2) Key Laboratory of Marine Environment and Ecology (Ocean University of China), Ministry of Education, Qingdao 266100, China
In recent years, aquaculture sector is developing rapidly in China. It is estimated that China aquaculture accounts for more than 60% of global aquaculture volume and roughly half of global aquaculture value (Cao et al., 2015). However, fisheries are facing great challenges due to overexploitation. Discharge of high nutrient loading such as residual feeding bait, metabolites and excrement, invariably leads to severe eutrophication (Wu et al., 2016). Specifically, sulfide is a key pollutant species which is generated from sulfate by sulfate-reducing bacteria in marine hypoxic habitats due to significant terrigenous organic matter loads (Asaoka et al., 2018). Considering its odor and toxic characteristics, sulfide pollution has resulted in the severe damage to the health of aquaculture, especially mariculture.
Traditional methods that remove sulfide or inhibit its production in mariculture consist of adsorption by coal ash (Asaoka et al., 2017) and precipitation with ferric hydroxide (Sun et al., 2014). However, comparing with chemical and physical methods, microbiological removal of sulfide is relatively less expensive and does not produce undesirable by-product, it thus has drawn an increasing attention of engineers during remediation of sulfide-rich environment (Cytryn et al., 2005; Zhao et al., 2016). Therefore, bio-oxidation of sulfide conducted by sulfur-oxidizing bacteria (SOB) is a promising alternative to bioremediate the deteriorated mariculture system.
This comprises a range of microorganisms with the ability of oxidizing sulfide to elemental sulfur, or further to sulfate (Pokorna and Zabranska, 2015). These organisms are divided into three groups based on their removal pathway of sulfide, that is, 1) phototroph, 2) chemolithotroph, 3) autotrophic denitrifying sulfur-oxidizing bacteria. Phototrophs obtain energy from light. Comparatively, chemolithotrophs, also known as colorless bacteria, excavate energy by oxidizing reaction. Compared to phototrophs, chemolithotrophs show a higher elemental sulfur production and faster growth rate. Thus, the chemolithotroph is a viable alternative for the biological treatment of aquatic sulfide-rich wastewater. Nowadays, considerable studies focus on gas desulfurization (Tsang et al., 2015) and industrial wastewater treatment (Fajardo et al., 2012). Microbiota dominated by Pseudomonas stutzeri and Microbacterium oxydans enhance the performance of biofilter for sulfide removal, with a high efficiency of 98% at high sulfide concentrations (0.2-4 mg L-1) (Rattanapan et al., 2009). Sulfide removal in an airlift bioreactor was recently studied (Lohwacharin and Annachhatre, 2010). They showed that over 93% sulfide removal was achieved by sulfur oxidizing microbiota at the volumetric sulfide loading rate of 4.0 kg S m-3 d-1. Although many marine sulfur-oxidizing strains were reported, there are lack of enough investigation or application in bioremediation of mariculture environment.
At present, some marine isolates that present promising application in bioremediation of mariculture have been obtained (Takai et al., 2004; Luo et al., 2013; Pokorna and Zabranska, 2015). These isolates generally present a long generation time due to their autotrophic property, double time usually reached 60 h, and show a weak tolerance of high sulfide concentration. Therefore, in order to be applied in the full-scale mariculture environment, it is necessary to enrich and isolate microorganisms with fast growth, higher sulfur-oxidizing ability and better environmental adaptability.
In this study, one mixtrophic strain TT with a good capability of sulfur removal was obtained from mariculture sediment in Jiaozhou Bay, and characteristics of sulfide removal in mariculture habitats was further explored.
2 Materials and Methods 2.1 Sampling Sites and InoculumJiaozhou Bay is a semi-enclosed estuary located in the southern part of Shandong Peninsula, China. The bay has undergone severe ecosystem deterioration due to overload aquaculture in the last few decades (Shi et al., 2011). The sediment used to be enriched with the microbes in this study was picked out from aquatic site (36°08.972xN, 120°19.685xE) for scallops and sea cucumber, being near to marine cage culture area with high sulfide concentration.
Sediment samples were obtained by using a vertical columnar and were immediately transported to laboratory and stored at 4℃. Referring to our previous study (Zhao et al., 2016), the surface sediments of 0-10 cm were adopted as the inoculum to isolate the SOB.
2.2 Culture MediaThe media used in this study were shown in Table 1. All media were prepared with the filtered and autoclaved seawater. The M1 medium was used to enrich SOB microbiota (Zhao et al., 2016).
|
|
Table 1 Ingredients in three types of media |
The pH of the medium was 7.0. Then the culture medium was aerated with nitrogen gas for 10 min to deoxygenate. Solid medium was prepared by adding 2% (w/v) agar.
To monitor biological sulfide removal under different conditions, the minimal medium (M2) contained the same compositions as the M1 with a small amendment. Since S2- is easy to form H2S and lost during the operation, it was substituted by Na2S2O3 in M2 medium (Zhao et al., 2016).
Some metals in the seawater and anion in the above media form precipitate during autoclaved process. Thereby these media cannot be used for the determination of growth curve by optical density method, the artificial seawater medium M3 with NaCl and trace element solution in 1 L deionized water was used.
2.3 Enrichment and Isolation of Microbes10 g sediment sample (wet weight) was mixed with 90 mL autoclaved seawater and shaken for 30 min to obtain the suspension. Then 10 mL suspension was moved to 90 mL M1 medium flask with 6.25 mmol L-1 Na2S and shaken at 150 r min-1 with amplitude of the agitation at 22 mm, 30℃ for 5 d. Then we repeated the above step twice by using M1 medium with increasing sulfide concentrations, i.e., 8.3 mmol L-1 and 10.42 mmol L-1 in sequence. After the enrichment, we obtained the sulfur-oxidizing microbiota with a stable sulfide removal ability and great tolerance of high sulfide concentrations.
The microbiota enrichment was diluted to 10-2-10-7 in series. Then the diluted liquid culture (150 μL) was spread onto the solid plates with sandwich structure and incubated at 30℃ for 48-72 h. Single colonies with different morphologies were picked up and cultured in M2 liquid medium. The colonies with great sulfur-oxidizing ability were further purified by sequential streak plating.
2.4 Identification and Characterization of the Isolates 2.4.1 Microscopic characterizationMorphologies of different colonies were observed. The motility and Gram staining results were examined with a microscope (XSP-11C, Changfang, China). The morphology and dimensions of the isolate were determined according to the picture of the isolates from transmission electron microscopy (JEM-1200EX, JEOL Japan) (Feng et al., 2012).
2.4.2 16S rRNA gene sequence and phylogenetic analysisThe bacterial 16S rRNA gene universal primer pair of BSF8/20 (5-AGAGTTTGATCCTGGCTCAG-3) and BSR1512/20 (5-GGTTACCTTGTTACGACTT-3) was used. PCR amplification was performed in 25 μL reaction volume including 75% of deionized water, 10% of PCR reaction buffer, 8% of dNTPs, 3% of each of the primers and 1% of rTaq DNA polymerase. The PCR amplification protocol was as follows: pre-denaturation of DNA at 94℃ for 5 min, followed by 25 cycles including denaturation at 94℃ for 30 s, annealing at 52℃ for 30 s and extension at 72℃ for 1 min 20 s. Finally, extension at 72℃ for 5 min. The PCR products were purified and subjected to determine the sequence with the same primer pair BSF8/20 and BSR1512/20 on the ABI 3730 platform (Boshi Biotechnology, Harbin China).
The 16S rRNA gene sequence of isolate was aligned with that obtained from NCBI database (https://www.ncbi.nlm.nih.gov/) by using multiple sequence alignment software Blast. A phylogenetic tree was constructed with MEGA 7.0 according to previous method (Feng et al., 2012).
The 16S rRNA gene sequence of isolate has been deposited in the Genbank under the accession number MG822786.
2.5 Physiological Characteristics of the Isolate 2.5.1 Growth kinetics of the isolateCulturing of isolates was conducted in 50 mL serum bottles sealed with butyl rubber stoppers. 5 mL of bacterial suspension was transferred to 50 mL M3 medium and then incubated at 30℃ and 150 r min-1. Controls were carried out with bacteria-free medium. The dry biomass and corresponding liquid pH were monitored simultaneously every 12 h. All experiments were conducted in triplicate.
2.5.2 Effects of carbon source, pH, temperature and initial thiosulfate concentration on thiosulfate removalTo assess the possibility of isolate growth on various carbon sources, sodium bicarbonate in M2 medium was replaced by the following organic carbons, that is, glucose, sodium citrate, sodium lactate, sodium acetate, peptone. A 5-mL bacterial suspension was inoculated in 45 mL of the above media at 30℃ for 24 h. To find the best condition of thiosulfate removal, we evaluated the removal rate at 30℃ for 24 h with a wide range of initial pH, that is, 1.5, 3.0, 5.0, 7.0, 9.0, 11.0, respectively. Afterwards, different incubation temperatures, i.e., 10, 20, 30, 40, 65℃ were investigated. To examine the tolerance of high thiosulfate concentrations, M2 media with various initial thiosulfate concentrations from 9 to 63 mmol L-1 were prepared. In these experiments, the bacteria-free medium was used as control to eliminate the possible chemical oxidization of thiosulfate by oxygen. Each experiment was conducted in triplicate. The thiosulfate was measured by using iodometric method according to a previous report (Zhao et al., 2016).
2.5.3 Effects of different electron acceptors on thiosulfate removalM3 media were prepared with different electron acceptors, that is, low-oxygen (dissolved oxygen, DO 0.024 mmol L-1), high-oxygen (DO 1.53 mmol L-1), 72 mmol L-1 nitrate and 120 mmol L-1 nitrite. 5 mL of bacterial suspension was inoculated into 50 mL M3 medium and then incubated at 30℃, 150 r min-1 for 24 h. When high-oxygen was used as electron acceptor, the serum bottles were covered by permeable film to ensure the supply of oxygen. The microaerobic medium was prepared as mentioned above. When the nitrate or nitrite was used as electron acceptors, the liquid medium was boiled and flushed with nitrogen gas to remove oxygen. 0.1 g L-1 resazurin was added into the medium as a redox indicator. At the same time, the DO in all media was measured by portable DO analyzer (AZ8403, Taiwan Hengxin, China).
2.5.4 Sulfide removing performance by the isolateThe bacterium in logarithmic growth phase was cultured in M1 medium containing 600 mmol L-1 S2- under the optimal growth condition with the initial DO 0.001 mmol L-1. The sulfide concentration was determined by using the methylene blue spectrophotometry (Tai and Zhou, 2012).
2.6 The Process of Thiosulfate OxidationThe bacterium in logarithmic growth phase was cultured in M2 medium under the optimal growth condition with the initial DO 0.0024 mmol L-1. The thiosulfate, sulfite, and sulfate in M2 culture medium were measured by using ion chromatograph (ICS-3000, Thermo USA) every 12 h, and pH was also examined. In addition, the yellow precipitate at the bottom of medium was analyzed by using X-ray diffraction (XRD) (D8 Advance, Bruker Germany) and Jade 6.0 software. Before being measured, the yellow precipitate in liquid culture was collected by centrifuged at 12000×g for 5 min. The precipitate was washed by 0.85% NaCl for three times and dried in oven for final analysis.
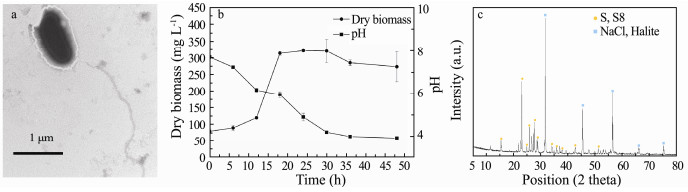
3 Results and Discussion 3.1 Characteristics of the Isolate from Mariculture SedimentSeveral isolates were obtained from solid plate and one fast-growth pure culture of them was designated as strain TT. Its colony on the agar sandwich plate was roundshaped, milky white, neat edges, smooth, moist and opaque. Cells of strain TT were Gram-negative, slightly curved with size of 1 ± 0.01 μm in length and 0.5 ± 0.01 μm in width (Fig. 1a). It has a polar flagellum. The isolate strain is able to produce yellow precipitate when feeding with thiosulfate in the liquid medium.
|
Fig. 1 Morphological characteristics under the transmission electron micrograph (a), growth curve in the thiosulfate liquid medium of the strain TT (b) and X-ray diffraction spectrogram for the insoluble yellow precipitate (c). Error bars in pattern b based on triplicate experiments are shown. |
As shown in Fig. 1b, the dry biomass of the culture was kept stable during the first 12 h, indicating that there is the lag phase during this stage. Then it came into exponential growth phase. At this stage, cells increased dramatically and the colorless culture turned to pale yellow. This suggests that the strain TT might be able to oxidize sulfide to elemental sulfur gradually during the growth. The colloidal elemental sulfur adhered to cells and formed aggregates. Fig. 1c shows that the compounds in the yellow precipitate were elemental sulfur (S8), indicating that part of the thiosulfate was oxidized to elemental sulfur. As the most common elemental sulfur, S8 was capable to exist stably in the environment, which guaranteed the high elemental sulfur recovery efficiency (Huang et al., 2018).
The isolate entered into stationary phase after 18 h. During this stage, the dry biomass reached its peak at 24 h. The whole culture process was followed by the continuous decrease in pH from 8.0 to 4.0, probably suggesting the thiosulfate oxidation is an acidogenic process according to the following equation (Houghton et al., 2016):
| $ 3{{\rm{S}}_2}{\rm{O}}_3^{2 - } + 3{{\rm{O}}_2} + {{\rm{H}}_2}{\rm{O}} \to 2{\rm{S}} + 4{\rm{SO}}_4^{2 - } + 2{{\rm{H}}^ + }. $ | (1) |
According to the Eq. (1), thiosulfate oxidizing process was accompanied by the release of H+. When pH of the culture medium dropped to approximately 4.0, this environment was not suitable for bacterial growth and further resulted in decline of dry biomass.
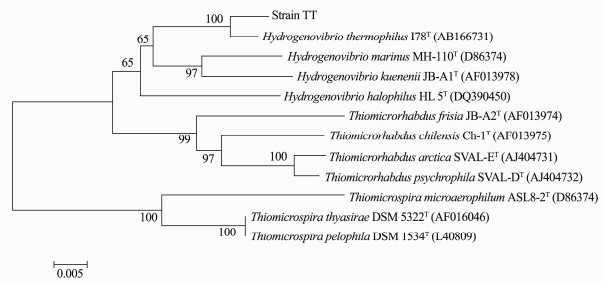
The partial 16S rRNA gene sequence of strain TT, comprising 1418 bp, was determined and a phylogenetic tree was conducted based on 16S rRNA gene sequences (Fig. 2). It was most closely related to the sequences of Hydrogenovibrio thermophilus I78T (Boden et al., 2017), previously called Thiomicrospira thermophila I78T (Takai et al., 2004), with identity up to 99%. Consequently, the isolate stain TT was proposed as H. thermophilus strain TT.
|
Fig. 2 Phylogenetic position of sulfur-oxidizing bacterium strain TT based on analysis of 16S rRNA gene sequences. Tree topology and evolutionary distances are given by the neighbor-joining method with Kimura 2-parameter. Numbers at nodes indicate percentages of bootstrap values for the clade of this group in 1000 replications. Values below 50% are not shown. |
According to a very recent report (Boden et al., 2017), most members of genus Hydrogenovibrio are obligately chemolithoautotrophic with heterotrophy occasionally observed in some species. Molecular oxygen is used as the sole terminal electron acceptor although most species grow optimally at oxygen partial pressures below atmospheric levels. Nitrate or nitrite can not be as terminal electron acceptor or nitrogen sources. The majority of the genus have been isolated from different marine habitats and play an important role in the sulfur cycle (Brazelton et al., 2006).
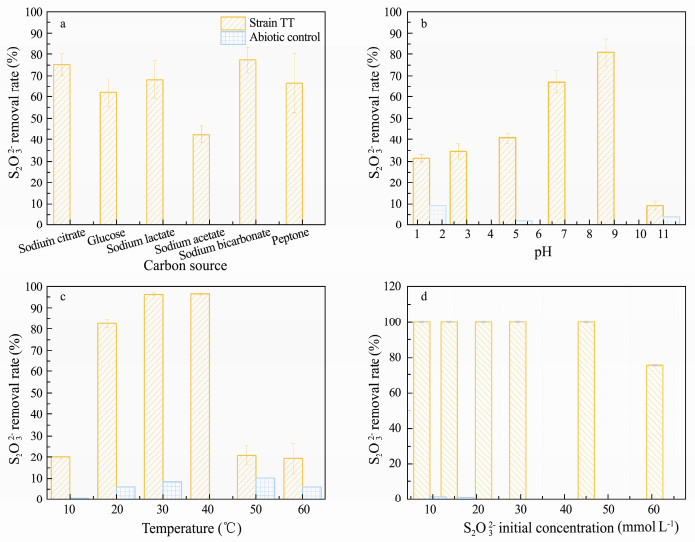
3.2 Effect of Carbon Source, pH, Temperature and Initial Thiosulfate Concentration on Thiosulfate RemovalThe SOB strain TT showed variable sulfur oxidizing performance under different carbon sources (Fig. 3a). It presented the highest thiosulfate removal with sodium bicarbonate as the carbon source. This might be ascribed to the increasing of CO2 from sodium bicarbonate under the continuous acidification in the culture medium. Meanwhile, the strain also showed a great removal ability using sodium citrate as a carbon source in the presence of sulfide, indicating the strain was capable of utilizing organic carbon source to grow, which is similar to the report on the H. thermophilus I78T by Takai et al. (2004).
|
Fig. 3 The influence of carbon sources (a), pH (b), temperatures (c) and initial thiosulfate concentrations (d) on the sulfur oxidizing ability of strain TT. |
Fig. 3b demonstrates that the optimal pH range for the strain to remove thiosulfate was from 7.0 to 9.0. Strain TT was able to grow and oxidize thiosulfate under acidic condition; however, the removal rate dropped dramatically to less than 10% when the pH approached to 9.0. The optima of pH range for strain TT was 7.0-9.0, while the optimal pH for H. thermophilus I78T growth was 6.0 (Takai et al., 2004). It suggested that the strain TT would perform better in marine aquaculture system since the seawater is a weak alkaline.
The thiosulfate removal rate of strain TT was improved with the increase of temperature (Fig. 3c). This isolate showed great capability of oxidizing thiosulfate between 20℃ and 40℃, which were similar to the optimal temperature range of H. thermophilus S5 (Jiang et al., 2017). Outside the optimal temperature range, the removal ability of strain TT was extremely weak. H. thermophilus I78T was able to grow at 55℃ (Takai et al., 2004), which was far beyond optimum temperature range of strain TT. Generally, the temperature of seawater in maricuture system was about 25℃, such as the average temperature in the Jiaozhou Bay is 27.8℃ in summer (Liu et al., 2008). Therefore, the isolate in our study presumably adapts well during the ecological restoration for the deteriorative mariculture habits.
The effect of initial thiosulfate concentration on the thiosulfate removal ability of strain TT is shown in Fig. 3d. With the increase of initial thiosulfate concentration in culture medium, the total removed thiosulfate rose gradually within 24 h, demonstrating that increasing thiosulfate provided the sufficient electron donor for strain TT. When the initial thiosulfate concentration reached approximately 45 mmol L-1, strain TT was capable of completely removing thiosulfate within 24 h. However, when the initial thiosulfate concentration in culture approached about 63 mmol L-1, this strain was still able to remove about 45 mmol L-1 thiosulfate within 24 h. This suggested that the removal performance was limited by the inoculum size if the electron donor was excess. However, this isolate revealed a slightly better performance than the SOB from sulfide removal bioreactor (Luo et al., 2013) or the sulfide-oxidizing microbiota from marine sediment (Zhao et al., 2016).
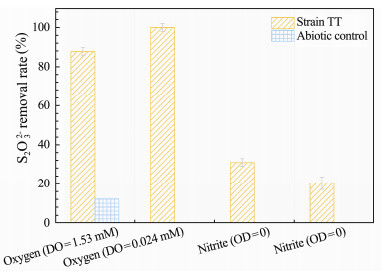
3.3 Effect of Different Electron Acceptor on the Thiosulfate RemovalThe effect of electron acceptor on the thiosulfate removal of the strain TT is shown in Fig. 4. This isolate grew well and presented high thiosulfate removal in the presence of low DO at about 0.024 mmol L-1, with the thiosulfate removal rate of 100% within 24 h.
|
Fig. 4 The effects of different electronic acceptors on the sulfur-oxidizing ability of the strain TT. |
Under aerobic condition that DO of the culture was 1.53 mmol L-1, the removal rate reached the same as under the microaerobic condition. However, under this condition, the control showed 12.2% removal rate, suggesting that thiosulfate can be partially chemically oxidized by oxygen under aerobic condition. Compared with that, the sulfur oxidizing ability was seriously restrained under strictly anaerobic condition, with 30.9% removal rate under nitrite-contained medium and 20.3% removal rate under nitrate-contained medium, respectively. These results indicated that this strain had the optimal thiosulfate removal ability under microaerobic condition. This result did not match with the previous study (Takai et al., 2004), where they found that H. thermophilus I78T could not grow with nitrite and nitrate as electron acceptor or with 20% oxygen partial pressure, i.e., DO = 1.53 mmol L-1. Comparably, the strain TT was capable of oxidizing thiosulfate with nitrite and nitrate as electron acceptor, which would be of benefit to the bioremediation of sulfide-rich mariculture due to the low oxygen and high nitrate feature at water-sediment interface. As such, strain TT actually showed promising application for biological remediation of sulfide-contaminated mariculture system.
3.4 Sulfide Removing Performance by the IsolateThe change of concentration of sulfide during the culturing process is shown in Fig. 5. Since sulfide is very unstable and easy to be oxidized by molecular oxygen, the concentration of sulfide in bacteria-free medium decreased steadily, while strain TT showed the greater sulfide removal ability of the maximum rate at 3.01 mmol L-1 h-1 during the culture process. Strain TT was capable to decrease sulfide from 18.52 to 0.46 mmol L-1 within 6 h under microaerobic conditions. After 12 h, there was no sulfide left in the medium. In addition, strain TT in this study performed almost two orders of magnitude better than the previous report of Alcaligenes faecalis T307 with sulfide removal rate of 0.068 mmol L-1 h-1 (Rattanapan et al., 2010), which showed that strain TT presented a very high tolerance of sulfide and a great sulfide removal ability in such a short time.
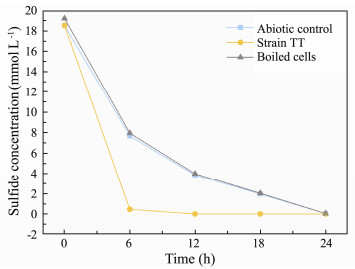
|
Fig. 5 Concentration curves of S2- during culturing the strain TT. |
The changes of thiosulfate, sulfite and sulfate and the variation of pH during the culturing process are shown in Fig. 6. These experiments were conducted at 35℃, 150 rmin-1 with initial DO at 0.024 mmol L-1. In the first 12 h, the removal rate of thiosulfate reached approximately 50%. The white precipitates, some carbonates formed by Ca2+ and Mg2+ existing in seawater, were dissolved by the H+ released during sulfur oxidizing process and the culture medium turn yellow due to the production of elemental sulfur. During this phase, the DO of the medium decreased to 0 mmol L-1, and the majority of thiosulfate was finally oxidized to sulfate. Sulfite did not change within 48 h, suggesting that there were no sulfite production and consumption.
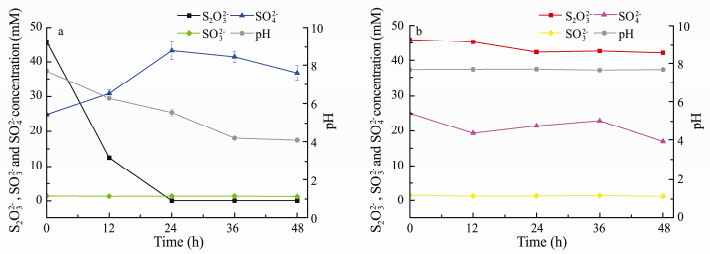
|
Fig. 6 Changes of S2O32-, SO32-, SO42- and pH in solutions culturing the strain TT (a) and abiotic control (b). |
The thiosulfate removal rate reached to 100% within 24 h, resulting in the further increase of the sulfate, and the yellow precipitate in the medium showed the increasing trend too. Compared with other chemolithoautotrophic bacteria (Ravichandra et al., 2007), strain TT can remove high concentration of sulfide in a short time. Moreover, strain TT performed much better at the removal rate of 45 mmol L-1d-1 thiosulfate than in the previous report of Rhodopseudomonas sp. (Luo et al., 2013). In their research, Rhodopseudomonas sp. obtained thiosulfate removal rate of 3.19 mmol L-1d-1.
According to the consumption of thiosulfate and the production of sulfate in Fig. 6a, 45 mmol L-1 thiosulfate was used up and 20 mmol L-1 sulfate produced within 24 h, which was obviously not accordant with the Eq. (1), where 1 mmol L-1 thiosulfate produced 1.3 mmol L-1 sulfate. Hence, thiosulfate might be transformed to other chemicals. According to the previous study on H. thermophilus strain EPR85, the tetrathionate might be formed during thiosulfate oxidation process as in the Eq. (2) (Houghton et al., 2016), suggesting that the decrease of thiosulfate was partially ascribed to the formation of the tetrathionate.
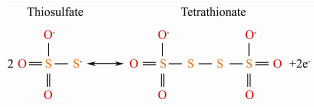
|
(2) |
After 24 h, there was no thiosulfate left in the medium, and the concentration of sulfate presented a slight decrease, while pH of the liquid medium decreased continuously. This indicated that there existed another reaction between 24 and 36 h, which resulted in the drop of pH value. When thiosulfate is completely consumed, tetrathionate is then oxidized to elemental sulfur by H. thermophilus EPR85, resulting in the accumulation of elemental sulfur accompanying with decreased pH (Houghton et al., 2016). The production of elemental sulfur reduced the requirement for the electron acceptors and it could also be recycled and reused as fertilizer or the raw material in many industries (Huang et al., 2018).
Although some SOB have been reported in many researches, few studies have addressed their possible application in biological restoration of sulfide-rich mariculture environment. Our attempt on SOB isolated from mariculture sediment provided a new strategy on remediation of sulfide-rich mariculture system.
4 ConclusionsA SOB named Hydrogenovibrio thermophilus was isolated from mariculture sediment in Jiaozhou Bay, China. It was mixtrophic and able to utilize both inorganic and organic carbon as carbon sources under microaerobic conditions. Besides oxygen, nitrite and nitrate could also be terminal electron acceptors. The isolate capable of growing in seawater and removing high sulfide under microaerobic, high nitrite and nitrate conditions was of benefit to sulfide control at the low-oxygen water-sediment interface. The oxidative product elemental sulfur was easy to be recycled and reused. The isolate demonstrated a promising application for biological remediation of sulfide-contaminated mariculture system.
AcknowledgementsThis work was financially supported by the Shandong Province Science and the Technology Research Projects (No. 2016GSF115004), and Fundamental Research Funds for the Central Universities (No. 201964004).
Asaoka, S., Okamura, H., Kim, K., Hatanaka, Y., Nakamoto, K., Hino, K., Oikawa, T., Hayakawa, S. and Okuda, T., 2017. Optimum reaction ratio of coal fly ash to blast furnace cement for effective removal of hydrogen sulfide. Chemosphere, 168: 384-389. DOI:10.1016/j.chemosphere.2016.10.070 (  0) 0) |
Asaoka, S., Umehara, A., Otani, S., Fujii, N., Okuda, T., Nakai, S., Nishijima, W., Takeuchi, K., Shibata, H., Jadoon, W. A. and Hayakawa, S., 2018. Spatial distribution of hydrogen sulfide and sulfur species in coastal marine sediments Hiroshima Bay, Japan. Marine Pollution Bulletin, 133: 891-899. DOI:10.1016/j.marpolbul.2018.06.042 (  0) 0) |
Boden, R., Scott, K. M., Williams, J., Russel, S., Antonen, K., Rae, A. W. and Hutt, L. P., 2017. An evaluation of Thiomicrospira, Hydrogenovibrio and Thioalkalimicrobium: Reclassification of four species of Thiomicrospira to each Thiomicrorhabdus gen. nov. and Hydrogenovibrio, and reclassification of all four species of Thioalkalimicrobium to Thiomicrospira. International Journal of Systematic and Evolutionary Microbiology, 67(5): 1140-1151. DOI:10.1099/ijsem.0.001855 (  0) 0) |
Brazelton, W. J., Schrenk, M. O., Kelley, D. S. and Baross, J. A., 2006. Methane- and sulfur-metabolizing microbial communities dominate the lost city hydrothermal field ecosystem. Applied & Environmental Microbiology, 72(9): 6257-6270. (  0) 0) |
Cao, L., Naylor, R., Henriksson, P., Leadbitter, D., Metian, M., Troell, M. and Zhang, W., 2015. China's aquaculture and the world's wild fisheries. Science, 347(6218): 133-135. DOI:10.1126/science.1260149 (  0) 0) |
Cytryn, E., van Rijn, J., Schramm, A., Gieseke, A., de Beer, D. and Minz, D., 2005. Identification of bacteria potentially responsible for oxic and anoxic sulfide oxidation in biofilters of a recirculating mariculture system. Applied and Environmental Microbiology, 71(10): 6134-6141. DOI:10.1128/AEM.71.10.6134-6141.2005 (  0) 0) |
Fajardo, C., Mosquera-Corral, A., Campos, J. L. and Mendez, R., 2012. Autotrophic denitrification with sulphide in a sequencing batch reactor. Journal of Environmental Management, 113: 552-556. DOI:10.1016/j.jenvman.2012.03.018 (  0) 0) |
Feng, S., Yang, H., Xin, Y., Zhang, L., Kang, W. and Wang, W., 2012. Isolation of an extremely acidophilic and highly efficient strain Acidithiobacillus sp. for chalcopyrite bioleaching. Journal of Industrial Microbiology and Biotechnology, 39(11): 1625-1635. DOI:10.1007/s10295-012-1174-1 (  0) 0) |
Houghton, J. L., Foustoukos, D. I., Flynn, T. M., Vetriani, C., Bradley, A. S. and Fike, D. A., 2016. Thiosulfate oxidation by Thiomicrospira thermophila: Metabolic flexibility in response to ambient geochemistry. Environmental Microbiology, 18(9): 3057-3072. DOI:10.1111/1462-2920.13232 (  0) 0) |
Huang, C., Liu, W. Z., Li, Z. L., Zhang, S. M., Chen, F., Yu, H. R., Shao, S. L., Nan, J. and Wang, A. J., 2018. High recycling efficiency and elemental sulfur purity achieved in a biofilm formed membrane filtration reactor. Water Research, 130: 1-12. DOI:10.1016/j.watres.2017.10.043 (  0) 0) |
Jiang, L., Lyu, J. and Shao, Z., 2017. Sulfur metabolism of Hydrogenovibrio thermophilus strain S5 and its adaptations to deep-sea hydrothermal vent environment. Frontiers in Microbiology, 8: 2513. DOI:10.3389/fmicb.2017.02513 (  0) 0) |
Liu, D., Sun, J., Zhang, J. and Liu, G., 2008. Response of the diatom flora in Jiaozhou Bay, China to environmental changes during the last century. Marine Micropaleontology, 66(3-4): 279-290. DOI:10.1016/j.marmicro.2007.10.007 (  0) 0) |
Lohwacharin, J. and Annachhatre, A. P., 2010. Biological sulfide oxidation in an airlift bioreactor. Bioresource Technology, 101(7): 2114-2120. DOI:10.1016/j.biortech.2009.10.093 (  0) 0) |
Luo, J., Tian, G. and Lin, W., 2013. Enrichment, isolation and identification of sulfur-oxidizing bacteria from sulfide removing bioreactor. Journal of Environmental Sciences, 25(7): 1393-1399. DOI:10.1016/S1001-0742(12)60179-X (  0) 0) |
Pokorna, D. and Zabranska, J., 2015. Sulfur-oxidizing bacteria in environmental technology. Biotechnology Advances, 33(6 Pt 2): 1246-1259. (  0) 0) |
Rattanapan, C., Boonsawang, P. and Kantachote, D., 2009. Removal of H2S in down-flow GAC biofiltration using sulfide oxidizing bacteria from concentrated latex wastewater. Bioresource Technology, 100(1): 125-130. DOI:10.1016/j.biortech.2008.05.049 (  0) 0) |
Ravichandra, P., Mugeraya, G., Rao, A. G., Ramakrishna, M. and Jetty, A., 2007. Isolation of Thiobacillus sp. from aerobic sludge of distillery and dairy effluent treatment plants and its sulfide oxidation activity at different concentrations. Journal of Environmental Biology, 28(4): 819-823. (  0) 0) |
Shi, J., Li, G. and Wang, P., 2011. Anthropogenic influences on the tidal prism and water exchanges in Jiaozhou Bay, Qingdao, China. Journal of Coastal Research, 27(1): 57-72. (  0) 0) |
Sun, J., Zhou, J., Shang, C. and Kikkert, G. A., 2014. Removal of aqueous hydrogen sulfide by granular ferric hydroxidekinetics, capacity and reuse. Chemosphere, 117: 324-329. DOI:10.1016/j.chemosphere.2014.07.086 (  0) 0) |
Tai, L. and Zhou, Y. X., 2012. Study of determining the concentration of hydrogen sulfide in air by methylene blue spectrophotometric method. Applied Mechanics & Materials, 110-116: 3086-3091. (  0) 0) |
Takai, K., Hirayama, H., Nakagawa, T., Suzuki, Y., Nealson, K. H. and Horikoshi, K., 2004. Thiomicrospira thermophila sp. nov., a novel microaerobic, thermotolerant, sulfuroxidizing chemolithomixotroph isolated from a deep-sea hydrothermal fumarole in the TOTO caldera, Mariana Arc, Western Pacific. International Journal of Systematic and Evolutionary Microbiology, 54(6): 2325-2333. DOI:10.1099/ijs.0.63284-0 (  0) 0) |
Tsang, Y. F., Wang, L. and Chua, H., 2015. Simultaneous hydrogen sulphide and ammonia removal in a biotrickling filter: Crossed inhibitory effects among selected pollutants and microbial community change. Chemical Engineering Journal, 281: 389-396. DOI:10.1016/j.cej.2015.06.107 (  0) 0) |
Wu, L., Shao, Y., Hu, Z. and Gao, H., 2016. Effects of soluble sulfide on zebrafish (Danio rerio) embryonic development. Environmental Toxicology and Pharmacology, 42: 183-189. DOI:10.1016/j.etap.2016.01.019 (  0) 0) |
Zhao, Y. G., Zheng, Y., Tian, W., Bai, J., Feng, G., Guo, L. and Gao, M., 2016. Enrichment and immobilization of sulfide removal microbiota applied for environmental biological remediation of aquaculture area. Environmental Pollution, 214: 307-313. DOI:10.1016/j.envpol.2016.03.028 (  0) 0) |
 2019, Vol. 18
2019, Vol. 18


