2) Key Laboratory of Marine Genetics and Breeding, Ministry of Education, College of Marine Life Science, Ocean University of China, Qingdao 266003, China
Saccharina japonica, which has been cultured with a large-scale in Northeast Asian countries, is one of the important economic seaweeds (Tseng, 2001; Buschmann et al., 2017; Liu et al., 2017). It exhibits an obligate heteromorphic life history that alternates between two generations of macroscopic sporophyte and microscopic gametophyte (Lüning, 1990; Schiel and Foster, 2006). In general, the microscopic gametophyte generation can be divided into three stages from the liberation of the zoospores to the formation of zygotes in 10 – 13 days, including gametophyte formation stage (about 2 – 3 d), gametophyte vegetative growth stage (about 6 d), and gametophyte reproduction stage (2 – 3 d) (Lüning, 1980; Tseng, 1987). Some studies have demonstrated that gametophyte of S. japonica can survive during extended periods of poor environmental quality (e.g., unsuitable irradiance and temperature, and poor nutrients), delaying growth and/or reproduction (Fang et al., 1978; Lüning, 1980; Yang et al., 2007). Thus, the delayed gametophytes can be considered as 'seed bank' and has evolved as an effective way for long-term preservation of the S. japonica germplasm (Carney et al., 2006; Yang et al., 2007). Moreover, the delayed gametophytes are the parental resources of the Saccharina variety and hybrid breeding, which have been often used in the commercial production of sporeling-raising of S. japonica (Cui et al., 2017). There is a trade-off between the vegetative growth and reproduction of kelp gametophyte, and the induction of gametogenesis showed a contrasting pattern to gametophyte vegetative growth (Izquierdo et al., 2002; Bartsch et al., 2008; Mohring et al., 2013; Karasov et al., 2017; Liu et al., 2017). For example, the optimum temperature range for gametophyte vegetative growth is wider than that for reproduction, and the minimal demand of light intensity and nutrient level for vegetative growth is lower than that for reproduction (Lüning and Neushul, 1978; Lüning, 1980; Martins et al., 2017; Wang et al., 2020). Many studies have reported that the transition between a vegetative growth phase and the reproduction phase in seaweed is a way to control the life cycle of seaweed (Cock et al., 2014; Charrier et al., 2017; Martins et al., 2017; Ratcliff et al., 2017).
Whether the kelp gametophyte reproduce sexually or delay development can be substantially influenced by abiotic factors such as light (e.g., light intensity, photoperiod, and light quality), temperature, and nutrient availability (Lüning and Neushul, 1978; Xu et al., 2009; Sui et al., 2011; Martins et al., 2017; Ratcliff et al., 2017). Gametogenesis was successfully induced by broad light intensity gradients (Lüning, 1980; Lee and Brinkhuis, 1988). Optimal light intensity for gametogenesis was different between kelp species, which is closely related to their geographical distribution (Lüning and Neushul, 1978; Lüning, 1980). Gametogenesis could not be induced under extremely low light intensity (e.g., 2 – 4.5 μmol photons m−2 s−1) in most cases in the kelp species (Bartsch et al., 2008). Studies have shown that blue light is an inductive condition necessary for the reproduction of some kelps (Lüning and Dring, 1972; Lüning and Neushul, 1978; Lüning, 1980). Only a small amount of gametophyte can reproduce at red light (Lüning and Dring, 1972). In a general way, long photoperiod is conducive to the vegetative growth of gametophytes, whereas optimal photoperiod for gametogenesis was different between kelp species. For example, long photoperiod was conducive to the gametogenesis of Laminaria digitata (Martins et al., 2017), while short photoperiod was beneficial to the gametogenesis of S. japonica (Zhang et al., 2008). Hence, light intensity, photoperiod, and light quality all are prime candidates as abiotic filters potentially capable of influencing the growth and reproduction of kelp gametophytes.
Delayed development of gametophytes is likely important for the recruitment of some kelp sporophyte (Carney and Edwards, 2006, 2010; Carney, 2011). It was reported that the meiospores releasing from sori of kelp sporophyte show a higher fecundity than pre-cultivated red-light grown gametophytes with delayed development (Izquierdo et al., 2002). Carney and Edwards (2010) found that delayed gametophytes can produce sporophytes 30% faster than gametophytes that had never been delayed once development is resumed. These results indicated that the optimal conditions for the reproduction of delayed gametophytes might be different from general gametophytes without delaying. How the terrestrial plants that form long-lived seed or seedling banks during unfavorable periods resume development when resources are renewed has been studied extensively (Grime, 2001; Makana and Thomas, 2005). However, the effects of environmental factors on the vegetative growth phase, reproduction phase, and the transition between these two phases of delayed gametophytes of S. japonica remain to be further researched.
Previous studies have commonly examined the effects of a single factor of light (e.g., light intensity), or dual factors of light (e.g., light intensity and light quality, or light intensity and photoperiod) on the vegetative growth and reproduction of kelp gametophytes (Choi et al., 2005; Ebbing et al., 2020). However, to the best of our knowledge, the combined effect of all light intensity, light quality, and photoperiod has never been investigated in an orthogonal design for the reproduction of delayed gametophytes. In addition, considering that the induction of gametogenesis showed a contrasting pattern to vegetative growth of gametophyte, culture conditions of light during the transition period may need to be further determined. Here, we designed an experiment to evaluate how the interaction of three environmental factors related to light influences the vegetative growth and reproduction of delayed gametophytes of S. japonica, aiming to seek the optimal light variables for commercial production of sporeling-raising of S. japonica.
2 Materials and Methods 2.1 Gametophyte CultureMature sporophyte of S. japonica 'Yudai No. 1' strain, which had a prolonged harvest period with higher yield, was collected from a S. japonica farm in Dalian city, China, in July 2018. Sporophyte blade in wet condition was put in sterile and sealable plastic bags in situ and transported to the laboratory in a cooler (< 10℃) in dark within 4 h. The sample was rinsed three times in plastic tray with filtered (0.22 μm pore size) and autoclaved seawater to remove any attached fouling organisms. Zoospore release was induced by immersing sori tissue in filtered seawater at 10℃. The zoospores attached to the glass microscope slides and germinated there. Female and male gametophytes were picked up separately when their gender was distinguishable. The selected gametophytes were transferred into NaNO3 (4 mg L−1 NO3−-N) and KH2PO4 (0.4 mg L−1 PO43−-P) enriched seawater, and cultured to a desirable amount of biomass. Then, the vegetative gametophytes were maintained in f/2 nutrient solution in artificial seawater without iron at low red light intensity (2 – 4 μmol photons m−2 s−1) under a 12 h: 12 h light/dark (L: D) cycle at 4℃ for at least 1 year to obtain reproduction-delayed S. japonica gametophytes.
2.2 Experiment 1Previous researches have reported that gametogenesis induction for most kelp gametophytes performed well in the light intensity range of 20 – 80 μmol photons m−2 s−1 within daylength of 8 – 16 h under white or blue light (Akiyama, 1965; Hsiao and Druehl, 1971; Choi et al., 2005; Sui et al., 2011). Therefore, based on the previous studies, an orthogonal experiment (experiment 1) was made to determine the tentative ranges of light intensity, light quality, and photoperiod in gametogenesis induction for the delayed S. japonica gametophytes, and the experimental levels were 3 light intensities (40, 50, and 60 μmol photons m−2 s−1), 3 light quality (white, red, and blue light), and 3 photoperiods (L: D = 8:16, 12:12, 16:8). An orthogonal array with totally 9 treatments was conducted as shown in Table 1. Three biological replicates were carried out for each treatment.
|
|
Table 1 Orthogonal design in gametogenesis induction for the delayed S. japonica gametophytes |
The female and male delayed gametophytes were mixed (fresh weight ratio of female: male = 2:1) and smashed by blender, then filtered with 200-mesh sieve. A total of 27 petri dishes (9 cm in diameter) were used for the above 9 treatments, each containing a glass microscope slide and enriched seawater (supplemented with 4 mg L−1 NO3−-N and 0.4 mg L−1 PO43−-P). The petri dishes were inoculated with the fragmented gametophytes (40 – 100 μm) to make a settlement density of 15 – 20 fragmented gametophytes in a field of view of 100× magnification and cultured at 10℃ for 12 days. The seawater was renewed with 50% fresh material every three days. The gametophytes were observed using the microscope (Nikon E200) at the beginning of culture and then on days 2, 4, 6, 8, 10, and 12 during the culture, and ten fields of view (100× magnification) were selected randomly and photographed for each microscope slide in each observation.
From the above microscopic observations, we found that the oogonium did not appear until the 8th day of culture. Therefore, we divided the process of gametogenesis induction into two phases: early vegetative growth phase (represented by Phase I, when delayed gametophytes enhance their number of cells and increase their cell volume, which is from the beginning to the 6th day of culture) and reproductive phase (represented by Phase II, when gametophytes form gametangium and gametes, finally resulting in sporophyte recruitment).
The photographs were analyzed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) to respectively measure the female and male vegetative gametophytes' sizes on the 6th day of culture. Then, the number of vegetative gametophyte (N1), oogonium (N2), eggs or zygotes (N3), and sporophytes (N4) were counted on the 12th day of culture.
The relative growth rates (RGR) of the female and male vegetative gametophytes were calculated using the cell area, respectively. The formula was as follows:
| $ R G R\left(\% \mathrm{~d}^{-1}\right)=\frac{\ln \left(S_2\right)-\ln \left(S_1\right)}{T_2-T_1} \times 100, $ |
where S2 and S1 were the gametophyte cell area at T2 and T1 respectively.
Reproduction percentage of S. japonica gametophyte was calculated by the formula:
| $ \text { Reproduction (%) }=\frac{N_2+N_3+N_4}{N_1+N_2+N_3+N_4} \times 100 . $ |
The results of the experiment 1 showed that the growth rate of delayed S. japonica gametophyte was the lowest under photoperiod of 8L: 16D and 40 μmol photons m−2 s−1, and increased with the increase of light time and light intensity in the Phase I, suggesting that longer daylength and higher light intensity may be more conducive to the growth rate in the Phase I. Therefore, in order to obtain the optimum light intensity and photoperiod in the Phase I for the delayed S. japonica gametophytes, more treatments with various light intensities (60, 80, and 100 μmol photons m−2 s−1) and different photoperiods (12L: 12D, 16L: 8D, and 24L: 0D) under white light were further conducted. Three replicates were carried out for each treatment. The other culture conditions and RGR calculation method were the same as those in Section 2.2.
2.4 Experiment 3In the experiment 1, the S. japonica gametophytes were maintained under a constant light condition from delayed state to gametogenesis during the 12-day gametogenesis induction process in each treatment. However, the previous studies showed that the light need between the early vegetative growth phase and the subsequent reproductive phase was different during gametogenesis induction process (Bartsch et al., 2008; Mohring et al., 2013; Liu et al., 2017). Thus, culture conditions of light in the transition period between Phase I and Phase II were changed in this experiment design. Based on the results of the experiment 1, the gametophytes were cultured at relatively appropriate light conditions (i.e., light intensity of 60 μmol photons m−2 s−1, photoperiod of 12L: 12D, and white light) for 6 days to make the delayed S. japonica gametophytes to achieve the optimal growth state in Phase I. Then, these delayed gametophytes were cultured at different light conditions for the following 6 days (Phase II). These experimental conditions were as follows: 1) three light intensities (60, 80, and 100 μmol photons m−2 s−1) with photoperiod of 12L: 12D under white light; 2) three light qualities (white, red, and blue light) with photoperiod of 12L: 12D at light intensity of 80 μmol photons m−2 s−1; 3) four photoperiods (8L: 16D, 12L: 12D, 16L: 8D, 24L: 0D) with light intensity of 80 μmol photons m−2 s−1 under white light. Three replicates were carried out for each treatment. The other culture conditions and reproduction rate calculation method were the same as those in Section 2.2.
2.5 Statistical AnalysisData were analyzed using the SPSS 19.0 statistical software packages. All values are presented as the means ± standard deviation (mean ± SD). Statistical variance analyses were performed using one-way ANOVA (Analysis of Variance) and compared with Duncan Multiple Comparisons Test. The statistical significance was set at P < 0.05.
3 Results 3.1 Microscopic Observation of Delayed Gametophytes During the Gametogenesis InductionThe morphological changes of delayed S. japonica gametophytes during the gametogenesis induction process were showed in Fig.1. During the stage of Phase I (from the beginning to the 6th day of culture), the cell number (from 2 – 3 to 7 – 9) and cell volume of the fragmented gametophytes increased as showed in Figs.1c and 1d. The color of cytoplasm deepened and cytoplasmic inclusions became more homogeneous. The width of the female gametophyte cells increased from 9.3 μm to 15.2 μm. In the stage of Phase II, the color of cytoplasm became deeper and the female gametophyte developed a single large oogonium which produced one egg (Figs.1e and 1f). The eggs were fertilized, producing the fertilized zygotes as shown in Fig.1g. The zygotes germinated and developed into young sporophytes (Fig.1h).

|
Fig. 1 Microphoto of delayed S. japonica gametophyte during the gametogenesis induction process at 60 μmol photons m−2 s−1 under a 12 h: 12 h white light/dark cycle at 10℃. Scale bar = 25 μm. (a), fragment of delayed female gametophyte; (b), fragment of delayed male gametophyte; (c), vegetative female gametophyte; (d), vegetative male gametophyte; (e), developing oogonium; (f), discharged egg attached to oogonium; (g), mature oogonium and zygote; (h), sporelings. |
ANOVA showed that both RGR of female and male gatophytes in Phase I were affected significantly by light innsity (P < 0.05) rather than light quality and photoperiod. Moreover, according to the max-min values in Table 2 and Table 3, it was found that the light intensity had a greater impact on RGR, followed by light quality. As Table 2 and Table 3 show, both RGR of female and male gametophytes increased with the increase of light intensity in Phase I, reaching a peak value at 60 μmol photons m−2 s−1 under white orblue light. When gametophytes were cultured at 40 μmol photons m−2 s−1 and with a photoperiod of 8L: 16D, both RGR of female and male gametophytes were significantly deeased (P < 0.05).
|
|
Table 2 RGR of female S. japonica gametophyte on the 6th day of culture in the orthogonal experiment |
|
|
Table 3 RGR of male S. japonica gametophyte on the 6th day of culture in the orthogonal experiment |
As Fig.2 shows, both RGR of female and male gamephytes reached a peak value at 80 μmol photons m−2 s−1 under photoperiod of 16L: 8D on the 6th day of culture. Under photoperiod of 12L: 12D, both RGR of female and male gametophytes decreased significantly (P < 0.05) at 100 μmol photons m−2 s−1 when compared with groups of 60 and 80 μmol photons m−2 s−1. There was no significant difference in RGR of female gametophyte among the treatment groups of 24L: 0D photoperiod (P > 0.05). Significant decreases in RGR of 24L: 0D photoperiod groups were observed as compared with 16L: 8D photoperiod groups (P < 0.05) when cultured at the same light intensity.
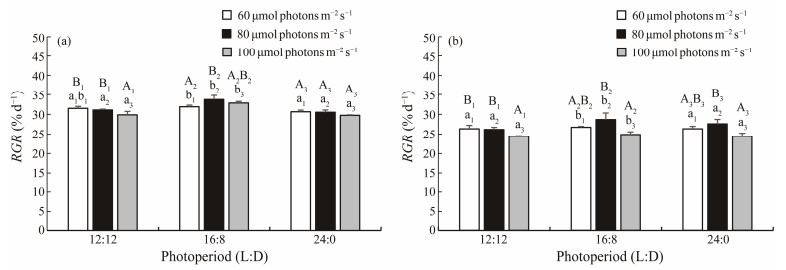
|
Fig. 2 RGR of female (a) and male (b) S. japonica gametophytes at different light intensities and photoperiods under white light on the 6th day of culture. The groups with the same subscript of uppercase letters were compared, and the groups with the same subscript of lowercase letters were also compared. The different letters represent significant differences (P < 0.05). Data are the mean ± SD (n = 30). |
ANOVA results showed that light intensity, light quality, and photoperiod all had a significant impact on the reproduction rate of delayed female gametophyte (P < 0.05). According to the max-min values in Table 4, it was found that the light quality had a greater impact on reproduction rate, followed by photoperiod. As Table 4 shows, the reproduction rate of female gametophyte increased with the increase of daylength. The reproduction rate of gametophyte reached a peak value when cultured at blue light, while it was zero under red light. Meanwhile, the reproduction rate of gametophyte was higher at 60 μmol photons m−2 s−1 than those at 40 and 50 μmol photons m−2 s−1.
|
|
Table 4 Reproduction rate of delayed female gametophyte of S. japonica on the 12th day of culture in the orthogonal experiment |
As Fig.3a shows, the reproduction rate of the group with light intensity of 100 μmol photons m−2 s−1 was significantly lower than those of the groups with light intensities of 60 and 80 μmol photons m−2 s−1 (P < 0.05). In addition, there was no significant difference in reproduction rate between the treatment groups with light intensities of 60 and 80 μmol photons m−2 s−1 (P > 0.05). As Fig.3b shows, the gametophyte cultured at blue light had significantly higher reproduction rate than that cultured at white light (P < 0.05). Moreover, the reproduction rate of gametophyte cultured at red light decreased significantly compared to that cultured at blue light or white light (P < 0.05). As Fig.3c shows, the reproduction rate increased as the daylength decreased and attained a peak value in group of 8L: 16D photoperiod.
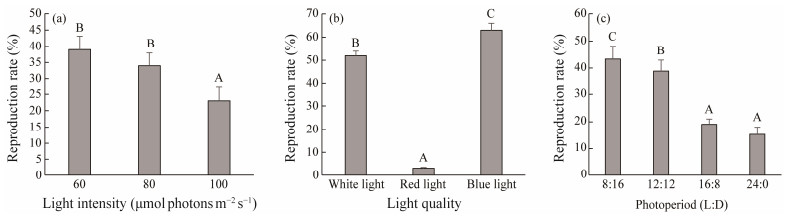
|
Fig. 3 Reproduction rate of S. japonica gametophyte at different light intensity (a), light quality (b), and photoperiod (c) on the 12th day of culture. Different letters above the bars indicate significant differences (P < 0.05). Data are the mean ± SD (n = 30). |
The technology of sporeling-raising using gametophyte clones has been developed in some kelp species such as S. japonica, Lessonia trabeculata and Macrocystis pyrifera recently (Li et al., 2002; Westermeier et al., 2006; Zhang et al., 2008; Xu et al., 2009), which needs two basic prerequisites for application in commercial production: 1) enough biomass of gametophyte accumulated by rapid vegetative growth; 2) efficient induction of gametogenesis by culture condition regulation (Zhang et al., 2008). The delayed gametophytes can remain viable for up to many years during conditions that are not conducive for sexual reproduction, and keep highly sensitive to changes in environment quality (Edwards, 2000; Carney and Edwards, 2006; Zhao et al., 2016). How environmental factors regulate the delay and resumption of microscopic development was one of the scientific mysteries that are necessary to investigate for seaweed (Hoffmann and Santelices, 1991). Moreover, the mechanism that regulates the trade-off between vegetative growth and reproduction in response to the changes of environmental conditions is critical to understand how seaweed integrate environmental signals and adjust its life cycle accordingly (Suda and Mikami, 2020).
Light exerts major impacts on the growth, photosynthetic activity, biochemical processes, and reproduction in seaweed, and seaweed have evolved to deal with the environmental fluctuations by regulating metabolism, changes in pigment concentration, enzyme activity, and so on (Kim et al., 2011; Bischof and Rautenberger, 2012). As a generic abiotic factor controlling gametogenesis, light has been the focus in many studies in regulating the delay and resumption of microscopicdevelopment. For example, the delayed Desmarestia gametophytes grown under low light levels and short-day photoperiods could resume growth when transferred to higher light and longer photoperiods (Edwards, 2000). The developments of most macroalgae at microscopic stages were able to be delayed for at least 60 days and resume growth due to increasing light intensity and daylength (Santelices et al., 2002). The interaction of temperature and light intensity can make the delayed gametophytes of S. latissima with more than one year of vegetative growth reproduce sexually reliably (Ebbing et al., 2021).
According to Table 2 and Table 3, we found thatlight intensity rather than light quality and photoperiod significantly affected the RGR of the delayed gametophytes, indicating that light intensity is the primary factor that determines whether the delayed S. japonica gametophytes can grow rapidly in the early vegetative growth phase of the process of gametogenesis induction. According to Fig.2, RGR of gametophytes reached a peak value when the light intensity was at about 80 μmol photons m−2 s−1, which was consistent with the findings of previous studies in other kelp species (Yang et al., 2002; Ebbing et al., 2020). Combining the results of Table 2, Table 3 and Fig.2, we proposed that the optimal light conditions in the early vegetative growth phase of gametogenesis induction for the delayed S. japonica gametophytes were 60 – 80 μmol photons m−2 s−1, 12L: 12D or 16L: 8D, and white light or blue light. Once sufficient biomass has accumulated, the initiation of reproductive structures development of gametophytes can be triggered (Ratcliff et al., 2017). It has been proved that blue light is a necessary factor for sexual reproduction in Laminaria gametophytes, and the gametophytes will delay in a vegetative state without blue light (Lüning and Dring, 1972; Lüning, 1981). The light quality results of this study (Table 4) showed that blue light had the greatest promotion effect on reproduction rate of S. japonica gametophyte as compared to white light and red light, and red light inhibited gametogenesis, which is consistent with the previous reports (Mizuta et al., 2007; Sui et al., 2011).
In the commercial production of sporeling-raising of S. japonica based on gametophyte, the delayed gametophytes might first undergo large-scale vegetative growth for a period of time, then the culture condition was changed to make them enter the transition period, and enter the gametogenesis stage afterwards. Therefore, it is crucial to understand the culture conditions of light during the transition period between the vegetative growth phase and reproduction phase. In the present study, we changed the light conditions in the transition period between Phase I and Phase II in the experiment of Section 2.4. It was interesting to find that the gametophytes which had received an inductive signal during white light cultivation were able to become fertile even transferred to non-inductive red light conditions (Fig.3), which was different from that in the orthogonal experiment with reproduction rate being zero in group of red light. Phytohormones act as vital switchers in the regulation of various aspects of development in plant (Weyers and Paterson, 2001; Saidi and Hajibarat, 2021). Mizuta et al. (2007) found that light quality influences internal IAA metabolism in the sporophytic stage of S. japonica. In Laminariales plants, the IAA content is higher in younger tissues or the vegetative parts (Williams, 1949; Kai et al., 2006). Therefore, we hypothesized that the interrelationship between light quality and internal IAA metabolism may also exist in the gametophytic stage, i.e., blue/white light probably reduced IAA content in the gametophyte thus promoting gametogenesis while IAA content was probably at a high level under red light. Phytohormone is regarded as an enduring message, which possibly remains active long after the immediate and possibly temporary stimulus has disappeared (Weyers and Paterson, 2001). This may be the reason why gametogenesis can still occur in some degree when the gametophytes are transferred from white light (cultured for 6 days) to red light (Fig.3). Pearson et al. (2019) found that a switch in culture irradiance from red to white light activated a core set of genes, involving rapid activation of ribosome biogenesis, as well as transcription- and translation-related pathways. These genes and pathways probably can provide foundation for the future understanding of the inhibition of red light on gametophyte reproduction.
The S. japonica gametophytes were generally maintained under a constant light condition from delayed state to gametogenesis in the whole gametogenesis induction process in some previous studies, which obtained different photoperiod conditions for gametogenesis. For example, Hsiao and Druehl (1971) found that long period of light (16L: 8D) was beneficial to the reproduction of Laminaria saccharina, but continuous light can significantly reduce the reproductive rate. Martins et al. (2017) also found that the reproductive rate of L. digitata gametophytes under long period of light (16L: 8D) was significantly higher than that under short term of light (8L: 16D). However, some studies have found that shortening light time was beneficial to the transition from vegetative growth to reproductive development for S. japonica gametophytes (Zhang et al., 2008; Xu et al., 2009). Moreover, short daylength has been applied to commercial production of sporeling-raising of S. japonica based on gametophyte (Xu et al., 2009).
When the S. japonica gametophytes were maintained under a constant light condition from delayed state to gametogenesis in the study (Tables 2, 3, 4), the beneficial photoperiod condition for vegetative growth and reproductive rate was both at photoperiod of 16L: 8D. We hypothesized that rapid vegetative growth may promote the gametogenesis since the delayed gametophyte need to undergo vegetative growth before gametogenesis. However, it was worth emphasizing here that, when the delayed S. japonica gametophytes achieve the optimal growth state in Phase I (first 6 days) and then they were cultured at different light conditions for the following 6 days, the reproduction rate increased as the daylength decreased and attained a peak value in group of 8L: 16D photoperiod as showed in Fig.3, which was different from that in the orthogonal experiment with reproduction rate increasing as the daylength increased (Table 4). Our results suggest that light time shortening at the transition period from vegetative growth phase to reproduction phase is crucial in the gametogenesis induction process of delayed gametophyte of S. japonica.
5 ConclusionsThe light intensity had the greatest impact on RGR of delayed gametophytes of S. japonica, followed by light quality. RGR reached a peak value at 60 – 80 μmol photons m−2 s−1 under white/blue light. The light quality had the greatest impact on reproduction rate of delayed gametophyte of S. japonica, followed by photoperiod. The gametophyte reproduction rate was considerably higher when cultured at the blue/white light than that at the red light. Rapid vegetative growth may promote the gametogenesis. When the delayed S. japonica gametophytes achieve the optimal growth state in vegetative growth phase, short daylength was beneficial for them during the transition from vegetative growth phase to reproduction phase.
AcknowledgementsThis study was financially supported by the National Key R & D Program of China (Nos. 2018YFD0900305, 2018YFD 0901500), the China Agriculture Research System of MOF and MARA, Central Public-interest Scientific Institution Basal Research Fund CAFS (No. 2020TD27), and the 'Young Talent of Fishery Sciences' project from Laboratory for Marine Fisheries and Aquaculture (No. 2018-MFS-T12).
Akiyama, K., 1965. Studies of ecology and culture of Undaria pinnatifida (Harv.) Sur. II. Environmental factors affecting the growing and maturation of gamtophyte. Bulletin of the Tohoku Regional Fisheries Laboratory, 25: 143-170. (  0) 0) |
Bartsch, I., Wiencke, C., Bischof, K., Buchholz, C. M., Buck, B. H., and Eggert, A., 2008. The genus Laminaria sensu lato: Re cent insights and developments. European Journal of Phycology, 43(1): 1-86. DOI:10.1080/09670260701711376 (  0) 0) |
Bischof, K., and Rautenberger, R., 2012. Seaweed responses to environmental stress: Reactive oxygen and antioxidative strategies. In: Seaweed Biology. Novel Insights into Ecophysiology, Ecology and Utilization. Ecological Studies, 219. Wiencke, C., and Bischof, K., eds., Springer, Berlin, 109-132.
(  0) 0) |
Buschmann, A. H., Camus, C., Infante, J., Neori, A., Israel, Á., Hernández-González, M. C., et al., 2017. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Journal of Phycology, 52: 391-406. DOI:10.1080/09670262.2017.1365175 (  0) 0) |
Carney, L. T., 2011. A multispecies laboratory assessment of rapid sporophyte recruitment from delayed kelp gametophytes. Journal of Phycology, 47: 244-251. DOI:10.1111/j.1529-8817.2011.00957.x (  0) 0) |
Carney, L. T., and Edwards, M. S., 2006. Cryptic processes in the sea: A review of delayed development in the microscopic life stages of marine macroalgae. Algae, 21(2): 161-168. DOI:10.4490/ALGAE.2006.21.2.161 (  0) 0) |
Carney, L. T., and Edwards, M. S., 2010. Role of nutrient fluctuations and delayed development in gametophyte reproduction by Macrocystis pyrifera (Phaeophyceae) in southern California. Journal of Phycology, 46: 987-996. DOI:10.1111/j.1529-8817.2010.00882.x (  0) 0) |
Charrier, B., Abreu, M. H., Araujo, R., Bruhn, A., Coates, J. C., De Clerck, O., et al., 2017. Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture. New Phytologist, 216(4): 967-975. DOI:10.1111/nph.14728 (  0) 0) |
Choi, H. G., Kim, Y. S., Lee, S. J., Park, E. J., and Nam, K. W., 2005. Effects of daylength, irradiance and settlement density on the growth and reproduction of Undaria pinnatifida gametophytes. Journal of Applied Phycology, 17: 423-430. DOI:10.1007/s10811-005-0432-2 (  0) 0) |
Cock, J. M., Godfroy, O., Macaisne, N., Peters, A. F., and Coelho, S. M., 2014. Evolution and regulation of complex life cycles: A brown algal perspective. Current Opinion in Plant Biology, 17: 1-6. DOI:10.1016/j.pbi.2013.09.004 (  0) 0) |
Cui, C., Li, Y., Liu, Y., Li, X., Luo, S., and Zhang, Z., 2017. Determination of genetic diversity among Saccharina germplasm using ISSR and RAPD markers. Comptes Rendus Biologies, 340: 76-86. DOI:10.1016/j.crvi.2016.11.005 (  0) 0) |
Ebbing, A., Pierik, R., Bouma, T., Kromkamp, J. C., and Timmermans, K., 2020. How light and biomass density influence the reproduction of delayed Saccharina latissima gametophytes (Phaeophyceae). Journal of Phycology, 56: 709-718. DOI:10.1111/jpy.12976 (  0) 0) |
Ebbing, A., Pierik, R., Fivash, G., van de Loosdrecht, N. C. J., Bouma, T., Kromkamp, J. C., et al., 2021. The role of seasonality in reproduction of multiannual delayed gametophytes of Saccharina latissima. Journal of Phycology, 57: 1580-1589. DOI:10.1111/jpy.13191 (  0) 0) |
Edwards, M. S., 2000. The role of alternate life-history stages of a marine macroalga: A seed bank analogue?. Ecology, 81: 2404-2415. DOI:10.1890/0012-9658(2000)081 (  0) 0) |
Fang, Z. X., Ou, Y. L., Cui, J. J., and Dai, J. X., 1978. Success in culturing clones of the gametophytes of Laminaria japonica. Science Bulletin, 23: 115-116 (in Chinese with English abstract). (  0) 0) |
Grime, J. P., 2001. Plant Strategies, Vegetation Processes, and Ecosystem Properties. Wiley, Chichester, UK, 417pp.
(  0) 0) |
Hoffmann, A. J., and Santelices, B., 1991. Banks of algal microscopic forms – Hypotheses on their functioning and comparisons with seed banks. Marine Ecology Progress Series, 79: 185-194. DOI:10.3354/meps079185 (  0) 0) |
Hsiao, S. I. C., and Druehl, L. D., 1971. Environmental control of gametogenesis in Laminaria saccharina. I. The effects of light and culture media. Canadian Journal of Botany, 49: 1503-1508. DOI:10.1139/b71-211 (  0) 0) |
Izquierdo, J. L., Pérez-Ruzafa, I. M., and Gallardo, T., 2002. Effect of temperature and photon fluence rate on gametophytes and young sporophytes of Laminaria ochroleuca Pylaie. Helgoland Marine Research, 55: 285-292. DOI:10.1007/s10152-001-0087-6 (  0) 0) |
Kai, T., Nimura, K., Yasui, H., and Mizuta, H., 2006. Regulation of sorus formation by auxin in Laminariales sporophyte. Journal of Applied Phycology, 18: 95-101. DOI:10.1007/s10811-005-9020-8 (  0) 0) |
Karasov, T. L., Chae, E., Herman, J. J., and Bergelson, J., 2017. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell, 29: 666-680. DOI:10.1105/tpc.16.00931 (  0) 0) |
Kim, J. H., Kang, E. J., Park, M. G., Lee, B. G., and Kim, K. Y., 2011. Effects of temperature and irradiance on photosynthesis and growth of a green-tide-forming species (Ulva linza) in the Yellow Sea. Journal of Applied Phycology, 23: 421-432. DOI:10.1007/s10811-010-9590-y (  0) 0) |
Lee, J. A., and Brinkhuis, B. H., 1988. Seasonal light and temperature interaction effects on development of Laminaria saccharina (Phaeophyta) gametophytes and juvenile sporophytes. Journal of Phycology, 24: 181-191. DOI:10.1111/j.1529-8817.1988.tb04232.x (  0) 0) |
Li, D. P., Lu, Y. H., and Wu, C. Y., 2002. The past and present status of Laminaria genetic breeding and sporophyte culture technology. Bulletin of Biology, 37: 1-3 (in Chinese with English abstract). (  0) 0) |
Liu, X., Bogaert, K., Engelen, A. H., Leliaert, F., Roleda, M. Y., and De Clerck, O., 2017. Seaweed reproductive biology: Environmental and genetic controls. Botanica Marina, 60: 89-108. DOI:10.1515/bot-2016-0091 (  0) 0) |
Lüning, K., 1980. Critical levels of light and temperature regulating the gametogenesis of three Laminaria species (Phaeophyceae). Journal of Phycology, 16: 1-15. DOI:10.1111/j.1529-8817.1980.tb02992.x (  0) 0) |
Lüning, K., and Dring, M. J., 1972. Reproduction induced by blue light in female gametophytes of Laminaria saccharina. Planta, 104: 252-256. DOI:10.1007/BF00387080 (  0) 0) |
Lüning, K., 1990. Seaweeds: Their Environment, Biogeography and Ecophysiology. John Wiley and Sons Inc., New York, 527pp. DOI:10.1002/aqc.3270010208
(  0) 0) |
Lüning, K., and Neushul, M., 1978. Light and temperature demands for growth and reproduction of Laminaria gametophytes in southern and central California. Marine Biology, 45: 297-309. DOI:10.1007/BF00391816 (  0) 0) |
Makana, J. R., and Thomas, S. C., 2005. Effects of light gaps and litter removal on the seedling performance of six African timber species. Biotropica, 37: 227-237. DOI:10.1111/j.1744-7429.2005.00030.x (  0) 0) |
Martins, N., Tanttu, H., Pearson, G. A., Serrao, E. A., and Bartsch, I., 2017. Interactions of daylength, temperature and nutrients affect thresholds for life stage transitions in the kelp Laminaria digitata (Phaeophyceae). Botanica Marina, 60: 109-121. DOI:10.1515/bot-2016-0094 (  0) 0) |
Mizuta, H., Kai, T., Tabuchi, K., and Yasui, H., 2007. Effects of light quality on the reproduction and morphology of sporophytes of Laminaria japonica (Phaeophyceae). Aquaculture Research, 38: 1323-1329. DOI:10.1111/j.1365-2109.2007.01809.x (  0) 0) |
Mohring, M. B., Kendrick, G. A., Wernberg, T., Rule, M. J., and Vanderklift, M. A., 2013. Environmental influences on kelp performance across the reproductive period: An ecological tradeoff between gametophyte survival and growth?. PLoS One, 8: e65310. DOI:10.1371/journal.pone.0065310 (  0) 0) |
Pearson, G. A., Martins, N., Madeira, P., Serro, E. A., and Bartsch, I., 2019. Sex-dependent and -independent transcriptional changes during haploid phase gametogenesis in the sugar kelp Saccharina latissima. PLoS One, 14: e0219723. DOI:10.1371/journal.pone.0219723 (  0) 0) |
Ratcliff, J. J., Soler-Vila, A., Hanniffy, D., Johnson, M. P., and Edwards, M. D., 2017. Optimisation of kelp (Laminaria digitata) gametophyte growth and gametogenesis: Effects of photoperiod and culture media. Journal of Applied Phycology, 29: 1957-1966. DOI:10.1007/s10811-017-1070-1 (  0) 0) |
Saidi, A., and Hajibarat, Z., 2021. Phytohormones: Plant switchers in developmental and growth stages in potato. Journal of Genetic Engineering and Biotechnology, 19: 89. DOI:10.1186/s43141-021-00192-5 (  0) 0) |
Santelices, B., Aedo, D., and Hoffmann, A., 2002. Banks of microscopic forms and survival to darkness of propagules and microscopic stages of macroalgae. Revista Chilena de Historia Natural, 75: 547-555. (  0) 0) |
Schiel, D. R., and Foster, M. S., 2006. The population biology of large brown seaweeds: Ecological consequences of multiphase life histories in dynamic coastal environments. Annual Review of Ecology, Evolution, and Systematics, 37: 343-372, https://doi.org/10.1146/annurev.ecolsys.37.091305.110251.
(  0) 0) |
Suda, M., and Mikami, K., 2020. Reproductive responses to wounding and heat stress in gametophytic thalli of the red alga Pyropia yezoensis. Frontiers in Marine Science, 7: 394. DOI:10.3389/fmars.2020.00394 (  0) 0) |
Sui, X. W., Ren, W., Yan, W. H., Yan, W. H., Liu, S. P., and Yang, N., 2011. Effects of light wavelength on growth and reproduction of the gametophytes of Laminaria japonica Aresch. Marine Science, 35: 33-36 (in Chinese with English abstract). (  0) 0) |
Tseng, C. K., 1987. Laminaria mariculture in China. FAO Fisheries Technical Paper 281. Food and Agriculture Organisation of the United Nations, Rome. http://www.fao.org/3/X5819E/x5819e00.htm.
(  0) 0) |
Tseng, C. K., 2001. Algal biotechnology industries and research activities in China. Journal of Applied Phycology, 13: 375-380. DOI:10.1023/A:1017972812576 (  0) 0) |
Wang, X., Liu, F. L., Liang, Z. R., Zhang, P. X., Yuan, Y. M., Wang, W. J., et al., 2020. Effects of iron on the growth and development of gametophyte clones in Saccharina japonica. Journal of Fishery Sciences of China, 9: 1052-1061 (in Chinese with English abstract). (  0) 0) |
Westermeier, R., Patiño, D., Piel, M. I., Maier, I., and Mueller, D. G., 2006. A new approach to kelp mariculture in Chile: Production of free-floating sporophyte seedlings from gametophyte cultures of Lessonia trabeculata and Macrocystis pyrifera. Aquaculture Research, 37: 164-171. (  0) 0) |
Weyers, J., and Paterson, N. W., 2001. Plant hormones and the control of physiological processes. New Phytologist, 152: 375-407. DOI:10.1046/j.0028-646X.2001.00281.x (  0) 0) |
Williams, L. G., 1949. Growth-regulating substances in Laminaria agardhii. Science, 110: 169. DOI:10.1126/science.110.2850.169 (  0) 0) |
Xu, B., Zhang, Q., S., Qu, S. C., Cong, Y. Z., and Tang, X. X., 2009. Introduction of a seedling production and method suing vegetative gametophytes to the commercial farming of Laminaria in China. Journal of Applied Phycology, 21: 171-178. DOI:10.1007/s10811-008-9347-z (  0) 0) |
Yang, G. P., Li, X. J., Cong, Y. Z., Qu, S. C., Li, Z. L., Zhang, Z. Z., et al., 2007. Trends in the cloning methodology of Laminaria gametophytes: Research and application. Periodical of Ocean University of China, 37: 569-572 (in Chinese with English abstract). (  0) 0) |
Yang, Y. X., Luo, S. J., Xie, Z. J., and Zhang, Z. Z., 2002. The effect of light intensity on growth of gametophytic clone of Laminaria japonica. Fisheries Science & Technology Information, 3: 114-116 (in Chinese with English abstract). (  0) 0) |
Zhang, Q. S., Qu, S. C., Cong, Y. Z., Luo, S. J., and Tang, X. X., 2008. High throughput culture and gametogenesis induction of Laminaria japonica gametophyte clones. Journal of Applied Phycology, 20: 205-211. DOI:10.1007/s10811-007-9220-5 (  0) 0) |
Zhao, X. B., Pang, S. J., Liu, F., Shan, T. F., Li, J., Gao, S. Q., et al., 2016. Intraspecific crossing of Saccharina japonica using distantly related unialgal gametophytes benefits kelp farming by improving blade quality and productivity at Sanggou Bay, China. Journal of Applied Phycology, 28: 449-455. DOI:10.1007/s10811-015-0597-2 (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


