2) Key Laboratory of Marine Environment and Ecology (Ocean University of China), Ministry of Education, Qingdao 266100, China
Nowadays, the requirement for high-quality protein is increasing. Seafood, as a protein-rich product, becomes more popular in our menu (Liu et al., 2017; Mo et al., 2017). To meet the increasing demand for seafood, more aquaculture or mariculture systems for culturing the fish either in freshwater or seawater, have to be built since there has been a gradual decrease of wild fishery resources. Thus, large amounts of wastewater containing suspended solids, nitrogen compounds, organic matter and bait are produced. Till now, the mariculture wastewater is usually discharged directly into nearby river of offshore without treatment due to high salinity, low carbon/nitrogen ratio (Zheng et al., 2016). China is the largest producer and exporter of seafood and contributes to 61.70% of the global aquaculture production (Liu et al., 2017). However, the wastewater from aquaculture is often in positive proportion to the production. As such, the increased discharge of mariculture wastewater and the pollution has drawn more attention. The efficient treatment for the high-salt wastewater containing high total nitrogen is urgently required. In addition, China is isussing more strictly aquaculture wastewater discharging standards, where more efficient wastewater treatment techniques and higher quality effluent are fundamental.
Infectious diseases are recognized to be one of the most frequent disasters in aquaculture (Lagana et al., 2011). Hence, antimicrobial compounds are often used in the baits to prevent fish diseases (Stolker and Brinkman, 2005; Saxena et al., 2018). The intensive farming practices in China are commonly accompanied by outbreaks of infectious diseases (Mo et al., 2017). Antibiotics are widely used in aquaculture to treat infections caused by a variety of bacterial pathogens, and even some prohibited antibiotics have also been found in recent years (Liu et al., 2017). As the largest producer and user of antibiotics, China consumed 162000 tons of antibiotics in 2013 (Zhang et al., 2015). Liu et al. (2017) found that the types of antibiotics in mariculture wastewater were more than those in freshwater aquaculture wastewater, indicating that the pollution of mariculture wastewater was even more serious. Antibiotics can inhibit the growth of pathogens, but they can also kill the beneficial microbes, which will finally disturb the balance of the ecosystem. The risks of antibiotics to human health are also not to be neglected due to adverse drug reactions and the potential epidemic of antibiotic resistance pathogens caused by selective pressure on bacteria (Xiong et al., 2015; Sun et al., 2016).
Long-term overuse of antibiotics resulted in rapid transfer of antibiotic resistance genes (ARGs) in the environment (Wang et al., 2018). In addition, Li et al. (2017) found that antibiotics reduced the diversity of microbial community and changed the bacterial community structure. There also many studies showed that antibiotics could block the nitrification and denitrification processes in wastewater treatment (Zheng et al., 2016; Li et al., 2017), which will bring more barriers to the biological processes. Hence, removal of the antibiotics by biological processes from the wastewater is a big challenge.
The biofilm-based wastewater treatment processes had more advantages over other methods, the advantages include higher biomass, longer sludge retention times, stronger resistance to shock loading, easier to separate sludge from water. In addition, the biofilm could provide more diverse ecological niches for different functional microbes to survive, so the microbes could cooperate with each other to completely degrade complex chemicals (William et al., 2003). Most importantly, once the microbes form biofilms, the biofilms presented higher resistance to antimicrobial agents (Wolcott et al., 2010), which made it possible to use the biofilm-based technique to treat the antibiotics-containing wastewater. Therefore, in this study, the anoxic/oxic (A/O) and mobile bed biofilm reactor (MBBR) were combined to treat mariculture wastewater containing high-nitrogen and antibiotics.
Sulfamethoxazole (SMX) is one of most commonly used sulfonamides antibiotics in mariculture. The SMX dose generally ranged from 100 to 200 mg kg−1 of fish body weight and SMX concentration in culturing pond was sometimes up to 25 mg L−1 (Liu et al., 2017). Therefore, this study adopted 10-30 mg L−1 SMX to explore the effect of the antibiotics on the performance of A/O-MBBR with salt-tolerant microbiota. Meanwhile, the response and change of microbial community were investigated and compared horizontally.
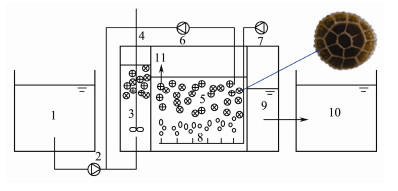
2 Materials and Methods 2.1 BioreactorThe integrated wastewater treatment bioreactor, A/O-MBBR was used in this study (Fig. 1). The bioreactor was consisting of three parts, that is, anoxic zone, oxic zone and sedimentary zone, and its total effective volume was 11.88 L. Firstly, the wastewater was pumped into the anoxic zone, where a mixing blender was installed. Then, the water flowed into the oxic zone, where the aerator device was set to provide enough oxygen. In order to sufficiently remove the nitrate in the water, a reflux system was built to pump the water back to the anoxic zone. About one third volume of carriers with a diameter of 25 mm and an effective surface area of more than 500 m2 m−3, were filled in the anoxic and oxic zones. The sedimentary zone with a volume of 0.84 L followed the oxic zone.
|
Fig. 1 The schematic diagram of the A/O-MBBR process. 1, Artificial wastewater tank; 2, Influent pump; 3, Anoxic zone; 4, Blender; 5, Oxic zone; 6, Reflux pump; 7, Aerating pump; 8, Microporous aerator; 9, Sedimentary zone; 10, Effluent; 11, Carrier (right top picture shows the carrier covered with biofilm). |
The seed sludge used in this study was taken from the secondary sedimentation tank of Tuandao wastewater treatment plant, China. In order to form the biofilm on the carriers, the batch mode operation was adopted at the startup stage of bioreactor in the first four weeks. One batch cycle contained 18 h of operation and 4 h of idling. During bioreactor operation, the artificial mariculture wastewater was sequentially pumped into the anoxic and oxic zones, where the water was completely mixed with activated sludge and carriers. The blender in the anoxic zone kept stirring during the operation; the microporous aerator at the bottom of the oxic zone kept the dissolved oxygen at about 5.00 mg L−1. After operation, all devices stopped working and kept stationary for 4 h and then the water was discharged. From the fifth week, the bioreactor was changed to continuous operating mode, where the Hydraulic Retention Time (HRT) was 8 h, and the reflux ratio was 100%. The outside temperature was 17-20℃.
The artificial mariculture wastewater was prepared according to the literature (Zheng et al., 2016), and the salinity was adjusted by adding the concentrated seawater (Yanrui New Materials, Qingdao, China). During the batch operation, the salinity of wastewater was initially set at 5‰ and it was then improved by 1‰ daily and finally to 30‰. As the bioreactor came into continuous mode, the salinity was kept stably at 30‰. The artificial wastewater composition contains (mg L−1): NH4Cl 18.15, NaNO3 52.15, NaNO2 3.54, KH2PO4 15.00, CH3COONa 190.00, Na2CO3 130.00, pH 6.50-7.50. The ratio of COD to total nitrogen was about 10.50, where the total inorganic nitrogen and COD were equal to 14.00 and 148.00 mg L−1.
Three A/O-MBBR bioreactors (named A, B and C) were operated synchronously to investigate the influence of SMX on the performance. After successful startup, 10, 20 and 30 mg L−1 SMX (Aladdin, Shanghai, China) was separately fed into the influents of three A/O-MBBR bioreactors A, B and C for eight consecutive days. The performance of bioreactors was monitored by analyzing the influent, as well as the effluent parameters. The biofilms attached to the carriers were obtained to reveal the changes of microbial community.
2.3 Analysis MethodsThe water quality parameters, including COD, NH4+-N, NO3−-N and NO2−-N, in the influent and effluent were examined daily referring to Chinese NEPA standard methods (Chinese NEPA, 2002).
The seeding sludge (Sl), biofilm samples in anoxic zone (AS0) and the oxic zone (OS0) at successful startup of bioreactors, and the biofilm samples in anoxic zone (AS10, AS20, AS30 for bioreactor A, B and C) and the oxic zone (OS10, OS20, OS30 for bioreactor A, B and C) at the end of operation were taken to analyze the diversity of microbial community by using 16S rRNA gene based high-throughput sequencing.
The total DNA of the sludge and biofilm samples was extracted by the Soil DNA Extraction Kit (Mobio, CA) according to manufacturer's instructions. Partial 16S rRNA gene based high-throughput sequencing was used to determine the diversity and composition of the bacterial communities in each sample. PCR amplifications were conducted in triplicate with the primer set 515F (5'-GTGCCAGCAGCCGCGGTAA-3') and 806R (5'-GGA CTACCAGGGTATCTAAT-3') targeting the V4 region of the bacterial 16S rRNA gene. The reverse primer contained a 6 bp error-correcting barcode unique to each sample. PCR was performed following the protocol described previously (Gao et al., 2014). Afterwards, the purified PCR products were subjected to high throughput sequencing on an Illumina MiSeq platform by Novogene (Tianjin, China). QIIME software package (http://qiime.org/) and UPARSE pipeline (http://drive5.com/uparse/) were used to analyze the reads and pick operational taxonomic units (OTUs). Sequencing results were filtered by splicing, and sequences with 97% or more similarities were classified into same OTU. The Rarefaction plots, Shannon index, Principal Component Analysis (PCA), and species composition and relative abundance of microbial community were analyzed by the methods as described in literature (Gao et al., 2014). All sequences obtained in this study were deposited in the MG-RAST database under the accession Nos. mgs751504-mgs751512.
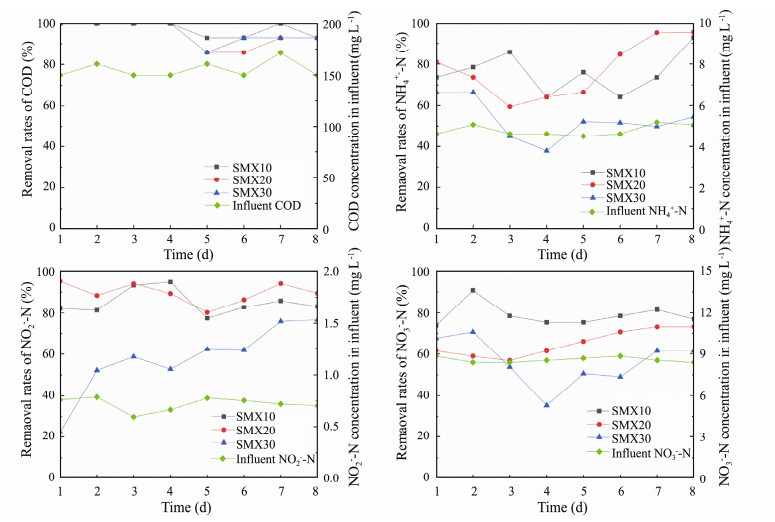
3 Results and Discussion 3.1 Effect of SMX on the Performance of the A/O-MBBRThe bioreactor efficiency at successful startup is listed in Table 1. The corresponding changes of bioreactors performance with SMX dosage are shown as Fig. 2. Almost all COD was removed as the bioreactors started up. When 10, 20 and 30 mg L−1 of SMX were added in influent, the average removal rates of COD reached 97.34%, 94.61% and 95.53% respectively, corresponding average COD 4.01, 8.02 and 6.68 mg L−1 in effluent, which showed that the COD removal was not heavily repressed by the high concentration of SMX. Similarly, Du et al. (2018) indicated that tetracycline exposure had little effect (P > 0.05) on the efficiency of COD removal during the whole period of lab-scale A-O systems.
|
|
Table 1 The performance of the bioreactors as they started up |
|
Fig. 2 Effects of different concentrations of SMX feeds on the removals of COD (a), NH4+-N (b), NO2−-N (c) and NO3−-N (d) in the A/O-MBBR. |
With the influent containing 0, 10, 20 and 30 mg L−1 SMX, the average removal rates of NH4+-N in bioreactors decreased from 83.69% to 76.11%, 77.50% and 53.02% respectively, resulting in 1.13, 1.06 and 2.22 mg L−1 NH4+-N in effluent. Interestingly, the average removal rates of NO2−-N even increased from 81.67% to 85.08%, 89.62% due to feeding of 10, 20 mg L−1 SMX; while it quickly went down to 57.80% due to feeding of 30 mg L−1 SMX. Tong et al. (2019) also found that low centration of ofloxacin increased the NH4-N removal efficiency from 72.62% to 80.66–82.13%. But the mechanism involved in should be further elucidated. The above results showed that the nitrifying process performed well as feeding 20 mg L−1 or less SMX. 30 mg L−1 SMX resulted in a significant decline for the removal of ammonia and nitrite, which finally led to accumulations of ammonia and nitrite.
The average removal rates for NO3−-N were 78.61%, 78.84%, 65.23% and 56.18% for the influent containing 0, 10, 20 and 30 mg L−1 SMX. Hence, compared with performance of bioreactors without SMX feed, 20-30 mg L−1 SMX presented an obvious inhibition on the denitrification. The denitrifying process revealed more sensitive to the SMX feeds than the nitrifying process and COD removal, which accorded well with the results of Zhu et al. (2017). They concluded that denitrification was more susceptible than nitrification and carbon oxidization processes under SMX stress.
The nitrogen removal efficiency presented a downward trend due to increased feeding concentrations of SMX. The reactor performance was then finally damaged in the removing the nitrogen when feeding 30 mg L−1 SMX. Zheng et al. (2016) reported that the bioreactor fed the mariculture wastewater containing oxytetracycline from 0 to 12 mg L−1 led to obvious decrease of the removal rates of NH4+-N and COD. Similarly, it was found that the removal rates of COD and NH4+-N decreased and the effluent NO2−-N and NO3−-N increased with the elevation of feeding florfenicol (Gao et al., 2018). Researchers demonstrated that the presence of antibiotics (such as erythromycin, tetracycline and SMX) impacted the nitrogen cycling and organic matter degradation by inhibiting the activity of key enzymes in different wastewater biological treatment processes (Cetecioglu et al., 2013; Katipoglu-Yazan et al., 2015). Comparing the control and the previous results, feeding 10 mg L−1 SMX presented almost no impact on the performance of mariculture wastewater by A/O-MBBR in this study. Moreover, 10-20 mg L−1 SMX even enhanced the removal of NO2−-N. As revealed by present study, 10-20 mg L−1 SMX feeds lightly inhibit the ammonia oxidation process and denitrification process, this would finally decrease the production of nitrite. Therefore, the part reason for increase of NO2−-N removal rate might be due to the decrease of production of NO2−-N. The removal rates of COD and nitrogen species responding differently to SMX feeds might be ascribed to that the different functional microbes revealed different sensitivity to the SMX and the biofilm in bioreactor provided diverse niches for the growth of microbes (Gui et al., 2017; Zhu et al., 2017).
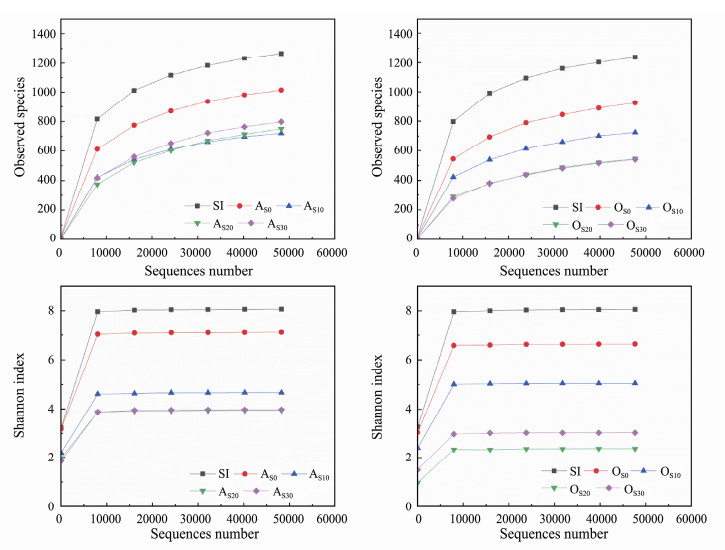
3.2 Effects of SMX on the Diversity of Microbial CommunitiesThe diversity of microbial community in the three reactors changed, especially after SMX dosage (Fig. 3). SMX feeding decreased the diversity of microbial communities in both the anoxic and oxic zones, which indicated that largely microorganisms were washed out under the SMX press and some species with high resistance to SMX were obviously enriched. Interestingly, in the oxic zones, the bioreactor feeding with 30 mg L−1 SMX presented higher diversity than that of bioreactor with 20 mg L−1 SMX. When the SMX concentration in the influent was 10, 20, 30 mg L−1, the number of microbial species in the anoxic zone decreased from 1014 (AS0) to 720 (AS10), 751 (AS20), 798 (AS30), and the corresponding diversity index decreased from 7.11 to 4.67, 3.95 and 3.98. Meanwhile, the number of microbial species in oxic zone decreased from 924 (OS0) to 724 (OS10), 545 (OS20), 540 (OS30), and the corresponding diversity index decreased from 6.64 to 5.05, 2.36 and 3.02.
|
Fig. 3 Rarefaction plots (a, b) and Shannon index (c, d) of microbial communities in seed sludge (SI) and biofilm samples from anoxic zone (AS0, AS10, AS20, AS30) and oxic zone (OS0, OS10, OS20, OS30). |
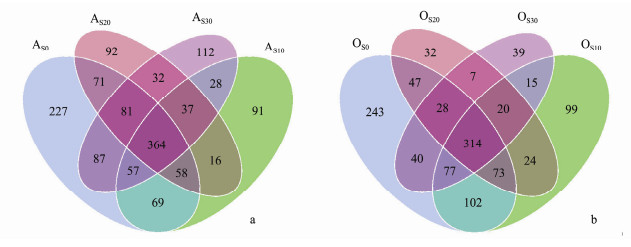
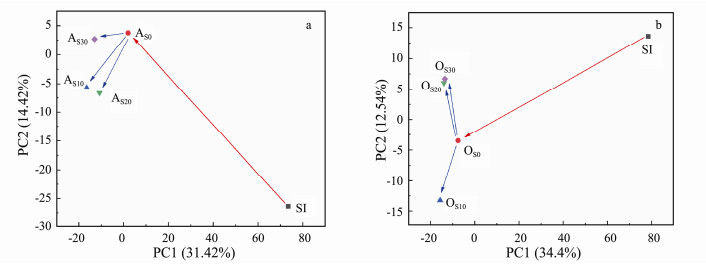
The shared or unique OTUs for different microbial samples was analyzed and the results are shown in Fig. 4. In the anoxic zones, the shared OTUs for the biofilm samples at the end of the fifth week (AS0) and the biofilm samples with 10, 20, 30 mg L−1 of SMX (AS10, AS20, AS30) were 364; while in the oxic zones, the shared OTUs was 324. As for the unique OTUs, the samples at the end of fifth week contained greater values, 227 OTUs for anoxic zone and 243 OTUs for the oxic zone. PCA for the microbial communities (Fig. 5) showed that the microbial populations of seed sludge (SI) was quite different from the biofilms, which indicated that the enrichment by wastewater in bioreactor impacted heavily on the microbial composition. Similarly, compared with the AS0 and OS0 samples as the bioreactor successful startup, the SMX feeds largely changed the microbial composition in biofilms. The SMX feeds presented some different influence on the biofilms in anoxic and the oxic zones. The sample in the oxic zone under the 10 mg L−1 SMX dosage went to the adverse direction as compared with the samples under the 20, 30 mg L−1 SMX feeds. These results showed that nitrification process under the 10 mg L−1 SMX feeds was quite different with that of 20, 30 mg L−1 SMX feeds.
|
Fig. 4 Venn map of the shared and unique OTUs for biofilm samples from anoxic zone (AS0, AS10, AS20, AS30) (a) and oxic zone (OS0, OS10, OS20, OS30) (b) at different concentrations of SMX feeds. |
|
Fig. 5 Principal Component Analysis (PCA) of microbial communities in seed sludge (SI) and biofilm samples from anoxic zone (AS0, AS10, AS20, AS30) (a) and oxic zone (OS0, OS10, OS20, OS30) (b). |
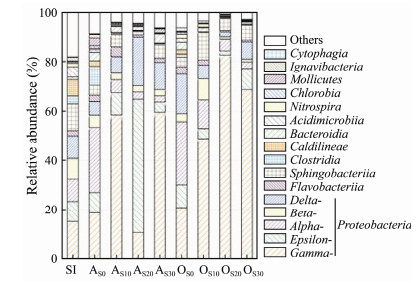
Fig. 6 shows the relative abundance of microbial communities in phylum or class. The microbial community of seeding sludge (Sl) presented the highest abundance with 12 phyla, among which Proteobacteria held the highest relative abundance. At the end of startup of the reactor, the relative abundance of Gammaproteobacteria in samples increased to 18.02% and 20.16% in the anoxic and oxic zones with the improvement of salinity. At the same time, the relative abundance of Alphaproteobacteria increased from 9.27% to 25.97%, and that of Epsilonproteobacteria remained stable at about 8.30%. In addition, Betaproteobacteria and Deltaproteobacteria gradually decreased slightly. Overall, Proteobacteria was gradually enriched with salinity improvement during startup, which indicated that most of the microorganisms in Proteobacteria were salt-tolerant.
|
Fig. 6 Microbial communities' composition and relative abundance in seed sludge (SI) and biofilm samples from anoxic zone (AS0, AS10, AS20, AS30) and oxic zone (OS0, OS10, OS20, OS30). |
In the anoxic zone of the reactor, with the increase of SMX concentration, the relative abundance of Gammaproteobacteria in biofilm increased from 17.85% to 58.22%, 10.41% and 59.19%, respectively. Deltaproteobacteria was also enriched slightly. On the contrary, Alphaproteobacteria and Betaproteobacteria gradually reduced. Proteobacteria tended to be gradually enriched.
In the oxic zone of the reactor, with the increase of SMX concentration, the relative abundance changes of microorganisms at the phylum level were similar with those in the anoxic zone. The relative abundance of Gammaproteobacteria in biofilm increased from 20.96% to 49.79%, 83.31% and 69.29%, respectively. Alpharoteobacteria, Betaproteobacteria, Deltaproteobacteria and Epsilonproteobacteria gradually decreased, which was different from previous report by Zheng et al. (2016), where they found out that the Betaproteobacteria increased largely with the rise of the concentration of norfloxacin. This might be due to the difference of antibiotics, as different antibiotics might present unique inhibition on some bacteria. In general, Proteobacteria in biofilm tended to be gradually enriched with the treatment of artificial mariculture wastewater containing SMX.
Previous research (Katipoglu-Yazan et al., 2015) showed that long-term SMX exposure gradually reduced the gene expression of nitrifying bacteria and impaired cell activity. Meanwhile, partial degradation of SMX by heterotrophic bacteria was observed. Based on the changes in the operation efficiency of the comprehensive reactor and the number of same and endemic OTU of microorganisms, it was speculated that with the increase of the concentration of SMX, some heterotrophic bacteria capable of degrading organic matter were enriched in the anoxic zone of the reactor. Therefore, the removal of COD in the reactor remained acceptable. However, some ammonia-oxidizing, nitrifying and denitrifying bacteria might reduce, consequently the removal rates of NH4+-N, NO2−-N and NO3−-N greatly decreased.
3.3 Salt-Tolerant Microbiota Contributing to Contaminants RemovalAs for the oxic zone of bioreactor when it started up, the genera Arcobacter and Thiothrixs were largely enriched in aerobic biofilm (OS0) and they reached to 9.27% and 6.24% of total bacteria (Table 2). Some other salttolerant bacteria, such as Marinobacter, Idiomarina, Pseudohongiella, Thalassospira, Hyphomonas, Paracoccus, Ilumatobacter, Cyclobacterium were also improved to above 1% of total bacteria, which indicated that high salt in the seawater played a key role in enriching the specific bacteria. The typical nitrifier Nitrosomonas presented a little increase to 0.67%. However, when the SMX was added, the profiles of dominant bacteria changed significantly. Almost all above mentioned enriched bacteria declined in relative abundance except the Thiothrixs, which was further improved to 40.72% under 10 mg L−1 SMX, 76.17% under 20 mg L−1 SMX, and 63.24% under 30 mg L−1 SMX. The genera Azoarcus, Nitrosomonas, Lewinella jumped to 5.01%, 2.55% and 6.70% in abundance as the reactor feeding with 10 mg L−1 SMX, but they decreased obviously as feeding with 20 mg L−1 and 30 mg L−1.
|
|
Table 2 Changes in relative abundance of the microbial communities in genus level |
Similar changes of microbial community in anoxic zone took place as the bioreactor started up (biofilm sample AS0). Compared with the sludge, the genera Arcobacter, Pseudomonas, Marinobacter, Idiomarina, Marinobacterium, Thiothrix, Celeribacter, Roseibaca, Fusibacter were largely enriched by high salt in biofilm sample AS0. However, most of the genera, except the Thiothrix, their abundance decreased with the increasing feed of SMX. The relative abundance of genera Desulfuromusa and Lewinella also presented an increase with the sudden feed of SMX which might suggest they were resistant to a low concentration of SMX (Zheng et al., 2016).
Undoubtedly, Arcobacter is a very important genus in the bioreactor due to its high abundance, from 1.63% to 53.52%, throughout the operation. Some of Arcobacter were considered as the pathogens with resistance to many antibiotics (Kabeya et al., 2004). However, we further retrieved the Arcobacter sequences in NCBI nucleotide database and found that the most similar isolates (> 99.90%) were the Candidatus Arcobacter sp. CpA_a5 (Genbank Accession No: FN397893) from anoxic subsurface mud breccia of the Capt Arutyunov mud volcanoa and Arcobacter nitrofigilis DSM 7299 (CP00 1999) from the roots of cordgrass growing in salty marshes at the East coast of Canada. Compared with many other Arcobacter species which are associated with warmlooded anmimals and tend to pathogenic, A. nitrofigilis is microaerophilic, chemoorganotrophic, halotolerant, antibiotics-resistant and symbiotic bacterium with the ablity of fixing nitrogen and reducing nitrate in a marine environment (Mcclung et al., 1983). In addition, many Arcobacter species were also one of the most commonly found groups in wastewater (Guo et al., 2019; Pishgar et al., 2019) and they were commonly identified as the key potential denitrifier under the anoxic condition (Pishgar et al., 2019). Considering the bioreactor performance and corresponding abundance of Arcobacter, our results suggested this genus might play an important role in removing nitrogen in the high antibiotics and salinity wastewater treatment system.
Thiothrix, as an important driver of nitrogen and sulfur cycling, was largely enriched throughout the bioreactor operation. It is amazing that its relative abundance reached to 76.17% in the oxic zone as the bioreactor feeding 20 mg L−1 SMX. Many reports showed this group took part in the nitrogen and sulfur biogeochemical cycle and were responsible for the formation of granular sludge, sludge bulking and presented strong resistance to environmental pressure, such as the antibiotics (van den Akker et al., 2015; Du et al., 2019). Howarth et al. (1999) found out that Thiothrix utilized different carbon sources to remove nitrite or nitrate in sewage treatment systems. Du et al. (2019) concluded that the abundance of Thiothrix significantly increased from 3.46% to 24.09% and sludge bulking was accelerated by feeding the 50 mg L−1 tetracycline and 10 mg L−1 SMX. Hence, the high abundance of Thiothrix, even not affected by SMX, may be the direct reason that the A/O-MBBR reactor presented acceptable removal efficiency on nitrogen in the treatment of aquaculture wastewater containing SMX.
Desulfuromusa was gradually enriched in anoxic zone by feeding the SMX. Previous research showed that they were salt tolerance, gram-negative, obligate anaerobic sulfur-reducing bacteria and exhibited complete oxidation of a wide range of electron donors (e.g., dicarboxylic acids and amino acids) linked to the reduction of sulfur (Liesack and Finster, 1994). However, till now, no results indicated that this genus could live or work under the antibiotics press. Hence, the presence of Desulfuromusa might contribute to the carbon and sulfur cycling.
Nitrosomonas, as one of important nitrifier, presented a high abundance (2.55%) in the biofilm of oxic zone with 10 mg L−1 SMX. They obtain energy from the oxidation of NH4+-N to NO2−-N. Silyn-Roberts and Lewis (2001) reported that Nitrosomonas presence rapidly increased as high as 7% of the total bacterial populations with the elevated ammonium concentrations. Kang et al. (2018) found that Nitrosomonas contained the genes capable of degrading SMX. Therefore, this genus was SMX-resistant and accelerated the oxidization of ammonia, which consequently enhanced the performance of the bioreactor.
4 ConclusionsThe feeding of 10-30 mg L−1 SMX in bioreactor presented no negative impact on the COD removal. Nitrifying of NH4+-N and NO2−-N demonstrated more tolerant to increasing SMX than denitrifying of NO3−-N. The dosage of SMX decreased the diversity of biofilm microbial communities, salt-tolerant Arcobacter, Thiothrix, Desulfuromusa and Nitrosomonas were extremely enriched. Combined the performance of bioreactor and their abundance, the cooperation of these bacteria played the fundamental role in COD removal, and nitrogen nitrification and denitrification. Finally, the process achieved the purpose of pollutants removal in the presence of low concentration of SMX.
AcknowledgementsWe thank Dr. Progress Mapindu in Ocean University of China for his carefully revising our manuscript. This work was supported by the Fundamental Research Funds for the Central Universities of China (No. 201964004) and the National Natural Science Foundation of China (No. 41977315).
Cetecioglu, Z., Ince, B., Gros, M., Rodriguez-Mozazc, S., Barcelo, D., Orhon, D. and Ince, O., 2013. Chronic impact of te-tracycline on the biodegradation of an organic substrate mixture under anaerobic conditions. Water Research, 47: 2959-2969. DOI:10.1016/j.watres.2013.02.053 (  0) 0) |
Chinese, NEPA, 2002. Water and Wastewater Monitoring Methods. 4th. Chinese Environmental Science Publishing House, Beijing.
(  0) 0) |
Du, B., Wang, R., Yang, Q., Hu, H., Li, X. and Duan, X., 2018. Impact of tetracycline on the performance and abundance of functional bacteria of a lab-scale anaerobic-aerobic wastewater treatment system. Biochemical Engineering Journal, 138: 98-105. DOI:10.1016/j.bej.2018.07.009 (  0) 0) |
D, u, B,, Ya, ng, Q,, L, i, X,, Yuan, W., Chen, Y. and Wang, R., 2019. Impacts of long-term exposure to tetracycline and sulfamethoxazole on the sludge granules in an anoxic-aerobic wastewater treatment system. Science of the Total Environment, 684: 67-77. DOI:10.1016/j.scitotenv.2019.05.313 (  0) 0) |
Gao, C., Wang, A., Wu, W.M., Yin, Y. and Zhao, Y., 2014. Enrichment of anodic biofilm inoculated with anaerobic or aerobic sludge in single chambered air-cathode microbial fuel cells. Bioresource Technology, 167: 124-132. DOI:10.1016/j.biortech.2014.05.120 (  0) 0) |
Gao, F., Li, Z., Chang, Q., Gao, M., She, Z., Wu, J., Jin, C., Zheng, D., Guo, L., Zhao, Y. and Wang, S., 2018. Effect of florfenicol on performance and microbial community of a sequencing batch biofilm reactor treating mariculture wastewater. Environmental Technology, 39: 363-372. DOI:10.1080/09593330.2017.1301567 (  0) 0) |
Gui, M., Chen, Q. and Ni, J., 2017. Effect of sulfamethoxazole on aerobic denitrification by strain Pseudomonas stutzeri PCN-1. Bioresource Technology, 235: 325-331. DOI:10.1016/j.biortech.2017.03.131 (  0) 0) |
Guo, B., Liu, C. and Gibson, C., 2019. Wastewater microbial community structure and functional traits change over short timescales. Science of the Total Environment, 662: 779-785. DOI:10.1016/j.scitotenv.2019.01.207 (  0) 0) |
Howarth, R., Unz, R.F., Seviour, E.M., Seviour, R.J., Blackall, L.L., Pickup, R.W., Jones, J.G., Yaguchi, J. and Head, I.M., 1999. Phylogenetic relationships of filamentous sulfur bacteria (Thiothrix spp. and Eikelboom type 021N bacteria) isolated from wastewater-treatment plants and description of Thiothrix eikelboomii sp.nov., Thiothrix unzii sp.nov., Thiothrix fructosivorans sp.International Journal of Systematic and Evolutionary Microbiology, 49: 1817-1827. (  0) 0) |
Kabeya, H., Maruyama, S., Morita, Y., Ohsuga, T., Ozawa, S., Kobayashi, Y., Abe, M., Katsube, Y. and Mikami, T., 2004. Prevalence of Arcobacter species in retail meats and antimicrobial susceptibility of the isolates in Japan. International Journal of Food Microbiology, 90: 303-308. DOI:10.1016/S0168-1605(03)00322-2 (  0) 0) |
Kang, A.J., Brown, A.K. and Wong, C.S., 2018. Variation in bacterial community structure of aerobic granular and suspended activated sludge in the presence of the antibiotic sulfamethoxazole. Bioresource Technology, 261: 322-328. DOI:10.1016/j.biortech.2018.04.054 (  0) 0) |
Katipoglu-Yazan, T., Merlin, C. and Pons, M.N., 2015. Chro-nic impact of tetracycline on nitrification kinetics and the activity of enriched nitrifying microbial culture. Water Research, 72: 227-238. DOI:10.1016/j.watres.2014.12.041 (  0) 0) |
Lagana, P., Caruso, G., Minutoli, E., Zaccone, R. and Santi, D., 2011. Susceptibility to antibiotics of Vibrio spp. and Photobacterium damsela ssp.piscicida strains isolated from Italian aquaculture farms.New Microbiologica, 34: 53-63. (  0) 0) |
Li, S., Zhang, S. and Ye, C., 2017. Biofilm processes in treating mariculture wastewater may be a reservoir of antibiotic resistance genes. Marine Pollution Bulletin, 118: 289-296. DOI:10.1016/j.marpolbul.2017.03.003 (  0) 0) |
Li, Z., Chang, Q. and Li, S., 2017. Impact of sulfadiazine on performance and microbial community of a sequencing batch biofilm reactor treating synthetic mariculture wastewater. Bioresource Technology, 235: 122-130. DOI:10.1016/j.biortech.2017.03.113 (  0) 0) |
Liesack, W. and Finster, K., 1994. Phylogenetic analysis of five strains of gram-negative, obligately anaerobic, sulfur-redu-cing bacteria and description of Desulfuromusa gen. nov., Including Desulfuromusa kysingii sp.nov., Desulfuromusa bakii sp.nov., and Desulfuromusa succinoxidans sp.nov.International Journal of Systematic and Evolutionary Microbiology, 44: 753-758. (  0) 0) |
Liu, X., Steele, J.C. and Meng, X.Z., 2017. Usage, residue, and human health risk of antibiotics in Chinese aquaculture:A review. Environmental Pollution, 223: 161-169. DOI:10.1016/j.envpol.2017.01.003 (  0) 0) |
Mcclung, C.R., Patriquin, D.G. and Davis, R.E., 1983. Campylobacter nitrofigilis sp. nov., a nitrogen-fixing bacterium associated with roots of Spartina alterniflora Loisel. International Journal of Systematic and Evolutionary Microbiology, 33: 605-612. (  0) 0) |
Mo, W.Y., Chen, Z., Leung, H.M. and Leung, A., 2017. Application of veterinary antibiotics in China's aquaculture industry and their potential human health risks. Environmental Science and Pollution Research International, 24: 8978-8989. DOI:10.1007/s11356-015-5607-z (  0) 0) |
Pishgar, R., Dominic, J.A., Sheng, Z. and Tay, J.H., 2019. De-nitrification performance and microbial versatility in response to different selection pressures. Bioresource Technology, 281: 72-83. DOI:10.1016/j.biortech.2019.02.061 (  0) 0) |
Saxena, S.K., Rangasamy, R., Krishnan, A.A., Singh, D.P., Uke, S.P., Malekadi, P.K., Sengar, A.S., Mohamed, D.P. and Gupta, A., 2018. Simultaneous determination of multi-residue and multi-class antibiotics in aquaculture shrimps by UPLC-MS/MS. Food Chemistry, 260: 336-343. DOI:10.1016/j.foodchem.2018.04.018 (  0) 0) |
Silyn-Roberts, G. and Lewis, G., 2001. In situ analysis of Nitrosomonas spp. in wastewater treatment wetland biofilms.Water Research, 35: 2731-2739. (  0) 0) |
Stolker, A.A.M. and Brinkman, U.A.T., 2005. Analytical strategies for residue analysis of veterinary drugs and growth-promoting agents in food-producing animals–A review. Jour-nal of Chromatography A, 1067: 15-53. DOI:10.1016/j.chroma.2005.02.037 (  0) 0) |
Sun, M., Chang, Z. and Rico, A., 2016. Environmental and human health risks of antimicrobials used in Fenneropenaeus chinensis aquaculture production in China. Environmental Science and Pollution Research, 23: 5689-5702. (  0) 0) |
Tong, X., Wang, X., He, X., X, u., K, . and Mao, F., 2019. Effects of ofloxacin on nitrogen removal and microbial community structure in constructed wetland. Science of the Total Environ-ment, 656: 503-511. DOI:10.1016/j.scitotenv.2018.11.358 (  0) 0) |
van den Akker, B., Reid, K., Middlemiss, K. and Krampe, J., 2015. Evaluation of granular sludge for secondary treatment of saline municipal sewage. Journal of Environmental Management, 157: 139-145. (  0) 0) |
Wang, J.H., Lu, J., Zhang, Y.X., Wu, J., Luo, Y.M. and Liu, H., 2018. Metagenomic analysis of antibiotic resistance genes in coastal industrial mariculture systems. Bioresource Technology, 253: 235-243. DOI:10.1016/j.biortech.2018.01.035 (  0) 0) |
William, C., Richard, V., Mark, S., Mark, P., Christopher, P. and Garth, E., 2003. The application of biofilm science to the study and control of chronic bacterial infections. Journal of Clinical Investigation, 112: 1466-1477. DOI:10.1172/JCI200320365 (  0) 0) |
Wolcott, R.D., Rhoads, D.D., Bennett, M.E., Wolcott, B.M., Gogokhia, L., Costerton, J.W. and Dowd, S.E., 2010. Chronic wounds and the medical biofilm paradigm. Journal of Wound Care, 19: 52-53. (  0) 0) |
Xiong, W.G., Sun, Y.X., Zhang, T., Ding, X.Y., Li, Y.F., Wang, M.Z. and Zeng, Z.L., 2015. Antibiotics, antibiotic resistance genes, and bacterial community composition in fresh water aquaculture environment in China. Microbial Ecology, 70: 425-432. DOI:10.1007/s00248-015-0583-x (  0) 0) |
Zhang, Q.Q., Ying, G.G., Pan, C.G., Liu, Y.S. and Zhao, J.L., 2015. Comprehensive evaluation of antibiotics emission and fate in the river basins of China:Source analysis, multimedia modeling, and linkage to bacterial resistance. Environmental Science & Technology, 49: 6772-6782. (  0) 0) |
Zheng, D., Chang, Q., Gao, M., She, Z.L., Jin, C.J., Guo, L., Zhao, Y.G., Wang, S. and Wang, X.J., 2016. Performance evaluation and microbial community of a sequencing batch biofilm reactor (SBBR) treating mariculture wastewater at different chlortetracycline concentrations. Journal of Environmental Management, 182: 496-504. DOI:10.1016/j.jenvman.2016.08.003 (  0) 0) |
Zheng, D., Chang, Q., Li, Z.W., Gao, M.C., She, Z.L., Wang, X.J., Guo, L., Zhao, Y.G., Jin, C.J. and Gao, F., 2016. Performance and microbial community of a sequencing batch biofilm reactor treating synthetic mariculture wastewater under long-term exposure to norfloxacin. Bioresource Technology, 222: 139-147. DOI:10.1016/j.biortech.2016.09.114 (  0) 0) |
Zheng, D., Gao, M., Wang, Z., She, Z.L., Jin, C.J. and Chang, Q.B., 2016. Performance comparison of biofilm and suspended sludge from a sequencing batch biofilm reactor treating mariculture wastewater under oxytetracycline stress. Environmental Technology, 37: 2391-2404. DOI:10.1080/09593330.2016.1150353 (  0) 0) |
Zhu, Y., Wang, Y., Jiang, X., Zhou, S., Wu, M., Pan, M. and Chen, H., 2017. Microbial community compositional analysis for membrane bioreactor treating antibiotics containing wa-stewater. Chemical Engineering Journal, 325: 300-309. DOI:10.1016/j.cej.2017.05.073 (  0) 0) |
 2020, Vol. 19
2020, Vol. 19


