近年来, 肺炎衣原体(CPn)与动脉粥样硬化(AS)和冠心病(CAD)的相关性受到广泛关注, 多项大型临床试验结果表明, 抗生素并不能有效减少心脏病的发生率〔1〕, 但这只能说明抗生素治疗不能逆转已形成的动脉粥样硬化损害, 不能否定感染在动脉粥样硬化发病过程中的可能作用。研究表明, 肺炎衣原体参与了动脉粥样硬化的发病〔2〕, 其导致动脉粥样硬化的作用有赖于血清胆固醇的水平〔3〕。鉴于临床上动脉粥样硬化发病的多因素性, 本文探讨了肺炎衣原体感染与高脂饮食协同作用在动脉粥样硬化发病过程中的作用。
1 材料与方法 1.1 材料 1.1.1 主要仪器和试剂(1)仪器: BIO-RAD MODEL550酶标仪; Labsystems Dragon Well wash 4MK2洗板机; Leica RM 2025切片机; ZMN-6802漂烘处理仪; Leica EG 1140H包埋机; Leica TP 1020脱水机; Olympus BX50显微镜; 北航图像中心病理图文管理系统4.0图像处理系统; JEM-1200EX透射电子显微镜; A0-型超薄切片机; BIOPAC十六道生理系统记录仪; Olympus BX 50显微镜。(2)试剂: Mouse TNF-α试剂盒、Mouse IL-6试剂盒、Mouse IL-10试剂盒、Mouse sICAM-1试剂盒、sVCAM-1试剂盒、sE-selectin试剂盒、细胞凋亡试剂盒(美国R & D公司); 硝酸甘油、盐酸肾上腺素(广州明兴制药厂); 乙酰胆碱(上海三爱思试剂有限公司); 氯化钾(西安利君制药有限公司)。
1.1.2 实验动物、药物和细胞株(1)实验动物:雄性C57BL/6J小鼠40只, 8周龄, SPF级(第一军医大学实验动物中心, 许可证号: 2002-009 2003B029 2003A075); (2)药物:黄芩提取物, 含90.8%黄芩苷(四川广汉市维康植化有限公司); 阿奇霉素(丽珠集团制药厂); (3)细胞株:肺炎衣原体AR-39(美国华盛顿大学)。
1.2 方法 1.2.1 实验分组小鼠饲养于广州中医药大学第一附属医院清洁级动物房(粤监证字2004C019号)。小鼠随机分为高脂感染组、高脂组、感染组、正常组。其中正常组和感染组喂普通饲料和水; 高脂组、高脂感染组喂高脂饲料, 正常饮水。高脂饲料含2%胆固醇, 10%猪油, 0.5%胆酸钠, 其他各成分按标准饲料配方〔4〕, 由广东省实验动物中心配制。
1.2.2 肺炎衣原体感染模型制作感染组、高脂感染组喂饲高脂饲料1周后, 开始接种肺炎衣原体, 方法参照文献〔5〕。第1次接种后第18周末处死全部动物。
1.2.3 检测(1)血清炎症因子、粘附分子检测:采用ELISA法分别测定血清肿瘤坏死因子-α(TNF-α)、白细胞介素-6(IL-6)、白细胞介素-10(IL-10)、可溶性细胞间粘附分子-1(sICAM-1)、可溶性血管细胞粘附分子-1 (sVCAM -1)、可溶性E-选择素(sE-selectin)。(2)小鼠主动脉斑块面积评价:参照文献〔6〕, 以动脉粥样斑块面积/管腔面积比值的平均值表示斑块面积指数(PAI, Plaque Area Index)。(3)内皮细胞超微结构观察:应用透射电镜参照细胞损伤程度分级标准〔7〕对其超微结构保护效果进行评价分级。(4)血管反应性测定:参照文献〔8〕方法。
1.3 统计分析采用SPSS11.5软件分析处理。内皮细胞损伤程度比较用等级资料秩和检验, 细胞因子和粘附分子等及PAI、血管最大反应性比较用单因素方差分析检验。
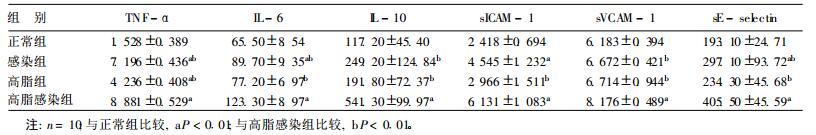
2 结果 2.1 各组小鼠细胞因子和粘附分子等水平变化(表 1)| 表 1 各组小鼠细胞因子和粘附分子水平比较(pg/ml, x ± s) |
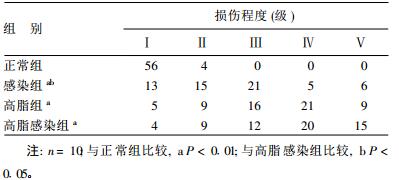
2.2 内皮细胞损伤程度比较(表 2)
| 表 2 各组60个内皮细胞不同级别损伤数量(个) |
正常内皮细胞呈条索状, 受损内皮细胞可出现细胞水肿、细胞核裸露或呈乳头状突起, 甚至整个细胞脱落。
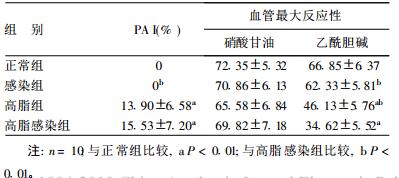
2.3 斑块面积指数(PAI)及血管反应性比较(表 3)| 表 3 各组PAI和血管最大反应性比较(%, x ± s) |
正常组、感染组无斑块形成, 高脂感染组有明显的动脉粥样硬化斑块形成。各组血管对硝酸甘油引起的非内皮依赖最大舒张反应差异无统计学意义; 各组对乙酰胆碱的内皮依赖舒张反应有明显差异。
3 讨论研究表明, 肺炎衣原体感染与动脉粥样硬化有关〔9, 10〕; 虽然颈动脉粥样硬化程度与肺炎衣原体感染无相关性, 但颈动脉粥样硬化斑块常可检测到肺炎衣原体感染〔11〕。有学者发现, 肺炎衣原体感染与外周血管的动脉粥样硬化也有关〔12〕。
本研究结果显示, 肺炎衣原体感染和高脂饮食均可使血清炎症因子TNF-α, IL-6和IL-10、sICAM-1水平升高, 两者有协同作用, 这与Kazmierski等〔13〕的临床研究结论相似。本研究发现, 肺炎衣原体感染可增加内皮细胞损伤, 而且与高脂饮食有协同作用。高脂饮食对斑块形成、血管的最大反应性影响显著, 肺炎衣原体感染可加强高脂饮食的这种作用, 提示肺炎衣原体感染具有促进高脂饮食致动脉粥样硬化病理进程的作用。本研究未发现单纯肺炎衣原体感染对动脉粥样硬化斑块的形成和血管的最大反应性有显著影响, 提示肺炎衣原体感染致动脉粥样硬化需其他协同因素并存, 其机制有待进一步研究。
| [1] | Eli V, Christopher P. Antibiotics for secondary prevention of coronary artery disease:an ACES hypothesis but we need to proveit[J]. American Heart Journal, 2004, 147(2) : 202. DOI:10.1016/j.ahj.2003.09.011 |
| [2] | Fong IW, Chiu B, Viira E, et al. Influence of clarithromycin on early atherosclerotic lesions after chlamydia pneumoniae in fection in a rabbit model[J]. Antmiicrobial Agents and Chemotherapy, 2002, 46(8) : 2321–2326. DOI:10.1128/AAC.46.8.2321-2326.2002 |
| [3] | Blessing E, Campbell LA, Rosenfeld ME, et al. Chlamydia pneumoniae and hyperlipidemia are co-risk factors for atherosclerosis:infection prior to induction of hyperlipidemia do not accelerate development of atherosclerotic lesions in C57BL/6J mice[J]. Infect Immun, 2002, 70(9) : 5332–5334. DOI:10.1128/IAI.70.9.5332-5334.2002 |
| [4] | Brunt EM, Jannery CG, DiBisceglie AM, et al. Nonaleoholic steato hepatits:a proposol for grading and staging the histological lesions[J]. Am J Gastroenterol, 1999, 94(9) : 2467–2474. DOI:10.1111/ajg.1999.94.issue-9 |
| [5] | Ridker PM, Rifai N, Stampfer JM, et al. Plasma ooncentration of in terleuk in-6 and the risk of future myocardial infarction among apparently healthy men[J]. Circutation, 2000, 101 : 1767–1772. |
| [6] | Mublestein JB, Anderson JL, Hammond EH, et al. Infection with Chlamydia pneumoniae accelerate the development of atherosclerosis and treamtent with azithromycin prevent it in a rabbit model[J]. Circulation, 1998, 97 : 633–636. DOI:10.1161/01.CIR.97.7.633 |
| [7] | Axford Gatley RA, Wilson G J, Feindel CM. Comparison of blood-based and asanguineous cardioplegic solutions administered at 4 degrees C.An ultrastru ctural morphometric study in the dog[J]. J Thorac Cardiovasc Surg, 1990, 100 : 400–409. |
| [8] | 张然, 贾国良, 林乐健, 等. 血管紧张素Ⅱ1型受体阻断对模拟失重大鼠动脉血管反应性的影响[J]. 中华航空航天医学杂志, 2007, 18(1) : 30–36. |
| [9] | Kmi DK, Kmi HJ, Han SH, et al. Chlamydia pneumoniae accompanied by in flammation is associated with the progression of athero sclerosis in CAPD patients:a prospective study for 3 years[J]. Nephrol Dial Transplant, 2008, 23(3) : 1011–1018. |
| [10] | Yavuz MT, Yavuz O, Yazici M, et al. Interaction between Chlamydia pneumoniae seropositivity, inflammation and risk factors for atherosclerosis in patients with severe coronary stenosis[J]. Scand J Clin Lab Invest, 2006, 66(6) : 523–534. DOI:10.1080/00365510600931114 |
| [11] | Kónya J, Molnár S, Magyar MT, et al. Severity of carotid atherosclerosis unrelated to Chlamydia pneumoniae infection in acute ischemic stroke patients:a clinicopathological study[J]. Cerebrovasc Dis, 2008, 25(1-2) : 170–175. DOI:10.1159/000113735 |
| [12] | Ustnsoy H, Sivrikoz C, Sirmatel F, et al. Is Chlamydia pneumoniae a risk factor for peripheral atherosclerosis?[J]. Asian Cardiovasc Thorac Ann, 2007, 15(1) : 9–13. DOI:10.1177/021849230701500103 |
| [13] | Kazmierski R, Podsiadly E, Tylewska-Wierzbanowska S, et al. Association between carotid atheroscleros is, inflammatory markers and Chlamydia pneumoniae infection[J]. Neurol Neurochir Pol, 2005, 39(4) : 277–286. |
 2009, Vol. 25
2009, Vol. 25