2) Liaoning Provincial Aquatic Products Analyzing, Testing and Processing Technology Scientific Service Center, Dalian 116023, China;
3) Key Laboratory of Biotechnology and Bioresources Utilization of Ministry of Education, Dalian Minzu University, Dalian 116600, China;
4) Collaborative Innovation Center of Seafood Deep Processing, Dalian Polytechnic University, Dalian 116034, China;
5) Dalian Key Laboratory of Marine Bioactive Substances Development and High Value Utilization, Dalian 116034, China
The bioaccumulation of heavy metals in shellfish has become more serious in recent years due to seawater pollution (Santos et al., 2013; Li et al., 2019). Some heavy metal ions, such as Pb, Cr, Cd, and Cu ions, are toxic and carcinogenic and harmful to human health at low concentrations due to long-term bioaccumulation (Bogdanovic et al., 2014; Drewnowska et al., 2017; Wang et al., 2020). More attention has been focused on bivalve mollusks due to their bioaccumulation of toxic trace elements and relative position in the food chain (Tapia et al., 2010; Heidari et al., 2013; Bogdanovic et al., 2014; Barbosa et al., 2018). Therefore, the removal of heavy metals in shellfish is important in food safety control (Bille et al., 2015; Mirlean et al., 2019). Many techniques, including precipitation, coagulation, ion exchange, and adsorption have been developed for the treatment of aquatic products containing heavy metal ions; however, most of these approaches are costly or ineffective (Hami Dindar et al., 2015; Yang et al., 2019).
A number of adsorbents, including activated carbon (Zacaroni et al., 2015), chitosan and alginate nanocomposites (Gokila et al., 2017), hybrid materials based on silica particles (Radi et al., 2016), magnetic nanoparticle-functionalized lactic acid bacteria cells (Li et al., 2020), montmorillonite (Liu et al., 2019), Fusarium-coated multi-walled carbon nanotubes (Moallaei et al., 2020), and cellulose nanofibrous mats (Zhang et al., 2020) have been applied to remove heavy metal ions from food and water. However, these materials usually exhibit inherent disadvantages, such as low adsorption capacity, low selectivity, long equilibrium time, or mechanical and thermal instability, particularly when the initial concentrations of metal ions are relatively low. The preparation of silica-based mesoporous materials has attracted considerable attention due to their uniquely large surface area, regular pore structure, high mechanical and thermal stability, and easily modified surface properties (Zhu et al., 2016; Jadhav et al., 2020). Surfacemodified mesoporous silicas are promising adsorbents with high adsorption capacities for heavy metals. Mesoporous silicas functionalized with organic compounds by grafting organosiloxane precursors have been widely studied (Lee et al., 2016; Vunain et al., 2016). Mesoporous materials modified with ligands containing nitrogen or sulfur exhibit high affinities for the adsorption of heavy metal ions, including Cu, Ni, Zn, Pb, Ag, Cr, and Co (Zhang and Li, 2013; Zhang et al., 2020). Ammonium pyrrolidine dithiocarbamate (APDC), a compound containing nitrogen and sulfur, has been used to modify mesoporous materials due to its high affinity for heavy metals (Goubert-Renaudin et al., 2009).
The reduction of nonspecific adsorption is another challenge in the removal of heavy metal ions from food, especially with respect to unexpected losses of nutrients, such as proteins and polysaccharides (Deere et al., 2003; Yan et al., 2011). The extraction of heavy metals from protein solutions is more challenging due to the interaction of metal ions with proteins, and decreasing heavy metal ion concentrations to regulatory standards is difficult. In addition, as proteins can be possibly adsorbed to sorbents, the extraction of heavy metal ions is accompanied by decreasing protein concentrations. Furthermore, the nonspecific adsorption of proteins occurs in the active sites involved in heavy-metal removal, resulting in the decreased efficiency of heavymetal-ion removal (Howard et al., 2008; Zhang et al., 2008). Thus, to efficiently extract metals from protein solutions, scientists need to develop sorbents with strong binding affinities toward metal ions and limited adsorption capabilities for proteins. However, the available studies are limited. Sulfonated polystyrene nanospheres have been reported to remove heavy metals from collagen solutions with adsorption capacities of 50.7, 15.0, 8.7, and 39.0 mg g−1 for Pb2+, Mn2+, Cr3+, and Cd2+, respectively, and a protein loss percentage of 15.2% at pH 4.5 (Peng et al., 2018). However, high removal rates of heavy metals and low losses of proteins are still expected. Therefore, increasing the removal rate of heavy metals while avoiding the nonspecific adsorption of other nutrients is an important topic in the study of heavy-metal removal. Our previous work demonstrated that alkyl-diol groups modified on the external surface of a mesoporous material can efficiently decrease the nonspecific adsorption of proteins (Qi et al., 2010).
In this study, we synthesized and characterized a new hybrid SBA15 mesoporous material modified with APDC on the inner surface and alkyl-diol groups on the outer surface, which was named as Diol-APDC-SBA15. The applicability of Diol-APDC-SBA15 for the removal of heavy metal ions, including Pb, Cr, Cd, and Cu, from shellfish processing liquids was systematically investigated.
2 Materials and Methods 2.1 Instruments and EquipmentPowder X-ray diffraction (XRD) patterns were determined using an X'pert Pro vertical goniometer (PANalytical, Holland). Nitrogen adsorption measurements were performed at 77 K with a NOVA 2200e surface area and pore size analyzer (Quantachrome, USA). Fourier transform infrared (FT-IR) spectroscopy was performed with a spectrum 100/100N system (PerkinElmer, USA) with a scan range of 4000 – 400 cm−1 at 25℃ ± 3℃. Each protein or polysaccharide concentration was determined using an ultraviolet-visible spectrometer (Lambda 35, PerkinElmer). The metal ion concentrations were measured by inductively coupled plasma atomic emission spectrometry (ICP-AES) on a PerkinElmer Optima 8000 spectrometer (PerkinElmer, USA).
2.2 Materials and ReagentsTEOS and [3-(2, 3-epoxypropoxy)propyl]trimethoxysilane were purchased from Meryer Chemical Technology Co. (Shenzhen, China). Potassium iodide and APDC were obtained from Solarbio Technology Co. (Beijing, China). PEGPPG-PEG (P123) was purchased from Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). Acetic acid and sodium acetate were obtained from Damao Chemical Reagent Factory (Tianjin, China). Standard stock solutions of Pb(II), Cr(III), Cd(II), and Cu(II) were obtained from Beijing Century Aoke Biological Technology Co. (Beijing, China). Bovine serum albumin (BSA) and trypsin were obtained from Sigma (St Louis, MO, USA). Deionized water (R > 18.2 MΩ) was purified by a Millipore (Billerica, MA, USA) purification system.
The cooking liquid of clams (Ruditapes philippinarum) was obtained from Liaoning Ande Food Co., Ltd., and the soluble solid content of the cooking liquid was adjusted to 10% in our laboratory before use. The oyster (Ostrea gigas Thunberg) hydrolysate liquid was prepared by hydrolyzing oysters with trypsin followed by ultra-filtration with a 3000 Da molecular weight cut-off. The low-molecularweight components were collected and lyophilized. The powder was dissolved in water at a ratio of 1:50 (w/v) before use. The polysaccharide solution was prepared by dissolving the dry powder of crude polysaccharides extracted from the concentrated cooking liquid of clam in water at a ratio of 1:50 (w/v) and stirring until complete dissolution without precipitation for use.
2.3 Mesoporous Material Preparation 2.3.1 Preparation of APDC-SBA15 mesoporous materialFirst, 72-mL water, 8.9-mL concentrated hydrochloric acid, and 3.56-g P123 were added to a round flask with stirring at 41℃. Then, after complete dissolution of P123, 8-mL TEOS was dropwise added slowly. After stirring the solution for 10 min, the formed gel was transferred to a Teflon bottle and maintained at 40℃ for 24 h. The resultant product was filtered, washed with ethanol and distilled water, and then dried at 100℃ for 5 h. The surfactant (P123) in the materials was extracted by acidified ethanol, and 100 mL 1.2 mol L−1 aqueous HCl and 100-mL absolute alcohol were added to 1 g material and refluxed for 24 h, followed by drying at 100℃ for 5 h (Zhao et al., 1998). The modification of APDC on the mesoporous material was performed as follows: 4 mL 3-chloropropyltriethoxysilane and 4.0 g potassium iodide were added to a round flask and stirred at 40℃ for 2 h. Then, 4.0 g APDC was added, and the solution was stirred at 40℃ for 3 h. Next, 80 mL absolute ethanol and 2.0 g SBA15 were added, and the solution was refluxed for 12 h. The resultant product was filtered, washed, and dried at 100℃ for 10 h (Jiang et al., 2013). The resultant materials were designated as APDC-SBA15.
2.3.2 Preparation of Diol-APDC-SBA15 mesoporous materialThe material Diol-APDC-SBA15 was prepared in accordance with the previous research (Qi et al., 2010) with some modifications. Briefly, 4.0 g P123 was suspended in a solution of 85 mL water and 10 mL concentrated hydrochloric acid at 41℃ and stirred until complete dissolution. Then, 10 mL TEOS, 4.0 mL 3-chloropropyltriethoxysilane, and 4.0 g APDC were added to the above mixture successively and stirred for 30 min. The mixture was transferred to an autoclave and then maintained at 80℃ for 12 h. The resultant product was filtered, washed with water, and then dried at 100℃ for 10 h.
Next, 2 g of the above sample was suspended in 80 mL ethanol and added with 3 mL [3-(2, 3-epoxypropoxy)propyl] trimethoxysilane. After refluxing under nitrogen protection for 24 h, the suspension was filtered, washed with ethanol, and dried at 100℃. The material was treated with acidified ethanol to remove the surfactant (P123) and to convert the (2, 3-epoxypropoxy)propyl group to an alkyl-diol group as previously described (Qi et al., 2010). The resultant material was designated as Diol-APDC-SBA15.
2.4 Material CharacterizationThe Diol-APDC-SBA15 material was characterized by powder XRD, nitrogen adsorption, and FT-IR analyses. XRD patterns were set for scanning in the 2θ range of 0.5˚ – 8˚. Nitrogen adsorption measurements were performed at 77 K with a NOVA 2200e surface area and pore size analyzer. FT-IR spectroscopy was performed with a spectrum 100/100N system (scan range, 4000 – 400 cm−1; 25℃ ± 3℃).
2.5 Heavy-Metal Adsorption from Standard HeavyMetal Solutions by Diol-APDC-SBA15A standard heavy-metal solution of Pb, Cr, Cd, and Cu metal ions was prepared, and the adsorption conditions were optimized. The solution pH is an important parameter for the heavy-metal removal efficiency. In the adsorption experiments, Pb, Cr, Cd, and Cu metal ion solutions were each mixed at a concentration of 4 mg L−1. Then, 30 mL of the metal ion solution was treated with acetate-sodium acetate solution to adjust the pH to 4 – 8, followed by mixing with 30 mg of Diol-APDC-SBA15 at 25℃ ± 3℃. After stirring for 30 min, the suspension was centrifuged, and the supernatant was collected. The metal ion concentrations of the initial and final solutions were measured by ICP-AES.
In the adsorption time investigation, Pb, Cr, Cd, and Cu metal ion solutions were each mixed at a concentration of 4 mg L−1 at optimum pH. Then, 30 mL of the metal solution was mixed with 30 mg Diol-APDC-SBA15 at 25℃ ± 3℃. After stirring for 3, 5, 10, 30, 60, or 120 min, the suspension was centrifuged, and the supernatant was collected. The metal ion concentrations of the initial and final solutions were also measured by ICP-AES.
In the adsorption concentration investigation, mixed metal ion solutions containing Pb, Cr, Cd, and Cu with concentrations of 2, 4, 8, 10, 15, 20, 30, 50, 100, 150, 300, 400, 600, and 800 mg L−1 were prepared first. Next, 30 mL of the mixed-metal solution at optimum pH in 0.2 mol L−1 acetatesodium acetate buffer solution was mixed with 30 mg of Diol-APDC-SBA15 at 25℃ ± 3℃. After stirring for 30 min, the suspension was centrifuged, and the supernatant was collected. The metal ion concentrations of the initial and final solutions were measured by ICP-AES.
2.6 Practical Application of Diol-APDC-SBA15 for Removal of Heavy Metals from Shellfish Processing Liquids 2.6.1 Removal efficiency of heavy metals from shellfish processing liquidsThe reliability and practicability of the proposed method were investigated by removing Pb, Cr, Cd, and Cu ions from two shellfish product processing liquids. First, 30 mL of the prepared sample solution was added to a centrifuge tube, in which 30 mg of APDC-SBA15 or Diol-APDC-SBA15 was added. After mixing, the mixture in centrifuge tubes were shaken for 30 min, and centrifuged for 5 min at 1500 r min−1, and the supernatant was collected. The removal efficiencies for heavy metal ions and the amounts of proteins and polysaccharides in the samples before and after heavymetal removal were measured, and the differences between the two mesoporous materials in the removal of aquatic products were compared.
Then, 5 mL of concentrated nitric acid was added to a 5 mL liquid sample, and the mixture was heated on a hot plate (150℃) until the whole solution became colorless and transparent. Then, the solution was filtered through a filter paper and finally diluted to a volume of 50 mL for further experiments. The metal removal efficiency was calculated using the following equation:
| $\text { Removal efficiency (%) }=\frac{C_0-C_1}{C_0} \times 100 \text {, }$ | (1) |
where C0 is the concentration of heavy metal ions before absorption (mg L−1), and C1 is the concentration of heavy metal ions after absorption (mg L−1).
To estimate the possible matrix effects on the low contents of heavy metal ions, the cooking liquid of R. philippinarum was also spiked with four heavy metal ions at a concentration of 10 mg L−1 and analyzed using the proposed method. The contents of Pb, Cr, Cd, and Cu before and after the removal of heavy metal ions in the samples were also determined by ICP-AES.
2.6.2 Losses of proteins and polysaccharides from shellfish processing liquidsIn this experiment, the losses of proteins and polysaccharides were determined to investigate the losses of proteins and polysaccharides caused by the removal of heavy metal ions from the samples. The protein content was determined by the biuret method using BSA as the standard (Lubran, 1978). The polysaccharide content was determined by the phenol-sulfuric acid method (A 490 nm) using glucose as the standard (Dubois et al., 1956). The expression of loss percentage is shown in Eq. (2):
| $\operatorname{Loss}(\%)=\frac{\left(C_0-C_1\right)}{C_0} \times 100,$ | (2) |
where C0 is the concentration of protein or polysaccharide before adsorption (mg L−1), and C1 is the concentration of protein or polysaccharide after adsorption (mg L−1).
All tests were performed in triplicate.
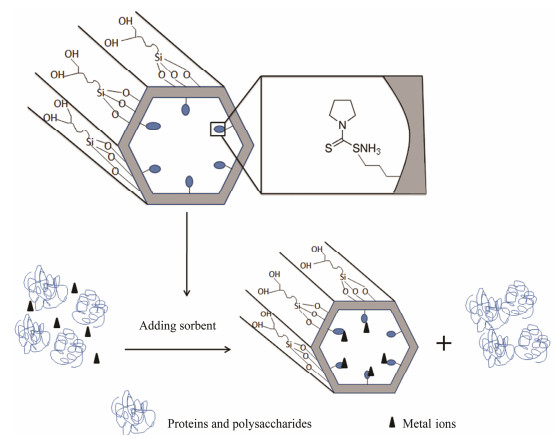
3 Results and Discussion 3.1 Preparation and Characterization of Diol-APDC-SBA15Scheme 1 shows the heavy metal ion removed by the Diol-APDC-SBA15 material. The APDC group was anchored to the mesoporous silica material via a co-condensation method, and alkyl-diol groups were grafted on the external surface of the mesoporous material by the post-graft method when the pore former was in the mesopores. The APDC groups of the Diol-APDC-SBA15 material were used for heavy metal ion removal. The alkyl-diol groups on the external surface of the mesoporous material reduced the nonspecific adsorption of proteins and polysaccharides.
|
Scheme1 Sorption schematic of heavy metal ions using Diol-APDC-SBA15. |
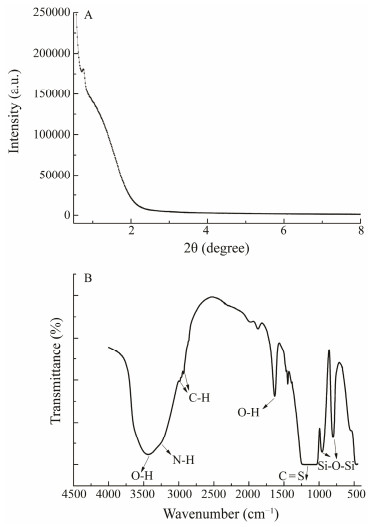
Fig.1A shows the XRD patterns of the Diol-APDC-SBA15 mesoporous sorbent. The sorbent exhibited an intense (100) peak near 1.0˚, indicating that the ordered hexagonal structure was not completely destroyed during the surface modification process. The pore diameter distributions of the samples were further studied by N2 adsorption-desorption analyses. The pore diameter of the mesoporous material was 33.0 Å, and its distribution was calculated from the adsorption branch of the isotherm by the Barrett-Joyner-Halenda method. The Brunauer-Emmett-Teller surface area was 133 m2 g−1, which was attributed to the SBA15 surface having undergone stepwise organic functionalization by APDC and alkyl-diol groups.
|
Fig. 1 Powder XRD patterns (A) and FT-IR spectra (B) of Diol-APDC-SBA15. |
Fig.1B shows the FT-IR spectrum of the Diol-APDC-SBA15 material. The bands near 800 and 960 cm−1 were assigned to the Si–O–Si stretching and bending modes of the mesoporous silica framework, respectively, and the wide band between 1200 and 1050 cm−1 was assigned to the C = S wagging vibration of the APDC group. The bands near 2900 cm−1 were assigned to C–H bonds. The bands near 3400 cm−1 corresponded to the O–H bonds of the alkyl-diol groups and the N–H stretching modes of the APDC group. These results indicated that alkyl-diol and APDC groups were successfully grafted onto the material surface.
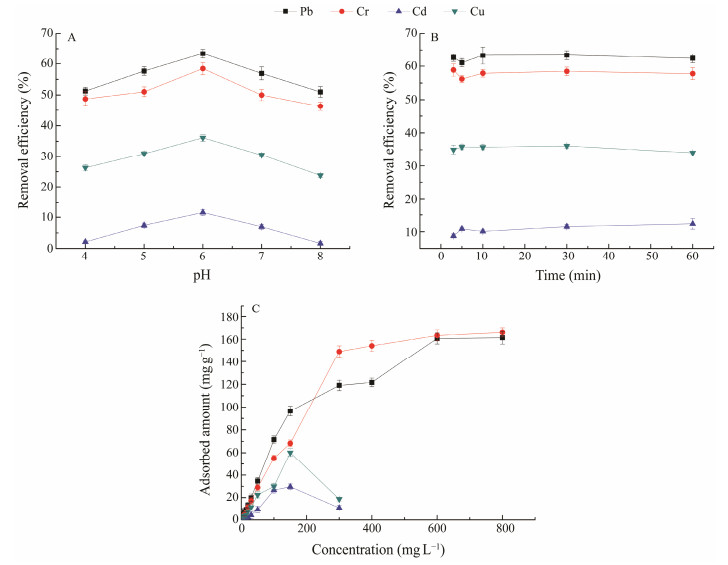
3.2 Optimization of the Adsorption Conditions of Heavy Metal Ions with Standard Heavy-Metal SolutionThe optimal pH of the standard heavy-metal solution for heavy-metal removal was pH 6 (Fig.2A). The removal efficiency was low at pH 4, and it increased with the increase of pH from pH 4 to 6. However, the removal efficiency decreased with the increase of pH from pH 6 to 8. In the removal experiment, the electron-donating elements (N and S) of the mesoporous material supported complexation reactions with metal ions. At the pH range of 4 – 8, the N and S atoms exhibited their chelating capabilities for heavy metal ions, and the empty orbit of the Pb ion chelated with N and S to form a stable ligand. Cr, Cd, and Cu ions with empty orbits followed the similar mechanisms. When the pH was low, the N and S atoms combined with H+ ions, but the two types of atoms could not coordinate the metal ions. At alkaline pH, metal ions may precipitate; thus, electrondonating atoms will not coordinate ions, and the removal efficiency decreases (Zhang et al., 2020). At pH 6.0, the N and S atoms exhibited their strong chelating capabilities, and the adsorption capacity for heavy metals was the highest.
|
Fig. 2 Investigation of (A) the efficiencies of removing heavy metal ions at different pH values; (B) adsorption kinetics of heavy metal ions; (C) amounts of heavy metal ions adsorbed at different concentrations. |
The adsorption kinetics of the Diol-APDC-SBA15 material were investigated, and the removal efficiencies for Pb, Cr, Cu, and Cd metal ions at different times were determined (Fig.2B). The equilibrium time was 10 min, which was considerably shorter than those of other materials for removing all four metal ions. Equilibration times of 120 min for clay and 360 min for activated charcoal in the removal of Cu were previously reported (Zacaroni et al., 2015). Our material required a short equilibrium time because the ordered pore channels of the Diol-APDC-SBA15 material allowed the heavy metal ions to easily reach the APDC groups.
The amounts of Pb and Cr ions adsorbed by the Diol-APDC-SBA15 material increased with the increasing metal ion concentrations from 2 to 800 mg L−1, but the adsorbed amounts for Cd and Cu ions reached the highest values at the concentration of 150 mg L−1. This result might have been due to the precipitation of Cd and Cu ions at high concentrations. The equilibrium adsorption amounts of four heavy metal ions, including Pb, Cr, Cd, and Cu, were 161.4, 166.1, 29.6, and 60.2 mg g−1, respectively, and the order of adsorption amounts was Pb > Cr > Cu > Cd (Fig.2C). The equilibrium adsorption amounts for Diol-APDC-SBA15 were slightly higher than those of the ethylenediaminepro-pyl-2-pyridylimine-modified mesoporous material (106.62 mg g−1 for Pb and 48.26 mg g−1 for Cu) (Zhang et al., 2020), and considerably higher than those of clay-, charcoal-, and magnetic nanoparticle-functionalized lactic acid bacteria cells. The maximum amounts of Pb and Cd adsorbed by magnetic nanoparticle-functionalized lactic acid bacteria cells were 0.17 and 0.57 mg g−1, respectively (Li et al., 2020); the maximum capacity of clay for absorbing Cu was 10.8 mg g−1, and that of charcoal was 5.9 mg g−1 (Zacaroni et al., 2015). The amounts of Pb and Cd adsorbed by Diol-APDC-SBA15 were 948 and 51 times greater than those by the functionalized lactic acid bacteria cell adsorbent, respectively. The amounts of Cu adsorbed by Diol-APDC-SBA15 were 5 and 9 times higher than those by clay and charcoal, respectively. The high surface area of the Diol-APDC-SBA15 material and high metal ion affinity of the APDC group might have contributed to the high adsorbed amounts of metal ions.
3.3 Removal of Heavy Metals from Shellfish Processing Liquids Using Mesoporous MaterialsTo evaluate the heavy-metal-ion removal efficiency from shellfish processing liquids, we removed the heavy metals in the cooking liquid of clams (R. philippinarum), hydrolysate liquid of oysters (Ostrea gigas Thunberg), and polysaccharides solution from the cooking liquid of R. philippinarum using Diol-APDC-SBA15 and APDC-SBA15 materials. The results are summarized in Table 1. The removal efficiencies of Diol-APDC-SBA15 and APDC-SBA15 materials for Pb in the cooking liquid were prominent and above 99.6%. The removal efficiencies of the APDC-SBA15 material for Pb and Cr ions from oyster hydrolysate liquid were 62.3% and above 99.9%, respectively. The removal efficiencies of the Diol-APDC-SBA15 material for Pb and Cr were 77.0% and above 99.9%, respectively. The removal efficiencies of Diol-APDC-SBA15 and APDC-SBA15 materials for Pb from the polysaccharide solution were 60.5% and 84.5%, respectively. The two mesoporous materials had high removal efficiencies for Pb and Cr. Diol-APDC-SBA15 showed a higher removal efficiency for Pb from oyster hydrolysate liquid than APDC-SBA15, but APDC-SBA15 had a higher removal efficiency for Pb from the polysaccharide solution than Diol-APDC-SBA15. However, the removal efficiencies of the two materials for Cd and Cu were below 3%, which might be due to uncertainties in the low concentrations of Cd and Cu in shellfish processing liquids.
|
|
Table 1 Efficiencies of removing heavy metal ions from shellfish processing liquid (%) |
To further investigate the efficiencies of removing the four heavy metal ions from the cooking liquid of clams (R. philippinarum), we added a standard heavy-metal-ion solution with a concentration of 10 mg L−1 for each heavy metal ion to the cooking liquid of clams. Table 2 shows the efficiencies of removing metal ions from this cooking liquid after the addition of standard heavy metal ions. The removal efficiencies of APDC-SBA15 for Pb, Cr, Cd, and Cu were 73.2%, 77.6%, 44.8%, and 33.3%, respectively. The corresponding removal efficiencies of Diol-APDC-SBA15 were 74.1% (Pb), 75.3% (Cr), 43.0% (Cd), and 32.0% (Cu). The two mesoporous materials exhibited nearly the same removal efficiencies for the four heavy metal ions, indicating that the external modification with alkyl-diol groups did not influence the adsorption of heavy metal ions.
|
|
Table 2 Efficiencies of removing heavy metal ions in spiked cooking liquid of clam |
The losses of polysaccharides and proteins from the shellfish processing liquids after heavy metal ion removal were also determined. The results are shown in Table 3. The losses of polysaccharides reduced from 7.3% to 5.5% when the absorbent was changed from APDC-SBA15 to Diol-APDC-SBA15. Given the low concentration of proteins and matrix interference of the cooking liquid of clams, the losses of proteins were undetected for the two materials. The losses of proteins from oyster (Ostrea gigas Thunberg) hydrolysate liquids were 14.6% and 1.2% for APDC-SBA15 and Diol-APDC-SBA15, respectively. The lower loss of protein with Diol-APDC-SBA15 was due to the repulsion of proteins of the alkyl-diol groups, which has been previously reported (Qi et al., 2010). The polysaccharide loss by Diol-APDC-SBA15 from the polysaccharide solution was 3.7%. This result indicated that the external modification of alkyl-diol groups can decrease the losses of protein and polysaccharides during heavy metal ion removal. In summary, the Diol-APDC-SBA15 material can efficiently remove heavy metal ions from shellfish processing liquids with low losses of proteins and polysaccharides.
|
|
Table 3 Loss rates of protein and polysaccharide in shellfish processing liquid (%) |
Mesoporous silica modified internally with APDC and externally with alkyl-diol groups, which was named as Diol-APDC-SBA15, was successfully prepared and applied to remove heavy metal ions (Pb, Cr, Cd, and Cu) from shellfish processing liquids. The results showed that the Diol-APDC-SBA15 material can efficiently remove metal ions from standard heavy metal ion solutions and shellfish processing liquids. In addition, alkyl-diol group modification of the external surface of the mesoporous material efficiently decreased the losses of proteins and polysaccharides during the removal process. Therefore, Diol-APDC-SBA15 is a promising sorbent material for the removal of metal ions from complex shellfish processing liquids.
AcknowledgementsThis work was supported by the National Key R & D Program of China (No. 2018YFD0901004), the National Natural Science Foundation of China (No. 31601538), the Key Science and Technology Program of Liaoning Province (No. 2020JH1/10200001), the Fundamental Research Foundation of Education Department of Liaoning Province (No. JL202008), and the Science & Technology Innovation Foundation of Dalian (No. 2019J12SN61).
Barbosa, I. S., Brito, G. B., Santos, G. L., Santos, L. N., Teixeira, L. S. G., Araujo, R. G. O., et al., 2018. Multivariate data analysis of trace elements in bivalve molluscs: Characterization and food safety evaluation. Food Chemistry, 273: 64-70. DOI:10.1016/j.foodchem.2018.02.063 (  0) 0) |
Bille, L., Binato, G., Cappa, V., Toson, M., Pozza, M. D., Arcangeli, G., et al., 2015. Lead, mercury and cadmium levels in edible marine molluscs and echinoderms from the Veneto Region (north-western Adriatic Sea – Italy). Food Control, 50: 362-370. DOI:10.1016/j.foodcont.2014.09.018 (  0) 0) |
Bogdanovic, T., Ujevic, I., Sedak, M., Listes, E., Simat, V., Petricevic, S., et al., 2014. As, Cd, Hg and Pb in four edible shellfish species from breeding and harvesting areas along the eastern Adriatic Coast, Croatia. Food Chemistry, 146: 197-203. DOI:10.1016/j.foodchem.2013.09.045 (  0) 0) |
Deere, J., Magner, E., Wall, J. G., and Hodnett, B. K., 2003. Adsorption and activity of proteins onto mesoporous silica. Catalysis Letters, 85: 19-23. DOI:10.1023/A:1022156405117 (  0) 0) |
Drewnowska, M., Falandysz, J., Chudzińska, M., Hanć, A., Saba, M., and Barałkiewicz, D., 2017. Leaching of arsenic and sixteen metallic elements from Amanita fulva mushrooms after food processing. LWT – Food Science and Technology, 84: 861-866. DOI:10.1016/j.lwt.2017.04.066 (  0) 0) |
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F., 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28: 350-356. DOI:10.1021/ac60111a017 (  0) 0) |
Gokila, S., Gomathi, T., Sudha, P. N., and Anil, S., 2017. Removal of the heavy metal ion chromiuim(VI) using chitosan and alginate nanocomposites. International Journal of Biological Macromolecules, 104: 1459-1468. DOI:10.1016/j.ijbiomac.2017.05.117 (  0) 0) |
Goubert-Renaudin, S., Gaslain, F., Marichal, C., Lebeau, B., Schneider, R., and Walcarius, A., 2009. Synthesis of dithiocarbamate-functionalized mesoporous silica-based materials: Interest of one-step grafting. New Journal of Chemistry, 33: 528-537. DOI:10.1039/B811780B (  0) 0) |
Hami Dindar, M., Yaftian, M. R., Pilehvari, M., and Rostamnia, S., 2015. SBA-15 mesoporous materials decorated with organic ligands: Use as adsorbents for heavy metal ions. Journal of the Iranian Chemical Society, 12: 561-572. DOI:10.1007/s13738-014-0513-8 (  0) 0) |
Heidari, B., Bakhtiari, A. R., and Shirneshan, G., 2013. Concentrations of Cd, Cu, Pb and Zn in soft tissue of oyster (Saccostrea cucullata) collected from the Lengeh Port Coast, Persian Gulf, Iran: A comparison with the permissible limits for public health. Food Chemistry, 141: 3014-3019. DOI:10.1016/j.foodchem.2013.06.002 (  0) 0) |
Howard, M. D., Jay, M., Dziubla, T. D., and Lu, X., 2008. PEgylation of nanocarrier drug delivery systems: State of the art. Journal of Biomedical Nanotechnology, 4: 133-148. DOI:10.1166/jbn.2008.021 (  0) 0) |
Jadhav, S. A., Patil, V. S., Shinde, P. S., Thoravat, S. S., and Patil, P. S., 2020. A short review on recent progress in mesoporous silicas for the removal of metal ions from water. Chemical Papers, 74: 4143-4157. DOI:10.1007/s11696-020-01255-6 (  0) 0) |
Jiang, F. M., Pu, Q. M., Ren, F. L., Huang, H. Q., Cao, F. Y., Li, Y., et al., 2013. Green graft process for preparation of functional mesoporous silica materials. Materials Research Innovations, 17: 122-128. DOI:10.1179/1433075X12Y.0000000041 (  0) 0) |
Lee, J. Y., Chen, C. H., Cheng, S., and Li, H. Y., 2016. Adsorption of Pb(II) and Cu(II) metal ions on functionalized large-pore mesoporous silica. International Journal of Environmental Science and Technology, 13: 65-76. DOI:10.1007/s13762-015-0841-y (  0) 0) |
Li, P., Pan, Y., Fang, Y., Du, M., Pei, F., Shen, F., et al., 2019. Concentrations and health risks of inorganic arsenic and methylmercury in shellfish from typical coastal cities in China: A simultaneous analytical method study. Food Chemistry, 278: 587-592. DOI:10.1016/j.foodchem.2018.11.085 (  0) 0) |
Li, X., Ming, Q., Cai, R., Yue, T., Yuan, Y., Gao, Z., et al., 2020. Biosorption of Cd2+ and Pb2+ from apple juice by the magnetic nanoparticles functionalized lactic acid bacteria cells. Food Control, 109: 106916. DOI:10.1016/j.foodcont.2019.106916 (  0) 0) |
Liu, Y., Luan, J., Zhang, C., Ke, X., and Zhang, H., 2019. The adsorption behavior of multiple contaminants like heavy metal ions and p-nitrophenol on organic-modified montmorillonite. Environmental Science and Pollution Research, 26: 10387-10397. DOI:10.1007/s11356-019-04459-w (  0) 0) |
Lubran, M. M., 1978. The measurement of total serum proteins by the Biuret method. Annals of Clinical & Laboratory Science, 8: 106. (  0) 0) |
Mirlean, N., Ferraz, A. H., Seus-Arrache, E. R., Andrade, C. F., Costa, L. P., and Johannesson, K. H., 2019. Mercury and selenium in the Brazilian subtropical marine products: Food composition and safety. Food Composition and Analysis, 84: 103310. DOI:10.1016/j.jfca.2019.103310 (  0) 0) |
Moallaei, H., Bouchara, J. P., Rad, A., Singh, P., Raizada, P., Tran, H. N., et al., 2020. Application of Fusarium sp. immobilized on multi-walled carbon nanotubes for solid-phase extraction and trace analysis of heavy metal cations. Food Chemistry, 322: 126757. DOI:10.1016/j.foodchem.2020.126757 (  0) 0) |
Peng, Y., Shen, Y., Ge, M., Pan, Z., Chen, W., and Gong, B., 2018. Efficient extraction of heavy metals from collagens by sulfonated polystyrene nanospheres. Food Chemistry, 275: 377-384. DOI:10.1016/j.foodchem.2018.09.111 (  0) 0) |
Pereira Santos, L. F., Sitonio Trigueiro, I. N., Lemos, V. A., Nóbrega Furtunato, D. M., and Cássia Vieira Cardoso, R., 2013. Assessment of cadmium and lead in commercially important seafood from São Francisco do Conde, Bahia, Brazil. Food Control, 33: 193-199. DOI:10.1016/j.foodcont.2013.02.024 (  0) 0) |
Qi, Y., Wei, J., Wang, H., Zhang, Y., Xu, J., Qian, X., et al., 2010. Improved selection of LMW over HMW proteins from human plasma by mesoporous silica particles with external modification. Talanta, 80: 703-709. DOI:10.1016/j.talanta.2009.07.050 (  0) 0) |
Qi, Y., Wu, D., Wei, J., Ding, K., Wang, H., Zhang, Y., et al., 2010. Selective extraction of low molecular weight proteins by mesoporous silica particles with modified internal and external surfaces. Analytical and Bioanalytical Chemistry, 398: 1715-1722. DOI:10.1007/s00216-010-4081-1 (  0) 0) |
Radi, S., Toubi, Y., El-Massaoudi, M., Bacquet, M., Degoutin, S., and Mabkhot, Y. N., 2016. Efficient extraction of heavy metals from aqueous solution by novel hybrid material based on silica particles bearing new Schiff base receptor. Journal of Molecular Liquids, 223: 112-118. DOI:10.1016/j.molliq.2016.08.024 (  0) 0) |
Tapia, J., Vargas-Chacoff, L., Bertrán, C., Carrasco, G., Torres, F., Pinto, R., et al., 2010. Study of the content of cadmium, chromium and lead in bivalve molluscs of the Pacific Ocean (Maule Region, Chile). Food Chemistry, 121: 666-671. DOI:10.1016/j.foodchem.2009.12.091 (  0) 0) |
Vunain, E., Mishra, A. K., and Mamba, B. B., 2016. Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the removal of heavy-metal ions: A review. International Journal of Biological Macromolecules, 86: 570-586. DOI:10.1016/j.ijbiomac.2016.02.005 (  0) 0) |
Wang, X., Wu, J., Yu, B., Dong, K. F., and Zhang, C., 2020. Heavy metals in aquatic products and the health risk assessment to population in China. Environmental Science and Pollution Research, 27: 22708-22719. DOI:10.1007/s11356-020-08685-5 (  0) 0) |
Yan, F., Sun, L., Li, F., Zhuang, J., Wang, H., and Yang, W., 2011. Mesoporous silica-coated superparamagnetic particles prepared by pseudomorphic transformation and their application in purification of plasmid DNA. Journal of Nanoparticle Research, 13: 6613-6620. DOI:10.1007/s11051-011-0569-7 (  0) 0) |
Yang, H., Hu, Y., Wang, X., Fu, W., Tian, H., and Alam, E., 2019. Investigation on synthesis of ion-imprinted mesoporous adsorbents by using ultrasound- and microwave-assisted preparation and their dynamic adsorption properties on heavy metals. Environmental Science and Pollution Research, 26: 10987-10999. DOI:10.1007/s11356-019-04436-3 (  0) 0) |
Zacaroni, L. M., Magriotis, Z. M., Graças Cardoso, M., Santiago, W. D., Mendonça, J. G., Vieira, S. S., et al., 2015. Natural clay and commercial activated charcoal: Properties and application for the removal of copper from cachaça. Food Control, 47: 536-544. DOI:10.1016/j.foodcont.2014.07.035 (  0) 0) |
Zhang, D., and Li, J. H., 2013. Ordered SBA-15 mesoporous silica with high amino-functionalization for adsorption of heavy metal ions. Chinese Science Bulletin, 58: 879-883. DOI:10.1007/s11434-012-5594-0 (  0) 0) |
Zhang, D., Xu, W., Cai, J., Cheng, S. Y., and Ding, W. P., 2020. Citric acid-incorporated cellulose nanofibrous mats as food materials-based biosorbent for removal of hexavalent chromium from aqueous solutions. International Journal of Biological Macromolecules, 149: 459-466. DOI:10.1016/j.ijbiomac.2020.01.199 (  0) 0) |
Zhang, L., Ma, Z. C., and Liu, J., 2008. Influence of bovine serum albumin on the adsorption equilibrium between Cu(2+) and δ-MnO2. Chinese Journal Ecology, 27: 756-761. (  0) 0) |
Zhang, Y., Cao, X., Sun, J., Wu, G., Wang, J., and Zhang, D., 2020. Synthesis of pyridyl Schiff base functionalized SBA-15 mesoporous silica for the removal of Cu(II) and Pb(II) from aqueous solution. Journal of Sol-Gel Science and Technology, 94: 658-670. DOI:10.1007/s10971-019-05205-x (  0) 0) |
Zhao, D. Y., Huo, Q. S., Feng, J. L., Chmelka, B. F., and Stucky, G. D., 1998. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. Journal of the American Chemical Society, 120: 6024-6036. DOI:10.1021/ja506344k (  0) 0) |
Zhu, J. Y., Zhu, X. Y., Gu, J. L., Zhao, L. M., Jiang, L. H., and Qiu, Y. J., 2016. Effective adsorption and concentration of carnosine by nickel species within mesoporous silica. LWT – Food Science and Technology, 74: 211-218. DOI:10.1016/j.lwt.2016.07.016 (  0) 0) |
 2023, Vol. 22
2023, Vol. 22


