In the mucosal fluid, cells of the mucosal epithelium secrete a variety of defensive compounds, including mucins, antibodies, defensins, protegrins, collectins, lysozyme, and histatin (Raj et al., 2002). Thereinto, mucins are a group of proteins with large molecular weight and high glycosylation and are main components of mucus. Mucins have been divided into two types according to its functional structure: transmembrane mucins and secretory mucins (Thornton et al., 2008). These proteins are detected on the surface of nearly all mucosal tissues and constitute an important barrier for the mucosa to defend against pathogenic infection. In fact, mucins can participate in multiple biological processes including affecting the bacteria rating and resisting pathogenic invasion, which provide the first physical, chemical, and biological line of host defense (Peterson and Artis, 2014). Specifically, they are produced by epithelial tissues and mucous membranes to protect mucosa tissues from pathogens invasion (Hattrup and Gendler, 2008; Esteban et al., 2012).
MUC2, as a secreted mucin, is the primary gel-forming mucin and mainly exists in the small and large intestines. It can influence the fundamental architecture of mucus-gel on the intestinal mucosal tissue, which is related to epithelial cells and mucus layer at the interface of the lumen in mammals (Hansson, 2012). As a barrier on the enterocyte surface, MUC2 protein isolates the gut microbiota and plays an important physiological role in intestine (Johansson et al., 2008). Furthermore, extensive O-glycosylation of MUC2 stimulates microbiota and host growth by providing nutrients (e.g., carbohydrate moieties), as well as maintains the gut by delivering tolerance signals to dendritic cells in the lamina propria steady state of intestine (Arike and Hansson, 2016). In mice, MUC2-knockout experiment reveals its influence on intestine homeostasis and host defense against pathogens (Velcich et al., 2002). Moreover, pathogenic bacteria may be able to access and invade intestinal epithelial cells via MUC2-definition in mucus layers (Bergstrom et al., 2010).
As a type of secretory mucin, MUC2 (Mucin 2) is highly expressed in the gastrointestinal tract of teleost. Studies on crap skin showed that MUC2 plays an important role in mucosal immune response (Adamek et al., 2017). Research indicates that a single nucleotide polymorphism in MUC2 may be associated with resistance to Aeromonas hydrophila infection in Labeo rohita (Robinson et al., 2014). In medaka, the downregulation of MUC2 in IL-22-KO was confirmed in both of larval and adult intestines (Takahashi et al., 2021). Recently, pool mucins have attracted considerable research interest due to their importantance to mucus in teleosts. However, the role of MUC2 in mucosal immunity of teleosts after pathogenic infection is rarely reported.
Japanese flounder (Paralichthys olivaceus) is a very important economic aquaculture fish distributed throughout northeast Asia, especially in China, Japan and Korea (Seikai et al., 2002). In the process of large-scale breeding, flounder disease problems have been becoming increasingly prevalent. With the rapid growth of marine farming, epidemic diseases have had a significant impact on olive flounder aquaculture (Nho et al., 2011). To ensure a healthy aquaculture industry, it is essential to learn how fish immune systems work together against pathogens.
Here, we reported two homologous genes of MUC2 identified from the Japanese flounder. Specifically, the conserved domains, phylogenetic relationships, structures, tissues-specific expression profiles in both healthy flounder and flounders under pathogenic bacteria Edwardsiella tarda challenge were characterized. Additionally, ISH was performed to reveal the expression pattern in tissue localization level. Finally, the siRNA-mediated knockdown of MUC2-1 and MUC2-2 and its effect were detected. These studies might give us an insight of MUC2 function in mucosal immunity in the Japanese flounder.
2 Materials and Methods 2.1 Ethics StatementAll experimental animal programs are reviewed and approved by the Animal Care and Use Committee of Qingdao Agricultural University, and all experimental animal programs are carried out in accordance with the guidelines of the Animal Care and Use Committee.
2.2 Collection and Processing of Experimental MaterialsThe flounders used in this experiment were purchased from the northern base of Mariculture seeds in Haiyang, Shandong, China, with an average weight of 400 g and a body length of 20 cm ± 2 cm. They were temporarily cultured in the laboratory seawater circulation system (22℃ water temperature, 12 h light/dark cycle, pH 7.4, dissolved oxygen 8.30 mg L−1). The cylinder block is about 47 cm long, 32 cm wide and 30 cm high. Flounders were fed with commercial feed twice a day. After feeding for a week, they were used for experiment.
2.3 Preparation of Flounder Challenge Test and Sample CollectionThree individuals of Japanese flounder were randomly selected and prepared as parallel samples. Healthy tissues, including blood, kidney, liver, spleen, gills, skin, intestine, brain, and muscle, were collected and intimately frozen in liquid nitrogen. Some P. olivaceus were used for bacterial challenge as experimental group.
To determine the gene expression of MUC2 profiles after bacterial challenge, the empirical Japanese flounder were challenged with E. tarda. The bacteria colony was inoculated in LB broth culture fleetly overnight (28℃, 180 r min−1), and all fish (n=70) were intraperitoneally injected with 1×106 CFUs (colony forming units) of E. tarda. Three fish were randomly selected as the experimental group and other three fish as the control group. The different tissues (e.g., blood, kidney, liver, spleen, gills, skin, intestine, brain, and muscle) were collected at different infection points (0, 6, 12, 24, and 48 h) and three samples were collected each time. Then, all tissues were instantly frozen in liquid nitrogen and were kept at −80℃ for further RNA extraction.
2.4 RNA Extraction and cDNA SynthesisTRIzol reagent (Invitrogen, Carlsbad, CA, USA) was applied to extract RNA from tisues. The integrity of RNA extraction was detected by electrophoresis, and Nanodrop 2000 (Thermo Electron North America LLC, FL, USA) was employed to check the concentration and extraction quality. Evo-M-MLV reverse transcription kit (Takara, Dalian, China) was used to remove genomic DNA and synthesize the first strand cDNA following the experimental procedure according to the manufacture's instruction.
2.5 Bioinformatics IdentificationThe predicted sequence of MUC2 gene of the flounder is obtained from whole genome sequences in NCBI website (https://www.ncbi.nlm.nih.gov/). HMMER was used to excerpt the homologous genes and conserved motifs using Simple Modular Architecture Research Tool (SMART) program (http://smart.embl-heidelberg.de/) to predict genes and protein sequences. According to the P. olivaceus genome information and predicted results, the numbers of exons, introns and position of MUC2-1, MUC2-2 on the chromosome were obtained. In addition, molecular weight (MW) and isoelectric point (PI) information of the two MUC2 genes are concluded by NovoPro (https://www.novopro.cn/tools/protein_iep.Html). Cell-PLoc 2.0 online subcellular localization website was used to predict positioning of subcellular fraction of MUC2. Then, the SMART Domain Analysis Database (http://smart.embl.de/) was used to build the conserved structural domain of MUC2-1 and MUC2-2 in the fish.
2.6 Phylogenetic AnalysisTo build the phylogenetic tree of the MUC2 genes in teleost fish and other mammals, the relationship was assembled using maximum likelihood method with 1000 bootstrap through software IQ-tree 2. At the same time, MUC 2 and its homologous proteins in other bony fishes including striped perch (Morone saxatilis), mandarin mandarin (Siniperca chuatsi), turbot (Scophthalmus maximus), Senegalese sole (Solea senegalensis), European plaice (Pleuronectes platessa), narrow flat (Hippoglossus stenolepis), and plaice (Hippoglossus hippoglossus), fish (Toxotes jaculatrix), perch perch (Lates calcarifer), bluefin tuna (Thunnus maccoyii), largemouth bass (Micropterus salmoides), perch (Dicentrarchus labrax) were searched. Furthermore, multiple sequences alignment of the MUC2 protein were conducted using MEGA 6 software to compare and search conserved domains.
2.7 In situ Hybridization AnalysisAccording to the obtained sequence of MUC2 gene, the primers were designed and amplified for probe sequences. In order to distinguish between MUC2-1 and MUC2-2 probe sequences, non-homologous 600 bp–1000 bp fragments of them were selected and were cloned into pBluescript Ⅱ SK vector with Ecor I and Hind Ⅲ enzyme cut sites to linearize the recombinant plasmid. The linearized products were determined to transcribe in vitro using T7 and SP6 RNA polymerase to obtain anti-sense and sense as control, respectively. In situ hybridization was performed using the identified probes according to the previous experimental process (Saúde et al., 2005).
2.8 siRNA Interference of MUC2-1 and MUC2-2To distinguish the functions of two MUC2 genes, three RNA interfering primers were designed at MUC2-1 cDNA sequence (6430 bp) 4229 bp, 5149 bp, 5321 bp sites, and two siRNA predicted sites for MUC2-2 (3894 bp) at 66 bp and 118 bp. The primers were synthesized by RiboBio Co., Ltd. (Guangzhou, Guangdong Province, China). In addition, the Japanese flounder gill cell lines were executed to determinate MUC2-1, MUC2-2, and NC (siRNA negative control) groups transfecting effects after cell were cultured for 48 h. The RNA was excerpted from above cells in different groups, respectively, then the cDNA was synthesized based on the abovementioned methods. Finally, qPCR was conducted to detect the expressions of MUC2-1 and MUC2-2, and the expressions of mucosal immune-related genes such as smads, galnt genes were evaluated.
3 Results 3.1 Identification of Homology Genes and the Structural and Phylogeny AnalysesIn this study, two MUC2 homology genes MUC2-1 and MUC2-2 were identified and characterized in Japanese flounder (XM_020092135.1, XM_020078576.1). The results predicted that MUC2-1 was 6430 bases long, coding 2166 amino acids. Its predicted MW and pI were 232.28 kDa and 5.63, respectively. MUC2-2 was with 3894 bp bases and can encode 1293 amino acids. Its predicted MW and pI were 143.38 kDa and 5.46, respectively. The subcellular localization of MUC2-1 and MUC2-2 were both in plasma membrane. As shown in Fig. 1A, two MUC2 proteins shared two common (vWF) D (VWD) and C8 domains. MUC2-1 has unique domains including von Willebrand factor type C (VWC) and Cystine-knot (CT), and MUC2-2 has an additional TIL domain. As shown in Fig. 1B, totally 12 conserved motifs were found in MUC2 of P. olivaceus according to PFAM database. A phylogenetic tree was constructed according to protein sequences of different species. The results showed that P. olivaceus MUC2-1 and MUC2-2 were clustered in two clades. Clade Ⅰ primarily contains MUC2-1 and some teleosts and other higher vertebrates, and Clade Ⅱ includes MUC2-2 and another homologous of MUC2 gene in other teleosts (Fig. 2).

|
Fig. 1 Structure and motif analysis of MUC2 proteins in P. olivaceus. (A) Domain pattern of mucin genes in P. olivaceus. Different colors and shapes indicate the different domains. The horizontal pink bars represent the signal peptide and the vertical blue bars represent transmembrane domains. (B) The boxes with different colors present the 12 predicted motifs. |

|
Fig. 2 Phylogenetic analysis of MUC2 obtained from P. olivaceus and other vertebrates. The tree was constructed with the neighbour-joining algorithm in MEGA 7.0. The relative genetic distances are explained by the scale bar and the branch lengths. |
The qPCR analysis revealed that MUC2-1 and MUC2-2 could be found in healthy Japanese flounder tissues, including blood, brain, gill, intestine, skin, spleen, stomach, heart, liver, muscle, and kidney. The results showed that both MUC2-1 and MUC2-2 were predominately expressed in intestine and gill tissues. Significantly higher transcripttion level of MUC2-1 was also observed in kidney, as shown in Fig. 3.

|
Fig. 3 Expression of MUC2 genes in P. olivaceus tissues examined by qPCR. The expression of MUC2-1 and MUC2-2 we detected in different tissues of healthy Japanese flounder, including blood, brain, gill, intestine, skin, spleen, stomach, heart, liver, muscle, kidney. The expression levels were normalized using β-actin as the internal control. The mean ± SEM values from three separate individuals (n = 3) are shown. Letters indicate statistically significant differences (P < 0.05). |
MUC2 expression levels were detected in gill, intestine, skin, liver, spleen, and kidney after E. tarda infection. MUC2-1 and MUC2-2 showed significantly different expression pattern in intestine, spleen, skin, and kidney. In gill, the two gene showed the similar expression trend of first downand then up-regulation. Interestingly, MUC2-2 exhibited fluctuation and up-regulated transcription level in mucosa-associated tissue intestine and skin. MUC2-1 showed significantly steadily up-regulated expression trend in liver and kidney (Fig. 4).
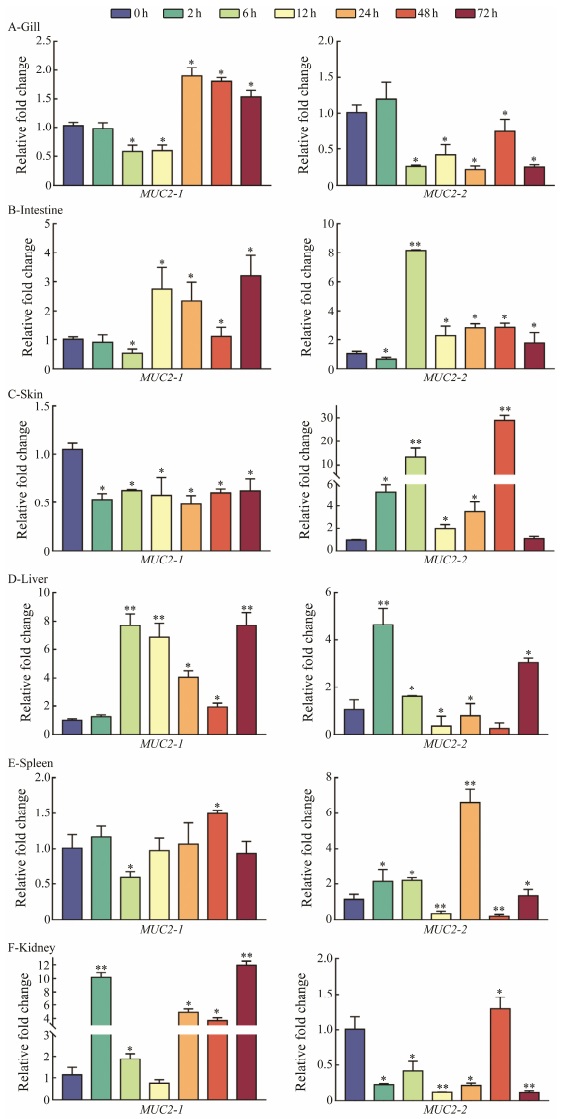
|
Fig. 4 Expression profile of MUC2-1 and MUC2-2 in P. olivaceus tissues at different time points (0, 2, 6, 12, 24, 48, and 72 h) after E. tarda challenge. The expression levels were normalized using β-actin as the internal control. The mean ± SEM values from three separate individuals (n=3) are shown. Asterisks mark the significant differences between control and experimental samples (* P < 0.05; ** P < 0.01). |
A further analysis of the immune response pattern of MUC2 was conducted by analyzing the expression changes of the MUC2 gene in gill cells in response to LPS, PGN, and polyI:C. In LPS-treated group, MUC2-1 showed persistently down-regulation trend and MUC2-2 was downregulated from 2 h to 6 h and rapidly up-regulated at 24 h. In PGN-treated group, it was down-regulated from 2 h to 12 h, then rapidly up-regulated at 24 h. In the poly I:C group, MUC2-1 and MUC2-2 all exhibited fluctuating expression levels (Fig. 5).
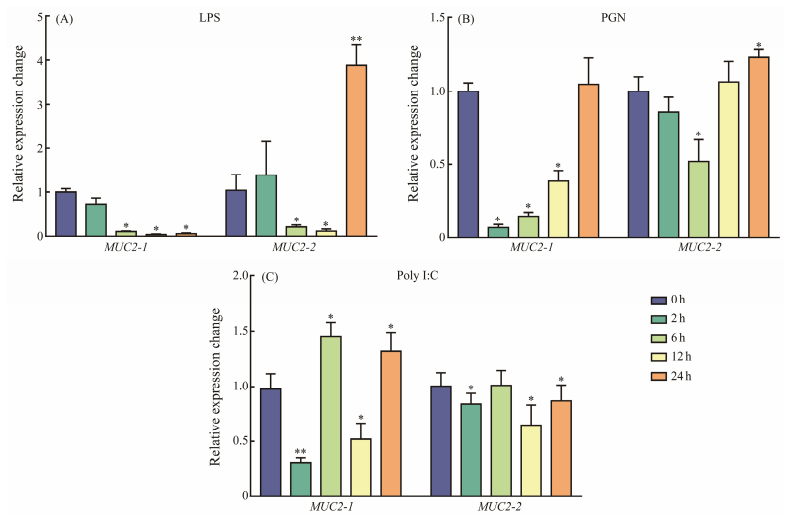
|
Fig. 5 Analysis of the in vitro stimulation of MUC2-1 and MUC2-2 in response to LPS, PGN, and poly: C in gill cell line of the flounder, respectively. The expression levels were normalized using β-actin as the internal control. The mean ± SEM values from three separate individuals (n=3) are shown. Asterisks mark the significant differences between control and experimental samples (*P < 0.05; ** P < 0.01). |
ISH was performed to detect the cellular localization of MUC2-1 and MUC2-2 in intestine and gill tissues. As shown in Fig. 6, the hybridization signals of MUC2-1 were detected primarily in the goblet cells of the gill and located in the lamina propria near the intestinal basement membrane. In the gill tissues, MUC2-2 was found both in goblet cells and epithelial cells, while its localization in intestinal tissues appeared similar to MUC2-1 in intestine (Fig. 7). Sense probes used as negative control exhibited no significant signals in both intestine and gill tissues.
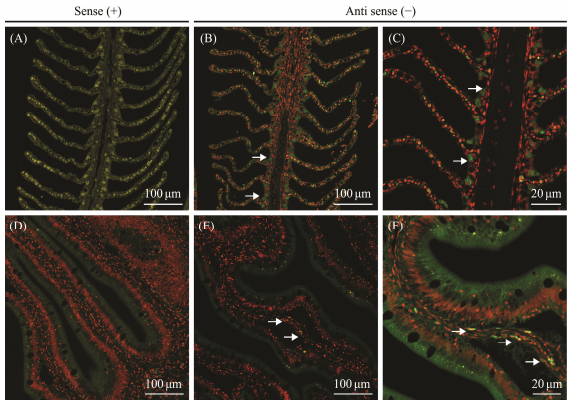
|
Fig. 6 Fluorescence in situ hybridization of P. olivaceus MUC2-1 in gill and intestine tissues. (A–C) ISH in gill with antisense probes corresponding to 100× and 200× magnification, in which (A) is as the sense to be defined as control. (D–F) ISH in the intestine tissues with antisense probes corresponding to 100× and 200× magnification, in which (D) is as the sense to be defined as control. The white arrows indicate the location of the fluorescent signal. |
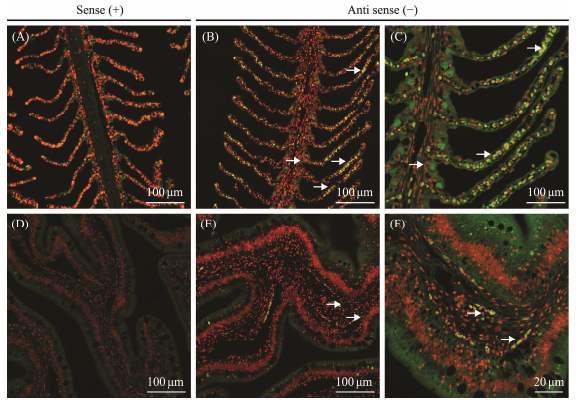
|
Fig. 7 Same as Fig. 6 but for P. olivaceus MUC2-2. |
To inhibit the expressions of MUC2-1 and MUC2-2, three and two siRNAs were designed for each gene, respectively, and the knockdown effect was detected. The siRNA with the most efficiency effect (siRNA 2 for MUC2-1, and siRNA 1 for MUC2-2) were singled out. After affirm the highest transcription efficiency, cells with three transfected siRNA were carried out for qPCR to detected the expression levels of MUC2-1 and MUC2-2, while cells with siRNA-nc was applied as control. Meanwhile, their network interaction genes (Figs. 8A, 8B), i.e., c1galt1, poc1b1, gcnt4, crebbpa, galnt and smad family members were detected after MUC2-1 and MUC2-2 were knocked down with siRNA (Figs. 8E, 8F).

|
Fig. 8 PPI (protein-protein interaction) network construction and the transcript levels of MUC2-1 and MUC2-2 and related protein in the gill cell line after RNAi for 48 h. (A), the predicted PPI network of MUC2-1; (B), the predicted PPI net-work of MUC2-2; (C), the knockdown effects of three siRNAs on P. olivaceus MUC2-1; (D), the knockdown effects of two siRNAs on P. olivaceus MUC2-2; (E), expressions of MUC2-1 and its related genes after MUC2-1 was knocked down by siRNA; (F), expression of MUC2-2 and its related genes after MUC2-2 was knocked down by siRNA. Asterisks indicate significant level between RNAi-treated group and negative control group (* P < 0.05). |
A secretory mucin, MUC2, is highly expressed in the teleost gastrointestinal tract (Perez-Sanchez et al., 2013). Mammalian MUC2 is secreted from goblet cells residing in the epithelial lining into the lumen (Gomez et al., 2013). The two homologous genes of MUC2-1 and MUC2-2 belong to secretory mucins in Japanese flounder. Similarly, there are two types of MUC2 named MUC2.1, MUC2.2 in Atlantic salmon (Salmo salar) and MUC2, MUC2-like in channel catfish (Ictalurus punctatus) (Peatman et al., 2013; Sveen et al., 2017). In fact, there are more homologous genes of MUC2 in other bony fish, such as, four types of MUC2 were identified in the zebrafish (Danio rerio), including MUC2.1, MUC2.2, MUC2.3 and MUC2.4, while four types were also identified in puffer fish (Fugu rubripes), which were named as MUC2A, MUC2B, MUC2C, and MUC2D (Lang et al., 2004, 2016). These results indicate that there are multiple homologous genes of MUC2 in teleost. As a secreted mucins protein, MUC2 has multiple characteristic domain structures: von Willebrand factor (vWF) D-type domain (VWD) at the N-terminus, a trypsin inhibitor-like cysteine-rich domain (TIL), C-terminal cysteine domain (Cys-knot), and von Willeb rand C domain (VWC) (Lang et al., 2007). From the structural perspective, the two homology P. olivaceus MUC2-1 and MUC2-2 contains common domains VWD and C8, while special VWC and CT domain appears in the MUC2-1, and TIL detected in MUC2-2, respectively. Interestingly, the N-terminus of the MUC2 contains VWD-C8-TIL and PTS domains. The PTS domain in mammals usually consists of a long fragment with a high repeat sequence rich in proline, threonine and serine residues (Strous and Dekker, 1992). However, the number, length, and amino acid sequence of PTS domains are poorly conserved across species (Lang et al., 2016). MUC2 of teleost usually consists of one or more VWD, TIL, and C8 domains. Compared with mammals, the PTS domain is not ubiquitous. such as, this study about P. olivaceus, S. salar and Cyprinus carpio L contain 2 VWD, C8 and 2 TIL domains (Van der Maria et al., 2012), However, MUC2.1 in D. rerio contains three VWD and a long PTS domain (Jevtov et al., 2014). Above all, compared to mammals, most of teleost MUC2 proteins have conserved VWD in N-terminal position, however, some domains such as PTS might disappear during evolution and chromosome replication process. The considerable desire of building the phylogenetic trees demonstrate the classification among different MUC2 in species. Interestingly, for the two MUC2 genes in the genomic data of P. olivaceus, phylogenetic tree analysis showed that MUC2-1 was more closely related to MUC2 in other higher vertebrates. MUC2-1 and MUC2-2 from the same fish were clustered in two different groups.
In this study, the analysis in healthy fish revealed that MUC2-1 and MUC2-2 both exhibited intestine- and gillbiased expressions. Moreover, MUC2-1 also showed significantly high expression level in kidney. As mucins are O-glycoproteins expressed by epithelial cells, high expression level of MUC2 gene was observed in intestine of different species, which was also detected in human normal kidney (Leroy et al., 2002; Woodfint et al., 2017). Notably, in situ hybridization experiments detected strong signals of MUC2-1 and MUC2-2 in gill and intestine tissues. Concretely, MUC2-1 and MUC2-2 located in the intestinal epithelium and lamina propria near the basal cells. However, MUC2-2 located in all goblet cells including gill filaments, while MUC2-1 is only detected in the goblet cells in the base of gill filaments. Analogously, mammalian MUC2 is excreted from goblet cells locating in the epithelial lining into the lumen of the parge intestine. In fact, MUC2 deficiency caused abnormal mucus layers in mice, which increased bacterial adhesion to surface epithelium and intestinal penetration (Kim et al., 2010). In fact, MUC2 is mainly expressed in mucosal tissues such as intestine, gill, and skin, and there are differences among different species and tissues of teleosts. For example, MUC2.1 and MUC2.2 have similar tissues distribution and are mainly found in the foregut in Atlantic salmon (Sveen et al., 2017). In crucian crap, only one MUC2 exists, and its mRNA is mainly detected in the intestine (Adamek et al., 2017). Exceptionally, MUC2-like mRNA is examined in gill, skin, and brain of C. carpio (Van der Maria et al., 2012). Above all, these results suggest that the two homology MUC2 genes are dominantly expressed in mucosal tissues.
In addtion, the MUC2-1 and MU2-2 expression pattern showed significant differences during E.tarda infection. One of the MUC2 homolog genes showed more clearly expression pattern in different tissues in response to bacterial infection, revealing that the different homolog gene of MUC2 might have synergistic function in most immune tissues especially in mucosal tissues in P. olivaceus. Interestingly, it has been reported that the expression of MUC2 in gilthead sea bream (Sparus aurata) infected by myxosporean Enterromyxum leei was significantly reduced (Perez-Sanchez et al., 2013), and after being challenged with enteric pathogen Edwardsiella ictalur, MUC2 of channel catfish (Ictalurus punctatus) was markedly upregulated (Liu et al., 2020). Generally, MUC2 homologous genes have expanded reply to diversified bacteria, highlighting the fact that they are innate immune components to defeat microorganism (Lang et al., 2004). Although mucins may not respond to infection in all hosts, it is possible that physicochemical properties of gill and skin mucus shift in response to gill parasites (Gomez et al., 2013). Interestingly, MUC2-2 showed significant up-regulation response to LPS stimulation in gill cell lines of flounder, as well as MUC2-1 to PGN and Poly: C. In addition, MUC2 showed strong immune responses but no pathological changes after SVCV invasion in intestine, which could lead to secondary infection in teleosts (Dong et al., 2019). Similarly, when MUC2 knockout mice were infected with Salmonella, they showed dramatic susceptibility to infection, developing significantly higher barrier involved in epithelial function (Zarepour et al., 2013). Above all, it demonstrated that different homology of MUC2 might play complicated rolesin immune response in teleosts.
MUC2-associated genes, including galnts, smads, crebbpa, and poc1b1, were studied to understand their expression changes in immune responses. The results showed that after in vitro knockdown of MUC2-1 gene, the expressions of galnt's family genes were down-regulated. As a wellknown galnt's family, galnt1 was predicted to target many mRNA and is responsible for glycosylation of MUC7. It is a large salivary protein containing central mucin domains, and functions in the lubrication of oral mucosa (Veeregowda et al., 2012). In contrast, in our study, MUC2-2 was postulated to participate in the Smad signaling pathway involved in immune response to bacterial infection. In fact, TGF-β-Smad signaling pathway was identified as a league, together with the TLR2-MyD88, to mediate NF-κB-dependent MUC2 transcription in bacterial pathogenesis (Jono et al., 2002). In fact, in medaka (Oryzias latipes), IL (Interleukin)-22-KO could significantly down-regulate MUC2 expression (Takahashi et al., 2021), which is significantly lower than in wild type fish. This study indicates that MUC2-1 and MUC2-2 might depend on Galnt and Smad pathway to function in the mucosal immune process, however, more studies are necessary to confirm the cooperation and the mechanism behind it.
In a conclusion, the profiles of two MUC2 genes were systematically identified in Japanese flounder genome, named MUC2-1 and MUC2-2. The functional domains, gene motif structures, and phylogeny were consummated depending on bioinformatics. These two genes were found to demonstrate a mucosal tissues-biased expression pattern. The expression profile of MUC2 genes in challenged by bacteria was analyzed, which indicated that MUC2-1 and MUC2-1mainly showed obvious change in mucosal related tissues, thereinto, MUC2-1 mainly expressed in gill and intestine, and MUC2-2 exhibited significant change in intestine and skin, respectively. In addition, In situ hybridization indicated that MUC2-1 and MUC2-2 were expressed differently in gill tissue. Moreover, qPCR was used to examine the knockdown effect and the expression profile of factors involved in mucosal immune process, which means it's probably related to the galnt family genes and it may work through the smad pathway. Based on this study, we gained valuable insight into the possible functions of MUC2 associated with mucosal immune system in response to the invasion of bacterial pathogen.
AcknowledgementsThis study was supported by the Natural Science Foundation of Shandong Province (No. ZR2022QC037), the Shandong Key R&D Program for Academician Team in Shandong (No. 2023ZLYS02), the High-Level Talents Research Fund of Qingdao Agricultural University (No. 663/1120033), the Young Experts of Taishan Scholars (No. 201909130), the Shandong Technical System of Fish Industry (No. SDAIT-12-03), and the Science and Technology Support Plan for Youth Innovation of Colleges and Universities in Shandong Province (No. 2019KJF003).
Adamek, M., Hazerli, D., Matras, M., Teitge, F., Reichert, M., and Steinhagen, D., 2017. Viral infections in common carp lead to a disturbance of mucin expression in mucosal tissues. Fish & Shellfish Immunology, 71: 353-358. DOI:10.1016/j.fsi.2017.10.029 (  0) 0) |
Arike, L., and Hansson, A., 2016. The densely O-glycosylated MUC2 mucin protects the intestine and provides food for the commensal bacteria. Journal of Molecular Biology, 428(16): 3221-3229. DOI:10.1016/j.jmb.2016.02.010 (  0) 0) |
Bergstrom, K. S. B., Kissoon-Singh, V., Gibson, D. G., Ma, C. M., Montero, M., Sham, H. P., et al., 2010. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathogens, 6(5): e1000902. DOI:10.1371/journal.ppat.1000902 (  0) 0) |
Dong, S. D., Ding, L., Cao, J. F., Liu, X., Xu, H. Y., Meng, K. F., et al., 2019. Viral-infected change of the digestive tract microbiota associated with mucosal immunity in teleost fish. Frontiers in Immunology, 10: 2878. DOI:10.3389/fimmu.2019.02878 (  0) 0) |
Esteban, M. A., 2012. An overview of the immunological defenses in fish skin. International Scholarly Research Notices, 2012: 1-29. DOI:10.5402/2012/853470 (  0) 0) |
Gomez, D., Sunyer, J. O., and Salinas, I., 2013. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish & Shellfish Immunology, 35(6): 1729-1739. DOI:10.1016/j.fsi.2013.09.032 (  0) 0) |
Hansson, G. C., 2012. Role of mucus layers in gut infection and inflammation. Current Opinion in Microbiology, 15(1): 57-62. DOI:10.1016/j.mib.2011.11.002 (  0) 0) |
Hattrup, C. L., and Gendler, S. J., 2008. Structure and function of the cell surface (tethered) mucins. Annual Review of Physiology, 70: 431-457. DOI:10.1146/annurev.physiol.70.113006.100659 (  0) 0) |
Jevtov, I., Samuelsson, T., Yao, G., Amsterdam, A., and Ribbeck, K., 2014. Zebrafish as a model to study live mucus physiology. Scientific Reports, 4(1): 6653. DOI:10.1038/srep06653 (  0) 0) |
Johansson, M., Phillipson, M., Petersson, J., and Hansson, G. C., 2008. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proceedings of the National Academy of Sciences of the United States of America, 105(39): 15064-15069. DOI:10.1073/pnas.0803124105 (  0) 0) |
Jono, H., Shuto, T., Xu, H. D., Kai, H., Lim, D. J., Gum, J. R., et al., 2002. Transforming growth factor-β-Smad signaling pathway cooperates with NF-κB to mediate nontypeable Haemophilus influenzae-induced MUC2 mucin transcription. Journal of Biological Chemistry, 277(47): 45547-45557. DOI:10.1074/jbc.M206883200 (  0) 0) |
Kim, Y. S., and Ho, S. B., 2010. Intestinal goblet cells and mu-cins in health and disease: Recent insights and progress. Current Gastroenterology Reports, 12: 319-330. DOI:10.1007/s11894-010-0131-2 (  0) 0) |
Lang, T., Alexandersson, M., Hansson, G. C., and Samuelsson, T., 2004. Bioinformatic identification of polymerizing and transmembrane mucins in the puffer fish Fugu rubripes. Glycobiology, 14(6): 521-527. DOI:10.1093/glycob/cwh066 (  0) 0) |
Lang, T., Hansson, G. C., and Samuelsson, T., 2007. Gel-forming mucins appeared early in metazoan evolution. Proceedings of the National Academy of Sciences of the United States of America, 104(41): 16209-16214. DOI:10.1073/pnas.0705984104 (  0) 0) |
Lang, T., Klasson, S., Larsson, E., Johansson, M. E. V., Hansson, G. C., and Samuelsson, T., 2016. Searching the evolutionary origin of epithelial mucus protein components–Mucins and FCGBP. Molecular Biology and Evolution, 33(8): 1921-1936. (  0) 0) |
Leroy, X., Copin, M. C., Devisme, L., Buisine, M. P., Aubert, J. P., Gosselin, B., et al., 2002. Expression of human mucin genes in normal kidney and renal cell carcinoma. Histopathology, 40(5): 450-457. DOI:10.1046/j.1365-2559.2002.01408.x (  0) 0) |
Liu, H., Xu, H., Shangguan, X., Wang, L., and Liu, X., 2020. Identification, annotation of Mucin genes in channel catfish (Ictalurus punctatus) and their expression after bacterial infections revealed by RNA-Seq analysis. Aquaculture Research, 51(5): 2020-2028. DOI:10.1111/are.14553 (  0) 0) |
Nho, S. W., Hikima, J., Cha, I. S., Park, S. B., Jang, H. B., del Castillo, C. S., et al., 2011. Complete genome sequence and immunoproteomic analyses of the bacterial fish pathogen Streptococcus parauberis. Journal of Bacteriology, 193(13): 3356-3366. (  0) 0) |
Peatman, E., Li, C., Peterson, B. C., Straus, D. L., Farmer, B. D., and Beck, B. H., 2013. Basal polarization of the mucosal compartment in Flavobacterium columnare susceptible and resistant channel catfish (Ictalurus punctatus). Molecular Immunology, 56(4): 317-327. DOI:10.1016/j.molimm.2013.04.014 (  0) 0) |
Perez-Sanchez, J., Estensoro, I., Redondo, M. J., Calduch-Giner, J. A., Kaushik, S., and Sitja-Bobadilla, A., 2013. Mucins as diagnostic and prognostic biomarkers in a fish-parasite model: Transcriptional and functional analysis. PLoS One, 8(6): e65457. DOI:10.1371/journal.pone.0065457 (  0) 0) |
Peterson, L. W., and Artis, D., 2014. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nature Reviews Immunology, 14(3): 141-153. DOI:10.1038/nri3608 (  0) 0) |
Raj, P. A., and Dentino, A. R., 2002. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiology Letters, 206(1): 9-18. DOI:10.1111/j.1574-6968.2002.tb10979.x (  0) 0) |
Robinson, N., Baranski, M., Mahapatra, K. D., Saha, J. N., Das, S., Mishra, J., et al., 2014. A linkage map of transcribed single nucleotide polymorphisms in rohu (Labeo rohita) and QTL associated with resistance to Aeromonas hydrophila. BMC Genomics, 15(1): 1-23. DOI:10.1186/1471-2164-15-541 (  0) 0) |
Saúde, L., Lourenço, R., Gonçalves, A., and Palmeirim, I., 2005. terra is a left-right asymmetry gene required for left-right synchronization of the segmentation clock. Nature Cell Biology, 7(9): 918-920. DOI:10.1038/ncb1294 (  0) 0) |
Seikai, T., 2002. Flounder culture and its challenges in Asia. Re-views in Fisheries Science, 10(3-4): 421-32. DOI:10.1080/20026491051721 (  0) 0) |
Strous, G. J., and Dekker, J., 1992. Mucin-type glycoproteins. Critical Reviews in Biochemistry and Molecular Biology, 27(1-2): 57-92. DOI:10.3109/10409239209082559 (  0) 0) |
Sveen, L. R., Grammes, F. T., Ytteborg, E., Takle, H. T., and Jør-gensen, S. M., 2017. Genome-wide analysis of Atlantic salmon (Salmo salar) mucin genes and their role as biomarkers. PLoS One, 12(12): e0189103. DOI:10.1371/journal.pone.0189103 (  0) 0) |
Takahashi, Y., Okamura, Y., Harada, N., Kono, T., Sakai, M., Hikima, J., et al., 2021. Interleukin-22 deficiency contributes to dextran sulfate sodium-induced inflammation in Japanese medaka, Oryzias latipes. Frontiers in Immunology, 12: 688036. DOI:10.3389/fimmu.2021.688036 (  0) 0) |
Thornton, D. J., Rousseau, K., and McGuckin, M. A., 2008. Structure and function of the polymeric mucins in airways mucus. Annual Review of Physiology, 70: 459-486. DOI:10.1146/annurev.physiol.70.113006.100702 (  0) 0) |
Van der Maria, M., Adamek, M., Gonzalez, S. F., Forst, P., Rombout, J. H., Wiegertjes, G. F., et al., 2012. Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after β-glucan feeding. Fish & Shellfish Immunology, 32(3): 494-501. DOI:10.1016/j.fsi.2011.12.008 (  0) 0) |
Veeregowda, D. H., Busscher, H. J., Vissink, A., Jager, D. J., Sharma, P. K., and Mei, H. C., 2012. Role of structure and glycolsylation of adsorbed protein films in biolubrication. PLoS One, 7(8): e42600. DOI:10.1371/journal.pone.0042600 (  0) 0) |
Velchich, A., Yang, W. C., Heyer, J., Fragale, A., Nicholas, C., Viani, S., et al., 2002. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science, 295(5560): 1726-1729. DOI:10.1126/science.1069094 (  0) 0) |
Woodfint, R. M., Chen, P. R., Ahn, J., Suh, Y., Hwang, S., Lee, S. S., et al., 2017. Identification of the MUC2 promoter as a strong promoter for intestinal gene expression through generation of transgenic quail expressing GFP in gut epithelial cells. International Journal of Molecular Sciences, 18(1): 196. DOI:10.3390/ijms18010196 (  0) 0) |
Zarepour, M., Bhullar, K., Montero, M., Ma, C., Huang, T., Vel-cich, A., et al., 2013. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infection and Immunity, 81(10): 3672-3683. DOI:10.1128/IAI.00854-13 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


