2) Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
bHLH gene family is a very diverse transcription factors superfamily with basic helix-loop-helix structural domain (Liu et al., 2014; Li et al., 2016; Tarczewska and Greb-Markiewicz, 2019). This domain is conserved with a approximate length of 60 amino acids, which may form homodimeric or heterodimeric complexes with each other to bind to specific DNA sequence (Zhang et al., 2017). The family members are involved in the development of numerous tissues including gonad, spleen, lung and heart (Wang et al., 2017; Chiba et al., 2019; Tan et al., 2019). Given the complexity and diversity of the family, it has been studied in multiple developmental processes including neurogenesis, mesoderm formation, cranial vault development and sex determination (Zhang et al., 2017; Blumel et al., 2019).
Figla, as a transcription factor gene belonging to bHLH family, plays an important role in the formation of primordial follicles in some vertebrates (Soyal et al., 2000; Bayne et al., 2004). Many researches have revealed some molecular mechanisms about the function of figla in mammals. In mouse, primordial follicles were not formed at birth once mutagenesis happened in embryonic stem cells. Subsequently, massive depletion of oocytes resulted in shrunken ovaries and female sterility. In addition, zona pellucida genes (Zp1, Zp2, and Zp3) are not expressed in figla null female mouse (Soyal et al., 2000), which indicates figla can regulate the expression of zona pellucida genes. Figla also functions as a key regulatory molecule in coordinating expression of the NALP family genes, as well as some genes related to oogenesis such as Serpinb6c, Arhgap20 and Pdzk1 (Joshi et al., 2007). In human, the expression of figla increases across mid-gestation and reaches the peak by the time of primordial follicle formation. Furthermore, Figla can heterodimerize with E12 protein and bind to the E-box of the human ZP2 promoter to regulate its expression level (Bayne et al., 2004).
In teleosts, the studies on figla gene were far less detailed than in mammals. Two figla homologues with different functions during sex differentiation were identified in tongue sole (Li et al., 2016). In Nile tilapia, figla was only expressed in ovary and over-expression of figla in XY fish resulted in the disruption of spermatogenesis along with the depletion of meiotic spermatocytes and spermatids in testis (Qiu et al., 2015), which was consistent with impaired meiosis and germ cell apoptosis due to the ectopic figla expression in testis of mouse (Hu et al., 2010). Although there have been some studies about figla in teleosts, studies examining its expression regulation involved signal pathways are limited. Japanese flounder (P. olivaceus) is an economically important cultured fish in China, Korea and Japan. Due to its remarkable sexual dimorphism during growth (females grow faster and have superior body length than males) (Yamamoto, 1999), finding ways to increase the proportion of female during the breeding process has been a research hotspot. In order to achieve this goal, exploring and understanding the regulatory mechanisms of genes that participate in gonad development will be fundamental and meaningful. Recently, Liang et al. (2020) have characterized figla and explored its role in the proliferation of oocytes in P. olivaceus.
Although there have been some researches about figla in flounder, studies examining its regulation of expression by upstream genes or signaling pathways are lacking. Interestingly, in our results, quantitative real-time polymerase chain reaction (qRT-PCR) and in situ hybridization (ISH) indicated that figla was not only expressed in the ovary, but also expressed in the testis in the early developmental stage, which was slightly different from the results of Liang et al. (2020). In addition, we made deletion constructs and sitedirected mutagenesis of the promoter regions, combined with dual luciferase assays to identify potential transcription factors that regulate figla transcription, and explored the regulation of canonical Wnt signaling pathway on figla. This is the first attempt to study the potential regulator of figla at molecular level in Japanese flounder, which could be helpful for understanding the gonad development, artificial breeding and further gene regulation studies of figla in other species.
2 Materials and Methods 2.1 Ethics StatementAll experimental protocols were performed in accordance with the Institutional Care and Use Committee of the Ocean University of China (Qingdao, China) and were conducted to fit in the guidelines of Chinese Government's Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training (China, 1988).
2.2 Fish and Embryo SamplingAll healthy fish samples and embryos were reared in the recirculating aquaculture system in Yellow Sea Aquatic Product Co., Ltd., Yantai, Shandong, China. Three females and three males of Japanese flounder (1-year-old) were selected randomly. Tissue samples including heart, liver, spleen, kidney, brain, gill, muscle, intestine, testis and ovary were collected for tissue distribution analysis. Testes and ovaries of 8-months, 1-year and 1.5 years old individuals were collected, respectively. Each sample was collected in triplicate. Embryo samples at twelve different developmental stages, including 1 cell, 16 cells, morula, high blastula, low blastula, early gastrula, late gastrula, neurula, tailbud formation, hearting-beating, before hatching and hatching stages were collected for gene expression analyses. Each sample, which contained 30 embryos, was collected in triplicate. All samples were immediately frozen using liquid nitrogen and stored in −80℃ for RNA extraction.
2.3 RNA Extraction and cDNA SynthesisTotal RNA was extracted from tissues and embryos using TRIzol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer's instructions, then genomic DNA was removed with RNase-free DNase I (TaKaRa, Dalian, China). RNA quality and quantity were assessed by electrophoresis detection and NanoDrop determination, respectively. The cDNA was synthesized with 1 μg high-quality total RNA and 25 μmol L−1 random primer using the Reverse Transcriptase M-MLV Kit (TaKaRa, Dalian, China) according to the manufacturer's instructions.
2.4 Isolation, Sequence Analysis of PofiglaThe available sequences of figla orthologs were identified in other teleosts from NCBI and Ensemble to obtain figla sequence of P. olivaceus by tBlastx. The core cDNA sequence of the Pofigla was amplified by PCR with degenerate primers figla-core-FW/RV (Table 1). PCR products were separated by agarose gel electrophoresis, purified using the Gel Extraction Kit (CWBIO, Beijing, China), and cloned and ligated into the pEASY-Blunt Simple Vector (TransGen Biotech, Beijing, China) for sequencing. Homologous protein sequences were confirmed through BLAST searches in NCBI and Ensemble (Table 2). Multiple sequence alignments were conducted using ClustalX 2.1. The amino acid Logos of alignments were generated with WebLogo (http://weblogo.berkeley.edu/logo.cgi). Structure of flounder figla gene was determined by the online Gene Structure Display Server 2.0 (http://gsds.cbi.pku.edu.cn). Chromosome synteny was performed according to the location of Pofigla and its flanking genes obtained from NCBI online genome database (MPLB00000000.1).
|
|
Table 1 Primers used in this study |
|
|
Table 2 Sequence used for alignments |
The primers (in Table 1) used for qRT-PCR were designed in the non-conserved regions by the online Integrated DNA Technologies (IDT) (http://sg.idtdna.com/Primerquest/Home/Index). qRT-PCR was performed using a LightCycler 480 (Roche, Forrentrasse, Switzerland) with a total 20 µL reaction volume, which contained 10 ng template cDNA, 0.4 µL of each primer (10 μmol L−1) and 10 µL 2×SYBR Green qPCR Master Mix (US Everbright Inc., Suzhou, China). The specificity of primers was validated according to the single peak of melting curves generated after amplification reaction. β-actin was selected as the reference gene according to the previous study (Zhang et al., 2013). All qRT-PCR assays were performed in triplicate. The relative expression levels of the target gene were calculated by the 2−ΔΔCt comparative Ct method.
2.6 In Situ HybridizationISH analyses in 8-month-old male and female gonads were performed using the probe spanning the non-conserved region of cDNA (Table 1). DIG-labeled RNA sense and antisense probes were synthesized using a DIG RNA Labeling Kit (SP6/T7) (Roche, Mannheim, Germany) according to the manufacturer's protocol. The experiment of tissue ISH has been described in our previous research (Gao et al., 2013). Briefly, gonad tissues were dehydrated with gradient methanol, then transited to xylene and embedded by paraffin ultimately. The ovary and testis blocks were sectioned at 7 and 5 μm thickness, respectively. And then all sections were fixed in the microscope (Solarbio, Shanghai, China) coated with polylysine for 12 h at 37℃. Each sample sections were incubated with 20 μg mL−1 protease K at 37℃ for 15 min and fixed with 4% PFA for 20 min. The hybridization was carried out at 60℃ for 15 h (3 h for pre-hybridization process and 12 h for hybridization process). After hybridization, all sample sections were washed twice in 2 × SSC, 1 × SSC and 0.2 × SSC for 15 min at 60℃, respectively. Then they were washed twice in maleic acid buffer at room temperature for 5 min each time. Samples were blocked in blocking buffer for 2 h, then incubated with blocking buffer mixed with anti-DIG alkaline phosphatase-conjugated F (ab)' 2 fragments for 12 h at 4℃. The positive signals were observed after reaction with NBT/BCIP substrate solution (Roche, Mannheim, Germany). Then the sample sections were stained with 1% neutral red for 20 – 30 min and covered with neutral resin. The sections were observed and photographed using a Nikon Eclipse Ti-U microscope (Nikon, Tokyo, Japan).
2.7 Plasmid Construction, Matinspector Analysis and Site-Directed MutagenesisFor the Yeast two-hybrid assay, the coding sequence of Pofigla was cloned and inserted into pGADT7 and pGBKT7 (Clontech, San Francisco, USA) with EcoRI and BamHI digestion method. For the analysis of promoter activity, the promoter regions with different lengths of Pofigla (−251/−44, −444/−44, −772/−44, −1137/−44, −1729/−44, −2126/−44, and −2966/−44) were amplified from Japanese flounder genome and cloned into KpnI and SmaI restriction enzyme sites of pEGFP-1 (Clontech, San Francisco, USA), respectively. For the analysis of cis-acting elements regulating figla promoter activity, the −772/−44 and −2966/−44 regions were cloned into KpnI and XhoI restriction enzyme sites of pGL3-basic vector (Clontech, San Francisco, USA). The potential transcription factor binding sites within these two regions were forecasted using the online program MatInspector (http://www.genomatix.de/matinspector.html) with the Matrix Family Library Version 9.3 (Cartharius et al., 2005). The transcription factor binding sites were deleted with the QuickChange Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, USA) using the wild-type constructs pGL3−2966/−44 and pGL3−772/−44 as the templates following the manufacturer's instructions. The coding sequences of β-catenin and lef1 were amplified and cloned into EcoRI and KpnI restriction enzyme sites of the pEGFP-N1 for the co-transfection and dual-reporter assays. The coding sequence of figla was amplified and cloned into EcoRI and BamHI sites of pmCherry-N1 for the colocalization assay. All constructed recombinant plasmids were proofed by sequencing (BGI, Shenzhen, China). The primers used in this study were listed in Table 1.
2.8 Yeast Two-Hybrid AssayThe recombinant plasmids PoFigla-pGADT7 and PoFigla-pGBKT7 were co-transformed into Y2HGold competent yeast cells (Weidi Biotechnology, Shanghai, China) according to the manufacturer's protocol. The transformed yeast cells were cultured in leucine and tryptophan auxotrophic media for 3 d at 29℃. Then the yeast colonies were transferred into adenine, histidine, leucine, and tryptophan auxotrophic media supplemented with aureobasidin A (AbA) and Alpha-galactosidase (X-α-Gal) for identification of the protein-protein interaction. pGADT7-T and pGBKT7-53 plasmids were co-transformed as positive control. pGADT7-T and pGBKT7-lam co-transformation was used as negative control. The self-activation of yeast twohybrid system was validated before experimental assay.
2.9 Transfection, Dual-Luciferase Reporter and Co-Localization AssayAbout 2×105 HEK 293T cells were seeded in 24-well plates, cultured in the Dulbecco modified Eagle medium (Biological Industries, Bet-Haemek, Israel) containing 10% fetal bovine serum (Gibco, Carlsbad, USA) and 1% penicillin-streptomycin (Gibco, Carlsbad, USA) for 12 h under 5% CO2 at 37℃, and transfected with the plasmids using Lipofectamine 3000 Reagent (Life Technologies, Carlsbad, USA) according to the manufacturer's protocol. For dualluciferase assay, the total 1000 ng plasmids including overexpression plasmids B-catenin or LEF1 with different doses (0, 50, 200 ng), 20 ng pRL-TK Vector (Promega, Madison, USA) and the remaining empty pEGFP-N1 were transfected into HEK 293T cells. At 36 h after transfection, the luciferase values were obtained with the Dual-Luciferase Reporter Assay System (Promega, Mannheim, Germany) according to the manufacturer's protocol. Each sample in the dual-luciferase reporter assays was performed in triplicates. For GFP reporter assay, the cells were transfected with the same amount of recombinant pEGFP-1 vectors (500 ng per well), the expression of GFP in transfected cells was observed with an inverted fluorescence microscope at 48 h after transfection. pEGFP-C1 was selected as a positive control for successful transfection. For co-localization assay, the recombinant PoLEF1-pEGFP-N1 and PoFigla-pmCherry-N1 were constructed and the different combinations of plasmids were transfected into HEK 293T cells. After 36 h of incubation, the cells were fixed with 4% paraformaldehyde for 20 min and washed 3 times with PBS, stained with DAPI (Sangon Biotech, Shanghai, China) for 15 min followed by washing 3 times. Lastly, the cell fluorescence photographs of each combination group were acquired through the laser confocal scanning microscope A1R (Nikon, Tokyo, Japan).
2.10 Expression of β-catenin, Lef1 and Figla in Ovarian Cells Treated with BIO and XAV-939Healthy Japanese flounder adults were obtained from a commercial hatchery located in Qingdao City of China. Adult fish were cultured for one week in seawater tanks at 20℃ ± 1℃ under laboratory conditions. The ovarian tissue was carefully dissected and cut into pieces (mean size = 5 mm3) in PBS (pH 7.4). To avoid the bacterial infections and remove the white ovarian walls, the ovary pieces were washed 5 to 6 times in PBS added with 5% penicillinstreptomycin. Finally, ovary tissue pieces were cultured in vitro at 24℃ in DMEM/F12 medium (Thermo Fisher Scientifc Inc, Waltham, USA) containing 15% FBS (Gibco, Carlsbad, USA), 10 mmol L−1 HEPES, 10 mmol L−1 non-essential amino acids (NEAA), 2 mmol L−1 L-glutamine (Solarbio, Beijing, China), 50 µg mL−1 streptomycin and 50 U mL−1 penicillin (Gibco, Carlsbad, USA).
In order to explore the influence of Wnt signaling pathway on the expression of figla, we selected the activator (BIO) (Cayman Chemical, Ann Arbor, USA) and inhibitor (XAV-939) (Cayman Chemical, Ann Arbor, USA) of canonical Wnt signaling pathway (Niu et al., 2018), to treat the cultured ovary tissue. The tissue was stimulated with 10 μmol L−1 BIO and 10 μmol L−1 XAV-939, respectively. The tissue treated with solvent DMSO (0.1%) was considered as a control. Total RNA was extracted and qRT-PCR of βcatenin, lef1 and figla was carried out at 24 h after treatment.
2.11 Expression Analysis of Genes in Canonical Wnt Signaling Pathway Basing on Transcriptome DataThe transcriptome of testis and ovary in our laboratory (Zhang et al., 2016) were applied to analyze the expression of genes lef1, β-catenin, frizzled-1, dsh, wnt1, lrp5, lrp6, rtk, ror2 and bcl9. The expression levels were evaluated by normalized Fragments Per Kilobase per Million (FPKM) value.
2.12 Statistical AnalysisAll experimental data were expressed as the mean ± SEM (standard error of mean) of three independent experiments. The data were statistically analyzed using one-way ANOVA followed by Games-Howell or Independent-Samples t-test using SPSS 20.0 (IBM, New York, USA) with P < 0.05 indicating significant difference.
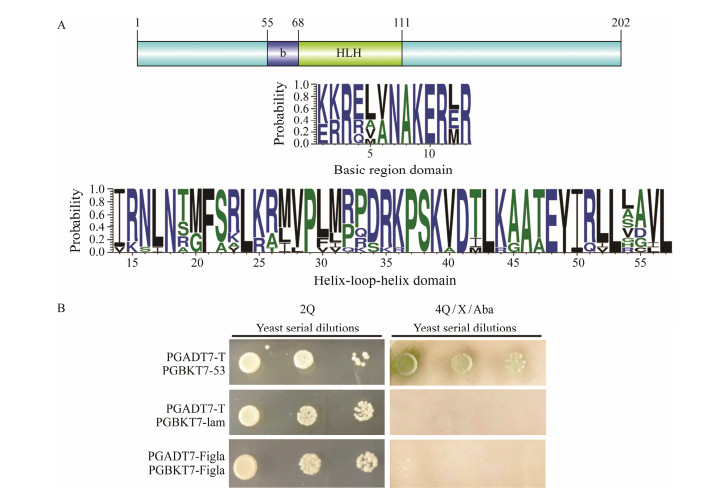
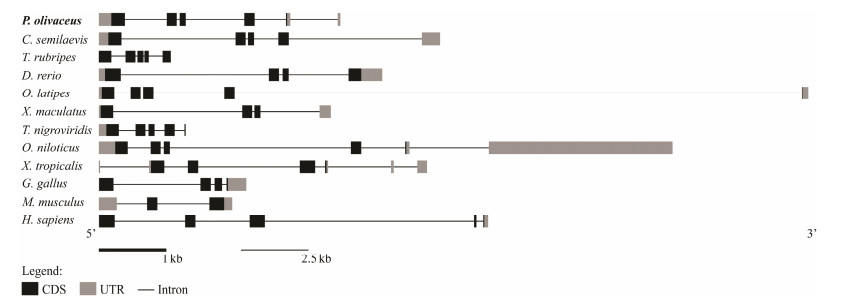
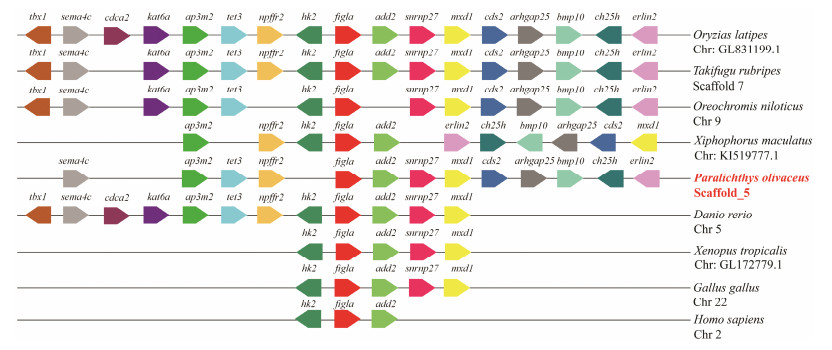
3 Results 3.1 Sequence Characteristics, Gene Structure and Evolution of PofiglaThe sequence of figla was identified from P. olivaceus transcriptome by tBlastx and verified by PCR. The figla cDNA sequence contains an open reading frame (ORF) of 609 bp, which encodes a polypeptide of 202 amino acids with a predicted molecular weight of 23.126 kDa. Gene figla contains two functional domains: basic region and helix-loop-helix (HLH) region (Fig.1A). In these regions, the percent identity of most amino acid in the alignments exceeded 60% (Fig.1A). Yeast two-hybrid assay showed that all groups could grow on 2Q deficient medium, which demonstrated plasmids were successfully transformed into yeast. In the positive control group (PGADT7-T and PGBKT7-53), a few green colonies could grow on the 4Q/X/Aba deficient medium. However, no yeast colonies could grow on the multiple defective medium in the experiment group (PGADT7-Figla and PGBKT7-Figla) and negative control group (PGADT7-T and PGBKT7-lam), which strongly suggested that PoFigla itself does not have the potential to interact and form homodimers (Fig.1B). So it may regulate downstream gene expression by combining other transcription factors to form heterodimers. Comparison of cDNA and genomic sequences showed that figla contains six exons, which differs from other teleost species such as tongue sole, fugu, zebrafish and medaka (Fig.2). A conserved syntenic relationship was obtained for figla and its surrounding genes in vertebrates from fish to tetrapods although fragment between elin2 and mxdl is reversed in Xiphophorus maculatus (Fig.3). These results together revealed that figla is greatly conserved during the evolution.
|
Fig. 1 Sequence characterization and verification of non-homodimer for PoFigla. (A) Above: Location information of conserved domains of PoFigla. Below: The consensus logos of basic region and HLH domain. The height of each letter indicates the percentage of corresponding amino acid residues at that position in the multiple sequence alignments. Default color scheme was selected for different amino acids. Multiple sequence alignment of Figla protein sequences from P. olivaceus and other vertebrates was performed by Clustal X (2.0). The abbreviated species names and GenBank accession numbers are available in Table 2. (B) Yeast two-hybrid assay to confirm PoFigla could not form homodimers. 2Q: leucine and tryptophan auxotrophic medium; 4Q/X/Aba: adenine, histidine, leucine, and tryptophan auxotrophic media were supplemented with aureobasidin A (AbA) and Alpha-galactosidase (X-α-Gal). |
|
Fig. 2 The genomic structure of figla in some teleosts and tetrapods. Coding sequence and UTRs are indicated by dark and gray boxes, respectively, whereas introns are represented by lines. |
|
Fig. 3 Chromosome synteny of Pofigla with those of other vertebrates. The pentagons with different colors represent various genes and their respective directions. Gene order was determined according to the relative position of genes in the same chromosome (Chr) or scaffold. |
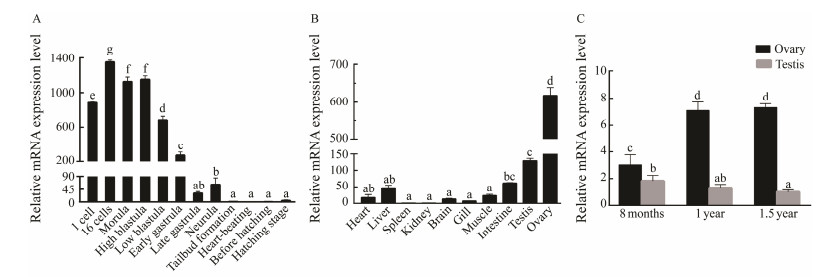
The temporal expression pattern of figla at the early stages of embryonic development was investigated via qRT-PCR. Pofigla transcripts showed a maternal expression pattern in view of the result that transcripts maintained a high level until blastula stage and then decreased significantly at gastrula stage and reached the undetectable level after neurula stage (Fig.4A).
|
Fig. 4 The relative expression patterns of Pofigla during embryonic development stage from 1 cell stage to the hatching (A); in different adult tissues (B); and in gonad tissues at different developmental stages (C). Data are shown as mean ± standard error of mean (n = 3). Different superscripts indicate significant differences P < 0.05. |
The distribution pattern of Pofigla in different tissues was shown in Fig.4B. Pofigla transcripts were specifically distributed in gonads, with a sexually dimorphic expression pattern observed. More specifically, a significantly higher expression level was found in the ovary. By contrast, figla was moderately expressed in liver and intestine and not expressed in spleen and kidney (Fig.4B). To better understand the expression change of figla during gonadal developmental stages, we performed qRT-PCR on gonads at different stages, including 8 months (immature gonads), 1 year (testis is typically mature) and 1.5 year (ovary is typically close to maturity).The expression rose in ovary from 8-month-old stage to 1-year-old and 1.5-year-old stage although there was no significant difference between 1 year and 1.5 year stages. Significant difference between ovary and testis sustained in these three phases with ovary showing higher expression than testis (Fig.4C).
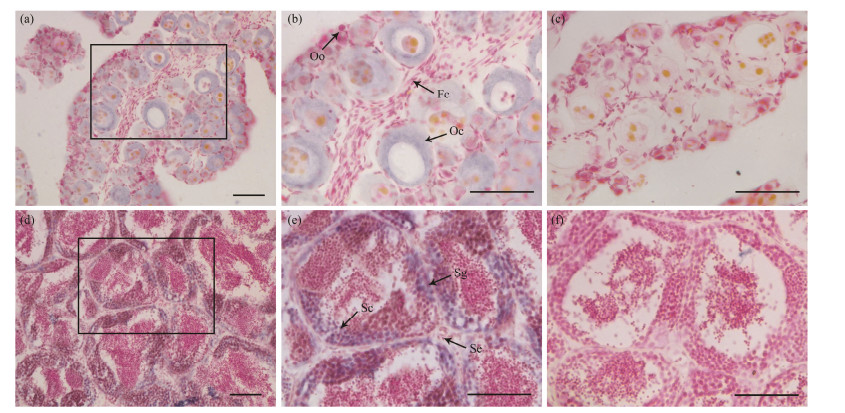
3.3 Localization of Pofigla in Gonad Tissues by ISHIn the ovary, figla mRNA signals could be specially found in the cytoplasm of oocytes but not in oogonia. In testis, the signals were mainly concentrated in the sperma-togonia and primary spermatocytes. No signals could be detected in the somatic cells around the germ cells both in ovary and testis, which indicate figla may be a germ cell specific transcription factor in P. olivaceus (as shown in Fig.5).
|
Fig. 5 The expression of Pofigla in gonad tissues determined by ISH. The positive signals were stained as purple or blue (a, b, d, e), while the negative control with sense probe hybridization was unstained (c, f). Panels (b) and (e) are magnifications of panels (a) and (d), respectively. The lower left cornerpart is magnifications of black box in panel (e). Oo, oogonia; Oc, oocytes; Fc, Follicular cell; Sg, spermatogonia; Sc, spermatocyte; Se, Sertoli cells. Scale bars = 50 μm. |
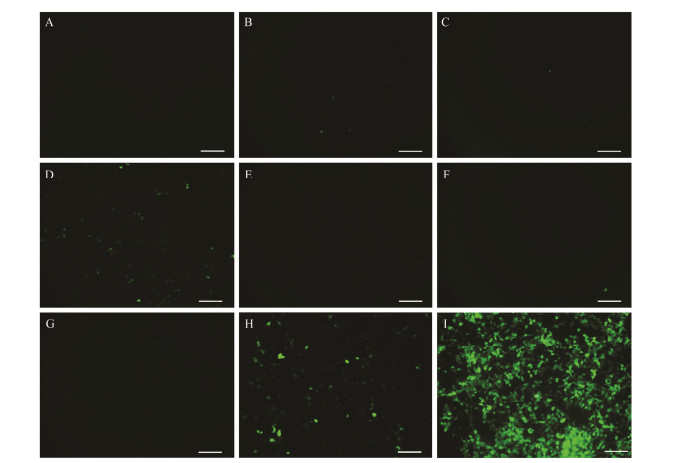
In order to find the cis-elements responsible for promoter activity of figla, we constructed a series of plasmids containing GFP driven by the sequentially deleted promoters of figla. A 2923 bp fragment (−2966 to −44) of the figla 5' flanking region was cloned and sequenced. These deletion constructs were transiently transfected into HEK 293T cells, and the green fluorescence intensity was observed after 48 h (Fig.6A – I). The results showed that the figla promoter fragments −251/−44 (Fig.6B), −444/−44 (Fig.6C), −1137/−44 (Fig.6E), −1729 /−44 (Fig.6F) and −2126 /−44 (Fig.6G) could hardly induce GFP expression in HEK 293T cells, while the region −772 /−44 (Fig.6D) and −2966 /−44 (Fig.6H) could evidently drive GFP expression. This indicated that there may be some strongly positively regulated cis-elements binding in the fragments −772 /−444 and 2966 /−2126. The fluorescence intensity of all figla promoter fragments were weaker than those of CMV promoters (Fig.6I).
|
Fig. 6 Functional analysis of Pofigla promoter. (A) HEK 293T cells transfected with pEGFP-1. (B – H) HEK 293T cells transfected with plasmids containing EGFP driven by the sequentially deleted promoters pEGFP-251/−44 (B), pEGFP −444/−44 (C), pEGFP −772/−44 (D), pEGFP −1137/−44 (E), pEGFP −1729/−44 (F), pEGFP −2126/−44 (G), and pEGFP −2966/−44 (H), respectively. (I) HEK 293T cells transfected with pEGFP-C1. Scale bars = 100 μm. |
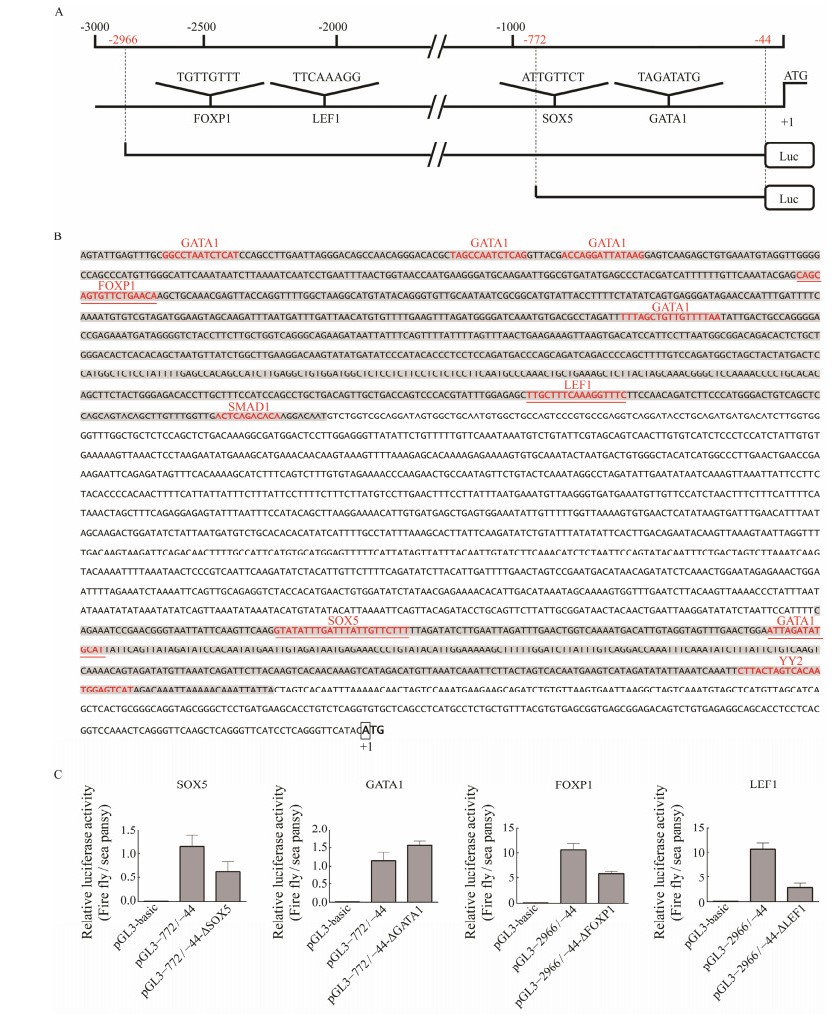
In the present study, −772/−444 and −2966/−2126 are two highly active promoter regions of figla. To identify regulatory cis-acting elements in these promoter regions, sequence analysis was performed by the MatInspector program with a cutoff value over 95%. Some putative binding sites of the transcription factor motifs, including GATA1, FOXP1, LEF1, SMAD1 in −2966/−2126 and SOX5, GATA1, YY2 in −772/−444, respectively, were identified (Fig.7A). Furthermore, by removing the motifs that lack experimental basis provided in MatInspector, we set the research targets at FOXP1 motif located at −2428 to −2412, LEF1 motif located at −2218 to −2202, SOX5 motif located at −738 to −716, and GATA1 motif located at −654 to −642 (Figs. 7A, B). To define the functional status of the four potential four regulatory elements within the promoter, we performed four site-directed plasmid mutations, namely, −2966/−44-ΔFOXP1, −2966/−44-ΔLEF1, −772/−44-ΔSOX5 and −772/−44-ΔGATA1, respectively (Fig.7A). Then, we evaluated the function of these cis-acting elements through dual-luciferase reporter assay in HEK 293T cells. The relative luciferase activities of −2966/−44-ΔFOXP1, −2966/−44-ΔLEF1 and −772/−44-ΔSOX5 exhibited statistically lower level compared with that of the wild-type construct pGL3−2966/−44 or pGL3−772/−44 (shown in Fig.7C). In contrast, −772/−44-ΔGATA1 showed the significantly higher relative luciferase level compared with construct pGL3−772/−44 (Fig.7C). These results indicated that FOXP1, LEF1 and SOX5 were effective to regulate figla promoter transcriptional activity, while GATA1 might play a negative regulatory role for figla transcription in −772/−44 region.
|
Fig. 7 Putative binding motif of SOX5, GATA1, FOXP1 and LEF1 and site-directed mutations assays in the figla promoter. (A) A schematic diagram of putative SOX5, GATA1, FOXP1 and LEF1 binding elements in the figla promoter. The initiation codon (ATG) is designated as +1. The scale is given above and the names of the potential TF binding sites are provided at the bottom. The core motif sequence of each transcription factor is shown just above their names. Extremities of the two control plasmid locations are marked with red numbers in the scale. (B) Nucleotide sequences and putative regulatory elements of the Pofigla promoter regions. The −2966/−2126 and −772/−444 regions are represented with a gray background. The putative regulatory element motif sequences are in red font. The candidate four motifs are underlined in red and ATG is defined as +1 position. (C) The effect of mutagenesis of transcription factor binding sites of SOX5, GATA1, FOXP1 and LEF1 on the figla promoter. Fragments indicated in red demonstrate the success of mutation assays. Data are shown as mean ± SEM (n = 3 for each group). Different superscripts indicate that P < 0.05. |
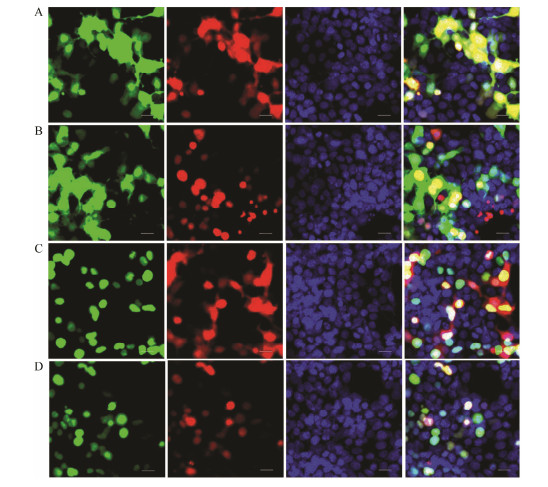
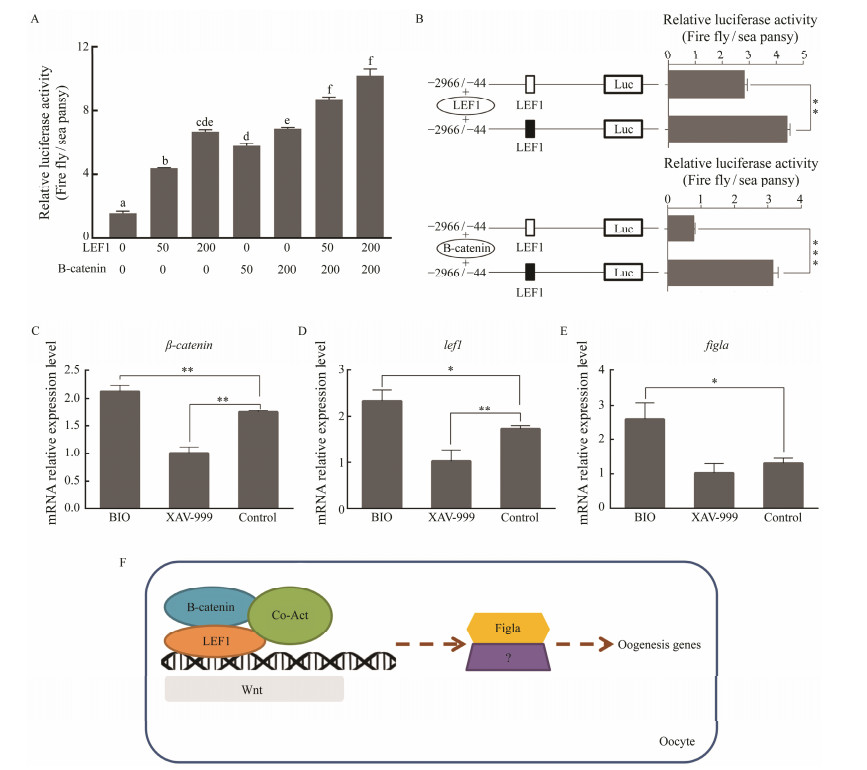
The above results illustrated that lef1 plays non-ignorable role in activating the transcription of figla. Given the positive transcription regulation effect of lef1 on figla, we explore whether there is a co-localization between them. When HEK 293T cells were transfected with LEF1pEGFP-N1 and Figla-pmCherry-N1, the green and red fluorescence were coincided mostly in the nuclear with each other. While in other groups (Fig.8), there was no apparent merge phenomenon of nuclear localization. These results indicated the close relationship between lef1 and figla. A previous study has shown β-catenin could regulate the mRNA level of figla in half-smooth tongue sole (Zhu et al., 2017). Given that β-catenin and lef1 are pivotal members of the canonical Wnt signaling pathway (Wong et al., 2002; Deroo et al., 2004; Pilon et al., 2006), it is reasonable to speculate that the transcription of figla may be regulated by this pathway. To test this hypothesis, we examined the effects of β-catenin and lef1 on the transcription activity of the figla promoter separately or by co-transfecting the overexpression vector into the HEK 293T cells. The dual-luciferase reporter assays showed that both B-catenin and LEF1 could enhance transcriptional activity of figla in a dose-dependent manner (Fig.9A). Mutant LEF1 binding site inhibited the lef1 or β-catenin overexpression-induced upregulation of the figla promoter activity (Fig.9B). Then we explored whether figla mRNA could change in ovarian cells with the activator and inhibitor of Wnt signaling pathway. qRT-PCR showed that BIO (activator) could apparently improve the mRNA expression of β-catenin, lef1 and figla (Figs.9C – E), while XAV-939 (inhibitor) could significantly inhibit the mRNA expression of β-catenin, lef1 and showed an insignificant inhibitory effect on figla expression (Figs.9C – E). These results suggested that the putative binding sites for LEF1 are required for the transcription activation of Pofigla and implied that the regulation of the canonical Wnt signaling pathway is significant (Fig.9F).
|
Fig. 8 Co-localization of LEF1 and Figla. HEK 293T cells were transfected with different combination of recombinant pEGFP and pmCherry plasmids. The left-hand panels depict GFP staining, the second panels from the left depict mCherry staining, the third panels from the left depict DAPI staining, and the right-hand panels depict merged GFP/mCherry/DAPI staining. The upper panels depict localization of the GFP and mCherry negative control (A), and the 2 – 4 panels depict localization of the pEGFP-N1 and PoFigla-pmCherry-N1 (B), PoLEF1-pEGFP-N1 and pmCherry-N1 (C), PoLEF1-pEGFPN1 and PoFigla-pmCherry-N1 proteins (D), respectively. The nuclei were stained as blue by DAPI. Scale bars = 20 μm. |
|
Fig. 9 The effect of Wnt signaling pathway on the transcription of Pofigla. (A) The luciferase activity of Pofigla promoter −2966/−44 region after overexpressing lef1 and β-catenin with different amount (ng). (B) Effects of lef1 or β-catenin overexpression on the activity of the Pofigla promoter −2966/−44 region with a mutation in the binding site for lef1. Black and white box indicated the normal and mutational binding motif of LEF1, respectively. (C – E) qRT-PCR analyses for expressions of β-catenin (C), lef1 (D), and figla (E) in Japanese flounder ovarian cells stimulated by BIO or XAV-939. (F) Hypothetical model of figla regulated by Wnt signaling in Japanese flounder oocytes. Data are shown as mean ± SEM (n = 3 for each group). * indicates significant difference (P < 0.05), while ** indicates highly significant difference (P < 0.01). |
Gonadal differentiation and development determine reproductive potential and concomitantly affect growth in some fishes. As an important gene associated with ovarian development and female fertility, figla has been cloned and characterized in many species, including mouse (Liang et al., 1997), bovine (Tripurani et al., 2013), zebrafish (Qin et al., 2018), half-smooth tongue (Li et al., 2016) and Nile Tilapia (Qiu et al., 2015). Although figla has been identified in some teleosts, the clues about its transcription regulation is still unclear. To date, it was found that Taf4b can directly target figla to regulate the meiotic process of oocytes in mouse (Grive et al., 2016). It is meaningful to explore the transcriptional mechanism of figla in teleosts, which helps us understand the internal molecular mechanisms of gonadal development from the perspective of gene regulation.
In this study, the core sequence of figla gene was obtained from ovary cDNA library in Japanese flounder. The deduced amino acid sequence of figla showed typical characteristic features of the bHLH family with conserved DNA-binding region (Liu and Zhao, 2010) and followed by a helix-loop-helix domain responsible for interacting with other bHLH proteins (Wang et al., 2015). Yeast two-hybrid assay indicated Figla probably regulates downstream genes as a heterodimers with other transcription factor in Japanese flounder. As an important gene for fertility, figla has been well studied in human (Bayne et al., 2004). Once there was one or several mutations in the exon of figla gene, most women will suffer the severe premature ovarian failure (Tosh et al., 2015). With the rapid development of high-throughput sequencing technology, figla has been considered as a female-biased gene in gonad of several teleosts, such as spotted scat (He et al., 2019), black porgy (Zhang et al., 2019), and Japanese eel (Jeng et al., 2018). In this study, figla was expressed extremely high in the ovary and it was specifically distributed in the cytoplasm of oocytes in Japanese flounder (Fig.5), which was consistent with the study by Liang et al. (2020). In bovine, figla might be a maternal gene with high mRNA expression from pronuclear to eight-cell stage but is barely detectable at morula and blastocyst stages (Tripurani et al., 2013). Here, figla was highly expressed from 1 cell stage to low blastula stage (Fig.4), which also showed a maternal expression pattern in Japanese flounder.
One interesting phenomenon was that figla was also highly expressed in testis compared with other tissues except for ovaries, which is different from the results by Liang et al. (2020). In their results, the expression of figla was nearly not detectable in the testis throughout the developmental cycle. While in our study, the higher expression of figla in testis was detected at 8-month-old stage, compared with 1-year-old and 1.5-year-old stage. And the smallest expression difference, less than a 2-fold gap, between testis and ovary was found at 8-month-old stage. It is worth reminding that in half-smooth tongue sole, figla_tv1 was uniquely expressed in the female ovary, while figla_tv2 was mainly expressed in the testis of pseudo male (Li et al., 2016). Therefore, different developmental backgrounds of the male fish (genetic background, presence of adulterated pseudo males, different sampling season) could contribute to these different results. We suspected that figla gene not only plays a role in the ovary, but also participates in the development of the testis at early developmental stage.
Here, we identified and characterized the highly active promoter regions of Pofigla and predicted the putative important transcription factor according to their binding motif sequences. In addition to the identified two promoter regions (−772/−444 and −2966/−2126) with positive regulatory cis-elements, the results also showed that there may be strong negative regulating elements in the fragments −1137/−772 and −444/−251. Lack of transcription factors that can activate the figla expression in the HEK 293T cells may also contribute to this result. By dual luciferase assay, we concluded that SOX5, LEF1 and FOXP1 may play important roles in the transcriptional activation of figla, while GATA1 may play a negative role in the regulation of figla expression. Sox5 was reported to regulate cyp19a1a expression to promote gonad development in protogynous hermaphrodite red-spotted grouper and Carassius auratus (Huang et al., 2009; Chen et al., 2014). In medaka, sox5 was involved in germ-cell regulation and sex determination (Schartl et al., 2018). We speculated that sox5 is also involved in the development of the gonads, through regulating figla in Japanese flounder.
Another transcription factor that has a significant positive regulatory effect on figla is lef1. As a target gene of Wnt, lef1 is generally considered to be an important indicator of whether Wnt is activated (Clevers, 2006). In mouse, there is a female-specific regulation of lef1 splicing, and this regulation may enhance Wnt signaling activity in female gonads (Zhao et al., 2018). In addition, it has been reported recently that the canonical Wnt signaling pathway regulated gonad differentiation and development in zebrafish (Sreenivasan et al., 2014; Safian et al., 2018). Once Wnt was inhibited during gonad differentiation in zebrafish, it will lead to more male individuals and decrease the expression of gonadal aromatase gene (cyp19a1a) (Sreenivasan et al., 2014). In our study, we proved that lef1 and β-catenin have positive regulatory effects on the promoter activity of figla. It is reasonable to think that there are some genes or other pathways that compensate for the expression of figla when Wnt was inhibited in Japanese flounder. β-catenin and lef1 indeed play an important role in the transcriptional activation of figla. Hence Wnt signaling may regulate the expression of figla gene in the ovary of Japanese flounder, which was similar to the 'pro-female' pathway of Wnt/β-catenin signaling in zebrafish (Sreenivasan et al., 2014). We have found many vital members of Wnt signaling pathway such as lef1, β-catenin, frizzled-1, dsh, wnt1 lrp5, lrp6, rtk, ror2 and bcl9, were ovary-biased genes in our previous RNA-seq data (Zhang et al., 2016). Taken together, all the results implied that transcription level of figla might be activated by canonical Wnt signaling pathway based on potential binding site of LEF1 on the promoter of figla in Japanese flounder (Fig.9F).
5 ConclusionsIn summary, the cDNA sequence of figla was identified in P. olivaceus. Amino acid alignment and chromosome synteny analysis showed that figla was conserved in evolution and held conserved structure between fish and tetrapods. qRT-PCR assay demonstrated that figla was significantly expressed in gonad, with a higher expression in ovary than in testis. ISH results revealed that figla was specifically distributed in the spermatogonia and primary spermatocyte of male testis and oocytes of female ovary. Screening and analyzing promoter regions of figla showed its transcription could be regulated by SOX5, GATA1, FOXP1, LEF1 and Wnt signaling pathway. All the results in this study provided the first evidence that figla may not only serves as a considerable role in the ovary development, but also plays some important functions in testis development and/or male germ cell differentiation. Moreover, Wnt signal induces figla expression in oocytes in Japanese flounder.
AbbreviationsP. olivaceus, Paralichthys olivaceus; figla, the factor in the gerline alpha; Pofigla, Paralichthys olivaceus figla; cDNA, complementary deoxyribonucleic acid; UTR, untranslated region; ORF, open reading frame; qRT-PCR, quantitative real-time polymerase chain reaction; ISH, in situ hybridization; Serpinb6c, serpin family B member 6c; Arhgap20, Rho GTPase activating protein 20; Pdzk1, PDZ domain containing 1; NALP, NACHT, LRR and pyrin domains containing protease; E12, helix-loop-helix protein E12; CMV, human cytomegalovirus; HEK, human embryonic kidney; GATA1, transcription factor GATA-1; FOXP1, Forkhead box P1; SOX5, SRY-box transcription factor 5; LEF1, lymphoid enhancer binding factor 1; SMAD1, mothers against decapentaplegic homolog 1; YY2, YY2 transcription factor; Frizzled-1, frizzled class receptor 1; Dsh, dishevelled; Wnt1, Wnt family member 1; Lrp5, LDL receptor related protein 5; Lrp6, LDL receptor related protein 6; RTK, receptor protein tyrosine kinase; ROR2, receptor tyrosine kinase like orphan receptor 2; BCL9, BCL9 transcription coactivator.
AcknowledgementThis study was supported by the National Key R & D Program of China (No. 2018YFD0901205).
Bayne, R. A., Martins da Silva, S. J., and Anderson, R. A., 2004. Increased expression of the FIGLA transcription factor is associated with primordial follicle formation in the human fetal ovary. Molecular Human Reproduction, 10(6): 373-381. DOI:10.1093/molehr/gah056 (  0) 0) |
Blumel, R., Zink, M., Klopocki, E., and Liedtke, D., 2019. On the traces of tcf12: Investigation of the gene expression pattern during development and cranial suture patterning in zebrafish (Danio rerio). PLoS One, 14(6): e0218286. DOI:10.1371/journal.pone.0218286 (  0) 0) |
Cartharius, K., Frech, K., Grote, K., Klocke, B., Haltmeier, M., Klingenhoff, A., et al., 2005. MatInspector and beyond: Promoter analysis based on transcription factor binding sites. Bioinformatics, 21(13): 2933-2942. DOI:10.1093/bioinformatics/bti473 (  0) 0) |
Chen, X. W., Jiang, S., Gu, Y. F., and Shi, Z. Y., 2014. Molecular characterization and expression of cyp19a gene in Carassius auratus. Journal of Fish Biology, 85(2): 516-522. DOI:10.1111/jfb.12418 (  0) 0) |
Chiba, Y., He, B., Yoshizaki, K., Rhodes, C., Ishijima, M., Bleck, C. K. E., et al., 2019. The transcription factor AmeloD stimulates epithelial cell motility essential for tooth morphology. Journal of Biological Chemistry, 294(10): 3406-3418. DOI:10.1074/jbc.RA118.005298 (  0) 0) |
Clevers, H., 2006. Wnt/beta-catenin signaling in development and disease. Cell, 127(3): 469-480. DOI:10.1016/j.cell.2006.10.018 (  0) 0) |
Deroo, T., Denayer, T., Van Roy, F., and Vleminckx, K., 2004. Global inhibition of Lef1/Tcf-dependent Wnt signaling at its nuclear end point abrogates development in transgenic Xenopus embryos. Journal of Biological Chemistry, 279(49): 50670-50675. DOI:10.1074/jbc.M408969200 (  0) 0) |
Gao, J., Wang, J., Jiang, J., Fan, L., Wang, W., Liu, J., et al., 2013. Identification and characterization of a nanog homolog in Japanese flounder (Paralichthys olivaceus). Gene, 531(2): 411-421. DOI:10.1016/j.gene.2013.08.030 (  0) 0) |
Grive, K. J., Gustafson, E. A., Seymour, K. A., Baddoo, M., Schorl, C., Golnoski, K., et al., 2016. TAF4b regulates oocyte-specific genes essential for meiosis. PLoS Genetics, 12(6): e1006128. DOI:10.1371/journal.pgen.1006128 (  0) 0) |
He, F. X., Jiang, D. N., Huang, Y. Q., Mustapha, U. F., Yang, W., Cui, X. F., et al., 2019. Comparative transcriptome analysis of male and female gonads reveals sex-biased genes in spotted scat (Scatophagus argus). Fish Physiology and Biochemistry, 45(6): 1963-1980. DOI:10.1007/s10695-019-00693-8 (  0) 0) |
Hu, W., Gauthier, L., Baibakov, B., Jimenez-Movilla, M., and Dean, J., 2010. FIGLA, a basic helix-loop-helix transcription factor, balances sexually dimorphic gene expression in postnatal oocytes. Molecular and Cellular Biology, 30(14): 3661-3671. DOI:10.1128/mcb.00201-10 (  0) 0) |
Huang, W., Zhou, L., Li, Z., and Gui, J. F., 2009. Expression pattern, cellular localization and promoter activity analysis of ovarian aromatase (Cyp19a1a) in protogynous hermaphrodite redspotted grouper. Molecular and Cellular Biology, 307(1-2): 224-236. DOI:10.1016/j.mce.2009.04.003 (  0) 0) |
Jeng, S. R., Wu, G. C., Yueh, W. S., Kuo, S. F., Dufour, S., and Chang, C. F., 2018. Gonadal development and expression of sex-specific genes during sex differentiation in the Japanese eel. General and Compapative Endocrinology, 257: 74-85. DOI:10.1016/j.ygcen.2017.07.031 (  0) 0) |
Joshi, S., Davies, H., Sims, L. P., Levy, S. E., and Dean, J., 2007. Ovarian gene expression in the absence of FIGLA, an oocytespecific transcription factor. BMC Developmental Biology, 7: 67. DOI:10.1186/1471-213x-7-67 (  0) 0) |
Li, H., Xu, W., Zhang, N., Shao, C., Zhu, Y., Dong, Z., et al., 2016. Two Figla homologues have disparate functions during sex differentiation in half-smooth tongue sole (Cynoglossus semilaevis). Scientific Reports, 6: 28219. DOI:10.1038/srep28219 (  0) 0) |
Liang, L., Soyal, S. M., and Dean, J., 1997. FIGalpha, a germ cell specific transcription factor involved in the coordinate expression of the zona pellucida genes. Development, 124(24): 4939-4947. (  0) 0) |
Liang, S., Wang, W., Wang, L., Wu, Z., Zou, Y., Tan, X., et al., 2020. Figla gene roles in the proliferation of oocytes in the olive flounder Paralichthys olivaceus. Aquaculture, 528: 735493. DOI:10.1016/j.aquaculture.2020.735493 (  0) 0) |
Liu, W. Y., and Zhao, C. J., 2010. Genome-wide identification and analysis of the chicken basic helix-loop-helix factors. Compapative and Functional Genomics, 2010: 682095. DOI:10.1155/2010/682095 (  0) 0) |
Liu, X. T., Wang, Y., Wang, X. H., Tao, X. F., Yao, Q., and Chen, K. P., 2014. A genome-wide identification and classification of basic helix-loop-helix genes in the jewel wasp, Nasonia vitripennis (Hymenoptera: Pteromalidae). Genome, 57(10): 525-536. DOI:10.1139/gen-2014-0171 (  0) 0) |
Niu, J., Guan, J., Li, R., Li, X., Zhai, J., Qi, J., et al., 2018. Cynoglossus semilaevis Rspo3 regulates embryo development by inhibiting the Wnt/beta-catenin signaling pathway. Intertional Journal of Molecular and Science, 19(7): 1915. DOI:10.3390/ijms19071915 (  0) 0) |
Pilon, N., Oh, K., Sylvestre, J. R., Bouchard, N., Savory, J., and Lohnes, D., 2006. Cdx4 is a direct target of the canonical Wnt pathway. Developmental Biology, 289(1): 55-63. DOI:10.1016/j.ydbio.2005.10.005 (  0) 0) |
Qin, M., Zhang, Z., Song, W., Wong, Q. W., Chen, W., Shirgaonkar, N., et al., 2018. Roles of Figla/figla in juvenile ovary development and follicle formation during zebrafish gonadogenesis. Endocrinology, 159(11): 3699-3722. DOI:10.1210/en.2018-00648 (  0) 0) |
Qiu, Y., Sun, S., Charkraborty, T., Wu, L., Sun, L., Wei, J., et al., 2015. Figla favors ovarian differentiation by antagonizing spermatogenesis in a teleosts, Nile tilapia (Oreochromis niloti cus). PLoS One, 10(4): e0123900. DOI:10.1371/journal.pone.0123900 (  0) 0) |
Safian, D., Bogerd, J., and Schulz, R. W., 2018. Igf3 activates beta-catenin signaling to stimulate spermatogonial differentiation in zebrafish. Journal of Endocrinology, 238(3): 245-257. DOI:10.1530/joe-18-0124 (  0) 0) |
Schartl, M., Schories, S., Wakamatsu, Y., Nagao, Y., Hashimoto, H., Bertin, C., et al., 2018. Sox5 is involved in germ-cell regulation and sex determination in medaka following co-option of nested transposable elements. BMC Biology, 16(1): 16. DOI:10.1186/s12915-018-0485-8 (  0) 0) |
Soyal, S. M., Amleh, A., and Dean, J., 2000. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development, 127(21): 4645-4654. (  0) 0) |
Sreenivasan, R., Jiang, J., Wang, X., Bartfai, R., Kwan, H. Y., Christoffels, A., et al., 2014. Gonad differentiation in zebrafish is regulated by the canonical Wnt signaling pathway. Biology of Reproduction, 90(2): 45. DOI:10.1095/biolreprod.113.110874 (  0) 0) |
Tan, X., Xu, P., Zhang, Y., and Zhang, P. J., 2019. Olive flounder (Paralichthys olivaceus) myogenic regulatory factor 4 and its muscle-specific promoter activity. Comparative Biochemistry and Physiology. B: Biochemistry and Molecular Biology, 236: 110310. DOI:10.1016/j.cbpb.2019.110310 (  0) 0) |
Tarczewska, A., and Greb-Markiewicz, B., 2019. The significance of the intrinsically disordered regions for the functions of the bHLH transcription factors. Intertional Journal of Molecular and Science, 20(21): 5306. DOI:10.3390/ijms20215306 (  0) 0) |
Tosh, D., Rani, H. S., Murty, U. S., Deenadayal, A., and Grover, P., 2015. Mutational analysis of the FIGLA gene in women with idiopathic premature ovarian failure. Menopause, 22(5): 520-526. DOI:10.1097/gme.0000000000000340 (  0) 0) |
Tripurani, S. K., Wee, G., Lee, K. B., Smith, G. W., Wang, L., and Yao, J. B., 2013. MicroRNA-212 post-transcriptionally regulates oocyte-specific basic-helix-loop-helix transcription factor, factor in the germline alpha (FIGLA), during bovine early embryogenesis. PLoS One, 8(9): e76114. DOI:10.1371/journal.pone.0076114 (  0) 0) |
Wang, L., Zhu, Y., Xu, W., Shao, C., Dong, Z., Li, H., et al., 2017. Molecular characterization of Pod1 during sex development in Chinese tongue sole (Cynoglossus semilaevis). Biochemical and Biophyical Research Communications, 494(3-4): 714-718. DOI:10.1016/j.bbrc.2017.10.126 (  0) 0) |
Wang, X. H., Wang, Y., Liu, A. K., Liu, X. T., Zhou, Y., Yao, Q., et al., 2015. Genome-wide identification and analysis of basic helix-loop-helix domains in dog, Canis lupus familiaris. Moleular Genetics and Genomics, 290(2): 633-648. DOI:10.1007/s00438-014-0950-1 (  0) 0) |
Wong, M. H., Huelsken, J., Birchmeier, W., and Gordon, J. I., 2002. Selection of multipotent stem cells during morphogenesis of small intestinal crypts of Lieberkuhn is perturbed by stimulation of Lef-1/beta-catenin signaling. Journal of Biological Chemistry, 277(18): 15843-15850. DOI:10.1074/jbc.M200184200 (  0) 0) |
Yamamoto, E., 1999. Studies on sex-manipulation and production of cloned populations in hirame, Paralichthys olivaceus (Temminck et Schlegel). Aquaculture, 173(1): 235-246. DOI:10.1016/S0044-8486(98)00448-7 (  0) 0) |
Zhang, D., Li, G., and Wang, Y., 2017. A genome-wide identification and analysis of basic helix-loop-helix transcription factors in cattle. Gene, 626: 241-250. DOI:10.1016/j.gene.2017.05.036 (  0) 0) |
Zhang, J., Hu, Y. H., Sun, B. G., Xiao, Z. Z., and Sun, L., 2013. Selection of normalization factors for quantitative real time RT-PCR studies in Japanese flounder (Paralichthys olivaceus) and turbot (Scophthalmus maximus) under conditions of viral infection. Veterinary Immunology and Immunopathology, 152(3-4): 303-316. DOI:10.1016/j.vetimm.2012.12.018 (  0) 0) |
Zhang, K., Xu, J., Zhang, Z., Huang, Y., Ruan, Z., Chen, S., et al, 2019. A comparative transcriptomic study on developmental gonads provides novel insights into sex change in the protandrous black porgy (Acanthopagrus schlegelii). Genomics, 111(3): 277-283. DOI:10.1016/j.ygeno.2018.11.006 (  0) 0) |
Zhang, W., Liu, Y., Yu, H., Du, X., Zhang, Q., Wang, X., et al., 2016. Transcriptome analysis of the gonads of olive flounder (Paralichthys olivaceus). Fish Physiology and Biochemistry, 42(6): 1581-1594. DOI:10.1007/s10695-016-0242-2 (  0) 0) |
Zhao, L., Wang, C., Lehman, M. L., He, M., An, J., Svingen, T., et al., 2018. Transcriptomic analysis of mRNA expression and alternative splicing during mouse sex determination. Molecular and Cellular Endocrinology, 478: 84-96. DOI:10.1016/j.mce.2018.07.010 (  0) 0) |
Zhu, Y., Hu, Q., Xu, W., Li, H., Guo, H., Meng, L., et al., 2017. Identification and analysis of the beta-catenin1 gene in halfsmooth tongue sole (Cynoglossus semilaevis). PLoS One, 12(5): e0176122. DOI:10.1371/journal.pone.0176122 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21

