2) The First Institute of Oceanography, State Oceanic Administration of China, Qingdao 266061, China
Polyunsaturated fatty acids (PUFAs) are indispensable components of biomembranes of all living cells and have become a research hotspot (Kendall et al., 2017). PUFAs are vital for human health and nutrition, and are used to alleviate symptoms of several diseases such as hypertension, depression and cancer (Montes et al., 2013). However, PUFAs cannot be synthesized by the body in sufficient amounts to satisfy metabolic demands. Therefore, PUFAs obtained from other sources are needed (Takeuchi et al., 2001; Rebecca et al., 2012). Nannochloropsis sp., a marine microalga, is an ideal source of PUFAs (Aslan et al., 2017) because it contains all the intermediate fatty acids in the biosynthetic pathways (Fig. 1) (Niu et al., 2009; Nicolás et al., 2017) and various elongases and desaturases that are critical for PUFA biosynthesis (Lina et al., 2017). There is great research interest in the fatty acid genes and metabolism of Nannochloropsis sp..

|
Fig. 1 Putative biosynthesis of PUFAs in marine microalgae. elo, elongase; des, desaturase; C18:1, elaidic acid; C18:2, linolelaidic acid; C18:3, linolentic acid; C20:2, eicosadienoic acid; C20:3, eicosatrienoic acid; C20:4, eicosatetraenoic acid; C20:5, eicosapentaenoate acid; C22:5, docosapentaenic acid; C22:6, docosahexaenoic acid. |
PUFAs can be synthesized through two reactions, i.e., chain elongation and desaturation (Leonie et al., 2017). The chain elongation of fatty acids is the most important aspect in PUFA biosynthesis since it determines the length of the carbon chain (Leonard et al., 2004). Therefore, elongases have been increasingly investigated and characterized. Long-chain fatty acid elongase (FAE) is a member of the Elovl fatty acid enzyme family, which is involved in the biosynthesis of PUFAs Nannochloropsis sp. naturally has one copy of the FAE gene that controls the conversion of short-chain fatty acids to long-chain fatty acids (Sushil et al., 2016). Consequently, isolation, functional expression and characterization of the FAE gene from Nannochloropsis sp. (NsFAE) is important since it will help with understanding the biosynthesis of PUFAs in this organism and may potentially be used to enhance the percentage of PUFAs in the total fatty acids.
In this study, we describe the cloning of long-chain fatty acid elongase gene NsFAE from Nannochloropsis sp. and the characteristics of the resulting enzymatic activity in transgenic yeast. NsFAE was expressed as an active enzyme with the ability to lengthen the carbon chains of fatty acids. Its heterologous expression in yeast will be a good foundation for genetic modification for the development of PUFA synthesis.
2 Materials and Methods 2.1 Strains and Culture ConditionsEscherichia coli DH5α was used for preparing and storing plasmids. Saccharomyces cerevisiae strain BY4742 was the host for gene expression. Nannochloropsis sp. was obtained from the Marine Microalgae Research Center, Chinese Academy of Ocean Sciences, kept in our laboratory, and used as a source of DNA to clone the NsFAE gene. E. coli DH5α was grown at 37℃ in Luria-Bertani medium (LB; 10 g L-1 tryptone, 5 g L-1 yeast extract, 10 g L-1 NaCl) containing 0.1 g L-1 ampicillin. S. cerevisiae BY4742 was cultivated in yeast extract-peptone-dextrose medium (YPD; 20 g L-1 peptone, 20 g L-1 dextrose, 10 g L-1 yeast extract) at 30℃ for 72 h with shaking at 250 r min-1. Nannochloropsis sp. was grown at 25℃ in f/2 medium (seawater filtered through a membrane of 0.45-μm pore size and sterilized at 121℃ for 20 min) containing 100 mg L-1 ampicillin and 100 mg L-1 kanamycin, illuminated with white light for 12 h every day. Yeast transformants were separated on medium lacking uracil and cultivated in YPD medium.
2.2 Identification of the NsFAE Gene from Nannochloropsis sp.Nannochloropsis sp. was harvested by centrifugation at 5000-6000 × g for 10 min, then frozen and ground in liquid nitrogen. Total RNA was extracted from Nannochloropsis sp. using a Plant RNA Kit R6827 (Omega Bio-tek) following the manufacturer's instructions. First-strand cDNA was synthesized using a PrimeScript™ II First-strand cDNA Synthesis Kit (TaKaRa) and adopted as the template for reverse transcription PCR amplification. To identify the long-chain fatty acid elongase gene, primers P1, P2, P3, and P4 were designed according to the conserved amino acid sequence, FLHWYHHV and HAIMYAYY, of the elongase found in N. gaditana and N. oculata (Corteggiani et al., 2014). Primers used in this study were designed using Primer Premier 5.0 software and are listed in Supplementary Table 1. PCR amplification was performed with initial denaturation at 98℃ for 2 min, followed by 25 cycles of 98℃ for 10 s, 55℃ for 10 s and 72℃ for 10 s, with a final extension at 72℃ for 5 min. The amplified PCR fragment was subcloned into vector Pclone007 (Tsingke) and sequenced. The fulllength 1068 bp gene was named NsFAE and the putative protein sequence compared with sequences in the NCBI database using the Fasta program.
|
|
Table 1 Major fatty acid composition and content of S. cerevisiae BY4742 cells expressing NsFAE |
The expression level of NsFAE in Nannochloropsis sp. was detected using qRT-PCR. qRT-PCR amplification reactions were performed using a LightCycler® 480 Real Time PCR System and SYBR Green Real Time PCR Master mix (Qiagen, California, USA). PCR amplification was performed with initial denaturation at 95℃ for 10 min, followed by 40 cycles of 95℃ for 5 s and 68℃ for 30 s. The housekeeping gene β-actin was used as an internal control. Expression level of NsFAE was calculated by 2-ΔΔCt method. Data are presented as the average of 3 independent experiments. SPSS statistics software was used to analysis the statistical significance, and the P value < 0.05 was considered to indicate statistical significance.
To generate the full-length cDNA, 5'-rapid amplification of the cDNA ends (RACE) was performed using a 5' -Full RACE Kit (Takara, Japan). The primers for 5' RACE are listed in Supplementary Table 1. All PCR products were excised, purified with MiniBEST Agarose Gel DNA Extraction Kit (Takara, Japan) and cloned into vector Pclone007 (Tsingke) and sequenced at Tsingke biotechnology Company (China).
2.4 Bioinformatics AnalysisOpen reading frames (ORFs) were predicted with ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) software online. Homology searching was conducted based on BLASTx (http://www.ncbi.nlm.nih.gov/) searching using the NCBI Blast server. The deduced amino acid sequence of NsFAE was aligned using the Clustal W program in DNAMAN, and the phylogenetic tree was constructed with the neighbor-joining algorithm in MEGA 5.0 software. The sequences applied in this study were downloaded from GenBank.
2.5 Vector Construction and Heterologous Expression in S. cerevisiaeThe pNsFAE-CRISPR plasmid was modified from the pCRCT plasmid described by Bao et al. (2015) to replace its guide RNA array with delta-specific guide RNA. The oligonucleotides delta. gRNA1_bsaIF and delta. gRNA1_ bsaIR encoding the delta-specific guide RNA were mixed and incubated to form duplex DNA. The resultant duplex and pCRCT plasmid were digested with BsaI and ligated to generate pNsFAE-CRISPR. The plasmid pNsFAE-CRISPR was sequenced and used for expression experiments in S. cerevisiae.
The open reading frame of NsFAE was amplified using the Phusion high-fidelity PCR kit (with primer pairs P1 and P2, and P3 and P4). The promoter ADH1 and the terminator CYC were amplified from the S. cerevisiae BY4742 genome using primer pairs P5 and P6 and P7 and P8, respectively. Then, a recombinant donor DNA fragment consisting of ADH1p, NsFAE and CYCt was generated through overlap PCR with primers P9 and P10. The resulting PCR fragment (ADH1p-NsFAE-CYCt) was subcloned into vector Pclone007 and sequenced.
For expression of Nannochloropsis sp. NsFAE in S. cerevisiae, the plasmid pNsFAE-CRISPR and the fragment ADH1p-NsFAE-CYCt were introduced into competent cells of strain BY4742 by the standard electroporation procedure (Guan et al., 2012), and transformants were selected on minimal medium plates lacking uracil. Selected transformants were first grown in 5 mL of liquid medium lacking uracil containing 2% glucose at 30℃. After overnight culture, the cells were then centrifuged, washed, and resuspended in distilled water and inoculated into 20 mL of liquid medium lacking uracil and grown for 72 h at 30℃ in an air shaker at 250 r min-1.
2.6 Fatty Acid Extraction and AnalysesFatty acids were extracted using the method of Robert (Robert et al., 2005) with some modifications. Nonadecanoic acid was used as an internal standard (Sigma-Aldrich). HCl (2 mL, 1 mol L-1) in methanol was added to 15 mg yeast powder and vortexed for 50 min with glass beads, and then heated to 80℃ for 60 min. After cooling to room temperature, 2 mL of 0.9% NaCl solution was added with 1.5 mL of hexane and vortexed for 5 min. The solution was then centrifuged at 1000×g for 5 min. Fatty acids were collected in the upper hexane layer, and the layer was transferred to and stored in a clean gas chromatography-mass spectrometry (GC/MS) vial. Fatty acid content was analyzed by GC/MS using an Agilent 5975 series MSD and an Agilent 7890A equipped with a HP-5 column (30 m × 0.25 mm, film thickness 0.25 m) at the College of Chemistry and Chemical Engineering, Qingdao University. The program used during the analysis was: 50℃ for 1 min; heating at 25℃ min-1 to 175℃; heating at 4℃ min-1 to 230℃; hold at 230℃ for 4 min; heating at 5℃ min-1 to 250℃; hold at 250℃ for 5 min; and the electron ionization 70 eV (Jun et al., 2016). The statistical analysis of fatty acid content between BY4742 and BY4742-NsFAE was then presented by t test and the P value < 0.05 was considered to indicate statistical significance.
3 Results 3.1 Isolation and Identification of the Elongase Gene from Nannochloropsis sp.A potential long-chain fatty acid elongase gene homologue was isolated from Nannochloropsis sp. using degenerate oligonucleotides targeting conserved amino acid motifs. A partial sequence of 805 bp was used to design gene specific primers that were used to amplify the 5'-end of the gene. Assembling the 5′-RACE PCR product sequences resulted in the identification of a cDNA clone with homology to known elongase genes. The full-length cDNA corresponding to the long-chain fatty acid elongase gene was named NsFAE and submitted to the GenBank database with accession number MF680548. The ORF of NsFAE was 1068 bp long, encoding a predicted protein of 355 amino acids.
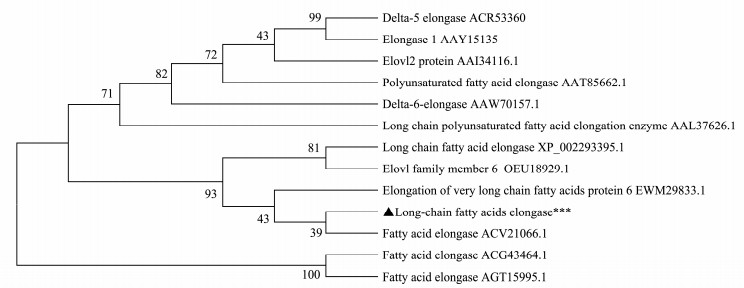
3.2 Bioinformatic AnalysisThe NJ-tree showing the phylogenetic relationship of FAE gene from different species (Twelve from the GenBank and one from the present study), which was constructed based on the alignment of selected FAE amino acid sequences (Fig. 2). The sequence comparison revealed that those species homologous to each other in the traditional taxonomic group clustered together, e.g. Zea mays and Saccharum; Pyramimonas cordata and Rebecca salina. Further, Nannochloropsis sp. had higher similarity to the microalgae Nannochloropsis oculata, Nannochloropsis gaditana, Thalassiosira pseudonana and Fragilariopsis cylindrus.
|
Fig. 2 Phylogenetic tree was constructed in the neighbor-joining method using the MEGA5.1 software. Boot-strap values are indicated on the branches (1000 repeats). The fatty acid elongases of Zea mays (ACG43464.1) and Saccharum (AGT15995.1) were used as the outgroup. Pyramimonas cordata (ACR53360), Rebecca salina (AAY15135), Danio rerio (AAI34116), Marchantia polymorpha (AAT85662), Phaeodactylum tricornutum (AAW70157), Isochrysis galbana (AAL37626.1), Thalassiosira pseudonana (XP002293395), Fragilariopsis cylindrus (OEU18929), Nannochloropsis gaditana (EWM29833.1), Nannochloropsis oculata (ACV21066). |
Amino acid sequence alignments of FAE gene from different species were performed (Fig. 3). The NsFAE shared the highest (43%) identity with fatty-acyl of Nannochloropsis gaditana, 43% with long chain fatty acid elongase of Thalassiosira pseudonana, and 42% with fatty acid elongase of Nannochloropsis oculata. The sequence comparison revealed that NsFAE from Nannochloropsis sp. clearly clustered with algal sequences. The NsFAE amino acid sequence contains four conserved motifs-a histidine box LHXXHH, a tyrosine box HXXM YXYY, and two other boxes LFXXF and KXXEXXDT, that are characteristic of the PUFA elongases. In addition, the conserved motif (LHXXHH) has been proved to be the active center of fatty acid elongases (Qi et al., 2002).

|
Fig. 3 Alignment of the deduced amino acid sequences of plant fatty acid elongase polypeptides by clustal W software. Genbank accession numbers are as follows: N. oculata (ACV21066), N. gaditana (EWM28304), I. galbana (AAL37626.1), T. pseudonana (XP002293395), F. cylindrus (OEU18929), D. rerio (AAI34116). |
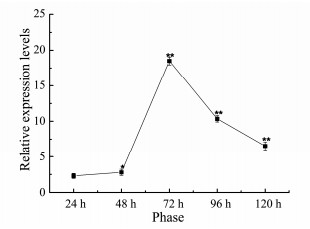
As the long-chain fatty acid elongase enzyme is involved in the process of converting linoleic acid into eicosadienoic acid, we were interested to determine the expression of NsFAE during different growth stages of Nannochloropsis sp. RT-PCR analysis was carried out to detect the relative levels of transcription of the NsFAE gene at five different growth stages of Nannochloropsis sp. using β-actin as an internal control. On the other hand, The NsFAE gene exhibited its highest accumulation at 72 h (i.e., in the third growth stage) and, despite there being different expression levels, the expression was significantly lower in the other four growth stages (Fig. 4).
|
Fig. 4 RT-PCR analysis of the NsFAE gene expressed in Nannochloropsis sp. at five different growth stages. Data are presented as the average of 3 independent experiments. Error bars represent means ± standard deviation. Significant differences were calculated using the t test (**P < 0.01, *P < 0.05). |
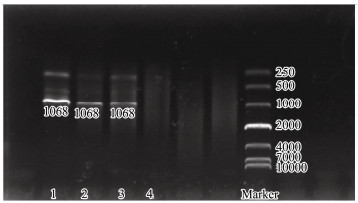
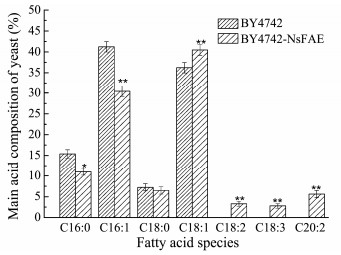
To characterize the elongase activity of NsFAE, the DNA fragment ADH1p-NsFAE-CYCt and the plasmid pNsFAE-CRISPR were introduced into competent S. cerevisiae BY4742 cells and transformants were selected on minimal medium plates lacking uracil. To identify the transformants, genomic DNA of transformants were identified by PCR with primers designed according to NsFAE gene sequences, PCR screening gave shown of approximately 1000 bp, the appearance of band revealed the expression of NsFAE in the recombinant strain (Fig. 5). The fatty acid profiles of the transformed yeast were evaluated by GC/MS analysis (each strain was analyzed in triplicate). The wild-type strain BY4742 showed the FA composition typical for S. cerevisiae (16:0, 16:1, 18:0, and 18:1) (Christopher et al., 2015). Expression of NsFAE resulted in the appearance of three additional peaks, which were identified as linoleic acid (18:2), linolenic acid (18:3) and eicosadienoic acid (20:2). The production of linoleic acid, linolenic acid and eicosadienoic acid in the yeast cells transformed with NsFAE was associated with a notable concomitant increase in oleic acid (18:1) and a sharp decrease in palmitoleic acid (16:1) (Fig. 6; Table 1).
|
Fig. 5 PCR analysis to confirm the presence of inserted genes in S. cerevisiae BY4742. Lanes 1, 2, and 3, The band of NsFAE gene was amplified from BY4742-NsFAE; Lane 4, The aseptic distilled water as negative control group. |
|
Fig. 6 Fatty acid composition of transformed S. cerevisiae. Data are presented as the average of 3 independent experiments. Error bars represent means ± standard deviation. Significant differences were calculated using the t test (**P < 0.01, *P < 0.05). |
In this study, we report the identification of a cDNA from the marine microalga Nannochloropsis sp. coding for a long-chain fatty acid elongase (NsFAE) that can introduce double bonds and extend the carbon chain, resulting in the production of linoleic acid (18:2), linolenic acid (18:3) and eicosadienoic acid (20:2). This result provides evidence of the effective functional expression of the PUFA elongase NsFAE in S. cerevisiae. Like other PUFA elongases, NsFAE contains four conserved motifs: a histidine box having three histidine residues (LHX XHH), a tyrosine box having three tyrosine residues (HXXMYXYY), and the motifs LFXXF and KXXEX XDT. These motifs are critical for elongase activity (Leonard et al., 2004; Sakuradani et al., 2009; Dong et al., 2010). The deduced protein encoded by NsFAE exhibits sequence homology to the fatty acid elongase of N. oculata (43%) and to the long-chain fatty acid elongase of Thalassiosira pseudonana (44%), both marine algae.
Marine microalgae are an important source of PUFAs for several types of applications (e.g., food, pharmaceuticals, nutraceuticals) (Petrie et al., 2010; Lieselot et al., 2018). In microalgae, PUFAs are mainly synthesized via an alternative series of chain elongation and desaturation reactions (Li et al., 2012). Recently, some elongases and desaturases have been identified: C20-fatty acid elongase from Pavlova viridis (Shi et al., 2013), C16-Δ9 elongases from Thalassiosira pseudonana (Junichiro et al., 2013), Δ6 fatty acid desaturase from Phaeodactylum tricornutum (Zhu et al., 2017), ω-3 fatty acid desaturase from Chlorella vulgaris (Norashikin et al., 2018), Δ9 desaturase from Cyanobacteria (Faten et al., 2017). These results suggest that elongases and desaturases are essential for the synthesis of PUFAs in marine algae. To date, elongases involved in conversion of C18:2 to C20:2 were detected in the several marine algaes of Isochrysis galbana, Emiliania huxleyi, Pavlova pinguis and Pavlova salina (Qi et al., 2002; Petrie et al., 2010) but not in Nannochloropsis sp.. Therefore, we report the identification of a long-chain fatty acid elongase NsFAE from Nannochloropsis sp.. We studied the expression of the NsFAE gene in different growth stages of Nannochloropsis sp.. A high relative level of the NsFAE transcript was found in the third growth stage, whereas in the other stages it was hardly detectable. The fatty acid composition in Nannochloropsis sp. revealed an increase in the C18:2 content during the third growth stage, consistent with the expression pattern of NsFAE (Fig. 7). It may be speculated that synthesis of other substances in the mid-growth stage of Nannochloropsis sp. consumed some of C18:1, leading to the production of C18:2. It is difficult to determine which metabolic pathway works, but in the logarithm growth phase of Nannochloropsis sp, there are some gene expressions associated with the biosynthesis of unsaturated fatty acids, leading to the transformation of unsaturated fatty acids into polyunsaturated fatty acids (Zheng et al., 2013).
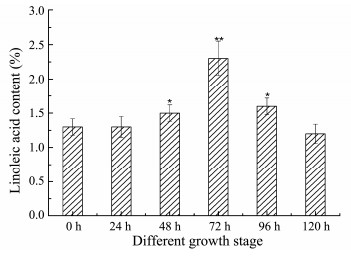
|
Fig. 7 The content of Linoleic acid in Nannochloropsis sp. at different growth stages. Data are presented as the average of 3 independent experiments. Error bars represent means ± standard deviation. Significant differences were calculated using the t test (**P < 0.01, *P < 0.05). |
Expression in S. cerevisiae is usually used for functional characterization of enzymes participating in PUFA biosynthesis (Shi et al., 2012; Chodok et al., 2013). Functional characterization of NsFAE in S. cerevisiae showed that this enzyme converted the palmitoleic acid (16:1) to oleic acid (C18:1) and linoleic acid (C18:2) to eicosadienoic acid (C20:2) (Xie et al., 2016). This has also been observed, for example, with a C18-△9 elongase from Thraustochytrium sp. (Junichiro et al., 2013) and Isochrysis galbana H29 (Li et al., 2012). Previous reports found different elongases exhibit different fatty acid substrates preferences (Shi et al., 2013). The HpELO2 from Hansenula polymorpha was found to recognize two substrates (containing 20 and 22 carbons) (Phatthanon et al., 2007); The PsELO5 from Pavlova sp. CCMP459 displayed unique substrate specificity for both ω-6 and ω-3 C20 PUFA substrates (Kaewsuwan et al., 2010); The Elovl6 from goat mammary epithelial cells involved in the elongation of various PUFA containing 16 and 18 carbons (Shi et al., 2017). In our experiments, an accumulation of oleic acid (18:1) and significant increase in the levels of C18:2 and C20:2, were observed in the transformed yeast. These resullts supported an idea that NsFAE encodes a fatty acid elongase involved in the elongation of C16:1 to C18:1 and C18:2 to C20:2 (Sun et al., 2014). Moreover, the contents of eicosadienoic acid (C20:2) and linolenic acid (C18:3) accumulated to 5.60% and 2.78% of the total fatty acids respectively, showing a higher conversion ratio of linoleic acid (C18:2) to eicosadienoic acid (C20:2) than to linolenic acid (C18:3) (Baoxiu et al., 2004). An enzyme that can convert fatty acids would clearly be useful in attempts to engineer PUFA pathways.
In summary, we have cloned and identified the function of an elongase gene, NsFAE, from Nannochloropsis sp., that is involved in the synthesis of essential fatty acids (linoleic acid and linolenic acid). Moreover, NsFAE possesses the ability to convert linoleic acid (C18:2) into eicosadienoic acid (C20:2). The accumulation of linoleic acid (C18:2), linolenic acid (C18:3) and eicosadienoic acid (C20:2) are very important for production of further PUFAs.
AcknowledgementThis work was supported by the Basic Scientific Fund for National Public Research Institutes of China (No. 2016Q07).
Aslan, M. A. and Ilmutdin, M. A., 2017. The study of microalgae Nannochloropsis salina fatty acid composition of the extracts using different techniques. SCF vs conventional extraction. Journal of Molecular Liquids, 239: 96-100. DOI:10.1016/j.molliq (  0) 0) |
Bao, Z., Xiao, H., Liang, J., Zhang, L., Xiong, X., Sun, N., Si, T. and Zhao, H., 2015. A Homology Integrated CRISPR-Cas (HI-CRISPR) system for one-step multi-gene disruptions in Saccharomyces cerevisiae. ACS Synthetic Biology, 4: 585-594. DOI:10.1021/sb500255k (  0) 0) |
Baoxiu, Q., Fraser, T., Mugford, S., Dobson, G., Sayanova, O., Butler, J., Napier, J. A., Stobart, A. K. and Lazarus, C. M., 2004. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nature Biotechnology, 22: 739-745. DOI:10.1038/nbt972 (  0) 0) |
Chodok, P., Eiamsaard, P., Cove, D. J., Quatrano, R. S. and Kaewsuwan, S., 2013. Identification and functional characterization of two Δ12-fatty acid desaturases associated with essential linoleic acid biosynthesis in Physcomitrella patens. Journal of Industrial Microbiology and Biotechnology, 40: 901-913. DOI:10.1007/s10295-013-1285-3 (  0) 0) |
Christopher, L., Brian, P., Ruben, F. M. and Nancy, A. D. S., 2015. Overproduction and secretion of free fatty acids through disrupted neutral lipid recycle in Saccharomyces cerevisiae. Metabolic Engineering, 28: 54-62. DOI:10.1016/j.ymben.2014.11.006 (  0) 0) |
Corteggiani, C. E., Telatin, A., Vitulo, N., Forcato, C., D'Angelo, M., Schiavon, R., Vezzi, A., Giacometti, G. M., Morosinotto, T. and Valle, G., 2014. Chromosome scale genome assembly and transcriptome profiling of Nannochloropsis gaditana in nitrogen depletion. Molecular Plant, 7: 323-335. DOI:10.1093/mp/sst120 (  0) 0) |
Dong, H. K., Periasamy, A., Young, S. J., Bidur, P. C., Jeong, W. S. and Byung, K. H., 2010. Identification and characterization of a novel enzyme related to the synthesis of PUFAs derived from Thraustochytrium aureum ATCC 34304. Biotechnology and Bioprocess Engineering, 15: 261-272. DOI:10.1007/s12257-009-0223-8 (  0) 0) |
Faten, B. A., Mohamed, B., Fatma, E., Nesrine, K., Mouna, D., Bruno, B., Pierre, V., Slim, A. and Imen, F., 2017. Cyanobacteria as source of marine bioactive compounds: Molecular specific detection based on Δ9 desaturase gene. International Journal of Biological Macromolecules, 105: 1440-1445. DOI:10.1016/j.ijbiomac.2017.07.139 (  0) 0) |
Guan, B., Lei, J., Su, S., Chen, F., Duan, Z., Chen, Y., Gong, X., Li, H. and Jin, J., 2012. Absence of Yps7p, a putative glycosylphosphatidylinositol-linked aspartyl protease in Pichia pastoris, results in aberrant cell wall composition and increased osmotic stress resistance. FEMS Yeast Research, 12: 969-979. DOI:10.1111/1567-1364.12002 (  0) 0) |
Jun, X., Ling, W. and Jian-bo, Z., 2016. Expression of Shewanella frigidimarina fatty acid metabolic genes in E. coli by CRISPR/cas9-coupled lambda red recombineering. Biotechnology Letters, 38: 117-122. DOI:10.1007/s10529-015-1956-4 (  0) 0) |
Junichiro, O., Keishi, S., Yuji, O., Nozomu, O. and Makoto, I., 2013. Two fatty acid elongases possessing C18-Δ6/C18-Δ9/C20-Δ5 or C16-Δ9 elongase activity in Thraustochytrium sp. ATCC 26185. Marine Biotechnology, 15: 476-486. DOI:10.1007/s10126-013-9496-1 (  0) 0) |
Kendall, A. C., Kiezel, T. M., Brownbridge, L. C., Harwood, J. L. and Nicolaou, A., 2017. Lipid functions in skin: Differential effects of n-3 polyunsaturated fatty acids on cutaneous ceramides, in a human skin organ culture model. BBA-Biomembranes, 1859: 1679-1689. DOI:10.1016/j.bbamem.2017.03.016 (  0) 0) |
Kaewsuwan, S., Bunyapraphatsara, N., Cove, D. J., Quatrano, R. S. and Chodok, P., 2010. High level production of adrenic acid in Physcomitrella patens using the algae Pavlova sp. Delta(5)-elongase gene. Bioresource Technology, 101: 4081-4088. DOI:10.1016/j.biortech.2009.12.138 (  0) 0) |
Leonard, A. E., Pereira, S. L. and Sprecher, H., 2004. Elongation of long-chain fatty acids. Progress in Lipid Research, 43: 36-54. DOI:10.1016/S0163-7827(03)00040-7 (  0) 0) |
Leonie, W., Tao, Y. and Florian, D., 2017. Establishing very long-chain fatty alcohol and wax ester biosynthesis in Saccharomyces cerevisiae. Biotechnology and Bioengineering, 114: 1025-1035. DOI:10.1002/bit.26220 (  0) 0) |
Li, M., Ou, X. Y., Yang, X. D., Guo, D. Q., Qian, X. Y., Xing, L. J. and Li, M. C., 2012. Cloning and identification of a novel C18-Δ9 polyunsaturated fatty acid specific elongase gene from DHA-producing Isochrysis galbana H29. Biotechnology and Bioprocess Engineering, 17: 22-32. DOI:10.1007/s12257-011-0037-3 (  0) 0) |
Lieselot, B., Charlotte, B., Koen, G., Céline, D. and Imogen, F., 2018. Influence of high pressure homogenization on free fatty acid formation in Nannochloropsis sp. European Journal of Lipid Science and Technology, 120: 1-19. DOI:10.1002/ejlt.201700436 (  0) 0) |
Lina, J. D., Camille, R., Giorgio, P., Guillaume, T., Richard, B., Marina, L., Tomas, M., Frédérique, T., Jean, D. F., Denis, F., Juliette, J., Olga, S., Frédéric, B. and Eric, M., 2017. A palmitic acid elongase affects eicosapentaenoic acid and plastidial monogalactosyldiacylglycerol levels in Nannochloropsis. Plant Physiology, 173: 742-759. DOI:10.1104/pp.16.01420 (  0) 0) |
Montes, R., Chisaguano, A. M. and Castellote, A. I., 2013. Fatty-acid composition of maternal and umbilical cord plasma and early childhood atopic eczema in a Spanish cohort. European Journal of Clinical Nutrition, 67: 658-663. DOI:10.1038/ejcn.2013.68 (  0) 0) |
Niu, Y., Kong, J., Fu, L., Yang, J. and Xu, Y., 2009. Identification of a novel C20-elongase gene from the marine microalgae Pavlova viridis and its expression in Escherichia coli. Mar Biotechnol, 11: 17-23. DOI:10.1007/s10126-008-9116-7 (  0) 0) |
Nicolás, L., Sebastian, M., María, P. C., Natalia, R., Dante, T., Alex, D. G., Natalia, G., Nicole, E. and Alejandro, M., 2017. Reconstruction of the microalga Nannochloropsis salina genome-scale metabolic model with applications to lipid production. BMC Systems Biology, 11: 2-17. DOI:10.1186/s12918-017-0441-1 (  0) 0) |
Norashikin, N., Loh, S. H., Aziz, A. and Cha, T. S., 2018. Metabolic engineering of fatty acid biosynthesis in Chlorella vulgaris using an endogenous omega-3 fatty acid desaturase gene with its promoter. Algal Research, 31: 262-275. DOI:10.1016/j.algal.2018.02.020 (  0) 0) |
Petrie, J. R., Mackenzie, A. M., Shrestha, P., Liu, Q., Frampton, D. F., Robert, S. S. and Singh, S. P., 2010. Isolation of three novel long-chain polyunsaturated fatty acid delta 9-elongases and the transgenic assembly of the entire Pavlova salina docosahexaenoic and pathway in Nicotiana benthamiana1. Journal of Phycology, 46: 917-925. DOI:10.1111/j.1529-8817.2010.00870.x (  0) 0) |
Phatthanon, P., Yoshinobu, K., Minetaka, S., Takeshi, B., Eiichiro, F., Akio, K. and Satoshi, H., 2007. Functional analysis of very long-chain fatty acid elongase gene, HpELO2, in the methylotrophic yeast Hansenula polymorpha. Applied Microbiology and Biotechnology, 76: 417-427. DOI:10.1007/s00253-007-1012-y (  0) 0) |
Qi, B., Beaudoin, F., Fraser, T., Stobart, A. K., Napier, J. A. and Lazarus, C. M., 2002. Identification of a cDNA encoding a novel C18-Δ9 polyunsaturated fatty acid-specific elongating activity from the docosahexaenoic acid (DHA)-producing microalga, Isochrysis galbana. FEBS Letters, 510: 159-162. DOI:10.1016/S0014-5793(01)03247-1 (  0) 0) |
Rebecca, M. L. and Brian, F. P., 2012. Engineering Escherichia coli to synthesize free fatty acids. Trends in Biotechnology, 30: 659-667. DOI:10.1016/j.tibtech.2012.09.006 (  0) 0) |
Robert, S. S., Singh, S. P. and Rong, Z. X., 2005. Metabolic engineering of Arabidopsis to produce nutritionally important DHA in seed oil. Functional Plant Biology, 32: 473-479. DOI:10.1071/FP05084 (  0) 0) |
Sakuradani, E., Nojiri, M., Suzuki, H. and Shimizu, S., 2009. Identification of a novel fatty acid elongase with a wide substrate specificity from arachidonic acid-producing fungus Mortierella alpina 1S-4. Applied Microbiology and Biotechnology, 84: 709-716. DOI:10.1007/s00253-009-1999-3 (  0) 0) |
Shi, T. L., Yu, A. Q., Li, M., Ou, X. Y., Xing, L. J. and Li, M. C., 2012. Identification of a novel C22-Delta 4-producing docosahexaenoic acid (DHA) specific polyunsaturated fatty acid desaturase gene from Isochrysis galbana and its expression in Saccharomyces cerevisiae. Biotechnology Letters, 34: 2265-2274. DOI:10.1007/s10529-012-1028-y (  0) 0) |
Shi, T. L., Yu, A. Q., Li, M., Zhang, M., Xing, L. J. and Li, M. C., 2013. Identification and characterization of a novel C20-elongase gene from the marine microalgae, Pavlova viridis, and its use for the reconstitution of two pathways of longchain polyunsatured fatty acids biosynthesis in Saccharomyces cerevisiae. Biotechnology Letters, 35: 1271-1282. DOI:10.1007/s10529-013-1194-6 (  0) 0) |
Shi, H. B., Wu, M., Zhu, J. J., Zhang, C. H., Yao, D. W., Luo, J. and Loor, J. J., 2017. Fatty acid elongase 6 plays a role in the synthesis of long-chain fatty acids in goat mammary epithelial cells. Journal of Dairy Science, 100: 4987-4995. DOI:10.3168/jds.2016-12159 (  0) 0) |
Sushil, B., Joon, N. L. and Young-Il, K., 2016. The fatty acid chain elongase, Elovl1, is required for kidney and swim bladder development during zebrafish embryogenesis. Organogenesis, 12: 78-93. DOI:10.1080/15476278.2016.1172164 (  0) 0) |
Sun, H. K., So, Y. K., Eui, K. K., Kyung, H. R., Jung, B. K. and Kwang, S. K., 2014. Identification and functional characterization of polyunsaturated fatty acid elongase (McELOVL5) gene from pike eel (Muraenesox cinereus). Biotechnology Letters, 36: 29-37. DOI:10.1007/s10529-013-1344-x (  0) 0) |
Takamiya, M., Sakurai, M. and Teranishi, F., 2016. Lead discovery for mammalian elongation of long chain fatty acids family 6 using a combination of high-throughput fluorescent-based assay and RapidFire mass spectrometry assay. Biochemical and Biophysical Research Communications, 480: 721-726. DOI:10.1016/j.bbrc.2016.10.103 (  0) 0) |
Takeuchi, T., 2001. A review of feed development for early life stages of marine finfish in Japan. Aquaculture, 200: 203-222. DOI:10.1016/S0044-8486(01)00701-3 (  0) 0) |
Xie, D. Z., Chen, F., Lin, S. Y., You, C. H., Wang, S. Q., Zhang, Q. H., Monroig, Ő., Tocher, D. R. and Li, Y. Y., 2016. Longchain polyunsaturated fatty acid biosynthesis in the euryhaline herbivorous teleost Scatophagus argus: Functional characterization, tissue expression and nutritional regulation of two fatty acyl elongases. Comparative Biochemistry & Physiology Part B, 198: 37-45. DOI:10.1016/j.cbpb.2016.03.009 (  0) 0) |
Zheng, M., Tian, J., Yang, G., Zheng, L., Chen, G., Chen, J. and Wang, B., 2013. Transcriptome sequencing, annotation and expression analysis of Nannochloropsis sp. at different growth phases. Gene, 523: 117-121. DOI:10.1016/j.gene..2013.04.005 (  0) 0) |
Zhu, B. H., Tu, C. C., Shi, H. P., Yang, G. P. and Pan, K. H., 2017. Overexpression of endogenous delta-6 fatty acid desaturase gene enhances eicosapentaenoic acid accumulation in Phaeodactylum tricornutum. Process Biochemistry, 57: 43-49. DOI:10.1016/j.procbio.2017.03.013 (  0) 0) |
 2019, Vol. 18
2019, Vol. 18


