2) Institute of Fisheries Science, Tibet Academy of Agricultural and Animal Husbandry Sciences, Lhasa 850002, China;
3) Key Laboratory of Tropical Marine Bio-Resources and Ecology, Chinese Academy of Sciences, Guangzhou 510301, China
Mitochondria are a subcellular organelle with important biochemical functions, which is the powerhouse of the eukaryotic cell. The mitochondrial genome is located within the organelle, which is independent of the nuclear genome but with a close relationship with each other (Wolstenholme, 1992). The typical animal mitogenome is a closed circular molecule of 14–20 kilobases (kb) containing 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs), two ribosomal RNA genes (12S and 16S), and an AT-rich region (also known as the control region, CR) (Boore, 1999). At present, mitochondrial DNA is considered an efficient and reliable molecular marker for phylogenetic studies (Wang et al., 2008). Given that amplification is non-recombining, fast evolving, and relatively easy, the circular mitochondrial genomes have been widely used in the studies of phylogenetics, population genetics, and evolutionary genomics (Ne et al., 2011; Tan et al., 2015). In addition, the number of complete mitochondrial genomes increases rapidly on the basis of next-generation sequencing technology, which allows a comprehensive understanding of the complete mitogenome (Fendt et al., 2009; Wang et al., 2020).
Branchiopoda, derived from the Cambrian of the Paleozoic, comprises two subclasses and three orders: Sarsostraca, including Anostraca; Phyllopoda, including Notostraca and Diplostraca (Joel, 1995). In addition, Brendonck et al. (2008) found that several Branchiopod species were endangered because of the reduced quantity and quality of temporary wetlands. Meanwhile, the inter-ordinal and evolutionary relationships of many Branchiopods with low-level taxa remain unclear (Brendonck et al., 2008). Previously, considerable research preferred to construct taxonomy and phylogenetics of Branchiopods using the morphology of sex symbols rather than mitogenomes (Linder, 1941). These factors have posed a great challenge to researchers in the study of Branchiopod species. Recently, several researchers have attempted to establish phylogenetic relationships to solve particularly systematic problems of high-level taxa in Branchiopods using mitogenomes (Olesen, 1998; Liu et al., 2015; Tokishita et al., 2017; Bellec et al., 2019; Tladi et al., 2020). However, these mitogenome phylogenetic studies have included only a few representatives of the diversity of Branchiopoda because of the meager mitogenome sequences for most order-level taxa in Branchiopoda. Therefore, re-establishing Branchiopod phylogenetic relationship using suitable species is necessary.
Daphniopsis tibetana belongs to Crustacea, Branchiopoda, Diplostraca, Cladocera, and Daphniidae. It is the dominant zooplankton and apex species in many high-altitude (4000 m) salt lakes (Zhao et al., 2016; Wang et al., 2019). In addition, D. tibetanacan adapt to saline water, whereas other species in Cladocera can only inhabit in freshwater (Zhao et al., 2004). Therefore, it plays a role in the study of Cladocerans (Zhao and Li, 2012). The phylogenetic status of D. tibetanawill provide a comprehensive understanding of the lake ecosystem. At present, the ecological distribution (Zhao et al., 2002), morphological characterization (Zhao and Wang, 2005), nutritional analysis (Zhao et al., 2006), oxygen consumption rate (Zhao et al., 2007), and chromosome karyotype (Zhao et al., 2004) of D. tibetana were investigated. Moreover, the molecular approach of investigation has focused on D. tibetana. Colbourne et al. (2006) examined the molecular genetic divergence and evolution of Daphnia and Daphniopsis on the basis of COI, 16S rDNA, and 12S rDNA sequences, indicating that D. tibetanashould be classified in the genus Daphnia. However, Zhao et al. (2011) constructed a phylogenetic tree based on 18S rDNA and found that the taxonomic level of D. tibetanawas closer to Daphniopsis than to Daphnia. Since then, the systematic classification of D. tibetana has been controversial for a long time.
As the largest order of the Branchiopoda class, the relationship among branches in Diplostraca is unclear (Olesen, 1998; Braband et al., 2002; Dewaard et al., 2006). Moreover, the phylogenetic significance based on the complete mitochondrial sequence of D. tibetanahas not been investigated because of the lack of mitogenome sequences in Diplostraca. In this study, the first complete mitogenome sequences of D. tibetanawere sequenced, annotated, and characterized. The phylogenetic tree (Bayesian inference (BI) and maximum likelihood (ML)) was constructed on the basis of 13 PCGs to assess the systematic position of D. tibetanain Diplostraca and provide new insights into the phylogenetic implications for Branchiopoda. Therefore, the complete mitogenome of D. tibetanawill provide a comprehensive understanding of the phylogenetic relationships within Branchiopoda.
2 Materials and Methods 2.1 Sample Collection and DNA ExtractionAn adult specimen of D. tibetanawas collected from a saline lake in Tibet, China (30°44'13.1''N, 90°33'33''E). The specimen was then stored in 95% ethanol until DNA extraction. Whole genomic DNA was extracted from a single specimen using an improved method and multi-well plates according to the manufacturer's instructions (Yue and Orban, 2010). Finally, the extracted DNA was stored at −20℃ until used for PCR amplification.
2.2 Mitogenome Sequencing and AssemblyThe complete mitogenome of D. tibetanawas sequenced using next-generation sequencing. First, iTru adaptors and primers and the KAPA Hyper Plus kit were used to construct an Illumin library following the manufacturer's protocol (Glenn et al., 2019). Then, the library was sequenced on an Illumina HisSeq 4000 PE150. In addition, the raw reads were assessed and cleaned using FastQC and Trimmomatic, respectively (Bolger et al., 2014; Brandine and Smith, 2019). Finally, NOVOPlasty (Dierckxsens et al., 2017) was used to assemble the paired reads. Moreover, De Novo Assemble, Map to Reference, and BLAST were used in accordance with Geneious 11.1.2 to verify the resulting mitogenome (Altschul et al., 1997; Ripma et al., 2014).
2.3 Sequence Annotation and AnalysisFor 37 mitochondrial genes, 13 PCGs were annotated using Sequin version 15.10 (http://www.ncbi.nlm.nih.gov/Sequin) and MITOS2 (Bernt et al., 2013) and compared with other Diplostraca mitochondrial genomes from Genbank. NCBI-BLAST (http://blast.ncbi.nlm.nih.gov) and tRNAscan-SE 1.21 (Schattner et al., 2005) were used to identify the boundaries of rRNA and tRNA genes, respectively. The mitochondrial genome map was constructed using OGDRAW (https://chlorobox.mpimp-golm.mpg.de/OGDraw.html) (Lohse et al., 2007). The secondary tRNA structure was predicted through the MITOS webserver (Bernt et al., 2013). MEGA (version 10.1.8) (Sudhir et al., 2018) was used to analyze the nucleotide composition and relative synonymous codon usage (RSCU). The bias of nucleotide composition was calculated using the following formulas (Perna and Kocher, 1995):
| $ {\text{AT - skew}} = ({\text{A}} - {\text{T}})/({\text{A}} + {\text{T}}), $ |
| $ {\text{GC - skew}} = ({\text{G}} - {\text{C}})/({\text{G}} + {\text{C}}). $ |
Phylogenetic analysis was conducted on the basis of the nucleotide sequences of 13 PCGs using BI and ML to assess the systematic position of D. tibetana. 27 complete mitogenome sequences of Branchiopoda downloaded from the GenBank database (https://www.ncbi.nlm.nih.gov/genbank) and one newly determined sequence (D. tibetana) were used to reconstruct the phylogenetic relationships among Branchiopoda (Table 1). In addition, two species from Archosauria were used as outgroups (Table 1). First, PhyloSuite (Zhang et al., 2020) was used to extract nucleotide sequences of 13 PCGs for each species from GenBank files. Then, the MAFFT program (Toh, 2010) integrated into PhyloSuite was used to align the multiple sequences in normal-alignment mode. Moreover, the alignment results were imported to Gblocks (Gerard and Jose, 2007) to identify and remove ambiguously aligned regions. Afterward, the sequences were concatenated and used to generate input files (phylip and nexus format) for phylogenetic analyses. ModelFinder (Kalyaanamoorthy et al., 2017) was used to select the best-fit model based on the Bayesian information criterion. The best-fit models, namely, TVM + F + R5 and GTR + F + I + G4, were selected to perform ML and BI analyses, respectively. Next, ML analysis was conducted in IQ-TREE (Lam et al., 2015) using an ML + rapid bootstrap algorithm with 1000 replicates (Guindon et al., 2010). Furthermore, BI analysis was performed in MrBayes 3.2.6 (Nylander et al., 2004) using default parameters and 3 × 106 Metropolis-coupled Markov Chain Monte Carlo generations and sampled every 100 generations with a burn-in of 25%. The average standard deviation of split frequencies below 0.01 was considered to reach convergence. Finally, the resulting phylogenetic trees were visualized through iTOL (https://itol.embl.de/) (Letunic and Bork, 1988).
|
|
Table 1 List of 27 Branchiopoda species and two outgroups used in this paper |
The complete mitogenome of D. tibetanais a closed circular molecule, which is 16196 bp long (GenBank accession number MW981579). This mitogenome contains 13 PCGs, two rRNAs, 22 tRNAs, and a putative CR, which is consistent with most published studies on Diplostraca (Fig.1, Table 2) (Crease, 1999; Liu et al., 2017). In addition, most of the mitochondrial genes are distributed on the heavy (H-) strand except for four PCGs (nad5, nad4, nad4l, and nad1), eight tRNAs (trnQ, trnC, trnY, trnF, trnH, trnP, trnL1, and trnV), and two rRNAs, which are encoded on the light (L-) strand (Table 2). Similar to other invertebrate mtDNAs, overlapping (nine overlaps totaling 48 bp) and non-coding bases among genes are found (Table 2) (Wang et al., 2020). The overall base composition of D. tibetana is 29.6% A, 33.2% T, 18.2% C, and 19.0% G. Calculations show that the GC-skew value and AT-skew value are 0.021 and −0.059, respectively (Table 3). The results suggest that AT base migration primarily occurs in the hysteretic chain, whereas GC base migration primarily occurs in the leading chain, which is similar to other Diplostraca species (Bellec et al., 2019). Moreover, the composition of nucleotide is highly A + T biased (62.80%), as exhibited in other Diplostraca mitogenomes (Tokishita et al., 2017). Furthermore, the A + T contents of PCGs, tRNAs, and rRNAs are 61.2%, 65.6%, and 68.2%, respectively (Table 3).
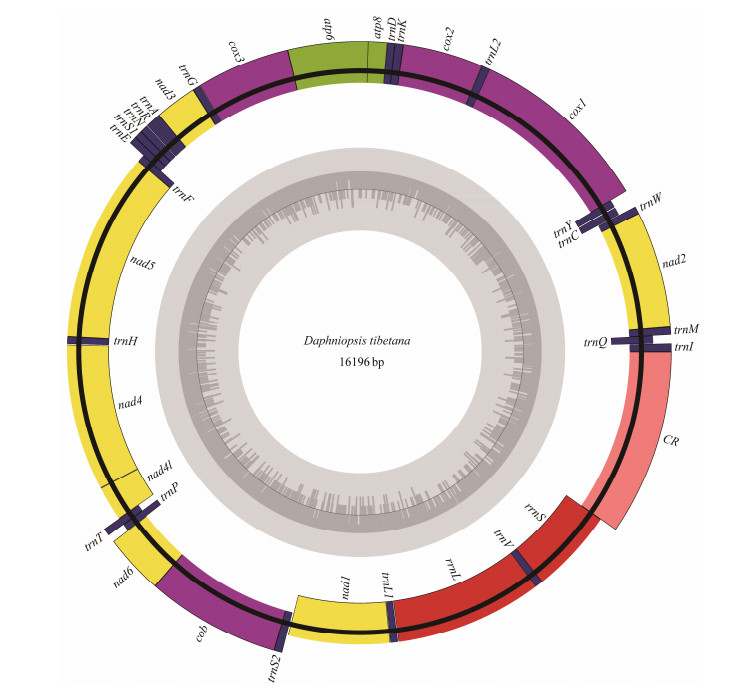
|
Fig. 1 Gene map of the D. tibetana mitogenome. Genes encoded on the heavy or light strands are shown at the outside or inside of the circular gene map, respectively. |
|
|
Table 2 Features of the mitochondrial genome of D. tibetana |
|
|
Table 3 Composition and skewness of D. tibetana mitogenome |
The 13 PCGs of this sequence is 11059 bp long, containing seven NADH dehydrogenases (nad1–nad6 and nad4l), three cytochrome c oxidases (cox1–cox3), two ATPases (atp6 and atp8), and one cytochrome b (cob), which were also found in previous studies on Diplostraca species (Table 2) (Crease, 1999; Liu et al., 2017). The length of the PCGs ranges from 162 bp (atp8) to 1707 bp (nad5) (Table 2), encoding a total of 3674 amino acids. The total AT bias of 13 PCGs in D. tibetanais 62.80%, ranging from 59.0% (cox1) to 71.0% (atp8) (Table 3). Furthermore, all AT-skew of 13 PCGs show notable negative values, and most GC-skew values are positive, indicating that Ts and Gs are more than As and Cs (Table 3). Of the 13 PCGs, six are initiated by the canonical start codon ATG (cox2, atp8, atp6, cox3, nad4, and cob), while two use GTG (nad2 and nad5), four use ATT (nad1, nad3, nad4l, and nad6), and one use TTG (cox1). These rare start codons have been found in other animals (Wolstenholme, 1992), and they are similar to some Daphnia species (Tokishita et al., 2017). For the stop codon, 10 PCGs perform the routine termination codon (TAA or TAG), whereas three other PCGs (cox2, nad5, and nad4) are with an incomplete stop codon T or TA (Table 2). Stop codons are consistent with other Diplostraca species (Liu et al., 2017).
Among the 3674 amino acids encoded by 13 PCGs, the frequently used amino acids include Leu (16.03%), Ser (9.03%), Phe (8.60%), and Val (8.21%). By contrast, the least common amino acids include Glu (2.06%), Arg (1.79%), Gln (1.79%), and Cys (0.10%) (Fig.2A, Table 4). In addition, RSCU analysis shows that the frequently used codons are UCU(S), UUA(L), and CCU(P), whereas CUC(L), ACG(T), CCG(P), and AGG(S) are the least often used codons (Fig.2B, Table 4). The preference for NNU codons can be found in the mitochondrial PCGs, which is similar to other invertebrate animals (Wang et al., 2015; Zhang et al., 2020).
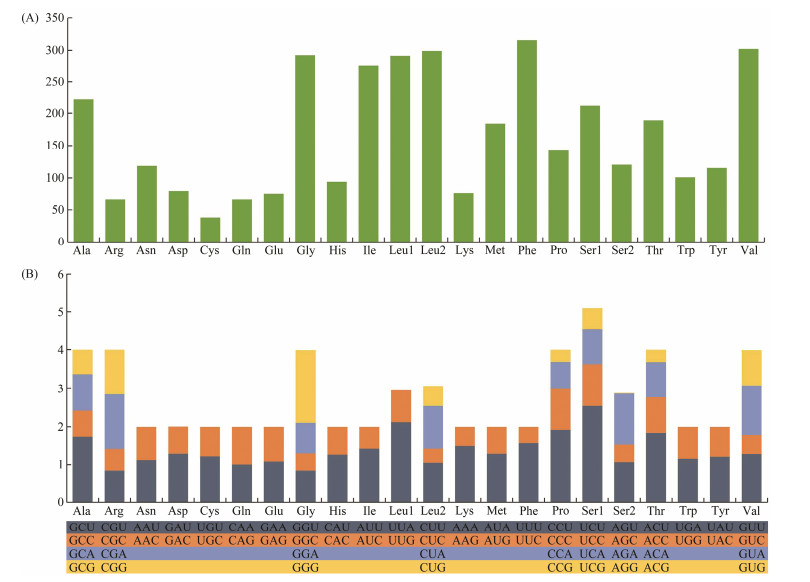
|
Fig. 2 Amino acid composition (A) and relative synonymous codon usage (B) in the mitogenome of D. tibetana. |
|
|
Table 4 Codon number and relative synonymous codon usage (RSCU) of 13 PCGs in D. tibetana mitogenome |
A total of 22 tRNAs (total length 1458 bp) are identified in the mitogenome of D. tibetana, ranging from 63 bp (trnG) to 71 bp (trnC and trnV) in length (Table 2). The AT bias of 22 tRNAs is high (65.6%). The AT-skew (−0.017) of tRNAs is lower than the GC-skew (0.064), indicating that Ts and Gs are more than As and Cs (Table 3). Eight tRNAs (trnQ, trnC, trnY, trnF, trnH, trnP, trnL1, and trnV) are encoded by the L-strand, whereas the other tRNAs are encoded by the H-strand. Moreover, only trnS1 (TCT) lacks a dihydrouridine arm when most tRNAs can fold into the typical cloverleaf structure (Fig.3), which is a common feature in vertebrate and invertebrate mitogenomes (Fig.3) (Zhang et al., 2019; Lu et al., 2020). Based on the Watson-Crick base pair (A–T and G–C) matches (Holbrook et al., 1991), a total of 15 unmatched base pairs (G–U pairs) are found in the D. tibetanamitochondrial tRNA genes (trnA, trnQ, trnR, trnD, trnC, trnQ, trnG, trnH, trnI, trnL1, trnM, trnP, trnS1, trnW, trnY, and trnV; Fig.3), forming a weak bond.
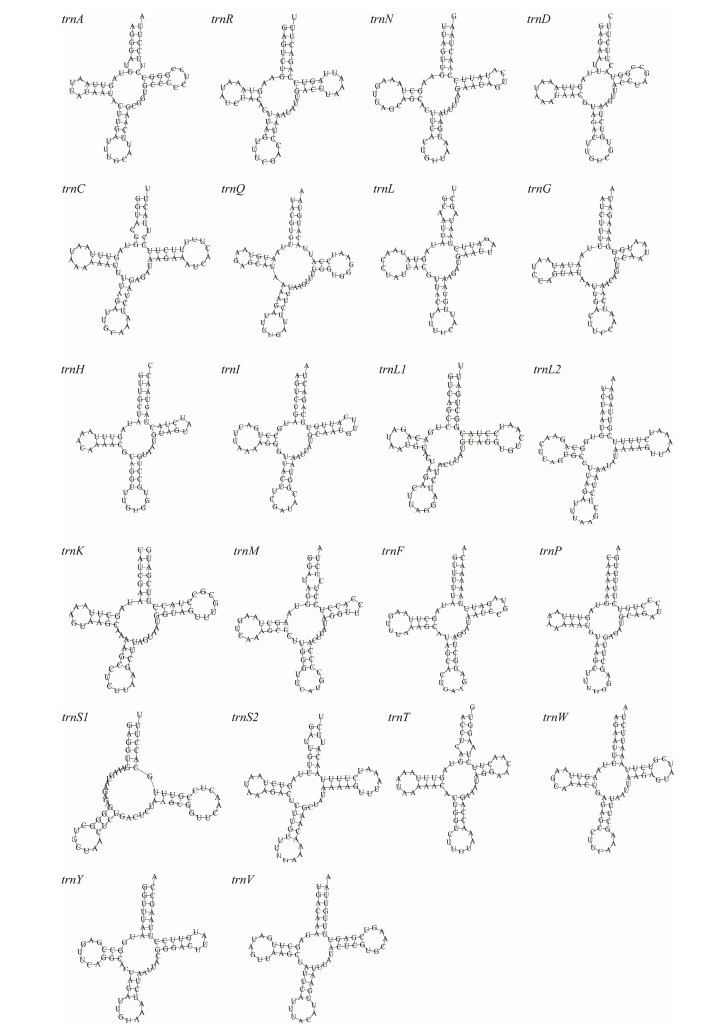
|
Fig. 3 Potential secondary structures of 22 inferred tRNAs in D. tibetana mitogenome. |
As shown in Table 2, the mitogenome of D. tibetana contains two rRNAs, rrnL and rrnS, which are 1343 and 756 bp long. They are typically separated by trnV, and both rRNAs are located on the L-strand (Table 2), which are consistent with most invertebrate mitogenomes (Kim et al., 2016; Lu et al., 2020). The total A + T content of two rRNAs is 68.2%, and the AT-skew and GC-skew exhibit a positive value (0.017 and 0.109, Table 3), indicating that As and Gs are more than Ts and Cs.
The CR of the mitogenome is located between rrnS and trnI. In comparison with rRNA and PCGs genes, CR is the dominant region for evaluating intraspecies genetic variations caused by the high variation and mutation rates throughout the mitogenome (Parsons et al., 1997; Norman et al., 2010). The 1569 bp CR is evidently AT-biased (64.2%), and the AT-skew and GC-skew in CR are −0.001 and −0.100, respectively, indicating an evident bias toward the use of Ts and Cs.
3.4 Phylogenetic AnalysisTwo phylogenetic trees (ML and BI) were constructed on the basis of the sequences of 13 PCGs, including 28 species of Branchiopoda and two outgroup species, to investigate the phylogenetic position of D. tibetanawithin Branchiopoda. ML and BI trees show an identical topology; thus, only one topology (ML) with both support values is displayed. However, the BI tree has a higher support value than the ML tree (Fig.4). The phylogenetic trees show that the class Branchiopoda is divided into three major clades (Diplostraca, Anostraca, and Notostraca) and nine families. First, the order Anostraca, including A. urmiana, A. tibetiana, A. salina, A. franciscana, A. sinica, B. kugenumaensis, S. cafer, S. sirindhornae, P. tserensodnomi, and E. grubii, belong to four families (Chirocephalidae, Thamnocephalidae, Streptocephalidae, and Artemiidae), which show a sister relationship with Notostraca and Diplostraca. Meanwhile, the relationship among families Chirocephalidae, Thamnocephalidae, and Streptocephalidae is closer than the relationship with Artemiidae. In this clade of Anostraca, most terminal relationships are well resolved, which is consistent with the previous studies (Daniels et al., 2015; Bellec et al., 2019). Then, Notostraca with its two representative genera Triops (Schrank, 1803) and Lepidurus (Leach, 1819), belonging to the family Triopsidae, is considered as a monophyletic group in the present mitogenomic phylogeny, which was also proven by Longhurst (1955) and Brendonck et al. (2008).
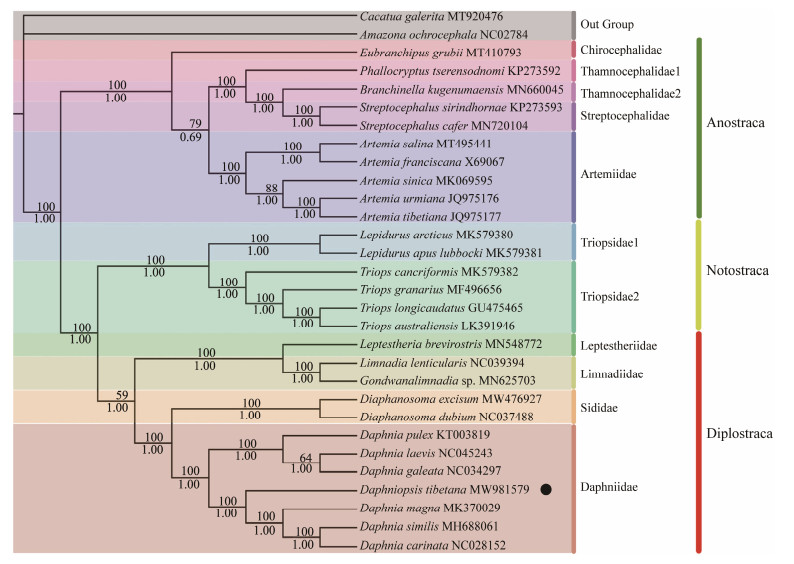
|
Fig. 4 Phylogenetic tree of Branchiopoda species inferred from the nucleotide sequences of 13 PCGs based on maximum likelihood (ML) and Bayesian inference (BI) analyses. The dots indicate the species studied in this paper. |
In addition, three clades (Anostraca, Notostraca, and Diplostraca) in this phylogenetic tree show high support values except for Diplostraca (posterior probabilities = 1; bootstrap = 59; Fig.4), indicating that the target species (D. tibetana) in Diplostraca is not highly differentiated. At the bottom of the tree, there are 12 Diplostraca species, including L. brevirostris, L. lenticularis, Gondwanalimnadia sp., D. excisum, D. dubium, D. pulex, D. laevis, D. galeata, D. tibetana, D. magna, D. similis, and D. carinata. They are separated into four clades, Daphniidae, Sididae, Limnadiidae, and Leptestheriidae. Seven species (D. pulex, D. laevis, D. galeata, D. tibetana, D. magna, D. similis, and D. carinata) cluster together as the main clade Daphniidae species. The results are similar to the previous phylogenetic tree of Bellec et al. (2019). BI and ML trees show that D. tibetanais close to D. magna, D. similis, and D. carinata, forming a sister group at the end of the trees, which belongs to Diplostraca and Daphniidae. On the contrary, Sars (1903) erected the genus Daphniopsis through his description of D. tibetanaand found that Daphniopsis was intermediate to Simocephalus and Daphnia. In 1936, Wagler assigned the genus to Daphnia and not to Daphniopsis, and a taxonomic debate on the designation of this genus began (Zhao and Wang, 2005). Later, other researchers recognized that the validity of the genus Daphniopsis was closer to Daphnia than to Simocephalus (Hann, 1986). Moreover, Zhao and Li (2012) found that the differences in mitochondrial 12S rRNA indicated that Daphniopsis tibetana and Daphnia tibetana were different species and that the two genera were highly similar, which is consistent with the results of this study using whole mitogenomes of D. tibetana. However, considering only 12 mitogenomes of this order, more sequences of Branchiopoda are needed to confirm the phylogenetic position of Diplostraca. In future studies, more taxa samplings are required to conclusively resolve the origin and evolutionary relationships among Branchiopoda.
4 ConclusionsIn this study, the complete mitogenome of D. tibetanawas sequenced and characterized, which is the first complete mitogenome of the genus Daphniopsis within Branchiopoda. The mitogenome of D. tibetanais 16196 bp long, which is a typical mitogenome of Branchiopoda with 37 genes and an AT-rich region. The overall base composition is 29.6% A, 33.2% T, 19.0% G, and 18.2% C, which contains a high AT bias (62.8%) and exhibits a negative AT-skew (−0.059) and a positive GC-skew (0.021). ML and BI phylogenetic trees show that Branchiopoda species are classified into three major clades (Anostraca, Notostraca, and Diplostraca) with high support values. Meanwhile, the tree shows that D. tibetanahas a close relationship with D. magna, D. similis, and D. carinata, but it belongs to the genus Daphniopsis, which is consistent with previous studies based on morphology. These results not only reconfirm the phylogenetic position of D. tibetanain Diplostraca, but also reconstruct the phylogenetic relationship between Diplostraca and other orders in Branchiopoda.
AcknowledgementsThis study was supported by the Zhejiang Provincial Natural Science Foundation of China (Nos. LY22D060001, LY20C190008), the National Natural Science Foundation of China (Nos. 41806156, 31702321), the Fund of Guangdong Provincial Key Laboratory of Fishery Ecology and Environment (FEEL-2021-8), the Open Foundation from Key Laboratory of Tropical Marine Bio-resources and Ecology, Chinese Academy of Sciences (No. LMB20201005), the Science and Technology Project of Zhoushan (No. 2020 C21016), the Open Foundation from Marine Sciences in the First-Class Subjects of Zhejiang (Nos. 20200201, 2020 0202), and the Starting Research Fund from the Zhejiang Ocean University.
Altschul, S. F., Thomas, L. M., Alejandro, A. S., Zhang, J. H., Zhang, Z., Webb, M., et al., 1997. Gapped BLAST (Basic Local Alignment Search Tool) and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25(17): 3389-3402. DOI:10.1093/nar/25.17.3389 (  0) 0) |
Bellec, L., Debruyne, R., Utge, J., and Rabet, N., 2019. The first complete mitochondrial genome of Limnadia lenticularis (Branchiopoda, Spinicaudata), with new insights on its phylogeography and on the taxonomy of the genus. Hydrobiologia, 826: 145-158. DOI:10.1007/s10750-018-3724-7 (  0) 0) |
Bernt, M., Donath, A., Juhling, F., Externbrink, F., Florentz, C., Fritzsch, G., et al., 2013. MITOS: Improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics & Evolution, 69(2): 313-319. (  0) 0) |
Bolger, A. M., Marc, L., and Bjoern, U., 2014. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30(15): 2114-2120. DOI:10.1093/bioinformatics/btu170 (  0) 0) |
Boore, J. L., 1999. Animal mitochondrial genomes. Nucleic Acids Research, 27(8): 1767-1780. DOI:10.1093/nar/27.8.1767 (  0) 0) |
Braband, A., Richter, S., Hiesel, R., and Scholtz, G., 2002. Phylogenetic relationships within the Phyllopoda (Crustacea, Branchiopoda) based on mitochondrial and nuclear markers. Molecular Phylogenetics & Evolution, 25(2): 229-244. (  0) 0) |
Brandine, G., and Smith, A. D., 2019. Falco: High-speed FastQC emulation for quality control of sequencing data. F1000 Research, 8: 1874. DOI:10.12688/f1000research.21142.1 (  0) 0) |
Brendonck, L., Rogers, D. C., Olesen, J., Weeks, S., and Hoeh, W. R., 2008. Global diversity of large branchiopods (Crustacea: Branchiopoda) in freshwater. Hydrobiologia, 595(1): 167-176. DOI:10.1007/s10750-007-9119-9 (  0) 0) |
Colbourne, J. K., Wilson, C. C., and Hebert, P., 2006. The systematics of Australian Daphnia and Daphniopsis (Crustacea: Cladocera): A shared phylogenetic history transformed by habitat-specific rates of evolution. Biological Journal of the Linnean Society, 89(3): 469-488. DOI:10.1111/j.1095-8312.2006.00687.x (  0) 0) |
Crease, T. J., 1999. The complete sequence of the mitochondrial genome of Daphnia pulex (Cladocera: Crustacea). Gene, 233(1-2): 89-99. DOI:10.1016/S0378-1119(99)00151-1 (  0) 0) |
Daniels, S. R., Hamer, M., and Rogers, C., 2015. Molecular evidence suggests an ancient radiation for the fairy shrimp genus Streptocephalus (Branchiopoda: Anostraca). Biological Journal of the Linnean Society, 82(3): 313-327. (  0) 0) |
Dewaard, J. R., Sacherova, V., Cristescu, M., Remigio, E. A., and Hebert, P., 2006. Probing the relationships of the branchiopod crustaceans. Molecular Phylogenetics & Evolution, 39(2): 491-502. (  0) 0) |
Dierckxsens, N., Mardulyn, P., and Smits, G., 2017. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res, 45(4): e18. (  0) 0) |
Fendt, L., Zimmermann, B., Daniaux, M., and Parson, W., 2009. Sequencing strategy for the whole mitochondrial genome resulting in high quality sequences. BMC Genomics, 10(1): 1-11. DOI:10.1186/1471-2164-10-1 (  0) 0) |
Gerard, T., and Jose, C., 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology, 56(4): 564-577. DOI:10.1080/10635150701472164 (  0) 0) |
Glenn, T., Nilsen, R. A., Kieran, T. J., Sanders, J. G., and Faircloth, B. C., 2019. Adapterama I: Universal stubs and primers for 384 unique dual-indexed or 147, 456 combinatorially-indexed Illumina libraries (iTru & iNext). PeerJ, 7: e7755. DOI:10.7717/peerj.7755 (  0) 0) |
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O., 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3. 0. Systematic Biology, 59(3): 307-321. DOI:10.1093/sysbio/syq010 (  0) 0) |
Hann, B. J., 1986. Revision of the genus Daphniopsis Sars, 1903 (Cladocera: Daphniidae) and a description of Daphniopsis chilensis, new species, from South America. Journal of Crustacean Biology, 6(2): 246-263. DOI:10.1163/193724086X00073 (  0) 0) |
Holbrook, S. R., Cheong, C., Tinoco, I., and Kim, S. H., 1991. Crystal structure of an RNA double helix incorporating a track of non-Watson-Crick base pairs. Nature, 353(6344): 579-581. DOI:10.1038/353579a0 (  0) 0) |
Joel, W. M., 1995. The Upper Cambrian Rehbachiella and the phylogeny of Branchiopoda and Crustacea. Earth Science Reviews, 38(1): 72-75. DOI:10.1016 (  0) 0) |
Kalyaanamoorthy, S., Minh, B. Q., Wong, T., Haeseler, A. V., and Jermiin, L. S., 2017. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14(6): 587-589. DOI:10.1038/nmeth.4285 (  0) 0) |
Kim, J. Y., Yoo, J. S., and Park, Y. C., 2016. The complete mitochondrial genome of the green crab spider Oxytate striatipes (Araneae: Thomisidae). Mitochondrial DNA Part A, 27(3): 1878-1879. (  0) 0) |
Lam, T. N., Schmidt, H. A., Arndt, V. H., and Quang, M. B., 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology & Evolution, 32(1): 268-274. (  0) 0) |
Letunic, I., and Bork, P., 1988. Interactive Tree of Life (iTOL): An online tool for phylogenetic tree display and annotation. FEBS Letters, 232(1): 78-82. DOI:10.1016/0014-5793(88)80390-9 (  0) 0) |
Linder, F., 1941. Contributions to the morphology and the taxonomy of the Branchiopoda Anostraca. Zoologiska Bidrag fran Uppsala, 20: 101-302. (  0) 0) |
Liu, P., Xu, S., Huang, Q., Dumont, H. J., Lin, Q., and Han, B. P., 2017. The mitochondrial genome of Diaphanosoma dubium with comparison with Daphnia magna. Mitochondrial DNA Part B, 2(2): 926-927. DOI:10.1080/23802359.2017.1413295 (  0) 0) |
Liu, X. C., Li, H. W., Jermnak, U., and Yang, J. S., 2015. The complete mitogenome of the freshwater fairy shrimp Streptocephalus sirindhornae (Crustacea: Anostraca: Streptocephalidae). Mitochondrial DNA Part A, 27(5): 3189-3191. (  0) 0) |
Lohse, M., Drechsel, O., and Bock, R., 2007. OrganellarGenome-DRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Current Genetics, 52(5-6): 267-274. DOI:10.1007/s00294-007-0161-y (  0) 0) |
Longhurst, A. R., 1955. A review of the Notostraca. Bulletin of the British Museum Natural History Zoology, 3(4): 143-145. (  0) 0) |
Lu, X., Gong, L., Zhang, Y., Chen, J., Liu, L., Jiang, L., et al., 2020. The complete mitochondrial genome of Calappa bilineata: The first representative from the family Calappidae and its phylogenetic position within Brachyura. Genomics, 112(3): 2516-2523. DOI:10.1016/j.ygeno.2020.02.003 (  0) 0) |
Ne, S. D., Archer, F. I., Vilstrup, J., Caballero, S., and Morin, P. A., 2011. Mitogenome phylogenetics: The impact of using single regions and partitioning schemes on topology, substitution rate and divergence time estimation. PLoS One, 6(11): e27138. DOI:10.1371/journal.pone.0027138 (  0) 0) |
Norman, J. A., Moritz, C., and Limpus, C. J., 2010. Mitochondrial DNA control region polymorphisms: Genetic markers for ecological studies of marine turtles. Molecular Ecology, 3(4): 363-373. (  0) 0) |
Nylander, J., Fredrik, R., Huelsenbeck, J. P., and Joséluis, N. A., 2004. Bayesian phylogenetic analysis of combined data. Systematic Biology, 53(1): 47-67. DOI:10.1080/10635150490264699 (  0) 0) |
Olesen, J., 1998. A phylogenetic analysis of the Conchostraca and Cladocera (Crustacea, Branchiopoda, Diplostraca). Zoological Journal of the Linnean Society, 122(4): 491-536. DOI:10.1111/j.1096-3642.1998.tb02161.x (  0) 0) |
Parsons, T. J., Muniec, D. S., Sullivan, K., Woodyatt, N., Alliston-Greiner, R., Wilson, M. R., et al., 1997. A high observed substitution rate in the human mitochondrial DNA control region. Nature Genetics, 15(4): 363. DOI:10.1038/ng0497-363 (  0) 0) |
Perna, N. T., and Kocher, T. D., 1995. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. Journal of Molecular Evolution, 41(3): 353-58. DOI:10.1007/BF01215182 (  0) 0) |
Ripma, L. A., Simpson, M. G., and Hasenstab-Lehman, K., 2014. Geneious! Simplified genome skimming methods for phylogenetic systematic studies: A case study in Oreocarya (Boraginaceae). Applications in Plant Sciences, 2(12): 1400062. DOI:10.3732/apps.1400062 (  0) 0) |
Sars, G. O., 1903. On the crustacean fauna of Central Asia. Part III. Copepoda and Ostracoda. Annuaire du Musée Zoologique de l'Académie Impériale des Sciences de St. Pétersbourg, 8: 157-194. (  0) 0) |
Schattner, P., Brooks, A. N., and Lowe, T. M., 2005. The tRNAs-can-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Research, 33: W686-W89. DOI:10.1093/nar/gki366 (  0) 0) |
Sudhir, K., Glen, S., Li, M., Christina, K., and Koichiro, T., 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology & Evolution, 35(6): 1547-1549. (  0) 0) |
Tan, M. H., Gan, H. M., Schultz, M. B., and Austin, C. M., 2015. MitoPhAST, a new automated mitogenomic phylogeny tool in the post-genomic era with a case study of 89 decapod mitogenomes including eight new freshwater crayfish mitogenomes. Molecular Phylogenetics & Evolution, 85: 180-188. (  0) 0) |
Tladi, M., Dalu, T., Rogers, D. C., Nyamukondiwa, C., and Wasserman, R. J., 2020. The complete mitogenome of the fairy shrimp Streptocephalus cafer (Lovén, 1847) (Crustacea: Branchiopoda: Anostraca) from an ephemeral pond in Botswana, southern Africa. Mitochondrial DNA Part B, 5(1): 623-625. DOI:10.1080/23802359.2019.1711222 (  0) 0) |
Toh, H., 2010. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics, 26(15): 1899. DOI:10.1093/bioinformatics/btq224 (  0) 0) |
Tokishita, S. I., Shibuya, H., Kobayashi, T., Sakamoto, M., Ha, J. Y., Yokobori, S. I., et al., 2017. Diversification of mitochondrial genome of Daphnia galeata (Cladocera, Crustacea): Comparison with phylogenetic consideration of the complete sequences of clones isolated from five lakes in Japan. Gene, 611: 38-46. DOI:10.1016/j.gene.2017.02.019 (  0) 0) |
Wang, C., Qin, C., Lu, G., Xu, J., Yang, Q., and Li, S., 2008. Complete mitochondrial genome of the grass carp (Ctenopharyngodon idella, Teleostei): Insight into its phylogenic position within Cyprinidae. Gene, 424(1-2): 96-101. DOI:10.1016/j.gene.2008.07.011 (  0) 0) |
Wang, M., Zhao, W., Wei, J., Wang, S., and Xie, X., 2019. Acute effects of UVB radiation on the survival, growth, development, and reproduction of Daphniopsis tibetana Sars (Crustacea: Cladocera). Environmental Science and Pollution Research, 26(11): 10916-10925. DOI:10.1007/s11356-019-04490-x (  0) 0) |
Wang, Q., Tang, D., Guo, H., Wang, J., Xu, X., and Wang, Z., 2020. Comparative mitochondrial genomic analysis of Macrophthalmus pacificus and insights into the phylogeny of the Ocypodoidea & Grapsoidea. Genomics, 112(1): 82-91. DOI:10.1016/j.ygeno.2019.12.012 (  0) 0) |
Wang, X., Huang, Y., Liu, N., Yang, J., and Lei, F., 2015. Seven complete mitochondrial genome sequences of bushtits (Passeriformes, Aegithalidae, Aegithalos): The evolution pattern in duplicated control regions. Mitochondrial DNA, 26(3): 350. DOI:10.3109/19401736.2014.1003821 (  0) 0) |
Wolstenholme, D. R., 1992. Animal mitochondrial DNA: Structure and evolution. International Review of Cytology, 141(6): 173-216. (  0) 0) |
Yue, G. H., and Orban, L., 2010. A simple and affordable method for high-throughput DNA extraction from animal tissues for polymerase chain reaction. Electrophoresis, 26(16): 3081-3083. (  0) 0) |
Zhang, D., Gao, F., Jakovli, I., Zou, H., and Wang, G. T., 2020. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources, 20(1): 348-55. DOI:10.1111/1755-0998.13096 (  0) 0) |
Zhang, Y., Gong, L., Lu, X., Jiang, L., and Zhang, X., 2020. Gene rearrangements in the mitochondrial genome of Chiromantes eulimene (Brachyura: Sesarmidae) and phylogenetic implications for Brachyura. International Journal of Biological Macromolecules, 162: 704-714. DOI:10.1016/j.ijbiomac.2020.06.196 (  0) 0) |
Zhang, Z., Cheng, Q., and Ge, Y., 2019. The complete mitochondrial genome of Rhynchocypris oxycephalus (Teleostei: Cyprinidae) and its phylogenetic implications. Ecology and Evolution, 9(13): 7819-7837. DOI:10.1002/ece3.5369 (  0) 0) |
Zhao, W., and Li, R., 2012. Molecular phylogeny of four strains of Daphniopsis tibetana Sars based on mitochondrial 12S rRNA gene sequences. Journal of Dalian Ocean University, 27(4): 300-305 (in Chinese with English abstract). (  0) 0) |
Zhao, W., and Wang, Q. H., 2005. The morphological redescription of Daphniopsis tibetana Sars (Crustacea: Cladocera: Daphnidae). Journal of Dalian Fisheries University, 20(3): 166-173 (in Chinese with English abstract). (  0) 0) |
Zhao, W., Bi, J. H., Han, T. T., Wei, J., Chai, X. J., Lu, J. X., et al., 2011. A preliminary study on genetic diversity of Daphniopsis tibetana Sars. Journal of Dalian Ocean University, 26(2): 108-113 (in Chinese with English abstract). (  0) 0) |
Zhao, W., Huo, Y. Z., and Gao, J., 2006. Analysis and appraisement of nutrient compositions for Daphniopsis tibetana Sars. Journal of Fishery Sciences of China, 13: 446-446 (in Chinese with English abstract). DOI:10.3321/j.issn:1005-8737.2006.03.018 (  0) 0) |
Zhao, W., Huo, Y. Z., and Xue, D. N., 2007. Effects of alkalinity and pH on the survival, growth and neonate production of Daphniopsis tibetana Sars. Acta Hydrobiologica Sinica, 31(3): 332-338 (in Chinese with English abstract). DOI:10.3321/j.issn:1000-3207.2007.03.006 (  0) 0) |
Zhao, W., Wang, Q. H., Zheng, Y. B., and Wang, H. L., 2002. A preliminary study on the biology of Daphniopsis tibetana Sars. Journal of Dalian Ocean University, 17(3): 209-214 (in Chinese with English abstract). (  0) 0) |
Zhao, W., You, Z. X., Wei, J., and Wang, S., 2016. Compensatory population growth in Daphniopsis tibetana Sars (Crustacea: Cladocera) following starvation. Limnology, 18: 167-174. (  0) 0) |
Zhao, W., Zhang, P., Huo, Y. Z., and Wang, H. L., 2004. Study of karyotype on Daphniopsis tibetana Sars (Cladocera: Daphniidae). Journal of Dalian Fisheries University, 19(3): 167-170 (in Chinese with English abstract). DOI:10.3969/j.issn.1000-9957.2004.03.002 (  0) 0) |
 2022, Vol. 21
2022, Vol. 21


