2) Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
Okadaic Acid (OA, C44H68O13, Mw. 805 g mol–1) has been recognized as a major toxic compound in ocean, causing diarrheic shellfish poisoning (DSP) by inhibiting the action of protein phosphatase (Ramalingam et al., 2019; Kong et al., 2021) and resulting in a series of symptoms of diarrheal poisoning, including nausea, vomiting, dizziness, diarrhea, and abdominal pain (Paredes et al., 2011; Gu et al., 2017). Derived from some algae such as Prorocentrum and Dynophysis, OA can be accumulated in the shellfish (Vanessa et al., 2013), exposing high risk to the consumer health. The European Union stipulates the maximum allowable OA content in shellfish at 160 µg kg–1, and the United States Food and Drug Administration has regulated a standard of total OA not higher than 200 µg kg–1.
To ensure the human health, effective methods for the detection and monitoring of OA content are always needed (Campàs et al., 2007; Masanori et al., 2014; Wang et al., 2020). The traditional detection methods to detect OA include mouse bioassays (MBAs) (Kjetil et al., 1988), high performance liquid chromatography-mass spectrometry (HPLC-MS) (Louppis et al., 2010), and immunoassays (Vdovenko et al., 2013; Kawatsu et al., 2014). Among them, MBAs were regarded as the standard method for OA quantification before 2011; however, these methods involve animal ethics issues and have been banned in some countries. Although HPLC-MS achieves accurate quantitative and qualitative analysis of OA (Wu et al., 2015), it requires complicated and professional operations, which makes the method more suitable for the final verification analysis, while it cannot meet the needs of rapid preliminary screening from multiple samples. The immunoassays are developed based on the specific binding between antigen and antibody and have good potential to be applied for rapid detection of OA; however, the complicated producing process and difficult storage of the antibodies bring some challenges to such methods (Sassolas et al., 2013; Garibo et al., 2014). Moreover, with a low molecular size and high toxicity, it is more difficult to produce the antibody of OA. Until now, there is still an urgent need of a simple and efficient method for rapid and preliminary detection of OA from food samples.
Aptamers are single-stranded DNA or RNA oligonucleotides, selected totally in vitro by Systematic Evolution of Ligands by Exponential Enrichment (SELEX) (Ellington and Szostak, 1990; Tuerk and Gold, 1990). Termed as chemical antibody (Toh et al., 2015), aptamers can fold into unique three-dimensional structures to bind specifically with various targets. In theory, aptamers towards all targets such as metal ions (Yu and Jiang, 2011; Wang et al., 2019), proteins (Zhao et al., 2020), and even whole cells (Ardjomandi et al., 2013; Hu et al., 2017) can be selected in vitro by SELEX, with no limitation by the immunogenicity, size, or toxicity of the targets. Moreover, compared with the general antibodies, the aptamers have advantages of good chemical stability, wide acceptability of modification, and low synthesis cost, and thus attracting more and more attention from the fields of food safety, environment safety, diseases diagnosis, and so on (Song et al., 2008; Nutiu and Li, 2010; Farzin et al., 2017).
In recent years, several aptasensors have been developed for OA since Eissa et al. (2013) reported the anti-OA aptamer. For example, Ramalingam et al. (2019) modified the screen-printed carbon electrode with lithium phosphorence-gold nanocomposite and proposed an electrochemical microfluidic chip to detect OA. Gu et al. (2017) described a competitive fluorescent aptasensor for OA, based on signal amplification by rolling circle amplification. Weng and Neethirajan (2018) developed a nano-materials enhanced multipurpose paper-based microfluidic aptasensor for the detection of OA. These aptasensors provide high sensitivity and selectivity. However, they need complex nanomaterials or additional modifications. In addition, for most cases of studies on developing novel aptasensors, aptamers were directly applied and combined with various biosensing transducers, without any investigations of the molecular recognition mechanism between the aptamer and the targets. The correlation between the molecular recognition mechanism of the aptamer and its practicality in the aptasensor is still in a vague exploration stage. Therefore, it is of great significance to understand how the aptamers bind to targets before designing and developing efficient aptasensors.
In this study, a simple label-free colorimetric aptasensor was developed for rapid detection of OA. Gold nanoparticles (AuNPs) were applied as the colorimetric transducer, as AuNPs have many inherent optical properties (Wang et al., 2016; Pan et al., 2017; Qi et al., 2018; Ling et al., 2019). No complicated materials or modifications were needed. Before the development of the aptasensor, the molecular recognition mechanism of the anti-OA aptamer and OA was thoroughly investigated by molecular docking, fluorescent assay, and biolayer interferometry to assist in the rational design. Then on the basis of understanding the recognition mechanism, the aptamer, AuNPs, and polydiallyl dimethyl ammonium chloride (PDDA) were combined to construct a colorimetric aptasensor for simple, rapid, and visual detection of OA in shellfish samples. To our best knowledge, this is the first time that the anti-OA aptamer is thoroughly studied, and a label-free colorimetric aptasensor is rationally designed in this way.
2 Materials and Methods 2.1 Materials and EquipmentsAll oligonucleotides were commercially synthesized by Sangon Biotech Co., Ltd. (Shanghai, China), manually diluted and stored at −20℃. The OA aptamer was selected by Eissa et al. (2013) with the following sequence: 5'-GGTCACCAACAACAGGGAGCGCTACGCGAAGGGTCAATGTGACGTCATGCGGATGTGTGG-3'. The aptamer was modified with polyA, BHQ, or biotin. OA, gonyautoxin 1/4 (GTX 1/4), saxitoxin (STX), neo saxitoxin (neoSTX) standards were purchased from National Research Council Canada. Polydiallyl dimethylammonium chloride (PDDA) and (2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were obtained from Sigma Aldrich. Tween-20 was obtained from Solarbio Life Sciences. Other reagents were all domestic analytical reagents. The binding buffer was prepared with 50 mmol L–1 Tris, 150 mmol L–1 NaCl, 2 mmol L–1 MgCl2 and 0.5% Tween-20 (pH 7.5). All chemicals were of analytical grade.
The computational molecular docking assay was performed with AutoDock Tools. The fluorescent signals were characterized with Shimadzu F-4600 fluorescence spectrophotometer (Shimadzu, Japan). The UV-Vis absorption spectrum was obtained with UV-2550 Spectrophotometer (Shimadzu, Japan) and Multiskan Sky (thermo scientific, USA). The TEM image was obtained by JEM-2100 (JEOL, Japan). The hydrodynamic diameter was measured by Zetasizer Nano ZS 90 (Malvern, England). Concentrated AuNPs were obtained by Centrifuge 5425 (Eppendorf, Germany). The dissociation constant Kd of the aptamer was obtained by Octet RED96e (Fortebio, USA).
2.2 Molecular DockingFirstly, the PDB format file of the aptamer obtained from the Mc-fold/sym (https://www.major.iric.ca/MC-Pipeline/) and mol2 file of the OA obtained from the Zinc website (http://zinc.docking.org/) were imported into the Autodock Tools software. Secondly, the two structures were preprocessed by setting the number of rotatable bonds, adding hydrogen and charge, and assigning atomic types. Thirdly, the grid parameters were set, with the active site box size of 32 × 38 × 104 Å, the grid spacing of 1 Å, and the center coordinates of the grid box of 1.433, 7.969, 44.208. The grid calculation parameter file was then built and run by the AutoGrid manipulated with cmd 4.0. Fourthly, the docking parameters were set. The number of GA Runs was set to be 100, the population size was set to be 150, and the maximum number of evals was set to be 2500000. Fifthly, molecular docking with AutoDock 4.0 software was carried out based on the Lamarckian genetic algorithmto obtain the corresponding output file. Finally, according to the minimum free energy, the binding interaction was analyzed and displayed by Pymol, and the key binding sites was revealed. The secondary structure predicted by Mfold was used as reference.
2.3 Fluorescent AssayThe quencher-modified aptamer (BHQ-aptamer) and the fluorophore-modified complementary DNA (FAM-cDNA, TGGTGACC or CCACACAT) with the same concentration (0.5 μmol L–1) were denatured by heating at 95℃ for 5 min and then slowly cooled to 25℃ to achieve hybridization. Different concentrations of OA (0, 125, 500, 1000, 2000 nmol L–1) were then added and incubated for 30 min. Then the fluorescent signals were measured with F-4600 fluorescence spectrophotometer, with the emission range of 500 – 650 nm under the 494 nm excitation light.
2.4 Biolayer InterferometryThe SA-coated chip was firstly immersed in a binding buffer for equilibration. Then 250 nmol L–1 of biotin-modified anti-OA aptamer was loaded and bound to the surface of the SA-coated chip. The anti-OA aptamer-functionalized chip was then immersed in a buffer solution and a sample solution containing OA (31.25 – 500 nmol L–1), respectively. Due to the specific binding between the aptamer and the target molecule, the biofilm layer thickness was further increased. Finally, the chip was immersed in a buffer solution for dissociation, with OA releasing from the chip surface, resulting in a decrease in the thickness of the biofilm layer. The assay process includes five steps: baseline, 120 s; loading, 180 s; baseline 2, 180 s; association, 300 s; dissociation, 300 s. The BLI works based on monitoring the light interference. Through real-time monitoring of the thickness of the biofilm layer and transducing it into BLI signal, and fitting the concentration-signal response curve, the dissociation constant of the aptamer and OA was thus obtained.
2.5 Preparation of the AuNPsThe 13 nm AuNPs were prepared according to the classical procedure, by reducing chloroauric acid with sodium citrate solution (Wang et al., 2016). The TEM image and hydrodynamic diameter of AuNPs were obtained by JEM-2100 and Zetasizer Nano ZS 90, respectively. And the final concentration of AuNPs was measured based on the absorbance at 520 nm by UV-2550 Spectrophotometer and calculated based on Lambert Beer's law. And the AuNPs were concentrated to 15 nmol L–1 before use.
2.6 Construction of the Label-Free Colorimetric AptasensorA final concentration of citrate buffer with a concentration of 10 mmol L–1 (pH 3.0) was used to protonate the polyA-aptamer, followed by adding it into the 15 nmol L–1 AuNPs solution and incubating for 10 min. Then 30 mmol L–1 of HEPES buffer (pH 7.6) was added and incubated for 10 min, adjusting the pH of the solution to be neutral. The polyA-aptamer-AuNPs complex was obtained thereof.
Then 20 μL of OA was added to 80 μL of polyA-aptamer-AuNPs solution and incubated for 30 min at room temperature. PDDA was added and incubated for another 10 min, after which the absorbance of the solutions was measured by a microplate reader. The ratio values of the peak absorbance at 520 nm and 620 nm were applied for the quantitation.
2.7 Pretreatment of Shellfish SamplesThe pretreatment method of scallop samples refers to the method of Ramalingam et al. (2019). The scallop tissue was taken out and washed thoroughly with deionized water to remove excess impurities. After 250 mg of scallop tissue was homogenized by a blender, 1 mL of 50% methanol was added and vortexed at room temperature for 5 min and centrifuged at 4000 r min–1 for 5 min. The supernatant was separated into a new centrifuge tube, heated at 75℃ for 5 min and centrifuged at 4000 r min–1 for 5 min. Finally, the supernatant was transferred to a new centrifuge tube and stored at −20℃ for further experiments.
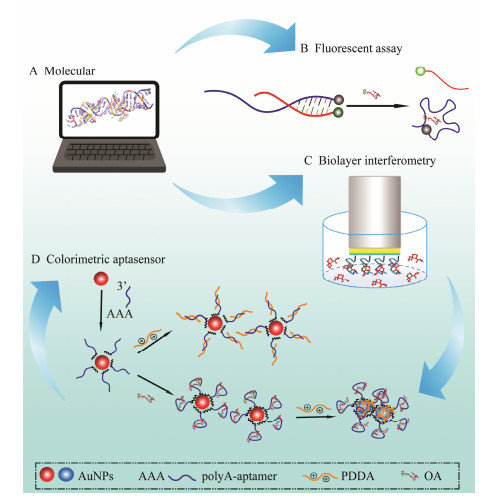
3 Results and Discussion 3.1 Schematic Illustration of the StudyFig.1 shows whole design of this study. To exploit the binding capability of the anti-OA aptamer as much as possible, the molecular docking (Fig.1A) was carried out to explore initially the binding interaction and recognition sites of anti-OA aptamer to OA, followed by the fluorescent assay (Fig.1B) and the biolayer interferometry (BLI) (Fig.1C) to further verify the simulation results. The label-free colorimetric aptasensor was developed, after the molecular recognition mechanism of the OA aptamer was revealed. As shown in Fig.1D, the aptasensor responds based on the unique optical signal generated by the distance-dependent AuNPs. One terminal of the aptamer was connected with polyA (12 nt). The protonated polyA immobilized the aptamer on the surface of the AuNPs, so that the binding site on the other terminal was fully exposed to OA. No labeling was needed in the whole process. Moreover, the poly A-based immobilization is much more conductive to the efficient, time-saving, and cost-effective preparation of AuNPs-aptamer complex. PDDA is a cationic polymer that can cause the aggregation of AuNPs (Shayesteh et al., 2019). The aggregation can be inhibited by free ssDNA aptamer, because PDDA can only bind with aptamer through electrostatic adhesion. However, in the presence of OA, the target will induce the adaptive conformational folding of the aptamer, leaving the PDDA free to be adsorbed onto the surface of the AuNPs, causing the aggregation of the AuNPs. Based on changes of color and absorbance, the rapid and visual detection was realized thereof.
|
Fig. 1 Schematic illustration the whole study. A, computational molecular docking; B, fluorescent assay; C, biolayer interferometry; D, schematic diagram of the label-free colorimetric aptasensor. |
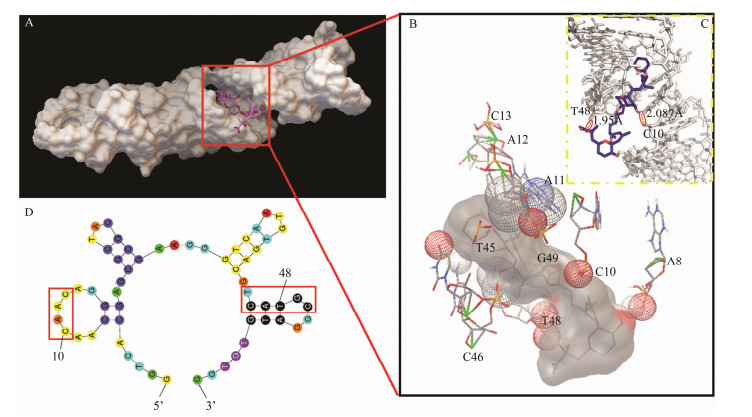
The molecular docking assay was performed to reveal the binding mechanism and binding sites. After the computational docking procedure, the optimal conformation was obtained, according to the principle of the lowest energy value, with an energy value of −3.34 kcal mol–1. As shown in Fig.2A, the white part represents the conformational structure of the aptamer, and the pink represents the target OA. The target OA embedded in the major groove formed by the aptamer. In the partial enlarged diagram in Fig.2B, the gray part represents the structure of OA, and the adjacent bases, A8, C10, A11, A12, C13, T45, C46, T48, and G49, were the key binding bases simulated in the molecular docking. Two hydrogen bonds were formed by C10-OA and T48-OA, with bond lengths of 2.087 Å and 1.95 Å (see the Fig.2C). Subsequently, in order to further confirm the anti-OA aptamer binding site, the secondary structure of the anti-OA aptamer was simulated by Mfold online server. As shown in Fig.2D, there were four stem-loops which are generally considered to have high possibility to participate in the binding. Considering with all of the information, it can be predicted that the stem-loops near the two terminals of the anti-OA aptamer play significant roles.
|
Fig. 2 Computational simulation results of (A) molecular docking of anti-OA aptamer with OA; B and C, partial enlarged diagram of the key binding bases and hydrogen bond; D, secondary structure of the anti-OA aptamer. |
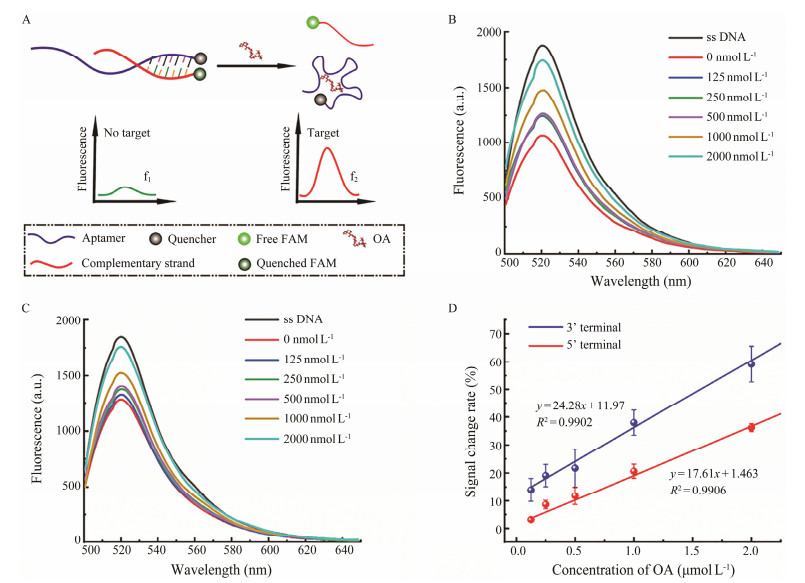
To further verify the prediction results, a competitive fluorescent assay was performed to compare the binding ability of the two terminals, i.e., 3' and 5' terminals. As shown in Fig.3A, when the aptamer modified with a quencher at the terminal was hybridized with its complementary sequence modified with a fluorophore, the fluorophore and the quencher were drew close to each other, resulting in quenching of the fluorescent signals. And in the presence of OA, the terminals would participate in conformational folding in priority, and the complementary sequence was thus released to trigger the fluorescent signals. Fig.3B and Fig.3C exhibit the fluorescent signal spectra obtained by complementary competition at the 3' and 5' terminals of the aptamer after adding OA with different concentration gradients. With the increment of OA concentration, the fluorescent intensity gradually increased (Figs.3A, 3B), indicating the releasing amount of the complementary sequences at the terminals of the aptamer, as well as the binding capability of the terminals. Obviously, both the 3' and 5' terminals of the OA aptamer could bind with the target, which is in consistent with the simulation results. The concentration-dependent change of fluorescent intensity was then used for comparison. The fluorescent signals before and after the target were measured, and the peak values were recorded as f1 and f2, respectively. The rate of change of fluorescent intensity was then calculated through (f2 − f1)/f1*100%. As shown in Fig.3D, the concentration-signal change rate curves were obtained. The slope of the curve (y = 24.28x + 11.97, R2 = 0.9902) obtained with the competition at the 3' terminal is obviously higher than that of the 5' terminal (y = 17.61x + 1.463, R2 = 0.9906), indicating the 3' terminal of the anti-OA aptamer exhibit better binding capability than the 5' terminal, with the slope representing the response sensitivity of the aptamer.
|
Fig. 3 Schematic diagram and results of the fluorescent assay. A, schematic diagram; B, C, fluorescent spectra of the competition at the 3' terminal and 5' terminal; D, comparison of the signal change rates. |
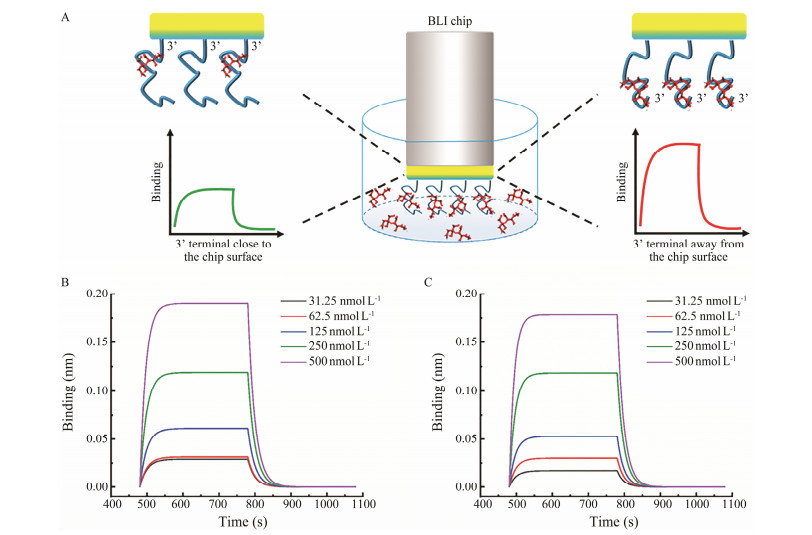
In order to further determine the binding ability of the two terminals, BLI was adopted for further verification. The anti-OA aptamer was immobilized with 3' terminal that was close or away from the chip surface (Fig.4A). The response intensity caused by the binding was monitored. As shown in Fig.4B and Fig.4C, the signal responses in the two ways both increased with the OA concentration increasing. However, the dissociation constant Kd measured with the 3' terminal close to the chip surface (Kd = 2.02 μmol L–1) was significantly higher than that (Kd = 1.320 μmol L–1) with the 3' terminal away from the chip surface, indicating that the 3' terminal exhibit more significant role than the 5' terminal, because the aptamer performed decreased binding ability when the 3' terminal was immobilized to meet with higher steric hindrance. And with the 3' terminal away from the chip surface and exposed fully to OA, the aptamer showed better binding capability.
|
Fig. 4 Schematic illustration and results of the biolayer interferometry. A, schematic illustration; B, C, the signal responses with 3' terminal close (B) or away (C) from the chip surface. |
On the basis of the above results, the 3' terminal was designed to be fully exposed to OA, and the 5' terminal was modified with polyA to be absorbed on the surface of the AuNPs. In the absence of OA, the PDDA would bind with the aptamer, the solution color remained red. While in the presence of OA, the free 3' terminal folded to bind with OA in priority, making the whole aptamer responded sensitively, and the PDDA would absorb on to the AuNPs to induce aggregation.
The key parameters of the aptasensor and PDDA were optimized. Even without PDDA, the ion concentration in the binding buffer would destroy the charge distribution on the surface of the AuNPs. Therefore, the protective effect of polyA in the polyA-aptamer on AuNPs was investigated. As shown in Fig.5A, when the concentration of polyA-aptamer increased (0 to 2000 nmol L–1), the color of the AuNPs solution gradually changed from blue to red. Finally, 500 nmol L–1 was selected for the subsequent experiments, as the A620/A520 in Fig.5B reached saturated with the 500 nmol L–1 aptamer, indicating the good protection of polyA against the ions.
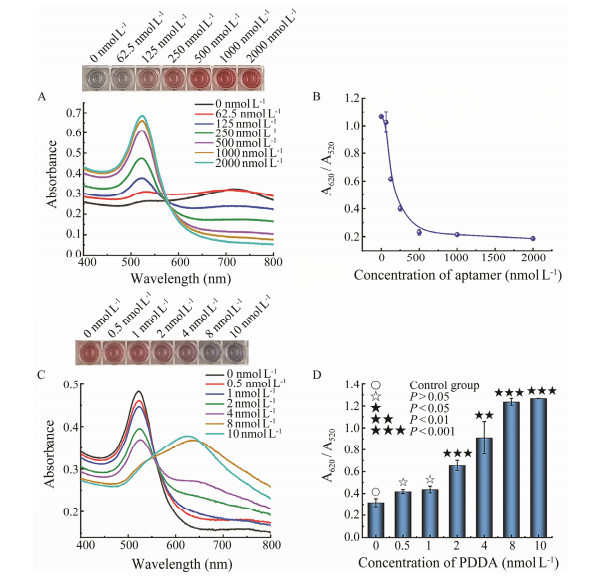
|
Fig. 5 Optimizations during the development of the colorimetric aptasensor. A, B, responses with different concentrations of polyA-aptamer; C, D, responses with different concentrations of PDDA. Significance analysis was characterized by P value using two-tailed T-tests. |
Next, the concentration of PDDA was optimized. When excessive PDDA was added to the system, the positive charge of PDDA would adsorb onto the surface of the negative charged AuNPs, causing the AuNPs to aggregate and turn blue (Shayesteh et al., 2020). The 500 nmol L–1 polyA-aptamers was used to protect the AuNPs. A suitable concentration of PDDA was chosen, with which the aptamer just protected the AuNPs from aggregation. As shown in Fig.5C, when the concentration of PDDA increased (0 to 10 nmol L–1), the solution gradually changed from red to blue. A significance analysis of the ratio of A620/A520 was performed (Fig.5D). Finally, 1 nmol L–1 PDDA was chosen as no significant difference was observed when compared with the control group.
3.5 Calibration Curves and LOD of the Label-Free Colorimetric AptasensorOA with a series of concentrations (0 – 1200 nmol L–1) was analyzed. As shown in Figs.6A, 6B, AuNPs aggregated gradually when the concentration of OA increased. The color of the solution changed from red to blue, and the response signal gradually increased. As shown in Fig.6C, A620/A520 is positively correlated with the OA concentration, and the linear equation is y = 0.8147x + 0.1747 (R2 = 0.9791), with a linear range of 100 – 1200 nmol L–1. The LOD of the colorimetric aptasensor was calculated to be 41.30 nmol L–1 by using the formula LOD = 3α/S, where α represents the standard deviation from the blank, S represents the slope of the linear curve (Wu et al., 2012). The LOD was much lower than the maximum allowable addition amount specified by the European Union. Moreover, compared with the existing methods, our developed aptasensor in this study showed great advantages. Immuno-sensors, such as the electrochemical immunoassays, immunochromatographic assays, enzyme-linked immunosorbent assays depend on antibodies. For these methods, there are some disadvantages, such as complicated generation of antibodies, high cost, high requirement of specific storage conditions, and potential invalidation of the binding capability of the antibodies. The aptamer-based colorimetric sensor designed in the present study has the advantages of high sensitivity and selectivity, simple operation, rapid response with visualization, and low cost, showing great potential to act as powerful tool in the efficient detection of OA from real world samples.
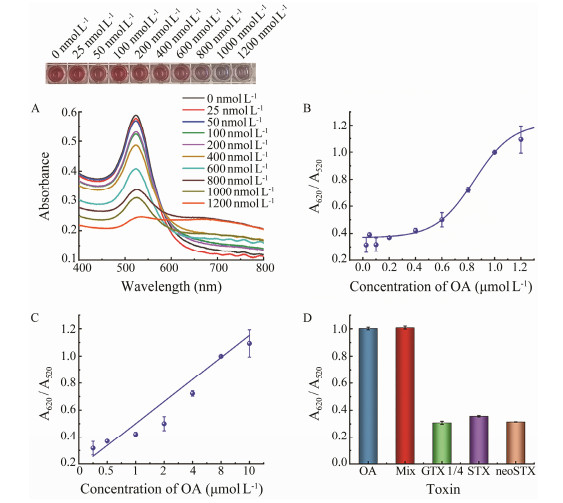
|
Fig. 6 Responses and evaluation of the colorimetric aptasensor. A, absorbance spectra and colorimetric response; B, response curve; C, linear curve; D, sensitivity assay results. |
In order to verify the accuracy and repeatability of the colorimetric aptasensor in detecting OA, three OA samples with different concentrations (200, 400, 800 nmol L–1) were added to shellfish samples and analyzed by the aptasensor. As shown in Table 1, the recovery rates of the aptasensor was between 91.6% – 106.2%, with RSD below 1.848%, indicating that the aptasensor had high accuracy and repeatability.
|
|
Table 1 Recovery assay results of the label-free colorimetric aptasensor |
In order to explore the selectivity of the designed colorimetric aptasensor for OA, this colorimetric aptasensor was tested with 1000 nmol L–1 different marine toxins such as GTX 1/4, STX, and neoSTX. The signal response values obtained are shown in Fig.6D. It can be clearly indicated that the colorimetric aptasensor showed a strong selectivity towards OA rather than towards other toxins, as only samples containing OA caused significant responses.
4 ConclusionsIn summary, a label-free colorimetric aptasensor towards OA was developed. The aptamer was rationally utilized in the aptasensor, based on the studies of the inherent molecular recognition mechanism by computational molecular docking, fluorescence assay, and biolayer interferometry. The 3' terminal showed a dominate role, and the fully exposed 3' terminal contributed greatly to the high sensitivity. The aptasensor provided a limit of detection of 41.30 nmol L–1, which is much lower than the maximum allowable amount of the European Union. It also showed a wide linear range of 100 – 1200 nmol L–1, excellent recovery rates of 91.6% – 106.2%, and good selectivity. The whole detection process could be completed within 1 h. Hopefully, this study not only provides a novel rapid detection method for the highly sensitive and specific detection of OA, but also provides a reference for the further application of novel aptasensors in the field of food analysis.
AcknowledgementThis research was funded by the National Natural Science Foundation of China (No. 31801620).
Ardjomandi, N., Niederlaender, J., Aicher, W. K., Reinert, S., and Alexander, D.. 2013. Identification of an aptamer binding to human osteogenic-induced progenitor cells. Nucleic Acid Therapeutics, 23(1): 44-61. DOI:10.1089/nat.2012.0349 (  0) 0) |
Campàs, M., Prieto-Simón, B., and Marty, J. L.. 2007. Biosensors to detect marine toxins: Assessing seafood safety. Talanta, 72(3): 884-895. DOI:10.1016/j.talanta.2006.12.036 (  0) 0) |
Eissa, S., Ng, A., Siaj, M., Tavares, A. C., and Zourob, M.. 2013. Selection and identification of DNA aptamers against okadaic acid for biosensing application. Analytical Chemistry, 85(24): 11794-11801. DOI:10.1021/ac402220k (  0) 0) |
Ellington, A. D., and Szostak, J. W.. 1990. In vitro selection of RNA molecules that bind specific ligands. Nature, 346(6287): 818-822. DOI:10.1038/346818a0 (  0) 0) |
Farzin, L., Shamsipur, M., and Sheibani, S.. 2017. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta, 174: 619-627. DOI:10.1016/j.talanta.2017.06.066 (  0) 0) |
Garibo, D., Campbell, K., Casanova, A., de la Iglesia, P., Fernández-Tejedor, M., Diogène, J., et al.. 2014. SPR immunosensor for the detection of okadaic acid in mussels using magnetic particles as antibody carriers. Sensors & Actuators B Chemical, 190: 822-828. DOI:10.1016/j.snb.2013.09.037 (  0) 0) |
Gu, H., Hao, L., Duan, N., Wu, S., and Wang, Z.. 2017. A competitive fluorescent aptasensor for okadaic acid detection assisted by rolling circle amplification. Microchimica Acta, 184(9): 1-7. DOI:10.1007/s00604-017-2293-1 (  0) 0) |
Hu, X., Wang, Y., Tan, Y., Wang, J., Liu, H., Wang, Y., et al.. 2017. A difunctional regeneration scaffold for knee repair based on aptamer-directed cell recruitment. Advanced Materials, 29(15): 1605235. DOI:10.1002/adma.201605235 (  0) 0) |
Kawatsu, K., Kanki, M., Harada, T., and Kumeda, Y.. 2014. A highly rapid and simple competitive enzyme-linked immunosorbent assay for monitoring paralytic shellfish poisoning toxins in shellfish. Food Chemistry, 162: 94-98. DOI:10.1016/j.foodchem.2014.04.038 (  0) 0) |
Kjetil, B., Wyman, J., Carmichael, W., and Dabholkar, A.. 1988. Isolated rat liver perfusion studies with cyclic heptapeptide toxins of Microcystis and Oscillatoria (freshwater cyanobacteria). Toxicon Official Journal of the International Society on Toxinology, 26(9): 827-837. DOI:10.1016/0041-0101(88)90324-8 (  0) 0) |
Kong, J., Li, D., Zhang, S., Zhang, H., and Zhu, X.. 2021. Okadaic acid promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells by inhibiting protein phosphatase 2A. Journal of Cellular Biochemistry, 122(9): 993-1002. DOI:10.1002/jcb.29629 (  0) 0) |
Ling, S., Li, X., Zhang, D., Wang, K., Zhao, W., Zhao, Q., et al.. 2019. Detection of okadaic acid (OA) and tetrodotoxin (TTX) simultaneously in seafood samples using colloidal gold immunoassay. Toxicon, 165: 103-109. DOI:10.1016/j.toxicon.2019.04.011 (  0) 0) |
Louppis, A. P., Badeka, A. V., Katikou, P., Paleologos, E. K., and Kontominas, M. G.. 2010. Determination of okadaic acid, dinophysistoxin-1 and related esters in Greek mussels using HPLC with fluorometric detection, LC-MS/MS and mouse bioassay. Toxicon, 55(4): 724-733. DOI:10.1016/j.toxicon.2009.10.026 (  0) 0) |
Masanori, K., Toshihiko, Y., Michio, M., Takeshi, Y., Marie, K., Patrick, L., et al.. 2014. Okadaic acid as the causative toxin of diarrhetic shellfish poisoning in Europe. Agricultural & Biolofical Chemistry, 50: 2853-2857. DOI:10.1080/00021369.1986.10867817 (  0) 0) |
Nutiu, R., and Li, Y.. 2010. In vitro selection of structure-switching signaling aptamers. Angewandte Chemie, 117(7): 1085-1089. DOI:10.1002/ange.200461848 (  0) 0) |
Pan, Y., Wan, Z., Zhong, L., Li, X., Wu, Q., Wang, J., et al.. 2017. Label-free okadaic acid detection using growth of gold nanoparticles in sensor gaps as a conductive tag. Biomedical Microdevices, 19(2): 33. DOI:10.1007/s10544-017-0162-7 (  0) 0) |
Paredes, I., Rietjens, I. M. C. M., Vieites, J. M., and Cabado, A. G.. 2011. Update of risk assessments of main marine biotoxins in the European Union. Toxicon, 58(4): 336-354. DOI:10.1016/j.toxicon.2011.07.001 (  0) 0) |
Qi, M., Tu, C., Dai, Y., Wang, W., Wang, A., and Chen, J.. 2018. A simple colorimetric analytical assay using gold nanoparticles for specific detection of tetracycline in environmental water samples. Analytical Methods, 10(27): 3402-3407. DOI:10.1039/C8AY00713F (  0) 0) |
Ramalingam, S., Chand, R., Singh, C. B., and Singh, A.. 2019. Phosphorene-gold nanocomposite based microfluidic aptasensor for the detection of okadaic acid. Biosensors Bioelectronics, 135: 14-21. DOI:10.1016/j.bios.2019.03.056 (  0) 0) |
Sassolas, A., Catanante, G. L., Hayat, A., Stewart, L. D., Elliott, C. T., and Marty, J. L.. 2013. Improvement of the efficiency and simplification of ELISA tests for rapid and ultrasensitive detection of okadaic acid in shellfish. Food Control, 30(1): 144-149. DOI:10.1016/j.foodcont.2012.05.028 (  0) 0) |
Shayesteh, O. H., and Ghavami, R.. 2019. Two colorimetric ampicillin sensing schemes based on the interaction of aptamers with gold nanoparticles. Microchimica Acta, 186(7): 485. DOI:10.1007/s00604-019-3524-4 (  0) 0) |
Shayesteh, O. H., and Khosroshahi, A. G.. 2020. A polyA aptamer-based label-free colorimetric biosensor for the detection of kanamycin in human serum. Analytical Methods, 12(14): 1858-1867. DOI:10.1039/D0AY00326C (  0) 0) |
Song, S., Wang, L., Jiang, L., Fan, C., and Zhao, J.. 2008. Aptamer-based biosensors. TrAC Trends in Analytical Chemistry., 27(2): 108-117. DOI:10.1016/j.trac.2007.12.004 (  0) 0) |
Toh, S. Y., Citartan, M., Gopinath, S. C. B., and Tang, T. H.. 2015. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosensors & Bioelectronics, 64: 392-403. DOI:10.1016/j.bios.2014.09.026 (  0) 0) |
Tuerk, C., and Gold, L.. 1990. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science, 249(4968): 505-510. DOI:10.1126/science.2200121 (  0) 0) |
Vanessa, V., María, P. F., Eduardo, P., Josefina, M., and Blanca, L.. 2013. Okadaic acid: More than a diarrheic toxin. Marine Drugs, 11(11): 4328-4349. DOI:10.3390/md11114328 (  0) 0) |
Vdovenko, M. M., Hung, C. T., Sakharov, I. Y., and Yu, F. Y.. 2013. Determination of okadaic acid in shellfish by using a novel chemiluminescent enzyme-linked immunosorbent assay method. Talanta, 116: 343-346. DOI:10.1016/j.talanta.2013.05.057 (  0) 0) |
Wang, J., Wang, Y., Hu, X., Zhu, C., Ma, Q., Liang, L., et al.. 2019. Dual-aptamer-conjugated molecular modulator for detecting bioactive metal ions and inhibiting metal-mediated protein aggregation. Analytical chemistry, 91(1): 823-829. DOI:10.1021/acs.analchem.8b03007 (  0) 0) |
Wang, S., Gao, S., Sun, S., Yang, Y., Zhang, Y., Liu, J., et al.. 2016. A molecular recognition assisted colorimetric aptasensor for tetracycline. RSC Advances, 6(51): 45645-45651. DOI:10.1039/C6RA08262A (  0) 0) |
Wang, Y., Rao, D., Wu, X., Zhang, Q., and Wu, S.. 2020. Aptamer-based microcantilever-array biosensor for ultrasensitive and rapid detection of okadaic acid. Microchemical Journal, 60(Part A): 105644. DOI:10.1016/j.microc.2020.105644 (  0) 0) |
Weng, X., and Neethirajan, S.. 2018. Paper-based microfluidic aptasensor for food safety. Journal of Food Safety, 38(1): e12412. DOI:10.1111/jfs.12412 (  0) 0) |
Wu, Y., Zhan, S., Wang, F., He, L., Zhi, W., and Zhou, P.. 2012. Cationic polymers and aptamers mediated aggregation of gold nanoparticles for the colorimetric detection of arsenic(Ⅲ) in aqueous solution. Chemical Communications, 48(37): 4459-4461. DOI:10.1039/c2cc30384a (  0) 0) |
Wu, Z. Q., Wang, B. C., Sun, Y. B., and Liu, Y.. 2015. Improvement of determination method of okadaic acid in shellfish by liquid chromatography-tandem mass spectrometry. Journal of Food Safety & Quality. DOI:10.19812/j.cnki.jfsq11-5956/ts.2015.01.046 (  0) 0) |
Yu, X., and Jiang, Z.. 2011. Aptamer-based fluorescence nanoprobe for sensitive and selective detection of potassium. Analytical Letters, 44(5): 898-907. DOI:10.1080/00032711003790031 (  0) 0) |
Zhao, L. P., Yang, G., Zhang, X. M., and Ou, F., 2020. Development of aptamer screening against proteins and its applications. Chinese Journal of Analytical Chemistry, 48 (5): 560-572, DOI: 10.1016.S1872-2040(20)60012-3.
(  0) 0) |
 2022, Vol. 21
2022, Vol. 21


