2) Laboratory for Marine Ecology and Environmental Science, Qingdao Marine Science and Technology Center, Qingdao 266237, China;
3) Frontiers Science Center for Deep Ocean Multispheres and Earth System, Ocean University of China, Qingdao 266100, China;
4) National Marine Hazard Mitigation Service, Ministry of Natural Resources, Beijing 100194, China;
5) Department of Life Sciences, National Natural History Museum of China, Beijing 100050, China
Jellyfish blooms have occurred frequently in coastal areas worldwide in recent years (Brotz et al., 2012; Condon et al., 2012). Jellyfish abundance increases in response to the combined effects of eutrophication, climate change, overfishing, and other anthropogenic activities (Purcell et al., 2007; Purcell, 2012). The blooms have many negative consequences, i.e., interfering with fisheries, clogging the cooling intakes of coastal power plants, causing severe damage to aquaculture, and stinging humans (Dong, 2019). The cascading impacts of jellyfish blooms pass from meso-zooplankton to microzooplankton and phytoplankton via the food web (Granéli and Turner, 2002; Pitt et al., 2007; Purcell et al., 2007; West et al., 2009; Xiao et al., 2019). After bloom, the decomposing jellyfish release nutrients into seawater, lowers pH and dissolved oxygen (DO) levels (e.g., DO levels in decomposing jellyfish treatments decreased from an initial average concentration of 261.5 ± 4.5 µmol L−1 to null within 40 h, Guy-Haim et al., 2020), and alters the benthic ecosystems (Sweetman et al., 2016; Tinta et al., 2021).
The life cycles of scyphozoan jellyfish which consist of sessile polyps and pelagic medusa, are among the most complex of any nonparasitic animals (Helm, 2018). In addition to sexual reproduction, a variety of asexual reproduction types have been observed in scyphozoans. The asexual budding of jellyfish includes typical budding (budding from the stalk of polyps), lateral budding (budding by stolon), fission budding, etc., through which vast numbers of daughter polyps are produced (Berrill, 1949a; Lucas, 2001; Adler and Jarms, 2009; Uye, 2011; Schiariti et al., 2014). Through strobilation, pelagic ephyra are released into the ambient seawater by sessile polyps and grow to medusae (Helm, 2018). Furthermore, jellyfish polyps can encyst as podocysts to survive in adverse environments (Thein et al., 2012). The recruitment of younger individuals (planula, polyp and ephyrae) determines the final abundance of adult jellyfish and contributes greatly to jellyfish bloom formation (Lucas et al., 2012).
Aurelia spp., usually called moon jellyfish, are a group of scyphozoans that commonly occur in oceans from temperate to tropical latitudes and are also bloom species in coastal waters (Dong, 2019). The asexual reproduction of Aurelia spp. has been well studied, including fission, budding, stolons, podocysts, and free-swimming propagules (Lucas, 2001; Adler and Jarms, 2009; Schiariti et al., 2014). In addition to the typical life cycle of scyphozoans, reverse development was also observed in Aurelia spp. Polyps can directly form from the ectoderm of degenerating juvenile medusae, cell masses, medusa tissue fragments, and the subumbrella of living medusae (He et al., 2015).
The ecological importance of tiny polyps to jellyfish blooms is usually difficult to estimate in the field, and most studies have been conducted in the laboratory (Miyake et al., 2002; Willcox et al., 2008; Malej et al., 2012). Temperature (Purcell, 2007; Han and Uye, 2010), salinity (Xing et al., 2020) and DO (Ishii et al., 2008) are suspected to be the key factors affecting the asexual reproduction of Aurelia polyps in laboratory cultures. However, its survival, growth, reproduction, and competition with other organisms in the field are scarce. Thus, the mortality of newly settled polyps in the field is supposed to be high, as tiny polyps are vulnerable to predation, sedimentation, and space competition (Watanabe and Ishii, 2001; Feng et al., 2017). Polyps could survive when competition decreased in autumn and proliferated their population by budding (Watanabe and Ishii, 2001).
While most field experiments on polyps have focused on population variation (Feng et al., 2017, 2018), the asexual reproduction modes of polyps are usually unnoticed. In the present study, the life history of A. coerulea polyps over the course of one year was observed in Jiaozhou Bay by deploying settlement plates in the field. Our objectives were: 1) investigate the life cycle of A. coerulea in Jiaozhou Bay, particularly polyp asexual reproduction; 2) assess polyps' abilities to form sedentary colonies; and 3) examine linkages between polyp populations and environmental factors.
2 Materials and Methods 2.1 Deployment of A. coerulea Polyp Plates in situFertilized A. coerulea medusae were captured in Jiaozhou Bay in August 2011. Planulae were taken from female brood sacs and transferred to a tank filled with seawater from Jiaozhou Bay. Corrugated polyethylene plates were placed on the bottom of the tank for planulae to settle on and metamorphose into polyps. Polyps were cultured at 18 ± 1℃ and a salinity of 31 PSU under natural light conditions with a 12:12 h light/dark cycle. Polyps were fed Artemia nauplii daily.
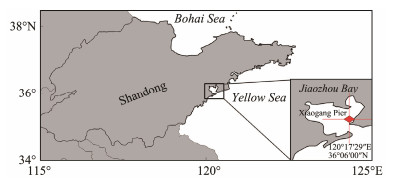
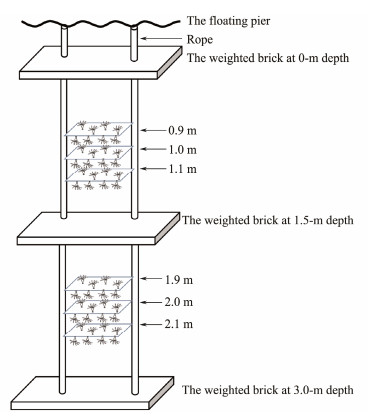
Plates settled with polyps were cut into 6 pieces with a square of 10 cm × 10 cm. Healthy polyps with a stalk length of about 200 μm were selected. In total, 30 polyps and 50 polyps remained on the upper surface and under surface of each plate, respectively (we aimed to maintain the highest possible number of healthy polyps on the plates for the field study. Consequently, the polyp counts on the two surfaces varied). Plates were placed beneath a floating bridge off the Xiaogang pier, Jiaozhou Bay (Fig.1). The seawater depth of the station was about 3.8 m. Two ropes were settled vertically in the seawater by the balance between the floating pier and weighed concrete bricks (Fig.2). Six plates with polyps were tied horizontally to the ropes. The six plates were plotted at two depths: plates 1 – 3 were placed at an average depth of 1 m (± 0.1) with a 10 cm interval, and plates 4 – 6 were placed at an average depth of 2 m (± 0.1) (The depths were referred from Watanabe and Ishii (2001), with little modification). Based on their placements, the polyps on the six plates were divided into four groups: upper surface and undersurface of plates at 1-m depth and upper surface and undersurface of plates at 2-m depth.
|
Fig. 1 Mooring station for polyp observations (red square) in Jiaozhou Bay. |
|
Fig. 2 Schematic diagram of the field experiment. The square of the plate is 10 cm × 10 cm, and the upper surface and lower surface of the plates were settled by Aurelia coerulea polyps. |
Plates were monitored from Oct 1, 2011 to Sep 3, 2012. Polyps on the plates were observed by transferring them in a plastic container filled with ambient seawater from the experiment site to the laboratory every 2 – 3 weeks. During the strobilation period, the polyps were monitored every 10 d. The plates were transferred to the laboratory in the evening, kept in the dark at the same temperature as the field, and sent to the field the next morning. Each plate was observed using an Olympus BX51 dissecting microscope (Olympus, Tokyo, Japan) for about 30 min. Biological characteristics (abundance, budding type, strobilation, and ephyra release) of the polyps were observed. New polyps were classified into three types (Adler and Jarms, 2009; Han and Uye, 2010): directly budded off the parental stalk, budded from the parent pedal stolon, or from longitudinal polyp fission. Hereafter, the three types of budding are abbreviated as 'direct budding', 'stolon budding' and 'fission budding', respectively.
2.3 Environmental ParametersTemperature, salinity and DO were measured using a multiparameter water quality meter (YSI HQ40d, HACH, USA). Surface seawater was collected by an organic glass hydrophore and then filtered through a cellulose acetate membrane with a pore size of 0.45 μm for chlorophyll a (Chl a) analysis. The Chl a of phytoplankton was extracted using acetone, and the concentration was determined with the fluorescence spectrophotometry method by a Turner Designs Model 7200 fluorometer. Phytoplankton were collected by an organic glass hydrophore. Zooplankton were collected by plankton net vertical tows (with a mesh size of 160 μm) from the bottom to the surface. The net samples were preserved immediately in 5% formaldehyde seawater solution. The biomasses of phytoplankton and zooplankton were counted under a microscope in the laboratory. The environmental parameter data were kindly provided by Jiaozhou Bay National Marine Ecosystem Research Station, Institute of Oceanology, Chinese Academy of Sciences.
2.4 Statistical AnalysisThe normality of distributions and homogeneity of variance of all experimental data were analyzed by the Kolmogorov-Smirnov test. The comparative analysis on the differentiation of polyp mean survivorship was tested using the Mann-Whitney U test due to unequal variances and nonnormal distribution of data. As most polyps at 2.0 m were dead before strobilation, the ratio of strobila was calculated for polyps at 1.0 m only. A T test was used to compare the difference in the ratio of strobila between polyps on the upper surface and undersurface at 1 m depth. The correlation between polyp survivorship and environmental factors was tested by Spearman correlation coefficient analysis. All statistical tests were conducted by SPSS 25.0.
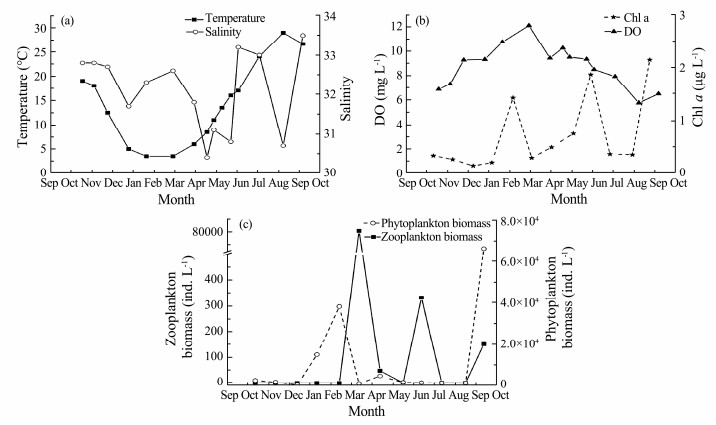
3 Results 3.1 Environmental Conditions in Jiaozhou BayThe changes in temperature, salinity, DO, Chl a, zooplankton, and phytoplankton biomass in the surface water of Jiaozhou Bay during the survey are depicted in Fig.3. The temperature of the surface water varied seasonally. The maximum temperature was 27℃ in August 2012, and the lowest temperature was 3.6℃ in February 2012. The concentration of DO in the seawater ranged from 5.74 mg L−1 to 12.08 mg L−1. From autumn to winter, the concentration of DO grew and then gradually fell in the following spring to summer. The seawater salinity was 29.6 to 32.7. The concentration of Chl a ranged from 0.14 μg L−1 (in December 2011) to 2.14 μg L−1 (in September 2012). The phytoplankton biomass rose from October to February and then dropped dramatically in the spring and summer before suddenly increasing to 6.64 × 104 ind L−1 in September 2012. The biomass of zooplankton ranged from 0.02 to 81008 ind L−1, with a peak in March.
|
Fig. 3 Variation in the environmental variables in Jiaozhou Bay. a, temperature and salinity; b, DO and Chl a; c, zooplankton and phytoplankton biomass. |
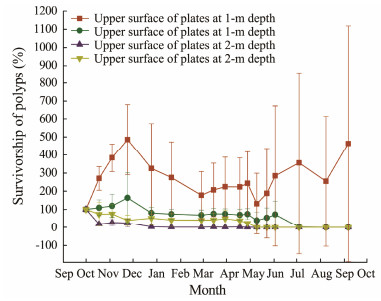
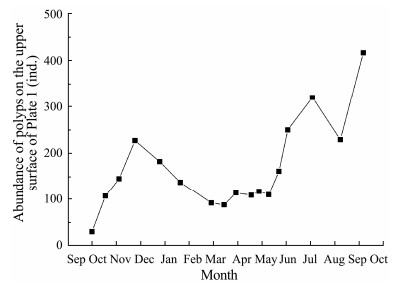
After deployment in the field, polyp abundance at 1.0-m depth increased and survived for over 8 months. The abundance of polyps at 2.0-m depth fell steadily, and all the polyps died within 6 months. The average polyp survivorship for the four groups was 270%, 69.2%, 10.6%, and 33.6%, respectively (Fig.4). The mean polyp survivorship at the 1.0-m depth was substantially greater than that at the 2.0-m depth (P < 0.01, Mann-Whitney U test). The mean polyp survivorship on the upper surfaces of plates at 1.0 m was significantly higher than those from the other three groups (P < 0.01, Mann-Whitney U test) and was approximately 245% higher than that on the upper surface at 2.0 m. Only polyps on the upper surface of Plate 1 survived in the end, exhibiting 4-stage dynamics: 1) an increase from October to December; 2) a declinefrom December to March; 3) a plateau with a low abundance from March to early May; and 4) an increase from May to September. The final abundance of polyps was 14 times higher than the initial amount (Fig.5).
|
Fig. 4 Survivorship of polyps on the upper surface and underside surface at 1 m and 2 m depths. |
|
Fig. 5 Polyp abundance on the upper surface of Plate 1. |
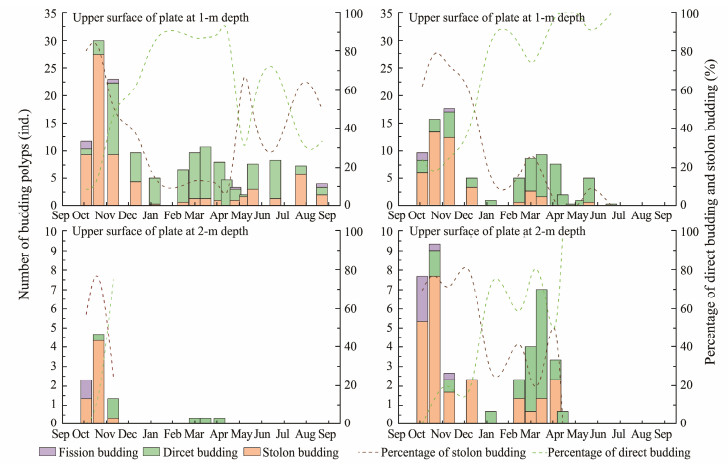
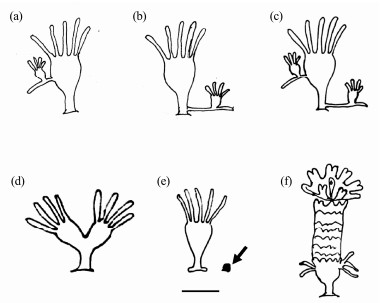
The abundance of budding polyps was higher in the first two months (in autumn) after being transferred to the field, was lower in winter, increased in spring and decreased in summer (Fig.6). The percentage of stolon budding and direct budding showed a significant shift over the survey period. Stolon budding was the most dominant budding type in October and November. Direct budding became prevalent from November and was dominant in winter and spring. The percentages of the two budding types were comparable in summer. Fission budding was rare during the whole period. Although very few polyps on the plates at 2.0-m depth survived, the budding types showed similar variation, with stolon budding predominating in the first two months and direct budding prevailing over in winter and spring.
|
Fig. 6 Proportions of three types of budding on the upper surface and underside surface at 1 m and 2 m depths. |
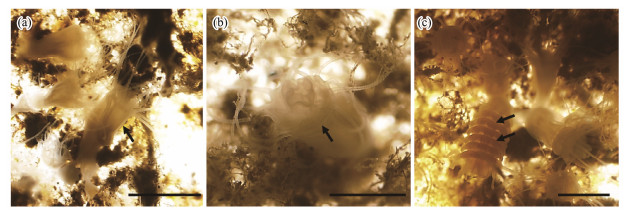
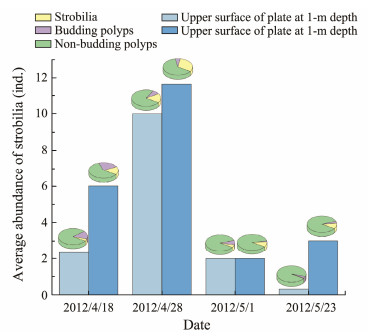
Strobilation was mainly observed in polyps at the 1.0-m depth, and most polyps at the 2.0-m depth died before strobilation. The process started on March 30, 2012, with a signal of the polyp stalk elongation (Fig.7a) and the deepening of grooves to several discs (Fig.7b). Ephyrae were formed systematically from the apical to the basal end of the polyp and progressively released starting on April 18, 2012. The strobilation lasted for 35 – 45 d and was completed on June 4, 2012. Usually, 1 – 8 discs were observed on each strobilia; however, the number would be undercounted due to the 10-day sampling interval. The amount of strobila grew substantially in April and then declined in May. The percentage of strobila on the lower surface of the plates was significantly higher than that on the upper surface (P < 0.05, T test).
|
Fig. 7 Strobilation of polyps of Aurelia coerulea. (a), prestrobilation. The stalk was elongated (arrow); (b), a strobila was forming, and the grooves were deepened (arrow); (c), the ephyrae developed orderly from the apical to basal end of the polyp (arrow). Scale bar = 1 mm. |
|
Fig. 8 Abundance and percentage of strobila on plates at 1.0-m depth. |
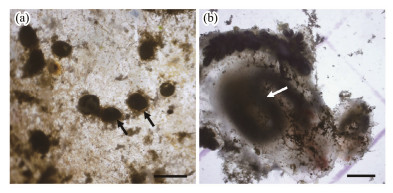
Podocysts were observed in winter (from December 2011 to January 2012), when the total number of polyps was drastically reduced (Fig.9a). The number of podocysts was not counted due to the sediment on the plates. Biofouling species, such as nudibranchs, covered the plates heavily at the same time (Fig.9b).
|
Fig. 9 Podocysts (a) of Aurelia coerulea and nudibranch (b) on the plate. Scale bar = 1 mm. |
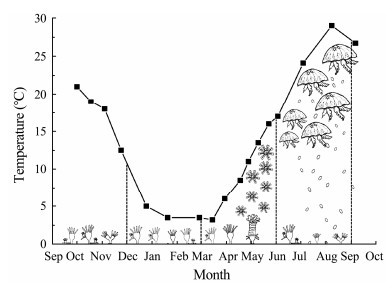
Because only polyps on the upper surface of Plate 1 survived during the entire study period, the relationships between environmental factors and polyp abundance on the upper surface of Plate 1 were investigated (Table 1). Polyp abundance increased with temperature (r = 0.618, P < 0.05) and decreased with DO concentration (r = −0.618, P < 0.05). There was no significant correlation between the abundance of polyps and salinity, zooplankton and phytoplankton biomass, or Chl a (P > 0.05). The life cycle of A. coerulea based on the temperature variations in Jiaozhou Bay is shown in Figs.10 – 11.
|
|
Table 1 Correlations between polyp survivorship on the upper surface of Plate 1 and environmental factors |
|
Fig. 10 Schematic illustration of the life cycle of Aurelia coerulea in Jiaozhou Bay, China. |
|
Fig. 11 Schematic illustration of asexual reproduction of A. coerulea (Scale bar = 1 mm). (a), direct budding off the parental stalk; (b), stolon budding from the parent pedal; (c), polyp with direct budding and stolon budding; (d), longitudinal fission budding; (e), podocyst (arrow); (f), strobiliation. |
Jellyfish use asexual reproduction to grow polyp colonies and expand their habitats. It is suggested that stolon budding aids in the rapid expansion of moon jellyfish polyps; direct budding and longitudinal fission are beneficial to daughter polyp formation (Sun et al., 2016). In the laboratory, A. coerulea polyps proliferated rapidly via direct budding in a suitable habitat and remained alive via stolon budding in food-limited conditions; daughter polyps produced by direct budding accounted for 78.6% to 94% of all polyps (Han and Uye, 2010; Lu et al., 2020). In the present study, stolon budding was the most dominant budding type in December and November. Direct budding became prevalent from November and was dominant in winter and spring. The percentages of the two budding types were comparable in summer. To compensate for mortality and maintain colonies, polyps stretch stolons into empty spaces on plates (Lucas, 2001; Miyake et al., 2002; Toyokawa et al., 2011; Feng et al., 2018). We infer that stolon budding is better than direct budding for occupying space when polyps first settle and that direct budding contributes to colony growth afterward. Budding in our study seemed to be related to the season, with stolon budding primarily occurring in the winter and direct budding primarily occurring in the spring and summer. Temperature and seasonality are associated with the proportions of direct budding and stolon buds to total buds in Koper port (Slovenia), with direct budding being highest in spring and stolon budding being most prevalent in summer (Hoevar et al., 2018; Marques et al., 2019).
The strobilation of A. coerulea usually occurs in winter and spring: in Thau (northwestern Mediterranean), it occurs from November to April (Marques et al., 2014, 2019); in Japan, it occurs primarily from December to March (Suzuki et al., 2019); and in the northern Adriatic Sea, it occurs from November to February (Di Camillo et al., 2010). A drop in temperature in the cold winter has been considered a trigger for A. coerulea strobilation. According to Marques et al. (2019), a decrease in water temperature to 8.3℃ causes strobilation in the Thau Lagoon, while a low temperature of 3.9℃ causes strobilation of A. coerulea in Jiaozhou Bay, as observed by Feng et al. (2018). Warming in the spring will hasten the process of strobilation and ephyra release (Liu et al., 2009; Feng et al., 2018). Following the yearly minimum temperature of 3.5℃, the strobilation of A. coerulea began in late February and ended in early June 2012 in the present study.
In our study, the presence of podocysts was in accordance with the population reduction, suggesting that it might be a mechanism for A. coerulea to cope with adverse environmental conditions. These cysts, which are nutrient-rich cells enclosed in a chitinous capsule, can protect themselves from predation and environmental stress, such as food scarcity (Ikeda et al., 2011). According to a laboratory study, the appearance of A. coerulea podocysts is generally accompanied by poor living conditions, such as food shortages (Arai, 2009; Thein et al., 2012). Most Scyphozoa engaged in blooming (e.g., Aurelia spp., Chrysaora spp., Cyanea spp., and Nemopilema species) can make podocysts, implying that podocysts contribute to population maintenance (Arai, 2009).
4.2 Polyp Dynamics and Their Relationship with Environmental FactorsUsually, there is a sharp decline in survivorship of newly settled polyps within the first few days, however, survivorship of polyps settled in October increased by budding up to 399% after two months observed in Tokyo Bay, Japan (Watanabe and Ishii, 2001). In the innermost of Tokyo Bay, the population of A. coerulea polyps was maintained from January to April, rapidly declined in May, and completely disappeared by June; while the population of A. coerulea polyps was maintained throughout the year in the mouth of the bay (Nagatsuka et al., 2024). In the present study, similar polyp dynamics were observed as in the innermost of Tokyo Bay. The polyps proliferated in the first two months after settled in October, declined sharply in May, and disappeared in June. However, polyps on the upper surface of plate at 1m-depth showed a trend like that in the mouth of Tokyo Bay, with polyps survived the whole year.
The number of polyps was favorably connected with temperature, according to a correlation test of the relationship between polyp survivability on the upper part of Plate 1 and environmental parameters. The seawater temperature in Jiaozhou Bay ranges from 3 – 30℃. Polyp reproduction increases within this temperature range. Several field investigations have revealed that the number of polyps increases in the summer and then steadily drops in the winter (Mutlu, 2001; Hoevar et al., 2018). Temperature influences not only polyp asexual reproduction but also polyp development indirectly by changing predator and prey abundances (Xing et al., 2020). The number of polyps in the laboratory frequently increases as the temperature rises (Han and Uye, 2010; Wang and Sun, 2015; Wang et al., 2015). Global warming has emerged as a critical factor influencing jellyfish bloom dynamics and coastal ecosystem health (Mills, 2001; Purcell, 2012). The climate change increased water temperatures, altered nutrient dynamics, and are expected to exacerbate jellyfish blooms, posing challenges to marine biodiversity, fisheries, and coastal communities. Effective management strategies, such as adaptive monitoring and predictive modeling, are essential to mitigate these impacts and maintain ecosystem resilience. Furthermore, integrating climate change considerations into jellyfish bloom management frameworks is crucial for sustainable coastal ecosystem management.
A. coerulea show a high hypoxic tolerance (Shoji et al., 2005a, b). The abundance of A. coerulea polyps was higher at the bottom of Tokyo Bay, where DO concentration was lower and seasonal hypoxia occurred (Ishii and Katsukoshi, 2010). In laboratory experiments, a reduced DO concentration to 0.2 mg L−1 increased the adhesion rate of A. coerulea planula larvae, but it resulted in polyp death due to hypoxia tolerance limits being exceeded; Under moderate hypoxic conditions (DO concentration was 2.0 – 4.5 mg L−1), the polyps were able to survive normally and continue their asexual budding reproduction (Ishii et al., 2008). Moreover, the space competitors and predators of the polyps exhibit lower tolerances to hypoxia, which enables the Aurelia aurita polyps not only to grow and reproduce normally but also to attain relatively more favorable conditions for growth and propagation (Shoji, 2008; Miller, 2009). In this study, the number of polyps was found to be negatively linked with the DO concentration. The DO concentration was at a normal level in Jiaozhou Bay. The negative correlation between the number of polyps and DO was not equivalent to stronger competitive ability under hypoxic conditions. DO in coastal and estuarine ecosystems appears to have a stratification pattern with increasing sea surface temperature (Orita et al., 2015). Therefore, the negative correlation between DO and polyp numbers may be due to temperature variations.
4.3 Polyp Survival of A. coerulea in the FieldThe polyp survival in the field is influenced by both biotic (competition, predator‒prey relationships, biofouling) and environmental factors (Lucas et al., 2012; Mineur et al., 2012). Over the last few decades, artificial structures such as pontoons and a cross-sea bridge have been built in Jiaozhou Bay, which not only provide appropriate substrates for polyps but are also frequently colonized by biofouling organisms (Dong et al., 2018). The survival of A. coerulea polyps was fluctuated with the abundance of biofouling organisms; Polyp numbers were higher in shallower waters with fewer nudibranchs, and greater nudibranch predation rates near the sea bed induced polyp extinction (Feng et al., 2017). Considerable biofouling creatures, such as nudibranchs, were observed on the plotting plates in August, in coincidence with a significant decrease in polyp abundance in present study, confirming that biofouling organisms could impact polyp survival. Nudibranch were observed settled on plates at 2.0 m depth, and not on plates at 1.0 m depth. While, polyps at 1.0 m depth grew better and survived longer than those at 2.0 m in present study. Therefore, we proposed that predator nudibranch was partially responsible for polyp death at 2.0 m depth.
Polyp survivorship was supposed to be influenced by the colony effect. The colony effect means that the polyps can eliminate open space for competitors. Polyps will die within one week if they cannot find a suitable substratum and start a new colony right away (Conley and Uye, 2015). If polyps continuously grow without any initial decrease due to competition, they are likely to survive over an extended period, and increased the population greatly (Watanabe and Ishii, 2001). In the present study, polyps at 1.0 m depth adapted to the environment successfully in the initial stage, and the colony proliferated. However, the abundance of polyps at 2.0 m depth decreased greatly after deployment to the field and ultimately died.
4.4 Life History of A. coeruleaA. coerulea has a very complex life history, using a variety of asexual reproduction methods to adapt to different environmental conditions. The life history of A. coerulea in the Thau lagoon, northwestern Mediterranean) displayed an apparent seasonality pattern. From February to April, the polyp grew following the increase of temperature and food abundance, and polyps budding led to an increase in population (Marques et al., 2019; Fernández-Alas et al., 2020). In Thau, the strobilation phase of A. coerulea lasted from early winter to early spring. A. coerulea transforms its life cycle between sexual type and asexual type in response to environmental variables (Suzuki et al., 2019).
Based on the results in this study and our early detection using molecular approaches (Wang et al., 2021), the life history of A. coerulea in Jiaozhou Bay was generalized (Fig.9). In summer (from July to August), mature medusae produce eggs and release planula. The planula searched for suitable habitats on natural hard substances or artificial structures to settle and developed into polyps. Polyps grow and enlarge populations predominantly by budding, with stolon budding occurring from October to February and direct budding occurring from March to July. The yearly lowest temperature in winter is a trigger for strobilation. Polyps began to strobilate in late February in Jiaozhou Bay, and strobilation was completed in June. The abundant zooplankton biomass peaks in May facilitates the quickly growth of the ephyra and medusae (Wan and Zhang, 2012). The medusae is matured in July, then carry out sexual reproduction.
5 ConclusionsThe seasonal dynamics of A. coerulea polyp populations in the field were investigated by deploying plates in Jiaozhou Bay. The abundance of polyps was positively correlated with seawater temperature. Direct budding and stolon budding were the main asexual reproduction types used by the polyps to proliferate their population. Stolon budding occurred dominantly in autumn, while direct budding occurred mostly in the winter and spring. The lowest temperatures in winter stimulate polyp strobilation. Polyps at a shallower depth thrived better than those at a deeper depth. The ratios of strobila to polyps on the plates' subsurfaces were much higher than those on the upper surface.
AcknowledgementsWe thank Dr. Yan Shi and Dr. Baozhou Fu for their kind help during the water sampling. Sincere thanks are given to staffs at the Jiaozhou Bay National Marine Ecosystem Research Station (http://jzb.cern.ac.cn) for providing the environmental data. The work was supported by the Scientific and Technological Innovation Project of the Laoshan Laboratory (No. LSKJ202203700) and the Beijing Natural Science Foundation (No. 8232026).
Adler, L., and Jarms, G., 2009. New insights into reproductive traits of scyphozoans: Special methods of propagation in Sanderia malayensis GOETTE, 1886 (Pelagiidae, Semaeostomeae) enable establishing a new classification of asexual reproduction in the class Scyphozoa. Marine Biology, 156: 1411-1420. DOI:10.1007/s00227-009-1181-6 (  0) 0) |
Arai, M. N., 2001. Pelagic coelenterates and eutrophication: A review. Hydrobiologia, 451(1-3): 69-87. DOI:10.1023/A:1011840123140 (  0) 0) |
Arai, M. N., 2009. The potential importance of podocysts to the formation of scyphozoan blooms: A review. Hydrobiologia, 616: 241-246. DOI:10.1007/s10750-008-9588-5 (  0) 0) |
Berrill, N. J., 1949. Developmental analysis of Scyphomedusae. Biological Reviews of the Cambridge Philosophical Society, 24(4): 393-410. DOI:10.1111/j.1469-185X.1949.tb00581.x (  0) 0) |
Brotz, L., Cheung, W. W. L., Kleisner, K., Pakhomov, E., and Pauly, D., 2012. Increasing jellyfish populations: Trends in large marine ecosystems. Hydrobiologia, 690: 3-20. DOI:10.1007/s10750-012-1039-7 (  0) 0) |
Condon, R. H., Graham, W. M., Duarte, C. M., Pitt, K. A., and Lucas, C. H., 2012. Questioning the rise of gelatinous zooplankton in the world's oceans. Bioscience, 62(2): 160-169. DOI:10.1525/bio.2012.62.2.9 (  0) 0) |
Conley, K., and Uye, S., 2015. Effects of hyposalinity on survival and settlement of moon jellyfish (Aurelia aurita) planulae. Journal of Experimental Marine Biology and Ecology, 462: 14-19. DOI:10.1016/j.jembe.2014.10.018 (  0) 0) |
Di Camillo, C. G., Betti, F., Bo, M., Martinelli, M., and Puce, S., 2010. Contribution to the understanding of seasonal cycle of Aurelia aurita (Cnidaria: Scyphozoa) scyphopolyps in the northern Adriatic Sea. Journal of the Marine Biological Association of the United Kingdom, 90(6): 1105-1110. DOI:10.1017/S0025315409000848 (  0) 0) |
Dong, Z. J., 2019. Blooms of the moon jellyfish Aurelia: Causes, consequences and controls. In: World Seas: An Environmental Evaluation. 2nd edition. Sheppard, C., ed., Academic Press, London, 163-171.
(  0) 0) |
Dong, Z. J., Sun, T. T., and Wang, L., 2018. The biogenic reefs formed by the alien polychaete Hydroides dianthus (Serpulidae, Annelida) favor the polyp stage of Aurelia coerulea (Cnidaria, Scyphozoa) in a coastal artificial lake. Marine Pollution Bulletin, 129(1): 86-91. DOI:10.1016/j.marpolbul.2018.02.016 (  0) 0) |
Feng, S., Wang, S. W., Sun, S., Zhang, F., and Zhang, G. T., 2018. Strobilation of three scyphozoans (Aurelia coelurea, Nemopilema nomurai, and Rhopilema esculentum) in the field at Jiaozhou Bay, China. Marine Ecology Progress Series, 591: 141-153. DOI:10.3354/meps12276 (  0) 0) |
Feng, S., Wang, S. W., Zhang, G. T., Sun, S., and Zhang, F., 2017. Selective suppression of in situ proliferation of scyphozoan polyps by biofouling. Marine Pollution Bulletin, 114(2): 1046-1056. DOI:10.1016/j.marpolbul.2016.10.062 (  0) 0) |
Fernández-Alías, A., Marco, S. C., Quispe, J. I., Sabah, S., and Pérez-Ruzafa, A., 2020. Population dynamics and growth in three scyphozoan jellyfishes, and their relationship with environmental conditions in a coastal lagoon. Estuarine, Coastal and Shelf Science, 243(5): 106901. DOI:10.1016/j.ecss.2020.106901 (  0) 0) |
Granéli, E., and Turner, J. T., 2002. Top-down regulation in ctenophore-copepod-ciliate-diatom-phytoflagenate communities in coastal waters: A mesocosm study. Marine Ecology Progress Series, 239(4): 57-68. DOI:10.3354/meps239057 (  0) 0) |
Guy-Haim, T., Rubin-Blum, M., Rahav, E., Belkin, N., and Guy, S. V., 2020. The effects of decomposing invasive jellyfish on biogeochemical fluxes and microbial dynamics in an ultraoligotrophic sea. Biogeosciences, 17: 5489-5511. DOI:10.5194/bg-17-5489-2020 (  0) 0) |
Han, C. H., and Uye, S. I., 2010. Combined effects of food supply and temperature on asexual reproduction and somatic growth of polyps of the common jellyfish Aurelia aurita s. l. Plankton & Benthos Research, 5(3): 98-105. DOI:10.3800/pbr.5.98 (  0) 0) |
He, J. R., Zheng, L. M., Zhang, W. J., and Lin, Y. S., 2015. Life cycle reversal in Aurelia sp. 1 (Cnidaria, Scyphozoa). PLoS One, 10(12): e0145314. DOI:10.1371/journal.pone.0145314 (  0) 0) |
Helm, R. R., 2018. Evolution and development of scyphozoan jellyfish. Biological Reviews, 93(2): 1228-1250. DOI:10.1111/brv.12393 (  0) 0) |
Hoevar, S., Malej, A., Boldin, B., and Purcell, J. E., 2018. Seasonal fluctuations in population dynamics of Aurelia aurita polyps in situ with a modelling perspective. Marine Ecology Progress Series, 591: 155-166. DOI:10.3354/meps12387 (  0) 0) |
Ikeda, H., Ohtsu, K., and Uye, S. I., 2011. Fine structure, histochemistry, and morphogenesis during excystment of the podocysts of the giant jellyfish Nemopilema nomurai (Scyphozoa, Rhizostomeae). Biological Bulletin, 221(3): 248-260. DOI:10.1086/BBLv221n3p248 (  0) 0) |
Ishii, H., and Katsukoshi, K., 2010. Seasonal and vertical distribution of Aurelia aurita polyps on a pylon in the innermost part of Tokyo Bay. Journal of Oceanography, 66(3): 329-336. DOI:10.1007/s10872-010-0029-5 (  0) 0) |
Ishii, H., Ohba, T., and Kobayashi, T., 2008. Effects of low dissolved oxygen on planula settlement, polyp growth and asexual reproduction of Aurelia aurita. Plankton & Benthos Research, 3: 107-113. DOI:10.3800/pbr.3.107 (  0) 0) |
Liu, W. C., Lo, W. T., Purcell, J. E., and Chang, H. H., 2009. Effects of temperature and light intensity on asexual reproduction of the scyphozoan, Aurelia aurita (L. ) in Taiwan. Hydrobiologia, 616: 247-258. DOI:10.1007/s10750-008-9597-4 (  0) 0) |
Lu, Y., Lucas, C. H., and Loveridge, A., 2020. Transgenerational acclimation influences asexual reproduction in Aurelia aurita jellyfish polyps in response to temperature. Marine Ecology Progress Series, 656: 35-50. DOI:10.3354/meps13517 (  0) 0) |
Lucas, C. H., 2001. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia, 451: 229-246. DOI:10.1023/A:1011836326717 (  0) 0) |
Lucas, C. H., Graham, W. M., and Widmer, C., 2012. Jellyfish life histories: Role of polyps in forming and maintaining scyphomedusa populations. Advances in Marine Biology, 63: 133-196. DOI:10.1016/B978-0-12-394282-1.00003-X (  0) 0) |
Malej, A., Kogovšek, T., Ramšak, A., and Catenacci, L., 2012. Blooms and population dynamics of moon jellyfish in the northern Adriatic. Cahiers de Biologie Marine, 53: 337-342. DOI:10.1063/1.446711 (  0) 0) |
Marques, R., Albouy-Boyer, S., Delpy, F., Carré, C., and Le Floc'h, É., 2014. Pelagic population dynamics of Aurelia sp. in French Mediterranean lagoons. Journal of Plankton Research, 37(5): 1019-1035. DOI:10.1093/plankt/fbv059 (  0) 0) |
Marques, R., Darnaude, A. M., Schiariti, A., Tremblay, Y., and Molinero, J. C., 2019. Dynamics and asexual reproduction of the jellyfish Aurelia coerulea benthic life stage in the Thau lagoon (northwestern Mediterranean). Marine Biology, 166(6): 1-14. DOI:10.1007/s00227-019-3522-4 (  0) 0) |
Miller, M. E. C., 2009. Settlement and survival of the scyphozoan Aurelia sp. in response to hypoxia in the northern Gulf of Mexico. PhD thesis. University of South Alabama.
(  0) 0) |
Mills, C. E., 2001. Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions?. Hydrobiologia, 451(1-3): 55-68. DOI:10.1023/A:1011888006302 (  0) 0) |
Mineur, F., Cook, E. J., Minchin, D., Bohn, K., and MacLeod, A., 2012. Changing coats: Marine aliens and artificial structures. Oceanography and Marine Biology, 50(50): 189-233. DOI:10.1201/b12157-5 (  0) 0) |
Miyake, H., Terazaki, M., and Kakinuma, Y., 2002. On the polyps of the common jellyfish Aurelia aurita in Kagoshima Bay. Journal of Oceanography, 58: 451-459. DOI:10.1023/A:1021628314041 (  0) 0) |
Mutlu, E., 2001. Distribution and abundance of the moon jellyfish (Aurelia aurita) and its food zooplankton in the Black Sea. Marine Biology, 138: 329-339. DOI:10.1007/s002270000459 (  0) 0) |
Nagatsuka, S., Ishii, H., Morimitsu, R., and Yasuda, A., 2024. In situ population dynamics of Aurelia coerulea polyps in Tokyo Bay. DOI: 10.21203/rs.3.rs-4113778/v1
(  0) 0) |
Orita, R., Umehara, A., Komorita, T., Choi, J. W., Montani, S., et al., 2015. Contribution of the development of the stratification of water to the expansion of dead zone: A sedimentological approach. Estuarine Coastal and Shelf Science, 164: 204-213. DOI:10.1016/j.ecss.2015.07.028 (  0) 0) |
Pitt, K. A., Kingsford, M. J., Rissik, D., and Koop, K., 2007. Jellyfish modify the response of planktonic assemblages to nutrient pulses. Marine Ecology Progress Series, 351: 1-13. DOI:10.3354/meps07298 (  0) 0) |
Pitt, K. A., Lucas, C. H., Condon, R. H., Duarte, C. M., and Stewart-Koster, B., 2018. Claims that anthropogenic stressors facilitate jellyfish blooms have been amplified beyond the available evidence: A systematic review. Frontiers in Marine Science, 5: 1-11. DOI:10.3389/fmars.2018.00451 (  0) 0) |
Purcell, J. E., 2012. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annual Review of Marine Science, 4: 209-235. DOI:10.1146/annurev-marine-120709-142751 (  0) 0) |
Purcell, J. E., 2007. Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Marine Ecology Progress Series, 348: 183-196. DOI:10.3354/meps07056 (  0) 0) |
Purcell, J. E., Bondyale-Juez, D. R., Romero-Kutzner, V., Martínez, I., and Caprioli, R., 2019. Food supply effects on the asexual reproduction and respiratory metabolism of Aurelia aurita polyps. Hydrobiologia, 846: 135-146. DOI:10.1007/s10750-019-04057-4 (  0) 0) |
Purcell, J. E., Uye, S. I., and Lo, W. T., 2007. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Marine Ecology Progress Series, 350: 153-174. DOI:10.3354/meps07093 (  0) 0) |
Schiariti, A., Morandini, A. C., Jarms, G., Paes, R. V. G., and Franke, S., 2014. Asexual reproduction strategies and blooming potential in Scyphozoa. Marine Ecology Progress Series, 510: 241-253. DOI:10.3354/meps10798 (  0) 0) |
Shoji, J., Masuda, R., Yamashita, Y., and Tanaka, M., 2005a. Effect of low dissolved oxygen concentrations on behavior and predation rates on Red Sea bream Pagrus major larvae by the jellyfish Aurelia aurita and by juvenile Spanish mackerel Scomberomorus niphonius. Marine Biology, 147: 863-868. DOI:10.1007/s00227-005-1579-8 (  0) 0) |
Shoji, J., Masuda, R., Yamashita, Y., and Tanaka, M., 2005b. Predation on fish larvae by moon jellyfish Aurelia aurita under low dissolved oxygen concentrations. Fisheries Science, 71(4): 748-753. DOI:10.1111/j.1444-2906.2005.01024.x (  0) 0) |
Shoji, J., 2008. Non-size-selective predation on fish larvae by moon jellyfish Aurelia aurita under low oxygen concentrations. Plankton & Benthos Research, 3: 114-117. DOI:10.3800/pbr.3.114 (  0) 0) |
Suzuki, K. S., Suzuki, K. W., Kumakura, E., Sato. K., and Oe, Y., 2019. Seasonal alternation of the ontogenetic development of the moon jellyfish Aurelia coerulea in Maizuru Bay, Japan. PLoS One, 14(11): 1-22. DOI:10.1371/journal.pone.0225513 (  0) 0) |
Sweetman, A. K., Chelsky, A., Pitt, K. A., Andrade, H., and Oevelen, D. V., 2016. Jellyfish decomposition at the seafloor rapidly alters biogeochemical cycling and carbon flow through benthic food-webs. Limnology and Oceanography, 61: 1449-1461. DOI:10.1002/lno.10310 (  0) 0) |
Thein, H., Ikeda, H., and Uye, S. I., 2012. The potential role of podocysts in perpetuation of the common jellyfish Aurelia aurita s. l. (Cnidaria: Scyphozoa) in anthropogenically perturbed coastal waters. Hydrobiologia, 690(1): 157-167. DOI:10.1007/s10750-012-1045-9 (  0) 0) |
Tinta, T., Klun, K., and Herndl, G. J., 2021. The importance of jellyfish-microbe interactions for biogeochemical cycles in the ocean. Limnology and Oceanography, 66: 2011-2032. DOI:10.1002/lno.11741 (  0) 0) |
Toyokawa, M., Aoki, K., Yamada, S., Yasuda, A., and Murata, Y., 2011. Distribution of ephyrae and polyps of jellyfish Aurelia aurita (Linnaeus 1758) sensu lato in Mikawa Bay, Japan. Journal of Oceanography, 67(2): 209-218. DOI:10.1007/s10872-011-0021-8 (  0) 0) |
Uye, S. I., 2011. Human forcing of the copepod-fish-jellyfish triangular trophic relationship. Hydrobiologia, 666(1): 71-83. DOI:10.1007/s10750-010-0208-9 (  0) 0) |
Wan, A., and Zhang, G., 2012. Annual occurrence of moon jellyfish Aurelia sp. 1 in the Jiaozhou Bay and its impacts on zooplankton community. Oceanologia et Limnologia Sinica, 43(3): 494-501 (in Chinese with English abstract). DOI:10.11693/hyhz201203014014 (  0) 0) |
Wang, J. Y., Mi, T. Z., Yu, Z. G., Wang, G. S., and Wei, Q. S., 2021. Species-specific detection and quantification of Scyphomedusae in Jiaozhou Bay, China, using a quantitative real-time PCR assay. Journal of Oceanology and Limnology, 39(4): 1360-1372. DOI:10.1007/s00343-020-0160-0 (  0) 0) |
Wang, Y. T., and Sun, S., 2015. Population dynamics of Aurelia sp. 1 ephyrae and medusae in Jiaozhou Bay, China. Hydrobiologia, 754: 147-155. DOI:10.1007/s10750-014-2021-3 (  0) 0) |
Wang, Y. T., Zheng, S., Sun, S., and Zhang, F., 2015. Effect of temperature and food type on asexual reproduction in Aurelia sp. 1 polyps. Hydrobiologia, 754: 169-178. DOI:10.1007/s10750-014-2020-4 (  0) 0) |
Watanabe, T., and Ishii, H., 2001. In situ estimation of ephyrae liberated from polyps of Aurelia aurita using settling plates in Tokyo Bay, Japan. Hydrobiologia, 451: 247-258. DOI:10.1023/A:1011856929443 (  0) 0) |
West, E. J., Pitt, K. A., Welsh, D. T., Koop, K., and Rissik, D., 2009. Top-down and bottom-up influences of jellyfish on primary productivity and planktonic assemblages. Limnology and Oceanography, 54(6): 2058-2071. DOI:10.4319/lo.2009.54.6.2058 (  0) 0) |
Willcox, S., Moltschaniwskyj, N. A., and Crawford, C. M., 2008. Population dynamics of natural colonies of Aurelia sp. Scyphistomae in Tasmania, Australia. Marine Biology, 154: 661-670. DOI:10.1007/s00227-008-0959-2 (  0) 0) |
Xiao, W. P., Zeng, Y., Liu, X., Huang, X. G., and Chiang, K. P., 2019. The impact of giant jellyfish Nemopilema nomurai blooms on plankton communities in a temperate marginal sea. Marine Pollution Bulletin, 149: 110507. DOI:10.1016/j.mar-polbul.2019.110507 (  0) 0) |
Xing, Y. Z., Liu, Q., Zhang, M., Zhen, Y., and Mi, T. Z., 2020. Effects of temperature and salinity on the asexual reproduction of Aurelia coerulea polyps. Journal of Oceanology and Limnology, 38(1): 133-142. DOI:10.1007/s00343-019-8337-0 (  0) 0) |
 2024, Vol. 23
2024, Vol. 23


