Reproduction is necessary for the perpetuation of living beings. As infertility happens occasionally in some animal individuals, the process and mechanism of gametogenesis of animals are investigated. In aquaculture industry, fish reproduction is involved in fry breeding that directly related to fish gametogenesis. Therefore, the process and mechanism of fish gametogenesis is an important field of research.
Mitochondria are required for male gametogenesis, namely spermatogenesis, the process of sperm production from spermatogonia. Mitochondrial defects caused by a gene mutation, hypoxia, or an environmental pollutant can result in abnormal spermatogenesis and even male infertility (Hales and Fuller, 1997; Huang et al., 2015; Quan et al., 2016). In addition, spermatogenesis is regulated by the mitochondria shape, which is dynamic and can adapt to the different metabolic levels in spermatogenic cells. For instance, in adult rat testes (Rattus norvegicus), orthodox mitochondria were observed in spermatogonia and leptotene spermatocytes, while condensed mitochondria were detected in spermatocytes at later stages and spermatids, possibly because it was more efficient in producing ATP (Ramalho-Santos et al., 2009; Rato et al., 2012). In Pelodiscus sinensis, mitochondria shape changes from rounded to an onion-like shape by elongation, fusion, and swelling during spermiogenesis (Haseeb et al., 2018). To understand the mitochondria dynamic during spermatogenesis, the functions of some mitochondrial proteins in spermatogenesis including GASZ, mitofusin 1 (MFN1), mitofusin 2 (MFN2), and dynamin-related protein 1 were investigated at a molecular level (Honda and Hirose, 2003; Zhang et al., 2016; Varuzhanyan et al., 2019). For instance, conditional deletion of the mouse mitofusin 1 gene in the testis that plays a role during mitochondrial fusion caused male infertility (Zhang et al., 2016; Varuzhanyan et al., 2019), indicating the importance of mitochondrial fusion in spermatogenesis. However, the molecular mechanism that regulates the mitochondrial dynamic and physiology during spermatogenesis is still not completely understood and needs further studied.
Prohibitin (PHB) is an important protein for the mitochondria morphology and physiology. PHB in the inner mitochondrial membrane (IMM) usually forms a complex with PHB2. The complex regulates the membrane protein degradation by the association with the m-AAA protease (Steglich et al., 1999); serves as a chaperone for newly synthesized proteins (Nijtmans et al., 2000, 2002); stabilizes the mtDNA by interacting with the TFAM protein, a component of the mitochondrial nucleoids (Wang and Bogenhagen, 2006; Bogenhagen et al., 2008); maintains the tubular cristae, and promotes mitochondrial fusion (Merkwirth et al., 2008; Merkwirth and Langer, 2009). These PHB functions are important for mitochondrial biogenesis, metabolic regulation, and cell migration, survival, and proliferation (Artal-San and Tavernarakis, 2009; Merkwirth and Langer, 2009). In addition, PHB was also detected in the plasma membrane, inhibiting inflammatory responses and mediating Ras-Raf signaling (Sharma and Qadri, 2004; Rajalingam and Rudel, 2005), and in cell nucleus, regulating the gene transcription (Mishra et al., 2005, 2006; Thuaud et al., 2013). Therefore, the PHB function depends on its location. In reproductive biology, PHB was involved in spermatogenesis and related to sperm quality. For instance, PHB deficiency resulted in abnormal spermatogenesis in Caenorhabditis elegans (Sanz et al., 2003). Low levels of PHB in Bubalus bubalis were associated with low-motility spermatozoa (Xiong et al., 2018). Meanwhile, in humans, they were related to asthenospermia and oligoasthenospermia (Wang et al., 2012). Furthermore, Chai et al. (2017) noted the low level of PHB increased the concentration of mitochondrial reactive oxygen species and lipid peroxidation in sperm, lowering the sperm quality. Thus, these results promoted the study of the PHB function in spermatogenesis.
The aquaculture production of Larimichthys crocea, an economically important species of marine fish of the order Perciformes and family Sciaenidae, has been ranked top one in the maricultured fishes in 2017 in China with a production of 176600 tons. For this reason, L. crocea is an important model fish in marine research and its reproductive biology has been widely researched, including the histological characteristics of gonadal development and its breeding cycle and artificial seedling production (Su et al., 1997; Fang et al., 2003; Liu, 2004; You et al., 2012), whereas the molecular mechanisms related to spermatogenesis are still unknown.
In the present study, we observed the ultrastructure characteristic of L. crocea spermatogenesis and analyzed the characteristic, expression, subcellular localization, and possible functions of PHB during spermatogenesis. This study serves as a basis for future research of the mechanisms of PHB in L. crocea spermatogenesis.
2 Materials and Methods 2.1 Animals and TissuesAll animal experiments in this paper were performed in accordance with the guidelines and procedures approved by the Animal Care and Use Committee of the Ningbo University and the Zhejiang Laboratory Animal Research Center.
Male L. crocea fish were sampled from the Xiangshan Fishery (Ningbo City, Zhejiang Province, China). Nine tissues (muscles, kidneys, testes, hearts, intestines, spleens, gills, livers, and brains) were harvested, immediately frozen in liquid nitrogen, and stored at −80℃ for RNA and protein extraction. Testis sections were fixed in 2.5% glutaraldehyde in 0.1 mol L−1 phosphate buffered saline (PBS; pH 7.4) for transmission electron microscopy (TEM) visualization, and in 4% paraformaldehyde in 0.1 mol L−1 PBS for fluorescent in situ hybridization (FISH) and immunofluorescence (IF) analyses.
2.2 Electron Microscopic Analysis of Spermato genesis in L. croceaThe TEM experiments characterized the ultrastructural features of spermatogenesis in L. crocea. The fixed testes in 2.5% glutaraldehyde 0.1 mol L−1 PBS at 4℃ for 24 h were rinsed in 0.1 mol L−1 PBS three times for 45 min, then fixed in 1% osmium tetroxide in the dark for 1 h, and finally washed in 0.1 mol L−1 PBS. The samples subsequently were dehydrated by immersion into increasing concentrations of ethanol and acetone, treated with an acetonearaldite mixture, and embedded in araldite. Ultrathin sections were obtained with the LKB-α ultramicrotome and stained with uranyl acetate and lead citrate. The ultrathin sections were examined and photographed with an H-7650 transmission electron microscope (Hitachi).
2.3 Full-Length cDNA Cloning of the phb GeneTotal RNA was extracted from Stage Ⅳ testis by using the TRIzol Reagent (Invitrogen, USA), according to the manufacturer instructions, subjected to 3' and 5' RACE reverse transcription by using the 3' full RACE Amplification (Takara, Japan) and Smart RACE cDNA Amplification Kits (Clontech, USA), respectively. The reverse transcribed products (cDNA) were stored at −25℃ for subsequent PCR.
The intermediate segment sequence of Lc-phb (GenBank accession number: XM_010740095.3) was confirmed by PCR and gene sequencing. Then, we designed the specific primers by using Primer Premier 5.0 software for RACE (Table 1). The touch-down PCR used for 3'/5' RACE was conducted as follows: 94℃ for 5 min; 8 cycles of 94℃ for 30 s, 62/69℃ for 30 s (decreased by 0.5℃/cycle) and 72℃ for 1 min; 27 cycles of 94℃ for 30 s, 58/65℃ for 30 s and 72℃ for 1 min; and then a final extension at 72℃ for 10 min. These PCR products were separated, identified, and sequenced, based on the method described by Zhang et al. (2017). We assembled the intermediate segment and the clonal 3' and 5' cDNA segment sequences and obtained the full-length cDNA of Lc-phb.
|
|
Table 1 Primer and probe sequence used in this study for phb cDNA full-length cloning and fluorescent in situ hybridization |
The predictions of Lc-PHB protein structure and property were conducted with online tools: the Sequence Manipulation Suite (http://www.bio-soft.net/sms/) was used for predicting the primary structure; the ProtParam tool (https://web.expasy.org/protparam/) was used for calculating the molecular weight and the isoelectric point; the online tool (https://embnet.vital-it.ch/software/TMPRED_form.html) was used for predicting transmembrane segments; the online tool (https://embnet.vital-it.ch/software/COILS_form.html) was used for detecting coiled-coil domains; and the I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) was used for predicting the tertiary structure. Sequence alignment and phylogenetic analyses were performed with the software Vector NTI and Mega 5.1, respectively. The amino acid (aa) sequences of PHB proteins were downloaded from National Center of Biotechnology Information (https://www.ncbi.nlm.nih.gov/), and their GenBank accession number were listed in Table 2.
|
|
Table 2 PHB proteins information and corresponding GenBank accession numbers used in this study |
Real-time quantitative PCR (qPCR) was used for analyzing the Lc-phb mRNA expression. The total RNA, extracted from muscles, kidneys, testes, hearts, intestines, spleens, gills, livers, and brains (collected from three male fish), was reversed transcribed by using the Prime-Script® RT reagent Kit (Takara, Japan). The reverse transcription products (cDNA) were stored in −25℃ until performing the qPCR experiment. The primers used for qPCR are listed in Table 1, and the β-actin gene was employed as housekeeping gene, based on Luo et al. (2019). The qPCR assay was conducted in the Rotor-Gene 6000 System. The reaction system contained 5 μL 1:25 diluted cDNA, 1 μL each primer (10 μmol L−1), 3 μL PCR-grade water, and 10 μL Master Mix (Genestar, China). The qPCR was conducted at 95℃ for 4 min, followed by 40 amplification cycles (denaturation at 95℃ for 10 s, annealing at 60℃ for 15 s, and extension at 72℃ for 15 s). All samples were run in triplicates on the same plate. The comparative delta-delta Ct method was applied for analyzing the gene expression of Lc-phb in different tissues. The liver was selected as the comparator samples for determining the relative mRNA expression levels of Lc-phb in different tissues. All data are expressed as the mean ± SD (standard deviation; n = 3). The one-way analysis of variance (ANOVA) with TukeyKramer Honest Significant Difference test was used to determine significant differences. P < 0.05 was considered statistically significant differences.
2.6 Fluorescence in situ Hybridization (FISH) AnalysisThe frozen testis sections were fixed in 4% paraformaldehyde in PBS at 4℃ for 20 h, then washed in 0.1 mol L−1 PBS, incubated with 0.5 mol L−1 sucrose-PBS (pH 7.4) overnight at 4℃, finally embedded in O.C.T. compound, and frozen at −80℃. The embedding blocks were sliced into 5-μm-thick frozen sections using a sliding microtome and placed on the RNase-free poly-lysine-coated slides, which were stored at −80℃ until further use. The probe (Table 1), conjugated to fluorescein isothiocyanate, was synthesized by Generay Biotech, Co., Ltd. (Shanghai, China). The hybridization experiments were conducted based on Xu et al. recommendations (2018). The negative control groups were incubated with a sense probe. The sections were observed and photographed with the Zeiss laser scanning confocal microscope (LSM880; Carl Zeiss, Germany).
2.7 Immunofluorescence (IF) AnalysisThe mouse anti-human PHB antibody (Abzoom, America) was applied in this experiment. Its specificity was determined by western blotting (WB) experiment according to Zhang et al. (2017). One single protein band (29 kDa) was detected in advance to certify the IF experiment can be conducted using this antibody. The experiments were conducted according to Zhang et al. (2017). The primary antibody (1:100 dilution) used in this study was mouse anti-human PHB antibody, and the secondary antibody (1:500 dilution) was Alexa Fluor 555-labelled donkey antimouse IgG (H+L; Beyotime, China). Meanwhile the mitochondrial dye (MitoTracker Deep Green FM; 1:2000 dilution) was added in the reaction solution. The negative control samples were incubated without the addition of primary antibodies. The fluorescence signal was then observed and photographed with the Zeiss laser scanning confocal microscope (LSM880; Carl Zeiss, Germany).
2.8 Extraction of Mitochondria and Detection of PHB ProteinMitochondria and cytoplasm were extracted from Stage Ⅳ testes with the tissue mitochondria isolation Kit (Beyotime, China) following the manufacturers' instructions. The WB experiments were cnducted according to Zhang et al. (2017) to detect PHB levels (with mouse anti-human PHB antibody) in the mitochondrial and cytoplasmic protein fractions. The cytochrome c oxidase subunit I (COX I) and β-tubulin proteins were used to confirm the efficient separation of mitochondria and cytoplasm, respectively. Rabbit anti-COX I and rabbit anti-β-tubulin antibodies were purchased from Abzoom (America) and Beyotime (China), respectively.
3 Results 3.1 Cytologic Characterization of SpermatogenesisL. crocea spermatogenesis included the proliferation and differentiation of spermatogonia, two cycles of meiosis, and spermiogenesis, during which spermatids develop into sperm. Cytological features of spermatogenic cells are described below.
3.1.1 SpermatogoniaSpermatogonia, usually are near the basement membrane and adhere to Sertoli cells, are the largest male germ cells with oval nuclei and with one or more nucleoli (Fig.1). Abundant mitochondria concentration was found in the cytoplasm of spermatogonia. The nuage, a unique germ-line organelle with high electron density in the cytoplasm, was observed near the nuclear membranes or randomly in cytoplasm (Fig.1). In addition, several mitochondria associated with a nuage, described as an intramitochondrial cement, formed a mitochondrial cluster. Mitochondria shapes were mainly circular or oval and infrequently clubbed with the tubular cristae (Fig.1).
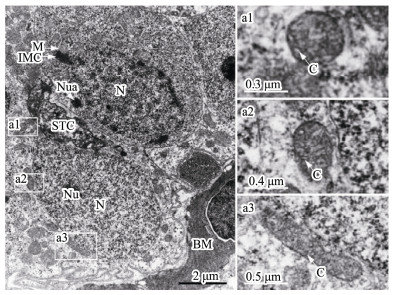
|
Fig. 1 Ultrastructure of spermatogonia. (a1– a3) Representative mitochondria in spermatogonia. BM, basement membrane; C, mitochondrial cristae; IMC, intramitochondrial cement; M, mitochondria; N, nucleus; Nu, nucleolus; Nua, nuage; STC, Sertoli's cells. |
Primary spermatocytes, arising from the differential spermatogonia, had a little volume than spermatogonia. The chromosome presented different features owing to the different developmental stages of the primary spermatocytes during meiosis (Figs.2A-E). During meiosis prophase, the leptotene, zygotene, pachytene, and diplotene cells were observed. Mitochondria were randomly distributed in the cytoplasm. In some cells, several mitochondria associated with intramitochondrial cement were observed, similarly to the findings obtained with spermatogonia (Figs.2B). The shapes of mitochondria were circular, elliptic, clubbed, and occasionally irregular with tubular cristae (Figs.2J-M).
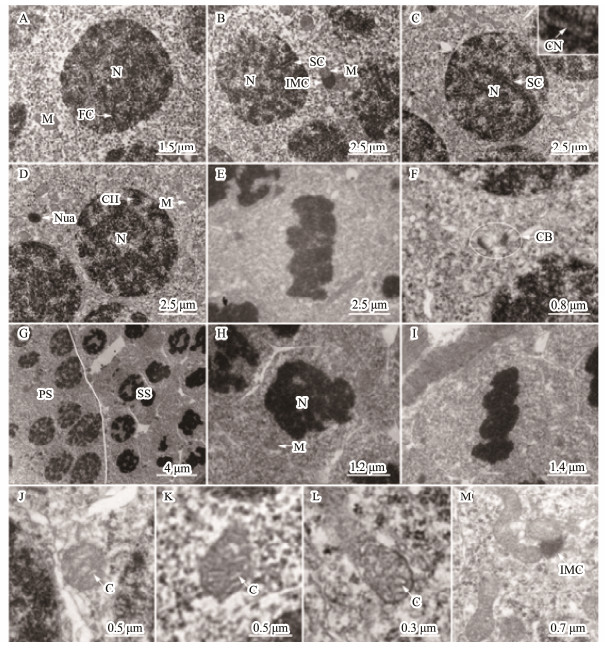
|
Fig. 2 Ultrastructure of spermatocyte. (A-F) Primary spermatocyte. The volume of primary spermatocytes smaller than spermatogonia. The chromosome has adopted different forms due to the different stages during meiosis. (A) Leptotene cell. The chromosome as a fibriform with one end linking the nuclear membranes. (B) Zygotene cell. The synaptonemal complex was formed by the pair of homologous chromosome and emerged near the nuclear membranes. (C) Pachytene cell. The synapsis was completed. A combination nodule was observed in the middle site of the synaptonemal complex. (D) Diplotene cell. The homologous chromosomes were separated and only associated by some chiasmas. (E) Metaphase I cell. The chromosome gathered in the equatorial plane. (F) Cytoplasmic bridge in primary spermatocytes. (G) Primary spermatocytes (PS) and secondary spermatocytes (SS). (H) Secondary spermatocytes. (I) Metaphase II cell. The volume, especially that of secondary spermatocytes was reduced, compared to that of primary spermatocytes (H). Secondary spermatocytes quickly performed the second meiosis. Therefore, the secondary spermatocytes were usually in metaphase II (J). Mitochondria were randomly distributed in the cytoplasm of spermatocytes and also associated with IMC in the cytoplasm. (J-L) Representative mitochondrial morphology in spermatocytes. (M) Occasional mitochondrial morphology observed in spermatocyte. Mitochondria consisted of circular, elliptic, clubbed, and occasionally irregularly shaped with the tubular cristae in spermatocyte. C, mitochondrial cristae; CB, cytoplasmic bridge; CH, chiasma; FC, fibriform chromosome; IMC, intermitochondrial cement; M, mitochondria; N, nucleus; Nua, nuage; Nu, nucleolus; SC, synaptonemal complex; CN, combination nodule. |
Secondary spermatocytes were smaller with a shorter life, and underwent meiotic division faster than the primary ones (Figs.2G-I).
3.1.3 Spermiogenesis and spermSpermiogenesis, the last stage of spermatogenesis, is a process by which spermatids mature into spermatozoids. Based on the cell size, nucleus shape, chromatin condensation degree, and mitochondrial distribution characteristics, we divided the spermatids into three developmental stages during spermiogenesis, including early spermatid, middle spermatid, and late spermatid. The early spermatid was smaller than the second spermatocyte after meiosis (Fig.3A1). The initial spermatid formed from the second meiosis had a high electron density in the nuclei (Fig.3A2). With development, the chromatin was well-distributed in the nuclei (Fig.3A3). Mitochondria, mainly oval or elliptic with the tubular cristae, were randomly distributed in the perinuclear cytoplasm (Figs.3A3-A4). Flagellum was also observed at this stage (Fig.3A2). The middle spermatid nucleus had high chromatin concentration, were uniformly distributed, and the posterior nuclear fossa was already formed (Fig.3B1). Cell polarity was detected. One side of the posterior nuclear fossa was abundant in cytoplasm, and contained merged mitochondria and vesicles (Figs.3B2-B3). Mitochondria were mainly oval (Figs.3 B3-B4). In addition, a cytoplasmic bridge among spermatids was detected (Fig.3B1). The late spermatid was significantly smaller than the middle spermatid, and its chromatin was highly concentrated with a graininess texture (Figs.3C1-C3). Mitochondria gathered in the future sperm midpiece in a rounded shape (Figs.3C3-C4). In fact, the morphology and volume of the late spermatids were all similar to those of the mature spermatozoa, except for having more residual cytoplasm. After reducing the cytoplasm, mature sperm were then generated. With the rupture of spermatocysts, mature sperm, composed of head and tail, were released into the lobular cavity; meanwhile, the sub-rounded mitochondria with thin tubular cristae gathered in the midpiece (Figs.3D1-D4).
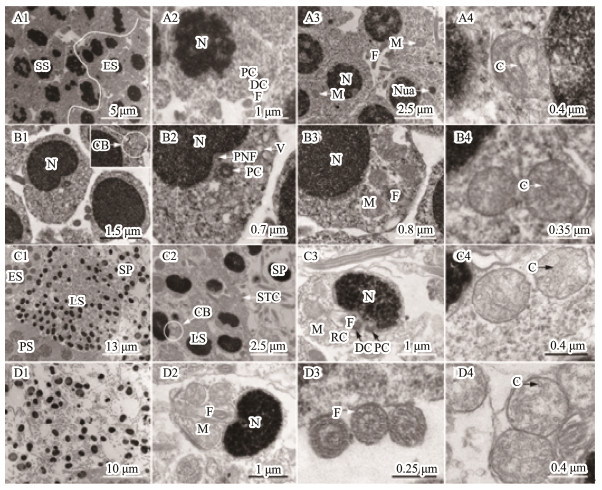
|
Fig. 3 Ultrastructure of spermatids and spermatozoon. (A1-A4) Early spermatid. The initial spermatid formed from the second meiosis had a high electron density in the nuclei (A1, A2). With development, the chromatin was well-distributed in the nuclei (A3). Mitochondria were randomly distributed in the cytoplasm mainly with circular shape and tubular cristae (A3, A4). The flagellum started to form (A2). (B1-B4) Middle spermatid. The chromatin was higher condensed than that in the early spermatids, and well-distributed in the nuclei. The posterior nuclear fossa was formed. The cytoplasm was mainly distributed on one side of the cell, where mitochondria and vesicles gathered. (C1-C4) Late spermatid. The chromatin was more condensed than observed in the middle spermatid with small particles. The volume of the nucleus was significantly small. The cytoplasm was not completely eliminated, and the spermatid package in the cysts by Sertoli's cells. (D1-D4) Sperm. Mature sperm was released in the lobular cavity (D1), consisting of head and tail (D2). The axial filament in the flagellum had a '9 + 2' structure (D3). The mitochondria gathered in the midpiece of the sperm tail with a circular shape and several tubular cristae (D4). C, mitochondrial cristae; CB, cytoplasmic bridge; DC, distal centriole; ES, Early spermatid; F, flagellum; LS, late spermatid; M, mitochondrion; N, nucleus; Nua, nuage; PC, proximal centriole; PNF, posterior nuclear fossa; RC, residual cytoplasm; SP, sperm; SS, secondary spermatocytes; STC, Sertoli's cells. |
Full-length cDNA of Lc-phb gene is 1625 bp (GenBank accession number: MG712297), which consists of a 77 bp 5' untranslated region (UTR), a 735 bp 3' UTR, and an 813 bp open reading frame sequence, encoding 271 aa (Fig.4A).
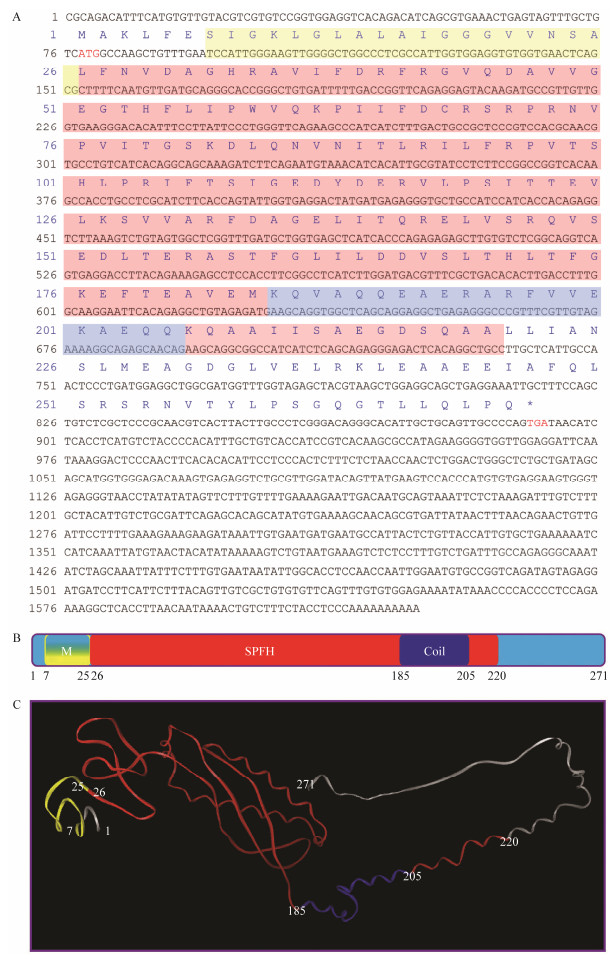
|
Fig. 4 Full-length cDNA and conservative structure of PHB. (A) Full-length cDNA of Lc-phb gene and primary structure of Lc-PHB protein. The initiation and termination codons were ATG and TGA (marked red word), respectively. The sequences labeled by the yellow, red, and light blue shades encoded the transmembrane/hydrophobic, SPFH, and coiled-coil domains, respectively. (B) Predicted function domains of Lc-PHB. The N-terminal transmembrane domain labeled in yellow corresponded to amino acids 7-25. The SPFH domain labeled in red corresponded to amino acids 26-220. The coiled-coil labeled in blue corresponded to amino acids 185-205. (C) Predicted tertiary structure of Lc-PHB. M, transmembrane domain; SPFH, SPFH domain; Coil, coiled-coil domain. |
Lc-PHB protein has a computed molecular weight of 29.8 kDa and theoretical isoelectric point of 5.39. Ala, Arg, Glu, Gly, Leu, Val, Ile, and Ser were dominant amino acids with percentages of 9.2%, 7.0%, 8.1%, 7.0%, 11.1%, 6.6%, and 6.6%, respectively. The Lc-PHB aa sequence analysis predicted three function domains: N-terminal transmembrane domain (7-25 aa), SPFH domain (26-220 aa), and coiled-coil domain (185-205 aa; Fig.4 B), and the tertiary structure is shown in Fig.4C.
In addition, the aa sequence alignment was conducted to analyze the conservation of the Lc-PHB primary structure. As shown in Fig.4D, the primary structure of PHB protein was highly conserved among vertebrates and invertebrates. The similarities of Lc-PHB with its homologues in H. sapiens, B. taurus, G. gallus, X. tropicalis, S. salar, D. rerio, E. sinensis, O. tankahkeei, P. esculenta, and C. elegans are 94.9%, 94.9%, 95.6%, 94.5%, 98.9%, 98.2%, 85.8%, 86.4%, 84.6%, and 78.9%, respectively. The result indicated that the PHB protein was conserved among animals.
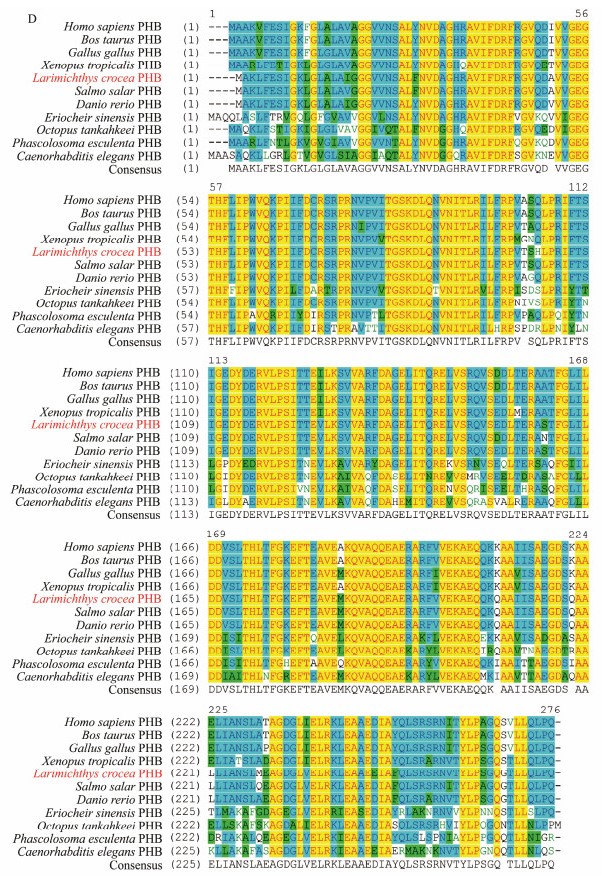
|
Fig. 4D (D) Sequence alignment of PHB amino acids among L. crocea and other species. The regions in yellow showed the same amino acids sequence; in light blue represented more than 50% similarity; in green represented a lower similarity. |
The phylogenetic analysis revealed an evolutionary relationship between PHB proteins (Fig.5). Lc-PHB together with its homologues in vertebrates formed a branch that was the closest to the fish PHB among the examined species. The evolutionary relationship of the Lc-PHB protein agreed with that of L. crocea.
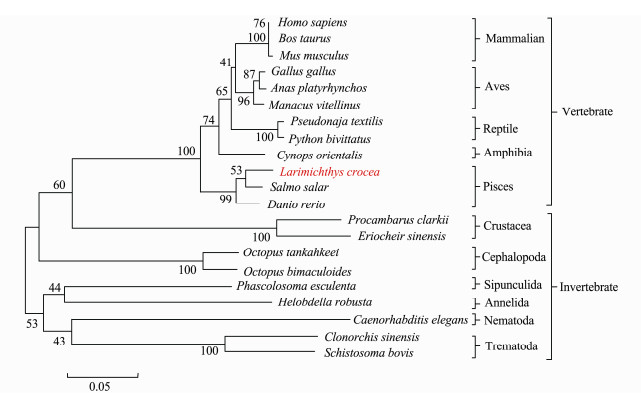
|
Fig. 5 Phylogenetic tree based on the amino acid sequences of PHB proteins. The Mega 5.1 software was used for constructing this NJ phylogenetic tree. Lc-PHB formed a sub-branch with fish PHB. |
As seen in Fig.6A, Lc-phb mRNA was expressed in liver, testis, kidney, spleen, brain, gill, intestine, muscle, and heart. However, according to qPCR, the muscle, liver, and heart contained the higher Lc-phb mRNA expression; while, testis, gill, and brain expressed intermediate levels, and the intestine, kidney, and spleen were correlated with the lower expression.
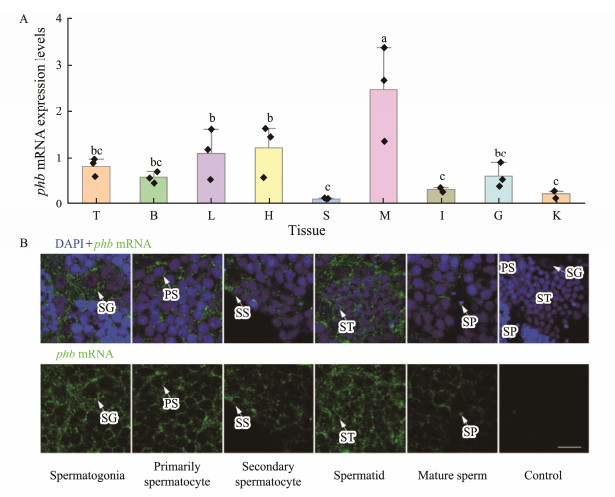
|
Fig. 6 Expression pattern of Lc-phb mRNA. (A) Expression of Lc-phb mRNA in different tissues (n = 3). Different letters above columns indicate significant differences (P < 0.05). T, testis; B, brain; L, liver; H, heart; S, spleen; M, muscle; I, intestine; G, gill; K, kidney. (B) FISH assay showing the expression of Lc-phb mRNA in spermatogenic cells. Blue indicates the nuclei strained by DAPI and green indicates the Lc-phb mRNA signals. Lc-phb mRNA was detected in spermatogonia, primarily spermatocyte, secondary spermatocyte, spermatid and sperm. No positive signals were detected in the control group. SG, spermatogonia; PS, primary spermatocyte; SS, secondary spermatocyte; ST, spermatid; SP, sperm. The scale bar is 10 µm. |
As observed in Fig.6B, FISH detected the expression of Lc-phb mRNA in spermatogenic cells. Lc-phb mRNA was detected in the perinuclear cytoplasm of spermatogonia, spermatocytes, and early spermatids, and in the sperm midpiece (Fig.6B).
3.6 Temporal and Spatial Distribution of Lc-PHB and Mitochondria During SpermatogenesisAs observed in Fig.7, IF assay showed that Lc-PHB protein was localized in the mitochondria present in the perinuclear cytoplasm of the spermatogonia (Figs.7A1-A4), primarily spermatocytes (Figs.7B1-B4), second spermatocytes (Figs.7C1-C4), and early spermatids (Figs.7D1-D4). With the development of spermatids, the Lc-PHB protein was mainly localized in the mitochondria present in one side of the middle spermatid (Figs.7E1-E4). In the sperm, the Lc-PHB and mitochondria were present in the midpiece (Figs.7F1-F5).
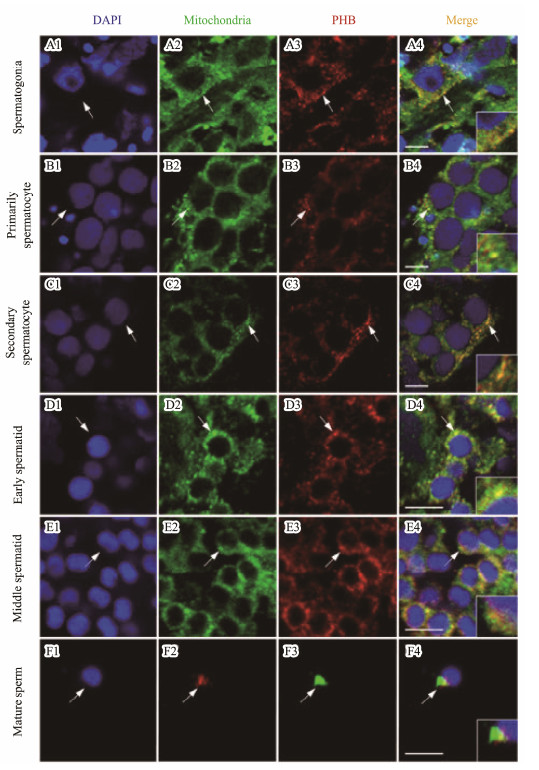
|
Fig. 7 IF assay showed the expression pattern of Lc-PHB during spermatogenesis. Lc-PHB protein was present in the mitochondria located in the cytoplasm around the nuclei in spermatogonia (A1-A4), primarily spermatocyte (B1-B4), secondary spermatocyte (C1-C4), and early spermatid (D1-D4), mainly on one side of nuclei in the cytoplasm of middle spermatid (E1-E4), and in the midpiece of mature sperm (F1-F4). No positive signals were detected in the control group (date not shown). The scale bars are 5 µm. |
To further confirm the Lc-PHB protein location in the mitochondria of spermatogenic cells, WB experiments were performed. As shown in Fig.8, COX I expression was detected only in the mitochondrial protein fraction. Contrarily, we detected tubulin protein in the cytoplasm but not in the mitochondrial fraction. This result indicated that the separation of mitochondria and cytoplasm protein fractions was successful. Subsequently, we only detected PHB protein in the mitochondrial protein fraction. This result and those from the IF experiment revealed the expression and mitochondria location of Lc-PHB during spermatogenesis.
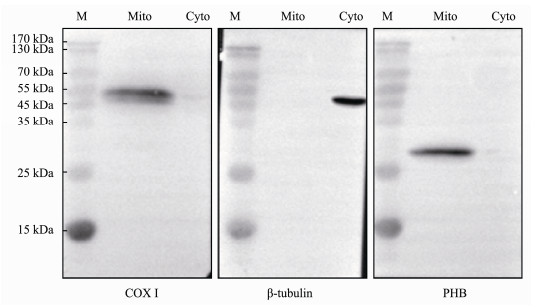
|
Fig. 8 WB assay showed the intracellular location of Lc-PHB in Stage Ⅳ testis. COX I protein was detected in Mito, and β-tubulin protein was detected in Cyto, characterizing both protein fractions and purities. PHB was detected in Mito, confirming that it was located in the mitochondria. M, protein marker; Mito, mitochondria protein fraction purified from Stage Ⅳ testis; Cyto, total protein extracted from cytoplasmic protein fraction (16 µg lane−1). |
Fish spermatogenesis included cystic and semi-cystic types (Mattei et al., 1993). In the cystic type, spermatogenic cells develop into sperm in cysts (spermatocysts). Meanwhile, in the semi-cystic type, cysts open before the sperm matures and therefore partial spermatogenic cells develop into sperm in the tubule lumen (Mattei et al., 1993). The cystic type spermatogenesis occurs in most teleosts, such as in the model organism Danio rerio (Rupik et al., 2011). The semi-cystic type is rare in fish but was observed in Bryconops affinis (Characidae; Andrade et al., 2001) and Ophidion sp. (Ophididae; Mattei et al., 1993). In L. crocea, the spermatogenic cells develop into cysts until they produce sperm. Spermatogenesis, therefore, is cystic type. In cystic type, the cysts are formed from Sertoli cells surrounding individual spermatogonia near the basement membrane. With the proliferation and differentiation of spermatogonia, the Sertoli cells enclose within a cyst (Uribe et al., 2014). In the presence of the same spermatocysts, the cytoplasmic bridge, a structure connecting adjacent cells, is possibly involved in the synchronism of spermatogenic cells by sharing RNA, protein, and other components (Ventelä et al., 2003). In our study, we observed the typical cytoplasmic bridge in spermatocytes and spermatids (Figs.2F, 3B1), indicating that this cytoplasmic bridge plays an important role in exchanging material and guaranteeing the synchronous development of spermatogenic cells in L. crocea. In addition, we proposed that the cytoplasmic bridge may be also necessary for the survival of spermatogenic cells in the testis. Spermatogenic cells mainly rely on Sertoli's cells for nutrition. For spermatogenic cells in the cysts that do not interact directly with Sertoli's cells, the cytoplasmic bridge may be an important way for feeding the spermatogenic cells.
Mitochondria play important roles in spermatogenesis by regulating the physiological status and providing energy for spermatogenic cells (Vertika et al., 2020). Mitochondria maintain their characteristic structures and regulate them during spermatogenesis. They regulate the spermatogenic cells' metabolism levels by promoting cell development and differentiation (Ramalho-Santos et al., 2009; Rato et al., 2012; Haseeb et al., 2018), a requirement for animal spermatogenesis and male fertility (Varuzhanyan et al., 2019). For instance, Varuzhanyan et al. (2019) found that depletion of MFN1 and MFN2 proteins inhibited a metabolic shift during meiosis and therefore arrested the spermatogenesis, highlighting the importance of the normal and dynamic mitochondrial morphology during spermatogenesis. In fish, although the fish spermatogenesis has been extensively reported (Stanley, 1969; Quagio-Grassiotto and Oliveira, 2008; Magalhaes et al., 2011; Rupik et al., 2011; Ribes et al., 2015), the detailed description of the dynamic mitochondria and the regulation of this mechanism were not reported. In this study, we observed the mitochondrial morphological changes during spermatogenesis in L. crocea. The mitochondria always maintained the tubular cristae; however, from spermatogonia to spermatocyte, the mitochondrial morphology became diverse in shape, and mainly was round in spermatid and sperm. A further understanding of the relationship between mitochondrial dynamic and spermatogenic cells' metabolism levels in L. crocea is required.
4.2 PHB Protein Structure and mRNA Expression CharacterizationPHB is an important protein that regulates the mitochondrial dynamic and was involved in animal spermatogenesis. In this study, we cloned the full-length cDNA of phb gene and analyzed its expression after investigating the cytological features of spermatogenesis in L. crocea. Through sequence alignment, we determined that the primary structure of PHB was conserved among different animals (Fig.4D). This result suggested that PHB may have conserved functions and play essential functions in animals; the mutation of the phb gene usually affects the survival of organisms. The importance of PHB was confirmed by previous research, while the knocking down of PHB expression can cause embryo death (Artal-Sanz and Tavernarakis, 2009b). We further predicted the function domain of PHB and detected the potential transmembrane, coiled-coil, and SPFH domains (Fig.4). These domains were also predicted in yeast PHB (Back et al., 2010), Pe-PHB (Hou et al., 2018), OtPHB (Mao et al., 2012), and Human PHB (Winter et al., 2007). In yeast, PHB forms PHB complex with PHB2. The transmembrane domain anchors this complex to the IMM (Tatsuta et al., 2005; Winter et al., 2007; Back et al., 2010), and the coiled-coil domain allows the PHB1 and PHB2 interaction (Tatsuta et al., 2005; Winter et al., 2007; Back et al., 2010). Based on the protein structure analysis, we hypothesized a conserved Lc-PHB function and location in the mitochondria.
According to qPCR experiment, high expression of phb was detected in multiple tissues, including muscle, liver, and heart. Similarly, the phb gene was ubiquitously expressed in multiple tissues of Octopus tankahkeei (Mao et al., 2012), Cynops orientalis (Jin et al., 2016), and P. esculenta (Hou et al., 2018). Artal-Sanz and Tavernarakis (2009a) summarized the PHB expression features and suggested its higher levels in tissues that heavily rely on mitochondrial function. Thus, the higher expression of phb gene in muscle, liver, and heart may indicate that these tissues rely heavily on mitochondrial function in L. crocea. In addition, in the present study, the detection of phb gene expression in spermatogenic cells at different stages by FISH indicated that PHB may play a role in the development of spermatogenic cells.
4.3 Intracellular Location and Putative Functions of PHB During SpermatogenesisA relationship between PHB and animals' fertility was reported. PHB deletion caused abnormal spermatogenesis in C. elegans and down-regulation of PHB expression may result in sperm mitochondrial damage and low sperm quality (Sanz et al., 2003; Wang et al., 2012; Chai et al., 2017). However, the molecular mechanism of PHB on spermatogenesis is inadequate understanding. To study the importance of the mitochondria during spermatogenesis and the multiple physiological functions of PHB in mitochondria, we investigated the spermatogenesis in L. crocea. Lc-PHB was detected in the cytoplasm of spermatogenic cells at different stages and localized within the mitochondria (Fig.7). The PHB and mitochondria were finally located in the sperm midpiece (Figs.7F1-F4). WB assay verified that Lc-PHB was located in the mitochondria extracted from Stage Ⅳ testis (Fig.8). These results confirmed the mitochondrial location of PHB and provided insights about the correlation of PHB absence with abnormal spermatogenesis, low sperm quality, and semen deficiency. 1) Are the tubular mitochondrial cristae and the long clubbed mitochondrial morphology related to the mitochondrial location of PHB in spermatogenic cells? In mouse embryonic fibroblasts (MEFs), Merkwirth et al. (2008) found the loss of PHB resulted in the swelling and even the disappearance of mitochondria cristae. Meanwhile, the lack of PHB caused mitochondrial fragment in C. elegans muscle cell (Sanz et al., 2003), MEFs (Merkwirth et al., 2008), HeLa cells (Kasashima et al., 2006), and PC12 neural cells (Anderson et al., 2018), indicating the importance of PHB in mitochondrial fusion. Therefore, our results about the expression and mitochondrial location of PHB provided evidence that PHB may associate with observed tubular mitochondrial cristae and long clubbed mitochondria in spermatogenic cells as observed in L. crocea. Given the necessity of mitochondrial fusion for meiosis of spermatocytes (Varuzhanyan et al., 2019), a deduction is that PHB may also be necessary for meiosis in spermatocytes by promoting mitochondrial fusion. 2) Is the PHB expression important for the proliferation and survival of spermatogenic cell? In MEFs and mouse endothelial cells, the loss of PHB impaired cell proliferation (Merkwirth et al., 2008; Schleicher et al., 2008). Similar conclusions were also reported in cancer cells (Sánchez-Quiles et al., 2010; Jiang et al., 2015). In addition, PHB plays roles in cell survival and inhibiting apoptosis (Peng et al., 2015). Spermatogonia are the only cells that can increase the sperm count by proliferation and differentiation during spermatogenesis. Thus, spermatogonia proliferation is a prerequisite to generate enough spermatozoa in animals (Uribe et al., 2014). Our results about the expression of PHB during spermatogenesis indicated a possible function of PHB in spermatogonia proliferation and spermatogenic cells survival. In humans, Wang et al. (2012) reported lower PHB expression in oligoasthenospermia. The lower expression of PHB could affect the proliferation of spermatogonia, maiosis of spermatocyte and increased the apoptosis of spermatogenic cells, and therefore resulted in a reduced sperm number and finally caused oligospermatism. The confirmation of these events is important for understanding the relationship among PHB, mitochondria, and male fertility.
5 ConclusionsWe performed an ultrastructural characterization of germ cells in L. crocea. In addition, we analyzed the expression pattern and possible functions of mitochondrial functional regulatory protein PHB during spermatogenesis. This gene was expressed in a testis. FISH revealed its expression in spermatogenic cells. Moreover, we detected PHB expression in mitochondria of spermatogenic cells, including spermatogonia, spermatocyte, spermatid, and sperm. These results indicated that 1) PHB may be a potential protein to regulate the mitochondrial morphology during spermatogenesis; 2) PHB may be involved in the proliferation and survival of spermatogenic cells. This conjecture can better explain the relationship between PHB absence with oligospermatism and abnormal spermatogenesis in animals. Further evidence about the conserved mitochondrial function of PHB in spermatogenic cells of L. crocea is currently under investigation.
AcknowledgementsThis research was funded by the Natural Science Foundation of Zhejiang Province (No. LY18C190007), the Natural Science Foundation of China-Zhejiang Joint Fund for the Integration of Industrialization and Informatization (No. U1809212), the Natural Science Foundation of Ningbo City (No. 2018A610228), the Scientific and Technical Project of Zhejiang Province (No. 2021C02069-1), the Scientific and Technical Project of Ningbo City (No. 2021Z002), the Scientific Research Foundation of Ningbo University (No. XYL19023), and the Collaborative Innovation Center for Zhejiang Marine High-efficiency and Healthy Aquaculture, the K. C. Wong Magna Fund in Ningbo University.
Anderson C. J., Kahl A., Qian L., Stepanova A., Starkov A., Manfredi G., et al. 2018. Prohibitin is a positive modulator of mitochondrial function in PC12 cells under oxidative stress. Journal of Neurochemistry, 146: 235-250. DOI:10.1111/jnc.14472 (  0) 0) |
Andrade R. F., Bazzoli N., Rizzo E., Sato Y.. 2001. Continuous gametogenesis in the neotropical freshwater teleost, Bryconops affinis (Pisces: Characidae). Tissue & Cell, 33(5): 524-532. (  0) 0) |
Artal-Sanz M., Tavernarakis N.. 2009a. Prohibitin and mitochondrial biology. Trends in Endocrinology and Metabolism, 20: 394-401. DOI:10.1016/j.tem.2009.04.004 (  0) 0) |
Artal-Sanz M., Tavernarakis N.. 2009b. Prohibitin couples diapause signalling to mitochondrial metabolism during ageing in C. elegans. Nature, 461(7265): 793-797. DOI:10.1038/nature08466 (  0) 0) |
Back J. W., Sanz M. A., de Jong L., de Koning L. J., Nijtmans L. G., de Koster C. G., et al. 2010. A structure for the yeast prohibitin complex: Structure prediction and evidence from chemical crosslinking and mass spectrometry. Protein Science, 11: 2471-2478. (  0) 0) |
Bogenhagen D. F., Rousseau D., Burke S.. 2008. The layered structure of human mitochondrial DNA nucleoids. Journal of Biological Chemistry, 283: 3665-3675. DOI:10.1074/jbc.M708444200 (  0) 0) |
Chai R. R., Chen G. W., Shi H. J., Martin-DeLeon P. A., Chen H.. 2017. Prohibitin involvement in the generation of mitochondrial superoxide at complex I in human sperm. Journal of Cellular and Molecular Medicine, 21: 121-129. DOI:10.1111/jcmm.12945 (  0) 0) |
Fang J. Z., Chu M. B., Xiao Q., Chen X. Z., Yu H.. 2003. Morphological studies on the early development of large yellow croaker, Pseudosciaena crocea (Richardson). Marine Sciences, 27(6): 1-6 (in Chinese with English abstract). DOI:10.3969/j.issn.1000-3096.2003.06.001 (  0) 0) |
Hales K. G., Fuller M. T.. 1997. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell, 90(1): 121-129. DOI:10.1016/S0092-8674(00)80319-0 (  0) 0) |
Haseeb A., Chen H., Huang Y. F., Yang P., Sun X. J., Iqbal A., et al. 2018. Remodelling of mitochondria during spermiogenesis of Chinese soft-shelled turtle (Pelodiscus sinensis). Reproduction Fertility and Development, 30(11): 1514-1521. DOI:10.1071/RD18010 (  0) 0) |
Honda S., Hirose S.. 2003. Stage-specific enhanced expression of mitochondrial fusion and fission factors during spermatogenesis in rat testis. Biochemical and Biophysical Research Communications, 311(2): 424-432. DOI:10.1016/j.bbrc.2003.10.008 (  0) 0) |
Hou C. C., Gao X. M., Ni J., Mu D. L., Yang H. Y., Liu C., et al. 2018. The expression pattern and potential functions of PHB in the spermiogenesis of Phascolosoma esculenta. Gene, 652: 25-38. DOI:10.1016/j.gene.2018.01.056 (  0) 0) |
Huang S. P., Cui Y. Q., Guo X. J., Wang L., Li S. Y., Lu Y., et al. 2015. 2, 2', 4, 4'-Tetrabromodiphenyl ether disrupts spermatogenesis, impairs mitochondrial function and induces apoptosis of early leptotene spermatocytes in rats. Reproductive Toxicology, 51: 114-124. DOI:10.1016/j.reprotox.2015.01.009 (  0) 0) |
Jiang L., Dong P., Zhang Z., Li C., Li Y., Liao Y., et al. 2015. Akt phosphorylates prohibitin 1 to mediate its mitochondrial localization and promote proliferation of bladder cancer cells. Cell Death & Disease, 6(2): e1660. (  0) 0) |
Jin J. M., Hou C. C., Tan F. Q., Yang W. X.. 2016. The potential function of prohibitin during spermatogenesis in Chinese fire-bellied newt Cynops orientalis. Cell & Tissue Research, 363: 805-822. (  0) 0) |
Kasashima K., Ohta E., Kagawa Y., Endo H.. 2006. Mitochondrial functions and estrogen receptor-dependent nuclear translocation of pleiotropic human prohibitin 2. Journal of Biological Chemistry, 281: 36401-36410. DOI:10.1074/jbc.M605260200 (  0) 0) |
Liu J. F.. 2004. A study on twice maturity characteristic of cultured large yellow croaker in one year. Journal of Jimei University (Natural Science), 3: 200-204 (in Chinese with English abstract). DOI:10.3969/j.issn.1007-7405.2004.03.002 (  0) 0) |
Luo S., Gao X., Ding J., Liu C., Du C., Hou C., et al. 2019. Transcriptome sequencing reveals the traits of spermatogenesis and testicular development in large yellow croaker (Larimichthys crocea). Genes (Basel), 10(12): 958. DOI:10.3390/genes10120958 (  0) 0) |
Magalhaes A. L. B., Andrade R. F., Gomes B. V. C., Perini V. R., Rizzo E., Bazzoli N.. 2011. Ultrastructure of the semicystic spermatogenesis in the South American freshwater characid Hemigrammus marginatus (Teleostei, Characiformes). Journal of Applied Ichthyology, 27(4): 1041-1046. DOI:10.1111/j.1439-0426.2011.01747.x (  0) 0) |
Mao H. T., Wang D. H., Lan Z., Zhou H., Yang W. X.. 2012. Gene expression profiles of prohibitin in testes of Octopus tankahkeei (ot-phb) revealing its possible role during spermiogenesis. Molecular Biology Reports, 39: 5519-5528. DOI:10.1007/s11033-011-1355-4 (  0) 0) |
Mattei X., Siau Y., Thiaw O. T., Thiam D.. 1993. Peculiarities in the organization of testis of Ophidian sp. (Pisces Teleostei). Evidence for two types of spermatogenesis in teleost fish. Journal of Fish Biology, 43(6): 931-937. (  0) 0) |
Merkwirth C., Langer T.. 2009. Prohibitin function within mitochondria: Essential roles for cell proliferation and cristae morphogenesis. Biochimica et Biophysica Acta-Molecular Cell Research, 1793: 27-32. DOI:10.1016/j.bbamcr.2008.05.013 (  0) 0) |
Merkwirth C., Dargazanli S., Tatsuta T., Geimer S., Löwer B., Wunderlich F. T., et al. 2008. Prohibitins control cell prolixferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes & Development, 22: 476-488. (  0) 0) |
Mishra S., Murphy L. C., Murphy L. J.. 2006. The prohibitins: Emerging roles in diverse functions. Journal of Cellular and Molecular Medicine, 10: 353-363. (  0) 0) |
Mishra S., Murphy L. C., Nyomba B. L., Murphy L. J.. 2005. Prohibitin: A potential target for new therapeutics. Trends in Molecular Medicine, 11: 192-197. (  0) 0) |
Nijtmans L. G. J., de Jong L., Sanz M. A., Coates P. J., Berden J. A., Back W. J., et al. 2000. Prohibitins act as a membranebound chaperone for the stabilization of mitochondrial proteins. EMBO Journal, 19(11): 2444-2451. DOI:10.1093/emboj/19.11.2444 (  0) 0) |
Nijtmans L. G. J., Sanz M. A., Grivell L. A., Coates P. J.. 2002. The mitochondrial PHB complex: Roles in mitochondrial respiratory complex assembly, ageing and degenerative disease. Cellular and Molecular Life Sciences, 59(1): 143-155. DOI:10.1007/s00018-002-8411-0 (  0) 0) |
Peng Y. T., Chen P., Ouyang R. Y., Song L.. 2015. Multifaceted role of prohibitin in cell survival and apoptosis. Apoptosis, 20(9): 1135-1149. DOI:10.1007/s10495-015-1143-z (  0) 0) |
Quagio-Grassiotto I., Oliveira C.. 2008. Sperm ultrastructure and a new type of spermiogenesis in two species of Pimelodidae, with a comparative review of sperm ultrastructure in Siluriformes (Teleostei: Ostariophysi). Zoologischer Anzeiger, 247(1): 55-66. DOI:10.1016/j.jcz.2007.07.002 (  0) 0) |
Quan C., Shi Y. Q., Wang C., Wang C. M., Yang K. D.. 2016. p, po-DDE damages spermatogenesis via phospholipid hydroperoxide glutathione peroxidase depletion and mitochondria apoptosis pathway. Environmental Toxicology, 31(5): 593-600. (  0) 0) |
Rajalingam K., Rudel T.. 2005. Ras-Raf signaling needs prohibitin. Cell Cycle, 4: 1503-1505. DOI:10.4161/cc.4.11.2142 (  0) 0) |
Ramalho-Santos J., Varum S., Amaral S., Mota P. C., Sousa A. P., Amaral A.. 2009. Mitochondrial functionality in reproduction: From gonads and gametes to embryos and embryonic stem cells. Human Reproduction Update, 15(5): 553-572. DOI:10.1093/humupd/dmp016 (  0) 0) |
Rato L., Alves M. G., Socorro S., Duarte A. I.. 2012. Metabolic regulation is important for spermatogenesis. Nature Reviews Urology, 9(6): 330-338. DOI:10.1038/nrurol.2012.77 (  0) 0) |
Ribes E., Cheema M., González-Romero R., Lloris D., Ausió J., Saperas N.. 2015. Spermiogenesis and biflagellate spermatozoon of the teleost fish Lampanyctus crocodilus (Myctophiformes, Myctophidae): Ultrastructure and characterisation of its sperm basic nuclear proteins. Cell Tissue Research, 361(2): 619-632. DOI:10.1007/s00441-015-2119-6 (  0) 0) |
Rupik W., Huszno J., Klag J.. 2011. Cellular organisation of the mature testes and stages of spermiogenesis in Danio rerio (Cyprinidae; Teleostei) – Structural and ultrastructural studies. Micron, 42(8): 833-839. DOI:10.1016/j.micron.2011.05.006 (  0) 0) |
Sánchez-Quiles V., Santamaría E., Segura V., Sesma L., Prieto J., Corrales F. J.. 2010. Prohibitin deficiency blocks proliferation and induces apoptosis in human hepatoma cells: Molecular mechanisms and functional implications. Proteomics, 10: 1609-1620. DOI:10.1002/pmic.200900757 (  0) 0) |
Sanz M. A., Tsang W. Y., Willems E. M., Grivell L. A., Lemire B. D., van der Spek H., et al. 2003. The mitochondrial prohibitin complex is essential for embryonic viability and germline function in Caenorhabditis elegans. Journal of Biological Chemistry, 278(34): 32091-32099. DOI:10.1074/jbc.M304877200 (  0) 0) |
Schleicher M., Shepherd B. R., Suarez Y., Fernandez-Hernando C., Yu J., Pan Y., et al. 2008. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. Journal of Cell Biology, 180: 101-112. DOI:10.1083/jcb.200706072 (  0) 0) |
Sharma A., Qadri A.. 2004. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proceedings of the National Academy of Sciences of the United States of America, 101: 17492-17497. DOI:10.1073/pnas.0407536101 (  0) 0) |
Stanley H. P.. 1969. An electron microscope study of spermiogenesis in the teleost fish oligocottus maculosus. Journal of Ultrastructure Research, 27(3): 230-243. (  0) 0) |
Steglich G., Neupert W., Langer T.. 1999. Prohibitins regulate membrane protein degradation by the m-AAA protease in mitochondria. Molecular and Cellular Biology, 19: 3435-3442. DOI:10.1128/MCB.19.5.3435 (  0) 0) |
Su Y. Z., Zheng Z. Y., You L., Shi X. W., Liu J. F., Wong Z. C., et al. 1997. A study on techniques of artificial propagation and breeding of large yellow croaker, Pseeudosciaeua crocea (Richardson). Modern Fisheries Information, 5: 21-27 (in Chinese with English abstract). (  0) 0) |
Tatsuta T., Model K., Langer T.. 2005. Formation of membrane-bound ring complexes by prohibitins in mitochondria. Molecular Biology of the Cell, 16: 248-259. DOI:10.1091/mbc.e04-09-0807 (  0) 0) |
Thuaud F., Ribeiro N., Nebigil C. G., Désaubry L.. 2013. Prohibitin ligands in cell death and survival: Mode of action and therapeutic potential. Chemistry & Biology, 20(3): 316331. (  0) 0) |
Uribe M. C., Grier H. J., Mejía-Roa V.. 2014. Comparative testicular structure and spermatogenesis in bony fishes. Spermatogenesis, 4(3): e983400. DOI:10.4161/21565562.2014.983400 (  0) 0) |
Van der Weyden L., Arends M. J., Chausiaux O. E., Ellis P. J., Lange U. C., Surani M. A., et al. 2006. Loss of TSLC1 causes male infertility due to a defect at the spermatid stage of spermatogenesis. Molecular and Cellular Biology, 26(9): 35953609. (  0) 0) |
Varuzhanyan G., Rojansky R., Sweredoski M. J., Graham R. L. J., Hess S., Ladinsky M. S., et al. 2019. Mitochondrial fusion is required for spermatogonial differentiation and meiosis. Elife, 8: e51601. DOI:10.7554/eLife.51601 (  0) 0) |
Ventelä S., Toppari J., Parvinen M.. 2003. Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: Mechanisms of haploid gene product sharing. Molecular Biology of the Cell, 14: 2768-2780. DOI:10.1091/mbc.e02-10-0647 (  0) 0) |
Vertika S., Singh K. K., Rajender S.. 2020. Mitochondria, spermatogenesis, and male infertility – An update. Mitochondrion, 54: 26-40. (  0) 0) |
Wang M. J., Ou J. X., Chen G. W., Wu J. P., Shi H. J., Chen H., et al. 2012. Does prohibitin expression regulate sperm mitochondrial membrane potential, sperm motility, and male fertility?. Antioxidants & Redox Signaling, 7(3): 513-519. (  0) 0) |
Wang Y., Bogenhagen D. F.. 2006. Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. Journal of Biological Chemistry, 281: 25791-25802. (  0) 0) |
Winter A., Kämäräinen O., Hofmann A.. 2007. Molecular modeling of prohibitin domains. Proteins, 68: 353-362. (  0) 0) |
Xiong Z., Zhang H., Huang B., Liu Q., Wang Y., Shi D., et al. 2018. Expression pattern of prohibitin, capping actin protein of muscle Z-line beta subunit and tektin-2 gene in Murrah buffalo sperm and its relationship with sperm motility. Asian-Australasian Journal of Animal Sciences, 31(11): 1729-1737. (  0) 0) |
Xu Y. R., Fan Y. S., Yang W. X.. 2017. Mitochondrial prohibitin and its ubiquitination during spermatogenesis of the swimming crab Charybdis japonica. Gene, 627: 137-148. (  0) 0) |
You X. R., Cai M. Y., Jiang Y. H., Wang Z. Y.. 2012. Histological observation on gonadal sex differentiation in large yellow croaker (Larimichthys crocea). Journal of Fisheries of China, 36(7): 1057-1064. (  0) 0) |
Zhang D. D., Gao X. M., Zhao Y. Q., Hou C. C., Zhu J. Q.. 2017. The C-terminal kinesin motor KIFC1 may participate in nuclear reshaping and flagellum formation during spermiogenesis of Larimichthys crocea. Fish Physiology and Biochemistry, 43: 1351-1371. (  0) 0) |
Zhang J. J., Wang Q., Wang M. S., Jiang M. X., Wang Y. S., Sun Y., et al. 2016. GASZ and mitofusin-mediated mitochondrial functions are crucial for spermatogenesis. EMBO Reports, 17(2): 220-234. (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


