Hydrogels, characterized by their three-dimensional, interconnected polymeric structures and significant water content, are well-known for their ability to absorb and retain substantial amount of water. The origins of hydrogels date back to 1960 when Wichterle and Lim developed the first hydrogel using poly (2-hydroxyethyl methacrylate) (HEMA). From then on, the field of hydrogels has rapidly evolved, garnering significant interest within the biomaterials science community. A diverse array of polymer compositions, encompassing both natural and synthetic types, has been extensively explored. Hydrogels are typically classified into three categories based on their polymer types: natural, synthetic, and hybrid or semi-synthetic. Natural polymers used in hydrogel formation include proteins such as collagen and gelatin, and polysaccharides, such as starch, alginate, and agarose.
Polysaccharide hydrogels, with their three-dimensional cross-linked networks, are particularly noteworthy. They can absorb and retain large volume of water without dissolving. Polysaccharide hydrogels's eco-friendliness, biocompatibility, unique functional attributes, and biodegradability make them increasingly appealing compared to conventional natural hydrogel materials. Notably, marine-derived natural polysaccharides are abundant and exhibit diverse structures, with different biological functions such as anti-inflammatory, antibacterial, antiviral, and anti-tumor properties. Algae, commonly known as seaweeds, are prominent natural sources of polysaccharides, and sulfated polysaccharides are prevalent components in seaweed cell walls.
Recently, sulfated polysaccharides derived from algae have attracted significant attention as functional additives in the pharmaceutical, food, and cosmetic industries. This review aims to explore the structures and functions of sulfated polysaccharides derived from seaweeds, specifically focusing on carrageenan, fucoidan, and ulvan. The mechanisms of sulfated polysaccharide hydrogel formation and their biomedical applications are introduced. The emphasis is the unique properties and the potential uses of these sulfated polysaccharide hydrogels in wound healing, tissue engineering, and drug delivery systems.
2 Sulfated Polysaccharides from Different SeaweedsSeaweed, a plentiful marine resource, is commonly di-vided into three groups according to its pigmentation: red algae (Rhodophyta), brown algae (Phaeophyta), and green algae (Chlorophyta) (Wassie et al., 2021). Seaweeds contain a variety of bioactive compounds, among which sulfated polysaccharides are of particular interest as an important class of biomacromolecules (Jiao et al., 2011). In red algae, Carrageenan is a commonly found sulfated polysaccharide (Khotimchenko et al., 2020; Pradhan and Ki, 2023), while in brown algae, fucoidan is a notable representative (Van Weelden et al., 2019; Gao et al., 2021). Green algae primarily contain polysaccharides such as ulvan (Fig.1). These sulfated polysaccharides derived from different seaweeds have different structural characteristics, biological functions, and potential applications in pharmaceuticals, food, and biomaterials.
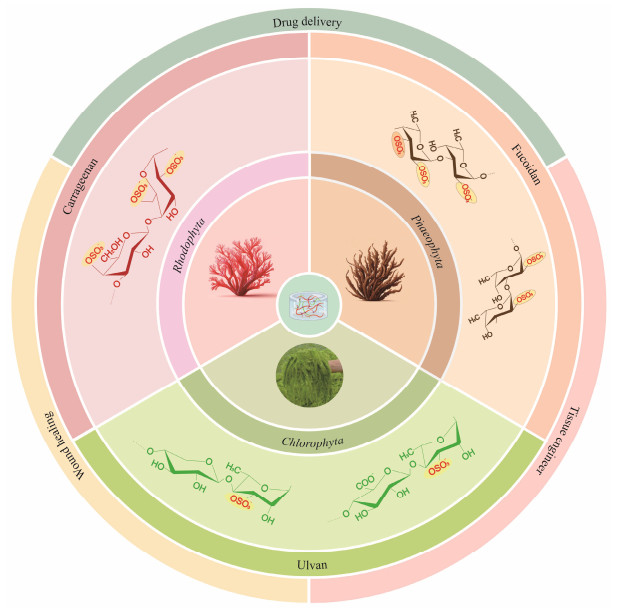
|
Fig. 1 Biomaterial sources and biomedical applications of sulfated polysaccharide-based hydrogels. |
Since the early 20th century, red seaweeds have been extensively consumed in Asian nations such as China, Japan, South Korea and Thailand, and were widely recognized for their health-promoting properties. With escalating demands from both food-related and industrial sectors, the cultivation of red seaweeds has notably expanded (Zhang et al., 2018). In China, the cultivation predominantly involves Kappaphycus, Eucheuma, Gracilaria, and Porphyra. These algae are particularly rich in polysaccharides, especially sulfated polysaccharides concentrated within in the cell wall matrix (Bhatia et al., 2013).
Carrageenan, a significant sulfated polysaccharide, is extracted from red macroalgae of the Rhodophyceae class, particularly from species like Chondrus, Gigartina, and Eucheuma (Liu et al., 2019). The fundamental monosaccharide unit of carrageenan is D-galactopyranose, whose chemical structure is with hydroxyl groups substituted by sulfate groups. These monosaccharides are linked together to form the long polysaccharide chains of carrageenan through alternating (1→3)-β-D- and (1→4)-α-D-glycosidic bonds (Jönsson et al., 2020). The position and number of sulfate substituents on carrageenans categorize them into γ-, β, δ-, α-, μ-, κ-, ν-, ι-, λ-, and θ-carrageenans (Liu et al., 2019). The κ, ι, and λ hydrogel forms have significant commercial value in the market (Cunha and Grenha, 2016) (Fig.2). Both κ-carrageenan and ι-carrageenan hold significant economic potential due to their gel and viscoelastic properties (Chen et al., 2019; Liu et al., 2019). They are capable of forming hydrogels and a three-dimensional double helix network through the crosslinking of neighboring sulfate groups. In contrast, λ-carrageenan does not form hydrogels as it lacks the cross-linking sulfate group that is necessary for such structures (Lester et al., 2020; Zhong et al., 2020).
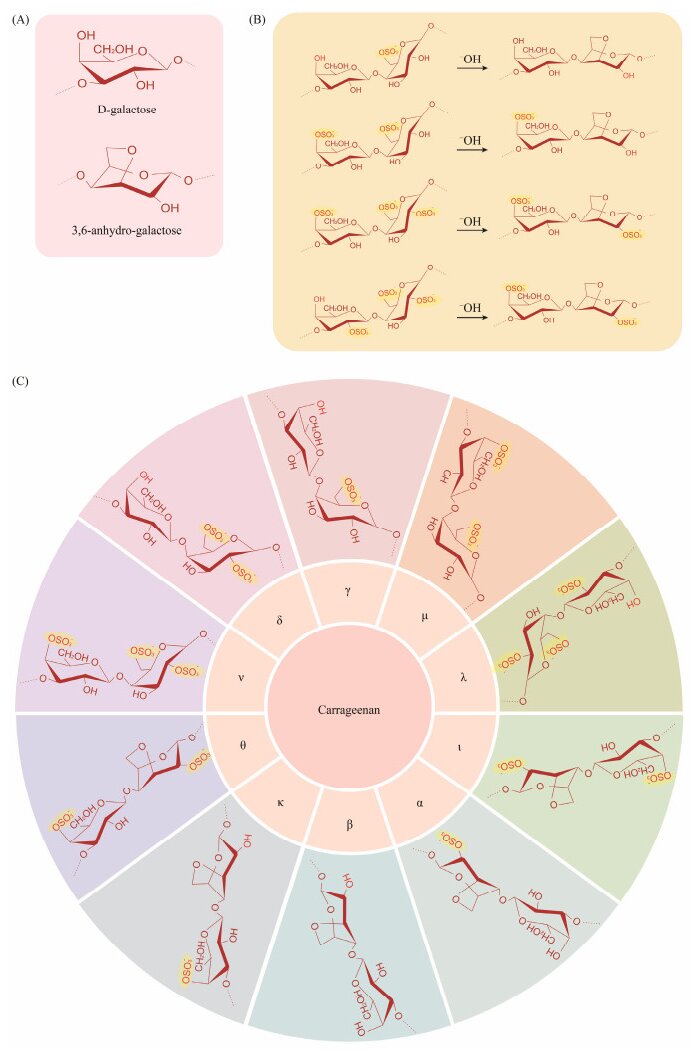
|
Fig. 2 Chemical structure of carrageenan. (A), monosaccharide composition; (B), conversion of various types of carragee-nan; (C), different types of carrageenan. |
Carrageenan can undergo physical or chemical cross-linking due to the hydroxyl and sulfate groups, enhancing its physicochemical properties and leading to novel activities and characteristics (Campo et al., 2009). As a vital biomaterial in the pharmaceutical field, carrageenan is used in drug formulations and has been documented to exhibit properties against respiratory diseases, tumor inhibition, and immune modulation (Pradhan and Ki, 2023). Researchers have extensively utilized carrageenan hydrogels in various biomedical and tissue generation applications, including drug delivery, wound dressing, and tissue engineering.
2.2 Fucoidan from Brown SeaweedBrown algae are a type of large seaweed belonging to the Phaeophyceae class within the algal phylum. They are predominantly found in cold marine environments, particularly around the Arctic and Antarctic regions (Coelho et al., 2020). The brown color of brown algae is due to the presence of fucoxanthin, a carotenoid pigment. Brown algae are crucial marine plants and serve as an important food source for humans and other marine organisms. The cell walls of brown algae are composed of various polysaccharides such as alginate, fucoidan, laminarin (also known as laminaran), and cellulose (Deniaud-Bouët et al., 2014).
Fucoidan is a type of sulfated polysaccharides predominantly found in brown algae and certain marine invertebrates, where it mainly functions as a structural polysaccharide (Fitton et al., 2015). Its chemical structure contains uronic acid, and is similar to vertebrate glycosaminoglycans (GAGs) which also consist of repetitive disaccharide units of an amino sugar and uronic acid. Its primary component is fucose, which is substituted with sulfate groups, It may also include other monosaccharides such as galactose, mannose, and xylose. Fucoidan can be broadly classified into two types. One type is composed of a backbone of α-1, 3-L-fucopyranose, while the second type consists of alternating α-L-fucopyranose residues linked by 1, 3- and 1, 4-bonds, some of which have branched structures. The majority of the sulfate groups are located at the C-2 position, with fewer at the C-3 and C-4 positions (Zayed et al., 2020) (Fig.3). The complexity of its structure varies significantly depending on the type of algae, its location, and the climate conditions.
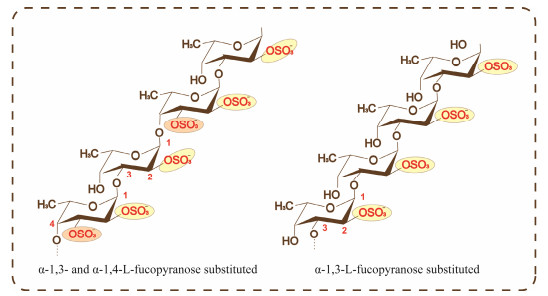
|
Fig. 3 Chemical structure of fucoidan. |
Fucoidan has attracted significant attention due to its diverse bioactive characteristics, such as anti-tumor (Van Weelden et al., 2019), anti-inflammatory (Li et al., 2021), antioxidative, anticoagulant, antithrombotic, and blood glucose-reducing properties (Li et al., 2008). However, despite these promising biological attributes, the application of fucoidan in biomedical scaffolds encounters challenges due to its high water solubility. This solubility hinders the creation of cohesive polymeric matrices without resorting to chemical crosslinking methods. Therefore, numerous studies advocate the incorporation of fucoidan with other materials such as chitosan, silk, gelatin, polycaprolactone, and hydroxyapatite to enhance its biomedical applications.
2.3 Ulvan from Green SeaweedUlvan, a water-soluble acidic polysaccharide, is derived from the cell walls of green seaweeds belonging to the Ulvales order, such as Ulva and Enteromorpha (formerly classified under Enteromorpha). It constitutes a significant portion, between 9% and 36%, of the dry weight of Ulva biomass (Tziveleka et al., 2019).
Ulvan primarily consists of sulfated rhamnose, uronic acids (including glucuronic acid and iduronic acid), and xylose. The main disaccharide units found in ulvans include ulvanobiuronic acid 3-sulfate β-D-GlcA (1→4) α-L-Rha 3S →1 (type A) and α-L-IdoA (1→4) α-L-Rha 3S→1 (type B), while minor variations sometimes feature glucuronic acid branches or partially sulfated xylose in place of uronic acid (Kidgell et al., 2019) (Fig.4).
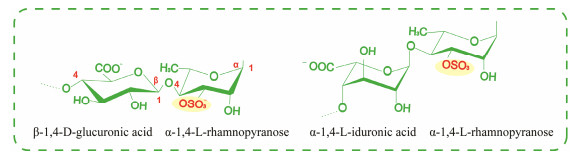
|
Fig. 4 Main repeating disaccharide unit of ulvan. |
Biologically, ulvan extracted from Ulva lactuca algae demonstrates a wide array of activities. It has been shown to possess immunoregulatory (Fernández-Díaz et al., 2017), anti-inflammatory (Kidgell et al., 2020), anticoagulant (Faggio et al., 2016), antitumor (Costa et al., 2010), antioxidant (Qi and Sun, 2015), and anti-hyperlipidemic properties (Qi et al., 2012). Structurally resembling mammalian glycosaminoglycans (GAGs), which are vital components of the extracellular matrix (ECM), ulvan holds promising potential in biomedicine, cosmetics, food, health products, and other industries (Shalaby et al., 2019).
3 Sulfated Polysaccharide HydrogelsCarrageenan, alginates, and agarose are natural sulfated polysaccharides derived from seaweeds, sharing similar structures characterized by sulfate groups. Therefore, they are classified as natural sulfated polysaccharides. These polysaccharides also contain hydroxyl groups, which are hydrophilic in nature, enabling them to form physically cross-linked hydrogels. Furthermore, chemical modifications can introduce additional functional groups, allowing the formation of chemically cross-linked hydrogels. Thus, sulfated polysaccharides from seaweed possess the potential to form hydrogels. Here, we briefly summarize their characterization methods and gelation mechanisms.
3.1 Characterization of Sulfated Polysaccharide Based Hydrogels 3.1.1 FTIRFourier transform infrared (FTIR) spectroscopy plays a crucial role in characterizing sulfated polysaccharide hydrogels, allowing for detailed analysis of their molecular structure and interactions. FTIR spectra provide insights into the presence of sulfate groups, hydroxyl groups, and other chemical functionalities within the polysaccharide backbone. Through FTIR analysis, researchers can discern the degree of sulfation, the types of bonding present (such as hydrogen bonding), and the overall conformational changes induced by gelation processes. This technique is indispensable for understanding the structural properties of sulfated polysaccharide hydrogels, thereby facilitating their development and application in various biomedical and industrial contexts (Arkin, 2013).
3.1.2 Scanning electron microscopy (SEM)Scanning electron microscopy (SEM) is an essential tool for characterizing sulfated polysaccharide hydrogels, offering detailed visualization of their microstructural features. SEM provides high-resolution images that reveal the surface morphology, pore structure, and overall architecture of hydrogels. Through SEM analysis, researchers can examine the uniformity of the gel network, the size and distribution of pores, and the effects of cross-linking on the gel structure. This technique is crucial for understanding the physical properties of sulfated polysaccharide hydrogels, which directly influence their mechanical strength, swelling behavior, and potential applications in various fields such as drug delivery, tissue engineering, and environmental remediation (Fischer et al., 2024).
3.1.3 Swelling degreesThe degree of swelling is a critical parameter for eva-luating the performance of sulfated polysaccharide-based hydrogels, as it directly relates to their capacity to absorb and retain water. This property is assessed by immersing the hydrogel sample in a solvent, typically water, and recording the increase in its weight over time until a state of equilibrium is reached. The swelling degree (SD) can be quantitatively expressed using the following formula (Ho et al., 2023):
| $ \text { Swelling degrees }=\frac{W_{\text {swollen }}-W_{\mathrm{dry}}}{W_{\mathrm{dry}}}, $ |
where Wswollen is the weight of the hydrogel after swelling, and Wdry is the initial dry weight of the hydrogel. This measurement offers valuable insights into the hydrogel's network structure and the density of its cross-linking, which are pivotal for its potential applications in areas such as drug delivery and tissue engineering.
3.1.4 RheologyRheological studies on sulfated polysaccharide hydro-gels are crucial for elucidating their viscoelastic properties, and are vital for applications in dynamic environments such as biomedical fields. By employing oscillatory shear tests, researchers can ascertain both the storage modulus (G') and the loss modulus (G''). The storage modulus (G') represents the hydrogel's ability to store energy and thus reflects its elastic behavior, while the loss modulus (G'') indicates the energy dissipated as heat, reflecting its viscous behavior. These parameters are integral in determining the hydrogel's gelling properties – how it transitions from a liquid to a gel under stress. Additionally, these tests help in assessing the hydrogel's viscosity, providing further insight into how it will flow and deform under applied forces. Understanding these properties is essential for predicting how the hydrogel will behave under physiological conditions, making it indispensable in the design of biomaterials (Noguchi and Kobayashi., 2022; Vo et al., 2022).
3.1.5 Mechanical propertiesThe mechanical properties of hydrogels, such as tensile strength, compressive strength, and elasticity, are fundamental for ensuring functionality in their intended applications. These properties are typically evaluated using standard material testing techniques, such as uniaxial tension or compression tests. For sulfated polysaccharide hydrogels, these measurements help in optimizing formulations for specific applications, such as scaffolds in tissue engineering or carriers in drug delivery systems (Dattilo et al., 2023).
3.2 Hydrogel Forming Mechanism of Sulfated Polysaccharide HydrogelsThe formation of hydrogel networks from sulfated polysaccharides can be classified into physical and chemical cross-linkings (Fig.5).
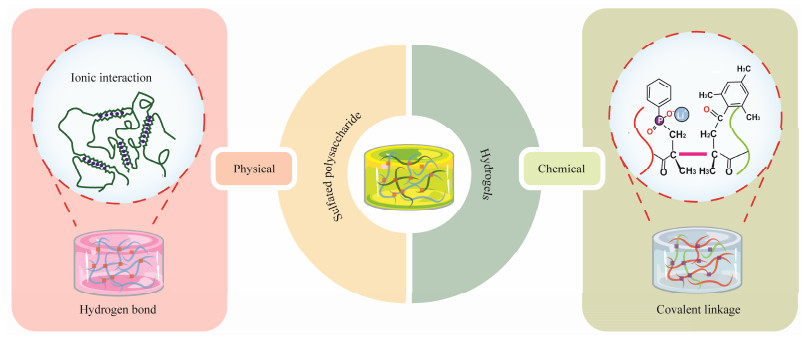
|
Fig. 5 Formation of hydrogel networks from sulfated polysaccharides. |
The principle of physical cross-linking in forming hydrogels from sulfated polysaccharides involves non-covalent interactions such as ionic bonds, hydrogen bonds, and hydrophobic interactions. This method does not require chemical reactions to form permanent bonds, making the process reversible and allowing for easy modification of the hydrogel properties in response to environmental stimuli (Hu et al., 2023).
Alginate hydrogels, for instance, are often cross-linked using calcium ions, which interact with the guluronic acid blocks of alginate to form a gel in a process known as the 'egg-box' model (Malektaj et al., 2023). This method is valued for its rapid gelation and ability to form hydrogels with varied mechanical properties and swelling behaviors, depending on the concentration and type of cations. Based on a similar principle, the sulfate groups of the aforementioned sulfated polysaccharides can form physical crosslinking structures with calcium ions, among others, which can serve as a method for forming hydrogels through physical cross-linking of sulfated polysaccharides (Liu et al., 2020).
The gelling capability of carrageenan is primarily attri-buted to the double helical structures within its polysaccharide molecules. These structures stabilize in the presence of appropriate ions, typically potassium or calcium, and under suitable temperature conditions, forming a crosslinked network. At lower temperatures, intermolecular hydrogen bonding among carrageenan molecules leads to the formation of double helices. When the temperature increases, these helical structures dissociate, causing the gel to melt. Upon cooling, the double helices reform, resulting in gelation of the solution. This thermoreversible nature is a key feature of carrageenan hydrogels (Pacheco-Quito et al., 2020). Additionally, the gelation of carrageenan can be enhanced through ionic cross-linking. Specific ions, such as calcium, interact with the sulfate groups in carrageenan to form a more stable network structure, thereby enhancing the mechanical properties and stability of the gel. This ionic interaction is critical for the robust applications of carrageenan hydrogels in both biomedicine and food industries.
Ulvan is a sulfated polysaccharide rich in hydroxyl groups within its structure. These hydroxyl groups can engage in complex interactions with borate ions, where borate ions can cross-link with two adjacent hydroxyl groups to form a stable diol structure. This interaction between borate and the hydroxyl groups leads to the formation of a stable crosslinked network among ulvan molecules. When such crosslinking occurs among multiple ulvan molecules, a three-dimensional gel network can be formed. If this is combined with polyacrylamide to create a double network hydrogel, the overall network structure of the hydrogel is enhanced. The polyacrylamide can intertwine with the ionically crosslinked ulvan network, and hydrogen bonding between them further strengthens the mechanical properties of the hydrogel (Jiang et al., 2021). This arrangement results in a hydrogel with robust mechanical characteristics.
3.2.2 Chemical methodsSulfated polysaccharides, characterized by their chemical structures rich in hydroxyl, carboxyl, and sulfate groups, can undergo various chemical modifications enabling covalent crosslinking reactions between sulfated polysaccharide derivatives to form three-dimensional hydrogels. Numerous chemical crosslinking methods exist, with hydrogel networks also potentially formed by dynamic covalent bonds, including established click chemistry reactions, Diels-Alder (DA) reactions, Michael addition reactions, Schiff base reactions, and free radical polymerization (Hu et al., 2019). Compared to physically crosslinked hydrogels, chemically crosslinked sulfated polysaccharide hydrogels are more stable, exhibit superior mechanical properties, and their degradation can be regulated.
Photopolymerization of hydrogels currently has a widespread impact in the field of 3D printing. The double bond sites of the methacrylate groups exhibit high reactivity, which can lead to the formation of radicals upon excitation by a photoinitiator, resulting in free radical polymerization to form hydrogel (Sun et al., 2020).
Click chemistry has attracted much attention in recent years due to its rapid reaction, high selection, and good biocompatibility. Through the modification of click groups on sulfated polysaccharide, the cross-linking of two sulfated polysaccharide derivatives can be triggered by chemical cross-linking to form hydrogels (Yu et al., 2015).
4 Biomedical Applications of Sulfated Polysaccharide Hydrogels Derived from SeaweedsSulfated polysaccharides derived from marine sources possess numerous biomedical activities. Based on their polysaccharide properties, they can be formulated into hydrogels, exhibiting unique efficacy in wound healing, drug delivery, and tissue engineering applications.
4.1 Biomedical Applications in Wound Healing 4.1.1 Carrageenan-based hydrogels for wound healingIn wound healing, carrageenan-based hydrogels can provide a moist environment and promote cell migration due to their water solubility, as well as absorb wound exudate. These hydrogels exhibit low cytotoxicity, antibacterial, and antioxidant properties, indicating their potential for healing both acute and chronic wounds (Liu et al., 2020).
Ulagesan et al. (2023) prepared a composite hydrogel using natural polysaccharide κ-carrageenan and phycobiliprotein from Porphyra yezoensis, which effectively promoted the migration of fibroblasts and accelerated wound healing. Raghunathan et al. (2024) developed nanofibers using natural microalgae-derived peptides, polycaprolactone (PCL), and κ-carrageenan through an electrospinning method. These nanofibers demonstrated good biocompatibility and significant self-healing characteristics, which effectively resisted bacteria, promoted cell proliferation, and accelerated wound healing (Fig.6A). Abou-Okeil et al. (2021) created a hyaluronic acid/oxidation κ-carrageenan electrospun nanofiber hydrogel with multifunctional properties. This hydrogel can inhibit both gram-positive and gram-negative microorganisms and promote wound healing. Gouda et al. (2021) prepared electrospun nanofiber scaffolds that was composed of polyvinyl alcohol/carrageenan and partially reduced graphene oxide. These scaffolds exhibited effective antibacterial and inhibitory activity, as well as promoted re-epithelialization, induced hair follicle growth, reduced scar formation, and successfully accelerated wound healing and skin repair. Sathuvan et al. (2023) developed a biopolymer composite film consisting of κ-carrageenan and coriander essential oil for wound healing (Fig.6B). This composite film can influence cell adhesion, F-actin tissue, and collagen synthesis, as well as promote mechanical induction activation in vitro and enhance wound healing in vivo. Khodaei et al. (2023) developed a new antibacterial and self-healing hydrogel made of aldehyde carrageenan (Fig.6C). They produced the hydrogels through NaIO oxidation, followed by the addition of dopamine and zinc ions. The hybrid H-OCA Dop Zn hydrogel exhibited excellent wound healing potential.
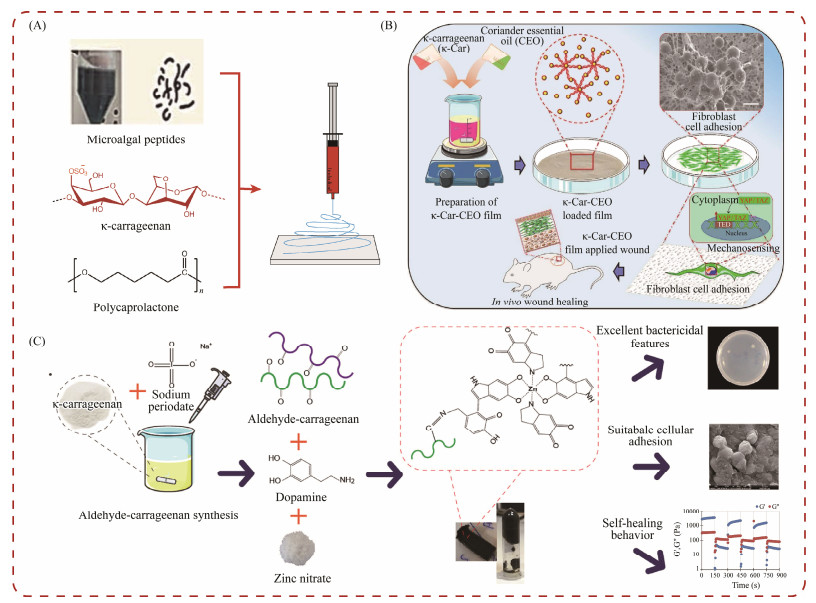
|
Fig. 6 Carrageenan hydrogels for wound healing. (A), carrageenan for nanofibers; (B), κ-Car composite film-based bioactive materials; (C), carrageenan for antibacterial and self-healing hydrogel. |
Fucoidan, a hydrophilic polysaccharide, is renowned for its significant physical properties, which makes it an ideal candidate for managing skin burns and wounds. These properties include high wound exudate absorption, strong adherence to mucosal surfaces, the capacity to attract and re-tain moisture, and oxygen permeability. Moreover, fucoidan exhibits pharmacological effects similar to heparin, such as anticoagulant, antithrombotic, and anti-inflammatory functions.
In a recent study, a hydrogel matrix was formulated using a combination of fucoidan, chitosan, alginate, carboxymethyl cellulose (CMC), and gellan gum (Fig.7A). Gellan gum was employed to stabilize the hydrogel in environments containing metallic ions, while CMC was employed to bolster the mechanical integrity of the matrix. The study indicated a significant acceleration in wound healing, characterized by increased expression of fibroblasts and collagen fibers. These findings highlight the potential of this fucoidan-based hydrogel as an effective therapeutic option for promoting rapid and efficient healing in the treatment of skin wounds (Karuppusamy et al., 2020).
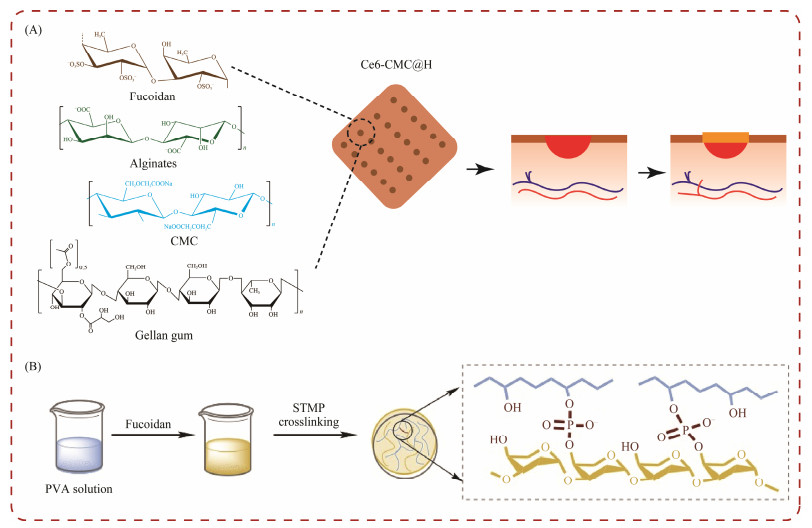
|
Fig. 7 Fucoidan hydrogels for wound healing. (A), fucoidan used in self-Ce6-CMC@H hydrogel; (B), fucoidan for PVA hydrogels. |
Yao et al. (2023) studied the application of fucoidan in enhancing the endothelialization and hemocompatibility of PVA hydrogels (Fig.7B). By integrating fucoidan, the researchers achieved a notable improvement in the adhesion and spread of endothelial cells on the PVA, while maintained its blood compatibility. The in vivo trials of these small diameters, fucoidan-enhanced PVA grafts showed favorable results, such as increased patency rates and a reducetion in the formation of intimal hyperplasia. This study underscores the versatility of fucoidan not only in wound healing but also as a surface-modifying agent for PVA grafts, demonstrating its broader potential in the development of vascular implants and devices (Yao et al., 2023).
4.1.3 Ulvan-based hydrogels for wound healingUlvan-based hydrogels have garnered significant attention for their potential in enhancing wound healing processes. These hydrogels, derived from marine sources like Ulva species, exhibit favorable properties that contribute to their therapeutic efficacy in treating various types of wounds (Jiang et al., 2022).
Researchers have explored diverse approaches to harness ulvan's benefits in wound healing. Ren et al. (2022) demonstrated the integration of human umbilical cord mesenchymal stem cells (hUC MSCs) into ulvan-based hydrogels. Their study highlighted ulvan's role in promoting cell proliferation and migration, which is crucial for accelerating wound closure, particularly in diabetic patients (Ren et al., 2022). Kikionis et al. (2022) developed non-woven nanofiber patches incorporating ulvan and polyethylene oxide (PEO). These patches exhibited potent anti-inflammatory properties, which is essential for reducing inflammation and facilitating effective wound healing without adverse reactions. Their innovative approach underscores ulvan's versatility in managing chronic wounds. Sulastri et al. (2023) formulated ulvan polysaccharide hydrogel films embedded with silver nanoparticles. This formulation can not only control inflammation but also promote re-epithelialization, angiogenesis, showing strong antibacterial effects. Such multifaceted benefits highlight ulvan's potential as a therapeutic agent for enhancing wound healing.
Ulvan-based hydrogels represent a promising avenue in wound care, leveraging their biological activities to address various aspects of the wound healing process. Further research on optimizing ulvan's properties and exploring new formulations holds promise for advancing clinical applications in wound management.
4.2 Biomedical Applications of in Drug Delivery Systems 4.2.1 Carrageenan-based hydrogels for delivery systemsCarrageenan has been increasingly employed as a beneficial compound in pharmaceutical formulations due to its unique physicochemical properties. These properties make carrageenan suitable as a delivery modifier in controlled-release systems, such as stabilizing agents, permeability enhancers, disintegrators, coatings, thickeners, and solubilizers. Additionally, carrageenan can facilitate sustained drug release by forming complexes with drugs and by its ability to form gels through heat-reversible gelation and ionic interactions. This is attributed to the strong anionic surface charge of carrageenan, which is helpful in the delivery of molecules such as anticancer drugs and proteins.
Santhamoorthy et al. (2023) developed a dual stimuli-responsive hydrogel using magnetic iron oxide nanoparticles and κ-carrageenan, achieving high drug loading and enhanced release with pH and temperature stimuli, demonstrating its biocompatibility for cancer therapy applications. Furthermore, Safarpour et al. (2023) introduced a red blood cell membrane vesicle-laden methacrylate kappa-carrageenan (KaMA) composite hydrogel, significantly enhancing compressive strength, toughness, and controlled drug release, while supporting fibroblast cell growth for soft tissue engineering applications.
These advancements highlight the versatility and efficacy of carrageenan-based hydrogels in drug delivery, offering promising solutions for sustained and controlled therapeutic applications.
4.2.2 Fucoidan-based hydrogels for delivery systemsRecent research has explored the application of fucoidan in drug delivery systems. In a study by Egle and his colleagues, self-assembled fucoidan/chitosan (FU_CS) hydrogels were engineered to counteract the rapid degradation and swift release of bioactive factors from Platelet-rich fibrin (PRF), a substance derived from human blood (Fig.7A). The findings from this study demonstrate that PRF/FU_ CS hydrogels facilitate sustained release of growth factors, significantly enhancing the potential of fucoidan/chitosan (FU_CS) hydrogels for wound healing and tissue regeneration (Egle et al., 2024).
Dou et al. (2023) developed a composite hydrogel using fucoidan and calcium alginate, incorporating probiotics to create bioactive patches for oral ulcers (Fig.8B). The material demonstrates excellent wet adhesion, appropriate swelling, and robust mechanical properties. It facilitates sustained release of probiotics and exhibits good storage stability. The inclusion of fucoidan significantly enhances the physicochemical properties of the composite hydrogel. This innovative approach holds considerable potential for the management of oral ulcers (Dou et al., 2023).
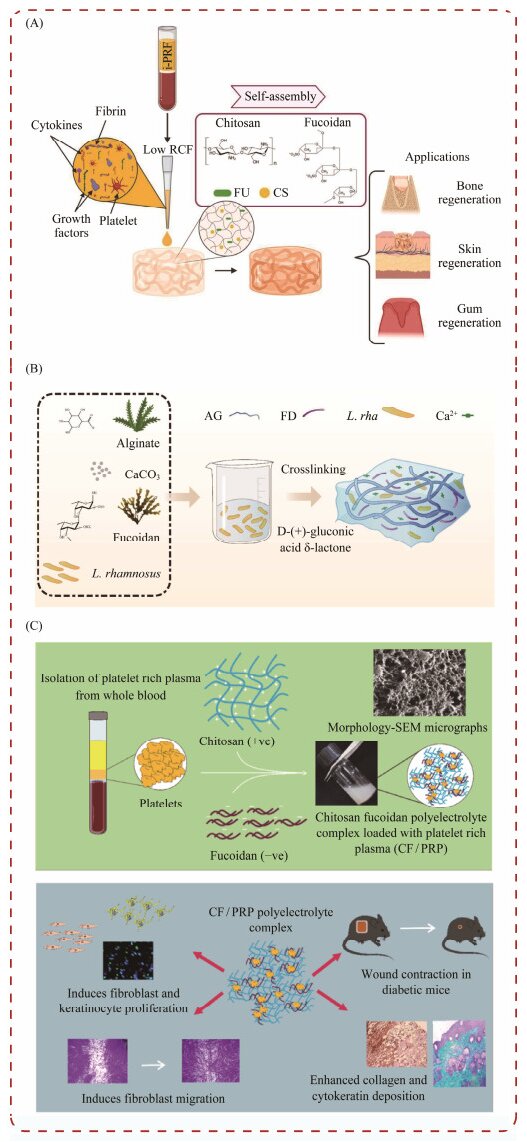
|
Fig. 8 Fucoidan hydrogels used in the drug delivery. (A), fucoidan is used in self-assembled fucoidan/chitosan (FU_CS) hydrogels; (B), fucoidan for bone tissue engineering scaffold preparation; (C), fucoidan for Nap-FFGRGD@FU hydrogel. |
Rao et al. (2022) engineered a polyelectrolyte complex matrix using chitosan and fucoidan extracted from Fucus vesiculosus, and specifically designed for diabetic wound therapy (Fig.8C). This matrix was utilized to administer platelet-rich plasma (PRP), which is enriched with growth factors and cytokines. The innovative formulation facilitated a sustained release of PRP for up to 72 h at 37℃, significantly promoting cell proliferation and collagen deposition in the in vivo experiments (Rao et al., 2022).
4.2.3 Ulvan-based hydrogels for delivery systemsThe hydrogel based on ulvan has been extensively studied in drug delivery. The efficacy of drugs or bioactive compounds depends on factors such as degradation, release rate, effective concentration, and interactions with targets (Chyzy et al., 2020). Polysaccharide gels, with their larger pores, can effectively load drugs with maintaining their activity and ensuring a slow-release effect (Goller and Turner, 2020).
The water insolubility of many bioactive compounds, including the polyphenol curcumin known for its diverse biological activities such as anti-tumor effects, limits their applications. Bang et al. (2019) modified ulvan through chemical modification, creating an acetylated ulvan nano-gel that can self-assembly to effectively carry and deliver water-insoluble bioactive compounds. Tziveleka et al. (2018) prepared lysozyme/ulvan complexes under physiological pH conditions with various charge ratios, which can effecttively preserve lysozyme's antibacterial activity. In another study, they incorporated ulvan into liposomes, enhancing the physical and chemical properties, as well as the stability of liposomes by interacting with neutral, negatively charged, or positively charged lipids (Tziveleka et al., 2022).
4.3 Biomedical Applications of Tissue Engineering 4.3.1 Carrageenan-based hydrogels for tissue engineeringIn recent years, natural carrageenan has demonstrated significant potential for the development of hydrogels with valuable properties such as high swelling rate, thermal stability, and mechanical qualities (Popa et al., 2011; Yegappan et al., 2018). These hydrogels can be applied in various biomedical fields, including tissue engineering and wound healing (Yegappan et al., 2018). For example, in bone tissue engineering, hydrogels composed of collagen-hydroxyapatite/κ-carrageenan have been utilized. κ-carrageenan has been employed as a crosslinker to enhance the composite's properties by reducing hygroscopicity and improving biodegradability (Feng et al., 2017). Another study focused on creating a porous bioactive hydrogel for bone tissue engineering (Fig.9A), which consisted of calcium silicate, carrageenan, and sodium alginate (Popa et al., 2011; Feng et al., 2017; Yegappan et al., 2018).
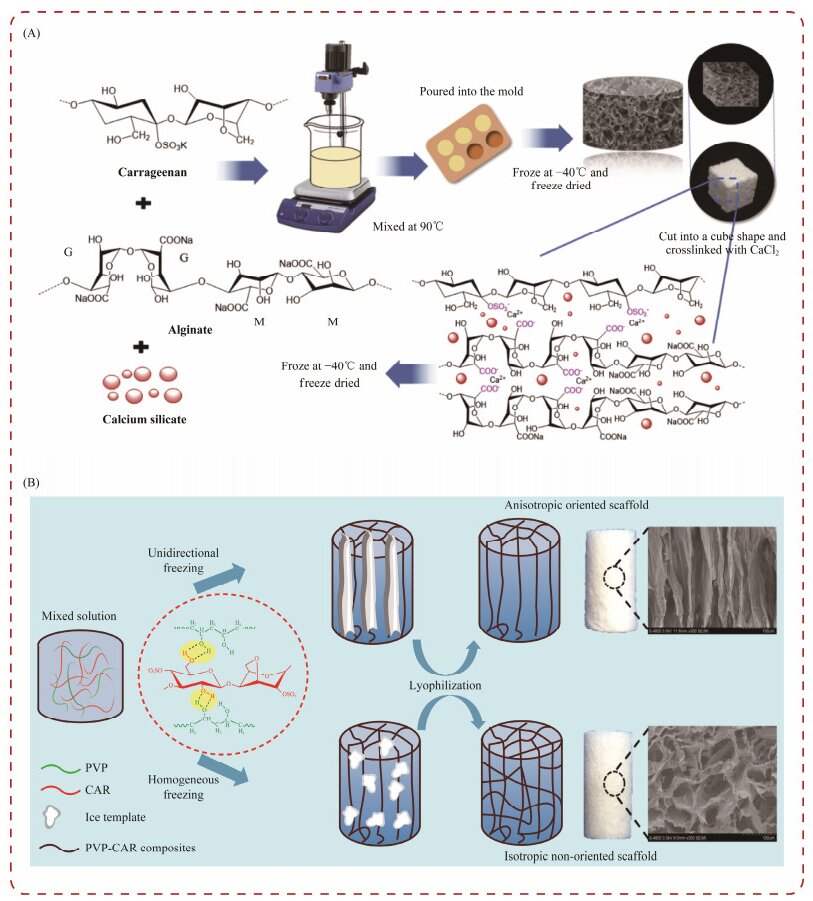
|
Fig. 9 Carrageenan hydrogels for tissue engineering. (A), carrageenan for a porous bioactive hydrogel; (B), carrageenan for the preparation of PVA-CAR scaffolds. |
Interestingly, the hydrogel showed the growth of hydroxyapatite crystals on its surface when immersed in simulated body fluid at 37℃. This hydrogel also demonstrated potential as a drug delivery platform for diclofenac, which can be used to treat postoperative acute inflammation during bone tissue engineering. In another study, an alginate/κ-carrageenan hydrogel was developed using 3D bioprinting. The incorporation of κ-carrageenan in the hydrogel increased its potential as a bio-ink, which can be used in the fabrication of 3D printing systems with excellent mechanical properties (Kim et al., 2019). Zhang et al. (2016) utilized polyvinyl alcohol (PVA) and carrageenan to create a biomimetic hydrogel system with a unique pore structure (Fig.9B). This hydrogel exhibited a hub pore structure, which is different with non-directional scaffolds prepared with traditional methods. The biomimetic hydrogel was able to mimic the biochemical and physical cues of the natural cartilage extracellular matrix, promoting the proliferation and differentiation of chondrocyte cells in vitro, thus showing potential for cartilage repair. Tytgat et al. (2019) also developed a scaffold using methacrylamide-modified gelatin and κ-carrageenan.
4.3.2 Fucoidan-based hydrogels for tissue engineeringResearchers have considered utilizing fucoidan to produce self-assembling hydrogels through co-assembly or conjugation with peptides and polysaccharides, as shown in Fig.10A. These hybrid hydrogels exhibited favorable mechanical strength and biological functions, making them suitable for applications in tissue engineering (Radvar and Azevedo, 2019).
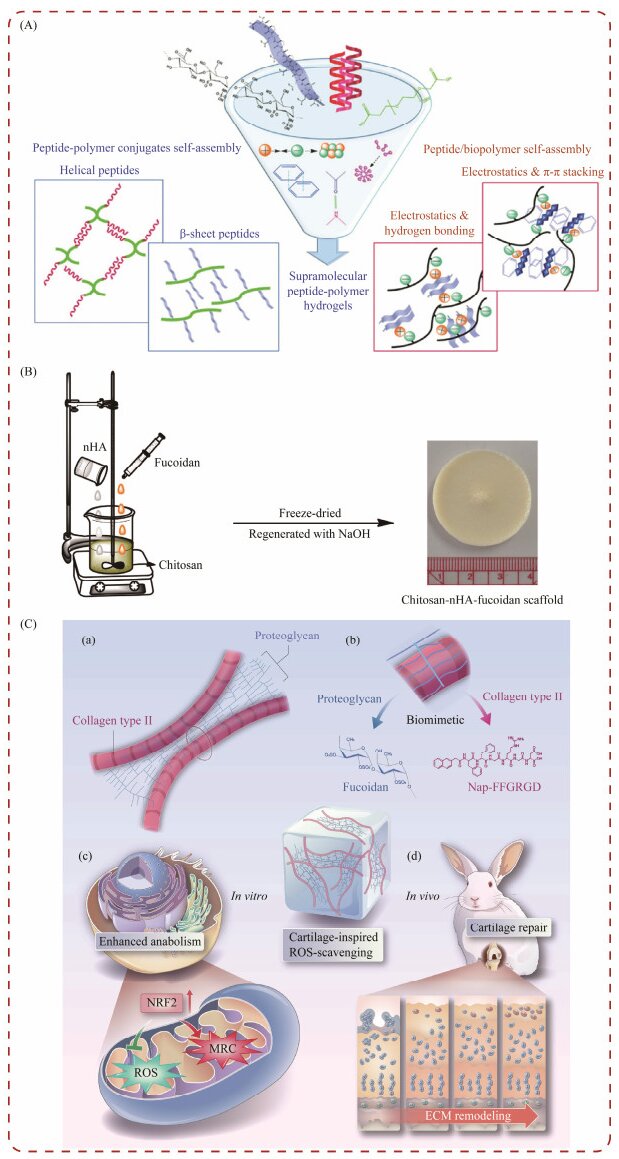
|
Fig. 10 Fucoidan hydrogels used in the field of tissue engineering. (A), fucoidan is used in self-assembling hydrogels; (B), fucoidan for bone tissue engineering scaffold preparation; and (C), fucoidan for Nap-FFGRGD@FU hydrogel. |
Lowe and colleagues synthesized scaffolds for bone tissue engineering (Fig.10B), incorporating chitosan, alginate, and fucoidan sourced from Fucus vesiculosus. Their findings indicate that integrating fucoidan significantly boosts the secretion of various biomarkers by human adipose tissue-derived stem cells. Furthermore, the team engineered three-dimensional scaffolds using fucoidan, nano-hydroxyapatite, and chitosan. This composition was pivotal in promoting the release of essential factors critical for osteoblast proliferation and tissue formation, highlighting fucoidan's vital role in enhancing bone regeneration (Lowe et al., 2016).
Zhao and colleagues engineered a hydrogel, termed Nap-FFGRGD@FU, which draws inspiration from the natural structure of cartilage (Fig.10C). This hydrogel comprises marine-derived fucoidan (FU) and a peptide known as Nap-FFGRGD, assembled through a simple self-assembling process. The architecture of Nap-FFGRGD@FU hydrogels mirrors the natural cartilage, featuring an interwoven network of collagen-like fibers and proteoglycans. Fucoidan not only mimics the structural attributes of cartilage but also imparts significant biological activity. Additionally, the inclusion of the Arg-Gly-Asp (RGD) sequence within the peptide furnishes cellular attachment sites, thereby enhances the hydrogel's functionality. This synergistic effect facilitates the production of the extracellular matrix (ECM) and the reduction of reactive oxygen species (ROS), which supports a conducive environment for chondrocyte interaction and ECM integration (Zhao et al., 2023).
4.3.3 Ulvan-based hydrogels for tissue engineeringUlvan-based hydrogels have shown remarkable potential in tissue engineering due to their versatile properties and biocompatibility. Mariia et al. (2021) developed a composite hydrogel combining chitosan and ulvan with cellulose nanocrystals (CNCs), significantly enhancing mechanical properties and cell proliferation capacity. This innovation underscores the mechanical robustness and bioactivity required for effective tissue engineering scaffolds. Terezaki et al. (2022) advanced this field by designing an electrospun nanofiber system incorporating ulvan and gelatin. Their system exhibited accelerated wound contraction and anti-inflammatory properties, which are crucial for tissue regeneration and healing. Jiang et al. further contributed by synthesizing a composite hydrogel (PEP-PAM) from Enteromorpha prolifera polysaccharides (Fig.11A). This hydrogel demonstrated strong mechanical strength, self-healing capabilities, and tissue adhesion, promoting cell proliferation and migration that is essential for tissue repair and regeneration (Jiang et al., 2021). Jiang et al. also developed an injectable double-network hydrogel (OPAB) characterized by adjustable gelation, pH-sensitive biodegradability, and efficient wound healing capabilities through curcumin nanoparticle delivery (Fig.11B). This hydrogel represents a significant advancement in providing adaptable and responsive scaffolds for tissue engineering (Jiang et al., 2024). Yang et al. created an antioxidant nanofiber facial mask using E. prolifera polysaccharides, showing superior antioxidant capacity and moisture retention, highlighting its potential for skin tissue applications (Yang et al., 2024).

|
Fig. 11 Ulvan-based hydrogels for tissue engineering. (A), PEP/PAM hydrogels; (B), an injectable double-network hydro-gel (OPAB) characterized by adjustable gelation, pH-sensitive biodegradability, and efficient wound healing capabilities through curcumin nanoparticle delivery. |
Collectively, these studies illustrate the versatile appli-cations and significant benefits of ulvan-based hydrogels in tissue engineering, paving the way for innovative solutions in regenerative medicine and therapeutic interventions.
5 Summary and ProspectMarine-derived sulfated polysaccharides, due to their structural similarity to the extracellular matrix (ECM) of human cells, represent a sustainable and natural alternative to mammalian polysaccharides for biomedical applications. These polysaccharides offer significant advantages, including reduced zoonotic disease risk and fewer social and religious constraints. Hydrogels derived from marine sulfated polysaccharides exhibit exceptional water retention, biocompatibility, biodegradability, and customizable structural and network morphologies. These properties enable them to replicate the complex architecture of natural ECM, thereby enhancing cellular functions and signaling pathways while inhibiting bacterial growth. Consequently, these hydrogels hold immense potential in the field of biomedical materials research and development. Despite their broad applicability and cost-effectiveness, the commercial application of marine-derived sulfated polysaccharide hydrogels faces several challenges. These include the potential introduction of harmful substances during hydrogel modification processes and difficulties in controlling the pore size, mechanical strength, swelling capacity, and responsiveness to external stimuli without grafting or utilizing singlecomponent formulations. Recent advancements in materials science and biomedicine have catalyzed interest in the de-velopment of composite, functionalized, and smart hydrogel materials. There is a growing focus on creating a controllable sulfated polysaccharide hydrogel system capable of mimicking the properties of skin, internal organs, bones, and other human tissues, thereby advancing the field of regenerative medicine. Marine sulfated polysaccharides are expected to play a pivotal role in the future exploration and development of innovative biomaterials, paving the way for new therapeutic strategies and methods.
AcknowledgementsThis work was funded by the Shandong Provincial Key Research and Development Program (No. 2019GSF107031). We gratefully acknowledge the financial support provided by this program, which was crucial for the successful completion of this work.
Abou-Okeil, A., Fahmy, H. M., Fouda, M. M. G., Aly, A. A., and Ibrahim, H. M., 2021. Hyaluronic acid/oxidized κ-carrageenan electrospun nanofibers synthesis and antibacterial properties. BioNanoScience, 11: 687-695. DOI:10.1007/s12668-021-00884-9 (  0) 0) |
Arkin, I., 2013. FTIR spectroscopy is a powerful tool. Biochimi-ca et Biophysica Acta, 1828(10): 2255. DOI:10.1016/j.bbamem.2013.06.027 (  0) 0) |
Bang, T. H., Van, T. T. T., Hung, L. X., Ly, B. M., Nhut, N. D., Thuy, T. T. T., et al., 2019. Nanogels of acetylated ulvan enhance the solubility of hydrophobic drug curcumin. Bulletin of Materials Science, 42(1): 1-7. DOI:10.1007/s12034-018-1682-3 (  0) 0) |
Bhatia, S., Rathee, P., Sharma, K., Chaugule, B. B., Kar, N., and Bera, T., 2013. Immuno-modulation effect of sulphated polysaccharide (porphyran) from Porphyra vietnamensis. International Journal of Biological Macromolecules, 57: 50-56. DOI:10.1016/j.ijbiomac.2013.03.012 (  0) 0) |
Campo, V. L., Kawano, D. F., da Silva, D. B., and Carvalho, I., 2009. Carrageenans: Biological properties, chemical modifications and structural analysis. Carbohydrate Polymers, 77(2): 167-180. DOI:10.1016/j.carbpol.2009.01.020 (  0) 0) |
Chen, Y., Sheng, Q., Hong, Y., and Lan, M., 2019. Hydrophilic nanocomposite functionalized by carrageenan for the specific enrichment of glycopeptides. Analytical Chemistry, 91(6): 4047-4054. DOI:10.1021/acs.analchem.8b05578 (  0) 0) |
Chyzy, A., Tomczykowa, M., and Plonska-Brzezinska, M. E., 2020. Hydrogels as potential nano-, micro- and macro-scale systems for controlled drug delivery. Materials, 13(1): 188. DOI:10.3390/ma13010188 (  0) 0) |
Coelho, S. M., Peters, A. F., Müller, D., and Cock, J. M., 2020. Ectocarpus: An evo-devo model for the brown algae. EvoDevo, 11: 19. DOI:10.1186/s13227-020-00164-9 (  0) 0) |
Costa, L. S., Fidelis, G. P., Cordeiro, S. L., Oliveira, R. M., Sabry, D. A., Camara, R. B. G., et al., 2010. Biological activities of sulfated polysaccharides from tropical seaweeds. Elsevier Masson, 64(1): 21-28. DOI:10.1016/j.biopha.2009.03.005 (  0) 0) |
Cunha, L., and Grenha, A., 2016. Sulfated seaweed polysaccha-rides as multifunctional materials in drug delivery applications. Marine Drugs, 14(3): 42. DOI:10.3390/md14030042 (  0) 0) |
Dattilo, M., Patitucci, F., Prete, S., Parisi, O. I., and Puoci, F., 2023. Polysaccharide-based hydrogels and their application as drug delivery systems in cancer treatment: A review. Journal of Functional Biomaterials, 14(2): 55. DOI:10.3390/jfb14020055 (  0) 0) |
Deniaud-Bouët, E., Kervarec, N., Michel, G., Tonon, T., Kloareg, B., and Hervé, C., 2014. Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Annals of Botany, 114(6): 1203-1216. DOI:10.1093/aob/mcu096 (  0) 0) |
Dou, X., Li, G., Wang, S., Shao, D., Wang, D., Deng, X., et al., 2023. Probiotic-loaded calcium alginate/fucoidan hydrogels for promoting oral ulcer healing. International Journal of Biological Macromolecules, 244: 125273. DOI:10.1016/j.ijbiomac.2023.125273 (  0) 0) |
Egle, K., Dohle, E., Hoffmann, V., Salma, I., Al-Maawi, S., Ghanaati, S., et al., 2024. Fucoidan/chitosan hydrogels as carrier for sustained delivery of platelet-rich fibrin containing bioactive molecules. International Journal of Biological Macromolecules, 262: 129651. DOI:10.1016/j.ijbiomac.2024.129651 (  0) 0) |
Faggio, C., Pagano, M., Dottore, A., Genovese, G., and Mora-bito, M., 2016. Evaluation of anticoagulant activity of two algal polysaccharides. Natural Product Research, 30(17): 1934-1937. DOI:10.1080/14786419.2015.1086347 (  0) 0) |
Feng, W. P., Feng, S. Y., Tang, K. Y., He, X. C., Jing, A. H., and Liang, G. F., 2017. A novel composite of collagen-hydroxyapatite/kappa-carrageenan. Journal of Alloys and Compounds, 693: 482-489. DOI:10.1016/j.jallcom.2016.09.234 (  0) 0) |
Fernández-Díaz, C., Coste, O., and Malta, E. J., 2017. Polymer chitosan nanoparticles functionalized with Ulva ohnoi extracts boost in vitro ulvan immunostimulant effect in Solea senegalensis macrophages. Algal Research, 26: 135-142. DOI:10.1016/j.algal.2017.07.008 (  0) 0) |
Fischer, E. R., Hansen, B. T., Nair, V., Hoyt, F. H., Schwartz, C. L., and Dorward, D. W., 2024. Scanning electron microscopy. Current Protocols, 4(5): e1034. DOI:10.1002/cpz1.1034 (  0) 0) |
Fitton, J. H., Stringer, D. N., and Karpiniec, S. S., 2015. Therapies from fucoidan: An update. Marine Drugs, 13(9): 5920-5946. DOI:10.3390/md13095920 (  0) 0) |
Gao, S. K., Yin, R., Wang, X. C., Jiang, H. N., Liu, X. X., Lv, W., et al., 2021. Structure characteristics, biochemical properties, and pharmaceutical applications of alginate lyases. Marine Drugs, 19(11): 628-628. DOI:10.3390/md19110628 (  0) 0) |
Goller, S., and Turner, N. J., 2020. The antimicrobial effective-ness and cytotoxicity of the antibiotic-loaded chitosan: ECM scaffolds. Applied Sciences, 10(10): 3446. DOI:10.3390/app10103446 (  0) 0) |
Gouda, M. H., Ali, S. M., Othman, S. S., Abd Al-Aziz, A., Abu-Serie, M. M., Elsokary, N. A., et al., 2021. Novel scaffold based graphene oxide doped electrospun iota carrageenan/polyvinyl alcohol for wound healing and pathogen reduction: In-vitro and in-vivo study. Scientific Reports, 11: 20456. DOI:10.1038/s41598-021-00069-0 (  0) 0) |
Ho, C. Y., Wang, C. C., Wu, T. C., Kuan, C. H., Liu, Y. C., and Wang, T. W., 2023. Peptide-functionalized double network hydrogel with compressible shape memory effect for intervertebral disc regeneration. Bioengineering & Translational Medicine, 8(2): e10447. DOI:10.1002/btm2.10447 (  0) 0) |
Hu, W., Wang, Z., Xiao, Y., Zhang, S., and Wang, J., 2019. Ad-vances in crosslinking strategies of biomedical hydrogels. Biomaterials Science, 7(3): 843-855. DOI:10.1039/c8bm01246f (  0) 0) |
Jiang, F., Chi, Z., Ding, Y., Quan, M., Tian, Y., Shi, J., et al., 2021. Wound dressing hydrogel of Enteromorpha prolifera polysaccharide-polyacrylamide composite: A facile transformation of marine blooming into biomedical material. ACS Applied Materials & Interfaces, 13(12): 14530-14542. DOI:10.1021/acsami.0c21543 (  0) 0) |
Jiang, F., Ding, Y., Tian, Y., Yang, R., Quan, M., Tong, Z., et al., 2022. Hydrolyzed low-molecular-weight polysaccharide from Enteromorpha prolifera exhibits high anti-inflammatory activity and promotes wound healing. Biomaterials Advances, 133: 112637. DOI:10.1016/j.msec.2021.112637 (  0) 0) |
Jiang, F., Li, L., Tian, Y., Su, Y., Zhao, T., Ren, R., et al., 2024. Enteromorpha Prolifera polysaccharide-derived injectable hydrogel for fast intraoperative hemostasis and accelerated postsurgical wound healing following tumor resection. Advanced Healthcare Materials, 13(10): e2303456. DOI:10.1002/adhm.202303456 (  0) 0) |
Jiao, G., Yu, G., Zhang, J., and Ewart, H. S., 2011. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Marine Drugs, 9(2): 196-223. DOI:10.3390/md9020196 (  0) 0) |
Jönsson, M., Allahgholi, L., Sardari, R. R. R., Hreggviðsson, G. O., and Nordberg Karlsson, E., 2020. Extraction and modifycation of macroalgal polysaccharides for current and next-generation applications. Molecules, 25(4): 930. DOI:10.3390/molecules25040930 (  0) 0) |
Karuppusamy, S., and Kim, H., 2020. Fucoidan-loaded hydro-gels facilitate wound healing using photodynamic therapy by in vitro and in vivo evaluation. Carbohydrate Polymers, 247: 116624. DOI:10.1016/j.carbpol.2020.116624 (  0) 0) |
Khodaei, T., Nourmohammadi, J., Ghaee, A., and Khodaii, Z., 2023. An antibacterial and self-healing hydrogel from aldehyde-carrageenan for wound healing applications. Carbohydrate Polymers, 302: 120371. DOI:10.1016/j.carbpol.2022.120371 (  0) 0) |
Khotimchenko, M., Tiasto, V., Kalitnik, A., Begun, M., Khotimchenko, R., Leonteva, E., et al., 2020. Antitumor potential of carrageenans from marine red algae. Carbohydrate Polymers, 246: 116568. DOI:10.1016/j.carbpol.2020.116568 (  0) 0) |
Kidgell, J. T., Glasson, C. R. K., Magnusson, M., Vamvounis, G., Sims, I. M., Carnachan, S. M., et al., 2020. The molecular weight of ulvan affects the in vitro inflammatory response of a murine macrophage. International Journal of Biological Macromolecules, 150: 839-848. DOI:10.1016/j.ijbiomac.2020.02.071 (  0) 0) |
Kidgell, J. T., Magnusson, M., de Nys, R., and Glasson, C. R. K., 2019. Ulvan: A systematic review of extraction, composition and function. Algal Research, 39: 101422. DOI:10.1016/j.algal.2019.101422 (  0) 0) |
Kikionis, S., Koromvoki, M., Tagka, A., Polichronaki, E., Strati-gos, A., Panagiotopoulos, A., et al., 2022. Ulvan-based nanofibrous patches enhance wound healing of skin trauma resulting from cryosurgical treatment of keloids. Marine Drugs, 20(9): 551. DOI:10.3390/md20090551 (  0) 0) |
Kim, M. H., Lee, Y. W., Jung, W. K., Oh, J., and Nam, S. Y., 2019. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. Journal of the Mechanical Behavior of Biomedical Materials, 98: 187-194. DOI:10.1016/j.jmbbm.2019.06.014 (  0) 0) |
Lester, C. G., Descallar, F. B. A., Du, L., Bacabac, R. G., Matsukawa, S., and Geonzon, L. C., 2020. Gelation mechanism and network structure in gels of carrageenan and their mixtures viewed at different length scales. Food Hydrocolloids, 108: 106039. DOI:10.1016/j.foodhyd.2020.106039 (  0) 0) |
Li, B., Lu, F., Wei, X., and Zhao, R., 2008. Fucoidan: Structure and bioactivity. Molecules (Basel, Switzerland), 13 (8): 1671-1695, DOI: 10.3390/molecules13081671.
(  0) 0) |
Li, Y. P., Zheng, Y. T., Zhang, Y., Yang, Y. Y., Wang, P. Y., Imre, B., et al., 2021. Brown algae carbohydrates: Structures, pharmaceutical properties, and research challenges. Marine Drugs, 19(11): 620. DOI:10.3390/md19110620 (  0) 0) |
Liu, S., Zhang, H., and Yu, W., 2020. Simultaneously improved strength and toughness in κ-carrageenan/polyacrylamide double network hydrogel via synergistic interaction. Carbohydrate Polymers, 230: 115596. DOI:10.1016/j.carbpol.2019.115596 (  0) 0) |
Liu, Z., Gao, T., Yang, Y., Meng, F., Zhan, F., Jiang, Q., et al., 2019. Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules, 24(23): 4286. DOI:10.3390/molecules24234286 (  0) 0) |
Lowe, B., Venkatesan, J., Anil, S., Shim, M. S., and Kim, S. K., 2016. Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. International Journal of Biological Macromolecules, 93(Pt B): 1479-1487. DOI:10.1016/j.ijbiomac.2016.02.054 (  0) 0) |
Malektaj, H., Drozdov, A. D., and deClaville Christiansen, J., 2023. Mechanical properties of alginate hydrogels cross-linked with multivalent cations. Polymers, 15(14): 3012. DOI:10.3390/polym15143012 (  0) 0) |
Mariia, K., Arif, M., Shi, J., Song, F., Chi, Z., and Liu, C., 2021. Novel chitosan-ulvan hydrogel reinforcement by cellulose nanocrystals with epidermal growth factor for enhanced wound healing: In vitro and in vivo analysis. International Journal of Biological Macromolecules, 183: 435-446. DOI:10.1016/j.ijbiomac.2021.04.156 (  0) 0) |
Noguchi, S., and Kobayashi, T., 2022. Ultrasound viscoelastic properties of biomass polysaccharide hydrogels as evaluated by rheometer equipped with sono-device. Gels, 8(3): 172. DOI:10.3390/gels8030172 (  0) 0) |
Pacheco-Quito, E. M., Ruiz-Caro, R., and Veiga, M. D., 2020. Carrageenan: Drug delivery systems and other biomedical applications. Marine Drugs, 18(11): 583. DOI:10.3390/md18110583 (  0) 0) |
Popa, E. G., Gomes, M. E., and Reis, R. L., 2011. Cell delivery systems using alginate-carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules, 12: 3952-3961. DOI:10.1021/bm200965x (  0) 0) |
Pradhan, B., and Ki, J. S., 2023. Biological activity of algal de-rived carrageenan: A comprehensive review in light of human health and disease. International Journal of Biological Macromolecules, 238: 124085. (  0) 0) |
Qi, H. M., and Sun, Y. L., 2015. Antioxidant activity of high sul-fate content derivative of ulvan in hyperlipidemic rats. Inter-national Journal of Biological Macromolecules: Structure, Function and Interactions, 76(5): 326-329. DOI:10.1016/j.ijbiomac.2015.03.006 (  0) 0) |
Qi, H. M., Huang, L. Y., Liu, X. L., Liu, D. M., Zhang, Q. B., and Liu, S. M., 2012. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydrate Polymers, 87(2): 1637-1640. DOI:10.1016/j.carbpol.2011.09.073 (  0) 0) |
Radvar, E., and Azevedo, H. S., 2019. Supramolecular peptide / polymer hybrid hydrogels for biomedical applications. Macromolecular Bioscience, 19(1): e1800221. DOI:10.1002/mabi.201800221 (  0) 0) |
Raghunathan, S., Kandasamy, S., Balakrishna Pillai, A., Senthi-lathiban, D. P., Thajuddin, N., Rasool Kamli, M., et al., 2024. Synthesis of biocomposites from microalgal peptide incorporated polycaprolactone/κ-carrageenan nanofibers and their antibacterial and wound healing property. International Journal of Pharmaceutics, 655: 124052. DOI:10.1016/j.ijpharm.2024.124052 (  0) 0) |
Rao, S. S., Venkatesan, J., Yuvarajan, S., and Rekha, P. D., 2022. Self-assembled polyelectrolyte complexes of chitosan and fucoidan for sustained growth factor release from PRP enhance proliferation and collagen deposition in diabetic mice. Drug Delivery and Translational Research, 12: 2838-2855. DOI:10.1007/s13346-022-01144-3 (  0) 0) |
Ren, Y., Aierken, A., Zhao, L., Lin, Z., Jiang, J., Li, B., et al., 2022. hUC-MSCs lyophilized powder loaded polysaccharide ulvan driven functional hydrogel for chronic diabetic wound healing. Carbohydrate Polymers, 288: 119404. DOI:10.1016/j.carbpol.2022.119404 (  0) 0) |
Safarpour, F., Kharaziha, M., Mokhtari, H., Emadi, R., Bakh-sheshi-Rad, H. R., and Ramakrishna, S., 2023. Kappa-carrageenan based hybrid hydrogel for soft tissue engineering applications. Biomedical Materials, 18(5): ace0ec. DOI:10.1088/1748-605X/ace0ec (  0) 0) |
Santhamoorthy, M., Thirupathi, K., Kumar, S. S. D., Pandiaraj, S., Rahaman, M., Phan, T. T. V., et al., 2023. k-Carrageenan based magnetic@polyelectrolyte complex composite hydrogel for pH and temperature-responsive curcumin delivery. International Journal of Biological Macromolecules, 244: 125467. DOI:10.1016/j.ijbiomac.2023.125467 (  0) 0) |
Sathuvan, M., Thangam, R., Cheong, K. L., Kang, H. M., and Liu, Y., 2023. κ-Carrageenan-essential oil loaded composite biomaterial film facilitates mechanosensing and tissue regenerative wound healing. International Journal of Biological Macromolecules, 241: 124490. DOI:10.1016/j.ijbiomac.2023.124490 (  0) 0) |
Shalaby, S., and Amin, H., 2019. Potential using of ulvan polysaccharide from Ulva lactuca as a prebiotic in synbiotic yogurt production. Journal of Probiotics & Health, 7: 1-8. DOI:10.4172/2329-8901.100208 (  0) 0) |
Sulastri, E., Lesmana, R., Zubair, M. S., Abdelwahab Mohammed, A. F., Elamin, K. M., and Wathoni, N., 2023. Ulvan/Silver nanoparticle hydrogel films for burn wound dressing. Heliyon, 9(7): e18044. DOI:10.1016/j.heliyon.2023.e18044 (  0) 0) |
Sun, G., Huang, Y., Li, D., Fan, Q., Xu, J., and Shao, J., 2020. Blue light induced photopolymerization and cross-linking kinetics of poly(acrylamide) hydrogels. Langmuir: The ACS Journal of Surfaces and Colloids, 36(39): 11676-11684. DOI:10.1021/acs.langmuir.0c02560 (  0) 0) |
Terezaki, A., Kikionis, S., Ioannou, E., Sfiniadakis, I., Tziveleka, L. A., Vitsos, A., et al., 2022. Ulvan/gelatin-based nanofibrous patches as a promising treatment for burn wounds. Journal of Drug Delivery Science and Technology, 74: 103535. DOI:10.1016/j.jddst.2022.103535 (  0) 0) |
Tytgat, L, Van Damme, L., Arevalo, M. D. O., Declercq, H., Thienpont, H., Otteveare, H., et al., 2019. Extrusion-based 3D printing of photo-crosslinkable gelatin and κ-carrageenan hydrogel blends for adipose tissue regeneration. International Journal of Biological Macromolecules, 140: 929-938. DOI:10.1016/j.ijbiomac.2019.08.124 (  0) 0) |
Tziveleka, L. A., Ioannou, E., and Roussis, V., 2019. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydrate Polymers, 218: 355-370. DOI:10.1016/j.carbpol.2019.04.074 (  0) 0) |
Tziveleka, L. A., Pippa, N., Georgantea, P., Ioannou, E., Demetzos, C., and Roussis, V., 2018. Marine sulfated polysaccharides as versatile polyelectrolytes for the development of drug delivery nanoplatforms: Complexation of ulvan with lysozyme. International Journal of Biological Macromolecules, 118(Pt A): 69-75. DOI:10.1016/j.ijbiomac.2018.06.050 (  0) 0) |
Tziveleka, L. A., Pippa, N., Ioannou, E., Demetzos, C., and Roussis, V., 2022. Development of ulvan-containing liposomes as antibacterial drug delivery platforms. Journal of Functional Biomaterials, 13(4): 186. DOI:10.3390/jfb13040186 (  0) 0) |
Ulagesan, S., Krishnan, S., Nam, T. J., and Choi, Y. H., 2023. The influence of κ-carrageenan-R-phycoerythrin hydrogel on in vitro wound healing and biological function. International Journal of Molecular Sciences, 24(15): 12358. DOI:10.3390/ijms241512358 (  0) 0) |
Van Weelden, G., Bobiński, M., Okła, K., Van Weelden, W. J., Romano, A., and Pijnenborg, J. M., 2019. Fucoidan structure and activity in relation to anti-cancer mechanisms. Marine Drugs, 17(1): 32. DOI:10.3390/md17010032 (  0) 0) |
Vo, T. M. T., Kobayashi, T., and Potiyaraj, P., 2022. Viscoelastic analysis of pectin hydrogels regenerated from citrus pomelo waste by gelling effects of calcium ion crosslinking at different pHs. Gels, 8(12): 814. DOI:10.3390/gels8030172 (  0) 0) |
Wassie, T., Niu, K., Xie, C., Wang, H., and Xin, W., 2021. Extraction techniques, biological activities and health benefits of marine algae Enteromorpha prolifera polysaccharide. Frontiers in Nutrition, 8: 747928. DOI:10.3389/fnut.2021.747928 (  0) 0) |
Yang, M., Zhao, T., Xia, W., Wei, K., Li, R., Jiang, W., et al., 2024. In-situ electrospinning with precise deposition of antioxidant nanofiber facial mask loaded with Enteromorpha prolifera polysaccharides. International Journal of Biological Macromolecules, 257(Pt 2): 128698. DOI:10.1016/j.ijbiomac.2023.128698 (  0) 0) |
Yao, Y., Zaw, A. M., Anderson, D. E. J., Hinds, M. T., and Yim, E. K. F., 2020. Fucoidan functionalization on poly(vinyl alcohol) hydrogels for improved endothelialization and hemocompatibility. Biomaterials, 249: 120011. DOI:10.1016/j.biomaterials.2020.120011 (  0) 0) |
Yegappan, R., Selvaprithiviraj, V., Amirthalingam, S., and Jaya-kumar, R., 2018. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydrate Polymers, 198: 385-400. DOI:10.1016/j.carbpol.2018.06.086 (  0) 0) |
Yu, F., Cao, X., Du, J., Wang, G., and Chen, X., 2015. Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining Diels-Alder click reaction and acylhydrazone bond. ACS Applied Materials & Interfaces, 7(43): 24023-24031. DOI:10.1021/acsami.5b06896 (  0) 0) |
Zayed, A., El-Aasr, M., Ibrahim, A. S., and Ulber, R., 2020. Fu-coidan characterization: Determination of purity and physicochemical and chemical properties. Marine Drugs, 18(11): 571. DOI:10.3390/md18110571 (  0) 0) |
Zhang, A., Wen, X., Yan, H., He, X., Su, H., Tang, H., et al., 2018. Response of microalgae to large-seaweed cultivation as revealed by particulate organic matter from an integrated aquaculture off Nan'ao Island, South China. Marine Pollution Bulletin, 133: 137-143. DOI:10.1016/j.marpolbul.2018.05.026 (  0) 0) |
Zhang, Y. B., Ye, L., Cui, J., Yang, B. G., Sun, H., Li, J. J., et al., 2016. A biomimetic poly(vinyl alcohol)-carrageenan composite scaffold with oriented microarchitecture. ACS Biomaterials Science & Engineering, 2: 544-557. DOI:10.1021/acsbiomaterials.5b00535 (  0) 0) |
Zhao, Z., Xia, X., Liu, J., Hou, M., Liu, Y., Zhou, Z., et al., 2023. Cartilage-inspired self-assembly glycopeptide hydrogels for cartilage regeneration via ROS scavenging. Bioactive Materials, 32: 319-332. DOI:10.1016/j.bioactmat.2023.10.013 (  0) 0) |
Zhong, H. W., Gao, X. R., Cheng, C., Liu, C., Wang, Q. W., and Han, X., 2020. The structural characteristics of seaweed polysaccharides and their application in gel drug delivery systems. Marine Drugs, 18(12): 658. DOI:10.3390/md18120658 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


