2) Co-Innovation Center of Jiangsu Marine Bio-Industry Technology, Jiangsu Ocean University, Lianyungang 222005, China;
3) College of Food Science & Technology, Shanghai Ocean University, Shanghai 201306, China
Oxidation is an important process during food deterioration that affects the safety, color, taste, and texture of food. Furthermore, oxidation during cellular metabolism can induce DNA mutations, modify the lipid components of cell membranes, and cause denaturation of cell membrane proteins. Antioxidants can prevent or decrease the harmful effects of free radicals in human body and delay the deterioration of fat, protein, and other food ingredients. Because of their potential teratogenicity, carcinogenicity, and mutagenicity, synthetic antioxidants are limited in their application. Numerous researches have been conducted on the preparation of natural and non-toxic antioxidants to replace the synthetic ones. These natural antioxidants include various plant- and animal-based extracts, such as proteins (Borawska et al., 2016; Yang et al., 2016), polysaccharides (Tang et al., 2016; Xu et al., 2016), flavonoids (Dulf et al., 2016; Chaaban et al., 2017), polyphenols (Shen et al., 2015; Plaza et al., 2016), lectins (Wu et al., 2015), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) (Sahari et al., 2017).
Antioxidant peptides, which consist of 2-20 amino acids, can be isolated from animal and plant protein by enzymatic hydrolysis. With their properties of easy absorption, intense activity, few side effects, excellent safety, and environment-friendly properties, antioxidant peptides can be used to prepare functional foods, protein supplements, and medications. Many studies have reported that hydrolyzed proteins from various animal and plant sources, such as bean (Marcela et al., 2016; Carlos et al., 2017), corn (Jin et al., 2016; Wang et al., 2016), shellfish (Wu et al., 2016; Jin et al., 2018), shrimp (Zhou et al., 2014; Kleekayai et al., 2015), fish (Ko et al., 2013; Qiu et al., 2014), and algae (Sheih et al., 2009; Yu et al., 2016), possess antioxidant activity.
Porphyra is one of the most important cultured seaweeds and a main exported sea product of China (Liu et al., 2017). The annual Chinese production of porphyra is 0.20 million tons, of which P. haitanensis accounts for 50% (Department of Fishery and Fishery Administration, Ministry of Agriculture and Rural Areas, 2019). It is rich with proteins, polysaccharides, flavonoids, polyphenols, frames, lectins, DHA, EPA, and other physiologically active components. The harvest period of P. haitanensis is generally divided into 4-5 parts. The nutritional quality and market price of P. haitanensis decreases gradually with a prolonged harvest period. In the final harvest period, P. haitanensis usually is discarded as a waste, and leads to considerable environmental pollution. Interestingly, this waste can be used as raw material to prepare nutrients and physiologically active ingredients such as proteins, polysaccharides, DHA, and EPA. It is of considerable significance to improve the economic value of P. haitanensis.
In the present study, the antioxidant activities of P. haitanensis hydrolysates produced with different hydrolysis time was investigated, and its antioxidant mechanism was explored by measuring the radical-scavenging activity of 1, 1-diphenyl-2-picrylhydrazyl (DPPH). The antioxidant peptide was separated from the hydrolysate by gel filtration chromatography. The HepG2 cell with oxidative damage was used to further evaluate the antioxidant activity of purified PHH and explore the antioxidant mechanism. The peptide sequence was identified using an electrospray mass spectrometer (ESI-MS/MS).
2 Materials and Methods 2.1 Materials and ChemicalsP. haitanensis was supplied by the Peilong seaweed culture area in Chenghai District, Shantou City, Guangdong Province, China. P. haitanensis was dried at 60℃ for 4 h, then ground and filtered through a 100-mesh screen, and finally stored dry. Acid protease (5 × 104 U g-1) was supplied by Hefei BoMei Biotechnology Incoporation (Hefei, China). HepG2 cells were provided by Guangzhou Yeshan Biotechnology Incoporation (Guangzhou, China). All other materials for cell culture were obtained from Hyclone (Logan, Utah, USA). Sephadex G-15 was purchased from Cool Chemical Technology (Beijing, China). Reduced glutathione (MW 307.3 Da) and bacitracin (MW 1422.69 Da) were obtained from Guangzhou Qiyun Biotechnology Incoporation (Guangzhou, China). Aprotinin (MW 6511.83 Da) and cytochrome C (MW 12400 Da) were obtained from Shanghai Maclean Biochemical Technology Incoporation (Shanghai, China). Glutathione (GSH), H2O2, and L-oxidized glutathione (MW 612.63 Da) were purchased from Sigma (St. Louis, MO, USA). All chemicals were of analytical grade.
2.2 Protein Extraction from P. haitanensisThe protein was extracted from P. haitanensis using an ultrasonic cell grinder (JY99LLDN, Ningbo Scientz Biotechnology Company, Jiangsu, China). P. haitanensis (1.0 g) and 100 mL of distilled water were mixed and stirred uniformly, and extracted at a power of 1260 W, room temperature for 30 min, and then centrifuged with 558.95 × g at 4℃ for 10 min to remove cell debris. The pH of the supernatant was adjusted to an isoelectric point of 4.2. After the solution was kept at room temperture for 1 h, it was centrifuged at 558.95 × g for 10 min to remove the supernatant. The precipitate was dissolved in distilled water, adjusted to neutral pH, and dialyzed in a dialysis bag with a molecular weight cutoff of 100 Da for 24-36 h. The protein solution was pre-chilled at -20℃ for 12 h, freezedried under vacuum at -44℃ for 36 h in an Alpha 1-4 lyophilizer (Marin Christ, Germany), and stored dry at room temperture.
2.3 Enzymatic Hydrolysis of P. haitanensisThe protein of P. haitanensis was hydrolyzed by acid protease for 2.0, 4.0, 6.0, 8.0, and 10.0 h at 54.4℃, pH 3.67, with 4240 U g-1 of the enzyme. Reactions were stopped by heating at 95℃ for 10 min, and samples were centrifuged to remove insoluble material. The degree of hydrolysis (DH) of P. haitanensis proteins was determined using the pH-stat method. Porphyra haitanensis hydrolysates (PHHs) were adjusted to neutral pH and dialyzed for 24-36 h in a 100 Da dialysis bag. PHHs were concentrated, and freeze-dried under vacuum at -44℃ for 36 h in an Alpha 1-4 lyophilizer (Marin Christ, Germany), and stored dry at room temperture.
2.4 Molecular Weight Distribution of PHHsThe molecular weight distribution of PHHs was determined using high-performance size-exclusion chromatography (Sallam et al., 2018). The column was TSK-GELG 2000SWXL (7.8 mm × 300 mm), and the mobile phase was acetonitrile containing 0.1% trifluoroacetic acid and double distilled water containing 0.1% trifluoroacetic acid. The detection wavelength was 214 nm, the sample volume was 10 μL, the flow rate was 0.5 mL min-1, and the elution length was 60 min. A molecular weight standard was dissolved in mobile phase at a concentration of 0.2 mg mL-1. By fitting time and molecular weight, a regression equation was obtained: log MW = -0.206t + 6.8009, R2 = 0.9874. The molecular weight distribution of PHHs was calculated by normalization of the peak area.
2.5 Purification of PHHsPurification of PHHs was performed using selective gel filtration chromatography (Mei et al., 1998). After lyophilization, the polypeptide was dissolved in deionized water at a concentration of 25 mg mL-1. The sample was loaded onto a Sephadex G-15 column (1.6 cm × 100 cm), eluted with deionized water at a flow rate of 0.6 mL min-1, and monitored at 214 nm. Fractions with strongest DPPH-free radical-scavenging ability were concentrated, lyophilized, and further analyzed.
2.6 Antioxidant Activity Assays 2.6.1 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity assayDPPH radical-scavenging activity was measured according to a previously described method (Jang et al., 2016) with some modifications. The sample (1.0 mL) was combined with 1.0 mL of DPPH (0.15 mmol L-1 in ethanol). The solution was immediately mixed and incubated in the dark for 30 min. The absorbance of the resulting mixture was measured at 517 nm. The control sample contained ethanol (1.0 mL) and DPPH (1.0 mL). DPPH free radical-scavenging activity was calculated as (1-As/Ac) × 100%, where As and Ac represent absorbance of the sample and control, respectively.
2.6.2 Hydroxyl radical-scavenging activity assayHydroxyl radical-scavenging activity was measured according to a previously described method (Chi et al., 2015) with some modifications. Firstly, 2 mL sample was mixed with 0.3 mL of 1, 10-phenanthroline (5 mmol L-1 in ethanol), 0.2 mL of 0.15 mol L-1 phosphate buffer (pH 7.4), and 0.3 mL of 0.75 mmol L-1 ferrous sulfate, and the solution was stirred vigorously. Then 0.2 mL of 0.1% H2O2 was added to the solution and incubated at 37℃ for 60 min. Finally, the absorbance of the sample As was measured at 510 nm. When an equivalent volume of deionized water was used instead of the sample and H2O2 solution, the absorbance value Ab was applied as the control. When Ddeionized water was used instead of the sample, the absorbance value A0 was applied as the blank. Hydroxyl radical-scavenging activity was calculated as (1-(AS-A0)/Ab) × 100%.
2.7 Antioxidant Activity Analysis in HepG2 Cells 2.7.1 Cell cultureHepG2 cells stored in liquid nitrogen were thawed in a water bath at 37℃. They were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U mL-1 penicillin, and 100 µg mL-1 streptomycin. Cells were propagated in a 25 cm2 tissue culture flask, harvested using trypsin during exponential growth, and seeded into 96-well plates at a density of 1 × 105 cells mL-1. Cells were incubated at 37℃ in 5% CO2, and the medium was changed every 2 d.
2.7.2 Cell viability assayHepG2 cells at Log-phase were seeded into 96-well plates with a density of 1 × 105 cells mL-1 and cultured for 24 h at 37℃ in a incubator containing 5% CO2. After the cells were cultured for 24 h, 20 µL of a 5 mg mL-1 methyl thiazolyl tetrazolium (MTT) solution was added to each well, and the cells were cultured for another 4 h. Then the supernatant was carefully discarded, 200 µL of dimethylsulfinic acid was added to each well. After the plate was shaken for 15 min, the absorbance value was measured at 490 nm. Cell viability was calculated based on absorbance (Jang et al., 2008).
2.7.3 Establishment of the cell modelIn drug screening experiments, an induction concentration with a survival rate of 50% to 70% is generally selected as the optimal concentration. When the concentration of inducer is too high, the cells will be susceptible to excessive damage and the survival rate will be very low. HepG2 cells were treated with different concentrations of H2O2 solutions (50, 100, 200, 400, 600, and 800 μmol L-1) for 4 h. Cell viability was determined using the MTT method to determine the appropriate H2O2 concentration and treatment time (Jang et al., 2016). Using the cell survival rate as the indicator, the optimal induction condition to obtain a survival rate of 60% was culturing the cells in 259 μmol L-1 H2O2 for 4 h. HepG2 cells were divided into a blank group, a control group, and a treatment group. The blank group was HepG2 cells without H2O2 treatment. The control group was HepG2 cells treated with 259 µmol L-1 H2O2 for 4 h. In the treatment group, the cells were treated with GSH or PHH-Ⅲ with a concentration of 100, 200, and 400 μg mL-1 for 24 h, respectively, and then were treated with H2O2. These cells were determined as low-, medium-, and high-dose groups.
2.7.4 Determination of superoxide dismutase (SOD) activity and malondialdehyde (MDA) contentHepG2 cells at Log-phase were prepared as single-cell suspensions and seeded into 96-well plates with a concentration of 1 × 104 cells per 200 µL. Following establishment of a cell model, different concentrations (100, 200, and 400 µg mL-1) of polypeptide and GSH were used to treat oxidatively damaged HepG2 cells, and the MDA content and SOD activity were determined. The SOD activity was determined using the Total Superoxide Dismutase Assay Kit with WST-8 according to the manufacturer's instructions. The MDA content in the cells was determined using the Lipid Peroxidation MDA Assay Kit according to the manufacturer's instructions.
2.8 Structure of PHHsStructure of PHHs was identified according to a previously described method (Lin et al., 2019) with some modifications. PHH-Ⅲ was dissolved in a mobile phase, filtered through a 0.22 µm filter, placed in a sample vial, and analyzed using ultra-high-pressure liquid chromatographyhigh-resolution mass spectrometry. PHH-Ⅲ were precipitated with an Agilent SB-C18 RRHD column (2.1 mm × 50 mm, 1.8 µm) with a flow rate of 0.2 mL min-1, 0.1% formic acid as phase A and acetonitrile as phase B, and an injection volume of 20.0 µL. The gradient elution conditions were as follows: 0-1 min, B phase remained unchanged at 15%; 1-4.5 min, volume fraction of phase B solution increased from 15% to 90%; 4.5-8 min, phase B maintained at 90%; and 8.5-10 min, phase B decreased from 90% to 15%. Scanning was performed in positive-ion mode with a scan range of m/z 100-1400.
2.9 Statistical AnalysisResults are presented as the mean and standard deviation of triplicates. Statistical analysis was performed using SPSS 20.0 software (SPSS, Chicago, IL, USA).
3 Results and Discussion 3.1 Antioxidant Activity and Molecular Weight Distribution of PHHsPHHs obtained by different hydrolysis times were tested for DPPH radical-scavenging activity. As shown in Table 1, PHHs with a hydrolysis time of 4 h were found to be more efficient than the others, and the resulting hydrolysates exhibited higher antioxidant capacity. This may result from hydrolysis producing more small peptides with antioxidant activity because of energetic electrons (Wang et al., 2013). However, excessive hydrolysis time increases may result in the degradation of these small fragments, which can cause down-rugulated antioxidant activity. PHHs prepared by 4 h of hydrolysis were then further isolated for the identification of antioxidant peptides.
|
|
Table 1 Comparison of DPPH radical-scavenging activity and molecular weight distribution of PHHs at different enzymatic hydrolysis times |
The chromatographic data (Table 1) showed that PHHs contained a significant amount (70%-80%) of oligopeptides below 1 kDa. The results indicate that the acid proteases are effective at producing short oligopeptides and removing large peptides or undigested proteins. Hydrolysates with different molecular weights have differences in antioxidant capacity (Li et al., 2013). Moreover, some research findings indicated that small molecular peptides possess more robust free radical-scavenging activity (Zhuang et al., 2010), further increasing the antioxidant function. Therefore, according to the molecular weight distribution of PHHs, an appropriate method can be selected to purify PHHs and further evaluate their antioxidant capacity.
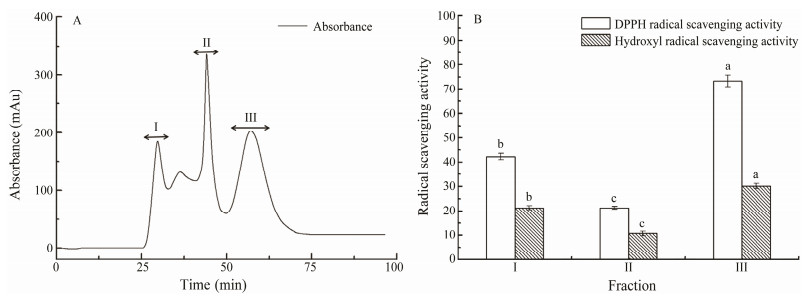
3.2 Purification of Different Fractions of PHHsGel filtration chromatography has been used for separation of biological extracts and protein hydrolysates (Knuckles et al., 2006). Considering the molecular weight of PHHs, a Sephadex G-15 column is suitable for further separation. PHHs were further separated into three fractions (Ⅰ-Ⅲ) by gel filtration chromatography. Each fraction was pooled, lyophilized, and tested for DPPH-scavenging ability. As shown in Fig. 1, fraction Ⅲ exhibited the most vigorous DPPH radical-scavenging activity with a rate of 73.32% at 1.0 mg mL-1. In this research, we found that the eluted fractions' antioxidant activity increased with increasing retention time. Consequently, the fraction of PHH-Ⅲ, which demonstrated the most potent activity, was selected for further analysis.
|
Fig. 1 (A) Chromatogram of PHH fractions purified by gel filtration column; (B) DPPH radical and hydroxyl radical-scavenging ability of each fraction (samples at 1.0 mg mL-1). Values within the same column, followed by the different letters (a, b, and c), are significantly different (P < 0.05). |
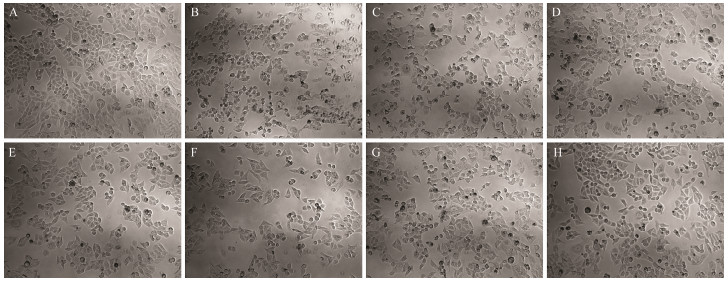
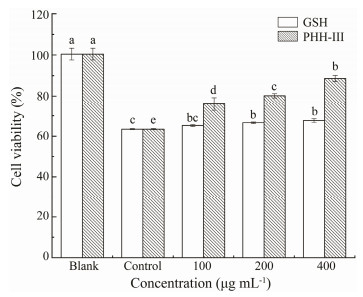
Oxidative stress refers to cell damage caused by high concentrations of reactive oxygen molecules or chemical derivatives of oxygen to cells. It is an essential mediator of the occurrence and development of obesity, diabetes, lipid deposition, and chronic inflammation (Skalicky et al., 2008). H2O2 is one of the commonly used substances for establishing oxidative damage in cells and has been used in osteoblasts, nerve cells, vascular endothelial cells, and hepatocytes (Zorov et al., 2000). HepG2 cells can fully express antioxidant enzymes and detoxification enzymes as in normal hepatocytes. Accordingly, They are often used for studying cytoprotection against exogenous chemicals and natural antioxidants (Lee et al., 2012). As shown in Figs. 2 and 3, compared with healthy cells (Blank), the viability of HepG2 cells in the injury group (Control) was reduced significantly. Moreover, the injured cells showed inter-adhesion, irregular shapes, and lower cell viability. Different doses of PHH-Ⅲ in each group can protect the cell and significantly increase cell viability (P < 0.05). Together with GSH, PHH-Ⅲ was better at improving the morphology and cell viability with a concentration of 100 µg mL-1. Additionally, the viability of cells cultured with different doses of PHH-Ⅲ was higher than that with different doses of GSH. The results of these experiments indicate that PHH-Ⅲ can increase the viability of cells damaged by H2O2.
|
Fig. 2 Effects of different concentrations of PHH-Ⅲ and GSH on the morphology of HepG2 cells treated with 259 µmol L-1 H2O2 for 4 h. (A) Blank, (B) Control, (C) 100 µg mL-1 GSH, (D) 200 µg mL-1 GSH, (E) 400 µg mL-1 GSH, (F) 100 µg mL-1 PHH-Ⅲ, (G) 200 µg mL-1 PHH-Ⅲ, and (H) 400 µg mL-1 PHH-Ⅲ. The blank group was HepG2 cells without H2O2 treatment. The control group was HepG2 cells treated with 259 µmol L-1 H2O2 for 4 h. |
|
Fig. 3 Effects of different concentrations of PHH-Ⅲ and GSH on the viability of HepG2 cells treated with 259 µmol L-1 H2O2 for 4 h. The blank group was HepG2 cells without H2O2 treatment. The control group was HepG2 cells treated with 259 µmol L-1 H2O2 for 4 h. The treatment group was treated with GSH or PHH-Ⅲ with a concentration of 100, 200, or 400 µg mL-1 for 24 h, respectively, as the low-, medium-, and high-dose groups. Then the H2O2 treatment was applied. Values within the same column, followed by different letters (a, b, c, d, and e) are significantly different (P < 0.05). Values are shown as mean±standard deviations from triplicates (n = 3). |
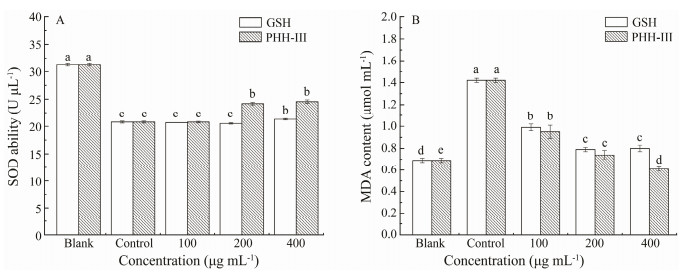
SOD catalyzes the disproportionation of superoxide anion to H2O2 and O2 to scavenge free radicals, and its vitality plays an important role in cellular oxidation and antioxidant balance (Miller, 2004). As shown in Fig. 4, compared with healthy cells (Blank), the SOD activity in injured HepG2 cells (Control) was significantly reduced. When medium- or high-dose PHH-Ⅲ was added in the culture media, the SOD activity in the cells could be increased significantly (P < 0.05), while only high dose GSH could significantly increase the SOD activity (P < 0.05).
|
Fig. 4 (A) Effects of different concentrations of GSH and PHH-Ⅲ on SOD activity in HepG2 cells treated with 259 µmol L-1 H2O2 for 4 h, (B) Effects of different concentrations of GSH and PHH-Ⅲ on MDA content in HepG2 cells treated with 259 µmol L-1 H2O2 for 4 h. The blank group was HepG2 cells without H2O2 treatment. The control group was HepG2 cells treated with 259 µmol L-1 H2O2 for 4 h. The treatment group was treated with GSH or PHH-Ⅲ with a concentration of 100, 200, and 400 µg mL-1 for 24 h, respectively, as the low-, medium-, and high-dose groups. Then H2O2 treatment, was applied. Values within the same column, followed by different letters (a, b, c, d, and e) are significantly different (P < 0.05). Values are shown as mean±standard deviations from triplicates (n = 3). |
MDA is an oxidation end product obtained by free radicals acting on lipids in the body (Bedoya-Ramírez et al., 2017). The MDA content in liver cells is generally considered to be an essential indicator of the degree of lipid peroxidation (Hu et al., 2017). As shown in Fig. 4B, compared with untreated cells (Blank), the MDA content in inhured HepG2 cells (Control) was significantly increased. The low-, medium-, and high-dose of PHH-Ⅲ or GSH significantly inhibited the increase of MDA content in the cells caused by H2O2 in a dose-dependent manner (P < 0.05). Compared with GSH, PHH-Ⅲ was better at reducing the MDA content in cells with low-, medium-, and high-dose. Thus PHH-Ⅲ can increase the SOD activity of H2O2-injured cells, inhibit the increase in MDA content, and enhance the antioxidant response of HepG2 cells.
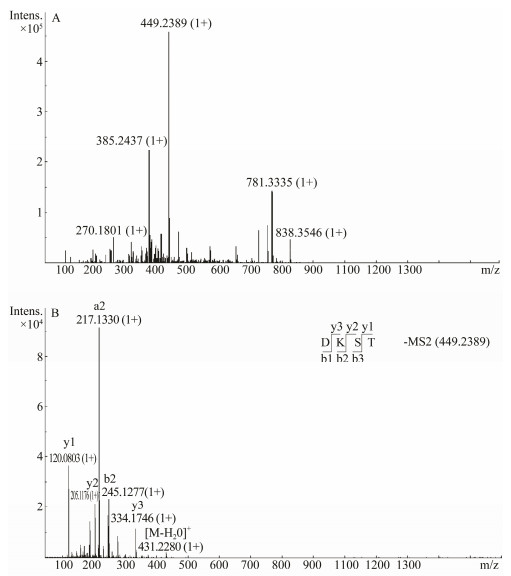
3.3.3 Mass spectrometry identification analysisMass spectrometry can accurately determine the molecular weight and amino acid sequence of peptides and has potential applications in proteomics analysis (Li et al., 2007). In order to identify the peptides exhibiting major antioxidant activity, the PHH-Ⅲ fractions were further sequenced by ESI-MS/MS. The MS spectrum of this fraction is shown in Fig. 5A, and the MS/MS spectrum of a single-charged ion with m/z 449 Da is shown in Fig. 5B. The molecular weight of the peptide in PHH-Ⅲ was determined to be 449 Da. Since each peptide matches a specific mass number and corresponding fragment map (Ma et al., 2012), the amino acid sequence of the peptide in PHH-Ⅲ was identified as Asp-Lys-Ser-Thr. According to the naming system proposed by Roepstorff and modified by Biemann, the N-terminal fragment ions are represented by the letters a, b, and c, and the C-terminal fragment ions are represented by the letters x, y, and z (Biemann, 1992). Since the amide bond in the peptide is relatively easy to be broken, the b- and y-type fragment series may have a higher frequency of occurrence on the mass spectrum. The y3 (m/z 334), y2 (m/z 205), b2 (m/z 245), and y1 (m/z 120) fragments are produced by cleavage of a peptide bond. The neutral molecule may also lose water or ammonia (Sun et al., 2008), resulting in a high abundance of m/z 431. It has been reported that residues of acidic amino acids (Asp, Glu, etc.) contribute to the antioxidant capacity of peptides (Saiga et al., 2003). Moreover, Lys contributes to the termination of a radical chain reaction, the interaction with free radicals, and the prevention of radical formation. In general, the number, type, and composition of amino acids play an essential role in antioxidative peptides.
|
Fig. 5 (A) Mass spectrum of the chromatographic peak Ⅲ; (B) MS/MS spectrum of ion m/z 449. |
In this study, the DPPH free radical-scavenging ability of PHHs was the strongest (59.28% at 1.0 mg mL-1) when hydrolyzed with an acidic protease for 4 h. Among the hydrolyzed products, PHHs with a molecular weight range of less than 1 kDa accounted for 70% to 80% of the total PHHs. Sephadex G-15 was used to separate and purify PHHs, and it was found that the PHH-Ⅲ fraction had the most vigorous activity. PHH-Ⅲ could alleviate the oxidative damage of HepG2 cells caused by H2O2, which might be realized through increasing SOD enzyme activity and reducing the production of MDA. The tetrapeptide Asp-Lys-Ser-Thr with a molecular weight of 448 Da was identified by high-resolution mass spectrometry in the PHH-Ⅲ. The specific signaling pathways and targets for the regulation of oxidative stress response in cells affected by algae peptides need to be further studied.
AcknowledgementsThis work was supported by the National Key R & D Program of China (No. 2018YFD0901102), the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (No. 2020KJ151), the Special Scientific Research Funds for Central Non-profit Institutes, Chinese Academy of Fishery Sciences (No. 2020 TD69), and the China Agriculture Research System (No. CARS-50).
Bedoya-Ramírez, D., Cilla, A., Contreras-Calderón, J. and Alegría-Torán, A., 2017. Evaluation of the antioxidant capacity, furan compounds and cytoprotective/cytotoxic effects upon Caco-2 cells of commercial Colombian coffee. Food Chemistry, 219: 364-372. DOI:10.1016/j.foodchem.2016.09.159 (  0) 0) |
Biemann, K., 1992. Mass spectrometry of peptides and proteins. Annual Review of Biochemistry, 61(1): 977-1010. DOI:10.1146/annurev.bi.61.070192.004553 (  0) 0) |
Borawska, J., Darewicz, M., Vegarud, G. E. and Minkiewicz, P., 2016. Antioxidant properties of carp (Cyprinus carpio L.) protein ex vivo and in vitro hydrolysates. Food Chemistry, 194: 770-779. DOI:10.1016/j.foodchem.2015.08.075 (  0) 0) |
Carlos, M. G. A., Walter, M. and Jonh, J. M. A., 2017. Antioxidant potential use of bioactive peptides derived from mung bean hydrolysates (Vigna radiata). African Journal of Food Science, 11(3): 67-73. DOI:10.5897/AJFS2016.1511 (  0) 0) |
Chaaban, H., Ioannou, I., Chebil, L., Slimane, M., Gérardin, C., Paris, C., Charbonnel, C., Chekir, L. and Ghoul, M., 2017. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. Journal of Food Processing and Preservation, 41(5): 1-12. (  0) 0) |
Chi, C. F., Hu, F. Y., Wang, B., Li, T. and Ding, G. F., 2015. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca Granosa) muscle. Journal of Functional Foods, 15: 301-313. DOI:10.1016/j.jff.2015.03.045 (  0) 0) |
Department of Fishery and Fishery Administration, Ministry of Agriculture and Rural Areas, 2019. China Fishery Statistical Yearbook, 23.
(  0) 0) |
Dulf, F. V., Vodnar, D. C. and Socaciu, C., 2016. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chemistry, 209: 27-36. DOI:10.1016/j.foodchem.2016.04.016 (  0) 0) |
Hu, N., Tu, X. R., Li, K. T., Liu, Y. K., Guo, A. M., Tu, G. Q. and Huang, L., 2017. Changes in antioxidant enzyme activities and malondialdehyde (MDA) content of rice with blast resistance induced by ag-antibiotic 702. Plant Diseases and Pests, 8(2): 36-40. (  0) 0) |
Jang, A., Jo, C., Kang, K. S. and Lee, M., 2008. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chemistry, 107(1): 327-336. DOI:10.1016/j.foodchem.2007.08.036 (  0) 0) |
Jang, H. L., Liceaga, A. M. and Yoon, K. Y., 2016. Purification, characterization and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. Journal of Functional Foods, 20: 433-442. DOI:10.1016/j.jff.2015.11.020 (  0) 0) |
Jin, D., Liu, X., Zheng, X., Wang, X. and He, J., 2016. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chemistry, 204: 427-436. DOI:10.1016/j.foodchem.2016.02.119 (  0) 0) |
Jin, J. E., Ahn, C. B. and Je, J. Y., 2018. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed ark shell (Scapharca subcrenata). Process Biochemistry, 72: 170-176. DOI:10.1016/j.procbio.2018.06.001 (  0) 0) |
Kleekayai, T., Harnedy, P. A., O'Keeffe, M. B., Poyarkov, A. A., Cunhaneves, A., Suntornsuk, W. and FitzGerald, R. J., 2015. Extraction of antioxidant and ace inhibitory peptides from thai traditional fermented shrimp pastes. Food Chemistry, 176: 441-447. DOI:10.1016/j.foodchem.2014.12.026 (  0) 0) |
Knuckles, B. E., Fremery, D. D. and Kohler, G. O., 1982. Gel filtration chromatography of protein extracts from lucerne (Alfalfa). Journal of the Science of Food and Agriculture, 33(2): 128-132. DOI:10.1002/jsfa.2740330204 (  0) 0) |
Ko, J., Lee, J., Samarakoon, K., Kim, J. and Jeon, Y., 2013. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food and Chemical Toxicology, 52: 113-120. DOI:10.1016/j.fct.2012.10.058 (  0) 0) |
Lee, M. S., Kim, J. I., Utsuki, T., Park, N. G. and Kim, H. R., 2012. Cytoprotective effects of phlorofucofuroeckol A isolated from Ecklonia stolonifera against tacrine-treated HepG2 cells. Fitoterapia, 83(6): 1060-1067. DOI:10.1016/j.fitote.2012.05.007 (  0) 0) |
Li, B., Chen, F., Wang, X., Ji, B. P. and Wu, Y. N., 2007. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chemistry, 102: 1135-1143. DOI:10.1016/j.foodchem.2006.07.002 (  0) 0) |
Li, X. H., Chen, Z. J., Liu, Y. L., Yu, J., Wang, F. X. and Wang, J. H., 2013. Molecular weight and antioxidant activity of enzymatic hydrolysates of silver carp. Food Science, 34(17): 28-32. (  0) 0) |
Lin, L., Zhu, Q. and Zhao, M., 2019. Preparation of antioxidant peptide from Moringa oleifera seeds and its protective effects on oxidatively damaged erythrocytes. Food Science, 40(7): 40-46. (  0) 0) |
Liu, Q. M., Xu, S. S., Li, L., Pan, T. M., Shi, C. L., Liu, H., Cao, M. J., Su, W. J. and Liu, Q. M., 2017. In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis. Carbohydrate Polymers, 165: 189-196. DOI:10.1016/j.carbpol.2017.02.032 (  0) 0) |
Ma, Z., Zhang, W., Yu, G., He, H. and Zhang, Y., 2012. The primary structure identification of a corn peptide facilitating alcohol metabolism by HPLC-MS/MS. Peptides, 37(1): 138-143. DOI:10.1016/j.peptides.2012.07.004 (  0) 0) |
Marcela, G. M., Eva, R. G., del Carmen, R. R. M. and Rosalva, M. E., 2016. Evaluation of the antioxidant and antiproliferative effects of three peptide fractions of germinated soybeans on breast and cervical cancer cell lines. Plant Foods for Human Nutrition, 71(4): 368-374. DOI:10.1007/s11130-016-0568-z (  0) 0) |
Mei, W., Dai, J. and Gu, W., 1998. Assay on the molecular weight distribution of oligo peptide in corn protein hydrolysate with gel filtration chromatography. Food and Fermentation Industries, 24(5): 25-27. (  0) 0) |
Miller, A. F., 2004. Superoxide dismutases: Active sites that save, but a protein that kills. Current Opinion in Chemical Biology, 8(2): 162-168. DOI:10.1016/j.cbpa.2004.02.011 (  0) 0) |
Plaza, M., Batista, A. G., Cazarin, C. B. B., Sandahl, M., Turner, C., Östman, E. and Júniorb, M. R., 2016. Characterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: A pilot clinical study. Food Chemistry, 211: 185-197. DOI:10.1016/j.foodchem.2016.04.142 (  0) 0) |
Qiu, X., Chen, S. and Dong, S., 2014. Effects of silver carp antioxidant peptide on the lipid oxidation of sierra fish fillets (Scomberomorus niphonius) during frozen storage. Journal of Food Biochemistry, 38(2): 167-174. DOI:10.1111/jfbc.12035 (  0) 0) |
Sahari, M. A., Moghimi, H. R., Hadian, Z., Barzegar, M. and Mohammadi, A., 2017. Physicochemical properties and antioxidant activity of α-tocopherol loaded nanoliposome's containing DHA and EPA. Food Chemistry, 215: 157-164. DOI:10.1016/j.foodchem.2016.07.139 (  0) 0) |
Saiga, A., Tanabe, S. and Nishimura, T., 2003. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. Journal of Agricultural & Food Chemistry, 51(12): 3661-3667. (  0) 0) |
Sallam, S., Dolog, I., Paik, B. A., Jia, X., Kiick, K. L. and Wesdemiotis, C., 2018. Sequence and conformational analysis of peptide-polymer bioconjugates by multidimensional mass spectrometry. Biomacromolecules, 19(5): 1498-1507. DOI:10.1021/acs.biomac.7b01694 (  0) 0) |
Sheih, I. C., Wu, T. K. and Fang, T. J., 2009. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresource Technology, 100(13): 3419-3425. DOI:10.1016/j.biortech.2009.02.014 (  0) 0) |
Shen, Y., Zhang, H., Cheng, L., Wang, L., Qian, H. and Qi, X., 2015. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chemistry, 194: 1003-1012. (  0) 0) |
Skalicky, J., Muzakova, V., Kandar, R., Meloun, M., Rousar, T. and Palicka, V., 2008. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clinical Chemistry and Laboratory Medicine, 46(4): 499-505. (  0) 0) |
Sun, S. W., Yu, C. G., Qiao, Y. T., Lin, Y., Dong, G. J., Liu, C. N., Zhang, L. F., Zhang, Z., Cai, J. J. and Zhang, H., 2008. Deriving the probabilities of water loss and ammonia loss for amino acids from tandem mass spectra. Journal of Proteome Research, 7(1): 202-208. DOI:10.1021/pr070479v (  0) 0) |
Tang, W., Lin, L., Xie, J., Wang, Z., Wang, H. and Dong, Y., 2016. Effect of ultrasonic treatment on the physicochemical properties and antioxidant activities of polysaccharide from Cyclocarya paliurus. Carbohydrate Polymers, 151: 305-312. DOI:10.1016/j.carbpol.2016.05.078 (  0) 0) |
Wang, B., Li, L., Chi, C., Ma, J., Luo, H. and Xu, Y., 2013. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chemistry, 138(2-3): 1713-1719. DOI:10.1016/j.foodchem.2012.12.002 (  0) 0) |
Wang, L., Ding, L., Yu, Z., Zhang, T., Ma, S. and Liu, J., 2016. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Research International, 90: 33-41. DOI:10.1016/j.foodres.2016.10.023 (  0) 0) |
Wu, H., Jin, W., Sun, S., Li, X. and Zhu, B., 2015. Identification of antioxidant peptides from protein hydrolysates of scallop (Patinopecten Yessoensis) female gonads. European Food Research and Technology, 242: 713-722. (  0) 0) |
Wu, M., Tong, C., Wu, Y., Liu, S. and Li, W., 2016. A novel thyroglobulin-binding lectin from the brown alga Hizikia fusiformis and its antioxidant activities. Food Chemistry, 201: 7-13. DOI:10.1016/j.foodchem.2016.01.061 (  0) 0) |
Xu, Y., Cai, F., Yu, Z., Zhang, L., Li, X., Yang, Y. and Liu, G., 2016. Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chemistry, 194: 650-658. DOI:10.1016/j.foodchem.2015.08.061 (  0) 0) |
Yang, S., Tang, Z., Tang, S., Zhang, T., Tang, F., Wu, Y., Wang, Y., Wang, L. and Liu, G., 2016. Purification and characterization of an antioxidant protein from fertilized eggs. Korean Journal for Food Science of Animal Resources, 36: 791-798. DOI:10.5851/kosfa.2016.36.6.791 (  0) 0) |
Yu, J., Hu, Y., Xue, M., Dun, Y. and Zhao, S., 2016. Purification and identification of antioxidant peptides from enzymatic hydrolysate of spirulina platensis. Journal of Microbiology and Biotechnology, 26(7): 1216-1223. DOI:10.4014/jmb.1601.01033 (  0) 0) |
Zhou, A., Feng, Z., Feng, Y., Liu, X., Xin, L. and Chen, Y., 2014. Research of antioxidant peptides produced from the waste of white shrimp (Penaeus Vannamei). Journal of Chinese Institute of Food Science and Technology, 14: 85-91. (  0) 0) |
Zhuang, Y., Sun, L. P., Zhao, X., Hou, H. and Li, B., 2010. Investigation of gelatin polypeptides of jellyfish (Rhopilema esculentum) for their antioxidant activity in vitro. Food Technology and Biotechnology, 48(2): 222-228. (  0) 0) |
Zorov, D. B., Filburn, C. R., Klotz, L. O., Zweier, J. L. and Sollott, S. J., 2000. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. Journal of Experimental Medicine, 192(7): 1001-1014. DOI:10.1084/jem.192.7.1001 (  0) 0) |
 2021, Vol. 20
2021, Vol. 20


