2) Sanmen Agricultural and Rural Bureau, Taizhou 317100, China;
3) Key Laboratory of Applied Marine Biotechnology, Ministry of Education, Ningbo University, Ningbo 315211, China
Portunus trituberculatus is widely distributed in India and the western Pacific waters (Carpenter and Niem, 1998; Hamasaki et al., 2006), which is popular with domestic coastal consumers and is one of the important marine fishing and aquaculture species in China. However, in the process of aquaculture, external environmental factors such as water salinity, temperature and pH have a very significant impact on its growth, especially the change of salinity. Salinity of aquaculture water is susceptible to short-term heavy rainfall and nearby river flow injection (Wang and Liu, 1992; Ye et al., 2008), which directly affects the osmotic pressure in P. trituberculatus, destroys its original balance system, and causes many physiological responses to osmotic adjustment. Therefore, it is necessary to pay attention to the effects of short-term salinity change on physiological metabolism disorder, immunity decline, disease occurrence and even death of P. trituberculatus during aquaculture (Moullac and Haffner, 2000; Wang and Pan, 2016).
At present, there have been reports on the effects of salinity on immune defense (Zheng et al., 2010), respiratory metabolism (Lu et al., 2012a), growth and development (Lu et al., 2012b) of P. trituberculatus, but few studies have been conducted on the osmotic physiological adaptability of P. trituberculatus. As for the osmotic adjustment mechanisms of other crustaceans, such as Eriocheir sinensis and Scylla paramamosain, researches are mainly carried out in the morphological structure of osmoregulatory organs (Li et al., 2006), hemolymph osmotic pressure regulation (Gilles and Péqueux, 1981), ion transport enzymes (Mcnamara and Faria, 2012; Qi et al., 2020) and molecular adaptation mechanisms (Wang et al., 2018a, 2018b, 2018c; Yao et al., 2020, 2021). Aquatic crustaceans mainly regulate hemolymph osmotic pressure to adapt to the changes of external salinity by changing the contents of water and osmotic pressure effectors in hemolymph and the permeability of inorganic ions (Chia and Chen, 1997; Lima et al., 1997; Wang et al., 2011). The gill is the main organ for osmotic pressure and ion regulation of crustaceans (Pan and Liu, 2005), while the hind gill is the main site for Na+ and Cl− transport (Zhou et al., 2001). The transmembrane transport of Na+ and Cl− is mainly completed in chlorine cells, and chlorine cells are widely distributed in the gill epithelium (Hirose et al., 2003). The plasma membrane contains a large number of ion transport enzymes Na+-K+-ATPase (Jiang and Xu, 2011). It was found that the activity of Na+-K+ATPase was significantly correlated with the osmotic adjustment ability (Castilho et al., 2001), and its activity could reflect the adaptability of crustaceans to external salinity changes. Carbonic anhydrase, as an important osmoregulatory enzyme, can provide H+ and HCO3− for the electroneutral exchange of Na+/NH4+ and Na+/H+ and participate in the exchange and transportation of Na+/H+ and Cl−/HCO3− during ion transport (Henry et al., 2003). In addition, studies have shown that the expression of V-ATPase subunit B in gill epithelial cells of some crustaceans increased or decreased significantly under high or low salinity stress, which may be due to differences in transcriptional efficiency or mRNA stability that affect the efficiency of enzyme synthesis (Weihrauch et al., 2001), and thereby affect osmotic regulation. Therefore, the research on the short-term low-salinity adaptation mechanism of P. trituberculatus is of great significance.
In this study, experiments of gradually decline and recovery and abrupt decline in salinity were carried out to analyze the physiological characteristics of P. trituberculatus under short-term low salinity stress, such as survival rate, changes in hemolymph osmotic pressure, morphological structure of gill filaments and activity of key ion transport enzymes. The enriched knowledge on salinity adaptation mechanism of P. trituberculatus can provide reference for the regulation of aquaculture water environment and healthy aquaculture of P. trituberculatus.
2 Materials and Methods 2.1 Grouping of Experimental AnimalsExperiment of gradual decline in salinity: a total of 180 healthy P. trituberculatus with a body weight of 45 g ± 5 g were randomly divided into 3 parallel groups, and 60 crabs in each group were placed into three buckets (0.4 m3). The initial salinity was 24, and the salinity decreased gradually every 24 h within 72 h. The salinity gradually decreased to 16, 12 and 8. The survival number of the 3 groups was respectively counted at 0 h, 24 h, 48 h and 72 h, and 12 crabs were sampled at each experimental point.
Experiment of recovery in salinity: after experiment of gradual decline in salinity, the water salinity had decreased to 8 and crabs were cultured for 12 h. Living crabs were restored to salinity every 12 h in 36 h, and the salinity gradually recovered to 12, 16 and 24. The survival numbers of the three groups were respectively counted at 12 h, 24 h and 36 h, and 12 crabs were sampled at each experimental time point.
Experiment of abrupt decline in salinity: a total of 270 healthy P. trituberculatus with a bodyweight of 5 g ± 0.5 g were randomly divided into 5 groups. The salinity of the control group was 24, and the salinity of the abrupt-decrease groups was 20, 16, 12 and 8. A total of 54 crabs in each group were set in 3 parallels and placed in 3 buckets (0.4 m3) in the same experimental environment. The survival numbers of the five groups were counted and sampled at 0 h, 6 h, 12 h, 24 h and 48 h, respectively.
In this study, the temperature of aquaculture water was controlled at 28℃ ± 1℃ by heat pump. The different shortterm low salinity stress treatments were conducted for analyzing different physiological characteristics of P. trituberculatus. In the experiment of the effect of low salt stress on the survival rate of P. trituberculatus, the experimental groups of gradual decline and recovery of salinity and abrupt decline of salinity were set. The experimental groups with gradual decline of salinity and abrupt decline of salinity were set up in the experiment on the morphological structure of gill filament. The salinity gradual decline and recovery groups were set up in the experiments on the changes of hemolymph osmotic pressure and the activity of key ion transport enzymes.
2.2 Calculation of Survival RateThe survival rate formula is:
| $ \text { Survival rate }=\left(N_t / N_{\mathrm{o}}\right) \times 100 \% \text {, } $ |
where Nt means the final number; No means the initial number.
2.3 Determination of Hemolymph Osmotic PressureSerum was prepared following the method of Yao et al. (2021): 1.5 mL whole blood was taken and placed over-night at 4℃. After 12 h, the clot was punctured and centrifuged at 20000 r min−1 for 5 min. The osmolality of serum and seawater samples was measured by Fiske 210 (ADV ANCED, USA) osmometer.
2.4 Observation on Morphological and Structural Changes of Gill FilamentIn the experiment of gradual decline in salinity, three crabs were randomly selected from four salinity experimental groups at the sampling points, and the hind gills were fixed with paraffin for microscopic observation.
In the experiment of abrupt decline in salinity, three crabs were randomly selected from the five salinity experimental groups at 48 h, and the hind gills were fixed with paraffin for microscopic observation.
Paraffin sections were prepared using hematoxylin-eosin staining, methods refer to Wang et al. (2018a). The removed hind gills were fixed in Bouin's fixative, embedded in paraffin, and cut into slices with thickness of 5 – 10 μm. Then, the sections were dewaxed with xylene and rehydrated in ethanol series. Finally, hematoxylin-eosin staining was performed.
2.5 Determination of the Activities of Na+-K+-ATPase, Carbonic Anhydrase ATPase and V-ATPaseFollowing the method of Yao et al. (2021), 0.1 g of rear gill tissue was diluted with saline (1:9) and homogenized for 1 min in an ice-water bath using an IKA micro-homogenizer. The homogenate was centrifuged for 10 min at 4℃ with 3000 r min−1. Afterwards, the supernatant was collected and the Na+-K+-ATPase activity was measured by ELISA assay kit (Nanjing Jiancheng Bioengineering Institute, China). The V-ATPase and CA activities were measured by ELISA assay kit (Qiaodu Biomart Inc., Shanghai, China). The experimental operation was performed according to the instructions of the manufactures.
2.6 Statistical Analysis of DataAll experimental data were expressed as mean ± SD. SPSS22.0 software was used for one-way ANOVA and Duncan test. Duncan test in one-way ANOVA was used to compare the difference of measurement indexes in different salinity groups, and the statistical significance level of data was set as P < 0.05.
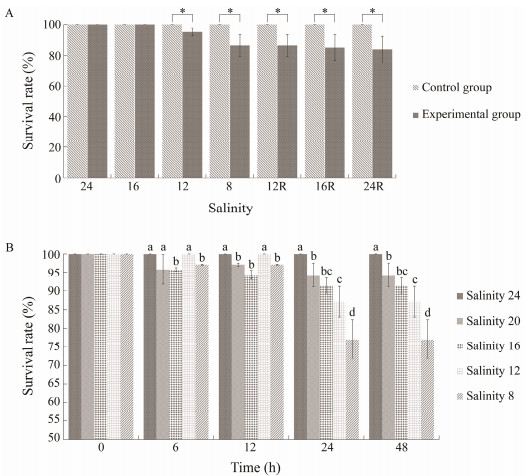
3 Results 3.1 Effect of Short-Term Low Salinity Stress on Survival Rate of P. trituberculatusWhen salinity decreased from 24 to 16, no death occurred in P. trituberculatus. When the salinity decreased to 12, the P. trituberculatus began to die. At salinity 8, survival rate of P. trituberculatus decreased by 7% to 93% compared with the control group (P < 0.05); however, when salinity returned to 24, the survival rate of P. trituberculatus was 92% (Fig.1A).
The survival status of P. trituberculatus under different salinity conditions for 48 h showed significant differences. There was no death in the control group. Compared with the control group, the survival rate of salinity 20 group decreased by 5.7% (P < 0.05), the survival rate of salinity 16 group decreased by 8.6% (P < 0.05), the survival rate of salinity 12 group decreased by 12.9% (P < 0.05), and the survival rate of salinity 8 group decreased by 22.9% (P < 0.05). With the extension of low salinity stress time, the lower the salinity, the higher the mortality rate. The crabs in the salinity 8 group began to die more from 24 h, and the survival rate was only 77% at 48 h, which was significantly lower than that of the control group (P < 0.05) (Fig.1B).
The above results suggested that salinity 12 might be the lowest salinity that P. trituberculatus can adapt to. The results also showed that the salinity should be greater than 12 as far as possible in the breeding process of P. trituberculatus, and the abrupt drop of salinity should be avoided.
|
Fig. 1 Survival rate differences of P. trituberculatus under short-term salinity stress. A, survival rate of P. trituberculatus under gradual low salinity and restoring stress; B, survival rate of P. trituberculatus in 48 h under abrupt low salinity stress. R refers to the salinity after recovery; * means significant difference (P < 0.05). Different letters show significant difference of survival rates under different salinity conditions (P < 0.05). |
During the decrease of salinity, the hemolymph osmotic pressure of P. trituberculatus continued to decrease from (713.93 ± 16.49) mOsg kg−1 at salinity 24 to (492.67 ± 24.38) mOsg kg−1 at salinity 8, and the decrease is remarkable (P < 0.05). Then, with the recovery of salinity from 8 to 24, the hemolymph osmotic pressure also increased, and recovered to (709.13 ± 7.94) mOsg kg−1, with a significant increase (P < 0.05), and was slightly lower than the original osmotic pressure (Fig.2A). Under different salinity conditions, the hemolymph osmotic pressure of P. trituberculatus was always higher than that of seawater (Fig.2B). It could be observed that short-term low salinity stress can significantly change the hemolymph osmotic pressure of P. trituberculatus and make it adapt to the osmotic pressure of the external environment.

|
Fig. 2 Changes of serum osmolality differences of P. trituberculatus under different salinity conditions. A, changes of serum osmolality of P. trituberculatus under different salinity conditions; B, comparison of seawater osmotic pressure and serum osmotic pressure of P. trituberculatus under different salinity. R refers to the salinity after recovery. Different letters show significant difference of serum osmolality and seawater osmolality under different salinity conditions (P < 0.05). |
Through the observation of tissue sections, the epithelial layer of gill filament in the control group showed a certain thickness, and the internal epithelial cells were closely arranged (Figs.3A, A'). In the group with salinity gradually decreasing to 16, it was found that the epithelial layer of the gill filament of P. trituberculatus became thinner and dissolved, and some epithelial cells separated from the cuticle to form vacuoles (Figs.3B, B'); When salinity gradually decreased to 12, epithelial cells arranged irregularly, filled with blood cells and vacuolated seriously (Figs.3C, C'). In the group with salinity gradually decreased to 8, the upper cortex seriously disintegrated, only a layer of cuticle, and the end of gill filament terminal expanded in a round bubble shape (Figs.3D, D'). In summary, with the decrease of salinity, the vacuoles in the gill filaments of P. trituberculatus increased, and the upper cortex was severely damaged, which caused the structure was incomplete.

|
Fig. 3 Effects of gradual low salinity stress on the gill of P. trituberculatus. A and A', salinity 24; B and B', salinity 16; C and C', salinity 12; D and D', salinity 8. |
Through the observation of tissue sections, the gill filaments in the control group were regular and the epithelial cells were closely arranged (Figs.4A, A'). The terminal of gill filaments of P. trituberculatus in salinity 20 group expanded slightly (Figs.4B, B'). In the group with salinity sharply dropped to 16, gill filaments showed regular thickening, gill chambers were relatively expanded, and the horny layer was wavy bulging (Figs.4C, C'). When the salinity sharply decreased to 12, the blood cells increased obviously, the epithelial cells arranged irregularly, and the terminal swelling became more obvious (Figs.4D, D'). When the salinity dropped to 8, gill filaments became shorter, the end of gill filaments became round, the upper cortex disintegrated seriously, and only one stratum corneum was left (Figs.4E, E'). In a summery, with the decrease of salinity, the blood cells gradually increased, the gill filaments became thicker and shorter, the upper cortex was destroyed, and the end of the gill filaments gradually expanded and showed a round bubble.
In general, the morphological structure of P. trituberculatus gill under short-term low salinity showed the phenomenon of gill filament terminal enlargement, gill filament thickening and shortening, epithelial cells thinning, destruction and even disintegration; especially with the further decrease of salinity, the above phenomenon is more obvious.

|
Fig. 4 Effects of abrupt low salinity stress on the gill of P. trituberculatus. A and A', salinity 24; B and B', salinity 20; C and C', salinity 16; D and D', salinity 12; E and E', salinity 8. |
In the short-term salinity gradual decline and recovery experiments, the Na+-K+-ATPase activity of P. trituberculatus increased at the beginning and then decreased (Fig.5A). When the salinity decreased from 24 to 8, the activity of Na+-K+-ATPase continued to increase remarkably (P < 0.05). The activity of Na+-K+-ATPase continued to increase after salinity recovery, and reached the maximum when salinity was 16 (P < 0.05). Then, the activity of Na+-K+-ATPase showed a downward trend. When the salinity was restored to 24, the enzyme activity was still significantly higher than that at initial salinity of 24 (P < 0.05).

|
Fig. 5 ATPase activity in gills of P. trituberculatus under different salinity conditions. R refers to the salinity after recovery. Different letters show significant difference of ATPase activity under different salinity conditions at the same time (P < 0.05). |
The CA activity of P. trituberculatus showed a remarkable upward trend when the salinity decreased to 8 (P < 0.05). Subsequently, the enzyme activity showed a steady downward trend when the salinity returned to 24, and it was slightly higher than that at the initial salinity of 24 (Fig.5B).
Similarly, the changes of V-ATPase activity and CA activity were basically the same. When the salinity decreased from 24 to 8, the V-ATPase activity increased significantly (P < 0.05). Then in the process of salinity recovery to 24, the enzyme activity showed a steady downward trend, and the enzyme activity at salinity 24 was slightly higher than that at initial salinity of 24 (Fig.5C).
In summary, the activities of key enzymes that play important role during osmotic adjustment in P. trituberculatus increased with the decrease of salinity. After salinity recovery, the enzyme activities were higher than those at the initial salinity of 24.
4 DiscussionChanges in water salinity can cause physiological dysfunction of P. trituberculatus and decrease its immunity, thus causing disease and even death. From theresults of this experiment, the survival rate of P. trituberculatus decreased with the decrease of salinity, but the effect of abrupt decrease of salinity on the survival rate of P. trituberculatus was greater than that of gradual decrease of salinity, indicating that its ability to adapt to gradual change of salinity was stronger than that of abrupt change, which was consistent with the results of Wang et al. (2010). During the gradual decline of salinity, when the salinity of water body decreased to 12, P. trituberculatus began to die, and it was speculated that 12 might be the lowest salinity that it could adapt to. However, during the gradual recovery of salinity, there was no significant change in the survival rate of P. trituberculatus, which was similar to the experimental results of Liao et al. (2007) on the recovery of Orithyia sinica after gradual salinity change. Therefore, it is speculated that salinity recovery can alleviate the damage caused by low salinity stress on P. trituberculatus, and it has strong adaptability to salinity recovery. In the experiment of abrupt decline in salinity, the survival rate of crabs in salinity 20 group remained above 90%, while crabs in salinity 8 group began to die obviously at 24 h, and the survival rate was only 77% at 48 h, which was lower with time. This result was similar to the result of Zhou et al. (2014) that the survival rate of P. trituberculatus was higher than 95% under the condition of water salinity of 20 and above, while the survival rate was lower than 45% under the condition of water salinity below 5. In addition, P. trituberculatus had a high survival rate in the water with salinity of 12 – 24, indicating that it had a wide tolerance range of salinity abrupt drop and could cope with the salinity abrupt drop caused by rainstorm during the breeding process.
Crustaceans have the ability to adapt to aquatic environmental salinity, and the normal life activities of the organism are maintained by regulating the osmotic pressure of hemolymph. According to the result, the osmotic pressure of P. trituberculatus decreased significantly with the decrease of salinity, and then increased significantly with the recovery of salinity, which was similar to the results of Li et al. (2012) on the osmotic regulation of Penaeus vannamei. Studies have shown that P. trituberculatus, as an osmoregulation species, has a strong ability to regulate the osmotic pressure in vivo (Long et al., 2019). Changes in external salinity can make it maintain the stability of the internal environment by regulating the osmotic pressure of its own hemolymph. Moreover, the experiment showed that the serum osmotic pressure of P. trituberculatus was always higher than that of seawater, and there was no isotonic phenomenon, indicating that P. trituberculatus belonged to the hyperosmotic regulation type (Ma et al., 2016). Salinity recovery after short-term low salinity stress restored the osmotic pressure of hemolymph which was a bit lower than the initial level, indicating that low salinity stress caused certain damage to osmotic adjustment function.
Salinity stress can damage gill tissue structure and affect its osmotic adjustment function (Lucu, 1990). In this study, after salinity stress, the gill cavity of P. trituberculatus enlarged, the gill filaments thickened and shortened, the end of gill filaments enlarged. Moreover, the epithelial cells became thinner, and were damaged or even disbanded. The effect of salinity abrupt-dropped on gill tissue structure was more intense than that of salinity gradual drop. Han et al. (2014) also proved that the decrease of salinity could increase the permeability of gill epithelial cells. A large amount of water infiltrated into the gill cavity, the volume of gill cavity increased, the gill filaments thickened, and further caused the swelling and proliferation of gill epithelial cells. Interestingly, this is also similar to the phenomenon of increased chlorine secretion cells and auxetic epidermal cells in gills of fish under low salinity stress, which can help the gills of fish to fully contact with water and increase the number of ion exchange (Pisam and Rambourg, 1991), and exchange substances with the external environment by active transport. Sun et al. (2016) found that when juvenile Takifugu rubripes were under low salinity stress, their gill filaments and gill lamellae width increased, gill lamellae spacing narrowed, gill lamellae cells round full, and chlorine secretion cells increased significantly. Qu et al. (2014) found that gill lamellae appeared swelling, shedding, large area smooth or wavy top opening under low salinity stress, which was also a way to adapt to low salinity environment for self-regulation. Therefore, it can be speculated that although the end of gill filaments of P. trituberculatus expanded into vacuoles, the shedding and disintegration of epithelial cells were the manifestations of gill tissue damage under salinity stress. These results may also increase the contact area with the environment, improve the ion exchange efficiency, and thus enhance its osmotic adjustment ability.
The ion-transporting gill epithelium of crustaceans is the main site for osmotic regulation and ion transport. The regulation of ion transport is mainly achieved through the role of Na+-K+-ATPase, V-ATPase, CA and other ion transport enzymes (Morris, 2001). Na+-K+-ATPase accounts for about 70% of the total ATPase activity, and plays a leading role in the osmotic pressure regulation of crustaceans (Furriel et al., 2000). Studies have shown that Na+-K+-ATPase activity showed an increasing trend and was significantly higher than the normal seawater group after low salinity stress treatment (Qi et al., 2011). Jiang and Xu (2011) found that Na+-K+-ATPase of P. trituberculatus also changed significantly with salinity. In this experiment, along with the decrease of salinity, the difference of osmotic pressure gradient between inside and outside of P. trituberculatus was large, resulting in the increase of osmotic adjustment and the enhancement of Na+-K+-ATPase activity, which was a response mode to adapt to low salinity changes. In addition, the Na+-K+-ATPase activity maintained an increase during salinity recovery, and began to decrease gradually after salinity recovered to 16. This is consistent with the results of Zhou et al. (2014) that the activity of Na+-K+-ATPase in gills of P. trituberculatus was inhibited in the low salinity environment for a long time, indicating that appropriate reduction of water salinity could stimulate and enhance the activity of Na+-K+-ATPase in the gill of P. trituberculatus in the short term. However, if the decline time of salinity exceeded its tolerance limit, the ATPase activity would be inhibited. Furthermore, CA and V-ATPase also play important roles in the osmotic adjustment and ion transport of P. trituberculatus. Henry et al. (2002) reported that the activity of CA in the gills of crustaceans was low in high salinity environment, but it could be increased by 5 – 10 times in low salinity environment. Serrano and Henry (2008) found that the expression levels of the two subtypes of carbonic anhydrase in Carcinus maenas increased significantly after low salinity stress, indicating that carbonic anhydrase was involved in the low salinity adaptation mechanism in Carcinus maenas. In the study of Sun et al. (2019), V-AT Pase played a certain role in the osmotic adjustment of P. trituberculatus. In this study, the activities of CA and VATPase increased significantly with the decrease of salinity. Subsequently, in the process of salinity recovery to 24, the enzyme activity showed a steady downward trend, and the enzyme activity at salinity recovery to 24 was slightly higher than the initial value. The main function of VATPase is to release energy. At the same time, transmembrane ATPase also inputs the substances needed for metabolism for cells, outputs wastes and toxins generated during metabolism, and participates in the regulation of cell osmotic pressure in the process of short-term salinity decline and recovery, providing the necessary energy for regulation (Lucu, 1990). This is also the possible reason why the enzyme activity test results are basically consistent with the change trend of hemolymph osmotic pressure.
5 ConclusionsIn this study, a sharp and gradual decrease in salinity in the short term significantly affected key physiological characteristics of P. trituberculatus. The results showed that P. trituberculatus could survive in a certain low salinity range in the short term, and salinity 12 was the lowest tolerable salinity. Hemolymph osmotic pressure decreased significantly with the decrease of salinity, and was always higher than seawater osmotic pressure. From the paraffin sections of gill tissue, it can be clearly observed that low salinity stress changes the morphological structure of gill tissue. The expansion of gill filament ends and epithelial cell shedding may also be conducive to osmotic adjustment to adapt to low salinity environment. At the same time, the activities of key osmotic adjustment enzymes Na+-K+-ATPase, CA and V-ATPase in gill increased with the decrease of salinity. Additionally, with the recovery of salinity, P. trituberculatus also had strong regulatory adaptability, which could make the physiological indexes tend to be stable, but the osmotic pressure after recovery was lower than the initial osmotic pressure level, and the enzyme activity after recovery was higher than the initial enzyme activity level.
Therefore, the results of this study can be used to formulate emergency measures to cope with the possible gradual and sudden decline of salinity in the breeding process of P. trituberculatus. At the same time, the salinity recovery experiment of P. trituberculatus after low salinity stress was carried out for the first time in this study. The results showed that the physiological damage caused by salinity stress could be alleviated by a certain salinity recovery after salinity decline, which provided a reference for the regulation of aquaculture water environment and healthy aquaculture of P. trituberculatus.
AcknowledgementsThis work was supported by the National Key R & D Program of China (No. 2020YFD0900203), the China Agriculture Research System of MOF and MARA, and the K. C. Wong Magna Fund in Ningbo University.
Carpenter, K. E., and Niem, V. H., 1998. Fao species identification guide for fishery purposes. The living marine resources of the western central Pacific. Journal of the Royal Geographical Society of London, 2003(1): 212-214. (  0) 0) |
Castilho, P. C., Martins, I. A., and Bianchini, A., 2001. Gill Na+, K+-ATPase and osmoregulation in the estuarine crab, Chasmagnathus granulata Dana, 1851 (Decapoda, Grapsidae). Journal of Experimental Marine Biology and Ecology, 256(2): 215-227. DOI:10.1016/S0022-0981(00)00315-4 (  0) 0) |
Chia, P. G., and Chen, J. C., 1997. Osmotic and ionic concentrations of Scylla serrata (Forskål) subjected to different salinity levels. Comparative Biochemistry and Physiology Part A: Physiology, 117(2): 239-244. DOI:10.1016/S0300-9629(96)00237-X (  0) 0) |
Furriel, R., Mcnamara, J. C., and Leone, F. A., 2000. Characterization of (Na+, K+)-ATPase in gill microsomes of the freshwater shrimp Macrobrachium olfersii. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 126(3): 303-315. DOI:10.1016/S0305-0491(00)00184-X (  0) 0) |
Gilles, R., and Péqueux, A., 1981. Cell volume regulation in crustaceans: Relationship between mechanisms for controlling the osmolality of extracellular and intracellular fluids. Journal of Experimental Zoology, 215(3): 351-362. DOI:10.1002/jez.1402150312 (  0) 0) |
Hamasaki, K., Fukunaga, K., and Kitada, S., 2006. Batch fecundity of the swimming crab Portunus trituberculatus (Brachyura: Portunidae). Aquaculture, 253(1-4): 359-365. DOI:10.1016/j.aquaculture.2005.08.002 (  0) 0) |
Han, X. L., Gao, B. Q., Wang, H. F., Liu, P., Chen, P., and Li, H., 2014. Effects of low salinity stress on microstructure of gill and hepatopancreas and family survival rate of Portunus trituberculatus. Progress in Fishery Sciences, 35(1): 104-110 (in Chinese with English abstract). (  0) 0) |
Henry, R. P., Garrelts, E. E., Mccarty, M. M., and Towle, D. W., 2002. Differential induction of branchial carbonic anhydrase and Na+/K+ ATPase activity in the euryhaline crab, Carcinus maenas, in response to low salinity exposure. Journal of Experimental Zoology, 292(7): 595-603. DOI:10.1002/jez.10075 (  0) 0) |
Henry, R. P., Gehnrich, S., Weihrauch, D., and Towle, D. W., 2003. Salinity-mediated carbonic anhydrase induction in the gills of the euryhaline green crab, Carcinus maenas. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 136(2): 243-258. (  0) 0) |
Hirose, S., Kaneko, T., Naito, N., and Takei, Y., 2003. Molecular biology of major components of chloride cells. Comparative Biochemistry and Physiology. Part B, Biochemistry and Molecular Biology, 136(4): 593-620. DOI:10.1016/S1096-4959(03)00287-2 (  0) 0) |
Jiang, S., and Xu, Q. H., 2011. Influence of salinity stress on the activity of gill Na+/K+-ATPase in swimming crab (Portunus trituberculatus). Journal of Fisheries of China, 35(10): 1475-1480 (in Chinese with English abstract). (  0) 0) |
Li, T., Roer, R., Vana, M., Pate, S., and Check, J., 2006. Gill area, permeability and Na+, K+-ATPase activity as a function of size and salinity in the blue crab, Callinectes sapidus. Journal of Experimental Zoology. Part A, Comparative Experimental Biology, 305A(3): 233-245. DOI:10.1002/jez.a.248 (  0) 0) |
Li, Y., Wang, F., Zhao, Z. Y., and Dong, S. L., 2012. Effects of salinity fluctuations on hemocyanins and glycolysis of Litopenaeus vannamei. Periodical of Ocean University of China, 42(9): 28-34 (in Chinese with English abstract). (  0) 0) |
Liao, Y. Y., Wu, L., Cai, K., and Pan, C. H., 2007. The effect of salinity and temperature on survivorship and food intake of tiger crab, Orithyia sinica. Acta Ecologica Sinica, 27(2): 627-639 (in Chinese with English abstract). (  0) 0) |
Lima, A. G., Mcnamara, J. C., and Terra, W. R., 1997. Regulation of hemolymph osmolytes and gill Na+/K+ ATPase activities during acclimation to saline media in the freshwater shrimp Macrobrachium olfersii (Wiegmann, 1836) (Decapoda, Palaemonidae). Journal of Experimental Marine Biology and Ecology, 215(1): 81-91. DOI:10.1016/S0022-0981(97)00016-6 (  0) 0) |
Long, X. W., Wu, R. F., Hou, W. J., Pan, G. P., Cheng, Y. X., and Wu, X. G., 2019. Effects of water salinity on the growth, ovarian development, osmoregulation, metabolism and antioxidant capacity of adult female swimming crab (Portunus trituberculatus). Journal of Fisheries of China, 43(8): 1768-1780 (in Chinese with English abstract). (  0) 0) |
Lu, Y. L., Wang, F., Gao, Q. F., and Dong, S. L., 2012a. Effects of salinity on the respiratory metabolism of preand post-maturity swimming crab (Portunus trituberculatus). Journal of Fisheries of China, 36(9): 1392-1399 (in Chinese with English abstract). (  0) 0) |
Lu, Y. L., Wang, F., Zhao, Z. Y., Dong, S. L., and Ma, S., 2012b. Effects of salinity on growth, molt and energy utilization of juvenile swimming crab Portunus trituberculat.. Journal of Fishery Sciences of China, 19(2): 237-245 (in Chinese with English abstract). (  0) 0) |
Lucu, Č., 1990. Ionic regulatory mechanisms in crustacean gill epithelia. Comparative Biochemistry and Physiology Part A Physiology, 97(3): 297-306. DOI:10.1016/0300-9629(90)90615-Y (  0) 0) |
Ma, J. W., Lv, J. J., Liu, P., Gao, B. Q., and Li, J., 2016. Effects of abrupt salinity stress on serum osmolarity and ion concentration of 'Huangxuan No. 1' Portunus trituberculatus. Progress in Fishery Sciences, 37(1): 58-62 (in Chinese with English abstract). (  0) 0) |
Mcnamara, J. C., and Faria, S. C., 2012. Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod crustacea: A review. Journal of Comparative Physiology B, 182(8): 997-1014. DOI:10.1007/s00360-012-0665-8 (  0) 0) |
Morris, S., 2001. Neuroendocrine regulation of osmoregulation and the evolution of air-breathing in decapod crustaceans. Journal of Experimental Biology, 204(5): 979-989. DOI:10.1242/jeb.204.5.979 (  0) 0) |
Moullac, G. L., and Haffner, P., 2000. Environmental factors affecting immune responses in crustacea. Aquaculture, 191(1-3): 121-131. DOI:10.1016/S0044-8486(00)00422-1 (  0) 0) |
Pan, L. Q., and Liu, H. Y., 2005. Review on the osmoregulation of crustacean. Journal of Fisheries of China, 29(1): 109-114 (in Chinese with English abstract). (  0) 0) |
Pisam, M., and Rambourg, A., 1991. Mitochondria-rich cells in the gill epithelium of teleost fishes: An ultrastructural approach. International Review of Cytology, 130(6): 191-232. (  0) 0) |
Qi, L., Jiang, K. J., Gu, X. L., and Qiao, Z. G., 2011. Growth, survival and Na+/K+-ATPase activity of early juvenile mud crabs (Scylla paramamosain) in low salinities. Modern Fisheries Information, 26(11): 20-23 (in Chinese with English abstract). (  0) 0) |
Qi, T. T., Liu, J., Zhao, P. S., Ge, B. M., and Zhang, D. Z., 2020. A novel modulation of physiological regulation in cultured Chinese mitten crab (Eriocheir japonica sinensis) in response to consistent salinity changes. Gene, 756: 144914. DOI:10.1016/j.gene.2020.144914 (  0) 0) |
Qu, Y. J., Lin, X. Z., Li, J. E., and Wen, J. F., 2014. The morphological structure of mitochondrion-rich cells in the gills of juveniles of the milkfish (Chanos chanos) and its changes in various salinities. Chinese Journal of Cell Biology, 36(12): 1622-1629 (in Chinese with English abstract). (  0) 0) |
Serrano, L., and Henry, R. P., 2008. Differential expression and induction of two carbonic anhydrase isoforms in the gills of the euryhaline green crab, Carcinus maenas, in response to low salinity. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 3(2): 186-193. DOI:10.1016/j.cbd.2008.02.003 (  0) 0) |
Sun, D. F., Lv, J. J., Gao, B. Q., Huan, P. P., Cai, Y., and Liu, P., 2019. Cloning of crustacean cardioactive peptide and its functional verification under low-salt adaptation in swimming crab (Portunus trituberculatus). Journal of Fishery Sciences of China, 26(2): 261-270 (in Chinese with English abstract). DOI:10.3724/SP.J.1118.2019.18207 (  0) 0) |
Sun, M. L., Jiang, J. L., Wang, L. P., Chen, F., Han, Y. Z., and Jiang, Z. Q., 2016. Structural changes in gill, kidney and intestine of juvenile Takifugu rubripes under low salinity treatment. Journal of Guangdong Ocean University, 36(6): 38-43 (in Chinese with English abstract). (  0) 0) |
Wang, C., Jiang, L. X., Wang, R. J., and Li, Y. Q., 2010. Effect of abrupt and gradual changes in salinity on development and feeding in juvenile swimming crab (Portunus trituberculatus). Fisheries Science, 29(9): 510-514 (in Chinese with English abstract). (  0) 0) |
Wang, F. G., and Liu, J. C., 1992. Studies on relations between the water environmental factors and diseases of Chinese shrimp. Journal of Marine Sciences, 10(4): 37-41 (in Chinese with English abstract). (  0) 0) |
Wang, H., Tang, L., Wei, H. L., Lu, J. K., Mu, C. K., and Wang, C. L., 2018a. Transcriptomic analysis of adaptive mechanisms in response to sudden salinity drop in the mud crab, Scylla paramamosain. BMC Genomics, 19(1): 421. (  0) 0) |
Wang, H., Wei, H. L., Tang, L., Lu, J. K., Mu, C. K., and Wang, C. L., 2018b. A proteomics of gills approach to understanding salinity adaptation of Scylla paramamosain. Gene, 677: 119-131. (  0) 0) |
Wang, H., Wei, H., Tang, L., Lu, J., Mu, C., and Wang, C., 2018c. Identification and characterization of miRNAs in the gills of the mud crab (Scylla paramamosain) in response to a sudden drop in salinity. BMC Genomics, 19(1): 609. (  0) 0) |
Wang, L., and Pan, L. Q., 2016. A study on penetration physiological adaptation of Portunus trituberculatus under low salinity. Transactions of Oceanology and Limnology, 2016(3): 106-112 (in Chinese with English abstract). (  0) 0) |
Wang, Y. R., Li, E. C., Long, L. N., Yu, N., Zhang, F. Y., and Chen, L. Q., 2011. Review on the osmoregulation and adaptation to salinity changes of Chinese mitten crab Eriocheir sinensis. Marine Fisheries, 33(3): 352-360 (in Chinese with English abstract). (  0) 0) |
Weihrauch, D., Ziegler, A., Siebers, D., and Towle, D. W., 2001. Molecular characterization of V-type H+-ATPase (B-subunit) in gills of euryhaline crabs and its physiological role in osmoregulatory ion uptake. The Journal of Experimental Biology, 204(1): 25-37. (  0) 0) |
Yao, H. Z., Li, X., Chen, Y. H., Liang, G. L., Gao, G., Wang, H., et al., 2021. Metabolic changes in Scylla paramamosain during adaptation to an acute decrease in salinity. Frontiers in Marine Science, 8: 734519. (  0) 0) |
Yao, H. Z., Li, X., Tang, L., Wang, H., Wang, C. L., Mu, C. K., et al., 2020. Metabolic mechanism of the mud crab (Scylla paramamosain) adapting to salinity sudden drop based on GCMS technology. Aquaculture Reports, 18(2): 100533. (  0) 0) |
Ye, J. S., Wang, X. Q., Ma, S., and Yan, B. L., 2008. The effect of abrupt change in salinity on the nonspecific immune factors of Litopenaeus vannamei. Progress in Fishery Sciences, 29(1): 38-43 (in Chinese with English abstract). (  0) 0) |
Zheng, P., Wang, C. L., Song, W., and Wu, D. H., 2010. Effect of salinity stress on serum non-specific immune factors in swimming crab Portunus trituberculatus. Fisheries Science, 29(11): 634-638 (in Chinese with English abstract). (  0) 0) |
Zhou, D., Mu, C. K., Song, W., Li, R. H., and Wang, C. L., 2014. Effects of low salinity stress on the antioxidant enzyme and ATPase activities in tissues of swimming crab Portunus trituberculatus. Ecological Science, 33(4): 698-703 (in Chinese with English abstract). (  0) 0) |
Zhou, S. L., Jiang, N. C., Lu, J. P., and Yang, W. X., 2001. Progress of the study on osmotic regulation in crustaceans I. The gill's structure and function and its' concerned factors. Journal of Marine Sciences, 19(1): 44-51 (in Chinese with English abstract). (  0) 0) |
Zhou, Y. H., Pan, G. P., Zhou, W. Y., Hou, W. J., and Zhang, G. N., 2014. Influences of different salinities on growth and survival rate of Portunus trituberculatus. Hunan Agricultural Sciences, 2014(10): 64-66 (in Chinese with English abstract). (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


