m−2, which shows a tendency to decrease as the tide level decreases (Fig.2c).
The average biomass of benthic macrofauna in three sampling sites over four seasons in the intertidal zone of Crocodile Island is (54.43 ± 10.72) g m−2. The average biomass of benthic macrofauna is the highest in winter with (84.31 ± 43.92) g m−2, followed by that in summer with (83.98 ± 45.09) g m−2, in spring with (29.96 ± 11.32) g m−2, and in autumn it is the lowest with (15.89 ± 8.03) g m−2. The average density of benthic macrofauna is the highest at site A with (65.16 ± 24.54) g m−2, at site B it is the second with (50.42 ± 21.11) g m−2, and at site C it is the lowest with (45.02 ± 34.57) g m−2, which shows a tendency to decrease as the tide level decreases (Fig.2d).
Two-way ANOVA tests on the species number, density, and biomass of benthic macrofauna, the species number and biomass of benthic macrofauna show significant seasonal variation. The density of benthic macrofauna is significantly different by the site, but it is only marginally significant (P = 0.049). Species number and biomass of benthic macrofauna are not significantly different by the site (Table 1).
|
|
Table 1 F and P values between seasons and sites for community parameters and biotic indices of benthic macrofauna in the intertidal zone of Crocodile Island |
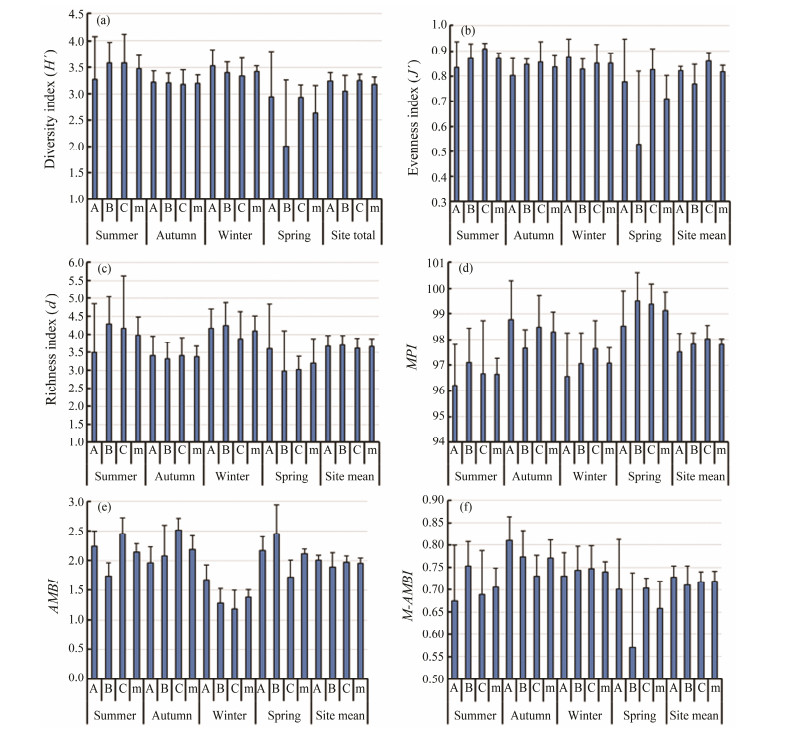
The average value of the Shannon-Wiener diversity index (Hˊ) of the macrozoobenthic community in Crocodile Island intertidal zone is 3.182, the average value of Pielou's evenness index (Jˊ) is 0.817, and the average value of Margalef's richness index (d) is 3.665, the average value of Macrozoobenthos Pollution Index (MPI) is 97.791, the average value of AZTI's Marine Biological Index (AMBI) is 1.957, and the average value of Multivariate AZTI's Marine Biological Index (M-AMBI) is 0.719 (Fig.3).
|
Fig. 3 Six biotic indices at three sites in the Crocodile Island intertidal zone over four seasons. Biotic indices data are presented as mean ± SD. |
A two-way ANOVA test shows that the Shannon-Wiener diversity index (Hˊ), Pielou's evenness index (Jˊ), Margalef's richness index (d), MPI, AMBI, and M-AMBI show significant seasonal changes. Jˊ shows significant site variation, but it is only marginally significant (P = 0.050). Hˊ, d, MPI, AMBI, and M-AMBI show no significant site variation (Table 1).
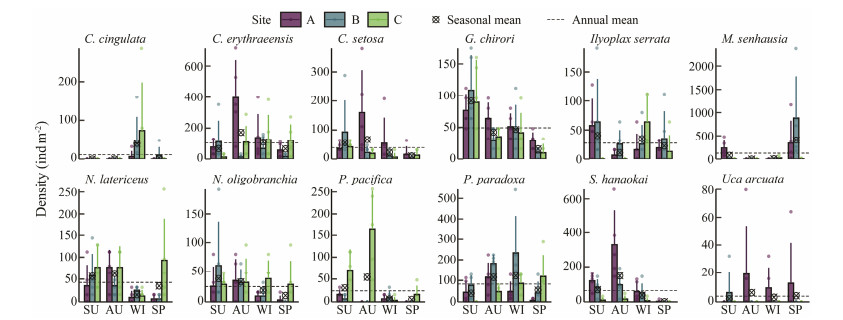
3.3 Density of Twelve Benthic Macrofauna Species at Three Sites in the Crocodile Island Intertidal Zone over Four SeasonsThe mean densities of Ceratonereis erythraeensis, Sigambra hanaokai, Chaetozone setosa, Glycera chirori, and Uca arcuata at site A were higher than those at site B and site C. The mean densities of Paralacydonia paradoxa, Musculista senhausia, and Ilyoplax serrata at site B were higher than those at site A and site C. The mean densities of Prionospio pacifica, Notomastus latericeus, Nephtys oligobranchia, and Cerithidea cingulate at site C were higher than those at site A and site B (Fig.4).
|
Fig. 4 The densities of twelve benthic macrofauna species at three sites in the Crocodile Island intertidal zone over four seasons. Density data are presented as mean ± SD. SU, summer; AU, autumn; WI, winter; SP, spring. The twelve benthic macrofauna are Cerithidea cingulata, Ceratonereis erythraeensis, Chaetozone setosa, Glycera chirori, Ilyoplax serrata, Musculista senhausia, Notomastus latericeus, Nephtys oligobranchia, Prionospio pacifica, Paralacydonia paradoxa, Sigambra hanaokai, Uca arcuata. |
A two-way ANOVA test were used to analyze the density of the twelve benthic macrofauna species. The densities of Ceratonereis erythraeensis, Chaetozone setosa, Glycera chirori, Paralacydonia paradoxa, Prionospio pacifica, Sigambra hanaokai, Notomastus latericeus, Musculista senhausia, and Cerithidea cingulata showed significant seasonal variation. The densities of Ceratonereis erythraeensis, Paralacydonia paradoxa, Prionospio pacifica, Sigambra hanaokai, and Notomastus latericeus showed significant site variation (Table 2).
|
|
Table 2 F and P values between seasons and sites for density of twelve benthic macrofauna species in the Crocodile Island intertidal zone |
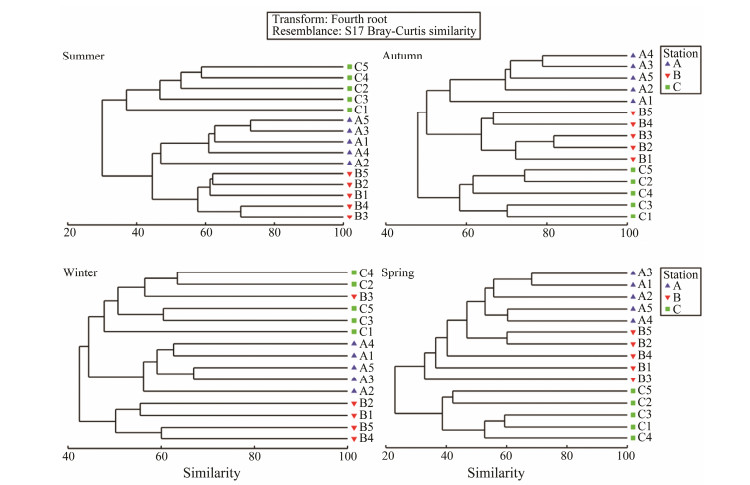
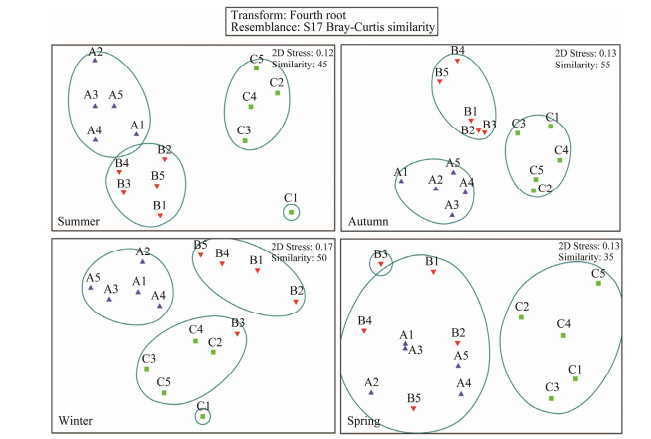
Cluster analysis showed that the Crocodile Island intertidal zone could be divided into three benthic macrofaunal communities in summer, autumn, and winter, and two benthic macrofaunal communities in spring (Fig.5). NMDS analysis results were similar to cluster analysis results (Fig.6).
|
Fig. 5 Cluster analysis showing the similarity of living sites of benthic macrofaunal community in the Crocodile Island intertidal zone over four seasons. 1 – 5, the sample number. |
|
Fig. 6 NMDS analysis showing the similarity of living sites of benthic macrofaunal community in the Crocodile Island intertidal zone over four seasons. 1 – 5, the sample number. |
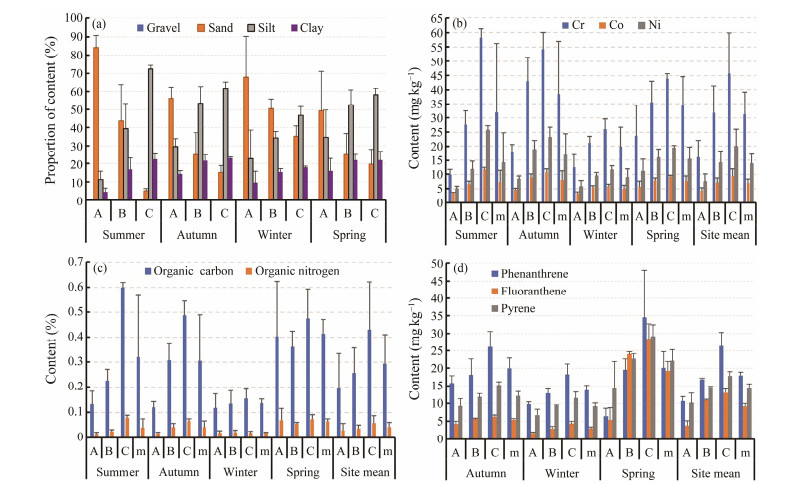
No gravel was collected at the three sites in the Crocodile Island intertidal zone in any of the four seasons. The percentage of sand content decreased from site A to site B to site C in all four seasons. The percentage of silt contents increased from site A to site B to site C in all four seasons. Except in spring, the percentage of clay contents increased from site A to site B to site C in autumn, winter, and summer (Fig.7a).
|
Fig. 7 Spatiotemporal variations of abiotic parameters in the Crocodile Island intertidal zone. (a), sediment grain size; (b), contents of Cr, Co, and Ni; (c), contents of organic carbon, organic nitrogen; (d), contents of phenanthrene, fluoranthene, and pyrene. |
The concentrations of Cr, Co, and Ni increased from site A to site B to site C over four seasons (Fig.7b). The organic carbon content increased from site A to site B to site C in summer, autumn and winter. The contents of organic nitrogen increased from site A to site B to site C in summer and autumn (Fig.7c). The contents of phenanthrene, fluoranthene, and pyrene increased from site A to site B to site C in autumn, winter, and spring (Fig.7d).
Two-way ANOVA test showed that the contents of sand, silt, clay, Cr, Co, Ni, organic carbon, organic nitrogen, phenanthrene, fluoranthene, and pyrene showed significant seasonal and site changes (Table 3).
|
|
Table 3 F and P values between seasons and sites for environmental parameters in the Crocodile Island intertidal zone, Xiamen |
There was a significant negative correlation between the densities of P. pacifica and N. latericeus and sand content, but there was no significant correlation between the densities of the other ten common benthic macrofauna and sand content. There was a significant negative correlation between the density of P. pacifica and the contents of phenanthrene, fluoranthene, and pyrene. There was a significant positive correlation between the densities of P. pacifica and N. latericeus and the contents of silt, Cr, Co, Ni, organic carbon. There was a significant negative correlation between the density of S. hanaokai and the contents of organic carbon and organic nitrogen fractions (Table 4).
|
|
Table 4 Correlation analyses matrix between densities of common benthic macrofauna and sediment environmental factors in the intertidal zone of Crocodile Island |
There was a significant negative correlation between the content of sand and the contents of silt, clay, Cr, Co, Ni, organic carbon, organic nitrogen, phenanthrene, fluoranthene, and pyrene, and a significant positive correlation among the contents of silt, clay, Cr, Co, Ni, organic carbon, organic nitrogen, phenanthrene, fluoranthene, and pyrene (Table 5).
|
|
Table 5 Correlation analyses among sediment environmental factors in the intertidal zone of Crocodile Island |
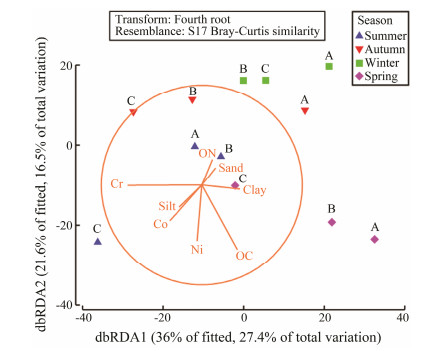
Because the phenanthrene, fluoranthene, and pyrene data were only available in three seasons, only eight environmental parameters, including sand, silt, and clay content, Cr, Co, Ni, organic carbon, organic nitrogen were available for the BIOENV analysis, which revealed that Co and organic carbon were the most important environmental factors (R = 0.438) correlated with the benthic macrofaunal community in the Crocodile Island intertidal zone. The dbRDA graph reflects the site differences among seasons in the Crocodile Island intertidal zone. Axis 1 explains 36.0% of the variation and axis 2 explains 21.6% of the variation (Fig.8).
|
Fig. 8 dbRDA plot of the relationships between benthic macrofaunal community and environmental factors. |
Cluster and NMDS analysis both showed that the Crocodile Island intertidal zone could be divided into three benthic macrofaunal communities in summer, autumn and winter, and two benthic macrofaunal communities in spring. Ceratonereis erythraeensis was the dominant benthic macrofauna at site A over four seasons. Glycera chirori was the dominant benthic macrofauna at site A in summer, winter and spring. Musculista senhausia was dominant benthic macrofauna at site A in summer and spring. Sigambra hanaokai was dominant at site A in autumn. Batillaria zonalis was dominant at site A in winter (Cai et al., 2021). The mean densities of Ceratonereis erythraeensis, Sigambra hanaokai, Chaetozone setosa, Glycera chirori, and Uca arcuata at site A were higher than those at site B and site C. The above results showed that the dominant species of benthic macrofaunal communities at site A in the Crocodile Island intertidal zone were polychaetes, bivalves, and crustaceans.
Juvenile horseshoe crabs are selective benthic feeders and subsist mainly on insect larvae, polychaetes, oligochaetes, small crabs, and thin-shelled bivalves (Zhou and Morton, 2004). The Indian horseshoe crab, Tachypleus gigas, is a benthic feeder which preys on mollusks, decays organic matter and polychaetes (Chatterji et al., 1992). Evidence of the δ13C and δ15N values in tissues of juvenile Chinese horseshoe crabs and their potential food sources suggested that the juveniles consumed a mixed diet mainly comprised a variety of polychaetes, crustaceans, and bivalves (Kwan et al., 2015). Polychaetes, crustaceans, bivalves, and others are available on muddy intertidal zones, which is a favorite habitat of juvenile horseshoe crabs (Hu et al., 2014; Kwan et al., 2015). We presume that polychaetes, especially the clamworm Ceratonereis erythraeensis, are therefore an important food source for horseshoe crabs in the Crocodile Island intertidal zone.
4.2 Sedimentary Environmental Characteristics with and Without Horseshoe Crab Presence in the Crocodile Island Intertidal Zone, XiamenOur results showed that the mean contents of Cr at sites A, B, C were 16.05, 31.83, and 45.57 mg kg−1 respectively, which indicates that the mean content of Cr at site with horseshoe crab (site A) was lower than that without horseshoe crab (sites B and C). In addition to Cr content, the contents of other metals such as Mg, Fe, Al, Li, Cu, Zn, and Pb were lower at the site with horseshoe crab (Fu, 2021). The mean content in the intertidal zone of Crocodile Island (31 mg kg−1) was lower than that in the surface sediments in western Xiamen Bay (75 mg kg−1). The Cr concentration in the surface sediments in western Xiamen Bay was below the Chinese National Standard of Marine Sediment Quality criteria (Zhang et al., 2007). The mean content of Cr at site A (16.05 mg kg−1) at Crocodile Island was lower than the historical mean (29 mg kg−1) (Dong et al., 2018). Cr is not considered as a significant influence on the substrate in the Xiamen sea area (Li et al., 2009). According to the Hˊ, MPI, AMBI, and M-AMBI analyses, the sedimentary environment in the Crocodile Island intertidal zone is slightly disturbed or undisturbed (Peng, 2021). This is consistent with good water quality and habitat conditions that are essential for horseshoe crabs (Hu et al., 2014; Kwan et al., 2015).
The distribution of different types of sediments and the variation of metal concentration are mainly affected by hydrodynamic conditions (Qu et al., 2004; Li et al., 2007a, b). Crocodile Island is located at the mouth of Tong'an Bay with strong hydrodynamic forcing and no fine sediment deposition.
Horseshoe crab juveniles have been recorded from sand and sandy-mud nursery beaches at Pak Nai (western New Territories), and San Tau and Shui Hau (Lantau Island), Hong Kong (Chiu and Morton, 2003). Horseshoe crab lives mostly in the bay where the wind and waves are relatively calm and the beach is sandy to muddy, for that juvenile horseshoe crab like to construct the habitat by drilling sand or mud (Weng et al., 2008). Our results showed that the density of polychaetes at the horseshoe crab presence site was higher than that at the site without horseshoe crab presence, which suggested that polychaetes prefer the higher 60% sand content. On the other hand, a high density of polychaetes in the northern Taiwan Strait was observed in a sedimentary environment with a silt content of about 60% (Sun and Chen, 1988).
In conclusion, the favorable sediment environment in the Crocodile Island intertidal zone is favorable for benthic macrofauna to inhabit. The grain size of sediment affected the community structure of benthic macrofauna but did not affect the quantity of benthic macrofauna in the Crocodile Island intertidal zone, Xiamen.
AcknowledgementsThis work was supported by the National Key Research and Development Program of China (No. 2016YFC0502 904). The authors thank Ms. Jinglin Liu, Mrs. Bingwen Chen, Zhangjian Zeng, Yiwei Jiang, Zhengwu Huyou and Wenhai Wang for their assistance in sample collection.
Anderson, M. J., Gorley, R. N., and Clarke, K. R.. 2008. PERMANOVA + for PRIMER: Guide to software and statistical methods. PRIMER-E, Plymouth, UK.
(  0) 0) |
Cai, L. Z.. 2003. Macrobenthos pollution index (MPI). Acta Scientiae Circumstantiae, 23(5): 625-629 (in Chinese with English abstract). (  0) 0) |
Cai, L. Z., Chen, X. W., Fu, S. J., Yang, D. Y., and Zhao, X. Y.. 2021. Population dynamics and benthic environment of Tachypleus tridentatus in the intertidal zone of Eyu Islet in Xiamen. Wetland Science & Management, 17(1): 14-18 (in Chinese with English abstract). (  0) 0) |
Chatterji, A., Mishra, J. K., and Parulekar, A. H.. 1992. Feeding behaviour and food selection in the horseshoe crab, Tachypleus gigas (Müller). Hydrobiologia, 246: 41-48. DOI:10.1007/BF00005621 (  0) 0) |
Chen, Q. M.. 2009. Research and the protection for the population of Chinese horseshoe crabs in Xiamen. Environmental Science and Management, 34(6): 9-11 (in Chinese with English abstract). (  0) 0) |
Chen, Z. B., Fan, H. Q., Liao, Y. Y., Qiu, Y. L., Xie, H. L., and Lin, W. Y.. 2015. Living fossil of animals facing survival dilemma – Horseshoe crabs. Science, 67(3): 60-62 (in Chinese). (  0) 0) |
Chiu, H. M., and Morton, B.. 2003. The sediment and hydrographic characteristics of three horseshoe crab nursery beaches in Hong Kong. Journal of Ocean University of Qingdao, 2: 35-43. DOI:10.1007/s11802-003-0023-2 (  0) 0) |
Dong, W. F., Zhang, Y., Dai, G. X., Su, R., Yuan, C. W., and Liu, Z. Y.. 2018. Spatial and temporal distribution and integrated ecological risk assessment of heavy metals in the surface sediments from Xiamen Sea area over past 40 years. Journal of Marine Science, 36(3): 89-95 (in Chinese with English abstract). (  0) 0) |
Fu, S. J., 2021. Ecology of meiofaunal assemblages in the intertidal zone along the southeastern coasts of China and notes of new species and new record species of marine nematodes. PhD thesis. Xiamen University (in Chinese with English abstract).
(  0) 0) |
Hu, M. H., Wang, Y. J., Cheung, S. G., and Shin, K. S.. 2014. Digestible dietary protein and energy requirements of juvenile Asian horseshoe crabs, Tachypleus tridentatus and Carcinoscorpius rotundicauda. Aquaculture Research, 45(10): 1621-1633. DOI:10.1111/are.12109 (  0) 0) |
Huang, Q., Lin, N. F., Gao, Y. S., You, H., and Lai, X. X.. 2003. The analysis of the reasons for the population reduces of Chinese horseshoe crabs in Pingtan. Fujian Environment, 20(1): 7-8 (in Chinese with English abstract). (  0) 0) |
Kwan, B. K. Y., Cheung, S. G., and Shin, P. K. S.. 2015. A dual stable isotope study for diet composition of juvenile Chinese horseshoe crab Tachypleus tridentatus (Xiphosura) on a seagrass-covered intertidal mudflat. Marine Biology, 162: 1137-1143. DOI:10.1007/s00227-015-2647-3 (  0) 0) |
Li, G. H., Gao, Z. M., Lan, D. Z., Xu, J., Wang, S. S., and Yin, W. H.. 2007a. Spatial variations in grain size distribution and selected metal contents in the Xiamen Bay, China. Environmental Geology, 52: 1559-1567. DOI:10.1007/s00254-006-0600-y (  0) 0) |
Li, G. H., Lan, D. Z., Cao, Z. M., Xu, J., Wang, S. S., and Lan, B. B.. 2007b. Specificity and potential ecological risks of heavy metals in the sediments of Xiamen Sea area. Journal of Marine Science Bulletin, 26(1): 67-72 (in Chinese with English abstract). (  0) 0) |
Li, Q. Z., Li, G. X., Luo, Z. X., Zhang, X., and Yan, C. Z.. 2009. Pollution characteristics and ecological risk assessment of heavy metals and polycyclic aromatic hydrocarbons (PSHs) in sediment from Xiamen Bay. Environmental Chemistry, 28(6): 869-875 (in Chinese with English abstract). (  0) 0) |
Peng, W. Q., 2021. Spatio-temporal variation of macrozoobenthic communities and sedimentary environment in the soft intertidal zone in Xiamen Crocodile Island. Master thesis. Xiamen University (in Chinese with English abstract).
(  0) 0) |
Qu, S. M., Zheng, J. H., Zheng, J. S., Richardson, B. J., and Lam Paul, K. S.. 2004. Petroleum hydrocarbons and polycyclic aromatic hydrocarbons in the surficial sediments of Xiamen Harbour and Yuan Dan Lake, China. Chemosphere, 56: 107-112. DOI:10.1016/j.chemosphere.2004.02.022 (  0) 0) |
Sun, D. Y., and Chen, B. D.. 1988. A preliminary study on the ecology of Polychaeta in northern Taiwan Strait. Marine Science, 2: 43-49 (in Chinese with English abstract). (  0) 0) |
Wang, Q., Mei, D. G., Chen, J. Y., Lin, Y. S., Liu, J. C., Lu, H. L., et al.. 2019. Sequestration of heavy metal by glomalin-related soil protein: Implication for water quality improvement in mangrove wetlands. Water Research, 148: 142-152. DOI:10.1016/j.watres.2018.10.043 (  0) 0) |
Weng, C. H., Xiao, Z. Q., Xie, Y. J., and Hong, S. G.. 2008. Construction of the nature reserve for horseshoe crab, Tachypleus tridentatus Leach in Xiamen. Journal of Jimei University (Natural Science), 13(1): 40-44 (in Chinese with English abstract). (  0) 0) |
Weng, Z. H., and Hong, S. G.. 2001. The distribution and habit of horseshoe crabs. Chinese Journal of Zoology, 36(5): 4-8 (in Chinese with English abstract). (  0) 0) |
Weng, Z. H., Xie, Y. J., Xiao, Z. Q., Huang, L. M., Li, J., Wang, S. H., et al.. 2012. Distribution and resource of Chinese horseshoe crab (Tachypleus tridentatus) in Fujian and other coast water of China. Chinese Journal of Zoology, 47(3): 40-48 (in Chinese with English abstract). (  0) 0) |
Zeng, G. S., He, M. H., and Chen, Z. D.. 1996. Monitoring and research in Lancelet Protected Area in Huangcuo, Xiamen. Journal of Oceanography in Taiwan Strait, 15(2): 174-181 (in Chinese with English abstract). (  0) 0) |
Zhang, L. P., Ye, X., Feng, H., Jing, Y. H., Tong, O. Y., Yu, X. T., et al.. 2007. Heavy metal contamination in western Xiamen Bay sediments and its vicinity, China. Marine Pollution Bulletin, 54(7): 974-982. DOI:10.1016/j.marpolbul.2007.02.010 (  0) 0) |
Zhou, H., and Morton, B.. 2004. The diets of juvenile horseshoe crabs, Tachypleus tridentatus and Carcinoscorpius rotundicauda (Xiphosura), from nursery beaches proposed for conservation in Hong Kong. Journal of Natural History, 38: 1915-1925. DOI:10.1080/0022293031000155377 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


