2) College of Life Sciences, Yantai University, Yantai 264005, China;
3) Yantai Institute of Marine Economy, Yantai 264003, China
With the rapid development of petrochemical, electric power, energy and other industries, more and more CO2 are discharged into the air, and some of it is dissolved into the seawater, resulting in the decrease of ocean pH value (Guinotte and Fabry, 2008; Doney et al., 2009; Heuer and Grosell, 2014; Kapsenberg and Cyronak, 2019). Ocean acidification caused by large amount of CO2 may have a significant impact on the growth, immunity, metabolism and other life activities of Marine organisms by reducing calcification rates (Stojkovic et al., 2013), changing fish behavior (Munday et al., 2009) and phytoplankton composition (Lohbeck et al., 2012; Matear and Lenton, 2018; Tan and Zheng, 2019; Baag and Mandal, 2022). It has been previously reported that CO2-induced Ocean acidification can lead to significantly reduced survival rate of Haliotis tuberculata in the early stages of life, reduced mineralization rate and retarded development during the shelled larval stage (Wessel et al., 2018). Gray et al. (2017) showed that longterm ocean acidification negatively affects the feeding activity and energy balance of mussels, leading to delayed development. It has also been reported that ocean acidification could adversely affect the neuroregulatory mechanisms of Lates calcarifer (Wang et al., 2021). Although the adverse effects of ocean acidification have been studied in a wide range of aquatic organisms, to date, they have been poorly studied in cephalopods, particularly in juvenile Sepia esculenta.
S. esculenta is a cephalopod mollusk with high nutritional value (Bai et al., 2020). It is widely farmed in the west coast of the Pacific Ocean (Bian et al., 2018; Bai et al., 2020). However, with the worsening marine environment, the cost of breeding S. esculenta is increasing year by year. In the face of the deteriorating marine environment, juvenile fish at the early stage of life are facing unprecedented survival challenges (Wang et al., 2023; Liu et al., 2024). For instance, ocean acidification leads to longer Doryteuthis pealeii incubation times and shorter mantle lengths. Although the differences are small, they can also alter larval behavior and survival (Kaplan et al., 2013). For the benign development of the S. esculenta aquaculture industry, it is necessary to investigate the altered life activity of S. esculenta under ocean acidification environment.
High-throughput transcriptome sequencing is a research technology that can find the differences at the molecular level of organisms, and it has been well developed (Morozova et al., 2009; Qian et al., 2014). In recent years, it has been widely used to study the molecular functions of mollusks. For example, transcriptome was used by Bao et al. (2022) to probe the immune feedback mechanism of S. esculenta larvae under Cu/Cd co-exposure. Castellanos-Martínez et al. (2014) used RNA-Seq to probe the response of Octopus vulgaris to gastrointestinal infection with Aggregata octopiana. Because of the high efficiency of transcriptome sequencing, this study used this technology to explore the altered physiological activities of S. esculenta in the ocean acidification environment.
In our study, RNA sequencing was performed on S. esculenta larvae that were kept in low pH seawater for 0 h, 4 h and 24 h. Then, based on the screened differentially expressed genes (DEGs), GO, KEGG and PPI networks were used to explore the key genes and signaling pathways that maintain the stability of life activities in cuttlefish under low pH environment. The findings provide a basis for exploring the effects of ocean acidification on mollusks and culturing S. esculenta with ocean acidification challenge.
2 Materials and Methods 2.1 Experimental SamplesS. esculenta adults (weight, 348.87 g ± 11.28 g; mantle length, 13.82 mm ± 0.23 mm) were obtained from the sea around Qingdao and kept in a workshop culture pond for a week. Eggs were subsequently collected daily from the pool using an attachment net and placed into plastic basins with holes. Eggs were subsequently collected daily from the pool using an attachment net and placed into plastic basins with holes. After collecting the eggs, the plastic basin was placed into another breeding pond to ensure that the seawater flow and oxygen supply were sufficient, and the pH value and other indicators were the same as those in the mother pond (salinity, 31 ± 0.4; pH, 8.1; dissolved oxygen, 5.7 mg L−1). Totally 100 L of seawater was added to each of the two square buckets whose capacity was 120 L. According to previous experiments, one group was set as the experimental group (low PH), and the other groups did not change anything. The pH was decreased to 7.5 by the addition of hydrochloric acid to normal seawater. In each group, 100 S. esculenta larvae were placed and sampled at 4 h and 24 h, respectively. The experiment was conducted using larvae that had reached two days post-hatch, with an average size of 7 mm ± 1 mm. Samples were placed into cryotubes and stored in liquid nitrogen.
2.2 RNA Preparation, Library Construction, and RNA-SeqNine larvae were randomly selected from per group at all set time points, and RNA was isolated by TRIzol (Thermo Fisher Scientific, USA) following the instruction of the manufacture. Five experimental groups were set up, including three normal growth controls (C_0 h, C_4 h and C_24 h), two experimental groups with low pH stress (pH_4 h and pH_24 h). Equimolar amounts of RNA from three juvenile fish randomly selected from each time point were used as the first copy of RNA-Seq. Three biological repetitions were conducted. Additional unused RNA samples were used for qRT-PCR validation. The acquired total RNA was enriched for mRNA with polyA tails by Oligo (dt) magnetic beads. Then these isolated mRNAs underwent random fragmentation using divalent cations. This step laid the foundation for constructing a library, employing the standard library construction method from NEB. The first strand of cDNA was synthesized in the M-MuLV reverse transcriptase system using fragmented mRNA as a template and random oligonucleotides as primers, followed by degradation of the RNA strand with RNaseH and the synthesis of the second strand of cDNA with dNTPs in the DNA polymerase I system. Following the purification of double-stranded cDNA, it underwent end-repair, A-tailing, and finally was connected to the sequencing junction. The cDNA fragments ranging from 250 to 300 bp were selected using AMPure XP beads. PCR amplification was carried out, and the resulting PCR products were purified once more using AMPure XP beads to ultimately obtain the library. The library construction kit was NEBNext® Ultra™ RNA Library Prep Kit for Illumina®. The Agilent 2100 BioAnalyzer was employed to ascertain the insert size, which guaranteed the library's quality (Masotti and Preckel, 2006). S. esculenta larvae were sequenced by Illumina NovaSeq 6000 (Illumina, USA). Sequencing by Synthesis (SBS) is a fundamental principle in sequencing technology. Within the flow cell used for sequencing, four fluorescently labeled deoxyribonucleotide triphosphates (dNTPs), DNA polymerase, and adapter primers are introduced for amplification. As each complementary strand in every sequencing cluster extends, the addition of a fluorescently labeled dNTP releases a corresponding fluorescent signal. The sequencing machine captures these fluorescence signals and utilizes computer software to convert the light signals into sequencing peaks, thereby obtaining the sequence information for the target fragment. The clean reads obtained after quality control were mapped to the reference genome. The reference genome of this species is recorded in our laboratory and has not been published yet.
2.3 Screening of DEGsDEGs were screened using the DESeq2 package for R as a negative binomial distribution model. First of all, data were imported for building the ddsmodel, and then the DESeq function was used to estimate the dispersion of the samples. Afterwards the difference in gene expressions was analyzed by this package. DEGs with P-value ≤ 0.05 were screened (Love et al., 2014).
2.4 Functional Enrichment Analysis of GenesThe DAVID v6.8 was used for GO and KEGG function enrichment analyses (Jiao et al., 2012). All genes annotated to the database were used as the background gene for functional enrichment analysis, and DEGs were used as the validation set for the analysis to explore the gene expression differences in S. esculenta when subjected to low pH stimulation.
2.5 Analysis of PPISTRING v11.5 with the default parameter settings was used to build PPI of S. esculenta under low pH stimulation (Szklarczyk et al., 2019). DEGs which were enriched to significantly different 11 KEGG signaling pathways were used to construct PPI.
2.6 Validation by Quantitative RT-PCRPrimer Premier 5.0 was used to design gene-specific primers of key genes (Table 1). Toensure the accuracy of the experiment, we compared the expression levels of three reference genes including β-actin, 18S, and GAPDH in S. esculenta larvae before experiment, and β-actin was selected because of its stable expression. Then, quantitative RTPCR was performed in a 20 µL solution containing 10 ng of template cDNA and SYBR Premix Ex Taq Ⅱ (TaKaRa) by using a LightCycler 480 at 95℃ for 5 min pre-incubation, followed by 45 cycles of 95℃ for 15 s and 60℃ for 45 s. Finally, the melting curve was analyzed to detect single amplification. Fluorescent signal accumulation was recorded at the 60℃ 45 s phase during each cycle by using LightCycler 480. The 2−ΔΔCt comparative Ct method was used to calculate the relative quantities of the target genes expressed as fold variation over β-actin. In addition, twelve genes that were significantly differentially expressed in S. esculenta larvae when subjected to low pH stimulation were selected to verify the quality of the sequencing results.
|
|
Table 1 Primers for qRT-PCR |
Sequencing of RNA from S. esculenta larvae after low pH stimulation was conducted. A total of 691664490 raw reads were generated, and 673136624 (97.32%) clean reads were remained after quality control, as shown in Table 2. An average of 87.99% of the clean reads can be mapped to the reference genome. The average Q20 of the clean reads was 96.96%, and the average Q30 of the clean reads was 92.03%.
|
|
Table 2 RNA-Seq data |
After analyzing the results of the volcano diagram, it can be concluded that 428 DEGs were identified, of which 222 DEGs were up-regulated and 206 DEGs were down-regulated in the samples collected at 4 h. In the samples collected at 24 h, 683 DEGs were identified. In between, 314 DEGs were up-regulated, and 369 DEGs were down-regulated (Fig. 1).
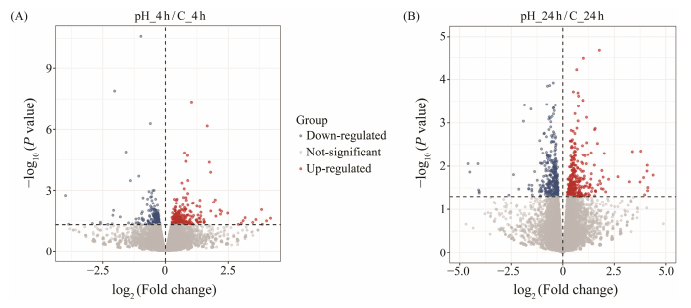
|
Fig. 1 Volcano diagram of DEGs. Each dot indicates a DEGs. Red dots indicate up-regulation of significant differential gene expression, blue dots indicate down-regulation of significant differential gene expression, and gray dots indicate non-significant differential gene expression. |
From the Venn diagram results, 1072 genes showed significant differential expressions in all samples, while 39 genes were differentially expressed at both 4 h and 24 h (Fig. 2).
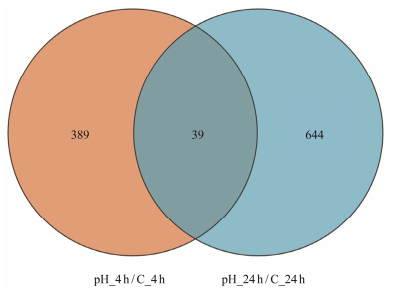
|
Fig. 2 Venn plot of DEGs. The blue and orange areas represent the quantities of genes significantly expressed at 4 h and 24 h, respectively. The co-expressed genes at the 4 h and 24 h are indicated by dark orange in the middle region. |
The cluster heatmap showed that DEGs expression were different at 4 h (pH_4 h) and 24 h (pH_24 h). Additionally, these DEGs in three control groups and two experimental groups all had different expression patterns (Fig. 3).
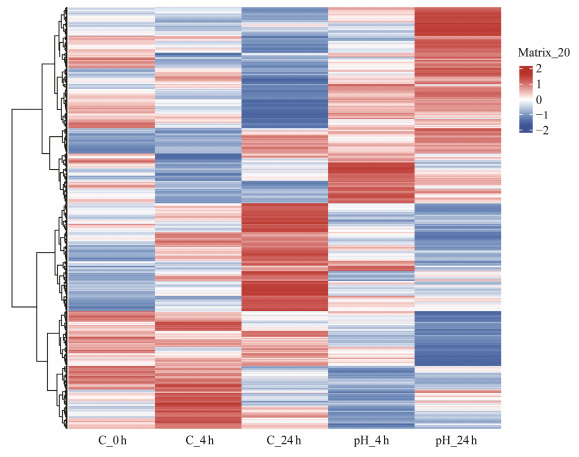
|
Fig. 3 Hierarchical clustering heatmap of DEGs. Each column represents a group and each row represents a gene. Colors ranging from blue to red indicating low to high expression levels. |
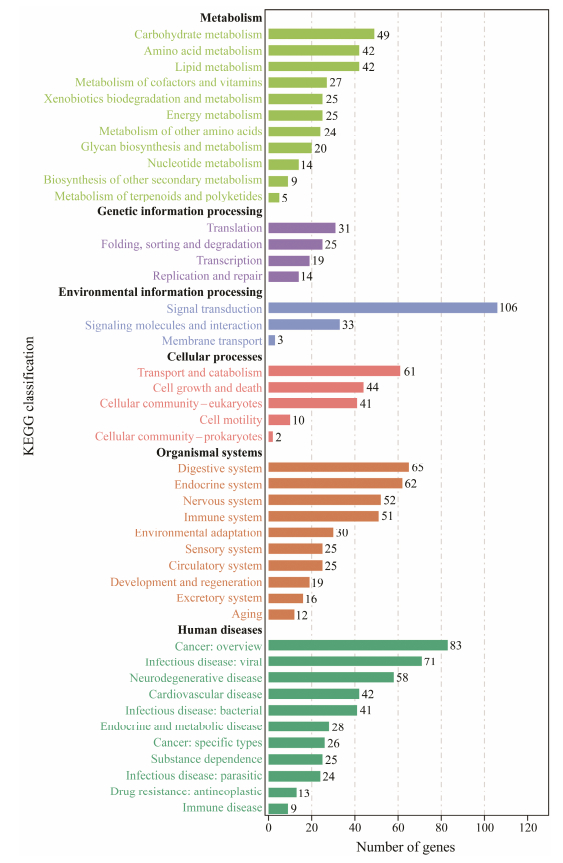
DEGs were employed for the enrichment analysis of KEGG and GO. In this study, a total of 88 GO terms included in three categories (biological process, cellular components, and molecular function) were remarkable enriched. First 20 GO terms in the biological process cluster and first 10 GO terms in the cellular component and molecular function cluster are presented in the figure (Fig. 4). Among these GO terms, ion transport, chloride transport and lipid storage in the biological process cluster suggest that larval S. esculenta may generate a stress response to maintain cell homeostasis at low pH. The KEGG analysis results indicated that several DEGs were enriched in metabolism-related level-2 KEGG signaling pathways (Fig. 5). Ocean acidification may affect metabolic activity and neural transduction in S. esculenta larvae. Eleven KEGG signaling pathways were identified (Table 3).
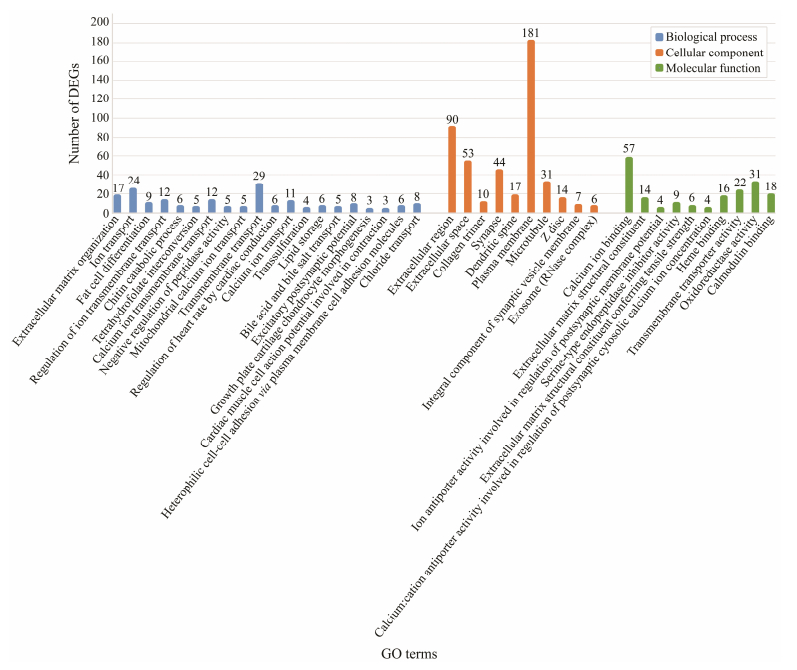
|
Fig. 4 GO enrichment analysis. |
|
Fig. 5 Level-2 KEGG signaling pathway annotation. |
|
|
Table 3 Eleven identified KEGG signaling pathways |
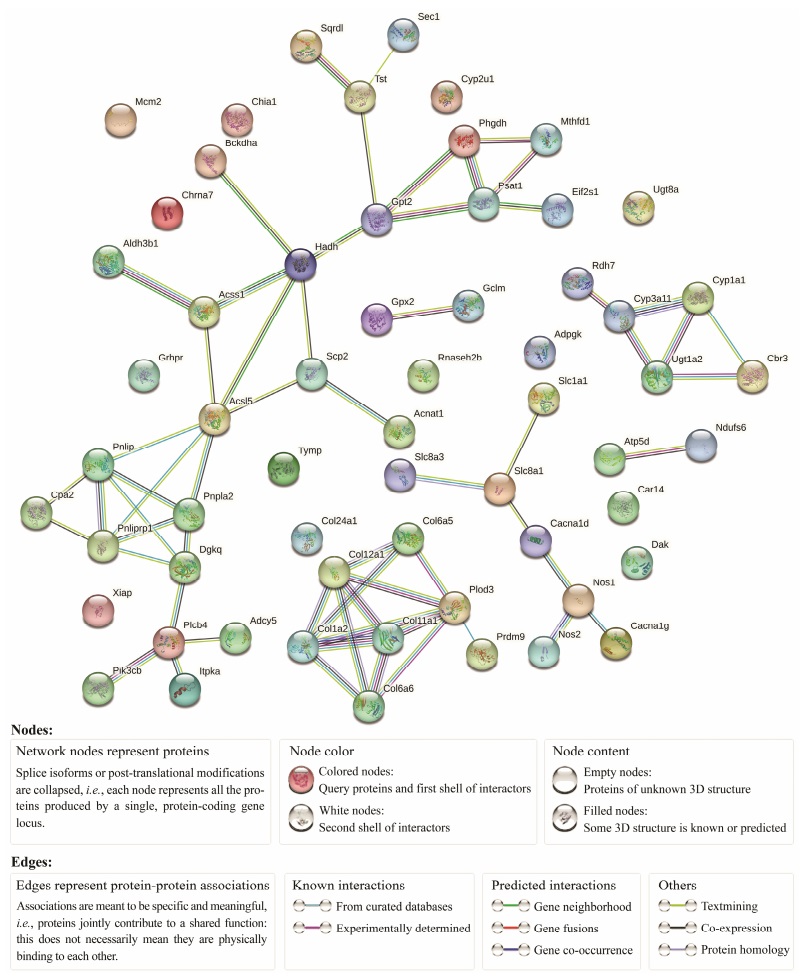
The ninety-six DEGs from Table 3 signaling pathway were used to construct the PPI (Fig. 6). The relevant network parameters of PPI are demonstrated in Table 4. The average node degree was 2.03, network clustering coefficient was 0.562, and the P-value was ≤ 2.34E−14. DEGs with a higher number of PPI interactions or KEGG signaling pathways are presented in Table 5. Among them, ACSL5, PLOD3 and COL1A2 had the highest numbers of PPI interactions and higher number of KEGG signaling pathways, and were identified as hub genes. The other 11 genes were designated as key genes. Previous studies have shown that these genes are involved in cellular life activities such as inflammatory response, stress response and metabolism. Further investigation of these genes will help to better understand the effects of low pH on S. esculenta larvae.
|
Fig. 6 Protein-protein interaction network. |
|
|
Table 4 Statistics of PPI data |
|
|
Table 5 Quantities of PPI and KEGG signaling pathways that include key DEGs |
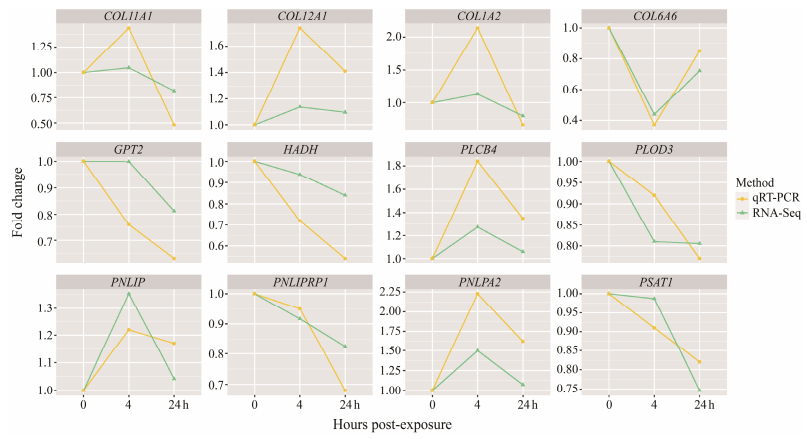
The precision of differential expression of 12 key genes in S. esculenta under low pH was employed in qRT-PCR. The expression patterns of the genes obtained by quantification comply with the trends of gene expression patterns of RNA-Seq analysis, confirming the confidence of the sequencing results (Fig. 7).
|
Fig. 7 qRT-PCR and RNA-Seq results. |
Ocean acidification is a typical destruction of the marine environment caused by human activities (Falkenberg et al., 2020). Lower pH water can have long-term effects on the normal development and reproduction of marine organisms (Christen et al., 1983; Dupont, 2010; Byrne and Przeslawski, 2013; Dorey, 2018). However, the artificial culture of S. esculenta is facing unprecedented challenges due to the more severe breeding environment in the future (Tan and Zheng, 2019). Therefore, it is essential to understand the physiological state and anti-stress mechanism of S. esculenta when it is grown in an acidic environment. The results of this study showed that a total of 1072 genes appeared to be differentially expressed in S. esculenta larvae when irritated by ocean acidification. The volcano and cluster thermogram results indicate that more genes showed different expression patterns with increasing exposure time. Since the experimental materials are larvae and develop rapidly, the differences in expression patterns between the control and treatment groups at the same time point can indicate that the organisms have undergone significant changes in gene expression patterns after stimulation. In the results of the Venn diagram, key genes such as GPT2, PLOD3, which were significantly expressed at both 4 h and 24 h, were previously reported to be involved in the onset and metastasis of cancer (Ewans et al., 2019; Kim et al., 2019; Mitra et al., 2021). We consider that 1072 genes are abnormally expressed in S. esculenta larvae when stimulated by low pH, leading to cellular pathology and dissemination within the organism.
4.2 Functional Enrichment AnalysesIn this study, 1072 DEGs were used for functional enrichment analysis of genes. The results of GO enrichment analysis showed that most of the first 20 GO terms in the biological process cluster were related to the self-regulation of organisms in response to external stimuli. Among them, the significantly enriched GO term 'transmembrane transport' plays a key role in alleviating the stimulation of the organism by enhancing the exchange of substances between organelles (Schendzielorz et al., 2018; Jones et al., 2019; Zhong and Zhao, 2019; Ming et al., 2020). Lipid storage is a biological process that occurs in most organisms. Fat is transformed into triglyceride for long-term storag to ensure the normal metabolic activities of the body in harsh environments (Pierron et al., 2007; Calvo et al., 2018; Farese and Walther, 2022). We hypothesized that ocean acidification might have some effects on the metabolism of S. esculenta larvae. In this study, 11 KEGG signaling pathways were significantly enriched. Among them, glycerol metabolism, protein digestion and absorption, and other signaling pathways have been reported to be involved in basic life activities such as growth regulation, development and reproduction of organisms (Huang et al., 2015; Zhang and Reue, 2017; He et al., 2021). This also indicated that ocean acidification had a significant effect on the metabolic activities of S. esculenta larvae.
4.3 Analysis of PPIThe metabolism and immunity of organisms, and other physiological functions are inseparable from the participation of proteins, which are the basis of all life activities (Ahmad et al., 2021; Chandhini et al., 2021; Li et al., 2021). Analysis of protein-protein interactions helps us to gain a deeper understanding of the changes in organisms in an acidized ocean environment. In this study, we constructed a PPI network using 69 DEGs involved in 11 significantly enriched signaling pathways. The results showed that there was a strong interaction between the proteins. For example, ACSL5 and PLOD3 have interactions with a variety of proteins. Previous studies indicated their involvement in the production and metastasis of tumor cells (Klaus et al., 2014; Ewans et al., 2019). Finally, 12 DEGs with higher number of protein interactions and KEGG signaling pathway involvement were identified for further exploration. These DEGs could be broadly divided into four categories: DEGs involved in immune, DEGs associated with inflammation, collagen protein family and some other key genes.
4.3.1 Analysis of DEGs in immuneUnlike common higher vertebrates, mollusks typically possess only innate immunity and lack the more defensive acquired immunity to combat external environmental stimuli (Canesi et al., 2022; Wu et al., 2023). The proteins within cells responsible for immune defense collectively form the immune system, which generates clearance responses against foreign substances, known as immune defense (Parkin and Cohen, 2001; Tomar and De, 2014; Hariyanto and Kurniawan, 2021). After the appearance of malignant cell, it will cause the organism to appear cell tissue bleeding, decreased vitality, decreased appetite and other adverse reactions (Karsch-Bluman et al., 2019; Hariyanto and Kurniawan, 2021; Bao et al., 2022). In this study, GPT2, HADH and PSAT1 were identified as key DEGs. Rapid proliferation of cells requires sufficient amounts of nutrients, which are mainly provided by the metabolism of mitochondrial glutamine metabolism (Kim et al., 2019). Glutamate pyruvate transaminase 2 (GPT2) catalyzes the reversible reaction between glucose-derived pyruvate and glutamine-derived glutamate to produce alanine and α-KG and is required for cell growth (Hao et al., 2016; Smith et al., 2016; Mitra et al., 2021). Abnormalities in energy metabolism can lead to abnormalities in the immune system in the organism (Wang et al., 2022). HADH is a key subunit of mitochondrial trifunctional protein, which is involved in fatty acid β-oxidation in vivo. Its abnormal expression is also one of the markers of metabolic abnormality (Diebold et al., 2019; Wang et al., 2022). Phosphoserine aminotransferase 1 (PSAT1) catalyzes the conversion of 3-phosphohydroxypyruvate and glutamate to 3-phosphatidylinic acid and α-ketoglutaric acid (Yang et al., 2023). PSAT1 provides anabolism and energy support for these immune cells, affecting their proliferation, survival, autophagy, migration, and invasion. These functions significantly contribute to the functioning of the immune system, providing substantial support to immune cells (Liu et al., 2016; Metcalf et al., 2020; Yang and Mottillo, 2020). Based on our results, it is hypothesized that ocean acidification leads to abnormal expression of genes involved in amino acid and fatty acid metabolism in S. esculenta larvae, providing the energy required for immunesystem formation and metastasis.
4.3.2 Analysis of DEGs in inflammationInflammation underlies a variety of physiological and pathological processes. Infection, tissue damage and stress all trigger the recruitment of white blood cells to the affected tissue sites to produce inflammation (Medzhitov, 2008; Yeung et al., 2018; Singh et al., 2019). Pancreatic triglyceride lipase (PNLIP) and pancreatic lipase-associated protein 1 (PNLIPRP1) are involved in the process of lipid catabolism in living organisms (Zhu et al., 2021). Its expression level is closely related to the occurrence of inflammation. When abnormal expressions of PNLIP and PNLIPRP1 occur, it represents the occurrence of inflammation in the organism (Maurin et al., 2022). In the results presented in this study, the expressions of two genes PNLIP and PNLIPRP1 differed significantly, suggesting that ocean acidification causes an inflammatory response to tissue damage in S. esculenta larvae. This suggests that we need to prevent the tissue damage caused by ocean acidification during the captive breeding of S. esculenta.
4.3.3 Collagen protein familyCollagen is a protein containing a triple helix structure that plays a central role in maintaining the structure of cellular tissue (Ricard-Blum and Ruggiero, 2005; Ricard-Blum, 2011). Collagen also interacts with cell surface receptors such as integrins, discoidal protein structural domain receptors and glycoprotein Ⅵ to regulate cell proliferation, differentiation and migration (Vogel, 2001). Abnormalities in intracellular physiological processes are usually accompanied by abnormal expression of collagen genes (Qi and Xu, 2018). In our results, four key DEGs were identified, including COL12A1, COL1A2, COL11A1 and COL6A6, which is a protofibril-associated collagen, can promote immune cell exercise function through positive feedback from the MAPK signaling pathway (Xiang et al., 2019). COL1A2 and COL11A1 belong to fibril-forming collagen, and the overexpression of this gene promotes the proliferation, migration and invasion of immune cells (Yu et al., 2018; Liu and Meng, 2022). COL6A6 has an important role in regulating cell function, and this gene can inhibit cancer cell proliferation and metastasis through the JAK signaling pathway (Qiao et al., 2021). In our results, the expression of collagen-related family genes, (including COL12A1, COL1A2, COL11A1 and COL6A6) showed significant differences, suggesting that the above genes regulate cell proliferation, differentiation and migration after S. esculenta larvae are stimulated by marine acidification through regulating MAPK signaling pathway, JAK signaling pathway and other signaling pathways.
4.3.4 Hub genes functional analysisIn this study, the effects of exposure to ocean acidification on the physiological function of S. esculenta were investigated through a comprehensive analysis of the KEGG signaling pathway and PPI network. Three DEGs with the highest number of protein interactions or the highest quantities of KEGG signaling pathways involved were identified as the core genes regulating the physiological processes of S. esculenta larvae after acidizing exposure, namely COL1A2, ACSL5 and PLOD3. The ACSL family is a family that plays a key role in fatty acid metabolism and cancer occurrence. They play a role in inhibiting cancer in vivo, and can also promote cell apoptosis (Soupene and Kuypers, 2008; Quan et al., 2021). ACSL5, which is identified as the hub gene, is one of the ACSL family. It is nuclear-coded and expressed in the mitochondria and physiologically participates in the pro-apoptotic sensing of cells (Quan et al., 2021). ACSL5 is mainly expressed in the intestine, and it induces the expression of mitochondrial death proteins, which enables the intestinal cells to quickly detect pro-apoptotic signals (Klaus et al., 2014). In pancreatic cancer cells, ACSL5 interacted with lipid metabolism-related enzymes such as long-chain Acyl-CoA dehydrogenase, carnitine palmitoyl transferase 1, fatty acid desaturase 2 and fatty acid synthase, suggesting that ACSL5 is involved in cancer progression by regulating lipid metabolic homeostasis (Klaus et al., 2014; Quan et al., 2021). The PLOD family has a high similarity. Collagen cross-link and deposition depend on lysyl hydroxylation, which is catalyzed by procollagenlysine, 2-oxoglutarate 5-dioxygenase (PLOD). PLOD3 activity is important for the biosynthesis of collagen types Ⅳ and Ⅵ (Qi et al., 2018; Qi et al., 2021). Aberrant regulation of PLOD3 leads to further cancer development and metastasis (Mitra et al., 2021). COL1A2 belongs to collagen proteins that can interact with cells to produce biochemical signals that induce tissue function and cancer progression. PLOD3 also regulates the synthesis of induced collagen (Qi et al., 2018). Based on our analysis, 3 hub genes were weakly expressed in the cells, suggesting that these genes work together to promote immune cell production and metastasis in S. esculenta larvae. Furthermore, PLOD3 can promote collagen synthesis to act together (Qi et al., 2018). In our experimental results, three pivotal genes COL1A2, ACSL5 and PLOD3 promoted immune cell production and metastasis in S. esculenta larvae subjected to ocean acidification stress by regulating lipid metabolism, collagen synthesis, and other regulation methods.
4.3.5 Other key DEGsIn the present study, in addition to the key genes and protein families mentioned above, other key genes that have been identified also have significant effects on physiological activities such as metabolism. Diacylglycerol kinase family member DGKQ is a key receptor that regulates lipid metabolism disorders and maintains cell stability after protein kinase Cε (PKCε) activation (Zheng et al., 2023). Phospholipase C beta 4 (PLCB4) is a subtype of phospholipase C-β that plays a role in the immune response and is able to activate immune cells to regulate the normal life activities of the organism after tumor development (Kawakami and Xiao, 2013). PNPLA2 can be activated by the interaction with α/β hydrolase domain containing protein 5 to ensure the normal transmission of cellular metabolic signals, promote communication between organs, and prevent the occurrence of cancer (Yang et al., 2020). DGKQ, PLCB4 and PNPLA2 are able to induce immune responses and ensure proper metabolic signaling. In the present study, the significantly enhanced expression of these three genes suggested that our ocean acidification would lead to tissue damage and abnormal metabolic signaling in S. esculenta larvae.
5 ConclusionsIn this study, transcriptome sequencing technology was used to explore the differential expression of genes in S. esculenta under ocean acidification. The results identified by functional enrichment analysis and protein interaction network analysis suggest that acidification stimulation leads to cancer production and migration in S. esculenta larvae. By analyzing the expression of key genes at different times, it can be concluded that long-term ocean acidification may lead to metabolic abnormalities and tissue damage among other responses in S. esculenta larvae. These results can be useful for further studies on marine acidification in mollusks. It can also prevent adverse reactions in cephalopods when stimulated by ocean acidification during artificial breeding and culture of cephalopods.
AcknowledgementThis research was funded by the Ministry of Agriculture of the People's Republic of China (No. CARS-49).
Ahmad, I., Ahmed, I., Fatma, S., and Peres, H., 2021. Role of branched-chain amino acids on growth, physiology and metabolism of different fish species: A review. Aquaculture Nutrition, 27: 1270-1289. DOI:10.1111/anu.13267 (  0) 0) |
Baag, S., and Mandal, S., 2022. Combined effects of ocean warming and acidification on marine fish and shellfish: A molecule to ecosystem perspective. Science of the Total Environment, 802: 149807. DOI:10.1016/j.scitotenv.2021.149807 (  0) 0) |
Bai, Q., Li, J., Xu, X., Zhang, Z., Li, W., and Zhang, X., 2020. Effects of social hierarchy on the behavioral phenotype and energy metabolism of Sepia esculenta in the reproductive period. Acta Ecologica Sinica, 40(15): 5408-5417. DOI:10.5846/stxb201905090947 (  0) 0) |
Bao, X., Wang, W., Chen, X., Feng, Y., Xu, X., Sun, G., et al., 2022. Exploration of immune response mechanisms in cadmium and copper co-exposed juvenile golden cuttlefish (Sepia esculenta) based on transcriptome profiling. Frontiers in Immunology, 13: 963931. DOI:10.3389/fimmu.2022.963931 (  0) 0) |
Bian, L., Liu, C., Chen, S., Zhao, F., Ge, J., and Tan, J., 2018. Transcriptome analysis of gene expression patterns during embryonic development in golden cuttlefish (Sepia esculenta). Genes & Genomics, 40(3): 253-263. DOI:10.1007/s13258-017-0588-6 (  0) 0) |
Byrne, M., and Przeslawski, R., 2013. Multistressor impacts of warming and acidification of the ocean on marine invertebrates' life histories. Integrative and Comparative Biology, 53(4): 582-96. DOI:10.1093/icb/ict049 (  0) 0) |
Calvo, N. S., Stumpf, L., Cortés-Jacinto, E., Castillo Díaz, F., and López Greco, L. S., 2018. Mobilization of energetic reserves during starvation in juveniles of different size of the redclaw crayfish Cherax quadricarinatus. Aquaculture Nutrition, 24: 952-960. DOI:10.1111/anu.12631 (  0) 0) |
Canesi, L., Auguste, M., Balbi, T., and Prochazkova, P., 2022. Soluble mediators of innate immunity in annelids and bivalve mollusks: A mini-review. Frontiers in Immunology, 13: 1051155. DOI:10.3389/fimmu.2022.1051155 (  0) 0) |
Castellanos-Martínez, S., Arteta, D., Catarino, S., and Gestal, C., 2014. De novo transcriptome sequencing of the Octopus vulgaris hemocytes using Illumina RNA-Seq technology: Response to the infection by the gastrointestinal parasite Aggregata octopiana. PLoS One, 9(10): e107873. DOI:10.1371/journal.pone.0107873 (  0) 0) |
Chandhini, S., Trumboo, B., Jose, S., Varghese, T., Rajesh, M., and Kumar, V. J. R., 2021. Insulin-like growth factor signalling and its significance as a biomarker in fish and shellfish research. Fish Physiology and Biochemistry, 47(4): 1011-1031. DOI:10.1007/s10695-021-00961-6 (  0) 0) |
Christen, R., Schackmann, R. W., and Shapiro, B. M., 1983. Metabolism of sea urchin sperm. Interrelationships between intracellular pH, ATPase activity, and mitochondrial respiration. Journal of Biological Chemistry, 258 (9): 5392-5399.
(  0) 0) |
Diebold, I., Schön, U., Horvath, R., Schwartz, O., Holinski-Feder, E., Kölbel, H., et al., 2019. HADHA and HADHB gene associated phenotypes – Identification of rare variants in a patient cohort by Next Generation Sequencing. Molecular and Cellular Probes, 44: 14-20. DOI:10.1016/j.mcp.2019.01.003 (  0) 0) |
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A., 2009. Ocean acidification: The other CO2 problem. Annual Review of Marine Science, 1: 169-192. DOI:10.1146/annurev.marine.010908.163834 (  0) 0) |
Dorey, N., 2018. Starfish larvae lose substantial energy to maintain digestion under ocean acidification conditions. Acta Physiologica, 224(2): e13169. DOI:10.1111/apha.13169 (  0) 0) |
Dupont, S., Ortega-Martínez, O., and Thorndyke, M., 2010. Impact of near-future ocean acidification on echinoderms. Ecotoxicology, 19(3): 449-462. DOI:10.1007/s10646-010-0463-6 (  0) 0) |
Ewans, L. J., Colley, A., Gaston-Massuet, C., Gualtieri, A., Cowley, M. J., McCabe, M. J., et al., 2019. Pathogenic variants in PLOD3 result in a Stickler syndrome-like connective tissue disorder with vascular complications. Journal of Medical Genetics, 56(9): 629-638. DOI:10.1136/jmedgenet-2019-106019 (  0) 0) |
Falkenberg, L. J., Bellerby, R. G. J., Connell, S. D., Fleming, L. E., Maycock, B., Russell, B. D., et al., 2020. Ocean acidification and human health. International Journal of Environmental Research and Public Health, 17(12): 4563. DOI:10.3390/ijerph17124563 (  0) 0) |
Farese, R. V., and Walther, T. C., 2022. The phase of fat: Mechanisms and physiology of lipid storage. The FASEB Journal, 36: 0I217. DOI:10.1096/fasebj.2022.36.S1.0I217 (  0) 0) |
Gray, M. W., Langdon, C. J., Waldbusser, G. G., Hales, B., and Kramer, S., 2017. Mechanistic understanding of ocean acidification impacts on larval feeding physiology and energy budgets of the mussel Mytilus californianus. Marine Ecology Progress Series, 563: 81-94. DOI:10.3354/meps11977 (  0) 0) |
Guinotte, J. M., and Fabry, V. J., 2008. Ocean acidification and its potential effects on marine ecosystems. Annals of the New York Academy of Sciences, 1134: 320-342. DOI:10.1196/annals.1439.013 (  0) 0) |
Hao, Y., Samuels, Y., Li, Q., Krokowski, D., Guan, B. J., Wang, C., et al., 2016. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nature Communications, 7: 11971. DOI:10.1038/ncomms11971 (  0) 0) |
Hariyanto, T. I., and Kurniawan, A., 2021. Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment. Cancer Treatment and Research Communications, 27: 100336. DOI:10.1016/j.ctarc.2021.100336 (  0) 0) |
He, S., You, J. J., Liang, X. F., Zhang, Z. L., and Zhang, Y. P., 2021. Transcriptome sequencing and metabolome analysis of food habits domestication from live prey fish to artificial diets in mandarin fish (Siniperca chuatsi). BMC Genomics, 22(1): 129. DOI:10.1186/s12864-021-07403-w (  0) 0) |
Heuer, R. M., and Grosell, M., 2014. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 307(9): R1061-R1084. DOI:10.1152/ajpregu.00064 (  0) 0) |
Huang, H. Y., Zhao, G. P., Liu, R. R., Li, Q. H., Zheng, M. Q., Li, S. F., et al., 2015. Brain natriuretic peptide stimulates lipid metabolism through its receptor NPR1 and the glycerolipid metabolism pathway in chicken adipocytes. Biochemistry, 54(43): 6622-6630. DOI:10.1021/acs.biochem.5b00714 (  0) 0) |
Jiao, X., Sherman, B. T., Huang D. W., Stephens, R., Baseler, M. W., Lane, H. C., et al., 2012. DAVID-WS: A stateful web service to facilitate gene/protein list analysis. Bioinformatics, 28(13): 1805-1806. DOI:10.1093/bioinformatics/bts251 (  0) 0) |
Jones, H. R., Johnson, K. M., and Kelly, M. W., 2019. Synergistic effects of temperature and salinity on the gene expression and physiology of Crassostrea virginica. Integrative and Comparative Biology, 59(2): 306-319. DOI:10.1093/icb/icz035 (  0) 0) |
Kaplan, M. B., Mooney, T. A., McCorkle, D. C., and Cohen, A. L., 2013. Adverse effects of ocean acidification on early development of squid (Doryteuthis pealeii). PLoS One, 8(5): e63714. DOI:10.1371/journal.pone.0063714 (  0) 0) |
Kapsenberg, L., and Cyronak, T., 2019. Ocean acidification refugia in variable environments. Global Change Biology, 25(10): 3201-3214. DOI:10.1111/gcb.14730 (  0) 0) |
Karsch-Bluman, A., Feiglin, A., Arbib, E., Stern, T., Shoval, H., Schwob, O., et al., 2019. Tissue necrosis and its role in cancer progression. Oncogene, 38(11): 1920-1935. DOI:10.1038/s41388-018-0555-y (  0) 0) |
Kawakami, T., and Xiao, W., 2013. Phospholipase C-β in immune cells. Advances in Biological Regulation, 53(3): 249-257. DOI:10.1016/j.jbior.2013.08.001 (  0) 0) |
Kim, M., Gwak, J., Hwang, S., Yang, S., and Jeong, S. M., 2019. Mitochondrial GPT2 plays a pivotal role in metabolic adaptation to the perturbation of mitochondrial glutamine metabolism. Oncogene, 38(24): 4729-4738. DOI:10.1038/s41388-019-0751-4 (  0) 0) |
Klaus, C., Kaemmerer, E., Reinartz, A., Schneider, U., Plum, P., Jeon, M. K., et al., 2014. TP53 status regulates ACSL5-induced expression of mitochondrial mortalin in enterocytes and colorectal adenocarcinomas. Cell and Tissue Research, 357(1): 267-278. DOI:10.1007/s00441-014-1826-8 (  0) 0) |
Klaus, C., Schneider, U., Hedberg, C., Schütz, A. K., Bernhagen, J., Waldmann, H., et al., 2014. Modulating effects of acyl-CoA synthetase 5-derived mitochondrial Wnt2B palmitoylation on intestinal Wnt activity. World Journal of Gastroenterology, 20(40): 14855-64. DOI:10.3748/wjg.v20.i40.14855 (  0) 0) |
Li, Z., Bao, X., Liu, X., Li, Y., Cui, M., Liu, X., et al., 2021. Transcriptome profiling based on protein-protein interaction networks provides a set of core genes for understanding the immune response mechanisms of the egg-protecting behavior in Octopus ocellatus. Fish & Shellfish Immunology, 117: 113-123. DOI:10.1016/j.fsi.2021.07.020 (  0) 0) |
Liu, B., Jia, Y., Cao, Y., Wu, S., Jiang, H., Sun, X., et al., 2016. Overexpression of phosphoserine aminotransferase 1 (PSAT1) predicts poor prognosis and associates with tumor progression in human esophageal squamous cell carcinoma. Cellular Physiology and Biochemistry, 39(1): 395-406. DOI:10.1159/000445633 (  0) 0) |
Liu, W., and Meng, K., 2022. COL11A1 is Downregulated by miR-339-5p and promotes colon carcinoma progression. Canadian Journal of Gastroenterology and Hepatology, 2022: 8116990. DOI:10.1155/2022/8116990 (  0) 0) |
Liu, X., Li, Z., Li, Q., Bao, X., Jiang, L., and Yang, J., 2024. Acute exposure to polystyrene nanoplastics induced oxidative stress in Sepia esculenta larvae. Aquaculture Reports, 35: 102004. DOI:10.1016/j.aqrep.2024.102004 (  0) 0) |
Lohbeck, K., Riebesell, U., and Reusch, T., 2012. Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geoscience, 5: 346-351. DOI:10.1038/ngeo1441 (  0) 0) |
Love, M. I., Huber, W., and Anders, S., 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology, 15(12): 550. DOI:10.1186/s13059-014-0550-8 (  0) 0) |
Masotti, A., and Preckel, T., 2006. Analysis of small RNAs with the Agilent 2100 Bioanalyzer. Nature Methods, 3: 658. DOI:10.1038/nmeth908 (  0) 0) |
Matear, R. J., and Lenton, A., 2018. Carbon-climate feedbacks accelerate ocean acidification. Biogeosciences, 15: 1721-1732. DOI:10.5194/bg-15-1721-2018 (  0) 0) |
Maurin, L., Marselli, L., Ning, L., Boissel, M., Boutry, R., Suleiman, M., et al., 2022. Epigenetics of PNLIPRP1 in human pancreas reveals a molecular path between type 2 diabetes and pancreatic cancer. medRxiv–Genetic and Genomic Medicine, DOI: 10.1101/2022.12.30.22284058
(  0) 0) |
Medzhitov, R., 2008. Origin and physiological roles of inflamemation. Nature, 454(7203): 428-35. DOI:10.1038/nature07201 (  0) 0) |
Metcalf, S., Dougherty, S., Kruer, T., Hasan, N., Biyik-Sit, R., Reynolds, L., et al., 2020. Selective loss of phosphoserine aminotransferase 1 (PSAT1) suppresses migration, invasion, and experimental metastasis in triple negative breast cancer. Clinical & Experimental Metastasis, 37(1): 187-197. DOI:10.1007/s10585-019-10000-7 (  0) 0) |
Ming, Z., Pang, Y., and Liu, J., 2020. Mechanical deformation mediated transmembrane transport. Macromolecular Rapid Communications, 41(2): e1900518. DOI:10.1002/marc.201900518 (  0) 0) |
Mitra, D., Vega-Rubin-de-Celis, S., Royla, N., Bernhardt, S., Wilhelm, H., Tarade, N., et al., 2021. Abrogating GPT2 in triplenegative breast cancer inhibits tumor growth and promotes autophagy. International Journal of Cancer, 148(8): 1993-2009. DOI:10.1002/ijc.33456 (  0) 0) |
Morozova, O., Hirst, M., and Marra, M. A., 2009. Applications of new sequencing technologies for transcriptome analysis. Annual Review of Genomics and Human Genetics, 10: 135-151. DOI:10.1146/annurev-genom-082908-145957 (  0) 0) |
Munday, P. L., Dixson, D. L., Donelson, J. M., Jones, G. P., Pratchett, M. S., Devitsina, G. V., et al., 2009. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proceedings of the National Academy of Sciences of the United States of America, 106(6): 1848-1852. DOI:10.1073/pnas.0809996106 (  0) 0) |
Parkin, J., and Cohen, B., 2001. An overview of the immune system. Lancet, 357(9270): 1777-1789. DOI:10.1016/S0140-6736(00)04904-7 (  0) 0) |
Pierron, F., Baudrimont, M., Bossy, A., Bourdineaud, J. P., Brèthes, D., Elie, P., et al., 2007. Impairment of lipid storage by cadmium in the European eel (Anguilla anguilla). Aquatic Toxicology, 81(3): 304-311. DOI:10.1016/j.aquatox.2006.12.014 (  0) 0) |
Qi, Q., Huang, W., Zhang, H., Zhang, B., Sun, X., Ma, J., et al., 2021. Bioinformatic analysis of PLOD family member expression and prognostic value in non-small cell lung cancer. Translational Cancer Research, 10(6): 2707-2724. DOI:10.21037/tcr-21-73 (  0) 0) |
Qi, Y., and Xu, R., 2018. Roles of PLODs in collagen synthesis and cancer orogression. Frontiers in Cell and Developmental Biology, 6: 66. DOI:10.3389/fcell.2018.00066 (  0) 0) |
Qian, X., Ba, Y., Zhuang, Q., and Zhong, G., 2014. RNA-Seq technology and its application in fish transcriptomics. OMICS: A Journal of Integrative Biology, 18(2): 98-110. DOI:10.1089/omi.2013.0110 (  0) 0) |
Qiao, H., Feng, Y., and Tang, H., 2021. COL6A6 inhibits the proliferation and metastasis of non-small cell lung cancer through the JAK signalling pathway. Translational Cancer Research, 10(10): 4514-4522. DOI:10.21037/tcr-21-2002 (  0) 0) |
Quan, J., Bode, A. M., and Luo, X., 2021. ACSL family: The regulatory mechanisms and therapeutic implications in cancer. European Journal of Pharmacology, 909: 174397. DOI:10.1016/j.ejphar.2021.174397 (  0) 0) |
Ricard-Blum, S., 2011. The collagen family. Cold Spring Harbor Perspectives in Biology, 3(1): a004978. DOI:10.1101/cshperspect.a004978 (  0) 0) |
Ricard-Blum, S., and Ruggiero, F., 2005. The collagen superfamily: From the extracellular matrix to the cell membrane. Pathologie Biologie, 53(7): 430-442. DOI:10.1016/j.patbio.2004.12.024 (  0) 0) |
Schendzielorz, A. B., Bragoszewski, P., Naumenko, N., Gomkale, R., Schulz, C., Guiard, B., et al., 2018. Motor recruitment to the TIM23 channel's lateral gate restricts polypeptide release into the inner membrane. Nature Communications, 9(1): 4028. DOI:10.1038/s41467-018-06492-8 (  0) 0) |
Singh, N., Baby, D., Rajguru, J. P., Patil, P. B., Thakkannavar, S. S., and Pujari, V. B., 2019. Inflammation and cancer. Annals of African medicine, 18(3): 121-126. DOI:10.4103/aam.aam_56_18 (  0) 0) |
Smith, B., Schafer, X. L., Ambeskovic, A., Spencer, C. M., Land, H., and Munger, J., 2016. Addiction to coupling of the warburg effect with glutamine catabolism in cancer cells. Cell Reports, 17(3): 821-836. DOI:10.1016/j.celrep.2016.09.045 (  0) 0) |
Soupene, E., and Kuypers, F. A., 2008. Mammalian long-chain acyl-CoA synthetases. Experimental Biology and Medicine, 233(5): 507-521. DOI:10.3181/0710-MR-287 (  0) 0) |
Stojkovic, S., Beardall, J., and Matear, R., 2013. CO2-concentrating mechanisms in three southern hemisphere strains of Emiliania huxleyi. Journal of Phycology, 49(4): 670-679. DOI:10.1111/jpy.12074 (  0) 0) |
Szklarczyk, D., Gable, A. L., Lyon, D., Junge, A., Wyder, S., Huerta-Cepas, J., et al., 2019. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research, 47(D1): D607-D613. DOI:10.1093/nar/gky1131 (  0) 0) |
Tan, K., and Zheng, H., 2019. Ocean acidification and adaptive bivalve farming. Science of the Total Environment, 701: 134794. DOI:10.1016/j.scitotenv.2019.134794 (  0) 0) |
Tomar, N., and De, R. K., 2014. A brief outline of the immune system. Methods in Molecular Biology, 184: 3-12. DOI:10.1007/978-1-4939-1115-8_1 (  0) 0) |
Vogel, W. F., 2001. Collagen-receptor signaling in health and disease. European Journal of Dermatology: EJD, 11(6): 506-514. (  0) 0) |
Wang, L., Sun, F., Wen, Y., and Yue, G., 2021. Effects of ocean acidification on transcriptomes in Asian seabass juveniles. Marine Biotechnology, 23(3): 445-455. DOI:10.1007/s10126-021-10036-5 (  0) 0) |
Wang, X., Song, H., Liang, J., Jia, Y., and Zhang, Y., 2022. Abnormal expression of HADH, an enzyme of fatty acid oxidation, affects tumor development and prognosis. Molecular Medicine Reports, 26(6): 355. DOI:10.3892/mmr.2022.12871 (  0) 0) |
Wang, Y., Chen, X., Xu, X., Yang, J., Liu, X., Sun, G., et al., 2023. Weighted gene co-expression network analysis based on stimulation by lipopolysaccharides and polyinosinic: polycytidylic acid provides a core set of genes for understanding hemolymph immune response mechanisms of Amphioctopus fangsiao. Animals, 14(1): 80. DOI:10.3390/ani14010080 (  0) 0) |
Wessel, N., Martin, S., Badou, A., Dubois, P., Huchette, S., Julia, V., et al., 2018. Effect of CO2-induced ocean acidification on the early development and shell mineralization of the European abalone (Haliotis tuberculata). Journal of Experimental Marine Biology and Ecology, 508: 52-63. DOI:10.1016/j.jembe.2018.08.005 (  0) 0) |
Wu, Y., Si, X., Qiu, L., Chen, X., Fu, P., Buttino, I., et al., 2023. Regulation of innate immunity in marine mussel Mytilus coruscus: MicroRNA Mc-novel_miR_196 targets McTLR-like1 molecule to inhibit inflammatory response and apoptosis. Fish & Shellfish Immunology, 138: 108868. DOI:10.1016/j.fsi.2023.108868 (  0) 0) |
Xiang, Z., Li, J., Song, S., Wang, J., Cai, W., Hu, W., et al., 2019. A positive feedback between IDO1 metabolite and COL12A1 via MAPK pathway to promote gastric cancer metastasis. Journal of Experimental & Clinical Cancer Research, 38(1): 314. DOI:10.1186/s13046-019-1318-5 (  0) 0) |
Yang, A., and Mottillo, E. P., 2020. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochemical Journal, 477(5): 985-1008. DOI:10.1042/BCJ20190468 (  0) 0) |
Yang, X., Li, C., and Chen, Y., 2023. Phosphoserine aminotransferase 1: A metabolic enzyme target of cancers. Current Cancer Drug Targets, 23(3): 171-186. DOI:10.2174/1568009622666220829105300 (  0) 0) |
Yeung, Y. T., Aziz, F., Guerrero-Castilla, A., and Arguelles, S., 2018. Signaling pathways in inflammation and anti-inflammatory therapies. Current Pharmaceutical Design, 24(14): 1449-1484. DOI:10.2174/1381612824666180327165604 (  0) 0) |
Yu, Y., Liu, D., Liu, Z., Li, S., Ge, Y., Sun, W., et al., 2018. The inhibitory effects of COL1A2 on colorectal cancer cell proliferation, migration, and invasion. Journal of Cancer, 9(16): 2953-2962. DOI:10.7150/jca.25542 (  0) 0) |
Zhang, P., and Reue, K., 2017. Lipin proteins and glycerolipid metabolism: Roles at the ER membrane and beyond. Biochimica et Biophysica Acta (BBA)–Biomembranes, 1859(9 Pt B): 1583-1595. DOI:10.1016/j.bbamem.2017.04.007 (  0) 0) |
Zheng, Z. G., Xu, Y. Y., Liu, W. P., Zhang, Y., Zhang, C., Liu, H. L., et al., 2023. Discovery of a potent allosteric activator of DGKQ that ameliorates obesity-induced insulin resistance via the sn-1, 2-DAG-PKCε signaling axis. Cell Metabolism, 35(1): 101-117.e11. DOI:10.1016/j.cmet.2022.11.012 (  0) 0) |
Zhong, J., and Zhao, X., 2019. Transcriptomic analysis of viable but non-culturable Escherichia coli O157: H7 formation induced by low temperature. Microorganisms, 7(12): 634. DOI:10.3390/microorganisms7120634 (  0) 0) |
Zhu, G., Fang, Q., Zhu, F., Huang, D., and Yang, C., 2021. Structure and function of pancreatic lipase-related protein 2 and its relationship with pathological states. Frontiers in Genetics, 12: 693538. DOI:10.3389/fgene.2021.693538 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


