2. 沈阳医学院附属中心医院病理科
研究显示,幽门螺杆菌(Helicobacter pylori, H.pylori)感染后可能引起一系列胃黏膜改变,包括胃炎、溃疡糜烂、肠上皮化生(intestinal metaplasia,IM)乃至胃癌[1];感染不同毒力因子的H.pylori可能出现不同的临床结局。细胞空泡毒素基因(vacuolating cytotoxin gene A,vacA)是H.pylori重要的致病相关基因之一,vacA基因序列上存在多态性位点,其中2个重要的变异区为信号区(s区)和中间区(m区)。由此,根据各种不同s区与m区的组合,可以将vacA基因分为s1m1、s1m2、s2m1和s2m2亚型。既往研究认为,仅s1m1型毒素具有高水平的毒素活性;近年来,s1m2型毒素的毒性作用已为学者们所逐步证实[2-3]。IM是胃癌重要的癌前病变,是肠型胃癌发生的唯一条件[4]。有文献报道,IM人群中胃癌的发病率为0%~10%不等,IM患者患胃癌的风险是正常人群的6倍[5]。本研究通过胃镜活检的石蜡包埋标本中H.pylori vacA基因型的检测,旨在探讨vacA s1m2基因型与胃黏膜IM的关系,为实现萎缩性胃炎的因型施治和胃癌的预防提供依据。结果报告如下。
1 材料与方法 1.1 主要试剂与仪器亚甲蓝染色试剂盒(福州迈新公司),石蜡包埋组织DNA提取试剂盒(北京天根公司),PCR试剂盒(大连宝生物公司);莱卡切片机(德国Leica公司),低温离心机(美国Thermo Fisher公司),PCR仪(德国Eppendorf公司),电泳仪(北京六一仪器厂)。
1.2 标本选取2011—2014年沈阳医学院附属中心医院胃镜活检的石蜡包埋标本396例,其中有271例为H.pylori阳性(PCR法),经病理诊断(苏木素-伊红染色、亚甲蓝染色)分为浅表性胃炎组126例、萎缩性胃炎组106例、胃溃疡组164例;其中男性200例、女性196例;年龄为28~86岁(平均年龄57.1岁);组间年龄、性别差异无统计学意义(P > 0.05)。
1.3 指标与方法 1.3.1 DNA提取采用TIANamp FFPE DNA试剂盒提取石蜡包埋标本中DNA,每个病例连续切18~20片(厚度为4 μm),放入1.5 mL离心管中,按试剂盒说明书操作提取总DNA。
1.3.2 H.pylori-PCR检测以提取的总H.pylori DNA为模版,利用H.pylori尿素酶基因对H.pylori进行PCR检测。
1.3.3 vacA s1m2基因型检测选用H.pylori s1和m2特异引物,通过巢式PCR分别对vacA s1和m2进行扩增[6],s1和m2的PCR反应体系均为20 μL,其中含模板DNA 2 μL、10 × PCR buffer 2.5 μL、dNTP 2 μL、引物1.25 μL、taq酶0.2 μL,去离子水补足至20 μL。(1)s1基因扩增:引物序列为s1上游引物1:5′GTG GAG CAA GCA CAG CTA AGG TTT TA 3′;s1下游引物1:5′ CAA AAT CGC TAC AAC ATT TTA TGG GT 3′;s1反应条件:95 ℃ 5 min,95 ℃ 1 min,54 ℃ 1 min,72 ℃ 1 min,72 ℃ 5 min,40个循环;再以第2对引物进行巢式PCR,s1上游引物2:5′CTG GTC TAA AGT CGC ACC CTT TGT GC 3′;s1下游引物2:5′CAA TGG CTG GAA TGA TCA CGG TTG TA 3′;反应条件与第1次相同,30个循环。(2)m2基因扩增: m2上游引物1:5′TTT GGA GCC CCA GGA AAC ATT G 3′;m2下游引物1:5′CCA CAC GCC CAT CTT GGA CAA 3′;m2反应条件:95 ℃ 5 min,95 ℃ 1 min,54 ℃ 1 min,72 ℃ 1 min,72 ℃ 5 min。再以第2对引物进行巢式PCR,m2上游引物2:5′ACC CTA AAC AGC AAC GCA AGC 3′;m2下游引物2:5′GAC AAA AAG ATT CAT CGT GCC TT 3′;反应条件与第1次相同,m2 2次PCR各进行35个循环。扩增产物经2%琼脂糖凝胶电泳分析,紫外凝胶成像系统成像观察。(3)基因型判定:s1产物片段为141 bp,m2产物片段为102 bp,同时出现s1和m2产物片段则判定为vacA s1m2亚型阳性,否则判定为vacA其它亚型[7]。
1.4 统计分析采用SPSS 16.0软件进行统计分析,组间比较采用χ2或Fisher精确概率法,P < 0.05为差异有统计学意义。
2 结果 2.1 H.pylori阳性判定HE染色,镜下可见H.pylori呈弯曲螺旋形、S型或海鸥状弯曲的短杆菌或球形体;亚甲蓝染色,镜下可见H.pylori呈螺旋形、S型或海鸥状弯曲的兰色杆状小体;以H.pylori尿素酶基因对H.pylori进行PCR检测,采用紫外凝胶成像系统进行观察,阳性条带为430 bp。2种染色方法任意一种观察到H.pylori且同时PCR方法检测阳性,则判定为H.pylori阳性;396例标本中有271例为H.pylori阳性,经病理学诊断确定76例为浅表性胃炎(伴IM 18例)、56例萎缩性胃炎(伴IM 37例)、139例胃溃疡(伴IM 30例)。
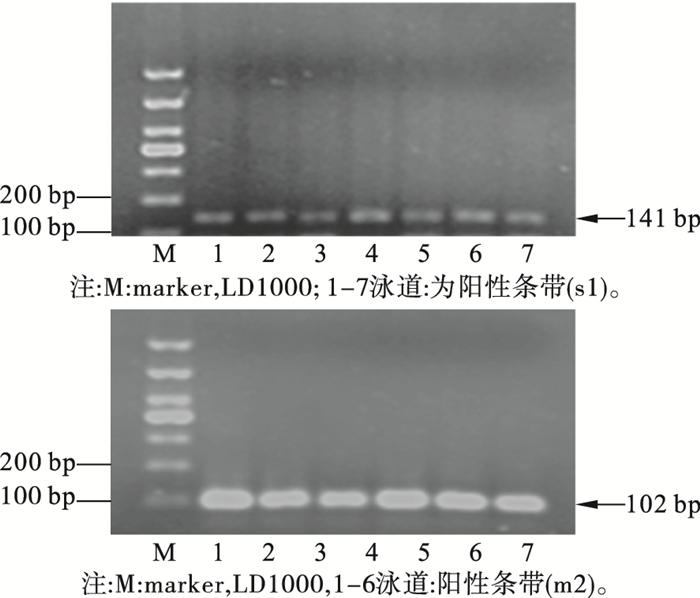
2.2 vacA s1m2基因型判定(图 1)
|
图 1 vacA基因s1m2型PCR产物琼脂糖凝胶电泳 |
s1片段长度为141 bp(图 1 A ),其中1~7泳道为s1的阳性条带;m2的片段长度为102 bp(图 1 B ),其中1~6道为m2的阳性条带。同时出现s1和m2阳性条带判定为vacA s1m2阳性。结果显示,在271例标本中,s1m2亚型阳性108例,阳性率为39.9%。
2.3 H.pylori vacA s1m2基因型与胃黏膜病变关系(表 1)| 表 1 H.pylori vacA s1m2基因型与胃黏膜病变关系 |
结果显示,在萎缩性胃炎病例组中,vacA s1m2亚型检出率为53.6%,明显高于浅表性胃炎组和胃溃疡组,差异有统计学意义(均P < 0.05)。
2.4 H.pylori vacA s1m2基因型与IM关系在全部271例H.pylori阳性病例中,伴IM组vacA s1m2亚型检出率为62.4%(53/85),显著高于无IM组的29.6%(55/186),差异有统计学意义(P < 0.01);萎缩性胃炎伴IM组病例中vacA s1m2亚型检出率为75.7%(28/37),明显高于萎缩性胃炎无IM组vacA s1m2亚型检出率10.5%(2/19),差异有统计学意义(P < 0.01);浅表性胃炎伴IM组和胃溃疡伴IM组vacA s1m2亚型检出率分别为50%(9/18)、53.3%(16/30),浅表性胃炎无IM组和胃溃疡无IM组vacA s1m2检出率分别为27.6%(16/58)、33.9%(37/109),差异无统计学意义(P > 0.05)。
3 讨论自VacA s1m2型毒素的毒性作用被证实以来,H.pylori vacA s1m2基因型与不同胃疾病的关系亦成为学者们关注的热点。Koehler等[8]研究显示,黏膜相关淋巴组织淋巴瘤中vacA s1m2亚型合并iceA1基因型者是慢性活动性胃炎的4.7倍。Marie[9]报道指出,在37株胃炎和31株消化性溃疡患者胃黏膜活检组织分离培养的H.pylori菌株中,vacA s1m2亚型分别占59%和16%,其与胃炎明显相关。Ghotaslou等[10]对非溃疡性消化不良和消化性溃疡患者胃黏膜活检组织分离培养的115株H.pylori菌株进行基因型分析,其中vacA s1m2亚型分别占52.8%和56.5%,均高于vacA s1m1和s2m2亚型。Pajavand等[11]利用胃黏膜活检组织分离培养出96株H.pylori菌株,胃溃疡组中vacA s1m2亚型的检出率为38.2%(13/34),认为vacA s1m2亚型与胃溃疡显著相关。本研究结果显示,萎缩性胃炎组病例中vacA s1m2亚型检出率为53.6%,明显高于浅表性胃炎组和胃溃疡组。提示,H.pylori vacA s1m2基因型与萎缩性胃炎的发生密切相关。
萎缩性胃炎的重要病理改变之一为胃黏膜IM。胃黏膜IM具有致癌风险。在中国进行的一项调查显示,IM的发病率在胃癌高发省份要比低发省份高很多[12-13]。日本利用胃癌风险指数评估胃癌前病变与胃癌的发生关系时得出结论,IM是肠型胃癌发生的唯一条件[14, 4]。本研究结果显示,在271例H.pylori阳性标本中,伴IM组vacA s1m2亚型检出率为62.4%,明显高于无IM组;萎缩性胃炎中伴IM组vacA s1m2亚型的检出率为75.7%,明显高于无IM组。提示,vacA s1m2基因型与IM密切相关,vacA s1m2基因型的H.pylori可导致胃黏膜IM;vacA s1m2基因型H.pylori为萎缩性胃炎相关高致病性菌株。
VacA毒素(vacA基因编码产物)可使胃上皮细胞离子转运蛋白的功能发生紊乱,通过影响离子转运,诱发靶细胞溶酶体及内质网损伤,破坏细胞的正常功能,导致靶细胞发生空泡变性[15-16]。有研究显示,在无H.pylori其他毒力因子情况下,单是纯化的VacA毒素即可诱导胃黏膜上皮细胞凋亡[17]。VacA毒素片段可插入线粒体膜引发细胞色素C释放,启动caspase-3途径介导的细胞凋亡,使胃腺体大量萎缩和减少,继而发生IM、异型增生乃至胃癌[18]。Junaid等[19]通过胃淋巴瘤患者胃黏膜活检组织分离培养获得vacA s1m2基因型菌株,而后将克隆的s1m2基因构建在大肠杆菌中表达,以纯化的表达产物作用于T84细胞和犬肾源性细胞(MDCK),结果这2种细胞均出现大量凋亡。有学者发现,H.pylori菌株培养滤液中的s1m2型VacA毒素可在较低浓度下(1.0 μg/mL)使人胃癌细胞(AGS)出现空泡变化[20]。推测VacA毒素及其中的s1m2型毒素导致萎缩性胃炎和肠上皮化生机制可能与胃黏膜细胞凋亡有关。
| [1] | Memon AA, Hussein NR, Miendje Deyi VY, et al. Vacuolating cytotoxin genotypes are strong markers of gastric cancer and duodenal ulcer-associated Helicobacter pylori strains:a matched case-control study[J]. J Clin Microbiol , 2014, 52 (8) : 2984–2989. DOI:10.1128/JCM.00551-14 |
| [2] | Santibáez M, Aguirre E, Belda S, et al. Relationship between tobacco, cagA and vacA i1 virulence factors and bacterial load in patients infected by Helicobacter pylori[J]. PLoS One , 2015, 10 (3) : 120444–120447. |
| [3] | Matsunari O, Miftahussurur M, Shiota S, et al. Rare Helicobacter pylori virulence genotypes in Bhutan[J]. Sci Rep , 2016, 2 (6) : 22584–22591. |
| [4] | Shimoyama T, Fukuda S, Tanaka M, et al. Evaluation of the applicability of the gastric carcinoma risk index for intestinal type cancer in Japanese patients infected with Helicobacter pylori[J]. Virchows Arch , 2000, 436 (6) : 585–587. DOI:10.1007/s004289900179 |
| [5] | Kim N, Park RY, Cho SI, et al. Helicobacter pylori infection and development of gastric cancer in Korea:long-term follow-up[J]. J Clin Gastroenterol , 2008, 42 (5) : 448–454. DOI:10.1097/MCG.0b013e318046eac3 |
| [6] | Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer[J]. N Engl J Med , 2001, 345 (11) : 784–789. DOI:10.1056/NEJMoa001999 |
| [7] | Cover TL, Tummuru MK, Cao P, et al. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains[J]. J Biol Chem , 1994, 269 (14) : 10566–10567. |
| [8] | Koehler CI, Mues MB, Dienes HP, et al. Helicobacter pylori geno-typing in gastric adenocarcinoma and MALT lymphoma by multiplex PCR analyses of paraffin wax embedded tissues[J]. J Clin Pathol:Mol Pathol , 2003, 56 (9) : 36–42. |
| [9] | Marie MA. Relationship between Helicobacter pylori virulence genes and clinical outcomes in Saudi patients[J]. J Korean Med Sci , 2012, 27 (2) : 190–193. DOI:10.3346/jkms.2012.27.2.190 |
| [10] | Reza Ghotaslou, Morteza Milani, Mohammad Taghi Akhi, et al. Diversity of Helicobacter pylori cagA and vacA genes and its relationship with clinical outcomes in Azerbaijan, Iran[J]. Adv Pharm Bull , 2013, 3 (1) : 57–62. |
| [11] | Pajavand H, Alvandi A, Mohajeri P, et al. High frequency of vacA s1m2 genotypes among Helicobacter pylori isolates from patients with gastroduodenal disorders in Kermanshah, Iran[J]. Jundishapur J Microbiol , 2015, 8 (11) : 25425–25429. |
| [12] | You WC, Zhang L, Gail MH, et al. Precancerous lesions in two counties of China with contrasting gastric cancer risk[J]. Int J Epidemiol , 1998, 27 (6) : 945–948. DOI:10.1093/ije/27.6.945 |
| [13] | 黄志刚, 宋春花, 范清堂. 幽门螺杆菌CagA基因对胃癌细胞的影响[J]. 中国公共卫生 , 2007, 23 (4) : 463–465. |
| [14] | 汪雪峰, 王克霞, 陈琳. 球形幽门螺杆菌vacA基因表达质粒构建及表达[J]. 中国公共卫生 , 2007, 23 (7) : 834–836. |
| [15] | Tomboia F, Del Giudice G, Papini E, et al. Blocks of vacA provide insights into the structure of the pore[J]. Biophys , 2000, 79 (2) : 863–873. |
| [16] | Kim JM, Kim JS, Kim N, et al. Helicobacter pylori vacuolating cytotoxin induces apoptosis via activation of endoplasmic reticulum stress in dendritic cells[J]. J Gastroenterol Hepatol , 2015, 30 (1) : 99–108. DOI:10.1111/jgh.12663 |
| [17] | Fox JG, Correa P, Taylor NS, et al. High prevalence and persistence of cytotoxin positive Helicobacter pylori strains in a population with high prevalence of atrophic gastritis[J]. Am J Gastroenterol , 1992, 87 : 1554–1560. |
| [18] | Phadnis SH, Ilver D, Janzon L, et al. Pathological significance and molecular characterization of the vacuolationg toxin gene of Helicobacter pylori][J]. Infect Immun , 1994, 63 (6) : 1557–1565. |
| [19] | Junaid M, Al-Gubare S, Yousef M, et al. Sequence and apoptotic activity of VacA cytotoxin cloned from a Helicobacter pylori Thai clinical isolate[J]. Biomed Res Int , 2014, 115 (10) : 398350–398354. |
| [20] | Radin JN, Gonzalez-Rivera C, Frick-Cheng AE, et al. Role of connexin 43 in:Helicobacter pylori VacA-induced cell death[J]. Infect Immun , 2014, 82 (1) : 423–432. DOI:10.1128/IAI.00827-13 |
 2016, Vol. 32
2016, Vol. 32


