2) School of Aquatic Sciences and Fisheries Technology, University of Dar es Salaam, Dar es Salaam, Tanzania;
3) Department of Natural Sciences, Mbeya University of Science and Technology, Mbeya, Tanzania
Freshwater systems, particularly rivers, play a pivotal role in supporting both human populations and ecological communities. These systems serve as crucial sources of drinking water for humans and provide vital habitats for diverse organisms, including fish (Zhou et al., 2020). The Qiantang River, holds significant importance as it represents a critical water area for the sustainable development of fishery resources and its complex water quality, plays a crucial role in both ensuring adequate water supply and supporting the fishery industry in coastal cities (Xiao et al., 2021). As one of the important freshwater fishery bases, the Qiantang River is recognized as one of the significant freshwater fishery bases due to its diverse ecological types of fishery resources. The fishery survey in the middle and lower reaches of the Qiantang River showed that the diversity of fishes included 13 orders, 24 families, 59 genera and 83 species, among which the order Carpiformes accounted for the largest proportion (Liu, 2021). The economic fish in Qiantang River include four major Chinese carps, indigenous economic fish (Xia et al., 1989). For example, the upper reaches of the Qiantang River have designated fish reservation areas aimed at preserving biodiversity. Additionally, stocking initiatives have been implemented in the middle and lower reaches to ensure the natural reproduction of economically valuable fish species (Hao et al., 2017). The Tonglu section of the Qiantang River serves as a provincial fishery resource area dedicated to fish breeding and releasing, while the Fuyang section is designated as a provincial conservation zone for aquatic germplasm resources. Releasing stock enhancement could increase the amount of fishery resources, and restore the ecological environment. The highest production of bighead and silver carp in Xin'an jiang Reservior reached 2580 t (Fang and Xiang, 2007). In addition, the occurrence of algal bloom was successfully prevented, and a number of water quality indicators were improved (Liu et al., 2007). However, environmental alterations such as water quality fluctuations, habitat degradation, and other factors can influence the overall health and productivity of fish communities in these sections of the river.
The composition of the bacterioplankton community is closely related to the ecological environment of the water column and is adaptable to environmental changes. In addition, the change of its community diversity can indicate the change in the water column environment. As the main bearer of the element cycle and energy flow in water environments (Newton et al., 2011; Liu et al., 2022), bacterioplankton play an important role in the degradation of organic matter and the material cycle, which in turn affect the function of ecosystems.
Due to natural and man-made influences, such as nutrient concentration, salinity and turbidity gradually transiting to the ocean, the distribution and composition of bacterioplankton in the ecosystem of the Qiantang River are susceptible to external disturbances (Mohapatra et al., 2020). The Qiantang River is located in a medium-low dimension area with great differences in temperature and rainfall in all four seasons. It has been found that nutrient concentration, water temperature (Staley et al., 2013; Read et al., 2015), salinity (Xuan et al., 2019) and other factors can affect bacterial community composition in the river. With the rapid economic development of cities along the river, environmental pollution has become a prominent problem in the Qiantang River, which can spread to the whole aquatic ecosystem (including land rivers and oceans) through rivers. Some terrestrial pollutants, such as domestic wastewater and industrial water, which contain a large number of nutrients, organic compounds and heavy metals, are imported into the water column, and these factors can then significantly affect the bacterioplankton community (Winter et al., 2007; Esteves et al., 2015; Read et al., 2015), and destroy its ecological stability.
Currently, studies on the bacterioplankton community focus on the ocean and lakes, while studies on most of the main stream basins of the Qiantang River are relatively few. Recently, researches on the ecological environment of Qiantang River mainly involve phytoplankton (Da et al., 2019) and benthos (Zhang et al., 2016), while researches on the planktonic bacterial communities are still weak. In view of the importance of plankton bacteria in rivers, the study of the distribution pattern of planktonic bacterial communities in Qiantang River Basin and the drivers of external environmental factors can help improve the ecological process of planktonic bacterial communities. Exploring the changes of bacterial communities can understand the health of water ecological environment, which is the key to the protection and utilization of fishery waters. In this study, surface water samples were collected from seven sites of the Qiantang River in four seasons, covering 5 cities: Jiande, Tonglu, Fuyang, Jiubao and Yanguan. The results of the study were able to analyze the bacterioplankton community of the Qiantang River on a large scale. We used the 16S rRNA gene high-throughput sequencing technology to analyze the composition and relative abundance of bacterioplankton communities in samples. Also, this study intends to provide a theoretical basis for the scientific conservation and utilization of aquatic ecosystems and resources in the Qiantang River. The research aims to examine the following aspects: 1) summarize the spatial and temporal distribution characteristics of planktonic bacterial communities in the investigated watershed of the Qiantang River; 2) explain the relationship between structural changes of planktonic bacterial communities and driving factors; and 3) discuss the potential effects of the plankton community structure on the growth and reproduction of fish in the Qiantang River.
2 Materials and Methods 2.1 Study Area and Sample CollectionIn July and October of 2018 and January and April of 2019, a total of seven sampling sites were selected along the Qiantang River for quarterly assessments. These sites cover 5 cities (Jiande, Tonglu, Fuyang, Jiubao and Yanguan), including one located downstream of the Xin'anjiang Reservoir (JD), two sites near the fork of the Fenshui River (TL1 and TL2), two sites on both sides of the Fuchunjiang Bridge (FY1 and FY2), and two sites (JB and YG) situated in the lower region of the Qiantang River (Fig.1). The JD site receives water discharged from Xin'anjiang Reservoir, the four sites of TL and FY are located in the important protected fishery resources areas of the Qiantang River Basin, and YG is the intersection of the fresh water of the Qiantang River and the seawater of the East China Sea. Each site had three samples, which were collected from the middle and on both sides of the river at a depth of 0.5 m simultaneously. From each sample, 500 mL water was pre-filtered with sterilized 100 μm mesh, and then filtered with a 0.2 μm polycarbonate membrane (Millipore, Boston, MA, USA) for subsequent DNA extraction. The tweezer was wiped with 75% alcohol and then clamped the filter membrane for filtration. The membranes were placed in sterile EP tubes and stored in an ultra-cold refrigerator at − 80℃. 500 mL water was stored at 4℃ for the determination of water quality physicochemical factors.
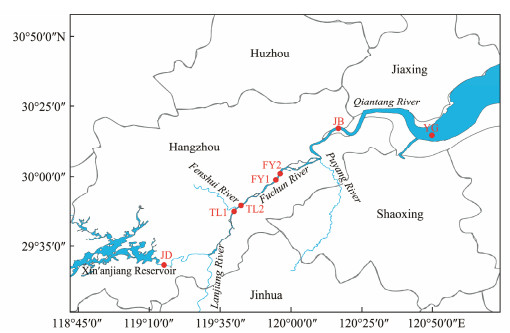
|
Fig. 1 Distribution map of sampling sites. |
The water temperature (WT), salinity (S), pH, dissolved oxygen (DO) of surface water were determined by YSI multifunctional water quality detector (YSI Inc., Yellow Springs, USA) on the sampling day. Transparency (SD) was measured using a black and white Secchi disk with a diameter of 0.2 m. FluoroProbe-Ⅲ (bbe Moldaenke, Schwentinental, Germany) was used for the content of chlorophyll a (Chl a) in water.
Chemical oxygen demand (COD) was determined using the acidic potassium permanganate method, and ammonia nitrogen (NH4+), nitrate (NO3−), nitrite (NO2−), PO43− (active phosphate), total nitrogen (TN), and total phosphorus (TP) in water were determined using an automated chemical analyzer (Smart-Chem 200 Discrete Analyzer, Westco Scientific Instruments, Brookfield, USA). The water samples for the determination of NH4+, NO3−, NO2−, and PO43− were filtered through a 0.45 μm pore size glass fiber membrane (GF/F, 50 mm). The NH4+ content was determined by sodium salicylate method, TN and NO3− content by cadmium-copper reduction method, NO2− content by diazo-azo method, TP and PO43− content by ascorbic acid reduction phosphomolybdenum blue method.
2.3 DNA Extraction and High Throughput Sequencing of 16S rRNAAfter cutting up the membranes by sterilized scissors, small pieces were incubated with 3 mL of lysis buffer and 75 μL of lysozyme at 37 ℃ for 45 min, and then with 75 μL of proteinase K and 300 μL of sodium dodecyl sulfate (SDS) 10% at 55℃ for 60 min. Using 5 mol L−1 NaCl and cetyl trimethyl ammonium bromide (CTAB) to incubate, then centrifugation with 128 μL of chloroform-isoamyl. Incubate with 256 μL 100% ethanol at − 20℃ for 15 min, then centrifuge. Then centrifuge with 125 μL 70% ethanol twice for 2 min each time. Finally, resuspended in ultrapure water (Mateus-Barros et al., 2019), and DNA concentration and purity were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). After obtaining the qualified DNA samples, appropriate amounts were taken and stored at − 80℃ for backup. The V3 − V4 variable region of the 16S rRNA gene was amplified using universal primers (341F: CCTAYGGGRBGCASCAG and 806R: GGA CTACNNGGGTATCTAAT), and PCR amplification was performed under a 50 μL reaction system. The PCR reaction conditions were as follows: predenaturation at 98℃ for 1 min; denaturation at 98℃, 10 s, annealing at 55℃ for 30 s, extension at 72℃ for 30 s, 30 cycles; extension at 72℃ for 5 min. The PCR amplification products were detected by 2% concentration agarose gel electrophoresis, and the target fragments that passed the detection were recovered by AxyPrep PCR Cleanup Kit (AXYGEN, Life Science Research), and then purified by PCR fragment purification kit (Takara Biotech, Japan). The PCR products of all samples were mixed in equimolar amounts and sent to Beijing NovoHorizon for high-throughput sequencing by Illumina MiSeq sequencing platform.
Paired sequences were combined by FLASH, then processed and chimeras were removed by QIIME 1.9.0; bacterial phylotypes were identified using Uclust, and sequences with > 97% similarity were classified into operational taxonomic units (OTUs), resulting a total of 24374 OTUs. Greengenes is the default database of software Qiime. Greengenes provids a consistent multiple-sequence alignment of bacterial 16S small-subunit rRNA genes to facilitate taxonomic placement (DeSantis et al., 2006). The most abundant sequences in each OTU were selected as representative sequences and compared with the Greengenes database (release 13.8) to obtain annotation information. The α-diversity of bacterial communities within samples was calculated, including richness (Chao1), Shannon diversity index (Shannon) and β-diversity index of bacterial communities between samples (Bray-Curtis dissimilarity).
2.4 Statistical AnalysisMeans of environmental factors and α-diversity were calculated for different seasons at different sites using Excel (2007) and plotted using Origin 8.5. One-way ANOVA followed by Tukey's multiple comparisons was performed to analyse significant differences by using SPSS 20. Prior to data analysis, all biological data were Hellinger transformed, while abiotic data were normalized using the function decostand() to improve their normality and homomorphism. Based on Bray-Curtis distances, relationships between samples were identified and differences in bacterial community structure were analyzed using principal coordinates analysis (PCoA) with the function cmdscale() in the 'vegen' package. Permutational variance (PERMANOVA) analysis was performed using the function adonis() in 'vegen' to assess the contribution of seasonal and cross-sectional changes in community structure. The bacterial species data were first subjected to Detrended correspondence analysis (DCA) and then selected for Redundancy analysis (RDA), and the environmental factors with strong multi strong multicollinearity were removed by the function ordiplot() in the 'vegen' package and the function vif.cca() in the 'fmsb' package. The results were verified by Monte Carlo permutation test. Spearman correlation analysis was performed and visualized using the 'corplot' package for the dominant taxa of bacterioplankton, α-diversity and water environmental factors. All the above visualizations were done using the 'ggplot2' package.
OTUs with mean relative abundance ≥ 0.1% of the total samples were selected, and samples from each of the four seasons were analyzed using the linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe) online tool (Galaxy (harvard.edu)). Taxa with significant differential relative abundance between sites were sought.
The Mantel and partial Mantel tests were conducted with the 'vegan' package. The function vegdist() was then used to calculate the Bray-Curtis similarity matrix of bacterial communities between sites, and the function distm() of the 'geosphere' package was used to calculate the geographic distance based on the latitude and longitude coordinates of each site for distance decay model analysis.
The sampling sites map of the study area was drawn using ARCGIS 10.8. The above analyses were done in R (3.5.2) unless otherwise indicated.
3 Results 3.1 Physical and Chemical Properties of Water Column in Qiantang RiverThere were obvious seasonal differences in environmental factors in all sites (Fig.2). For example, except in spring, ammonia nitrogen content in JB and YG was higher than that in TL, FY and JD at the same time. The chemical oxygen demand, salinity, active phosphate content and total phosphorus content of YG were higher than those of the remaining sites in the whole year, and in TL and FY were basically the same. Except for JB in summer, the total nitrogen content of the other sites generally showed an increasing trend along the river flow direction, and the total nitrogen content in spring was higher than the rest of the months, the chlorophyll a content fluctuated in autumn and winter, and the Chl a content was basically the same in spring and summer.
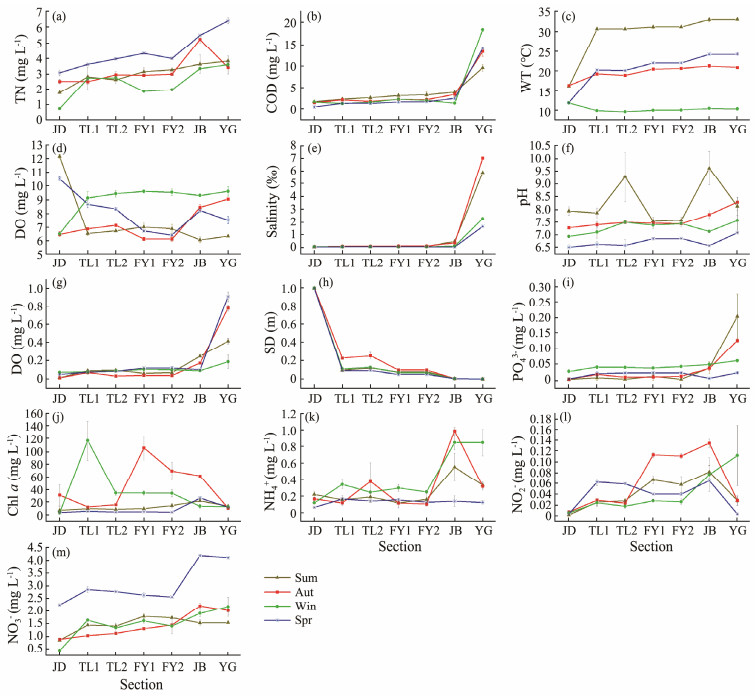
|
Fig. 2 Spatiotemporal variation of physical and chemical factors in Qiantang River. Sum, summer; Aut, autumn; Win, winter; Spr, spring. |
α-diversity focuses on the number of species in an individual community which can be described by community richness and diversity indices. The higher the Chao1 index, the richer the species variety. The Shannon index is used to characterize community diversity and is proportional to biodiversity. Both Chao1 index and Shannon index were significantly higher in autumn than in other seasons. The highest value of Chao1 index was found in JB in autumn and the lowest value was found in FY2 in summer (Fig.3). The highest value of Shannon index was found in YG in autumn and the Shannon index in JB in spring was significantly lower than other sites (P < 0.05). Chao1 index and Shannon index were significantly higher in autumn (Chao1: 3680.19 ± 404.85; Shannon: 7.56 ± 0.52) than in other seasons. Correlation analysis of the planktonic bacterial diversity index with environmental factors showed that several environmental factors had significant effects on the α-diversity of bacterioplankton in Qiantang River (Table 1).
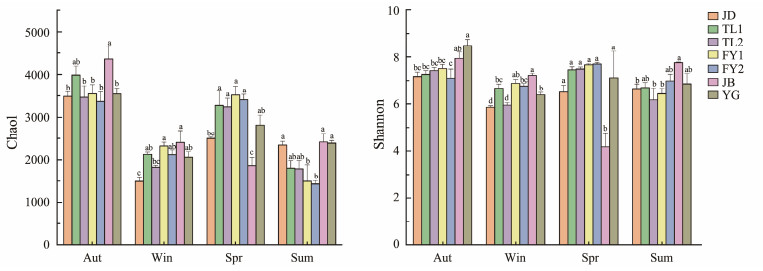
|
Fig. 3 Seasonal change of planktonic bacteria α diversity in Qiantang River. Different lowercase letters on the error line indicate significant differences between different sites in the same season. Sum, summer; Aut, autumn; Win, winter; Spr, spring. |
|
|
Table 1 Correlation between bacterioplankton α diversity and environmental factors |
A total of 57 bacterial phyla were detected in the surface layer of the Qiantang River, with the main dominant taxa being Proteobacteria (average relative abundance 50.42%), Actinobacteria (23.79%), Bacteroidetes (15.34%), Cyanobacteria (3.03%), Verrucomicrobia (2.28%), Chlorobi (1.85%), Acidobacteria (0.82%), Chloroflexi (0.72%), Firmicutes (0.48%) and Bacillariophyta (Gemmatimonadetes, 0.31%), which together accounted for more than 99.01% of all communities (Fig.4a).
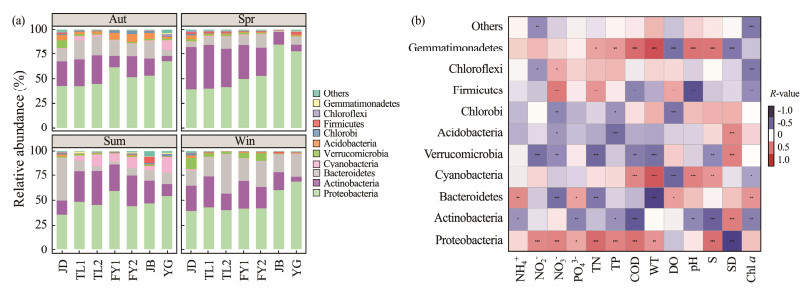
|
Fig. 4 Relative abundance of the dominant bacterial phyla and Spearman correlation between relative abundance of dominant planktonic bacteria and water environmental factors. Data in the graph are means; ***, P < 0.001; **, P < 0.01; *, P < 0.05. Color shades represent correlations, the darker the color, the stronger the correlation. Sum, summer; Aut, autumn; Win, winter; Spr, spring. |
There was significant spatial and temporal variation in the composition of these dominant bacterial taxa, with highly significant differences based on analysis of similarity (ANOSIM) across seasons (P = 0.001) and sites (P = 0.001). The relative abundance of Proteobacteria was the highest in all four seasons in FY1, FY2, JB and YG, while the relative abundance of Bacteroidetes in JD, TL1 and TL2 in spring and Actinobacteria in Jiande in summer were significantly higher and exceeded that of Proteobacteria. The relative abundance of Actinobacteria increased and then decreased along the river flow direction in summer, reaching a maximum in TL2, while in spring the relative abundance of Actinobacteria showed a decreasing trend along the river flow direction. The relative abundance of Cyanobacteria in all sites reached the highest in summer, and the relative abundance of Cyanobacteria in YG was the highest in all sites.
Correlation analysis of the dominant taxa with environmental factors showed that the relative abundance of the dominant bacterial taxa was significantly correlated with several environmental factors (Fig.4b), among which the relative abundance of the Proteobacteria was significantly negatively correlated with transparency and significantly positively correlated with nutrient salt concentration, salinity and water temperature. However, the Actinobacteria showed a significant negative correlation with nutrient salt concentration and a significant positive correlation with transparency. The Bacteroidetes showed significant positive correlations with ammonium salts, reactive phosphate, dissolved oxygen, and Chl a, while nitrate, total nitrogen, and temperature showed significant negative correlations. Cyanobacteria was significantly and positively correlated with chemical oxygen demand, temperature, pH and salinity, and negatively correlated with dissolved oxygen and Chl a.
3.3 Spatial and Temporal Distribution of Bacterioplankton Communities in the Qiantang RiverIn order to understand the differences in bacterial community structure, Principal coordinates analysis (PCoA) based on Bray-Curtis distances was performed (Fig.5). The first two axes explained a total of 35.22% of the differences. The results showed that the community structure of the four sites in Tonglu and Fuyang in four seasons were closely clustered on the PCoA diagram, so the four sites were discussed together subsequently. Except for JD, the bacterial community structure of the remaining sites changed with the river course in four seasons. The significance of spatial-temporal differences was tested by Permutational variance (PERMANOVA) analysis, and the results showed that the differences were significant for both sampling seasons and sampling sites, and the seasonal differences (R2 = 0.262, P = 0.001) were slightly more significant than the spatial differences (R2 = 0.248, P = 0.001), indicating that the sampling seasons had a slightly greater influence on the changes in the structure of the bacterioplankton community in the Qiantang River relative to the sampling sites.
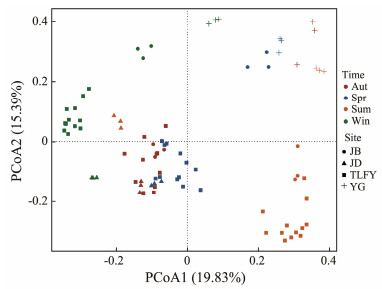
|
Fig. 5 Plot of Principal coordinate analysis (PCoA) based on the Bray-Curtis distance. Sum, summer; Aut, autumn; Win, winter; Spr, spring. |
To further determine the differences in abundance of bacterioplankton populations across sampling sites and seasons, LEFse analysis was performed by setting LDA > 4. The results showed that species relative abundance ≥ 0.1% had significant signature taxa in all four sites for four seasons (Fig.6). From the composition of the signature taxa in different sites in four seasons, most of the significantly different signature taxa in each site changed in four seasons.
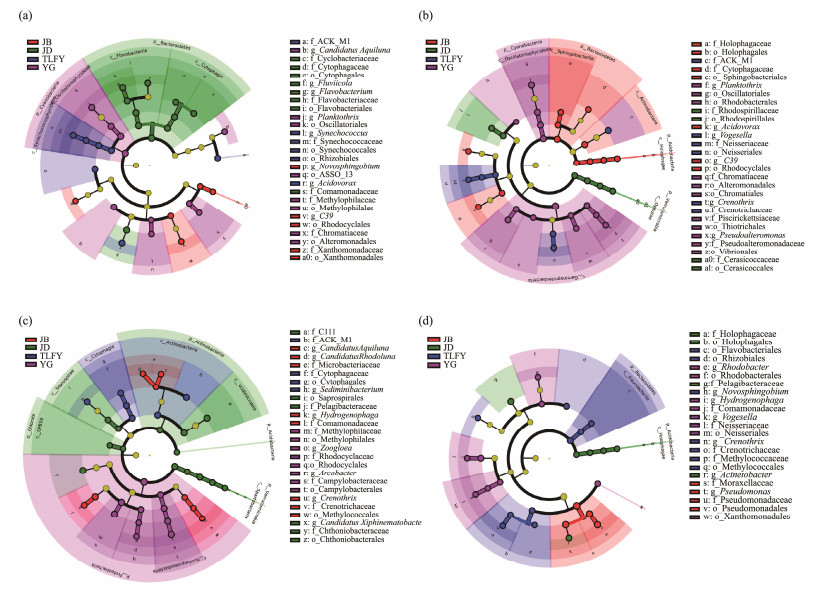
|
Fig. 6 LeFSe results of the bacterioplankton communities of four seasons in the Qiantang River (LDA score = 4.0, P < 0.05). a, LEfSe analysis of bacterioplankton in summer; b, LEfSe analysis of bacterioplankton in autumn; c, LEfSe analysis of bacterioplankton in winter; d, LEfSe analysis of bacterioplankton in spring. |
YG was rich in bacterial community characteristic taxa in all four seasons, and the Cytophagaceae became the signature taxa in JD, JB and TLFY in summer, autumn and winter, respectively. Synechococcus became the marker species in TLFY in summer, and the new Novosphingobium in JB were significantly different from other sites. Flavobacteriaceae were the marker taxa in JD in summer, while became the marker taxa in TLFY in spring. It is worth noting that Rhizobiales and Novosphingobium became the potential biomarkers of TLFY in spring.
3.4 Environmental Variables and Geographic Distance Affecting Bacterial Communities in the Qiantang RiverThe Mantel test on the composition of bacterioplankton community showed that the variation of bacterioplankton community structure in the Qiantang River was significantly influenced by both space and environment. The results showed the contribution of environmental variables (Mantel r = 0.6739, P < 0.001; partial Mantel r = 0.5070, P = 0.001) and geographical distance (Mantel r = 0.5322, P < 0.001; partial Mantel r = 0.1563, P = 0.001) to the variation of bacterioplankton community structure (Fig.7a), but according to the result of partial Mantel the importance of environmental variables in the process of building microbial communities was stronger than geographical distance.
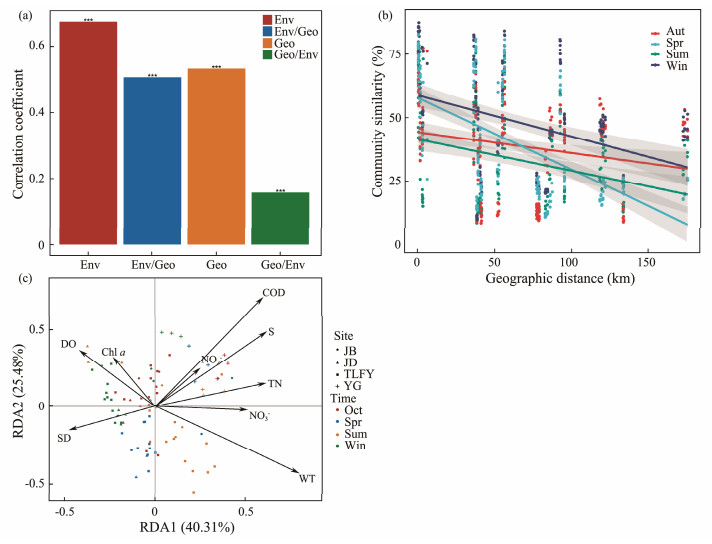
|
Fig. 7 Environmental variables and geographic distance affecting bacterial communities. a, (partial) Mantel analysis of the effects of environmental factors and geographical distance on bacterioplankton communities; b, distance attenuation relationship of bacterioplankton community similarity in the Qiantang River during four seasons; c, the plot of redundancy analysis (RDA) of bacterial community in Qiantang river. Geo/Env, relationship between bacterioplankton community and geographic distance under controlled environmental variables; Env/Geo, relationship between bacterioplankton community and environmental variables under controlled geographic distance; *** indicates P < 0.001. Sum, summer; Aut, autumn; Win, winter; Spr, spring. |
The results of the one-dimensional linear regression between the community composition similarity matrix based on OTU levels and the geographic distance matrix for each site showed that the bacterioplankton communities showed a significant distance decay pattern with increasing geographic distance for all four seasons (Fig.7b). The similarity of bacterioplankton community in spring (Radj2 = 0.319, P < 0.001) and winter (Radj2 = 0.1128, P < 0.001) decreased most significantly with the increase of geographical distance. Although there was a distance decline pattern in summer (Radj2 = 0.07314, P < 0.001) and autumn (Radj2 = 0.03625, P < 0.01), R2 was small and the distance decline relationship was relatively weak.
The de-trend correspondence analysis (DCA) results indicated Lengths of gradient first axis length < 3 (1.13), so the RDA linear model was chosen to perform a constrained ranking analysis of environmental factors and the relative abundance at the phylum level of bacterioplankton in the Qiantang River (Fig.7c). Nine environmental drivers with significant effects (P < 0.05) on the variability of the bacterioplankton community in the Qiantang River were screened using forward selection, including transparency, dissolved oxygen, chlorophyll a, chemical oxygen demand, nitrite, salinity, total nitrogen, nitrate, and water temperature. RDA 1 explained 40.31% of the variation between samples and was positively correlated with COD, nitrite, salinity, total nitrogen, nitrate and water temperature, while RDA 2 explained the variation between samples and 25.48% and was negatively correlated with transparency and water temperature.
4 Discussion 4.1 Spatial and Temporal Variation of Environmental Factors in the Qiantang RiverThe Qiantang River is a freshwater ecosystem that experiences considerable impacts from human activities. While the water resources in the river are abundant, their spatial and temporal distribution is uneven. Environmental factors appear to change significantly with seasons and cross-sections, while some environmental factors appear to change significantly with river flow direction such as salinity. Yanguan is at the location where salt and fresh water meet, and at high tide time, seawater enters Yanguan with the tide, so the salinity of YG is the highest. The water quality in the Xin'an River section is better with low nutrient concentrations (Zhang et al., 2022), and the total nitrogen, chemical oxygen demand, total phosphorus and reactive phosphorus levels increase with river flow, while the water transparency decreases with river flow. Chlorophyll a content is an important indicator of phytoplankton primary productivity and is often used as a major reference factor in evaluating the degree of eutrophication in water bodies and in predicting water blooms (Tan et al., 2014). Except for a few sites, the chlorophyll a content increased first and then decreased from summer to the following spring. With changes in water temperature or light, phytoplankton growth is less affected by nutrients, the biomass of green algae and other algae sensitive to temperature changes decreases simultaneously, Cyanobacteria resume growth due to less competitive pressure from green algae, and cryptophytes and other algae can still grow and constitute most of the chlorophyll sources until winter they decline (Zhou, 2018). There is basically no difference between most environmental factors in Tonglu and Fuyang, and the seasonal variation is exactly the same. However, the differences in environmental factors between Yanguan, Jiubao, Jiande and Tonglu Fuyang are obvious.
4.2 Spatial-Temporal Distribution of Bacterioplankton Community Patterns in Qiantang River and Their DriversIn dynamic rivers, bacteria are influenced by terrain and human activity (Liu et al., 2018; Zhang et al., 2021). The Qiantang River has the characteristics that the upper reaches of the river come from fresh water and the lower reaches lead to the East China Sea, and the hydrology process in different seasons will cause the fluctuation of the plankton bacterial community. Bacterial is an ideal variable for monitoring the ecology of river water environment (Lear et al., 2009). For example, studies have shown that actinomycetes have bioremediation capabilities and can serve as indicator organisms for the health of aquatic environments (Wang et al., 2023), and harmful algal bloom outbreaks significantly reduce the diversity and function of the other microbial communities (Huang et al., 2022). The presence of Arcobacter in rivers was significantly correlated with bacterial indicators of fecal contamination, suggesting a potential risk to the aquatic environment and human health (Collado et al., 2008). This study revealed significant spatial and temporal variations in the α-diversity of bacterioplankton communities across different sections of the Qiantang River. The α-diversity was notably higher during autumn compared to other seasons, with the YG, JB, JD, and FY1 sites displaying the highest levels during this period. In summer, characterized by high temperatures, the proliferation of algae blooms was observed. Consequently, the growth of certain heterotrophic bacteria groups was inhibited by toxins secreted by cyanobacteria, including cyclic peptides, alkaloids, lipopolysaccharides, and other compounds (Rastogi et al., 2015).
Proteobacteria, Actinobacteria and Bacteroidetes are the dominant phylum in Qiantang River, which is consistent with the results of studies on bacterial communities in other typical freshwater rivers (Liu et al., 2015; Bi et al., 2022; Siriarchawatana et al., 2024), but the relative composition is different. In this study, the YG site is a mix of freshwater to ocean, so the plankton bacterial communities in this area come from multiple bodies of water. In addition, salinity contributes to the formation of density gradients that separate water bodies and microbial communities, which may be the reason why it is different from other sections (Fortunato et al., 2011). Proteobacteria is the most representative bacterial category in freshwater ecosystems (Newton et al., 2011; de Oliveira and Margis, 2015; Kurilkina et al., 2016). Most Actinobacteria are widespread in freshwater habitats due to their high adaptability (Ghai et al., 2014; Neuenschwander et al., 2018). In this study, the bacterial community was dominated by the phyla Proteobacteria and Actinomycetes, accounting for 50.42% and 23.79% of all sequences, respectively. The annual relative abundance of Proteobacteria in YG was significantly higher than that in other sites. The water quality data showed that the concentrations of COD, total nitrogen and total phosphorus increased along the river trend, and the nutrient concentration in YG was the highest. Correlation analysis with environmental factors showed that the relative abundance of Proteobacteria was significantly positively correlated with salinity and nutrient concentration (Figs.4a, b), which may be the reason for the difference in the relative abundance of Proteobacteria between YG and other sites. Actinobacteria was the bacterial category with the second highest relative abundance. Correlation analysis showed that the Actinobacteria was negatively correlated with salinity. In summer, the relative abundance of Actinobacteria first increased and then decreased in the flow direction of the river, reaching the maximum at TL2, while decreased along the flow direction of the river in spring (Figs.4a, b). Actinobacteria was abundant in fresh waters, which is consistent with the results of the studies on the salinity gradient of estuarine waters of the salinity gradient in estuaries (Kirchman et al., 2005; Zhang et al., 2006). Studies have shown that salinity drives microbial community structure on a global scale (Lozupone and Knight, 2007), and Actinobacteria have different salinity preferences and ecological strategies to cope with salinity changes, which are manifested as preferentially occurring in low-salinity water (Mohapatra et al., 2020). Therefore the gradient change of salinity may be the cause of the gradient change of actinomyces in spring and summer.
Cyanobacteria, as a planktonic microalgae, will evolve into water blooms and red tides when it exists in large numbers in water bodies, so it can be used as a feature of eutrophication in water bodies (Ji et al., 2019). Meanwhile, Cyanobacteria have been found to be one of the most abundant bacterial species in freshwater (Lee et al., 2016). The water temperature in summer was within the optimal growth temperature range of Cyanobacteria (25 – 35℃), and the relative abundance of Cyanobacteria also reached the highest in summer. Correlation analysis with environmental factors showed that the content of Cyanobacteria was significantly and positively correlated with temperature, which also coincided with the above results. Studies have shown that both elevated water temperatures and nutrient enrichment from human inputs lead to Cyanobacteria blooms in inland waters (Guo et al., 2018), and the TL-FY section is near a residential area that receives various pollutants from human activities, creating a water environment preferred by Synechococcus (Zheng et al., 2023), which we speculate may be responsible for Synechococcus becoming the signature taxon of the TL-FY section in summer.
It is now become clear that microbial population abundance and distribution show a seasonal pattern (Hullar et al., 2006). On the temporal scale, the bacterioplankton community structure in each site of the Qiantang River evolved seasonally, but there were differences in the process of seasonal succession of bacterioplankton community structure in different sites (Fig.5). In the PCoA diagram, the bacterioplankton community structures in spring and autumn clustered closer together, while in winter and summer are obviously separated. This may be influenced by the temperature, which was closer in spring and autumn and significantly different in winter and summer. The inconsistent succession trends in JD may due to the fact that it received the water release from the Xin'anjiang reservoir on the day of the summer sampling, thus causing large changes in the structure of the planktonic bacterial community. In general, the bacterioplankton community structure of YG had little seasonal variation, which was different from other sites. This may be related to the differences in eutrophication degree and salinity of different sites, as the trophic state of the water column can significantly affect the changes in the structure of the bacterioplankton bacterial community (Gao et al., 2017).
The Mantel and partial Mantel analysis showed that the structure of the bacterioplankton community in the Qiantang River is influenced by both environmental factors and geographic distance, and previous studies have indicated that there is a distance decay pattern in the riverine bacterial community, which is important for determining the spatial similarity of the bacterioplankton community structure (Bell, 2010; Isabwe et al., 2018; Liu et al., 2018). In this study, under the premise of controlling environmental differences, geographical distance still significantly influenced the similarity of planktonic bacterial communities, indicating that the dispersal of bacterioplankton was limited. Precipitation data in the Qiantang River showed low precipitation in July and October 2018 compared to the multi-year average, and high precipitation in January and May 2019. The increase of rainfall may lead to strong scour effect on the microorganisms in the surrounding ecosystem, which makes the plankton species disperse better in the rainy season and increases the diversity of the community (Isabwe et al., 2018). It is noteworthy that the maximum runoff in the Qiantang River occurred from March to July affected by rainfall (Pan et al., 2023). The increased river flow enhanced river connectivity and habitat homogeneity, facilitating microbial transport within a limited range, which is consistent with the fact that the similarity of bacterioplankton community in spring presented the most significant geographic distance decay. Therefore, both environmental factors and spatial differences should be considered when assessing the structural changes of planktonic bacteria in Qiantang River.
Spatial and temporal differences between different ecosystems and within the same ecosystem can alter the drivers of biodiversity that dominate bacterioplankton communities. Studies have shown that water environmental factors, such as WT, DO, COD, pH and TN, are the main driving factors causing the differences in microbial diversity. Numerous studies have demonstrated that temperature is a strong overall correlate with planktonic bacterial community (Zhang et al., 2012; Gifford et al., 2014). In this study, environmental factors such as WT, COD, TN and salinity were all related to bacterial community structure, and the temperature was the strongest driver of bacterial community structure changes (Fig.7c).
4.3 Potential Impact of Bacterioplankton on Fish Survival and Reproduction in Qiantang RiverGiven the superior geographical location and good natural ecological environment, Qiantang River is rich in fishery resources, which breeds a variety of biological species. However, fish resources of Qiantang River are widely affected by anthropogenic activities. Water pollution and overfishing have contributed to ever-decreasing fish stocks and the construction of Xin'anjiang dam also obstructed fish migration. In order to restore and protect the fishery resources of Qiantang River, breeding and releasing activities were carried out in different waters. There are many economic fishes such as silver carp, bighead carp and carp in Qiantang River, and bacterioplankton in the environment affect the health of fish through stick to the skin and gills of fish, through water and food into the digestive tract of fish (Ghanbari et al., 2015). The microbial community structure of fish is strongly influenced by the environment, and aquatic microbial community is considered to be the main source of gut and skin microbial community of fish (Jing et al., 2021; Sadeghi et al., 2023). In addition, the initial colonization of gut microbes in some fish was derived from microbes in the surrounding water (Giatsis et al., 2015). Previous study has shown that the number of shared OTUs between fish and the aquatic environment before the first feeding is high (Burgos et al., 2018), so it is necessary to pay attention to the aquatic bacterial communities in fish breeding sites. Diet and physicochemical variations of water can adjust microbial diversity, affecting environmental adaptability of fish. Therefore, the analysis of fish-related microbial changes in water environment provides a new perspective to optimizing the survival and reproduction environment of fish.
In this study, Flavobacteriaceae became the signature group of JD in summer. Many Flavobacterium-like populations play an important role in degrading biological macromolecules especially after algal blooms (Mohapatra et al., 2020). They adapt to high-nutrient conditions and are favored during periods of high heterotrophic activity (Newton et al., 2011). Under the condition of high dissolved oxygen content, they can multiply in large numbers, increase denitrification activity, and then heterotrophic nitrification and metabolism of the organic matter. However, it should be noted that the large number of Flavobacteriaceae also has a certain ecological risk. Flavobacterium, a biomarker of environmental stress, frequently colonizes the skin of fish and can cause severe damage to fish (Damasceno et al., 2022). Previous studies point out that Flavobacterium columnare belonging to Flavobacteriaceae is the causative agent of columnaris disease in freshwater fish (Declercq et al., 2013; Loch and Faisal, 2014; Isabwe et al., 2018; Bo-Hyung et al., 2023).
The carp, Carassius carassius and Abramis in the lower reaches of Qiantang River spawn in Tonglu section from April to June, and concentrate in Fuyang section for fattening and overwintering in autumn and winter (Xia et al., 1989). The pollutant accumulation and eutrophication caused by pollutant discharge, reservoir construction and dam construction in Qiantang River Basin will adversely affect the survival of fish. In addition, studies have shown that nutrient load can drive changes in the composition and function of riverine bacterial community structure (Xie et al., 2021). In this study, the α diversity of the bacterial community in Qiantang River in autumn was the highest, which helped to resist the adverse effects of the water environment and provide a stable environment for the growth and reproduction of fish. LEfSe results showed that the bacterial community characteristic taxa were also richer in the spring 2019 TL-TF section, with the genera Novosphingobium and Rhizobiales becoming characteristic taxa. It has been confirmed that Novosphingobium is involved in the degradation of a variety of organic pollutants and has the ability to degrade microcystin produced after cyanobacteria outbreaks (Jiang et al., 2011; Li et al., 2020). Rhizobium can purify water, facilitate nutrient digestibility and improve resistance to pathogens, which contributes to the maintenance of good fishery productivity and has a positive impact on the growth and reproduction of fish.
5 ConclusionsThis study aimed to investigate the bacterioplankton communities in the Qiantang River across seven sections. It provides a detailed insight into the spatio-temporal distribution of bacterioplankton communities in the Qiantang River and their underlying environmental drivers. The results revealed that while the Qiantang River possesses abundant water resources, the distribution of high-quality water resources is heterogeneous both spatially and temporally. Furthermore, the α-diversity of plankton bacteria in the Qiantang River showed obvious seasonal fluctuations, which was significantly higher in autumn than that in other seasons. The dominant bacterial groups identified in the Qiantang River were mainly composed of Proteobacteria, Bacteroidetes, Actinobacteriota and Cyanobacteria, and these dominant bacterial groups were significantly correlated with some environmental factors. Additionally, the composition of microbial communities associated with fish health varied among different seasons and river sections. These findings contribute valuable insights for understanding changes in the planktonic bacterial community and promoting the sustainable development of fishery resources within the Qiantang River.
AcknowledgementsThis research was financially supported by the Fisheries Species Conservation Program of the Agricultural Department of China (Nos. 171821303154051044, 17190 236), the Natural Science Foundation of Zhejiang Province (No. LQ20C190003), the Natural Science Foundation of Ningbo Municipality (Nos. 2019A610421, 2019A610443), and the K. C. Wong Magna Fund in Ningbo University.
Bell, T., 2010. Experimental tests of the bacterial distance-decay relationship. ISME Journal, 4(11): 1357-1365. DOI:10.1038/ismej.2010.77 (  0) 0) |
Bi, S., Lai, H., Guo, D. L., Liu, X. G., Wang, G. P., Chen, X. L., et al., 2022. Spatio-temporal variation of bacterioplankton community structure in the Pearl River: Impacts of artificial fishery habitat and physicochemical factors. BMC Ecology and Evolution, 22(1): 19-25. DOI:10.1186/s12862-022-01965-3 (  0) 0) |
Bo-Hyung, L., Pierre, N., Izzet, B. S., Benjamin, F., Jean-François, B., Dimitri, R., et al., 2023. Investigation of the genus Flavobacterium as a reservoir for fish-pathogenic bacterial species: The case of Flavobacterium collinsii. Applied and Environmental Microbiology, 89(4): e02162-02122. DOI:10.1128/aem.02162-22 (  0) 0) |
Burgos, F. A., Ray, C. L., and Arias, C. R., 2018. Bacterial diversity and community structure of the intestinal microbiome of channel catfish (Ictalurus punctatus) during ontogenesis. Systematic and Applied Microbiology, 41(5): 494-505. DOI:10.1016/j.syapm.2018.04.006 (  0) 0) |
Collado, L., Inza, I., Guarro, J., and Figueras, M. J., 2008. Presence of Arcobacter spp. in environmental waters correlates with high levels of fecal pollution. Environmental Microbiology, 10(6): 1635-1640. DOI:10.1111/j.1462-2920.2007.01555.x (  0) 0) |
Da, W. Y., Zhu, G. W., Wu, Z. X., Li, Y. X., Xu, H., Zhu, M. Y., et al., 2019. Long-term variation of phytoplankton community and driving factors in Qiandaohu Reservoir, Southeast China. Journal of Lake Sciences, 31(5): 1320-1333 (in Chinese with English abstract). DOI:10.18307/2019.0522 (  0) 0) |
Damasceno, M. R. A., Lemes, C. G. D., Braga, L., Tizioto, P. C., Montenegro, H., Paduan, M., et al., 2022. Hatchery tanks induce intense reduction in microbiota diversity associated with gills and guts of two endemic species of the Sao Francisco River. Frontiers in Microbiology, 13: 966436. DOI:10.3389/fmicb.2022.966436 (  0) 0) |
de Oliveira, L. F. V., and Margis, R., 2015. The source of the river as a nursery for microbial diversity. PLoS One, 10(3): 120608. DOI:10.1371/journal.pone.0120608 (  0) 0) |
Declercq, A. M., Haesebrouck, F., Van den Broeck, W., Bossier, P., and Decostere, A., 2013. Columnaris disease in fish: A review with emphasis on bacterium-host interactions. Veterinary Research, 44: 27. DOI:10.1186/1297-9716-44-27 (  0) 0) |
DeSantis, T. Z., Hugenholtz, P., Larsen, N., Rojas, M., Brodie, E. L., Keller, K., et al., 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology, 72(7): 5069-5072. DOI:10.1128/aem.03006-05 (  0) 0) |
Esteves, K. E., Lôbo, A. V. P., and Hilsdorf, A. W. S., 2015. Abiotic features of a river from the upper Tietê River Basin (SP, Brazil) along an environmental gradient. Acta Limnologica Brasiliensia, 27(2): 228-237. DOI:10.1590/s2179-975x5914 (  0) 0) |
Fang, Z. P., and Xiang, G. C., 2007. Countermeasures and measures for the protection of fishery ecological resources in Thousand-island Lake. Water Conservancy and Fisheries, 4: 50-52. (  0) 0) |
Fortunato, C. S., Herfort, L., Zuber, P., Baptista, A. M., and Crump, B. C., 2011. Spatial variability overwhelms seasonal patterns in bacterioplankton communities across a river to ocean gradient. The ISME Journal, 6(3): 554-563. DOI:10.1038/ismej.2011.135 (  0) 0) |
Gao, Y., Wang, C. C., Zhang, W. G., Di, P. P., Yi, N., and Chen, C. R., 2017. Vertical and horizontal assemblage patterns of bacterial communities in a eutrophic river receiving domestic wastewater in Southeast China. Environment Pollution, 230: 469-478. DOI:10.1016/j.envpol.2017.06.081 (  0) 0) |
Ghai, R., Rodriguez-Valera, F., McMahon, K. D., Toyama, D., Rinke, R., Tereza, C. S., et al., 2014. Metagenomics of the water column in the pristine upper course of the Amazon River (vol 6, e23785, 2011). PLoS One, 9(5): 97393. DOI:10.1371/journal.pone.0097393 (  0) 0) |
Ghanbari, M., Kneifel, W., and Domig, K. J., 2015. A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture, 448: 464-475. DOI:10.1016/j.aquaculture.2015.06.033 (  0) 0) |
Giatsis, C., Sipkema, D., Smidt, H., Heilig, H., Benvenuti, G., Verreth, J., et al., 2015. The impact of rearing environment on the development of gut microbiota in tilapia larvae. Scientific Reports, 5(1): 18206. DOI:10.1038/srep18206 (  0) 0) |
Gifford, S. M., Sharma, S., and Moran, M. A., 2014. Linking activity and function to ecosystem dynamics in a coastal bacterioplankton community. Frontiers in Microbiology, 5: 185. DOI:10.3389/fmicb.2014.00185 (  0) 0) |
Guo, C. X., Zhu, G. W., Paerl, H. W., Zhu, M. Y., Yu, L., Zhang, Y. B., et al., 2018. Extreme weather event may induce Microcystis blooms in the Qiantang River, Southeast China. Environmental Science and Pollution Research, 25(22): 22273-22284. DOI:10.1007/s11356-018-2216-7 (  0) 0) |
Hao, Y. B., Liu, J. D., Guo, A. H., Zhang, A. J., and Ni, M., 2017. Current status of fishery resouces in Fuyang section of Qiantang River. Freshwater Fisheries, 47(3): 45-51 (in Chinese with English abstract). DOI:10.13721/j.cnki.dsyy.2017.03.007 (  0) 0) |
Huang, Z. H., Jiang, C. C., Xu, S. J., Zheng, X. X., Lv, P., Wang, C., et al., 2022. Spatiotemporal changes of bacterial communities during a cyanobacterial bloom in a subtropical water source reservoir ecosystem in China. Scientific Reports, 12(1): 17788. DOI:10.1038/s41598-022-17788-7 (  0) 0) |
Hullar, M. A. J., Kaplan, L. A., and Stahl, D. A., 2006. Recurring seasonal dynamics of microbial communities in stream habitats. Applied and Environmental Microbiology, 72(1): 713-722. DOI:10.1128/aem.72.1.713-722.2006 (  0) 0) |
Isabwe, A., Yang, J. R., Wang, Y. M., Liu, L. M., Chen, H. H., and Yang, J., 2018. Community assembly processes underlying phytoplankton and bacterioplankton across a hydrologic change in a human-impacted river. Science of the Total Environment, 630: 658-667. DOI:10.1016/j.scitotenv.2018.02.210 (  0) 0) |
Ji, B., Liang, J. C., Ma, Y. Q., Zhu, L., and Liu, Y., 2019. Bacterial community and eutrophic index analysis of the East Lake. Environmental Pollution, 252: 682-688. DOI:10.1016/j.envpol.2019.05.138 (  0) 0) |
Jiang, Y. G., Shao, J. H., Wu, X. Q., Xu, Y., and Li, R. H., 2011. Active and silent members in the mlr gene cluster of a microcystin-degrading bacterium isolated from Lake Taihu, China. FEMS Microbiology Letters, 322(2): 108-114. DOI:10.1111/j.1574-6968.2011.02337.x (  0) 0) |
Jing, X. J., Su, S. Y., Zhang, C. F., Zhu, J., Hou, Y. R., Li, Z. X., et al., 2021. Dynamic changes in microbial community structure in farming pond water and their effect on the intestinal microbial community profile in juvenile common carp (Cyprinus carpio L.). Genomics, 113(4): 2547-2560. DOI:10.1016/j.ygeno.2021.05.024 (  0) 0) |
Kirchman, D. L., Dittel, A. I., Malmstrom, R. R., and Cottrell, M. T., 2005. Biogeography of major bacterial groups in the Delaware Estuary. Limnology and Oceanography, 50(5): 1697-1706. DOI:10.4319/lo.2005.50.5.1697 (  0) 0) |
Kurilkina, M. I., Zakharova, Y. R., Galachyants, Y. P., Petrova, D. P., Bukin, Y. S., Domysheva, V. M., et al., 2016. Bacterial community composition in the water column of the deepest freshwater Lake Baikal as determined by next-generation sequencing. FEMS Microbiology Ecology, 92(7): 94. DOI:10.1093/femsec/fiw094 (  0) 0) |
Lear, G., Boothroyd, I. K. G., Turner, S. J., Roberts, K., and Lewis, G. D., 2009. A comparison of bacteria and benthic invertebrates as indicators of ecological health in streams. Freshwater Biology, 54(7): 1532-1543. DOI:10.1111/j.1365-2427.2009.02190.x (  0) 0) |
Lee, C. S., Kim, M., Lee, C., Yu, Z. T., and Lee, J. Y., 2016. The microbiota of recreational freshwaters and the implications for environmental and public health. Frontiers in Microbiology, 7: 1826. DOI:10.3389/fmicb.2016.01826 (  0) 0) |
Li, S. Y., Liu, J., Sun, K., Yang, Z. Y., and Ling, W. T., 2020. Degradation of 17β-estradiol by Novosphingobium sp. ES2-1 in aqueous solution contaminated with tetracyclines. Environmental Pollution, 260: 114063. DOI:10.1016/j.envpol.2020.114063 (  0) 0) |
Liu, J. W., Fu, B. B., Yang, H. M., Zhao, M. X., He, B. Y., and Zhang, X. H., 2015. Phylogenetic shifts of bacterioplankton community composition along the Pearl Estuary: The potential impact of hypoxia and nutrients. Frontiers in Microbiology, 6: 64. DOI:10.3389/fmicb.2015.00064 (  0) 0) |
Liu, P. F., 2021. Status of fish resources in the lower reaches of Qililongba of Qiantang River. PhD thesis. Nanjing Agricultural University.
(  0) 0) |
Liu, Q. G., Chen, L. Q., and Chen, Y., 2007. Correlation between biomass reduction of silver carp and bighead carp and the occurrence of algal blooms in Lake Qiandao. Transactions of Oceanology and Limnology, 21: 117-124. DOI:10.13984/j.cnki.cn37-1141.2007.01.017 (  0) 0) |
Liu, T., Zhang, A. N., Wang, J. W., Liu, S. F., Jiang, X. T., Dang, C. Y., et al., 2018. Integrated biogeography of planktonic and sedimentary bacterial communities in the Yangtze River. Microbiome, 6: 16. DOI:10.1186/s40168-017-0388-x (  0) 0) |
Liu, Y., Liu, Q., Tian, Y. L., Dong, M. Y., Xu, X., Guan, M. X., et al., 2022. Characteristics of bacterioplankton community with relations to environmental parameters in upstream and midstream of the Luanhe River, China. Acta Ecologica Sinica, 42(12): 5103-5114 (in Chinese with English abstract). (  0) 0) |
Loch, T. P., and Faisal, M., 2014. Deciphering the biodiversity of fish-pathogenic Flavobacterium spp. recovered from the Great Lakes Basin. Diseases of Aquatic Organisms, 112(1): 45-57. DOI:10.3354/dao02791 (  0) 0) |
Lozupone, C. A., and Knight, R., 2007. Global patterns in bacterial diversity. Proceedings of the National Academy of Sciences of the United States of America, 104(27): 11436-11440. DOI:10.1073/pnas.0611525104 (  0) 0) |
Mateus-Barros, E., Meneghine, A. K., Bagatini, I. L., Fernandes, C. C., Kishi, L. T., Vieira, A. A. H., et al., 2019. Comparison of two DNA extraction methods widely used in aquatic microbial ecology. Journal of Microbiological Methods, 159: 12-17. DOI:10.1016/j.mimet.2019.02.005 (  0) 0) |
Mohapatra, M., Behera, P., Kim, J. Y., and Rastogi, G., 2020. Seasonal and spatial dynamics of bacterioplankton communities in a brackish water coastal lagoon. Science of the Total Environment, 705: 134729. DOI:10.1016/j.scitotenv.2019.134729 (  0) 0) |
Neuenschwander, S. M., Ghai, R., Pernthaler, J., and Salcher, M. M., 2018. Microdiversification in genome-streamlined ubiquitous freshwater Actinobacteria. ISME Journal, 12(1): 185-198. DOI:10.1038/ismej.2017.156 (  0) 0) |
Newton, R. J., Jones, S. E., Eiler, A., McMahon, K. D., and Bertilsson, S., 2011. A guide to the natural history of freshwater lake bacteria. Microbiology and Molecular Biology Reviews, 75(1): 14-49. DOI:10.1128/mmbr.00028-10 (  0) 0) |
Pan, C. H., Wang, Q. S., Pan, D. Z., and Hu, C. F., 2023. Characteristics of river discharge and its indirect effect on the tidal bore in the Qiantang River, China. International Journal of Sediment Research, 38(2): 253-264. DOI:10.1016/j.ijsrc.2022.10.0021001-6279 (  0) 0) |
Rastogi, R. P., Madamwar, D., and Incharoensakdi, A., 2015. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Frontiers in Microbiology, 6: 254. DOI:10.3389/fmicb.2015.01254 (  0) 0) |
Read, D. S., Gweon, H. S., Bowes, M. J., Newbold, L. K., Field, D., Bailey, M. J., et al., 2015. Catchment-scale biogeography of riverine bacterioplankton. ISME Journal, 9(2): 516-526. DOI:10.1038/ismej.2014.166 (  0) 0) |
Sadeghi, J., Chaganti, S. R., Johnson, T. B., and Heath, D. D., 2023. Host species and habitat shape fish-associated bacterial communities: Phylosymbiosis between fish and their microbiome. Microbiome, 11(1). DOI:10.1186/s40168-023-01697-6 (  0) 0) |
Siriarchawatana, P., Harnpicharnchai, P., Phithakrotchanakoon, C., Kitikhun, S., Mayteeworakoon, S., Chunhametha, S., et al., 2024. Elucidating potential bioindicators from insights in the diversity and assembly processes of prokaryotic and eukaryotic communities in the Mekong River. Environmental Research, 243: 117800. DOI:10.1016/j.envres.2023.117800 (  0) 0) |
Staley, C., Unno, T., Gould, T. J., Jarvis, B., Phillips, J., Cotner, J. B., et al., 2013. Application of Illumina next-generation sequencing to characterize the bacterial community of the upper Mississippi River. Journal of Applied Microbiology, 115(5): 1147-1158. DOI:10.1111/jam.12323 (  0) 0) |
Tan, L., Cai, Q. H., Zhang, H. Y., Shen, H. L., and Ye, L., 2014. Trophic status of tributary bay aggregate and their relationships with basin characteristics in a large, subtropical dendritic reservoir, China. Fresenius Environmental Bulletin, 23(3): 650-659. (  0) 0) |
Wang, L., Wang, S. K., Zuo, J. E., Li, X. J., and Chen, Y., 2023. Urban river health assessment based on biotic integrity bacterioplankton-index of biotic integrity: A case study of Shenzhen River Basin. Chinese Journal of Environmental Engineering, 17(6): 2007-2014. DOI:10.12030/j.cjee.202211054 (  0) 0) |
Winter, C., Hein, T., Kavka, G., Mach, R. L., and Farnleitner, A. H., 2007. Longitudinal changes in the bacterial community composition of the Danube River: A whole-river approach. Applied and Environmental Microbiology, 73(2): 421-431. DOI:10.1128/aem.01849-06 (  0) 0) |
Xia, W. C., Xie, L. N., Tong, Y. Y., and Ma, X. N., 1989. Survey and research on the main economic fish and bait organism resources in the lower Qiantang River. Reservoir Fisheries, (2): 32-35. (  0) 0) |
Xiao, S. S., Hao, Y. B., Liu, J. D., Zhang, A. J., Wang, J., Luo, W., et al., 2021. Aquatic ecological health assessment of Qiantang River Basin in Zhejiang Province by phytoplankton index of biotic integrity. Fisherise Science, 40(5): 740-749. DOI:10.16378/j.cnki.1003-1111.20031 (  0) 0) |
Xie, G. J., Tang, X. M., Shao, K. Q., Zhu, G. W., and Gao, G., 2021. Bacterial diversity, community composition and metabolic function in Lake Tianmuhu and its dammed river: Effects of domestic wastewater and damming. Ecotoxicology and Environmental Safety, 213: 112069. DOI:10.1016/j.ecoenv.2021.112069 (  0) 0) |
Xuan, L. X., Sheng, Z. L., Lu, J. Q., Qiu, Q. F., Chen, J., and Xiong, J. B., 2019. Bacterioplankton community responses and the potential ecological thresholds along disturbance gradients. Science of the Total Environment, 696: 134015. DOI:10.1016/j.scitotenv.2019.134015 (  0) 0) |
Zhang, A. J., Liu, J. D., Yang, Y. J., Guo, A. H., and Gu, Z. M., 2016. Analysis of community characteristics of macrozoobenthos in enhancement and releasing zone in Tonglu section of Qiantang River. Acta Agriculturae Zhejiangensis, 28(8): 1323-1331 (in Chinese with English abstract). (  0) 0) |
Zhang, L., Cheng, Y., Zhou, Y., Lu, W. X., and Li, J., 2021. Effect of different types of anthropogenic pollution on the bacterial community of urban rivers. Water Environment Research, 93(8): 1322-1332. DOI:10.1002/wer.1517 (  0) 0) |
Zhang, M. L., Yu, N., Chen, L. Q., Jiang, C. H., Tao, Y. J., Zhang, T., et al., 2012. Structure and seasonal dynamics of bacterial communities in three urban rivers in China. Aquatic Sciences, 74(1): 113-120. DOI:10.1007/s00027-011-0201-z (  0) 0) |
Zhang, P., Guo, C. X., Yu, J., Quan, Q. M., Yao, J. L., Wang, J. Y., et al., 2022. Characteristics of phytoplankton community structure and its response to hydro-meteorology in summer of Qiantang River. Journal of Lack science, 34(02): 418-432 (in Chinese with English abstract). (  0) 0) |
Zhang, Y., Jiao, N. Z., Cottrell, M. T., and Kirchman, D. L., 2006. Contribution of major bacterial groups to bacterial biomass production along a salinity gradient in the South China Sea. Aquatic Microbial Ecology, 12: 233-241. (  0) 0) |
Zheng, Q., He, B. W., Shi, W. Q., Chen, Q., Lin, D. H., and Wang, Y., 2023. Ecological research progress on marine picocyanobacterial Synechococcus. Journal of Xiamen University (Natural Science), 62(3): 301-313. (  0) 0) |
Zhou, L., Chen, W. Y., Sun, J. J., Liu, L., and Huang, X. D., 2020. Spatial variation in bacterioplankton communities in the Pearl River, South China: Impacts of land use and physicochemical factors. Microorganisms, 8(6): 8060814. DOI:10.3390/microorganisms8060814 (  0) 0) |
Zhou, X. M., 2018. Study on dynamics of phytoplankton community in Jiangdong reservior and Jiulong River Estuary by high frequency observation. PhD thesis. Xiamen University.
(  0) 0) |
 2024, Vol. 23
2024, Vol. 23


