2) Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266235, China
Mollusks comprise approximately 23% of all known marine organisms, which are highly diverse in terms of size, anatomical structure, behavior, and habitat (Rosenberg, 2014; Yao et al., 2019). Similar to other invertebrates, mollusks lack adaptive immunity and have developed a highly effective and non-specific innate immune response system to adapt to the challenge of pathogens or alien materials (Wang et al., 2013; Yang et al., 2021). Invertebrate innate immunity consists of cellular and humoral responses, of which the latter involves a battery of hemolymph-based bioactive compounds (Tassanakajon et al., 2018).
In invertebrates, phenoloxidases (POs) are generated from a reaction cascade called the prophenoloxidase (pro-PO) activating system, which can be activated by a pathogen agent (Ma et al., 2014; Monwan et al., 2017; Gu et al., 2019). They can catalyze phenols into unstable quinones, which in turn can polymerise to form melanin (Muñoz et al., 2006; Anjugam et al., 2017; Stączek et al., 2020). The melanin and intermediate metabolites generated in the reactions are involved in melanization, encapsulation, wound healing, phagocytosis, and pathogen extermination (Antonio Luna-González et al., 2003; Palmer et al., 2011; Panigrahi et al., 2020). Based on the activities, there are three distinct types in the group of POs (Liu et al., 2006; Luna-Acosta et al., 2017), including tyrosinase (EC 1.14.18.1), catecholase (EC 1.10.3.1) and laccase (EC 1.10.3.2). All three types can catalyze the oxidation of o-diphenols, while only tyrosinase can catalyze the orhtohydroxylation of monophenols, and only laccase can catalyze the oxidation of mand p-diphenols (Luna-Acosta et al., 2017).
In bivalves, POs distribute across different tissue, such as haemocytes, plasma, gills, foot and mantle, and are likely to play various roles (Bharathi and Ramalingam, 1983; Thomas-Guyon et al., 2009; Yang et al., 2017). POs may be involved in antiviral and bactericidal defenses and play a crucial immune role (Xing et al., 2012; Bris et al., 2015; 2008). When contaminant enters the organism, POs can be considerate as a certain type of stressor, enhancing the release of hormones, neurotransmitters and especially catecholamines (Luna-Acosta et al., 2017). In the clam Ruditapes philippinarum, PO is copper-containing tyrosinasetype PO that can be inhibited by Ca2+, Mg2+, Cu2+, thiourea, EDTA and DETC (Cong et al., 2005). In the scallop Argopecten irradians, PO is identified as a copper-containing laccase-type PO with a molecular mass of 555 kDa and can be inhibited by sodium sulfite, EDTA and DETC (Jiang et al., 2011). In the Sydney rock oyster Saccostrea glomerata, two copper-containing tyrosinase-type POs are identified with molecular masses of 219 and 192 kDa respectively (Aladaileh et al., 2007). Moreover, hemocyanin isolated from the gastropods Helix pomatia and Rapana venosa (synonym of Rapana thomasiana) have been demonstrated to exhibit o-diphenoloxidase activity (o-diPO) (Idakieva et al., 2009).
Studying the biochemical characteristics of POs is very important for better understanding their roles in the invertebrates. With the aim of providing preliminary data on the POs characteristics in the mollusks, three cultured species were selected for POs biochemical characteristics analyses in the present research. Scallop Chlamys farreri, abalone Haliotis discus hannai and clam Scapharca subcrenata, two bivalves and one gastropod, are three important commercial marine mollusk species in China. In this study, their POs were isolated, and the substrate specificity, PO kinetics, and the effects of metal ions and other inhibitors on POs were investigated. The results can be the reference for further research of PO roles in the mollusks.
2 Materials and Methods 2.1 Experimental AnimalsThe adult scallop C. farreri (5.8 cm ± 0.44 cm shell length), abalone H. discus hannai (6.2 cm ± 0.17 cm shell length) and clam S. subcrenata (4.2 cm ± 0.21 cm shell length) were purchased from local farm in Qingdao, Shandong Province, China, and maintained in the aerated recirculating seawater system with sponge-filtering at 16℃ ± 2℃ for two weeks. The water was renewed at a volume of 1/2 tank daily. The abalone was fed with Laminaria Japonica (Taihua, China) and the scallop and clam were fed with Spirulina (Ruikang, China) once a day.
Totally, about 400 individuals of each species were used for hemolymph collection.
2.2 Hemocyte Lysate Supernatant PreparationAbout 100 mL hemolymph was withdrawn from each adductor muscle sinus using sterilized syringes. Then they were pooled and centrifuged at 700 × g for 10 min at 4℃. The haemocyte pellets were collected and suspended in phosphate-buffered saline (PBS, 2.7 mmol L−1 KCl, 0.137 mol L−1 NaCl, 1.47 mmol L−1 KH2PO4, 8.09 mmol L−1 Na2HPO4·12H2O, pH 7.6). The haemocyte suspensions were sonicated and the cell homogenates were then centrifuged at 15000 × g for 30 min at 4℃. The resulting supernatants, representing hemocyte lysate supernatant (HLS), were collected and stored at −80℃ for further purification.
2.3 Zymography and Isolation of POsThe HLS was subjected to linear-gradient native-PAGE (6%–20%) in Tris-Glycine buffer (0.025 mol L−1 Tris, 0.2 mol L−1 Glycine, pH 8.0) for 12 h at 3 W, using high molecular weight markers ranging from 67 to 669 kDa (GE Healthcare). After the electrophoresis, one lane of the gel was cut and stained with 1% (W/V) catechol to label the PO-containing bands. Based on the catechol staining band, the relevant unstained lanes of the gel were excised for PO-containing bands, which were successively sonicated in PBS and centrifuged at 17000 × g for 30 min at 4℃. The collected fractions of the six PO bands were condensed and desalted separately, using centrifugal concentrators (Amicon Ultra-15 mL, Millipore), and then used as crude POs for the subsequent assay respectively.
2.4 PO Activity AssayPO activity was measured spectrophotometrically by the formation of dopachrome with L-3, 4-dihydroxyphenylalanine (L-DOPA) method. In brief, 100 μL of the sample was added to 2.0 mL L-DOPA (15 mmol L−1 in 100 mmol L−1 Tris-HCl buffer, pH 8.0). Then the formation of dopachrome was measured with an UV-2100 Spectrophotometer (Unico, China) at 490 nm every 3 min for 30 min. An increase of 0.001 absorbance value per min was considered as one PO activity unit in the assay, expressed as 1 U (A490 10−3 min−1).
The protein concentrations were determined according to Bradford method using bovine serum album (BSA; Sigma, USA) as the protein standard.
2.5 Substrate Specificity and Kinetic AnalysisKinetic parameters were determined using the Lineweaver-Burk-plot method (Sajid-ur-Rehman et al., 2018). To 100 μL of the crude PO solution, 2.0 mL of different concentrations of L-DOPA, catechol, hydroquinone, dopamine and tyrosine dissolved in 100 mmol L−1 Tris-HCl buffer (pH 8.0) was added, respectively. And the enzymatic activities were measured by the spectrophotometry at 490 nm.
2.6 Effects of Divalent Metal Ions on POsThe effects of divalent metal ions on the activities of POs were assayed by mixing with divalent metal ions, including Fe2+, Mg2+, Zn2+, Mn2+, Cu2+ and Ca2+ from FeCl2, MgSO4, ZnSO4, MnCl2, CuSO4 and CaCl2 (Songon, China). The crude POs were adjusted to the same U mL−1. A total of 100 µL of each crude PO solution was incubated with 100 μL of divalent metal ions at different concentrations for 20 min at 4℃, followed by the addition of 1.9 mL of 15 mmol L−1 L-DOPA. The divalent metal ions and L-DOPA were both dissolved in 100 mmol L−1 Tris-HCl buffer (pH 8.0). In contrast, the control samples were performed by incubating the samples with 100 μL of 100 mmol L−1 Tris-HCl buffer (pH 8.0) without divalent metal ions. Then the PO activities were measured spectrophotometrically at 490 nm.
2.7 Effects of Inhibitors on POsPO inhibition assay was performed by incubating 100 µL of each crude PO solution with 100 μL of PO inhibitors dissolved in 100 mmol L−1 Tris-HCl buffer (pH 8.0) at different concentrations for 20 min at 4℃. The PO inhibitors used in the assay include cysteine, ascorbic acid, sodium sulfite, citric acid, thiourea, sodium azide, ethylenediaminetetraacetic acid disodium (EDTA) and sodium diethyldithiocarbamate (DETC). The crude POs were adjusted to the same U mL−1. The control samples were performed by incubating the samples with 100 μL of 100 mmol L−1 Tris-HCl buffer (pH 8.0) without the PO inhibitors. Then the solution was added with 1.9 mL of 15 mmol L−1 L-DOPA and the PO activities were determined spectrophotometrically as described above.
2.8 Statistical AnalysisAll of the experiments from section 2.5 to 2.7 were performed in triplicate, and the data from section 2.6 and 2.7 were presented as the means ± standard deviations. Statistical analysis was performed using SPSS 11.5.
3 Results 3.1 Molecular Mass of POsAfter the linear-gradient native PAGE, C. farreri HLS and H. discus hannai HLS showed only one band reacted to catechol, with a molecular mass of 576 kDa and 228 kDa respectively. However, in the HLS of S. subcrenata, four bands reacted to catechol. The first three were with molecular masses of 391 kDa, 206 kDa and 174 kDa respectively, named as 391-PO, 206-PO and 174-PO. The smallest one was with a molecular mass lower than 67 kDa, named as s-PO in this paper (Fig.1).
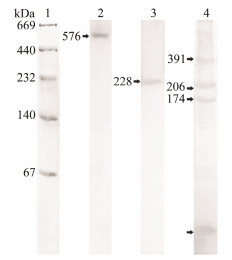
|
Fig. 1 Linear-gradient native PAGE of HLS. Lane 1 was stained with CBB, whereas lane 2 to lane 4 were stained with catechol. Lane 1, marker of protein; lane 2, C. farreri HLS; lane 3, H. discus hannai HLS; and lane 4, S. subcrenata HLS. |
Using the Lineweaver-Burk model, Km values of the C. farreri PO for catechol, L-DOPA, hydroquinone and dopamine were 0.2, 0.61, 0.84 and 0.88 mmol L−1 respectively (Fig.2A), that of H. discus hannai PO were 0.57, 0.58, 3.57 and 1.6 mmol L−1 (Fig.2B), and in S. subcrenata, that of 391-PO were 1.49, 0.77, 1.24 and 2.17 mmol L−1 (Fig.2C), that of 206-PO were 0.41, 0.65, 1.69 and 1.51 mmol L−1 (Fig.2D), that of 174-PO were 1.29, 0.55, 3.3 and 1.94 m mol L−1 (Fig.2E), and that of s-PO were 0.52, 0.44, 10.50 and 0.81 mmol L−1 (Fig.2F). However, no reaction with tyrosine was detected using the POs obtained from the three mollusks.
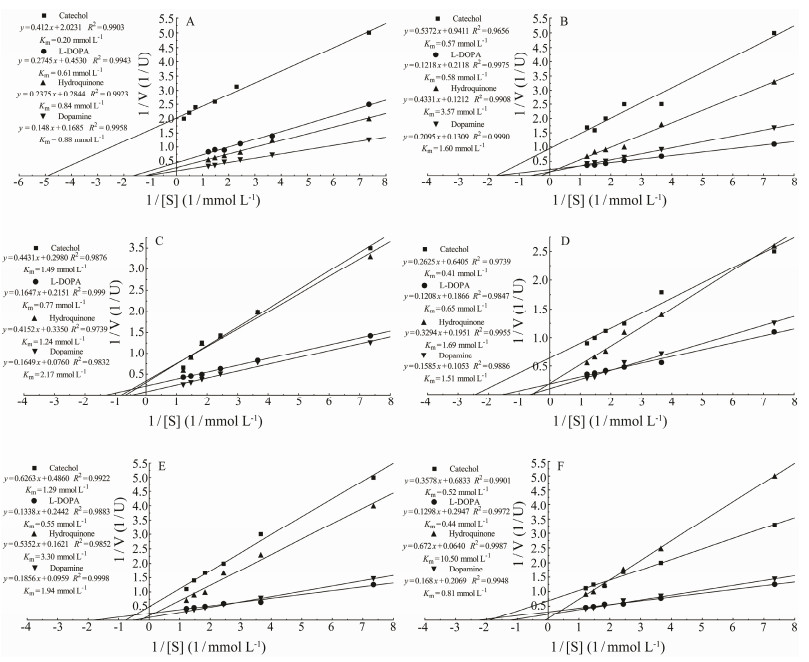
|
Fig. 2 Kinetic analyses of isolated POs. The Km values were determined using L-DOPA, catechol, hydroquinone and dopamine as substrates, and were calculated according to the Lineweaver-Burk model. A, C. farreri PO; B, H. discus hannai PO; C, 391-PO; D, 206-PO; E, 174-PO; F, s-PO. |
In C. farreri, Mg2+ and Ca2+ stimulated while Fe2+ inhibited the PO activity at all of the determined concentrations. Zn2+ stimulated at concentrations of 5 and 10 mmol L−1, but obviously inhibited at 50 mmol L−1. Cu2+ had stimulative effects at 5 mmol L−1, and inhibitory effects at 20 and 30 mmol L−1, which was opposite to Mn2+ (Fig.3A).
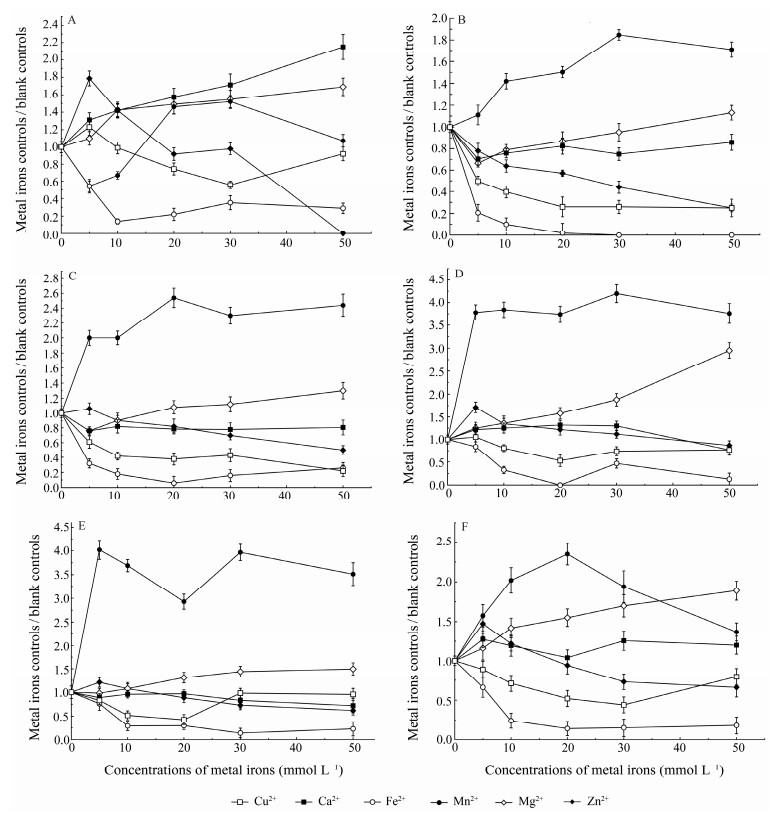
|
Fig. 3 Effects of 6 metal ions on the activity of isolated POs. The PO activity was assayed using L-DOPA (15 mmol L−1) as a specific substrate. A, C. farreri PO; B, H. discus hannai PO; C, 391-PO; D, 206-PO; E, 174-PO; F, s-PO. |
In H. discus hannai, Mn2+ stimulated while Cu2+, Zn2+, Fe2+ and Ca2+ inhibited the PO activity at all of the determined concentrations, and Mg2+ had inhibitory effect at 5 mmol L−1 and stimulative effect at 50 mmol L−1 (Fig.3B).
In S. subcrenata, as for 391-PO, Mn2+ stimulated while Cu2+, Fe2+ and Ca2+ inhibited the PO activity at all of the determined concentrations, and Zn2+ showed stimulative effect at 5 mmol L−1 and inhibitory effect at 20, 30 and 50 mmol L−1, which was opposite to Mg2+ (Fig.3C). For 206-PO, Mn2+, Mg2+ and Ca2+ stimulated while Fe2+ inhibited at all of the determined concentrations, Zn2+ and Cu2+ had stimulative effects at 5 mmol L−1 and inhibitory effects at 50 mmol L−1 (Fig.3D). For 174-PO, Mn2+ and Mg2+ stimulated while Fe2+ inhibited at all of the determined concentrations; Zn2+ had a stimulative effect at 5 mmol L−1, and Cu2+ showed obvious inhibition at the concentrations of 5, 10 and 20 mmol L−1 (Fig.3E). For s-PO, Mn2+, Mg2+ and Ca2+ stimulated while Cu2+ and Fe2+ inhibited PO activity at all of the determined concentrations, and Zn2+ showed a stimulative effect at 5 mmol L−1 and inhibitory effect at 50 mmol L−1 (Fig.3F).
In general, all POs of the three species were inhibited by Fe2+ at various determined concentrations. Except for scallop C. farreri PO, other five POs were stimulated by Mn2+ at various determined concentrations. Furthermore, Mg2+, Cu2+, Zn2+ and Ca2+ inhibited the PO in H. discus hannai, relatively different from what they did in C. farreri PO and S. subcrenata PO.
3.4 Effects of Inhibitors on POsWhen the inhibitors were adjusted to 50 mmol L−1, critric acid, ascorbic acid, DETC, cysteine and sodium sulfite showed 100% inhibition to the activities of POs, while the inhibition of EDTA varies from 30% to 90%. Furthmore, the inhibition of critric acid and EDTA are dose-dependent. Additionally, sodium azide only showed slightly inhibition to H. discus hannai PO and 391-PO, while thiourea only showed slightly inhibition to H. discus hannai PO, 391-PO and 174-PO (Fig.4).
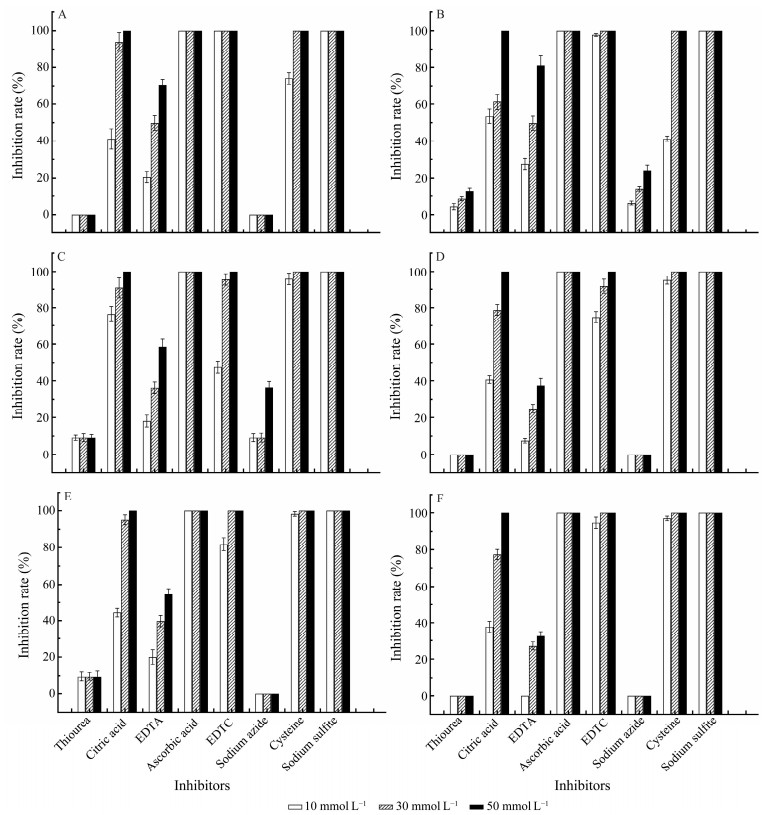
|
Fig. 4 Effects of inhibitors on the activity of isolated POs. The PO activity was assayed using L-DOPA (15 mmol L−1) as a specific substrate. A, C. farreri PO; B, H. discus hannai PO; C, 391-PO; D, 206-PO; E, 174-PO; F, s-PO. |
The function of POs has close relationship to the biochemical and enzymatic characteristics, and the characterization of POs has been reported in some seashells, such as the clam R. philippinarum, the scallop A. irradians, the Sydney rock oyster S. glomerata, and the Pacific oyster Crassostrea gigas (Cong et al., 2005; Aladaileh et al., 2007; Jiang et al., 2011; Luna-Acosta et al., 2011). Here, we isolated POs from the hemocytes of scallop C. farreri, abalone H. discus hannai, and calm S. subcrenata, and characterized POs based on the kinetic parameters, the effects of divalent metal ions and inhibitors on enzymatic activities.
The results of zymography showed that only one PO was detected in C. farreri and H. discus hannai, while four POs were detected in the S. subcrenata. The C. farreri PO (576 kDa) had a larger molecular mass than H. discus hannai PO (228 kDa) and S. subcrenata POs (391 kDa, 206 kDa, 174 kDa, < 67 kDa). Among the reported marine mollusk POs, the molecular mass of PO is parallel between C. farreri (576 kDa) and A. irradians (555 kDa) (Xing et al., 2012), H. discus hannai (228 kDa) and S. glomerata (219 kDa) (Aladaileh et al., 2007), S. subcrenata 391-PO (391 kDa) and mussel M. edulis (381 kDa) (Renwrantz et al., 1996), S. subcrenata s-PO (< 67 kDa) and Pacific oyster Crassostrea gigas (< 40 kDa) (Luna-Acosta et al., 2011). Column chromatography is a preferred method for protein isolation and mass spectrometry should be important. However, homogenization was not achieved, which could be caused by the complicated generation of PO. Since PO is produced by the proPO system and the content is very low in vivo (Monwan et al., 2017). It is a relatively complicated process and different forms of POs may be produced, which need to be fully studied. We have also tried de novo sequencing. However, the N-terminal of the POs was blocked. Additionally, conservation or specific genes for the laccase type PO in the three species have not been studied thoroughly.
All of the POs obtained in this study were capable of oxidizing L-DOPA, catechol and hydroquinone effectively, but failed to oxidize tyrosine. Based on the classification of POs (Luna-Acosta et al., 2017), we suggested that the three POs are all laccase-type phenoloxidase, similar to that of silkworm Bombyx mori (Yamazaki, 1972), oyster Crassostrea virginica (Jordan and Deaton, 2005), A. irradians (Jiang et al., 2011) and Plutella xylostella (Wang et al., 2018). Kinetic analysis indicated that the C. farreri PO, H. discus hannai PO, 206-PO, 174-PO and s-PO in S. subcrenata had higher affinity to L-DOPA and catechol than hydroquinone and dopamine, while the 391-PO of S. subcrenata had higher affinity to L-DOPA and hydroquinone than to catechol and dopamine. All of the POs had the highest affinity to L-DOPA. Considering the affinity from high to low, POs can be listed as s-PO, 174-PO, H. discus hannai PO, C. farreri PO, 206-PO and 391-PO.
The POs were inhibited by Fe2+ at all determined concentrations for the three species, which is also found in A. irradians (Jiang et al., 2011) and clam R. philippinarum (Jiang et al., 2012). The C. farreri PO was strongly stimulated in the presence of Ca2+ and Mg2+, similar to A. irradians (Xing et al., 2012), which may be due to the close relationship between the two species. In H. discus hannai, Mn2+ Cu2+, Zn2+ and Ca2+ inhibited the PO activity at all of the determined concentrations, absolutely different from C. farreri PO and S. subcrenata PO. The possible reasons may be that H. discus hannai and the other two species belong to two largely divergent classes. Furthermore, for C. farreri PO and 206-PO, Cu2+ had stimulative effects at 5 mmol L−1, and inhibitory effects at high concentrations. Meanwhile, for other four POs, Cu2+ had inhibitory effects at all concentrations. As we have known, Cu2+ is the active center of POs in most invertebrates. However, the extra copper ions, even at 5 mmol L−1, may inhibit the enzyme activity, which is also found in Ruditapes philippinarum (Jiang et al., 2012). Some studies speculated that metal ions may activate electrophile and nucleophile binding, then release electrons to modulate PO activity or divalent cations, which may change the secondary structure of certain peptides of PO to influence the activity (Feng et al., 2008; Zibaee et al., 2011). However, the detailed mechanisms behind this study require further investigation.
The common antioxidants including citric acid, ascorbic acid, cysteine, and sodium sulfite can strongly inhibit the activities of all of the POs obtained in this research. EDTA and DETC-a divalent cation and specific copper chelator can also inhibit the activities of POs. Meanwhile, sodium azide only inhibited the activities of H. discus hannai PO and 391-PO, while thiourea only showed inhibition to H. discus hannai PO, 391-PO and 174-PO. These results indicated that the construction of protein differed obviously among some POs.On the other hand, C. farreri PO, 206-PO and s-PO were relatively similar to each other and H. discus hannai PO was similar to 391-PO. Although POs are divided into three types according to the substrate specificity, the inhibitors do not have high specificity for the inhibition of the three types of POs. The mechanism of inhibiting POs of antioxidants, including citric acid, ascorbic acid, cysteine, sodium sulfite and thiourea, is causing a strong reduction reaction. EDTA and DETC can chelate Cu2+ in the PO molecule to change the active conformation of the enzyme, thereby producing inhibition effects, while sodium azide exerts an inhibitory effect through oxidation (Luna-Acosta et al., 2017).
In conclusion, POs were isolated from three cultured mollusk species, including scallop Chlamys farreri, abalone Haliotis discus hannai and clam Scapharca subcrenata. The biochemical characteristics of the separated enzymes were studied. Further study with gene cloning and monoclonal antibody production are expected to deeply investigate the function of POs.
AcknowledgementsThis research was supported by the Qingdao National Laboratory for Marine Science and Technology (No. QNLM 2016ORP0307), the National Key Research and Development Program of China (No. YFD0900504), the National Basic Research Program of China (No. 2012CB114405), and the Taishan Scholar Program of Shandong Province.
Aladaileh, S., Rodney, P., Nair, S. V., and Raftos, D. A.. 2007. Characterization of phenoloxidase activity in Sydney rock oysters (Saccostrea glomerata). Comparative Biochemistry and Physiology, Part B, 148(4): 470-480. DOI:10.1016/j.cbpb.2007.07.089 (  0) 0) |
Anjugam, M., Vaseeharan, B., Iswarya, A., Amala, M., Govindarajan, M., Alharbi, N. S., et al.. 2017. A study on β-glucan binding protein (β-GBP) and its involvement in phenoloxidase cascade in Indian white shrimp Fenneropenaeus indicus. Molecular Immunology, 92: 1-11. DOI:10.1016/j.molimm.2017.09.013 (  0) 0) |
Antonio, L. G., Maeda, M. A. N., Vargas, A. F., Ascencio, V. F., and Robles, M. M.. 2003. Phenoloxidase activity in larval and juvenile homogenates and adult plasma and haemocytes of bivalve molluscs. Fish and Shellfish Immunology, 15(4): 275-282. DOI:10.1016/S1050-4648(02)00165-1 (  0) 0) |
Bharathi, N., and Ramalingam, K.. 1983. Electrophoretic study of the enzyme phenoloxidase from the enzyme gland in the foot of Perna viridis Linnaeus. Journal of Experimental Marine Biology and Ecology, 70(2): 123-128. DOI:10.1016/0022-0981(83)90126-0 (  0) 0) |
Bris, C. L., Richard, G., Paillard, C., Lambert, C., Seguineau, C., Gauthier, O., et al.. 2015. Immune responses of phenoloxidase and superoxide dismutase in the manila clam Venerupis philippinarum challenged with Vibrio tapetis–Part Ⅰ: Spatio-temporal evolution of enzymes' activities post-infection. Fish and Shellfish Immunology, 42(1): 16-24. DOI:10.1016/j.fsi.2014.10.021 (  0) 0) |
Cong, R., Sun, W., Liu, G., and Fan, T.. 2005. Purification and characterization of phenoloxidase from clam Ruditapes philippinarum. Fish and Shellfish Immunology, 18(1): 61-70. DOI:10.1016/j.fsi.2004.06.001 (  0) 0) |
Feng, C., Song, Q., Lü, W., and Lu, J.. 2008. Purification and characterization of hemolymph prophenoloxidase from Ostrinia furnacalis (Lepidoptera: Pyralidae) larvae. Comparative Biochemistry and Physiology, Part B, 151(2): 139-146. DOI:10.1016/j.cbpb.2008.05.012 (  0) 0) |
Gu, Q., Zhou, S., Zhou, Y., Huang, J., Shi, M., and Chen, X.. 2019. A trypsin inhibitor-like protein secreted by Cotesia vestalis teratocytes inhibits hemolymph prophenoloxidase activation of Plutella xylostella. Journal of Insect Physiology, 116: 41-48. DOI:10.1016/j.jinsphys.2019.04.009 (  0) 0) |
Idakieva, K., Siddiqui, N. I., Meersman, F., De Maeyer, M., Chakarska, I., and Gielens, C.. 2009. Influence of limited proteolysis, detergent treatment and lyophilization on the phenoloxidase activity of Rapana thomasiana hemocyanin. International Journal of Biological Macromolecules, 45(2): 181-187. DOI:10.1016/j.ijbiomac.2009.04.022 (  0) 0) |
Jiang, J., Xing, J., and Zhan, W.. 2012. Purification and characterization of laccase-type phenoloxidase from the clam Ruditapes Philippinarum. Oceanologia et Limnologia Sinica, 43(2): 294-298 (in Chinese with English abstract). (  0) 0) |
Jiang, J., Xing, J., Sheng, X., and Zhan, W.. 2011. Characterization of Phenoloxidase from the Bay Scallop Argopecten irradians. Journal of Shellfish Research, 30(2): 273-277. DOI:10.2983/035.030.0212 (  0) 0) |
Jordan, P. J., and Deaton, L.. 2005. Characterization of phenoloxidase from Crassostrea virginica hemocytes and the effect of Perkinsus marinus on phenoloxidase activity in the hemolymph of Crassostrea virginica and Geukensia demissa. Journal of Shellfish Research, 24: 477-482. (  0) 0) |
Liu, G., Yang, L., Fan, T., and Cong, R.. 2006. Purification and characterization of phenoloxidase from crab Charybdis japonica. Fish and Shellfish Immunology, 20(1): 47-57. DOI:10.1016/j.fsi.2005.03.012 (  0) 0) |
Luna, A., Breitwieser, M., Renault, T., and Thomas, H.. 2017. Recent findings on phenoloxidases in bivalves. Bioorganic & Medicinal Chemistry Letters, 122: 5-16. DOI:10.1016/j.marpolbul.2017.06.031 (  0) 0) |
Luna, A., Thomas, H., Amari, M., Rosenfeld, E., Bustamante, P., and Fruitier, I.. 2011. Differential tissue distribution and specificity of phenoloxidases from the Pacific oyster Crassostrea gigas. Comparative Biochemistry and Physiology, Part B, 159: 220-226. DOI:10.1016/j.cbpb.2011.04.009 (  0) 0) |
Ma, T. H. T., Benzie, J. A. H., He, J. G., Sun, C. B., and Chan, S. F.. 2014. PmPPAF is a pro-phenoloxidase activating factor involved in innate immunity response of the shrimp Penaeus monodon. Developmental & Comparative Immunology, 44: 163-172. DOI:10.1016/j.dci.2013.12.007 (  0) 0) |
Monwan, W., Amparyup, P., and Tassanakajon, A.. 2017. A snakelike serine proteinase (PmSnake) activates prophenoloxidaseactivating system in black tiger shrimp Penaeus monodon. Developmental & Comparative Immunology, 67: 229-238. DOI:10.1016/j.dci.2016.09.016 (  0) 0) |
Muñoz, P., Meseguer, J., and Esteban, M., Á.. 2006. Phenoloxidase activity in three commercial bivalve species. Changes due to natural infestation with Perkinsus atlanticus. Fish and Shellfish Immunology, 20(1): 12-19. DOI:10.1016/j.fsi.2005.02.002 (  0) 0) |
Palmer, C. V., Bythell, J. C., and Willis, B. L.. 2011. A comparative study of phenoloxidase activity in diseased and bleached colonies of the coral Acropora millepora. Developmental & Comparative Immunology, 35(10): 1098-1101. DOI:10.1016/j.dci.2011.04.001 (  0) 0) |
Panigrahi, A., Sivakumar, M. R., Sundaram, M., Saravanan, A., Das, R. R., Katneni, V. K., et al.. 2020. Comparative study on phenoloxidase activity of bio floc-reared Pacific white shrimp Penaeus vannamei and Indian white shrimp Penaeus indicus on graded protein diet. Aquaculture, 518: 734654. DOI:10.1016/j.aquaculture.2019.734654 (  0) 0) |
Renwrantz, L., Schmalmack, W., Redel, R., Friebel, B., and Schneeweiß, H.. 1996. Conversion of phenoloxidase and peroxidase indicators in individual haemocytes of Mytilus edulis specimens and isolation of phenoloxidase from haemocyte extract. Journal of Comparative Physiology B, 165: 647-658. DOI:10.1007/BF00301133 (  0) 0) |
Rosenberg, G.. 2014. A new critical estimate of named specieslevel diversity of the recent mollusca. American Malacological Bulletin, 32(2): 308-322. DOI:10.4003/006.032.0204 (  0) 0) |
Sajid, R., Saeed, A., Saddique, G., Ali, C. P., Ali, L. F., Abbas, Q., et al.. 2018. Synthesis of sulfadiazinyl acyl/aryl thiourea derivatives as calf intestinal alkaline phosphatase inhibitors, pharmacokinetic properties, lead optimization, Lineweaver-Burk plot evaluation and binding analysis. Bioorganic & Medicinal Chemistry, 26(12): 3707-3715. DOI:10.1016/j.bmc.2018.06.002 (  0) 0) |
Stączek, S., Zdybicka-barabas, A., Pleszczyńska, M., Wiater, A., and Cytryńska, M.. 2020. Aspergillus niger α-1, 3-glucan acts as a virulence factor by inhibiting the insect phenoloxidase system. Journal of Invertebrate Pathology, 171: 1-5. DOI:10.1016/j.jip.2020.107341 (  0) 0) |
Tassanakajon, A., Rimphanitchayakit, V., Visetnan, S., Amparyup, P., Somboonwiwat, K., Charoensapsri, W., et al.. 2018. Shrimp humoral responses against pathogens: Antimicrobial peptides and melanization. Developmental & Comparative Immunology, 80: 81-93. DOI:10.1016/j.dci.2017.05.009 (  0) 0) |
Thomas, G. H., Gagnaire, B., Bado, N. A., Bouilly, K., Lapègue, S., and Renault, T.. 2009. Detection of phenoloxidase activity in early stages of the Pacific oyster Crassostrea gigas (Thunberg). Developmental & Comparative Immunology, 33: 653-659. DOI:10.1016/j.dci.2008.11.011 (  0) 0) |
Wang, L., Qiu, L., Zhou, Z., and Song, L.. 2013. Research progress on the mollusc immunity in China. Developmental & Comparative Immunology, 39: 2-10. DOI:10.1016/j.dci.2012.06.014 (  0) 0) |
Wang, Z., Hu, R., Ye, X., Huang, J., Chen, X., and Shi, M.. 2018. Laccase 1 gene from Plutella xylostella (PxLac1) and its functions in humoral immune response. Journal of Insect Physiology, 107: 197-203. DOI:10.1016/j.jinsphys.2018.04.001 (  0) 0) |
Xing, J., Jiang, J., and Zhan, W.. 2012. Phenoloxidase in the scallop Chlamys farreri: Purification and antibacterial activity of its reaction products generated in vitro. Fish and Shellfish Immunology, 32(1): 89-93. DOI:10.1016/j.fsi.2011.10.025 (  0) 0) |
Xing, J., Lin, T., and Zhan, W.. 2008. Variations of enzyme activities in the haemocytes of scallop Chlamys farreri after infection with the acute virus necrobiotic virus (AVNV). Fish and Shellfish Immunology, 25(6): 847-852. DOI:10.1016/j.fsi.2008.09.008 (  0) 0) |
Yamazaki, H. I.. 1972. Cuticular phenoloxidase from the silkworm, Bombyx mori: Properties, solubilization, and purification. Insect Biochemistry and Molecular Biology, 2: 431-444. DOI:10.1016/0020-1790(72)90023-6 (  0) 0) |
Yang, B., Pu, F., Li, L., You, W., Ke, C., and Feng, D.. 2017. Functional analysis of a tyrosinase gene involved in early larval shell biogenesis in Crassostrea angulata and its response to ocean acidification. Comparative Biochemistry and Physiology, Part B: Biochemistry and Molecular Biology, 206: 8-15. DOI:10.1016/j.cbpb.2017.01.006 (  0) 0) |
Yang, L., Wang, Z., Zuo, H., Geng, R., Guo, Z., Niu, S., et al.. 2021. The LARK protein is involved in antiviral and antibacterial responses in shrimp by regulating humoral immunity. Developmental & Comparative Immunology, 114: 103826. DOI:10.1016/j.dci.2020.103826 (  0) 0) |
Yao, T., Zhao, M., He, J., Han, T., Peng, W., and Zhang, H.. 2019. Gene expression and phenoloxidase activities of hemocyanin isoforms in response to pathogen infections in abalone Haliotis diversicolor. International Journal of Biological Macromolecules, 129: 538-551. DOI:10.1016/j.ijbiomac.2019.02.013 (  0) 0) |
Zibaee, A., Bandani, A. R., and Malagoli, D.. 2011. Purification and characterization of phenoloxidase from the hemocytes of Eurygaster integriceps (Hemiptera : Scutelleridae). Comparative Biochemistry and Physiology, Part B, 158(1): 117-123. DOI:10.1016/j.ijbiomac.2019.02.013 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


