2) Pilot National Laboratory for Marine Science and Technology, Qingdao 266000, China;
3) National Vegetable Quality Standard Center, Shouguang 262700, China;
4) College of Food Science and Technology, Hebei Agricultural University, Baoding 071000, China;
5) Qingdao Institute of Marine Bioresources for Nutrition & Health Innovation, Qingdao 266000, China
Dietary proteins provide the source of energy and ami no acids for the body (Ma et al, 2017). During the nutritional evaluation of dietary proteins, not only quantity, but also quality should be considered. The quality measures the content and pattern of essential amino acids in the food proteins as well as the degree to which the body digests and utilizes, while the quantity measures the amount of protein in the food. Dietary protein quality is commonly defined by the bioavailability of essential amino acids, a function of amino acid composition and protein digestibility (Szepe et al., 2021). Previous studies on the quality of protein main- ly focused on the protein content and essential amino acid patterns, while the digestion, absorption and utilization of the proteins were often ignored. The amino acid composition of some proteins can also be affected by the protein sources (animals, plants), nonprotein components (dietary fiber, trypsin inhibitors), and physiological factors. The degree of digestion, absorption, and utilization of these proteins in the body may be low, resulting in low nutritional value. Protein digestibility refers to the ratio of nitrogen absorbed from protein to nitrogen intake, which reflects the degree of food proteins decomposed and absorbed by the digestive enzymes (Ashwath Kumar et al., 2019). Therefore, when evaluating the quality of protein, the digestibility of the protein should be measured and analyzed, which is of great significance for the comprehensive evaluation of food nutrition. Differences in dietary structure may lead to differences in human nutritional intake, which will directly af- fect human health (Scheijen et al., 2018). If the protein can meet the needs of the human body, it can be considered as a new source of protein.
The method for measuring protein digestibility based on the direct uptake of protein samples in animals are called the in vivo protein digestibility assay. It is a good method to use animal experiments to determine digestibility in vivo. The rat fecal nitrogen balance experiment can provide the best human protein digestibility index (de Oliveira Sousa et al., 2011). The main steps of digesting a protein in the body are as follows. First, the protein is digested by the pepsin in the stomach and then digested by the protease in the intestine. Subsequently, the protein components that cannot be digested and absorbed, together with a small amount of fecal metabolism nitrogen of microorganisms in the body and exfoliated cells are excreted from the feces (fecal nitrogen) (Lee, 1995). Fecal metabolic nitrogen produced by rats under the condition of no protein intake is the endogenous nitrogen in feces. The protein digestibility measured by subtracting endogenous nitrogen from feces is close to the actual value of the actual digestibility of protein (Wong et al., 2004; Yu et al., 2018). The protein quality of cookies developed for undernourished adolescents was investigated by calculating the biological value, Net Protein Utilization, Protein Efficiency Ratio and True Protein Digestibility to indicate that the product had better quality (Szepe et al., 2021; Cinu et al., 2022).
The total abundance, biomass and production of Antarctic krill (Euphausia superba) are large (Atkinson et al., 2009). Protein can directly affect energy metabolism and protein metabolism (Bjorndal et al., 2014). Therefore, Antarctic krill has been regarded as the largest animal source of protein in the whole world, and its proteins contain all essential amino acids of human beings. Antarctic krill contains 16.31% of crude protein in its nutrition components, and the essential amino acids (EAA) account for 25.88% of the total amino acids (Wang et al., 2021). The krill products meet the FAO/WHO protein requirement for human, and their biological values are higher than casein (Chen et al., 2009; Li et al., 2020). In this study, a high-quality protein- casein was used as a control to analyze the bioavailability and nutritional metabolism of the proteins in krill meat (KM), krill surimi (KS), ordinary krill powder (OKP), skim krill powder (SKP), and defluorination krill powder (DKP) prepared from frozen Antarctic krill by the rat growth test. The digestibility and absorption of five Antarctic krill product proteins were investigated to provide a theoretical basis for the development and rational utilization of the Antarctic krill protein.
2 Materials and Methods 2.1 Materials and ReagentsAntarctic krill (Euphausia superba) was captured in July 2016 by China's Aquatic Products Co., Ltd. After being captured, it was extruded and frozen on a ship, and the specification was 20 kg per plate. It was transported to the laboratory and stored at −18℃ for future analyses. All the chemicals and reagents were of analytical grade or with higher quality.
2.2 Preparation of the Antarctic Krill ProductsThe frozen Antarctic krill were thawed, husked, and freezedried to obtain krill meat. The krill surimi was obtained by rinsing the Antarctic krill, followed by husking, rin- sing, draining, and then freeze-drying; the rinsing water temperature should be controlled at about 10℃ (to ice cooling). The ordinary krill powder was obtained by thawing the Antarctic krill at 100℃ and steam-treated by adding the the water with a ratio of 2:1 (w/v). Then it was extruded, drained, hot air-dried for 8 h at 60℃, and finally crushed (Liu et al., 2018). The ordinary krill powder was degreased with 95% ethanol (1/10, w/v) degreasing treatment for 10 h, filtered to remove the alcohol solution, and finally the residue was dried in cold air for 10 h to obtain skim krill pow- der (Wang et al., 2019). Skim krill powder was treated with 0.3 mol L−1 HCl (1/10, w/v) for 1 h and then centrifuged. The precipitate was treated repeatedly thrice, and was dried to obtain the defluorinated krill powder (Wang, 2013). The prepared krill product was stored in a refrigerator at −20℃ until further analyses. The protein contents of these five krill products were determined by Kjeldahl method (Li et al., 2019).
2.3 Animal StudyThe approach of feeding required for rat growth experiments was prepared using the method reported by Wong et al. (2004) with a protein content of 10%. The diet composition uses the AIN-93G standard, casein, KM, KS, OKP, SKP, and DKP as the sole protein source of the six experimental groups respectively. They were added to the diet at a final protein concentration of 10% to prepare the diets with different protein resources. The proteome-free diet (PF) replaced starch with 10% protein in the 10% LP diet. The required feed ingredients were purchased from Hua Fukang Biotechnology Co., Ltd. (Beijing, China).
Forty Sprague-Dawley rats with an average weight of 90 g ± 10 g were purchased from the Weitonglihua Experimental Animal Technology Co., Ltd. (Beijing, China). The rats were housed in cages at a room temperature of 21 – 25℃ under 55% ± 10% humidity conditions with a 12 h: 12 h light: dark cycle. All the rats were allowed free access to the diets and water, limiting the food supply to 20 g d−1. All aspects of the animal studies were conducted according to guidelines provided by the Ethical Committee of Experimental Animal Care of Ocean University of China (Qingdao, China), and the experimental procedures were approved by the Animal Care and Ethics Committee (No. AE20171023-2).
The digestion and absorption of proteins were carried out following the method of Wong et al. (2004). On the first three days, all rats received a casein-controlled diet. After this period, the rats were randomly divided into seven groups with PF, LP, KM, KS, OP, SKP, and DKP pectively (n = 10). The normal control group was fed a casein- controlled diet, the no-protein group was fed a protein-free diet, and the remaining five groups were fed an Antarctic krill product protein. The first three days of the respective diets were performed for metabolic experiments, during which fresh feces and urine samples were collected. The fecal and urine samples were immediately frozen in liquid nitrogen and stored at −80℃ for further analysis. After the end of the metabolic test, each group of animals was fed until 28 days. Food intake was recorded daily, and the body weight and body length were measured twice a week.
After feeding and fasting for 8 h, the rats were sacrificed by 10% chloral hydrate anesthesia and blood was collected by executing a cardiac puncture. The liver, kidneys, and fat pad were excised and weighed, colon tissues were collected, and then they were frozen with liquid nitrogen and stored at −80℃.
2.4 Determination of Nutritional Metabolism IndexThe feed nitrogen, fecal nitrogen, and urine nitrogen were determined by the Kjeldahl method for nitrogen determination (Zou et al., 2012; Zhang et al., 2016). According to the results of the nitrogen metabolism test on the 3rd day, various indices of digestion and absorption were calculated. The calculation formula is as follows:
| $ \text { Nitrogen retention }(\mathrm{NR}): \mathrm{NR}=\mathrm{I}-(\mathrm{FN}+\mathrm{UN}) \text {, } $ | (1) |
| $ \text { True digestibility (TD): } \mathrm{TD}(\%)=\frac{\mathrm{I}-(\mathrm{FN}-\mathrm{EFN})}{\mathrm{I}} \times 100 \text {, } $ | (2) |
| $ \text { Net protein utilization }(\mathrm{NPU}) \text { : NPU }(\%)=\frac{\mathrm{I}-(\mathrm{FN}-\mathrm{EFN})-(\mathrm{UN}-\mathrm{EUN})}{\mathrm{I}} \times 100 \text {, } $ | (3) |
Amino acid score corrected by protein digestibility (PDCAAS):
| $ \text { PDCAAS }=\text { True digestibility } \times \text { Minimum amino acid score, } $ | (4) |
| $ \text { Protein efficiency ratio }(\mathrm{PER}): \quad \mathrm{PER}=\frac{\mathrm{BW}}{\mathrm{I} \times 6.25} \text {, } $ | (5) |
Net protein ratio (NPR):
| $ N P R=\frac{\text { Average weight gain of animals in the test group }(\mathrm{g})+\text { Mean weight loss of animals without protein }- \text { Based diet }(\mathrm{g})}{\text { Food protein ingested by experimental animals }(\mathrm{g})}, $ | (6) |
where I is nitrogen intake (g), FN is fecal nitrogen, UN is urine nitrogen, EFN is endogenous fecal nitrogen in the rats of nitrogen-free group (g), EUN is endogenous urinary nitrogen in the rats of nitrogen-free group (g), BW is rats body weight gain (g).
2.5 Analysis of Biochemical Indicators in BloodThe plasma levels of total protein (TC), albumin (ALB), hemoglobin (HGB), AKP energy, calcium, and creatinine content, total cholesterol (TC), triglyceride (TG), high-den- sity lipoprotein cholesterol (HDL-C), low-density lipopro- tein cholesterol (LDL-C), alanine aminotransferase (ALT), and glutamic-oxaloacetic transaminase (GOT) concentrations in rats were determined using analytical reagent kits (Nanjing Jiancheng Bioengineering Institute, China).
2.6 Liver and Colonic HistomorphologyThe liver and colon tissue samples of rats were embedded in paraffin. All the 9 samples were cut into 5 μm slices. Then the samples were stained with hematoxylin-eosin. The sections were viewed under an optical microscope at × 400 magnification. Olympus light microscope BX41 (Olympus, Japan) was used for observing the villus length and crypt depth.
2.7 Determination of Liver Fatty AcidsThe extraction of rat liver lipids was carried out according to the method of Folch et al. (Zou et al., 2012) and was improvised. About 0.1 g of the liver was homogenized in a stoppered test tube and was shaken in a 37℃ water bath for 45 min to undergo full leaching. After cooling, the leachate was filtered into a stoppered tube, and the filtrate volume was recorded. Double-distilled water that was equivalent to 20% of the volume of the filtrate was added and mixed into an emulsion. The mixture was allowed to stand at room temperature overnight. The lower layer was col- lected, then dried under nitrogen, and dissolved in 10 mL of petroleum ether, with shaking. The mark was noted and stored at −40℃.
Methylation of liver lipids was performed according to the method of Cui et al. (2014). Liver fat (1 mL) was added to a methyl esterified tube, while 100 µg C15:0 (triglyceride) was used as an internal standard, and 2 mL of the methyl esterified reagent (hydrochloric acid: methanol = 1:5) was subjected to methyl esterification. After cooling, the layers were extracted with n-hexane. About 1 mL of the upper layer solution was concentrated by vacuum centrifugation and reconstituted with 50 μL of n-hexane, and 1 μL was taken for GC analysis.
2.8 Statistical AnalysisAnalyses were evaluated by the one-way ANOVA follow- ed by Duncan's multiple comparison test using the SPSS 18.0. All results are expressed as mean ± SEM (standard error of the mean, indicated by error bars). When variances were not homogeneous, the non-parametric Kruskal-Wallis test and the Mann-Whitney U test were conducted for pairwise comparisons. The difference was considered significant when P < 0.05.
3 Results 3.1 Food Intake, Body Weight Organ Indicators ParametersDietary protein intake is a background material or reference material for evaluating the nutritional status of protein in the body, and it is combined with the index of nutritional status of the protein in the body to assess the nu- tritional status of the protein in the body correctly (Tieland et al., 2018). The food intake in different groups fed with different diets is shown in Fig.1(A).
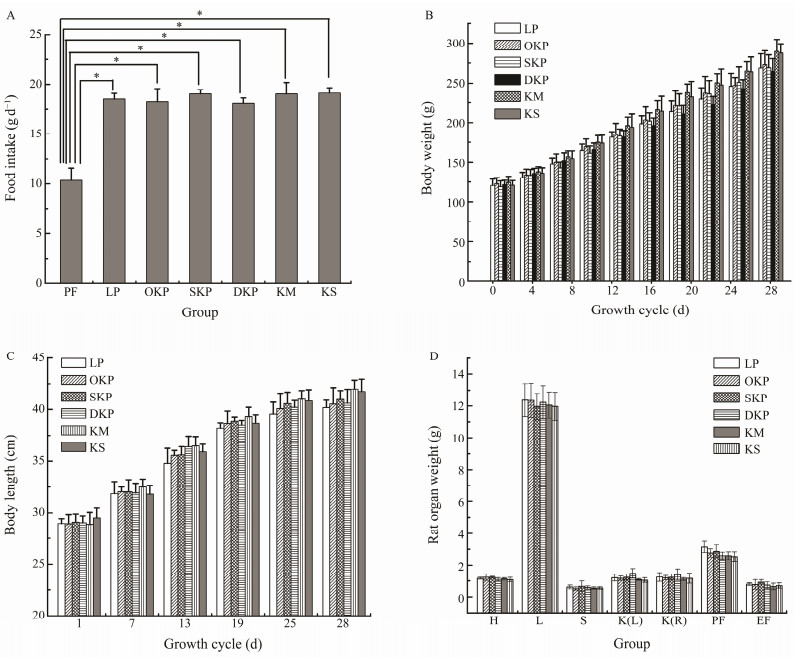
|
Fig. 1 Effects of different protein on the food intake (A), the body weight gain (B), the body length (C) and the organs weight (D) of the rats fed the 10% LP (LP group), 10% KM (KM group), 10% KS (KS group), 10% OKP (OKP group), 10% SKP (SKP group) and 10% DKP (DKP group) for 4 weeks. H, heart; L, liver; K(L), kidney (left); K(R), kidney (right); S, spleen; PF, perirenal fat; EF, epididymal fat. Values were expressed as mean ± SD (n = 6). Significant differences were denoted by * (P < 0.05). |
As described, the rats in the nitrogen-free group had the lowest food intake, only (10.35 ± 0.68) g d−1, which was related to long-term protein deficiency leading to the loss of appetite. The food intake of the OKP, SKP, DKP, KM, and KS groups ((18.22 ± 1.32), (19.04 ± 0.41), (18.06 ± 0.57), (19.02 ± 1.16), (19.12 ± 0.53) g d−1, respectively) (P < 0.05) was significantly higher than the nitrogen-free group, but not significantly different from the casein group. This also indicated that the flavor of the Antarctic krill protein itself and the changes of the protein flavor during the processing did not affect the appetite of the rat.
The body measurement of the experimental animals is an important reference for identifying the nutritional status of proteins. The body measurement indicators used in the as- sessment of growth and development status include weight, body length, etc. (Kurtoglu et al., 2012). The effects of casein and the proteins from the different Antarctic krill products on the bodyweight of rats were studied, and the results are shown in Fig.1(B).
As shown in Fig.1(B), in the first three days, to adapt the weaned rats to the environment and food, the basal feed containing casein was fed, and the weight of all the rats increased from (90 ± 10.87) g to (121 ± 3.62) g. Three days later, the feed supplemented with different proteins was administered. The bodyweight of the rats showed a difference, and the difference in the nitrogen-free group was the most significant (P < 0.05) compared to the casein group. The bodyweight of the nitrogen-free group rats showed a down- ward trend with time, reduced from (120.26 ± 3.94) g to (97.38 ± 1.19) g after feeding for 10 days. The weight of the KM group rats increased the fastest, from (124.86 ± 4.52) g to (289.61 ± 3.15) g, followed by the KS group, which increased from (120.91 ± 4.52) g to (287.87 ± 11.62) g, and the weight of OKP group rats increased from (123.02 ± 4.26) g to (273.2 ± 7.43) g. The weight gain of the rats was significantly higher (P < 0.05) than that of the casein group (from (120.75 ± 4.26) g increased to (268.82 ± 7.43) g). The trend of the weight gain of the SKP and DKP groups was consistent with that of the casein group. This may be related to the fact that degreasing and defluorination have changed the structure of the protein during product processing, thereby affecting the degree of protein utilization by the body. However, it can be seen from the figures that they are the only proteinogens in the diet, respectively, which did not cause protein malnutrition in rats, and there was no significant difference compared to the control group with casein as the sole proteinogen.
From the perspective of traditional nutrition, casein has the highest food conversion rate and is a high-quality protein that promotes growth and development (Huber et al., 2018). The changes in body weight of the rats indicated that the effect of SKP and DKP on promoting growth and development is similar to casein. The growth-promoting effects of KM, KS, and OKP are better than that of casein, and their nutritional value is higher.
The effect of the proteins from the five Antarctic krill products on the body length of rats was studied in the experiment. Fig.1(C) showed the growth difference of rats fed different Antarctic krill protein products. As shown in Fig.1(C), the body length of rats fed with the proteins from five Antarctic krill products increased with time, and the increases were significantly better than those of the casein group. Among them, KM and KS were more effective than others (P < 0.05).
The effect of different proteins on the organ weights of rats is shown in Fig.1(D). Changes in the organ weight of the rats were important evidence stating the role of proteins in rat growth and development. Compared to the casein group, there was no abnormality in the visceral tissues of rats who were fed the protein of five Antarctic krill products. It means that krill proteins have the effect on rat organs as casein.
3.2 NPU, NPR, TD, PDCAAS Indicators ParametersThe NPU, NPR, TD, and PDCAAS in rats fed different diets for 4 weeks are presented in Fig.2(B). CinuVarghese et al. (2022) investigated the protein quality of cookies developed for undernourished adolescents by calculating the Biological Value, Net Protein Utilization and True Protein Digestibility to indicate that the products had better qua- lity (Szepe et al., 2021). The TD of KM was highest for 97.79%, followed by 96.67% for KS, 95.2% for OKP, and 94.7% for SKP, all were significantly (P < 0.05) higher than 92.2% of casein. DKP has the lowest TD (93.8%) and is still higher than casein. There was no significant (P < 0.05) difference in the nitrogen intake between the rats fed An- tarctic krill protein and casein, but the protein from the An- tarctic krill product significantly (P < 0.05) reduced the urinary nitrogen and fecal nitrogen excretion in rats, and significantly improved in NPU group. The PDCAAS method is the most effective method for evaluating food proteins because it takes into account the amino acid score and digestibility required by the human body (Stodkilde et al., 2018). Hughes et al. (2011) analyzed the protein quality of four kinds soy products by using PDCAAS. As shown in Fig.2(B), the processing of products such as heating, degreasing, and defluorination did not show significantly (P < 0.05) affect the PDCAAS value of krill protein, which was 0.67 ± 0.04. There was also no significant (P < 0.05) difference between the PDCAAS value of the protein from the krill product and casein.
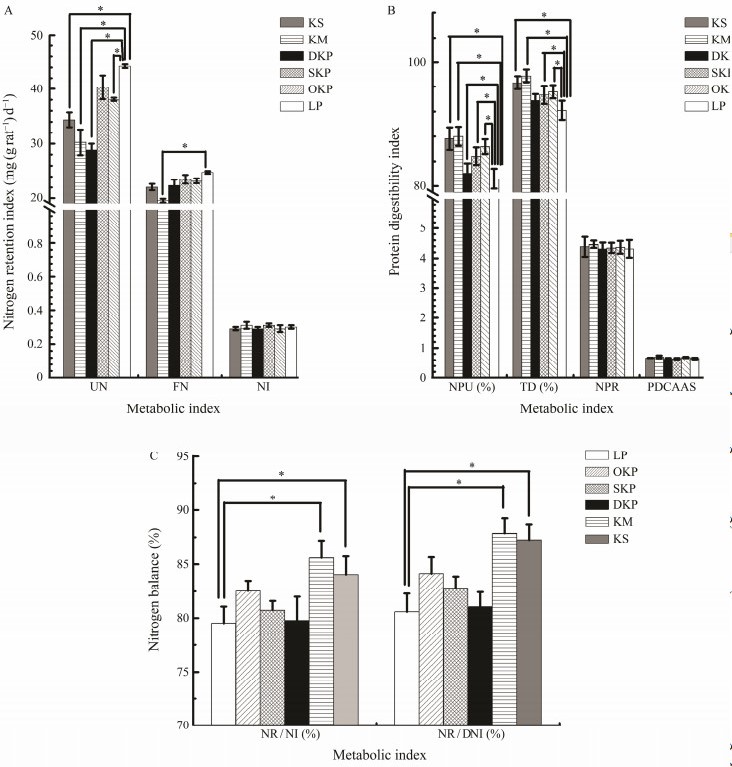
|
Fig. 2 NI, FN, UN, PDCAAS, NPR, TD, NPU, NR/DI and NR/DNI in rats fed the 10% LP (LP group), 10% KM (KM group), 10% KS (KS group), 10% OKP (OKP group), 10% SKP (SKP group) and 10% DKP (DKP group) for 4 weeks. Va- lues were expressed as mean ± SD (n = 8). Different letters in the same line indicate significant differences (P < 0.05). |
As shown in Figs.2(A) and (C), there was no significant difference in the NI of rats ingested by krill protein products compared with casein (P < 0.05), but krill protein products significantly reduced the excretion of UN and FN, and significantly increased the NPU. These data indicated that the protein availability of the krill protein products is higher than casein, and the results of KS and KM groups were more significant (P < 0.01).
3.3 Rat Blood Indicators ParametersPathological changes in any constituents in the blood will affect tissues and organs throughout the body; conversely, changes in tissues or organs will also cause changes in blood components, so hematological analysis is very helpful for understanding the health of the body (Stogdale, 1981; Lo et al., 2011). The levels of plasma TP, ALB, HGB, HDL- C, LDL-C, TC, TG, and blood indexes in rats fed different diets for 28 days are shown in Fig.3.
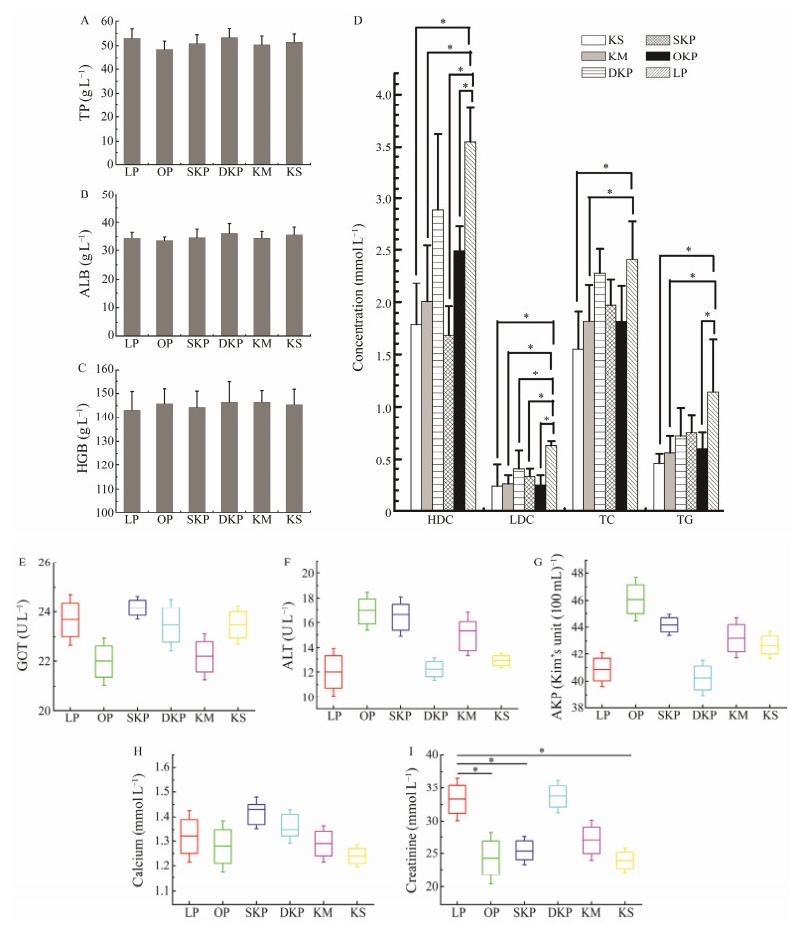
|
Fig. 3 Levels of plasma TP (A), ALB (B), HGB (C), HDL-C, LDL-C, TC and TG (D), GOT(E), ALT (F), AKP energy (G), calcium content (H), creatinine content (I) in rats fed the 10% LP (LP group), 10% KM (KM group), 10% KS (KS group), 10% OKP (OKP group), 10% SKP (SKP group) and 10% DKP (DKP group) for 4 weeks. Values were expressed as mean ± SD (n = 6). Significant differences were denoted by * (P < 0.05). |
It is generally believed that the contents of serum total protein and albumin are related to the anabolic metabolism of proteins in the body, which can reflect the deposition of proteins in tissues and organs to a certain extent (Williams Stahly et al., 1997; Litvak et al., 2013). Xia et al. (2003) reported that when the serum total protein and albumin content increased, the piglet's digestion and absorption, protein metabolism strengthened, and its immunity were improved. In contrast, malabsorption caused by chronic protein deficiency or chronic intestinal disorders can lead to low total protein levels. In addition, albumin content will be significantly reduced in the absence of protein, and an important reason for the body's edema is lack of albumin in the body.
The results are shown in Fig.3(A) and Fig.3(B) show that the total protein and albumin content of rats fed with the different diets were not significantly different (P < 0.05) in comparison with the high-quality protein-casein-fed rats (53.08 g L−1, 34.48 g L−1), which indicated that the protein from the Antarctic krill products did not affect the absorption and metabolism of protein in rats, and its absorption and metabolism in the body was comparable to casein. Therefore, all five Antarctic krill products were found to be capable of providing the rats with nitrogen sources for protein absorption and metabolism.
The content of hemoglobin can represent the hemato- poietic function of the body to a certain extent (Wei et al., 2018). As shown in Fig.3(C), the hemoglobin content in rats fed with the proteins from the Antarctic krill products was mildly higher than that in rats fed high-quality proteincasein (143.2 g L−1), followed by the SKP group for 144.37 g L−1, the KS group for 145.58 g L−1, the OKP group for 145.86 g L−1, the DKP group for 146.46 g L−1 and the KM group at 146.52 g L−1. This indicated that the proteins from the Antarctic krill did not affect the hematopoietic function of rats, and its hematopoietic capacity was slightly better than casein.
As shown in Fig.3(D), Fig.3(G), Fig.3(H), compared with casein, the Antarctic krill product protein significantly reduced (P < 0.05) the high and low-density cholesterol and triglyceride in the rat serum, and reduced the urea nitrogen content. In addition, the Antarctic krill protein had no significant effect on AKP activity and calcium content in rats (P < 0.05).
The liver is not only an important organ for the metabolism of proteins, lipids, and sugars in animals, but also an important organ for the body to secrete bile (Wei et al., 2018). Serum alanine aminotransferase, triglycerides, total cholesterol, total protein, etc. are important indicators for evaluating liver damage. When the liver is damaged, serum alanine aminotransferase can be increased (Shim et al., 2018).
3.4 Liver Histomorphology and Total Fatty Acid Composition in LiverThe liver sections of rats fed different diets for four weeks are presented in Fig.4. Among them, the liver big lobe and liver cells of the rats fed with casein did not show degenerations or necrosis, or the structure of the hepatic junction area was not abnormal. No fat turbidity, degeneration, and hepatocyte degeneration and necrosis were found, and no lipid droplets were observed in the cytoplasm of the liver big lobe and liver cells of the rats in the OKP, SKP, DKP, and KS groups. The central hepatic vein and the structure of the hepatic junction area were also normal.
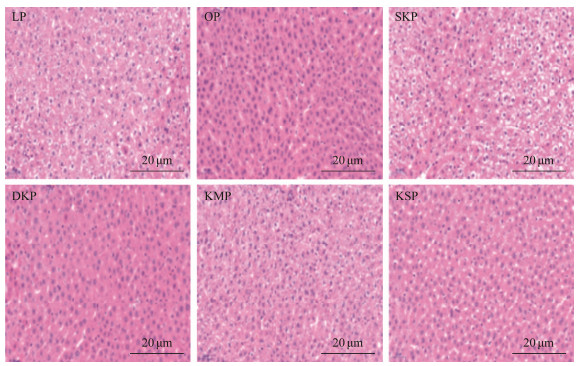
|
Fig. 4 HE staining of the liver of rats fed with different protein diets. |
The liver lobe and liver cells of rats in the KM group showed mild turbidity, and mild steatosis was also observed. However, no hepatocyte degeneration or necrosis was found, and no abnormalities were found in the liver central structure. Suspicious adipocyte infiltration and lymphocytic foci were observed between the large leaves of the liver of most animals in each experimental group (a common spontaneous disease in experimental rats) (Hanley et al., 2017).
As shown in Fig.4, the structure of the liver big leaf of the rats in each experimental group was normal. The ar- rangement of hepatocyte cords was normal and there was no dilatation or congestion of the hepatic sinus. Therefore, the experimental results show that the Antarctic krill proteins do not cause pathological damage to the rat liver, and they can improve liver steatosis and reduce lipid-containing vacuoles.
The fat contents in the liver of rats fed different diets are shown in Table 1. Compared to the casein group, the ratio of arachidonic acid in the total liver lipids of rats fed with Antarctic krill proteins decreased significantly (P < 0.05), while the ratios of EPA and DHA increased significantly (P < 0.01), which suggested that feeding the Antarctic krill protein increased liver lipid EPA and DHA levels in rats.
|
|
Table 1 Effects of Antarctic krill proteins on total fatty acid composition in rat liver |
The colon sections of rats fed different diets for four weeks are presented in Fig.5(A). The morphological observation of colon tissue showed that compared to the caseinfed rats, the colonic structure of rats fed with the Antarctic krill protein was complete, the villi were slender and closely arranged, and there was no significant difference in their morphology (P < 0.05). From Fig.5(A), the cells of each layer of the colon of the rats in each experimental group were arranged normally, and no bleeding and edema were observed in the lamina propria and muscular. Intestinal mucosa epithelial cells showed no obvious degeneration, necrosis, or ulcer formation. Therefore, the experimental results show that the proteins from the Antarctic krill products do not cause pathological damage to the colon of the rats.
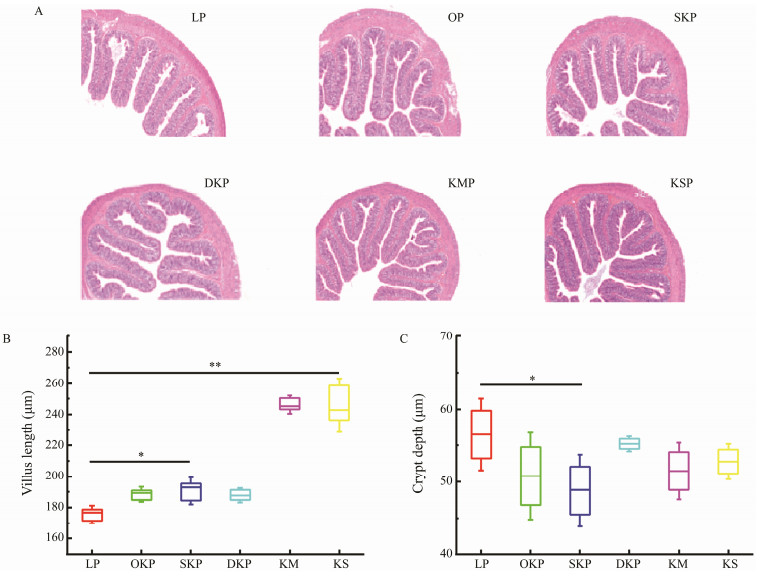
|
Fig. 5 A photomicrograph of colon histological sections (original magnification × 400) stained with hematoxylin-eosin (A), the villus height (B) and crypt depth (C) of colonic tissue in rats fed the 10% LP (LP group), 10% KM (KM group), 10% KS (KS group), 10% OKP (OKP group), 10% SKP (SKP group) and 10% DKP (DKP group) for 4 weeks. Values were expressed as mean ± SD (n = 8). Significant differences were denoted by * (P < 0.05). |
The morphological observation of colon tissue showed that compared to the casein-fed rats, the colonic structure of rats fed with the Antarctic krill protein was complete, the villi were slender and closely arranged, and there was no significant difference in their morphology (P < 0.05). From Fig.5(A), the cells of each layer of the colon of the rats in each experimental group were arranged normally, and no bleeding and edema were observed in the lamina propria and muscular. Intestinal mucosa epithelial cells showed no obvious degeneration, necrosis, or ulcer formation. There- fore, the experimental results showed that the proteins from the Antarctic krill products do not cause pathological dam- age to the colon of the rats.
The length of the colonic villi and the depth of the crypt were measured using the image processing software Case Viewer, and the ratio (V/C value) was calculated. The results are shown in Figs.5(B) and 5(C). As shown in Figs. 5(B) and 5(C). Compared to the normal group, the rats ingesting the proteins from the Antarctic krill product had increased villus length and increased V/C value. Among them, rats in the KM group showed significantly increased V/C values (P < 0.01). Therefore, the experimental results show that ingesting these five Antarctic krill product proteins can improve intestinal digestion, absorption, immune defense, and other comprehensive functional damage, and the effect is better than that of casein.
4 DiscussionOur results of the rat balance tests indicated that the proteins in these five Antarctic krill products could significantly (P < 0.05) promote the growth and development of rats when compared to casein, a well the recognized high-quality animal protein (Fernandez-Tome et al., 2017; Thakur, et al., 2020). The flavor changes of the Antarctic krill protein during processing did not affect the appetite, protein absorption, and metabolism of the rat. The TD of the five Antarctic krill products were all higher than 92.2% of casein, the highest TD of KM was 97.79%, followed by 96.67% for KS, 95.2% for OKP, 94.7% for SKP, and 93.76% for DKP. There was no significant difference in the nitrogen intake between the rats fed Antarctic krill protein and casein (P < 0.05), but the Antarctic krill product protein significantly reduced the urinary nitrogen and fecal nitrogen excretion, and significantly improved NPU in rats. Some studies in- dicated that compared to a casein control, different dehydration methods of krill could cause apparent urinary nitrogen losses and lower net protein utilization. At the end of the twentieth century, the FAO/WHO Expert Consultation on Protein Quality Evaluation recommended the use of the Protein Digestibility Corrected Amino Acid Score (PDCAAS) method, which considers both the indispensable amino acid content of the test protein and its digestibility (El and Ka-vas, 1996; Tavano et al., 2016).The processing had no sig- nificant (P > 0.05) effect on the PDCAAS value of the krill protein. The proteins from the five types of krill products have a slightly better hematopoietic ability than rat casein. They did not cause pathological changes in the rat visceral tissue, and had certain positive effects in maintaining the health of the rats.
The measurement results of rat blood showed that there was no significant difference of total protein and albumin content between the rats fed with the proteins from the Antarctic krill product the high-quality protein casein (P > 0.05), indicating that the proteins from the Antarctic phos- phorus krill product does not affect the absorption and metabolism of protein in rats, and its absorption and metabolism in the body is similar to those of casein. Therefore, these five types of Antarctic krill products can provide a nitrogen source for protein absorption and metabolism in rats, and the hematopoietic ability is slightly better than that of casein. Compared to casein, the proteins from the Antarctic krill product did not adversely affect the liver function of rats and significantly reduced the total cholesterol and triglyceride contents in the rat serum.
The results of the liver and colon section analysis in rats showed that the protein of Antarctic krill did not cause pathological changes in rat tissues and organs, and could improve liver steatosis and reduce lipid droplets in the liver. In addition, the proteins from the Antarctic krill product increased the rat villus length and increased the V/C value. Among them, the V/C value of the KM group was the most significant (P < 0.01). Feeding these five Antarctic krill protein products can maintain the normal health of rats, and the effects are better than those of casein. Different protein products differed in their digestible amino acid profile, offering a range of amino acid patterns that can be used to formulate specialized diets for which the digestible amino acid content must match closely the requirement of the end user (Cui et al., 2013; Bessada et al., 2019). Therefore, KM, KS, OKP, SKP, and DKP prepared from the Antarctic krill could be used as a potential novel protein resource in the food in- dustry.
5 ConclusionsFive kinds of the Antarctic krill protein products (KM, KS, OKP, DKP, SKP) were evaluated, while casein was employed as the control. The actual digestibility (TD) of these five protein products were higher than that of casein (92.2%). They significantly improve the net protein utilization (NRU). The results of pathological analysis showed that krill products could improve the comprehensive function damage of intestinal digestion, absorption and immune defense, and maintain the healthy state of intestinal tract. Therefore, these five Antarctic krill product can be used as potential new pro- tein resources for the food industry.
AcknowledgementsThis work was financially supported by the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (No. 2022 QNLM030002), the National Key R&D Program of China ‘Formation Mechanism of Antarctic Krill Fishery and Key Technologies for Efficient Utilization of Resources’: Antarctic Krill High Value Product Creation and Industrialization Demonstration (No. 2018YFC1406806), and the Technology Innovation Project of Qingdao Marine Science and Technology Pilot National Laboratory, Shandong Provincial Marine Science, the Technology Fund Major ‘Dark Blue Fishery’ 4-2: Construction of Antarctic Krill Processing Technology Process System (No. 2018SDKJ0304-2).
Ashwath Kumar, K., Sharma, G. K., and Anilakumar, K. R., 2019. Influence of multigrain premix on nutritional, in-vitro and in- vivo protein digestibility of multigrain biscuit. Journal of Food Science and Technology, 56(2): 746-753. DOI:10.1007/s13197-018-3533-z (  0) 0) |
Atkinson, A., Siegel, V., Pakhomov, E. A., Jessopp, M. J., and Loeb, V., 2009. A re-appraisal of the total biomass and annual production of Antarctic krill. Deep-Sea Research Part I – Ocea- nographic Research Papers, 56(5): 727-740. DOI:10.1016/j.dsr.2008.12.007 (  0) 0) |
Bjorndal, B., Berge, K., Barger, J. L., Berge, R. K., and Burri, L., 2014. A krill powder-diet reduces fatty acid and amino acid catabolism while increasing mitochondrial oxidative phosphorylation, a study of the hepatic transcriptome in mice. Journal of Functional Foods, 6: 623-630. DOI:10.1016/j.jff.2013.11.003 (  0) 0) |
Chen, Y. C., Tou, J. C., and Jaczynski, J., 2009. Amino acid and mineral composition of protein and other components and their recovery yields from whole Antarctic krill (Euphausia superba) using isoelectric solubilization/precipitation. Journal of Food Science, 74(2): H31-H39. DOI:10.1111/j.1750-3841.2008.01026.x (  0) 0) |
Cinu, V., Prem, P. S., and Roopesh, M. S., 2022. High-energy cookies for undernourished adolescents: In vivo rat assay of protein quality and evaluation of storage conditions on cookies shelflife. Future Foods, 6: 100154. DOI:10.1016/j.fufo.2022.100154 (  0) 0) |
Cui, J., Chong, B., Rutherfurd, S. M., Wilkinson, B., Singh, H., and Moughan, P. J., 2013. Gross and true ileal digestible amino acid contents of several animal body proteins and their hydrolysates. Meat Science, 94(3): 349-354. DOI:10.1016/j.meatsci.2013.03.002 (  0) 0) |
Cui, J., Liu, X., Dong, Z., Xue, Y., Xue, C. H., and Wang, Y. M., 2014. Effects of DHA-enriched phospholipids on lipid metabolism in diet-induced obese C57 BL/6 J mice model. China Oils and Fats, 39: 27-31. (  0) 0) |
de Oliveira Sousa, A. G., Fernandes, D. C., Alves, A. M., de Frei- tas, J. B., and Naves, M. M. V., 2011. Nutritional quality and protein value of exotic almonds and nut from the Brazilian Savanna compared to peanut. Food Research International, 44(7): 2319-2325. DOI:10.1016/j.foodres.2011.02.013 (  0) 0) |
El, S. N., and Kavas, A., 1996. Determination of protein quality of rainbow trout (Salmo irideus) by in vitro protein digestibility-corrected amino acid score (PDCAAS). Food Chemistry, 55(3): 221-223. DOI:10.1016/0308-8146(95)00111-5 (  0) 0) |
Fernandez-Tome, S., Martinez-Maqueda, D., Tabernero, M., Largo, C., Recio, I., and Miralles, B., 2017. Effect of the long-term intake of a casein hydrolysate on mucin secretion and gene expression in the rat intestine. Journal of Functional Foods, 33: 176-180. DOI:10.1016/j.jff.2017.03.036 (  0) 0) |
Hanley, J., Dhar, D. K., Mazzacuva, F., Fiadeiro, R., Burden, J. J., Lyne, A. M., et al., 2017. Vps33b is crucial for structural and functional hepatocyte polarity. Journal of Hepatology, 66(5): 1001-1011. DOI:10.1016/j.jhep.2017.01.001 (  0) 0) |
Huber, L. A., Rudar, M., Trottier, N. L., Cant, J. P., and de Lange, C. F. M., 2018. Whole-body nitrogen utilization and tissue protein and casein synthesis in lactating primiparous sows fed low-and high-protein diets. Journal of Animal Science, 96(6): 2380-2391. DOI:10.1093/jas/sky047 (  0) 0) |
Hughes, G. J., Ryan, D. J., Mukherjea, R., and Schasteen, C. S., 2011. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: Criteria for evaluation. Journal of Agricultural and Food Chmistry, 59(23): 12707-12712. DOI:10.1021/jf203220v (  0) 0) |
Kurtoglu, S., Hatipoglu, N., Mazicioglu, M. M., Akin, M. A., Coban, D., Gokoglu, S., et al., 2012. Body weight, length and head circumference at birth in a cohort of Turkish newborns. Journal of Clinical Research in Pediatric Endocrinology, 4(3): 132-139. DOI:10.4274/Jcrpe.693 (  0) 0) |
Lee, M. H., 1995. Official Methods of Analysis of AOAC International (16th edition); Official and Standardized Methods of Analysis (3rd edition). Trends in Food Science & Technology, 6(11): 382-383. (  0) 0) |
Li, Y. F., Zeng, Q. H., Liu, G., Chen, X. W., Zhu, Y. H., Liu, H. Q., et al., 2020. Food-grade emulsions stabilized by marine Antarctic krill (Euphausia superba) proteins with long-term phy- sico-chemical stability. LWT – Food Science and Technology, 128: 109492. DOI:10.1016/j.lwt.2020.109492 (  0) 0) |
Li, Z., and Zheng, L., 2019. Effect of different digestion conditions of two kinds of digestion instruments on the determination results of Kjeldahl method. Acta Agriculturae Boreali- Occidentalis Sinica, 28(9): 1485-1491. (  0) 0) |
Litvak, N., Htoo, J. K., and de Lange, C. F. M., 2013. Restricting sulfur amino acid intake in growing pigs challenged with lipopolysaccharides decreases plasma protein and albumin synthesis. Canadian Journal of Animal Science, 93(4): 505-515. DOI:10.4141/cjas2013-014 (  0) 0) |
Liu, Y. Z., Cong, P. X., Li, B. J., Song, Y., Liu, Y. J., Xu, J., et al., 2018. Effect of thermal processing towards lipid oxidation and non-enzymatic browning reactions of Antarctic krill (Euphausia superba) meal. Journal of the Science of Food and Agri- culture, 98(14): 5257-5268. DOI:10.1002/jsfa.9064 (  0) 0) |
Lo, K. S., Wilson, J. G., Lange, L. A., Folsom, A. R., Galarneau, G., Ganesh, S. K., et al., 2011. Genetic association analysis highlights new loci that modulate hematological trait variation in Caucasians and African Americans. Human Genetics, 129(3): 307-317. DOI:10.1007/s00439-010-0925-1 (  0) 0) |
Ma, N., Tian, Y., Wu, Y., and Ma, X., 2017. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Current Protein & Peptide Science, 18(8): 795-808. (  0) 0) |
Scheijen, J., Hanssen, N. M. J., van Greevenbroek, M. M., van der Kallen, C. J., Feskens, E. J. M., Stehouwer, C. D. A., et al., 2018. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clinical Nutrition, 37(3): 919-925. DOI:10.1016/j.clnu.2017.03.019 (  0) 0) |
Shim, J. J., Kim, J. W., Oh, C. H., Lee, Y. R., Lee, J. S., Park, S. Y., et al., 2018. Serum alanine aminotransferase level and liverrelated mortality in patients with chronic hepatitis B: A large national cohort study. Liver International, 38(10): 1751-1759. DOI:10.1111/liv.13705 (  0) 0) |
Stodkilde, L., Damborg, V. K., Jorgensen, H., Laerke, H. N., and Jensen, S. K., 2018. White clover fractions as protein source for monogastrics: Dry matter digestibility and protein digestibility-corrected amino acid scores. Journal of the Science of Food and Agriculture, 98(7): 2557-2563. DOI:10.1002/jsfa.8744 (  0) 0) |
Stogdale, L., 1981. Correlation of changes in blood chemistry with pathological changes in the animal's body: I. Serum nutrients and proteins. Journal of the South African Veterinary Association, 52(1): 57-63. (  0) 0) |
Szepe, K. J., Dyer, P. S., Johnson, R. I., Salter, A. M., and Avery, S. V., 2021. Influence of environmental and genetic factors on food protein quality: Current knowledge and future directions. Current Opinion in Food Science, 40(1): 94-101. (  0) 0) |
Thakur, N., Chauhan, G., Mishra, B. P., Mendiratta, S. K., Patta- naik, A. K., Singh, T. U., et al., 2020. Comparative evaluation of feeding effects of A1 and A2 cow milk derived casein hydrolysates in diabetic model of rats. Journal of Functional Foods, 75: 104272. DOI:10.1016/j.jff.2020.104272 (  0) 0) |
Tieland, M., Beelen, J., Laan, A. C. M., Poon, S., de Groot, L., Seeman, E.,et al, 2018. An even distribution of protein intake daily promotes protein adequacy but does not influence nutritional status in institutionalized elderly. Journal of the American Medical Directors Association, 19(1): 33-39. (  0) 0) |
Wang, L., 2013. New technologies of deep processing of Antarctic krill (Euphausia superba) proteins. PhD thesis. Ocean University of China, Qingdao.
(  0) 0) |
Wang, L., Deng, S., Li, Y., Huo, J., Xie, Z., and Xu, Y., 2019. A preparation process to produce Antarctic krill protein powder. Food and Fermentation Industries, 45(6): 133-138. (  0) 0) |
Wang, R. H., Wang, J. J., Guo, X. B., Li, Y. F., Wu, Y., Liu, H. Q., et al., 2022. Physicochemical and functional properties of the Antarctic krill proteins modified by succinylation. LWT – Food Science and Technology, 154: 112832. DOI:10.1016/j.lwt.2021.112832 (  0) 0) |
Wei, B., Duan, Z. G., Zhu, C. H., Deng, J. J., and Fan, D. D., 2018. Anti-anemia effects of ginsenoside Rk3 and ginsenoside Rh4 on mice with ribavirin-induced anemia. Food & Function, 9(4): 2447-2455. (  0) 0) |
Williams, N. H., Stahly, T. S., and Zimmerman, D. R., 1997. Effect of chronic immune system activation on the rate, efficiency, and composition of growth and lysine needs of pigs fed from 6 to 27 kg. Journal of Animal Science, 75(9): 2463-2471. DOI:10.2527/1997.7592463x (  0) 0) |
Wong, K. H., Cheung, P. C. K., and Ang, P. O., 2004. Nutritional evaluation of protein concentrates isolated from two red seaweeds Hypnea charoides and Hypnea japonicain growing rats. Hydrobiologia, 512L: 271-278. (  0) 0) |
Xia, X., Yuan, S. L., Yang, Y., and Song, C., 2003. Effects of Chinese herbal medicine additives and different diet types on blood biochemical indexes of growing-finishing pigs. Swine Industry Science, 20: 42-43. (  0) 0) |
Yu, C. P., Cha, Y., Wu, F., Fan, W. W., Xu, X. B., and Du, M., 2018. Effects of ball-milling treatment on mussel (Mytilus edulis) protein: Structure, functional properties and in vitro digestibility. International Journal of Food Science and Technology, 53(3): 683-691. (  0) 0) |
Zhang, L. X., Du, G. H., Ge, X. H., and Liu, J., 2016. Improvement of the determination method for protein content standard in foods. Baoji Supervision and Testing Institute of Product Quality, 36: 36-39 (in Chinese with English abstract). (  0) 0) |
Zou, X. J., Yang, H. Y., Luo, H., and Liao, J., 2012. Evaluation and utilization of mouse protein nutrition. Heilongjiang Animal Science and Veterinary Medicine, 17: 147-149 (in Chinese with English abstract). (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


