2) College of Environmental Science & Engineering, Yangzhou University, Yangzhou 225127, China;
3) Laboratory for Marine Drugs and Bioproducts, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
4) College of Pharmaceutical Sciences, Hebei University, Baoding 071002, China
Marine-derived fungi have been researched for decades due to their prospective reservoir for structurally multitudinous and biologically significant secondary metabolites (Carroll et al., 2019; Nagabhishek and Madankumar, 2019). Fungal secondary metabolites include alkaloids, polyketides, meroterpenoids, terpenoids, steroids, and peptides, which have a wide range of biological activities, such as antimicrobial, antiviral, antioxidant, anti-fouling, cytotoxicity, inhibition of various enzyme, etc. (Hafez Ghoran et al., 2022; Zhang et al., 2022). Enzymes that build secondary metabolites are encoded by colocalized biosynthetic gene clusters (BGCs). A lot of genome sequencing results have recently revealed that many BGCs are 'silent' or conveyed at low levels under standard laboratory conditions (Tomm et al., 2019; Xu et al., 2021). For exploring this incompletely tapped trove, it is a significant method to activate the silent BGCs of marine-derived fungi by modification of culture conditions, such as chemical epigenetics (Zarins-Tutt et al., 2016; Zhang et al., 2022). Nowadays, it is demonstrated that chemical epigenetic manipulation is an effective technique to remodel the fungal epigenome to acquire more latent fungal secondary metabolites (Guo et al., 2020; Zhao et al., 2018), which may encounter large manipulation in a pathway-specific regulator and these changes give an access to the production of novel secondary metabolite (Bharatiya et al., 2021).
In our previous work, a series of 14-membered resorcylic acid lactones (RALs) have been isolated from marine invertebrate-derived fungi collected from the South China Sea (Shao et al., 2011; Liu et al., 2014; Zhang et al., 2017; Xu et al., 2021). For example, seven RALs with potent antifouling activities, including cochliomycins A–C, zeaenol, LL-Z1640-1, LL-Z1640-2 and paecilomycin F, were firstly isolated from a gorgonian-derived fungus Cochliobolus lunatus (Shao et al., 2011). Eleven RALs with antifouling and fungicidal activities, cochliomycins D –F together with eight analogues, were obtained from a sea anemone-derived fungus C. lunatus (TA26–46) (Liu et al., 2014). RALs are a family of benzannulated macrolides, and constitute a 14-membered macrocyclic ring fused to a β-resorcylic acid moiety (Lai et al., 2016). Our previous investigation revealed a variety of biological properties of the isolated RALs and their semisynthetic derivatives, including anntifuling, antimalarial, cytotoxic, antiparasitic, antiviral, fungicidal and kinase inhibitory activities (Xu et al., 2019). In order to explore the products of silent secondary metabolic pathways, chemical epigenetic manipulation has been applied to the fungal strain C. lunatus (TA26–46), while sodium butyrate and SAHA lead to the isolation of brominated resorcylic acid lactones (Zhang et al., 2014), 5-azacytidine lead to the isolation of diethylene glycol phthalate esters (Chen et al., 2016) and α-pyrones (Wu et al., 2019). Many inducers inactivate the enzyme histone deacetylase and DNA containing gene material to transfer is transcribed and translated for expression of a gene. So, the addition of external inducer reinforced the transcription process (Bharatiya et al., 2021). In the present study, another type of chemical epigenetic agent, a histone deacetylation modifier, nicotinamide (100 μmolL−1), was added to the fermentation of C. lunatus (TA26–46), which resulting in remarkable difference in the secondary metabolites compared with the control. From the treated broth, a new RAL, 7′(Z)-zeaenol (1), together with six known analogues (2–7), as shown in Fig.1, were isolated. Herein, we report the isolation, structural characterization, and bioactivity evaluation of these RALs.

|
Fig. 1 Structures of compounds 1−7. |
Optical rotations were measured on an MCP300 automatic polarimeter (Anton Paar, Austria) at 20℃. ECD spectra were performed on a J-810 Circular Dichroism Spectrometer (JASCO, Japan). IR experiments were conducted on a Cary 610/670 spectrometer (Agilent, America) using KBr pellets. NMR spectra were measured with an AV-ANCE 600 NMR spectrometer (Bruker, Germany) (600 MHz for 1H and 150 MHz for 13C), using TMS as internal standard. HRESIMS spectra were acquired from Agilent 1290 Infinity II UHPLC/6530 Q-TOF MS (Waters, America). Semi-preparative HPLC was performed on a HITA-CHI system using a semi-preparative C18 (Kromasil, 5 μm, 10 mm × 250 mm) column coupled with a 2400 UV detector. Silica gel (Qing Dao Hai Yang Chemical Group Co.; 100–200 and 200–300 mesh), octadecylsilyl silica gel (Unicorn; 45–60 μm) and Sephadex LH-20 (Amersham Biosciences) were used for column chromatography (CC). Precoated silica gel plates (Yan Tai Zi Fu Chemical Group Co.; G60, F-254) were employed for thin layer chromatography (TLC).
2.2 Fungal Material and Culture ConditionsThe separation and authentication of the zoanthid-derived fungus Cochliobolus lunatus (TA26–46) have been reported previously (GenBank JF819163) (Liu et al., 2014). The fungal strain was inoculated in a malt extract broth (malt 30 g L−1, peptone 5 g L−1, artificial sea salt 30 g L−1) containing a kind of histone deacetylation modifier (100 μmolL−1 nicotinamide). The cultivation was incubated for 30 days at room temperature.
2.3 Extraction and IsolationThe cultivation was percolated to separate the broth from the mycelium. The mycelium was extracted three times with ethyl acetate (EtOAc) (200 mL for each flask) and two times with CH2Cl2–MeOH (1:1, v/v) (200 mL for each flask). Meanwhile the fermented broth was extracted with a three-fold volume of EtOAc for three times. The mycelium extraction and the fermented broth extraction were combined and concentrated in vacuo pump to produce a crude extract (60.7 g). Then the crude extract was separated by silica gel column chromatography (CC) using a step gradient elution of petroleum ether (PET)–EtOAc mixtures with increasing polarity (100:0–0:100) to produce six subtractions (Fr.1–Fr.6). Fr.4 and Fr.5 were purified respectively by silica gel Sephadex LH-20, octadecyl silane CC, and semipreparative HPLC to yield compounds 1 (5.3 mg), 2 (15.4 mg), 3 (6.1 mg), 4 (6.6 mg), 5 (3.6 mg), 6 (4.3 mg), and 7 (1.65 mg).
2.4 Physical-Chemical Property7′(Z)-zeaenol (1): white powder;
|
|
Table 1 NMR Spectroscopic Data for 1 (CDCl3) |
Cytotoxic activity was measured by using A549 (human pulmonary adenocarcinoma), HCT116 (human colorectal adenocarcinoma), HT-29 (human colorectal adenocarcinoma), Hela (human cervical carcinoma), MCF-7 (Human breast carcinoma) and K562 (Chronic myeloid leukemia) cell lines. The assessment was conducted through the 96-well microtitre plates (Skehan et al., 1990; Repetto et al., 2008), with adriamycin as positive control, and DMSO as negative control. The inhibition ratios of adriamycin were 79.75%, 61.19%, 75.02%, 69.10%, 82.97% and 62.27% respectively against the tested cell lines when its concentration is 1 μmolL−1.
2.5.2 Antibacterial activityAntibacterial activity was assayed on 13 bacterial strains, including Gram-negative Pseudomonas aeruginosa, Candida albicans, Escherichia coli, E. cloacae, E. hormaechei, Pelagibacterium halotolerans, P. angustum, Aeromonas hydrophila, A. salmonicida, Pseudomonas fulva, Vibrio anguillarum, Gram-positive Staphylococcus aureus, and Methicillin-resistant S. aureus. The evaluation was conducted through 96-well microtitre plates (Appendino et al., 2008), with ciprofloxacin and SeaNine 211 as positive controls, DMSO as negative control, and Luria-Bertani broth as blank control.
2.5.3 Microalgae growth inhibition activityThe microalgae growth inhibition activity was evaluated on Navicula exigua, N. leavissima, Chaetoceros socialis, Chlorella vulgaris and Phaeodactylum tricornutum Bohlin. The measurements were conducted using 24-well cell culture plates (Eisentraeger et al., 2003), with SeaNine 211 as a positive control and DMSO as negative control.
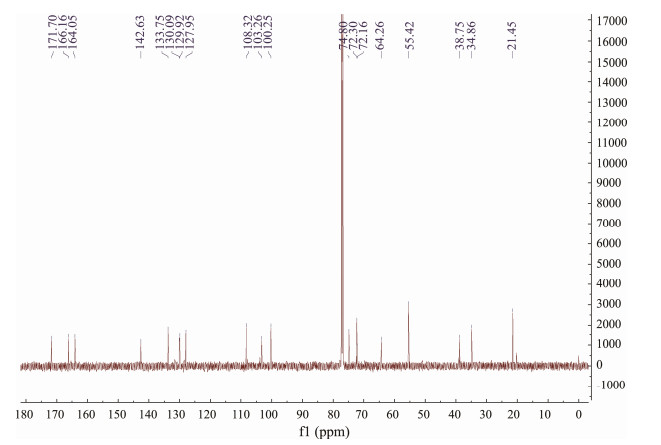
3 Results and Discussion 3.1 Structure DeterminationCompound (1) was isolated as a white, amorphous powder. Its molecular formula was confirmed as C19H24O7 (eight degrees of unsaturation) based on the analysis with HR-ESI-MS ion (Fig.2) at m/z 387.1405 [M + Na]+ (calcd for C19H24NaO7, 387.1414). Careful inspection of the 1D and 2D NMR (Table 1) spectra of 1 revealed that 1 is a RAL possessing the same planar structure as that of zeaenol (2), the major product of C. lunatus (TA26–46). The significant differences between 1 and 2 were the respective configuration of double bond at C-7′. In the 1H NMR spectrum (Fig.3), the small coupling constant J = 11.3 Hz between H-7′ (δH 5.84, dd, J = 11.3, 9.7 Hz) and H-8′ (δH 5.60, ddd, J = 11.3, 4.2, 2.4 Hz) suggested that the double bond at C-7′ is Z-configuration in 1, which is different from the 7′E-configuration in 2. Careful analysis of the 13C NMR spectrum (Fig.4) showed that the primary differences between 1 and 2 were the chemical shifts of C-6′ (δC 64.3 in 1 vs. 73.1 in 2), hence it seems that the stereogenic center C-6′ in 1 was inverted to the corresponding center in 2.

|
Fig. 2 HRESIMS spectrum of compound 1. |

|
Fig. 3 A part of 1H NMR (600 MHz, CDCl3) spectrum of compound 1. |
|
Fig. 4 13C NMR (150 MHz, CDCl3) spectrum of compound 1. |
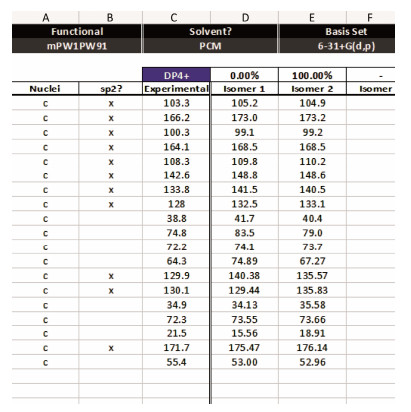
The absolute configuration of 1 was determined by its ECD spectrum, 13C NMR shift calculations, and biogenetic considerations. The absolute configuration of C-10′ was assigned by the analysis of ECD spectra (Fig.5), in which the appearance of a negative Cotton effect around 275 nm implied the 10′S configuration (Liu et al., 2014). It is a challenge to determine the absolute configurations of C4′, C5′, and C6′ of 1. The acetonide reaction of 1 failed due to the complex products which were difficult to be separated. Fortunately, two known 4′, 5′, 6′-triol RALs, zeaenol (2) (Sugawara et al., 1992) and 7-epi-zeaenol (3) (Ayers et al., 2011) with 4′S, 5′S configurations were isolated simultaneously from C. lunatus (TA26–46). On account of biogenetic considerations, the configurations at C-4′ and C-5′ of 1 were 4′S, 5′S. The absolute configuration of C-6′ was difficult to be determined as the molecule conformational flexibility reflect two kinds of possible absolute configurations (Fig.6, 1a with 4′S, 5′S, 6′R, 10′S and 1b with 4′S, 5′S, 6′S, 10'S). Consequently, the absolute configuration of 1 was determined by using calculation of GIAO NMR shift (Cao et al., 2019a, 2019b) at the B3LYP/6-311+G(d, p) level. The absolute configuration of 1b was more likely than 1a to be the real configuration, (100.00% 1b vs. 0.00% 1a in both unscaled shift data and shielding tensor data) (Fig.7) when the parameter of DP4 plus probability was considered (Grimblat et al., 2015). Therefore, the absolute configuration of 1 was proposed as 4′S, 5′S, 6′S, 10′S. The significant up-field chemical shift of C-6′ may be caused by the shielding effect of the double bond at C-7′. Thus, compound 1 was elucidated as 7′(Z)-zeaenol.

|
Fig. 5 ECD spectrum of compound 1. |

|
Fig. 6 Two possible absolute configurations (1a and 1b) for 1. |
|
Fig. 7 Summary of the parameters of DP4plus probability for 1a (isomer 1) and 1b (isomer 2). |
The known 14-membered RALs 2–7 have been determined to be zeaenol (2) (Sugawara et al., 1992), 7-epi-zeaenol (3) (Ayers et al., 2011), LL-Z1640-1 (4), LL-Z1640-2 (5) (Ellestad et al., 1978), paecilomycin G (6) (Bujaranipalli and Das, 2016), and deoxy-aigialomycin C (7) (Bajwa and Jennings, 2008) based on their NMR data, and by the comparison with the data previously reported.
It is interesting that when 100 μmolL−1 nicotinamide was added into the malt medium, multiple new peaks emerged in the HPLC profile between 35 and 50 min (Fig.8). Through further analysis, we found that RALs were focused on 45 to 50 min.

|
Fig. 8 HPLC profiles of EtOAc extracts of C. lunatus (TA26–46) cultured in malt extract broth with 100 μmolL−1 nicotinamide. |
More than 130 naturally occurring 14-membered RALs have been described from many fungal genera since the first-discovered RAL radicicol was isolated in 1953 (Xu et al., 2022). A literature survey revealed that many 14-membered RALs showed a variety of bioactivities, including but not limited to cytotoxic (Ayers et al., 2011) and antifouling (Xu et al., 2019) functions. Based on these reports, all isolated RALs (1–7) were first detected for their cytotoxic activity towards A549, HCT116, HT-29, Hela, MCF-7 and K562 cell lines. The new compound (1) displayed weak cytotoxic activity to inhibit HT-29 cell line growing. Compound 5 exhibited potent cytotoxic activity against A549, HCT116, HT-29, Hela, MCF-7 and K562 cell lines with the IC50 values of 6.97, 2.54, 4.95, 4.25, 6.22 and 7.44 μ mol L−1, respectively. However, the very similar compound 4 showed no such activities. Comparing the cytotoxic activities of 4 and 5, the configuration of C-5′ plays a critical role in the cytotoxic activities. Compounds 1–7 were also tested with their antibacterial activity against 13 pathogenic bacteria strains, and antifouling activity against 5 microalgaes. Unfortunately, no activity was found for these RALs.
4 ConclusionsIn conclusion, by introducing chemical epigenetic manipulation on the zoanthid-derived fungus C. lunatus (TA26–46) with a histone deacetylation modifier (100 μmolL−1 nicotinamide), a new 14-membered RAL 7′(Z)-zeaenol (1) and six analogues (2–7) can be isolated from the malt extract broth. Compound 5 shows strong cytotoxic activity and is worthy of further study. The results indicated once again that chemical epigenetic manipulation is an effective tool for exploring the products of fungal silent secondary metabolic pathways.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (Nos. 81673350, 81703411, 417 76156), the National Science and Technology Major Project for Significant New Drugs Development, China (No. 2018ZX09735-004), the Fundamental Research Funds for the Central Universities of China (No. 201962002), and the Taishan Scholars Program, China.
Appendino, G., Gibbons, S., Giana, A., Pagani, A., Grassi, G., Stavri, M., et al., 2008. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. Journal of Natural Products, 71(8): 1427-1430. DOI:10.1021/np8002673 (  0) 0) |
Ayers, S., Graf, T. N., Adcock, A. F., Kroll, D. J., Matthew, S., Carcache de Blanco, E. J., et al., 2011. Resorcylic acid lactones with cytotoxic and NF-κB inhibitory activities and their structure-activity relationships. Journal of Natural Products, 74(5): 1126-1131. DOI:10.1021/np200062x (  0) 0) |
Bajwa, N., and Jennings, M. P., 2008. Syntheses of epi-aigialomycin D and deoxy-aigialomycin C via a diastereoselective ring closing metathesis macrocyclization protocol. Tetrahedron Letters, 49(2): 390-393. DOI:10.1016/j.tetlet.2007.11.038 (  0) 0) |
Bharatiya, P., Rathod, P., Hiray, A., and Kate, A. S., 2021. Multifarious elicitors: Invoking biosynthesis of various bioactive secondary metabolite in fungi. Applied Biochemistry and Biotechnology, 193(3): 668-686. DOI:10.3390/md20050302 (  0) 0) |
Bujaranipalli, S., and Das, S., 2016. First stereoselective total synthesis of paecilomycin G. Tetrahedron Letters, 57(25): 2800-2802. DOI:10.1016/j.tetlet.2016.05.046 (  0) 0) |
Cao, F., Meng, Z. H., Mu, X., Yue, Y. F., and Zhu, H. J., 2019a. Absolute configuration of bioactive azaphilones from the marine-derived fungus Pleosporales sp. CF09-1. Journal of Natural Products, 82(2): 386-392. DOI:10.1021/acs.jnatprod.8b01030 (  0) 0) |
Cao, F., Sun, T. T., Yang, J. K., Zhao, G. Z., Liu, Q. A., Hu, L. D., et al., 2019b. The absolute configuration of anti-Vibrio citrinin dimeric derivative by VCD, ECD and NMR methods. Natural Product Reports, 33(15): 2192-2199. DOI:10.1080/14786419.2018.1493590 (  0) 0) |
Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R., 2019. Marine natural products. Natural Product Reports, 36(21): 122-173. DOI:10.1039/C9NP00069K (  0) 0) |
Chen, M., Zhang, W., Shao, C. L., Chi, Z. M., and Wang, C. Y., 2016. DNA methyltransferase inhibitor induced fungal biosynthetic products: Diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus. Marine Biotechnology, 18(3): 409-417. DOI:10.1007/s10126-016-9703-y (  0) 0) |
Eisentraeger, A., Dott, W., Klein, J., and Hahn, S., 2003. Comparative studies on algal toxicity testing using fluorometric microplate and Erlenmeyer flask growth-inhibition assays. Ecotoxicology and Environmental Safety, 54(3): 346-354. DOI:10.1016/S0147-6513(02)00099-4 (  0) 0) |
Ellestad, G. A., Lovell, F. M., Perkinson, N. A., Hargreaves, R. T., and McGahren, W. J., 1978. New zearalenone related macrolides and isocoumarins from an unidentified fungus. Journal of Organic Chemistry, 43(12): 2339-2343. DOI:10.1021/jo00406a007 (  0) 0) |
Grimblat, N. S., Zanardi, M. M., and Sarotti, A. M., 2015. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. Journal of Organic Chemistry, 80(24): 12526-12534. DOI:10.1021/acs.joc.5b02396 (  0) 0) |
Guo, D. L., Qiu, L., Feng, D., He, X., Li, X. H., Cao, Z. X., et al., 2020. Three new ɑ-pyrone derivatives induced by chemical epigenetic manipulation of Penicillium herquei, an endophytic fungus isolated from Cordyceps sinensis. Natural Product Research, 34(7): 958-964. DOI:10.1080/14786419.2018.1544974 (  0) 0) |
Hafez Ghoran, S., Taktaz, F., Ayatollahi, S. A., and Kijjoa, A., 2022. Anthraquinones and their analogues from marine-derived fungi: Chemistry and biological activities. Marine Drugs, 20(8): 1660-3397. DOI:10.1007/s12010-020-03423-6 (  0) 0) |
Lai, D., Mao, Z., Dan, X., Zhang, X., and Yang, L., 2016. Hyalodendriellins A-F, new 14-membered resorcylic acid lactones from the endophytic fungus Hyalodendriella sp. Ponipodef12. RSC Advances, 6(110): 108989-109000. DOI:10.1039/C6RA24009G (  0) 0) |
Liu, Q. A., Shao, C. L., Gu, Y. C., Blum, M., Gan, L. S., Wang, K. L., et al., 2014. Antifouling and fungicidal resorcylic acid lactones from the sea anemone-derived fungus Cochliobolus lunatus. Journal of Agricultural and Food Chemistry, 62(14): 3183-3191. DOI:10.1021/jf500248z (  0) 0) |
Nagabhishek, S. N., and Madankumar, A., 2019. A novel apoptosis-inducing metabolite isolated from marine sponge symbiont Monascus sp. NMK7 attenuates cell proliferation, migration and ROS stress-mediated apoptosis in breast cancer cells. RSC Advances, 9(11): 5878-5890. DOI:10.1039/C8RA09886G (  0) 0) |
Repetto, G., Del Peso, A., and Zurita, J. L., 2008. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nature Protocols, 3(7): 1125-1131. DOI:10.1038/nprot.2008.75 (  0) 0) |
Shao, C. L., Wu, H. X., Wang, C. Y., Liu, Q. A., Xu, Y., Wei, M. Y., et al., 2011. Potent antifouling resorcylic acid lactones from the gorgonian-derived fungus Cochliobolus lunatus. Journal of Natural Products, 74(4): 629-633. DOI:10.1021/np100641b (  0) 0) |
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., et al., 1990. New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute, 82(13): 1107-1112. DOI:10.1093/jnci/82.13.1107 (  0) 0) |
Sugawara, F., Kim, K. W., Kobayashi, K., Uzawa, J., Yoshida, S., Murofushi, N., et al., 1992. Zearalenone derivatives produced by the fungus Drechslera portulacae. Phytochemistry, 31(6): 1987-1990. DOI:10.1016/0031-9422(92)80346-G (  0) 0) |
Tomm, H. A., Ucciferri, L., and Ross, A. C., 2019. Advances in microbial culturing conditions to activate silent biosynthetic gene clusters for novel metabolite production. Journal of Industrial Microbiology & Biotechnology, 46: 1381-1400. DOI:10.1007/s10295-019-02198-y (  0) 0) |
Wu, J. S., Shi, X. H., Zhang, Y. H., Yu, J. Y., Fu, X. M., Li, X., et al., 2019. Co-cultivation with 5-azacytidine induced new metabolites from the zoanthid-derived fungus Cochliobolus lunatus. Frontiers in Chemistry, 7: 763. DOI:10.3389/fchem.2019.00763 (  0) 0) |
Xu, F., Wu, Y., Zhang, C., Davis, K. M., Moon, K., Bushin, L. B., et al., 2019. A genetics-free method for high-throughput discovery of cryptic microbial metabolites. Nature Chemical Biology, 15(2): 161. DOI:10.1038/s41589-018-0193-2 (  0) 0) |
Xu, W. F., Wu, N. N., Wu, Y. W., Qi, Y. X., Wei, M. Y., Pineda, L. M., et al., 2022. Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Marine Life Science & Technology, 4: 88-97. DOI:10.1007/s42995-021-00103-0 (  0) 0) |
Xu, W. F., Xue, X. J., Qi, Y. X., Wu, N. N., Wang, C. Y., and Shao, C. L., 2021. Cochliomycin G, a 14-membered resorcylic acid lactone from a marine-derived fungus Cochliobolus lunatus. Natural Product Research, 35(3): 490-493. DOI:10.1080/14786419.2019.1633646 (  0) 0) |
Zarins-Tutt, J. S., Barberi, T. T., Gao, H., Mearns-Spragg, A., Zhang, L., Newman, D. J., et al., 2016. Prospecting for new bacterial metabolites: A glossary of approaches for inducing, activating and upregulating the biosynthesis of bacterial cryptic or silent natural products. Natural Product Research, 33(1): 54-72. DOI:10.1039/c5np00111k (  0) 0) |
Zhang, R., Wang, H., Chen, B., Dai, H., Sun, J., Han, J., et al., 2022. Discovery of anti-MRSA secondary metabolites from a marine-derived fungus Aspergillus fumigatus. Marine Drugs, 20(5): 1660-3397. DOI:10.3390/md20050302 (  0) 0) |
Zhang, W., Shao, C. L., Chen, M., Liu, Q. A., and Wang, C. Y., 2014. Brominated resorcylic acid lactones from the marinederived fungus Cochliobolus lunatus induced by histone deacetylase inhibitors. Tetrahedron Letters, 55(35): 4888-4891. DOI:10.1016/j.tetlet.2014.06.096 (  0) 0) |
Zhang, X. Q., Spadafora, C., Pineda, L. M., Ng, M. G., Sun, J. H., Wang, W., et al., 2017. Discovery, semisynthesis, antiparasitic and cytotoxic evaluation of 14-membered resorcylic acid lactones and their derivatives. Scientific Reports, 7(1): 11822. DOI:10.1038/s41598-017-12336-0 (  0) 0) |
Zhao, M., Yuan, L. Y., Guo, D. L., Ye, Y., DaWa, Z. M., Wang, X. L., et al., 2018. Bioactive halogenated dihydroisocoumarins produced by the endophytic fungus Lachnum palmae isolated from Przewalskia tangutica. Phytochemistry, 148: 97-103. DOI:10.1016/j.phytochem.2018.01.018 (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


