2) Dean's Office, Hainan Provincial Party School, Haikou 571100, China;
3) School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China
The rapid development of aquaculture industries in China has led to the shortage of feed resources and environment pollution, which has been a major problem to be solved. In addition, the demand for compound aquatic feeds is rising because of high-density farming techniques (Liu et al., 2017). However, concomitant growth in the use of compound aquatic feeds easily results in poor growth performance and low feed utilization efficiency, mainly because of the low palatability and attractiveness (Nunes et al., 2006; Qiu and Davis, 2018). The use of dietary feeding attractants in aqua feeds has, therefore, received considerable attentions in recent years. A small quantity of feeding attractant can improve feed intake and, as a consequence, increase the animal's growth and survival (Tusche et al., 2013; Olsén and Lundh, 2016; Kim and Cho, 2019).
Previous studies have demonstrated that various feed attractants including amino acids and their mixtures, sulfur-containing organic compounds, alkaloids and nucleic acids have high attractiveness for aquatic animals (Nunes et al., 2006; Silva-Neto et al., 2012). Most of these attractants are isolated from different marine organisms, such as shrimp, worms, krill, squid and mussels (Murai et al., 1983; Daniel and Bayer, 1987; Kolkovski et al., 2010; Alam et al., 2012). To our knowledge, attractants obtained thorough fermentation of lactic acid bacteria (LAB) are rarely reported.
Lactic acid bacteria represent the majority of probiotics used in aquaculture (Gatesoupe, 2008). Species such as Lactobacillus plantarum, Lactococcus lactis, Leuconostoc lactis, Pediococcus pentosaceus, and Enterococcus faecium are commonly used in aquaculture due to their positive effects (RingØ and Gatesoupe, 1998; Sun et al., 2012; Xing et al., 2013; Zhang et al., 2013; Zheng et al., 2017). In particular, the application of E. faecium to aquatic organisms has yielded beneficial effects, mainly in improving growth performance and reducing the mortality rate by inhibiting pathogens, regulating intestinal flora and stimulating the immune system (Bogut et al., 2000; Swain et al., 2009; Avella et al., 2011). These benefits may result from its ability to produce organic acids, exopolysaccharides and functional oligopeptides such as bacteriocin (Herranz et al., 2001; Abdhul et al., 2014; Abdel-Rahman et al., 2015). It has also been reported that LAB fermentation may produce flavor substances such as amino acids, flavor peptides, organic acids, aldehydes and ketones (Han et al., 2007; Coolbear et al., 2011). They may become attractants and thus improve the palatability of the feed. However, the attractant effect ofLAB, especially that of E. faecium, on aquatic animalshas not been thoroughly investigated. The main aim of this study was to isolate E. faecium from the shrimp intestine and evaluate the attractant effects of its fermentation broth on shrimp (Litopenaeus vannamei).
2 Materials and Methods 2.1 Culture MediaTo prepare screening medium, 0.75% CaCO3 was added to the Man Rogosa Sharpe (MRS) agar medium. MRS, 2216E and thiosulfate citrate bile salts sucrose (TCBS) medium were purchased from Beijing Luqiao Technology Co., Ltd. (Beijing, China).
2.2 Sampling and LAB IsolationFresh shrimp (L. vannamei) individuals were collected from high intensive shrimp culture ponds in Jiangsu, Fujian and Guangdong Provinces, China, respectively. The samples were stored in the ice box during transportation to laboratory, and analyzed at the same day. Shrimp body surfaces were washed and sterilized with 70% ethanol. Then the whole intestine, together with the contents, were sampled and homogenized in sterile saline (1:9, w/v) with a sterile glass homogenizer on ice. The homogenates were serially diluted in sterile saline (from 10−1 to 10−6), and 100 μL of the diluted homogenate was spread onto triplicate plates of screening medium. The inoculated plates were cultured at 37℃ for 48 h in an incubator sett at a constant temperature (Yiheng, Shanghai, China). After incubation, colonies in the form of clear transparent circles with smooth surfaces and a diameter of 0.5–1 mm were selected and streaked onto MRS agar to obtain the isolates. The isolates were subjected to safety evaluation using the hemolytic assays (Bernheimer, 1988). Each single colony was placed on the blood agar plate (Merck, Germany) and incubated at 37℃ for 24 h, 48 h and 72 h for hemolysis observation. Purified strains (without hemolysis) were suspended in MRS broth containing 20% (v/v) sterile glycerol and stored at −80℃ for further analysis in the future.
2.3 Antimicrobial Activity AssaysThe antibacterial tests were carried out using a simple filter paper (Gould, 1952). Vibrio parahaemolyticus (laboratory preservation) were used as the indicator, which was isolated and identified from diseased shrimp during the early mortality syndrome (EMS) outbreaks in the high intensive shrimp culture ponds in Guangdong Province, China. A dilution of the V. parahaemolyticus was spread on TCBS plates. After impregnated and saturated with 50 μL putative LAB fermentation broth (MRS medium at 37℃ for 20 h), paper discs (7 mm in diameter) were placed on the agar plates. Plates were incubated at 28℃ for 24 h, and the strains with obvious zone of inhibition were screened. All tests were performed in triplicate.
2.4 Assessment of the Attractant Effect of Different StrainsShrimps(L. vannamei) were obtained from Charoen Pokphand Group (Hainan, China) for the experiments. The healthy shrimps (8 g ± 1.00 g) were pre-incubated in tanks containing aerated sea water (salinity 20, pH 7.2) at 26℃ for 1 week for acclimation. The animal care protocol in this study was approved by the Institutional Animal Care and Use Committee of Ocean University of China. The attractant effect of different strains was evaluated using a pellet test as described by Cai and Ye (2005) and Hidaka et al. (2000) with some modifications.
The LAB fermentation broth was prepared using the following method. Fifty microliters of LAB strain storage solution was inoculated into 5 mL MRS broth, and statically cultured at 37℃ for 20 h in an incubator set at a constant temperature. Then the pellets were prepared using a mixture composed of 35% flour (crude protein: 9%– 11%, Luwang Group, Jining, China), 25% gluten (keep the insoluble pellets, crude protein > 75%, Baipin biotechnology, Xingtai, China), 39% water and 1% LAB strain fermentation broth. The mixture with different materials was pressed into individual pellets with a size of 0.5 cm × 0.3 cm and a weight of 30 mg. The shrimps were fed basal pellets (without attractant) for three days to acclimate to eat the pellets. Twenty pellets supplemented with LAB strain fermentation broth were placed in the same position in an indoor tank (50 cm × 40 cm × 50 cm) containing 10 healthy shrimps. The pellets remaining were counted after 10 min. Pellets with 1% (v/w) MRS broth served as the control. The trial was repeated three times, and the status of the shrimp was consistent each trial (3 h fasted). Ten LAB strains (named L1, L2, L3, L4, L5, L6, L7, L8, L9 and SC-01) were isolated from L. vannamei intestine. The attractant index was calculated as follows:
| $ {\rm{Attractant\ \ index = 1 - }}\frac{{{\rm{The\ \ number\ \ of\ \ remaining\ \ pellets}}}}{{{\rm{The\ \ number\ \ of\ \ total\ \ pellets}}}}. $ |
Initial identification schemes were performed with biochemical tests as suggested by the Bergey's Manual of Determinative Bacteriology (Holt, 1994). The molecular taxonomy of the isolates was determined by 16S rRNA gene sequencing. The total DNA was extracted using the DNA Extraction Kit (Sangon Biotech, Shanghai, China). The 16S rRNA gene fragment (V3V4 region) was amplified by polymerase chain reaction (PCR) using bacterial universal primers 27F (5'-AGAGTTTGATCCTGGCTCAG-3') and 1492R (5'-GGTTACCTTGTTACGACTT-3'), and sequenced by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China). The partial 16S rRNA gene sequence was compared with other bacterial sequence data using the Basic Local Alignment Search Tool (BLAST). A phylogenetic tree of strain SC-01 was constructed based on the comparison of the sequences with those of other reference bacteria through Neighbor-Joining (NJ) analysis with 1000 boot-strap replicates (Molecular Evolutionary Genetics Analysis, MEGA 7.0 version).
2.6 Assessing the Biosecurity of Strain SC-01 for ShrimpShrimps (L. vannamei) were obtained from Charoen Pokphand Group (Hainan, China). The healthy shrimps (1.5 cm ± 0.1 cm) were pre-incubated in tanks containing aerated sea water (salinity 20, pH 7.2) at 26℃ for 1 week for acclimation. To assay the safety of strain SC-01, 90 shrimps with similar body weight were randomly assigned to 3 groups, 3 replicate tanks each group, 10 shrimps each tank (28 cm × 18 cm × 17 cm), in a recirculating system. Three treatment groups included: 1) control, without SC-01; 2) 104, with SC-01 at a concentration of 104 cfu mL−1; 3) 106, with SC-01 at a concentration of 106 cfu mL−1. During the experiment, half of the water in each tank was replaced with the fresh and filtrated sea water daily and shrimps were fed with the commercial pellet feed (Charoen Pokphand Group, Hainan, China) daily. Shrimp mortality was recorded periodically at 24 h, 48 h, 72 h and 96 h after the exposure, respectively. The mortality rate was measured at the end of cultivation.
Mortality rate was calculated using the following equations:
| $ {\rm{Mortality\ \ rate }}\left({\rm{\% }} \right){\rm{ = }}\frac{{{\rm{Initial\ \ shrimp\ \ count - Final\ \ shrimp\ \ count}}}}{{{\rm{Initial\ \ shrimp\ \ countt}}}}{\rm{ \times 100\% }} $ |
The attractant effects of strain SC-01 fermentation broth and other ingredients were evaluated based on the above method with some modifications, and different ingredients were tested simultaneously in each tank (100 cm × 40 cm × 50 cm). In addition, the preparation method of SC-01 fermentation broth was similar with that of LAB fermentation broth. Pellets were prepared using a mixture composed of flour, gluten, water and different attractant agents (a weight ratio of 1%, marked with different food colorings; Wilton, Chicago, USA), and we found that there was no difference on the number of remaining pellets of different colors. Sixty pellets derived from three attractant agents of different colors (20 pellets each color) and 20 pellets from the control group for each test were placed in the same position in a tank containing 40 healthy shrimps (8 g ± 1.00 g). In test 1, SC-01 fermentation broth, MRS medium, peptone and control were analyzed. In test 2, SC-01 fermentation broth, acetic acid, lactic acid (Sinopharm Chemical Reagent, Beijing, China) and control were analyzed. In test 3, SC-01 fermentation broth, trimethylamine oxide (TMAO) (purity > 99%, Zhonghong Biotechnology, Wuhan, China), dimethyl-beta-propiothetin (DMPT) (purity > 98%, DE Kay Biotechnology, Wuhan, China) and control were analyzed. Pellets without supplement served as the control. The pellets remaining were counted after 10 min. The trial was repeated three times, and the state of the shrimp used in each experiment was consistent (3 h fasted). The attractant index was calculated using the equation below:
| $ {\rm{Attractant\ \ index = 1 - }}\frac{{{\rm{The\ \ remaining\ \ number\ \ of\ \ certain\ \ color\ \ pellets}}}}{{{\rm{The\ \ total\ \ number\ \ of\ \ certain\ \ color\ \ pellets}}}}{\rm{.}} $ |
Analysis of lactic acid, acetic acid and inosine-5'-monophosphate (IMP) in SC-01 fermentation broth by LC3000 chromatograph (Luchuang Analytical Instrument, Shandong, China). Standards of the lactic acid, acetic acid and IMP (purity ≥ 99.8%) were purchased from Sigma (Sigma Chemical, St Louis, USA). Other chemicals (analytical grade) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). All chemicals were of analytical grade except methanol and acetonitrile (HPLC grade).
Chromatographic analysis was performed using an analytical Knaur C18 reversed phase column (150 mm × 4.6 mm I. D.) with a particle size of 5.0 μm (Agilent Technologies, Palo Alto, USA). The mobile phase was 0.01 mol L−1 phosphate buffer solution (pH 2.6) for analysis of lactic acid and acetic acid, and the methanol and phosphoric acid (2:98, v/v) buffer solution (containing 0.005 mol L−1 tetrabutyl bromoethane, pH 6.5) for analyzing IMP at a flow rate 1.0 mL min−1. The injection volume of each sample was 2 μL. The column was thermostated at 30℃ for analyzing lactic acid and acetic acid and 50℃ for analyzing IMP. All samples were detected at 210 nm. Standard stock solutions of lactic acid (1.992 g L−1), acetic acid (2.432 g L−1) and IMP (6.1767 g L−1) were prepared in ultrapure water. Working solutions were prepared by diluting the stock solutions with ultrapure water. The linear regression equation of the three analytes was described in Table 1. SC-01 fermentation broth was diluted with deionized water and filtered by a 0.45 μm cellulose acetate membrane, and injected into the chromatograph and repeated 3 times.
|
|
Table 1 Linear regression equation for three analytes |
Healthy shrimps(L. vannamei) were obtained from Charoen Pokphand Group (Hainan, China) and were acclimated at 26℃ ± 1℃ and a salinity of 20 in tanks, fed the basal diet three times a day for 1 week. The basal feed was purchased from Charoen Pokphand Group (commercial shrimp feed, Hainan, China). The nutrient contents include crude protein 42.85%, ether extract 8.41%, moisture 8.53%, ash 10.16%, Ca 1.94% and total phosphorus 1.02%.
To prepare the SC-01 fermentation broth, 100 μL of E. faecium SC-01 storage solution was inoculated in 10 mL MRS broth at 37℃. After 20 h, it was inoculated into 1 L fresh MRS broth, and statically incubated for another 20 h at 37℃. After incubation, the SC-01 fermentation broth was harvested and stored at 4℃, and the viable counts of SC-01 fermentation broth (5.7×109 cfu mL−1) were determined by plating on MRS agar.
Feeding experiment was conducted in 12 indoor tanks (2.0 m × 1.2 m × 1.0 m) in Pearl River Fisheries Research Institute, Guangzhou, China. Six hundred shrimps with an initial average weight of 5.55 g ± 0.20 g were randomly assigned to three groups, 50 each tank, 4 tanks each group. The diets in three treatment groups included the basal diet without SC-01 fermentation broth (control); the supplemented with 1% (v/w) SC-01 fermentation broth in the basal diet (SC-01 (1%)); the supplemented with 2% (v/w) SC-01 fermentation broth in the basal diet (SC-01 (2%)). The basal diet and SC-01 fermentation broth were mixed immediately before feeding. Shrimps were fed four times a day at 5% of body weight at 06:00, 12:00, 17:00 and 23:00 for 60 days, and uneaten feed was collected after 1 h of feeding and then immediately dried. The feed intake was calculated by subtracting the uneaten portion and recorded daily. Throughout the experimental period, temperature was ranged from 25℃ to 27℃, pH was 7.5–8.5, dissolved oxygen content was approximately 6.0 mg L−1, and total ammonia nitrogen content was < 0.2 mg L−1.
At the end of the experiment, shrimps were fasted for 24 h and then weighed. Three shrimps from each tank were randomly selected. Their whole intestines were aseptically dissected using sterilized surgical scissors, and immediately homogenized for counting the number of intestinal bacteria. The counts of the total culturable bacteria (TCB), LAB and Vibrio sp. in intestines were conducted as described by Zhang et al. (2010) using 2216E, MRS and TCBS agar plates, respectively. The weight gain rate (WGR), specific growth rate (SGR), feed conversion rate (FCR) and survival rate (SR) were calculated as follows:
| $ WGR\left(\% \right) = \frac{{Wt - Wi}}{{Wi}} \times 100, $ |
| $ SGR\left({\% {\rm{ }}{{\rm{d}}^{ - 1}}{\rm{ }}} \right){\rm{ }} = \frac{{{\rm{ln }}Wt - {\rm{ln}}\;Wi}}{T} \times 100, $ |
| $ FCR = \frac{{{\rm{Feed\ \ intake}}}}{{Wt{\rm{ }}Wi + {\rm{Death\ \ shrimps\ \ weight}}}}, $ |
| $ SR(\%)\frac{{{\rm{Survival\ \ shrimp}}}}{{{\rm{Total\ \ shrimp}}}} \times 100, $ |
where Wt means final weight; Wi means initial weight; and T means total experimental days.
2.10 Statistical AnalysesAll data were analyzed by one-way ANOVA using Statistical Analysis Systems (SAS). Significant difference was assessed at P < 0.05.
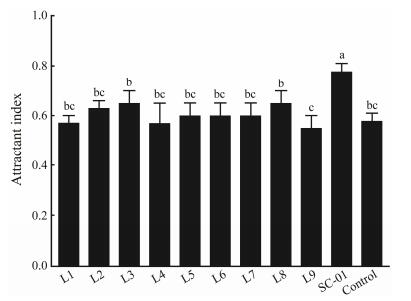
3 Results 3.1 Isolation of LAB StrainsCultured on MRS plates at 37℃ for 48 h, 10 isolates with clear transparent circles, smooth surfaces, hemolytic assays negative and round colony diameter 0.5–1 mm were screened out. The results of re-screening of pellet test showed that the attractant index of SC-01 strain was significantly higher than that of other strains and control (Fig. 1), thus, its attractant effect on shrimp was better than that of other strains.
|
Fig. 1 Attractant index of 10 selected strains. Values are expressed as the mean (n= 3) and the error bars represents standard deviation (SD). Means with different letters a, b, c are significantly different (P < 0.05). |
In the antibacterial test, V. parahaemolyticus, a common pathogen in aquaculture, was selected as an indicator. The test result showed that the strain SC-01 had a significant inhibitory effect on V. parahaemolyticus (Table 2), and an obvious inhibition zone (diameter 14 mm) was observed.
|
|
Table 2 Antibacterial activities of different strains (n = 3) |
SC-01 showed the typical characteristics of LAB species, which were gram-positive, catalase test-negative, VP test-positive, Arg production of ammonia test-positive, H2S test-negative, gelatin test-negative, indole test-negative, and no spore-forming bacteria (Table 3). Strain SC-01 was also able to grow with 6.5% NaCl at 10–45℃ and pH 4.5–9.6, which suggests that this strain is suitable for using in marine aquaculture.
|
|
Table 3 Physiological and biochemical characteristics of strain SC-01 |
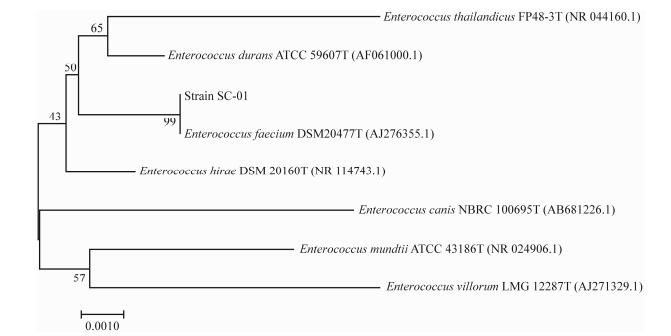
The BLAST program was used to identify homologous sequence in GenBank (http://www.ncbi.nlm.nih.gov/). The MEGA7.0 was used to construct a phylogenetic tree as is shown in Fig. 2. The 16S rRNA gene phylogenetic analysis revealed that the SC-01 strain shows 99% similarity to Enterococcus faecium DSM 20477T (accession number: AJ276355.1). Based on the above results, the strain SC-01 was identified as E. faecium.
|
Fig. 2 Phylogenetic tree based on 16S rRNA gene sequence of strain SC-01. |
SC-01 presented negative results in the hemolytic assays (Fig. 3). When the culture of SC-01 was added to shrimp sink, no shrimp death was observed in any test arrangement. Thus SC-01 is safe for aquaculture farming (Table 4).
|
Fig. 3 Hemolytic analysis of strain SC-01. |
|
|
Table 4 The effect of strain SC-01 on mortality of shrimp |
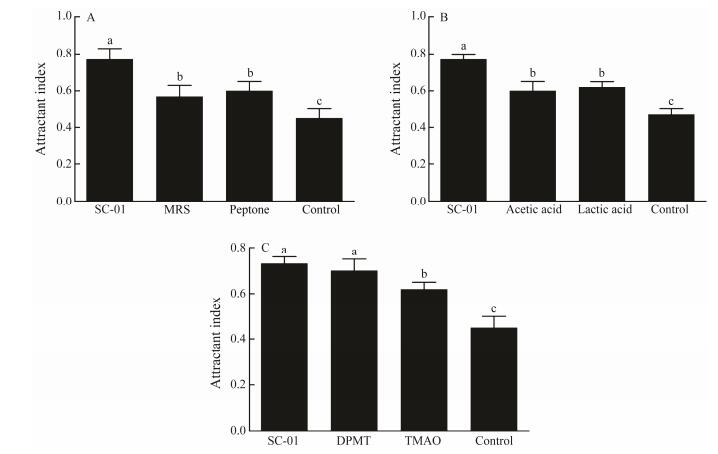
Comparison of the attractant effect of the strain SC-01 fermentation broth, medium components, organic acid and chemical attractants is shown in Fig. 4. The attractant index of SC-01 group was significantly higher than that of MRS medium, peptone, acetic acid, lactic acid, TMAO and control group (P < 0.05) (Figs. 4A, B and C), which had no significant difference with that of the DMPT group (Fig. 4C). The result indicated that the fermentation broth of strain SC-01 has the potential to be developed into a biological attractant.
|
Fig. 4 Attractant index of different attractant ingredients. Values are expressed as the mean (n= 3) and the error bars represent standard deviation (SD). Means with different letters a, b, c are significantly different (P < 0.05). MRS, Man Rogosa Sharpe medium; DMPT, dimethyl-beta-propiothetin; TMAO, trimethylamine oxide. |
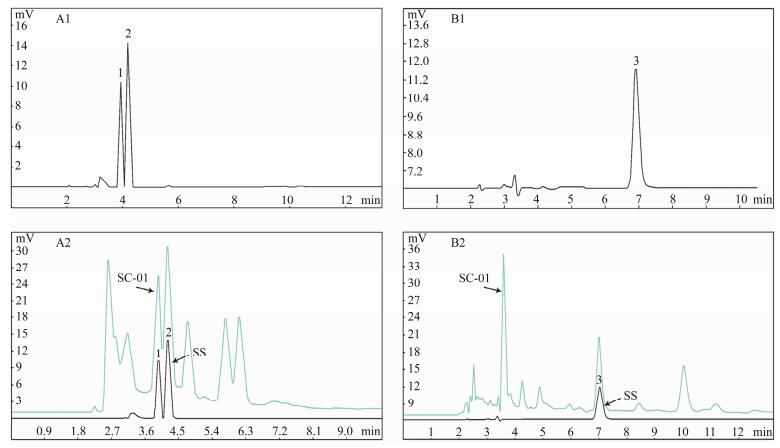
HPLC analysis of SC-01 fermentation broth composition is shown in Fig. 5. The retention time of lactic acid, acetic acid and IMP were 3.952, 4.197 and 6.972 min respectively (Figs. 5A1, B1). The components of SC-01 fermentation broth including lactic acid, acetic acid and IMP were confirmed by comparing the chromatogram peaks between SC-01 fermentation broth and the standard solutions (Figs. 5A2, B2), with average contents of 5.79, 0.72 and 0.18 g L−1, respectively.
|
Fig. 5 HPLC chromatograms of a mixture of standard solutions of lactic acid and acetic acid (volume ratio 1:1) (A1), standard solutions of inosine-5'-monophosphate (IMP) (B1), SC-01 with standard solutions of lactic acid and acetic acid (A2), and SC-01 with standard solutions of IMP (B2). Peak 1, lactic acid; peak 2, acetic acid; and peak 3, IMP. SS, standard solutions. |
The effects of SC-01 fermentation broth on the growth performance are shown in Table 5. There was no significant difference in initial weight among three groups. The final weight in the two treatment groups was significantly higher than that in the control group (P < 0.05). The values of WGR, feed intake and SGR in SC-01 (2%) treatment group were significantly higher than those in the control group (P < 0.05), and these indicators showed a higher trend in the SC-01 (1%) group. No significant difference was recorded in FCR and SR among three groups (P > 0.05). These results indicated that SC-01 fermentation broth can improve growth performance of shrimps.
|
|
Table 5 Effects of SC-01 fermentation broth on growth performance of shrimp |
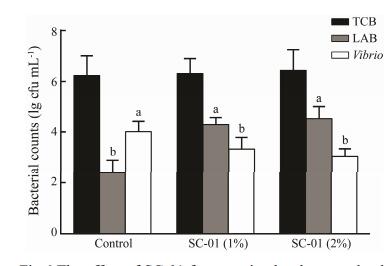
The result of the intestinal bacterial counts in the shrimp is shown in Fig. 6. There was no significant difference in the number of total TCB in the intestines among three groups (P > 0.05). Compared with the control group, the two treatment groups significantly increased the count of LAB (P < 0.05) and decreased the count of Vibrio sp. (P < 0.05) in the intestine of shrimp.
|
Fig. 6 The effect of SC-01 fermentation broth on total culturable bacteria (TCB), lactic acid bacteria (LAB) and Vibrio sp. counts in the intestine of shrimp. Values are expressed as the mean (n = 4) and the error bars represent standard deviation (SD). Means with different letters a, b are significantly different (P < 0.05). |
The results demonstrated that the LAB strain isolated from shrimp (L. vannamei) intestine had antibacterial activity and attractant effect. The strain was identified as E. faecium SC-01, based on morphological and biochemical features as well as on 16S rRNA gene sequence. Strain SC-01 is able to grow with 6.5% NaCl at 10 – 45℃ and pH 4.5–9.6, which suggested that it may be suitable for marine aquaculture. The biosecurity evaluation revealed negative results in the hemolytic assays and no shrimp mortality resulted from SC-01 fermentation broth challenge. Therefore, SC-01 is safe as a potent agent for feeding stimulants in aquatic farming.
The pellet test in this study is an improvement of the feeding behavior method and strain SC-01 showed the greatest attractant potential compared with other strains. This method does not require special equipment and has several favorable characteristics including simple operation, short screening period and high accuracy (Cai and Ye, 2005). A previous study showed that most E. faecium strains have a good antibacterial activity, and are used as probiotics to antagonize cohesive Escherichia coli in clinical medicine (Miyazaki et al., 2010). Furthermore, E. faecium can also have a good inhibitory activity against common aquatic pathogens such as V. parahaemolyticus, V. metschnikovi and V. harveyi (Sun et al., 2010), and protect shrimp (L. monodon) from attacks by V. parahaemolyticus (Swain et al., 2009). The present study revealed that E. faecium SC-01 had an inhibitory activity against V. parahaemolyticus. This suggests that strain SC-01, as a bioinducer, can promote shrimp feeding, inhibit pathogens and improve intestinal health.
The use of attractants can improve the palatability of feeds, enhance animal appetite and feed intake, reduce feed waste and improve growth performance in aquaculture (Papatryphon and Joseph, 2000; Pratoomyot et al., 2010). Attractant substances are mainly synthesized chemicals, or extracts from animals and plants. They comprise amino acids, alkaloids, sulfur compounds, Chinese herbal medicines, animal and plant extracts, and so on (Smith et al., 2005; Silva-Neto et al., 2012). Some attractants are obtained from microbial extracts, fermentation feeds and fermentation broths (Mato et al., 1977; Guerenstein et al., 1995). These attractants not only have attractant effects, but also have other functions, including regulating intestinal flora balance, improving host immunity, and reducing intestinal diseases (Banerjee and Ray, 2017). Gueren stein et al. (1995) reported that a volatile substance produced by Saccharomyces cerevisiae has a high attractant effect in Triatoma infestans. In aquaculture, fermented soybean meal is the most researched fermented feed, which can significantly improve the food intake of aquatic animals and enhance production performance ( Rombensoet al., 2013; Azarm and Lee, 2014).
Previous studies have shown that probiotic strains of E. faecium has been widely using in aquaculture mainly due to their excellent effects in inhibiting pathogens, regulating intestinal flora and enhancing immunity (Bogut et al., 2000; Swain et al., 2009; Avella et al., 2011). However, the attractant effect of the fermentation broth of E. faecium is rarely reported. Our study revealed that the SC-01 fermentation broth can promote the feed intake of shrimp. Its attractant effect is significantly better than that of MRS medium, peptone, acetic acid, lactic acid and the attractant TMAO, and is equivalent to that of the DMPT, thus it has the potential of being developed into a biological attractant. The similar result has been obtained in a previous study showing that fermentation broths of yeast and sugar can be attractants to moths (Utrio and Eriksson, 1977).
Microbial fermentation broths and fermented raw materials can promote the feed intake of aquatic animals. This may be partially due to the flavor substances (e.g., amino acids, small peptides and nucleotides) produced during microbial fermentation (Han et al., 2007; Coolbear et al., 2011). In the present study, the attractant effect of SC-01 fermentation broth was significantly better than that of the MRS medium and peptone, suggesting that the attractant ingredients may be some metabolites produced by the microorganisms. The active components of attractants can be assessed by carrying out an efficacy comparison test and a product detection test (Millar et al., 1992). Millar et al. (1992) analyzed a fermented Bermuda grass infusion by liquid chromatography and identified that the main attractant components included phenol, 4-methylphenol, 4-ethylphenol, indole and 3-methylindole. Another report showed that IMP can enhance the feeding activity of jack mackerel Trachurus japonicus ( Ikeda et al., 1988 ) . In this study, a small amount of IMP was also detected by HPLC, indicating that this component may participate in the attractant effect to shrimp. However, LAB fermentation can also produce small peptides, aldehydes, ketones and other flavor substances (Coolbear et al., 2011), which may also have important attractant effects. Further research is needed to reveal the components having attractant efficacy by means of liquid chromatograph-mass spectrometer (LC-MS) and gas chromatography-mass spectrometer (GC-MS).
Many studies have showed that an E. faecium supplement had many positive effects in aquatic animals, including reducing the feed coefficient, promoting growth, inhibiting pathogenic bacteria (such as V. parahaemolyticus, V. harveyi and Staphylococcus aureus), and improving immunity and survival rate (Wang et al., 2008; Swain et al., 2009; Avella et al., 2011; Sun et al., 2012). In the present study, a higher final weight, WGR and SGR, and a significantly increased feed intake were observed in the treatment groups compared with the control group. This was particularly marked in the SC-01 (2%) group, which suggested that SC-01 fermentation broth can improve the growth performance and may partly contribute to its attractant effect. Similarly, Wang et al. (2008) reported that E. faecium ZJ4 (1×107 cfu g−1) in feed significantly improved final weight and daily weight gain in tilapia. Bogut et al. (2000) reported that 2×108 cfu g−1 of E. faecium significantly improved the daily weight gain (up to 10.85%) in sheat fish (Silurus glanis).
It is well known that the gastrointestinal microflora of aquatic animals play an important role in the growth and health of the animals (Nayak, 2010). The components and amounts of gastrointestinal microbes in aquatic animals are easily affected by culture environment, supplementation of feed and some additives (Zhang et al., 2011). Generally, probiotics supplements can exert beneficial effects on the host by inhibiting pathogenic bacteria, improving the microbiological balance and maintaining intestinal health (Banerjee and Ray, 2017). The present study showed that the number of detected Vibrio sp.was significantly decreased by feeding E. faecium SC-01, which may be related to its antibacterial activity. Our findings agree with those of Adel et al. (2017) who found that a dietary P. pentosaceus probiotics significantly decreased the number of Vibrio sp. in the intestine of shrimp.
Our results indicated that E. faecium SC-01 fermentation broth may inhibit V. parahaemolyticus, increase feed intake and improve the growth performance of shrimp (L. vannamei). Hence, E. faecium SC-01 fermentation broth has the potential to be developed into a bioattractant. Further studies should be carried out using LC-MS and GC-MS to identify the possible attractant ingredients, and analyze the relationship between attractant ingredients and improved growth performance.
AcknowledgementThis research was supported by the Special Fund for Qingdao Marine Biomedical Science and Technology Innovation Center, China (No. 2017-CXZX01-3-13).
Abdel-Rahman, M. A., Tashiro, Y., Zendo, T., Sakai, K. and Sonomoto, K., 2015. Enterococcus faecium QU 50: A novel thermophilic lactic acid bacterium for high-yield L-lactic acid production from xylose. Fems Microbiology Letters, 362(2): 1-7. (  0) 0) |
Abdhul, K., Ganesh, M., Shanmughapriya, S., Kanagavel, M., Anbarasu, K. and Natarajaseenivasan, K., 2014. Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecium (BDU7) from Ngari. International Journal of Biological Macromolecules, 70(8): 450-454. (  0) 0) |
Adel, M., Yeganeh, S., Dawood, M. A. O., Safari, R. and Radhakrishnan, S., 2017. Effects of Pediococcus pentosaceus supplementation on growth performance, intestinal microflora and disease resistance of white shrimp, Litopenaeus vannamei. Aquaculture Nutrition, 23(6): 1401-1409. (  0) 0) |
Alam, M. S., Watanabe, W. O., Sullivan, K. B., Rezek, T. C. and Seaton, P. J., 2012. Replacement of menhaden fish meal protein by solvent-extracted soybean meal protein in the diet of juvenile black sea bass supplemented with or without squid meal, krill meal, methionine, and lysine. North American Journal of Aquaculture, 74(2): 251-265. (  0) 0) |
Avella, M. A., Olivotto, I., Silvi, S., Ribecco, C., Cresci, A., Palermo, F., Polzonetti, A. and Carnevali, O., 2011. Use of Enterococcus faecium to improve common sole (Solea solea) larviculture. Aquaculture, 315(3): 384-393. (  0) 0) |
Azarm, H. M. and Lee, S. M., 2014. Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, amino acid and biochemical parameters of juvenile black sea bream Acanthopagrus schlegeli. Aquaculture Research, 45(6): 994-1003. (  0) 0) |
Banerjee, G. and Ray, A. K., 2017. The advancement of probiotics research and its application in fish farming industries. Research in Veterinary Science, 115: 66-77. (  0) 0) |
Bernheimer, A. W., 1988. Assay of Hemolytic Toxins, Methods in Enzymology. Academic Press, Amsterdam, 213-217.
(  0) 0) |
Bogut, I., Milaković, Z., Kristek, S., Novoselić, D. and Bukvić, Ž., 2000. Effect of Enterococcus faecium on the growth rate and content of intestinal microflora in sheat fish (Silurus glanis). Veterinární Medicína, 45(4): 107-109. (  0) 0) |
Cai C. F., and Ye Y. T., 2005. A screening method for an aquatic animal attractant. CN 1672553A, China.
(  0) 0) |
Coolbear, T., Weimer, B. and Wilkinson, M. G., 2011. Lactic acid bacteria in flavor development. Encyclopedia of Dairy Sciences, 73(2): 160-165. (  0) 0) |
Daniel, P. C. and Bayer, R. C., 1987. Attraction of predatorily naive postlarval lobsters to extracts of metabolites of common prey: Mytilus edulis, Mya arenaria, Cancer irroratus, and Asterias vulgaris. Journal of Chemical Ecology, 13(5): 1201-1215. (  0) 0) |
Gatesoupe, F. J., 2008. Updating the importance of lactic acid bacteria in fish farming: Natural occurrence and probiotic treatments. Journal of Molecular Microbiology & Biotechnology, 14(1-3): 107-114. (  0) 0) |
Gould, J. C., 1952. The determination of bacterial sensitivity to antibiotics. Edinburgh Medical Journal, 59(4): 178-200. (  0) 0) |
Guerenstein, P. G., Lorenzo, M. G., Nunez, J. and Lazzari, C. R., 1995. Baker's yeast, an attractant for baiting traps for Chagas' disease vectors. Experientia, 51(8): 834-837. (  0) 0) |
Han, X. Y., Sun, D. Q., Xiang, L., Huo, G. C. and Jiang, Y. J., 2007. Gene regulation to lactic acid bacteria for increasing production of flavor metabolite. Acta Microbiologica Sinica, 47(6): 1105-1109. (  0) 0) |
Herranz, C., Casaus, P., Mukhopadhyay, S., Martı́nez, J. M., Rodrı́guez, J. M., Nes, I. F., Hernández, P. E. and Cintas, L. M., 2001. Enterococcus faecium P21: A strain occurring naturally in dry-fermented sausages producing the class II bacteriocins enterocin A and enterocin B. Food Microbiology, 18(2): 115-131. (  0) 0) |
Hidaka, I., Kohbara, J., Araki, T., Morishita, T., Miyajima, T., Shimizu, S. and Kuriyama, I., 2000. Identification of feeding stimulants from a jack mackerel muscle extract for young yellowtail Seriola quinqueradiata. Aquaculture, 181: 115-126. (  0) 0) |
Holt J., 1994. Bergey's Manual of Determinative Bacteriology. 9th edition. Lippincott Williams & Wilkins, Philadelphia, 787pp.
(  0) 0) |
Ikeda, I., Hosokawa, H., Shimeno, S. and Takeda, M., 1988. Studies on feeding stimulants for jack mackerel-I. Identification of feeding stimulant for jack mackerel in its muscle extract. Nippon Suisan Gakkaishi, 54(2): 229-233. (  0) 0) |
Kim, H. S. and Cho, S. H., 2019. Dietary inclusion effect of feed ingredients showing high feeding attractiveness to rockfish (Sebastes schlegeli Hilgendorf 1880) on the growth performance, feed utilization, condition factor and whole body composition of fish (II). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 231: 66-73. (  0) 0) |
Kolkovski, S., Czesny, S. and Dabrowski, K., 2010. Use of krill hydrolysate as a feed attractant for fish larvae and juveniles. Journal of the World Aquaculture Society, 31(1): 81-88. (  0) 0) |
Liu, X., Steele, J. C. and Meng, X. Z., 2017. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environmental Pollution, 223: 161-169. (  0) 0) |
Mato, J., van Haastert, P. J., Krens, F. A. and Konijn, T. M., 1977. An acrasin-like attractant from yeast extract specific for Dictyostelium lacteum. Developmental Biology, 57(2): 450-453. (  0) 0) |
Millar, J. G., Chaney, J. D. and Mulla, M. S., 1992. Identification of oviposition attractants for Culex quinquefasciatus from fermented bermuda grass infusions. Journal of the American Mosquito Control Association, 8(1): 11-17. (  0) 0) |
Miyazaki, Y., Kamiya, S., Hanawa, T., Fukuda, M., Kawakami, H., Takahashi, H. and Yokota, H., 2010. Effect of probiotic bacterial strains of Lactobacillus, Bifidobacterium, and Enterococcuson enteroaggregative Escherichia coli. Journal of Infection & Chemotherapy Official Journal of the Japan Society of Chemotherapy, 16(1): 10-18. (  0) 0) |
Murai, T., Sumalangcay, A. and Piedadpascual, F., 1983. Supplement of various attractants to a practical diet for juvenile Penaeus monodon Fabricius. Fisheries Research Journal of the Philippines, 8(2): 61-67. (  0) 0) |
Nayak, S. K., 2010. Role of gastrointestinal microbiota in fish. Aquaculture Research, 41(11): 1553-1573. (  0) 0) |
Nunes, A. J. P., Sá, M. V. C., Andriola-Neto, F. F. and Lemos, D., 2006. Behavioral response to selected feed attractants and stimulants in Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 260(1): 244-254. (  0) 0) |
Olsén, K. H. and Lundh, T., 2016. Feeding stimulants in an omnivorous species, crucian carp Carassius carassius (Linnaeus 1758). Aquaculture Reports, 4: 66-73. (  0) 0) |
Papatryphon, E. and Joseph, H. S., 2000. The effect of dietary feeding stimulants on growth performance of striped bass, Morone saxatilis, fed-a-plant feedstuff-based diet. Aquaculture, 185(3-4): 329-338. (  0) 0) |
Pratoomyot, J., Bendiksen, E., Bell, J. G. and Tocher, D. R., 2010. Effects of increasing replacement of dietary fishmeal with plant protein sources on growth performance and body lipid composition of Atlantic salmon (Salmo salar L.). Aquaculture, 305(1): 124-132. (  0) 0) |
Qiu, X. and Davis, D. A., 2018. Evaluation of dried fermented biomass as a feed ingredient in plant-based practical diets for juvenile Pacific white shrimp Litopenaeus vannamei. Aquaculture Nutrition, 24(1): 383-391. (  0) 0) |
RingØ, E. and Gatesoupe, F. J., 1998. Lactic acid bacteria in fish: A review. Aquaculture, 160(3-4): 177-203. (  0) 0) |
Rombenso, A., Crouse, C. and Trushenski, J., 2013. Comparison of traditional and fermented soybean meals as alternatives to fish meal in hybrid striped bass feeds. North American Journal of Aquaculture, 75(2): 197-204. (  0) 0) |
Silva-Neto, J. F., Nunes, A. J. P., Sabry-Neto, H. and Sá, M. V. C., 2012. Spirulina meal has acted as a strong feeding attractant for Litopenaeus vannamei at a very low dietary inclusion level. Aquaculture Research, 43(3): 430-437. (  0) 0) |
Smith, D. M., Tabrett, S. J., Barclay, M. C. and Irvin, S. J., 2005. The efficacy of ingredients included in shrimp feeds to stimulate intake. Aquaculture Nutrition, 11(4): 263-272. (  0) 0) |
Sun, Y. Z., Yang, H. L., Ma, R. L., Huang, K. P. and Ye, J. D., 2012. Culture-independent characterization of the autochthonous gut microbiota of grouper Epinephelus coioides following the administration of probiotic Enterococcus faecium. Aquaculture International, 20(4): 791-801. (  0) 0) |
Sun, Y. Z., Yang, H. L., Ma, R. L. and Song, K., 2010. Survival of lactic acid bacteria isolated from gut of Epinephelus coioides in mimic gastrointestinal environments. Journal of Fishery Sciences of China, 17(1): 128-136 (in Chinese with English abstract). (  0) 0) |
Sun, Y. Z., Yang, H. L., Ma, R. L., Song, K. and Li, J. S., 2012. Effect of Lactococcus lactis and Enterococcus faecium on growth performance, digestive enzymes and immune response of grouper Epinephelus coioides. Aquaculture Nutrition, 18(3): 281-289. (  0) 0) |
Swain, S. M., Singh, C. and Arul, V., 2009. Inhibitory activity of probiotics Streptococcus phocae PI80 and Enterococcus faecium MC13 against Vibriosis in shrimp Penaeus monodon. World Journal of Microbiology & Biotechnology, 25(4): 697-703. (  0) 0) |
Tusche, K., Nagel, F., Arning, S., Wuertz, S., Susenbeth, A. and Schulz, C., 2013. Effect of different dietary levels of potato protein concentrate supplemented with feed attractants on growth performance of rainbow trout (Oncorhynchus mykiss). Animal Feed Science and Technology, 183(3): 202-209. (  0) 0) |
Utrio, P. and Eriksson, K., 1977. Volatile fermentation products as attractants for Macrolepidoptera. Annales Zoologici Fennici, 14(2): 98-104. (  0) 0) |
Wang, Y. B., Tian, Z. Q., Yao, J. T. and Li, W. F., 2008. Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture, 277(3): 203-207. (  0) 0) |
Xing, C. F., Hu, H. H., Huang, J. B., Fang, H. C., Kai, Y. H., Wu, Y. C. and Chi, S. C., 2013. Diet supplementation of Pediococcus pentosaceusin cobia (Rachycentron canadum) enhances growth rate, respiratory burst and resistance against photobacteriosis. Fish & Shellfish Immunology, 35(4): 1122-1128. (  0) 0) |
Zhang, L., Mai, K. S., Tan, B. P., Ai, Q. H., Qi, C. Z., Xu, W., Zhang, W. B., Fu, Z. G., Wang, X. J. and Ma, H. M., 2010. Effects of dietary administration of probiotic Halomonas sp. b12 on the intestinal microflora, immunological parameters, and midgut histological structure of shrimp, Fenneropenaeus chinensis. Journal of the World Aquaculture Society, 40(1): 58-66. (  0) 0) |
Zhang, Q., Tan, B. P., Mai, K. S., Zhang, W. B., Ma, H. M., Ai, Q. H., Wang, X. J. and Liufu, Z. G., 2011. Dietary administration of Bacillus (B. licheniformis and B. subtilis) and isomaltooligosaccharide influences the intestinal microflora, immunological parameters and resistance against Vibrio alginolyticus in shrimp, Penaeus japonicus (Decapoda: Penaeidae). Aquaculture Research, 42(7): 943-952. (  0) 0) |
Zhang, W., Liu, M. Q. and Dai, X. J., 2013. Biological characteristics and probiotic effect of Leuconostoc lactis strain isolated from the intestine of black porgy fish. Brazilian Journal of Microbiology, 44(3): 685-691. (  0) 0) |
Zheng, X. T., Duan, Y. F., Dong, H. B. and Zhang, J. S., 2017. Effects of dietary Lactobacillus plantarum in different treatments on growth performance and immune gene expression of white shrimp Litopenaeus vannamei under normal condition and stress of acute low salinity. Fish & Shellfish Immunology, 62: 195-201. (  0) 0) |
 2020, Vol. 19
2020, Vol. 19


