Exploring the antineoplastic potential of α-mangostin in breast cancer
Abstract
Among women, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related mortality globally. Despite improvements in early detection and diagnosis, some risk factors have been on the rise, including the decline in birth rate, the use of oral contraceptives, and the escalation in alcohol consumption and obesity. Thus, there is an imperative urgent need to expand accessible prevention and treatment options for breast cancer. Regarding these tumors, several natural compounds have shown efficacy in slowing or preventing their progression, offering a promising therapeutic alternative. Among these, α-mangostin, a xanthone derived from mangosteen, has demonstrated promising antitumor effects against different malignancies, particularly breast cancer. The mechanisms involved in α-mangostin´s therapeutic effects include downregulation of oncogenic ion channels, modulation of cell cycle progression, suppression of oncogene expression, and interference with steroid and growth factor receptors signaling. This review thoroughly explores these mechanisms, as well as updates information on α-mangostin chemical structure and its potential as a coadjuvant to conventional breast cancer therapies. Furthermore, we provide scientifically supported insights for the development of clinically applicable α-mangostin-based treatments, highlighting the robust body of evidence supporting its cancer-fighting properties, despite the absence of clinical studies to date.Graphical Abstract
Keywords
α-Mangostin Breast cancer Antineoplastic effects Garcinia mangostana Xanthone1 Introduction
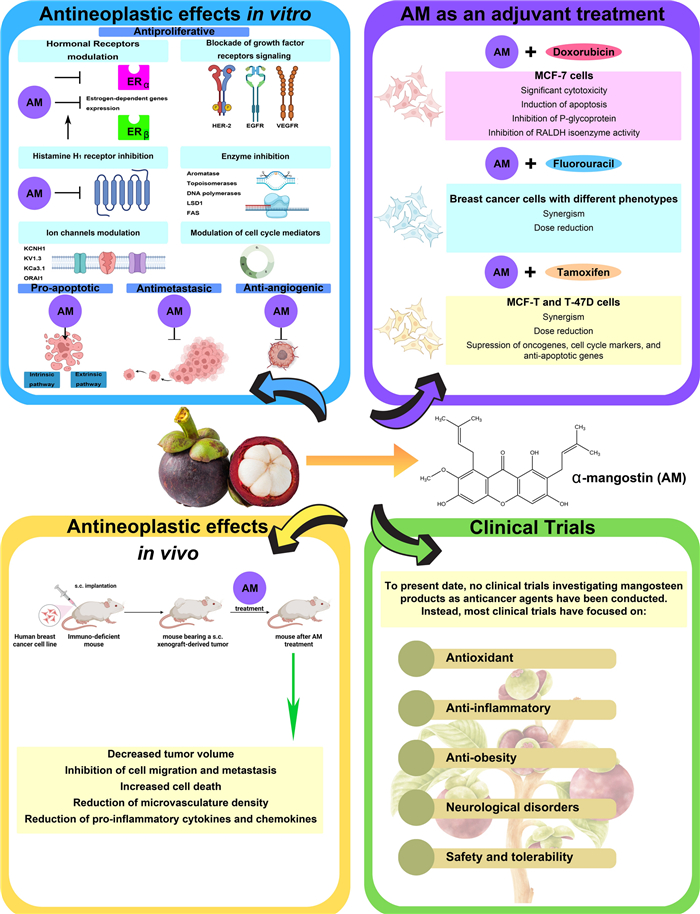
As estimated by GLOBOCAN 2022, breast cancer represents the most frequently diagnosed cancer type among women and the leading cause of cancer-related mortality worldwide, which accounts for one in six cancer deaths [1]. Moreover, mortality rates caused by this malignancy are higher in less developed countries, posing a heavy burden for healthcare systems and representing a clinical concern worldwide [1]. This underscores the urgent need for accessible therapeutic strategies with low toxicity, alongside early detection and timely diagnosis. Plant-derived bioactive molecules have been extensively studied for their therapeutic potential, with the mangosteen tree (Garcinia mangostana) emerging as a notable source of antineoplastic phytochemicals, particularly xanthones. Among these, α-mangostin has gained significant attention due to its ability to inhibit the proliferation of various breast cancer cell types in in vitro studies and xenografted murine models. In these preclinical studies, α-mangostin has demonstrated the potential to enhance the efficacy of conventional cancer therapies, such as doxorubicin, 5-fluorouracil, and tamoxifen, by allowing dose reductions while improving therapeutic outcomes [2-4]. This combination of low cost, reduced toxicity, and diverse antineoplastic mechanisms makes α-mangostin a promising candidate for developing novel breast cancer treatments. Notably, α-mangostin, exhibits significant anticancer properties across various cancer types, as reviewed elsewhere [5-8]. Beyond its antineoplastic effects, α-mangostin offers additional health benefits, including antibacterial, antifungal, and antiparasitic activities, as well as antioxidant, antidiabetic, antiobesity, and antiallergic effects. These attributes position α-mangostin as a valuable natural health product for improving overall health and supporting the treatment of various medical conditions [5].
This review provides an updated overview of the anticancer effects of α-mangostin, particularly in breast cancer, emphasizing its cellular and molecular mechanisms. The antineoplastic effects of α-mangostin include modulation of breast hormonal receptors, inhibition of growth factor and histamine H1 receptor (H1R) signaling, and targeting key enzymes and cell cycle mediators. α-Mangostin promotes apoptosis and inhibits cancer cell adhesion, invasion, and metastasis through mechanisms such as epithelial-mesenchymal transition (EMT) and focal adhesion kinase (FAK) inhibition. Additionally, α-mangostin exhibits antiangiogenic and immunomodulatory effects. Preclinical studies and clinical trials supporting its potential as an adjuvant therapy are also discussed.
1.1 Taxonomy of mangosteen
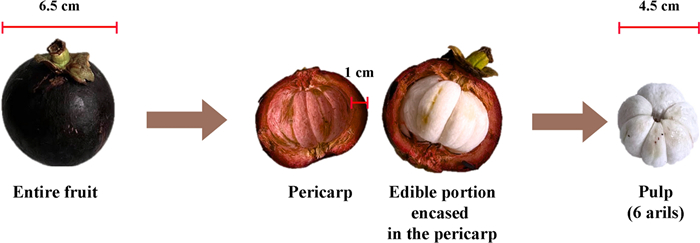
The mangosteen tree (Garcinia mangostana) belongs to the Garcinia genus within the Clusiaceae family, commonly known as Guttiferae. It can grow up to 25 m, featuring a central trunk, symmetrical branches, and dense foliage. Its leaves measure 15–25 cm in length and 4.5–13 cm in width. The flowers have four petals, measuring 2–5 cm long, and are either yellow-green with red edges or predominantly red [9-11]. These flowers produce mangosteen fruits, which are encased in a thick, hard, deep-red pericarp that encloses 4 to 7 arils (Fig. 1). Mangosteen trees grow slowly, requiring 6 to 9 years for the first harvest [9, 10].
Mangosteen fruit. The mangosteen fruit has a thick outer rind, known as the pericarp (rind or peel), which measures approximately 1.0 cm in thickness and encases the inner white pulp. The pulp, about 4.5 cm in diameter, consists of 4 to 7 soft, juicy arils with a sweet yet slightly acidic flavor. The entire fruit has an average diameter of 6.5 cm
The mangosteen tree, native from Southeast Asia, is extensively cultivated in Malaysia, Indonesia, Thailand, the Philippines, and certain regions of India and Sri Lanka. It has also been introduced to tropical regions in Australia, Africa, and America. Particularly in this last Continent, the mangosteen tree can be found in South and Central America, including Mexico [10, 12, 13].
1.2 Traditional medicinal applications of mangosteen and its fruit-derived supplements
Globally, mangosteen has a long history of use in traditional medicine, particularly in Southeast Asia. Various parts of the tree, including the leaves, bark, roots, heartwood, seeds, pericarp, and fruit, have been utilized in different forms, such as powders, ointments, tinctures, and teas to treat numerous medical conditions. As an antimicrobial agent, mangosteen has been employed to treat bacterial infections and parasitic diseases, including dysentery and cholera [14]. It also addresses other gastrointestinal issues like aphthae, abdominal pain, hemorrhoids, and diarrhea, and is considered a digestive aid. Externally, it treats skin infections, wounds, chronic ulcers, and suppurations [12, 15]. Additionally, mangosteen is used to treat genito-urinary diseases such as urethra suppuration, cystitis, leucorrhea, gonorrhea, vaginal thrush, and menstrual disorders, as well as respiratory diseases like tuberculosis [12, 15]. Its potent anti-inflammatory properties are effective for arthritis, skin disorders such as acne, eczema, hyperkeratosis, and psoriasis, as well as allergic reactions [12, 16]. In addition to traditional uses, mangosteen fruit, pericarp and whole fruit have been incorporated into a variety of dietary supplements, including capsules, tablets, powders, and beverages (Supplementary Table 1). However, it is important to note that these formulations have not been tested in clinical studies as anticancer agents.
Mangosteen is rich in bioactive compounds, including xanthones, terpenes, anthocyanins, and tannins. Xanthones are the predominant bioactive secondary metabolites, responsible for the most significant biological activities attributed to this fruit. Xanthones have been identified in different parts of the plant; however, most are concentrated in the pericarp of mangosteen fruit [12, 15].
2 Xanthones from Garcinia mangostana
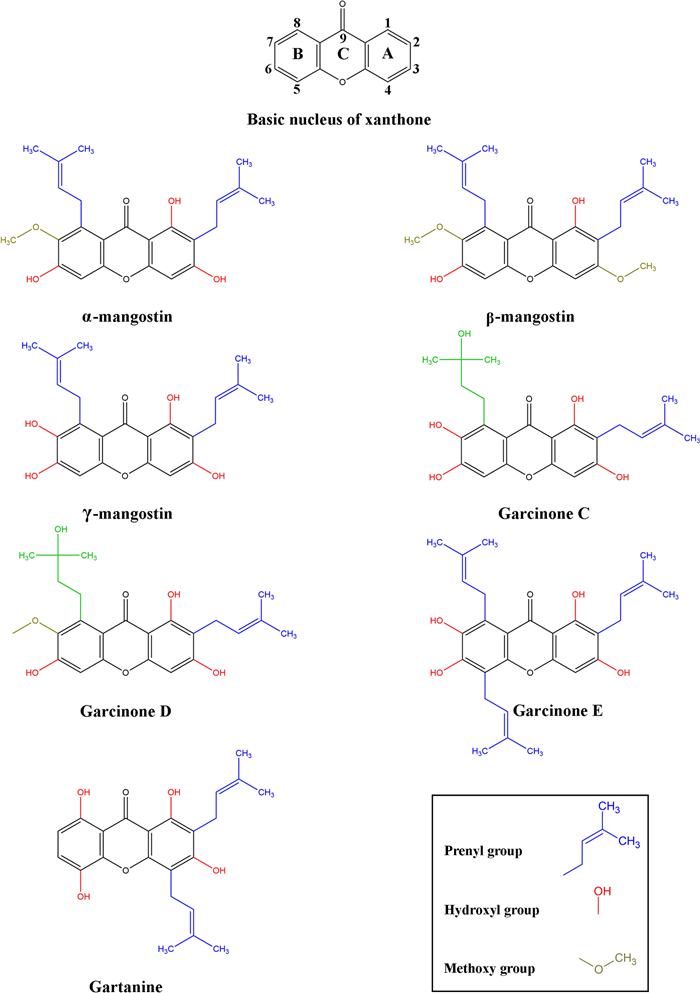
The pericarp exudes a yellow resin, hence the term “xanthones”, since the word xanthone is derived from the Greek word xanthós, meaning yellow. Xanthone is an aromatic oxygenated heterocyclic molecule with a planar, symmetric and tricyclic structure named dibenzo-γ-pyrone or 9H-xanthen-9-one. Xanthone has the molecular formula C13H8O2, its structure includes two aromatic rings: A-ring and B-ring, connected by γ-pyrone ring (C-ring), as shown in Fig. 2 [17].
Chemical structure of the major prenylated xanthones from mangosteen pericarp. The xanthone nucleus is an aromatic oxygenated heterocyclic molecule with a planar, symmetric, and tricyclic structure, comprising two aromatics rings: A-ring (carbons 1–4) and B-ring (carbons 5–8), connected by the C ring (γ-pyrone ring, carbon 9). The prenylated xanthones feature prenyl groups and vary in the number and position of these groups, as well as the hydroxyl and methoxy groups attached to the xanthone nucleus. These variations are believed to significantly influence the biological activities of the xanthones. The structures were created in ChemDraw version 20.0.0.41 (1998–2000 PerkinElmer informatics, Inc)
The xanthone nucleus is considered a “privileged structure” due to its ability to interact with various biological targets. This capability arises from its planar and rigid heteroaromatic tricyclic ring system that can accommodate a diverse group of substituents around these rings, the central carbonyl group capable of multiple interactions, and the biaryl ether group (two aromatic rings connected by an oxygen atom) contributing to the electronic system [18]. The xanthone nucleus can be substituted with various functional groups through processes such as alkylation, hydroxylation, prenylation, alkoxylation, and acylation, among others. Prenylated xanthones are the major group of naturally occurring xanthones, recognized for their anti-proliferative effects, attributed to the presence of prenyl groups (chains of carbon atoms derived from isoprene units) at key positions on the xanthone nucleus (Fig. 2) [18]. These prenyl groups facilitate the internalization of the molecule and influence protein interactions [15].
Structure–activity relationship studies indicate that both prenyl and hydroxyl groups on the xanthone nucleus are essential for promoting anti-proliferative effects [19]. By comparing the cytotoxicity of different xanthones and analyzing their structures, it has been concluded that the hydroxyl group at the C1 position and prenyl substituents at the C2 position in the xanthone nucleus are crucial for its cytotoxic effects [19, 20]. Hydroxylation at the C3 position enhances cytotoxicity, while its replacement by the methoxy group decreases the potency to cause mitochondrial dysfunction [15]. The cytotoxicity of xanthones is generally reduced by the hydroxylation of the prenylated chain in the C8, as observed in garcinone C and D, compared to α-mangostin and γ-mangostin [20]. The synthesis of α-mangostin derivatives highlights the significance of hydroxyl groups on C3 and C6 for cytotoxic activity. Di-substitution of these groups diminishes cytotoxicity against cancer cell lines, emphasizing the importance of retaining these hydroxyl groups. Additionally, the hydroxyl group at C7 plays a crucial role, replacing it with a methoxy group decreases cytotoxicity. Therefore, the number and locations of hydroxyl groups have varied effects on cytotoxicity, whereas oxidation of the prenyl group at C2 has limited impact. Moreover, Structure–activity relationship studies determined that maintaining the prenyl group at C8 is essential for retaining the cytotoxicity of xanthones; however, oxidizing the prenyl group at C8 results in the loss of this cytotoxicity [21, 22].
Interestingly, it was determined that certain xanthones could inhibit the cyclin-dependent kinase-4 (CDK4) protein, requiring specific functional groups for that, such as isoprenyl groups at the C2 and C8 positions, with hydroxyl groups extending from 3 and 7 positions, as well as the lack of isoprenyl group at the C4 position. The inhibition of CDK4 prevents the phosphorylation of downstream targets, which is crucial for stopping uncontrolled cell cycle progression. Vemu et al. hypothesized that the isoprenyl group at the C2 position of α-mangostin can bind deep within the ATP-binding pocket of CDK4. However, when the isoprenyl group is at C4, and absent at C8, as in gartanine, there is no inhibition of CDK4. It is possible that an isoprenyl group at C4 obstructs the binding of the xanthone to the CDK4 ATP-binding domain [22]. Hydroxylation of key groups is critical for inhibiting CDK4. Among α-mangostin, β-mangostin, and γ-mangostin, hydroxylation at the C3 and C7 positions resulted in the most potent inhibition, with γ-mangostin being the most effective. However, the in vitro assay did not consider phase Ⅰ metabolism, which may occur in vivo. It is possible that methoxy groups, such as those in α-mangostin (C7) and β-mangostin (C3 and C7), might undergo O-demethylation to generate γ-mangostin in vivo. When comparing γ-mangostin to garcinone D, hydroxylation of the isoprenyl group appeared to counteract the methoxy group at C7, thereby restoring CDK4 inhibition [22].
The most abundant mangostins in mangosteen fruit are α-mangostin, followed by γ-mangostin and β-mangostin. Other notable xanthones include garcinone E, gartanin, and 8-deoxygartanin. Their relative abundance varies depending on the extraction method used, as shown in Table 1.
Major xanthones extracted from mangosteen fruit tissues
The α-mangostin was the first xanthone isolated from the mangosteen pericarp, identified by W. Schmid in 1855. Between 1930 and 1932, Dragendorf and Murakami elucidated its structure, followed by Yates and Stout, who established its molecular formula in 1958 [12].
3 Therapeutic benefits of α-mangostin: antineoplastic properties
The α-mangostin has a wide range of medicinal properties, including antioxidant [23], antineoplastic [30], anti-inflammatory [31, 32], antihistamine [33], antibacterial [34], antifungal [35], antiviral [36-44], antimalarial [45], antidiabetic [46], antihyperlipidemic [46], cardioprotective [47, 48], hepatoprotective [49], neuroprotective [50], and immunomodulatory activities [51], among others.
In terms of its antineoplastic properties, α-mangostin has shown chemopreventive and antitumoral effects against various cancers, including cholangiocarcinoma [52], pheochromocytoma [53], glioblastoma [54], osteosarcoma [55], head and neck cancer [56], prostate cancer [57, 58], gastric cancer [59], pancreatic cancer [60-62], lung cancer [63], cervical cancer [43, 64], colon cancer [65], ovarian cancer [66, 67], skin cancer [68], renal cancer [69], and breast cancer [3, 70-79].
3.1 Mechanisms of α-mangostin involved in inhibiting breast cancer cell proliferation
In the context of breast cancer, while personalized therapeutic strategies have been developed and applied based on specific tumor phenotypes, α-mangostin has shown antiproliferative effects regardless of the tumor molecular expression profile. This includes its effectiveness in breast cancer cells representing tumor phenotypes such as Estrogen Receptor-positive (ER +), Human Epidermal Growth Factor Receptor Type 2 (HER-2) enriched, and Triple Negative (TN), which lacks expression of ER, Progesterone Receptor (PR), and HER-2 (Table 2).
Antiproliferative effects of α-mangostin or mangosteen extract on breast cancer cell lines
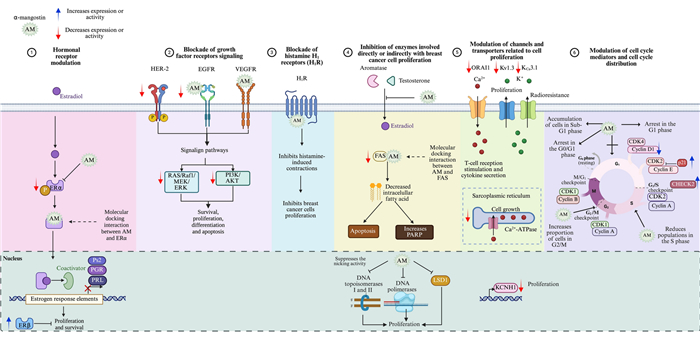
On the other hand, α-mangostin has demonstrated the ability to suppress breast cancer cell growth and the development of pre-neoplastic lesions induced by 7,12-dimethylbenzo (α) anthracene in a mouse mammary organ culture assay, with an IC50 of 2.44 µM [23]. Numerous studies highlight the impact of α-mangostin on breast cancer, identifying several mechanisms through which it may exert its antiproliferative effects (Fig. 3).
Antiproliferative mechanisms of α-mangostin. α-Mangostin (AM) exhibits diverse mechanisms to inhibit breast cancer cell proliferation, including: (1) Hormonal receptor modulation: AM reduces ERα expression and phosphorylation, increases ERβ levels, and inhibits estrogen-dependent gene expression. Docking studies indicate strong interactions with ERs, and derivatives of AM act as potential ER antagonists. (2) Blockade of growth factor receptor signaling: AM inhibits HER-2 phosphorylation and EGFR, and VEGFR binding, downregulating key oncogenic pathways (RAS/RAF1/MEK/ERK and PI3K/AKT). (3) Histamine H1 receptor (H1R) inhibition: AM blocks H1R, linked to tumor growth, and reduces breast cancer histamine levels. (4) Enzyme inhibition: AM targets aromatase, topoisomerases, DNA polymerases, Lysine-Specific Demethylase 1 (LSD1), and fatty acid synthase (FAS), disrupting proliferation, DNA processes, and lipid synthesis. (5) Ion channel modulation: AM inhibits oncogenic potassium channels (KCNH1, Kv1.3, KCa3.1), calcium transport (ORAI1, Ca2+ ATPase), impacting cell signaling and viability. (6) Cell cycle modulation: AM induces G1-phase arrest and downregulates cyclin-CDK complexes, preventing uncontrolled cell division. Figure created using BioRender.com
3.1.1 Modulation of hormone receptors by α-mangostin in breast cancer
In the canonical mechanism of action, ERα functions as a transcription factor upon ligand binding, driving the transcription of genes involved in cell proliferation. One mechanism through which α-mangostin inhibits cancer cell proliferation is by inhibiting ERα (Fig. 3). Specifically, at a concentration of 10 µM, α-mangostin suppresses ERα protein expression and decreases the estrogen-dependent gene Ps2 in MCF-7 cells. Notably, siRNA-mediated knockdown of ERα levels, followed by subsequent treatment with α-mangostin, reduces its effects, suggesting that the xanthone exerts its activity, at least in part, through ERα [76]. Additionally, ERα phosphorylation is reduced in T-47D cells treated with 30 µM of α-mangostin, suggesting that α-mangostin may additionally inhibit cell proliferation and survival by suppressing ERα signaling pathway [74]. Moreover, treatment of MCF-7 cells with mangosteen rind extract (50 and 100 µg/mL) decreased ERα gene expression in a concentration-dependent manner while increasing ERβ levels [80]. ERβ, in particular, plays a significant role in breast cancer, often acting as a tumor suppressor; while ERα promotes cancer cell proliferation, ERβ tends to inhibit it, and high levels of this protein are associated with better prognosis and improved survival rates [90].
Given the significance of estrogen signaling in breast cancer progression, blocking this pathway is a key therapeutic strategy in ER-positive tumors. In this context, it has been demonstrated that α-mangostin at 3.5 µM reduces the expression of estrogen-regulated genes, such as those encoding prolactin and PR, in MCF-7 cells, suggesting the interference with estrogen signaling [4].
In an additional study, the anti-proliferative effects of four xanthones, including α-mangostin, β-mangostin, mangostenol, mangaxanthone B, along with three benzophenones and one sterol, were evaluated. Initially, the antiproliferative activity of these compounds was assessed in the breast cancer cell lines MCF-7 and MDA-MB-231, identifying α-mangostin as the most potent compound, with IC50 values of 4.43 µM and 3.59 µM, respectively. Subsequently, a molecular docking simulation study was conducted to evaluate the interactions of xanthones with 2OCF (Protein Data Bank ID for the protein structure of ER). The analysis regarding α-mangostin revealed key interactions contributing to its potential biological activity. Specifically, the prenyl group at C8 of α-mangostin interacts with the Leu320, Trp393, Phe445, and Val446 residues of 2OCF at various distances (Å) through hydrophobic interactions. The aromatic ring A of α-mangostin interacts with Arg394, while rings B and C with Ile326 and Arg394 through hydrophobic interactions. Furthermore, the hydroxyl group at C1 and the carbonyl group (C = O) interact with Trp393 through hydrogen bonding. Additionally, a molecular docking simulation of xanthones was performed with 4PIV (Protein Data Bank ID for the β-ketoacyl part of Fatty Acid Synthase, FAS), which will be discussed in detail in the section regarding FAS inhibition [19].
Other studies have also explored α-mangostin and its derivatives as potential ERα antagonists. In one of them, α-mangostin was chemically modified by substituting the hydroxyl group at C6 with benzoyl derivatives. Computational analyses predicted that the resulting derivatives AMB-1, AMB-2, and AMB-10 may function as potential ERα antagonists. Of note, these findings were based solely in silico approaches and were not validated through experimental assays [71]. More recently, another study explored the conjugation of glycine to the hydroxyl groups at positions C3 and/or C6 of the α-mangostin, leading to the development of glycine-conjugated α-mangostin derivatives. Among these, the Am1Gly, with glycine attached to the hydroxyl group at C3, demonstrated promising potential as an ERα antagonist. Further pharmacophore modeling revealed that Am1Gly1 and Am2Gly2 possess structural features comparable to those of active drugs targeting ERα, reinforcing their potential for therapeutic development. Molecular docking analyses indicated that Am1Gly1 interacts with key residues essential for functioning as a 4-hydroxytamoxifen-like ERα antagonist, exhibiting dynamic interactions similar to those observed with 4-hydroxytamoxifen. These findings were supported by molecular dynamics simulations, which confirmed consistent amino acid variation patters over 200 ns. Additionally, binding affinity analysis, molecular docking, dynamic simulations, and pharmacophore modeling collectively supported the potential of Am1Gly1 as an ERα antagonist. This study was not accompanied by experimental validation [91].
3.1.2 Inhibition of growth-factor receptors signaling by α-mangostin in breast cancer
HER-2 is a receptor with intrinsic tyrosine kinase activity, comprising an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. While HER-2 does not have a known ligand that directly binds to it, it acts as the preferred heterodimerization partner for other members of the epidermal growth factor receptor (EGFR) family (ErbB). Thus, upon ligand binding to other ErbB members, HER-2 is recruited, leading to the autophosphorylation of its intracellular tyrosine kinase domains. This activation triggers the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB, also known as AKT) and mitogen-activated protein kinase (MAPK) signaling pathways, which are crucial for processes closely related to breast tumorigenesis such as cell survival, proliferation, differentiation, and apoptosis [92]. Notably, treatment of T-47D breast cancer cells with 30 µM of α-mangostin resulted in reduced phosphorylation of HER-2 at the Tyr1221/1222 within 30 min, leading to the deactivation of RAS/RAF1/MEK/ERK and PI3K/AKT signaling pathways, and consequently, an anti-proliferative and pro-apoptotic effect (Fig. 3) [74].
Additionally, molecular docking analysis provided evidence that garcinone E, another prenylated xanthone, which differs from α-mangostin by an isoprenyl group at the C5 position and hydroxyl group at the C7 position (Fig. 2), binds tightly to the EGFR and the vascular endothelial growth factor receptor (VEGFR), inhibiting their kinase activity with IC50 values of 315.4 nM and 158.2 nM, respectively. Furthermore, garcinone E blocks the endothelial growth factor (EGF)- and vascular endothelial growth factor (VEGF)-induced phosphorylation of EGFR and VEGFR, respectively. Although α-mangostin also binds to these receptors, it does so with less affinity than garcinone E. Beyond its molecular interactions, garcinone E demonstrated potent anti-cancer activity, inhibiting cancer cell proliferation, endothelial cell migration, invasion, and tube formation. Its dose-dependent suppression of angiogenesis was confirmed in ex vivo and in vivo models. In an MDA-MB-231 xenograft model, garcinone E (2 mg/kg) significantly reduced tumor growth, weight, and microvessel density, correlating with downregulation of VEGFR2, EGFR, and Ki-67 [93]. However, mangosteen rind extract (50 and 100 µg/mL) decreased the gene expression of EGFR in MCF-7 cells in a concentration-dependent manner [80]. In the TN breast cancer phenotype, EGFR is highly expressed, which is associated with cancer progression, proliferation, metastasis, and drug resistance, making EGFR a promising pharmacological target [94]. Therefore, α-mangostin could be considered a potential compound for targeting this receptor.
3.1.3 Inhibition of H1R by α-mangostin in breast cancer
α-Mangostin has been identified as an inhibitor of the H1R. Specifically, it exhibits a concentration-dependent inhibition of [3H]mepyramine binding, a selective H1 receptor antagonist, in rat aortic smooth muscle cells. Kinetic analysis indicates that α-mangostin acts as a competitive inhibitor of the H1R, preventing [3H]mepyramine from binding effectively with an IC50 value of 2.27 µM. Scatchard plot analysis further revealed that [3H]mepyramine binds to a high affinity receptor site with a dissociation constant (Kd) of 11.72 nM and a maximum binding capacity (Bmax) of 275.95 fmol/mg. Treatment with α-mangostin increased the Kd value to 38.02 nM, indicating reduced affinity, while the Bmax value remained unchanged. These findings confirm that α-mangostin function as a specific H1R antagonist [33] (Fig. 3). This is relevant because there is a direct correlation between endogenous histamine levels and breast cancer development. In particular, higher histamine concentrations have been observed in tumors and adjacent tissue of breast cancer patients compared to the unaffected tissue of healthy individuals [95]. Additionally, there is a significant increase in histamine levels in the plasma and tissues of patients with ductal breast cancer. The plasma histamine concentrations in women with ductal breast carcinoma depend on the number of involved lymph nodes and the grade of histologic malignancy [96]. Additionally, H1R is overexpressed in basal-type and HER-2-enriched breast tumors, which correlates with a shorter overall survival [97]. This supports the idea that histamine and its receptors are involved in cell growth. Furthermore, several antihistamines have been shown to inhibit breast cancer cell proliferation both in vitro and in vivo [98-101].
3.1.4 Inhibition of enzymatic activities associated with breast cancer cell proliferation by α-mangostin
3.1.4.1 Aromatase
Aromatase plays a pivotal role in converting androgens into estrogen through aromatization. Notably, breast cancer tissues exhibit higher levels of aromatase compared to healthy tissues [102]. Consequently, one of the therapeutic strategies in the clinical practice involves aromatase inhibitors to reduce breast cancer growth. These inhibitors hamper the enzyme’s function, affecting estrogen synthesis and, consequently, blocking breast cancer cell proliferation. Interestingly, a non-cellular, enzyme-based microsomal assay, revealed that garcinone D, garcinone E, α-mangostin, and γ-mangostin exhibited a dose-dependent aromatase inhibitory activity. Among these compounds, garcinone D (IC50 = 5.2 µM) and γ-mangostin (IC50 = 6.9 µM) demonstrated the strongest inhibitory effects in this assay. In contrast, the two other xanthones, α-mangostin (IC50 = 20.7 µM) and garcinone E (IC50 = 25.1 µM), displayed moderate inhibition of aromatase activity [103] (Fig. 3).
3.1.4.2 DNA polymerases and topoisomerases
DNA polymerase synthesizes new DNA strands during replication, while topoisomerases Ⅰ and Ⅱ play critical roles in cutting single-stranded or double-stranded DNA to relieve tension during replication or transcription events [104]. Interestingly, α-mangostin exhibits a strong inhibitory effect on both DNA polymerases (IC50 values 14.8 µM–25.6 µM) and topoisomerases Ⅰ and Ⅱ (15.0 µM and 7.5 µM, respectively). Notably, α-mangostin completely suppresses the nicking activity of topoisomerase Ⅰ at concentrations greater than 20 µM, while at 10 µM, it fully inhibits topoisomerase Ⅱ. Interestingly, as determined by thermal transition analysis, α-mangostin does not directly bind to double-stranded DNA. This suggests that the decreased proliferation observed with α-mangostin may be partially due to the inhibition of these enzymes [105] (Fig. 3).
3.1.4.3 Lysine-specific demethylase 1 (LSD1)
LSD1 is an enzyme that plays a crucial role in gene expression regulation through histone modification. It is frequently overexpressed in several human cancers, including breast cancer, where it promotes tumor progression by supporting cancer cell survival and creating a pro-oncogenic environment. As a histone demethylase, LSD1 removes methyl groups from specific lysine residues on histone proteins, mainly histone H3 at lysine 4 (H3K4) and lysine 9 (H3K9). This chromatin remodeling process, directly influences transcriptional activity, either repressing or activating gene expression [106]. In ERα-positive breast cancer, LSD1 demethylates H3K9 at ERα target genes in an estrogen-dependent manner, facilitating transcriptional activation. This regulatory mechanism is reinforced by Proline, Glutamate, and Leucine Rich Protein 1 (PELP1), a co-activator that links LSD1 to ERα, forming the LSD1-PELP1-ERα axis implicated in hormone resistance. Additionally, LSD1 indirectly promotes CYP19A1 expression, encoding aromatase, through its interaction with HER-2 signaling [107]. Conversely, LSD1 demethylates non-histone proteins, such as p53, leading to the repression of its activity [108]. Moreover, LSD1 downregulates CDH1 expression, the gene encoding E-cadherin, which may result in decreased E-cadherin levels, compromising cell adhesion, thereby facilitating EMT, a critical step in metastasis [109]. This dual regulatory function underscores LSD1´s central role in tumor progression and endocrine resistance, making it a promising therapeutic target. Regarding this, α-mangostin is a specific inhibitor of LSD1, with an IC50 value of 2.81 ± 0.44 µM. Bioactivity studies and molecular docking analysis indicated that this xanthone could inhibit the migration and invasion of MDA-MB-231 cells by targeting intracellular LSD1 activity. Moreover, α-mangostin increases the expression of the epithelial cell marker E-cadherin, while downregulating the mesenchymal cell marker N-cadherin, indicating its potential role in modulating EMT [110] (Fig. 3).
3.1.4.4 FAS inhibition
FAS is a metabolic enzyme synthesizing long-chain saturated fatty acids, essential for membrane formation in proliferating cells. In cancer cells, including breast cancer, FAS overexpression leads to de novo lipogenesis, incorporating lipids into the lipid rafts of tyrosine kinase membrane receptors, such as the EGFR family, triggering oncogenic signaling pathways that promote cell survival, proliferation, migration, and invasion. Therefore, inhibiting FAS reduces phospholipid synthesis and cell proliferation while increasing apoptosis. Additionally, FAS inhibition can disrupt lipid raft assembly and impair EGFR localization to the membrane of breast cancer cells. Due to its extensive and high expression in many human cancers, FAS has been proposed as a potential molecular target for developing antineoplastic drugs. In this context, α-mangostin (1–4 µM) has been shown to suppress the expression and activity of FAS in MCF-7 and MDA-MB-231 cells, leading to a decreased intracellular fatty acid accumulation (Fig. 3). This reduction can lower cell viability, induce apoptosis in breast cancer cells, increase levels of the Poly (ADP-ribose) polymerase (PARP) activation product, and affect the balance between anti-apoptotic BCL-2 and pro-apoptotic BAX proteins [75]. Additionally, treating breast cancer cells with α-mangostin decreases the phosphorylation of AKT, which suggests that PI3K/AKT is related to the downregulation of FAS expression by the xanthone. A molecular docking simulation study of α-mangostin with 4PIV determined that different groups of α-mangostin interact with several residues of 4PIV. The aromatic ring A of α-mangostin interacts with Leu1971 and Ile2063 through hydrophobic interactions. The hydroxyl groups at C1, C3, and C6 form hydrogen bonds with Ile 2068, Leu1971, and Trp2060 residues of 4PIV. The prenyl group at C2 interacts with Val1973 and Ile2068 residues, while the prenyl group at C8 interacts with Leu1975, Tyr2034, and Leu2069 residues through hydrophobic interactions. The methoxyl group at C7 interacts with Ser2021 and Gly2061 residues through hydrogen interactions. Additionally, ring C and the C = O interact with Ile2068 through hydrophobic and hydrogen interactions, respectively. These findings were supported by cell proliferation experiments, which demonstrated that α-mangostin exhibited strong anti-proliferative activity against breast cancer cell lines MCF-7 and MDA-MB-231, with IC50 values of 4.43 µM and 3.59 µM, respectively [19].
3.1.5 Modulation of ion channels by α-mangostin in breast cancer
The oncogenic voltage-gated potassium channel subfamily H member 1 (KCNH1), encodes the Ether-à-go-go 1 (EAG1, Kv10.1) potassium channel, which is overexpressed in various cancers, while inhibiting its expression or activity decreases cancer cell proliferation [111]. Interestingly, α-mangostin at concentrations of 3.5 µM and 7 µM significantly reduced KCNH1 gene expression in MCF-7 and T-47D breast cancer cells, respectively [4] (Fig. 3). Additionally, it was demonstrated that α-mangostin decreased KCNH1 gene expression in cervical cancer cells, both in vitro and in vivo [43]. These findings suggest that inhibiting KCNH1 expression could be another mechanism by which α-mangostin decreases cancer cell proliferation.
Furthermore, cytosolic calcium levels regulate cell growth. It has also been demonstrated that α-mangostin decreases the activity of Ca2+-ATPase and the calcium transport of the sarcoplasmic reticulum from rabbit skeletal muscle in a concentration-dependent manner, with an IC50 of 5 µM [112]. Additionally, the generation of intracellular calcium signaling is crucial for T-cell receptor stimulation and cytokine secretion. This signaling is mediated by the calcium release-activated calcium modulator 1 (ORAI1) channel and potassium ion channels, which provide the electrical driving force to generate sufficient calcium ion influx. α-Mangostin inhibited ORAI1 in a concentration-dependent manner with an IC50 of 1.27 ± 1.144 µM, and at 3 µM, suppressed the activity of the voltage-dependent potassium channel Kv1.3 and the intermediate-conductance calcium-activated KCa3.1 by 41.38 ± 6.19 % and 51.16 ± 5.38 %, respectively [113]. Noteworthy, the overexpression of Kv1.3 and KCa3.1 channels is also associated with aberrant cancer cell proliferation; indeed, the overexpression of Kca3.1 confers radioresistance to breast cancer cells [114, 115].
Although studies on α-mangostin modulation of ion channels in cancer are limited, an electrophysiological investigation explored its analgesic effects through ion channels modulation in dorsal root ganglion neurons. Specifically, α-Mangostin (1–3 µM) influenced neuronal excitability by hyperpolarizing the resting membrane potential, likely due to an increased K+ conductance, which ultimately suppressed action potential generation. This xanthone activated TREK-1, TREK-2, and TRAAK channels. Additionally, at 0.43 ± 0.27 µM, α-mangostin inhibited capsaicin-induced TRPV1 currents and partially reduced tetrodotoxin-sensitive voltage-gated Na+ (Nav) channel activity. Molecular docking analyses suggest that α-mangostin´s oxygen atoms establish hydrogen bonds with these channels [116].
3.1.6 Modulation of the cell cycle by α-mangostin in breast cancer
The inhibition of cell proliferation is closely related to cell cycle blockade. Numerous cell cycle mediators, including cyclins, CDKs, and CDK inhibitors (CDKI), play a crucial role in controlling different phases of the cell cycle. Disruption of their balance can alter cell cycle distribution, finally affecting cell proliferation [117].
In this context, α-mangostin (20 µM) led to G1-phase cell cycle arrest in MDA-MB-231 cells by increasing the expression of p21, a CDK inhibitor. Additionally, there was a tendency for increased expression of the cell cycle checkpoint regulator CHEK2. This rise in p21 and CHEK2 expression decreased CDKs and cyclin expression, resulting in G1-phase arrest and inhibition of cell proliferation [77]. In another study involving MDA-MB-231 cells, treatment with 5 µM α-mangostin resulted in an increased proportion of cells in the G2/M and S phases, while the proportion of cells in the G1-phase decreased. This effect was also associated with decreased Cyclin D1 expression (CCND1) [70]. Similarly, treatment with of α-mangostin (3.5 µM and 7.1 µM) in MCF-7 and T-47D cells reduced CCND1 gene expression [4].
The active complex CDK4/Cyclin D1 phosphorylates retinoblastoma (Rb) protein, which releases the transcription factor E2F and activates the expression of GI/S phase genes. Therefore, the up-regulation of the CDK4/Cyclin D1-Rb pathway increases the mitogenic potential of neoplastic cells, contributing to resistance to endocrine therapy in hormone-dependent cancers, including breast cancer. Interestingly, it has been determined, by a cell-free biochemical assay, that α-mangostin (IC50 value of 8.5 µM), as well as other isoprenylated xanthones, inhibited CDK4/Cyclin D1 complex, preventing the phosphorylation of downstream targets, therefore inhibiting uncontrolled cell cycle progression [22].
α-Mangostin at 1 µM, 5 µM and 10 µM resulted in a concentration-dependent accumulation of cells in the Sub-G1 phase, indicating xanthone-induced apoptosis in MCF-7 cells [76]. In T-47D cells, similar effects were observed, treatment with α-mangostin at 15 µM and 30 µM promoted Sub-G1 phase arrest with a decrease in G1-phase cells [74]. Additionally, α-mangostin treatment at 4.36 µM and 5.23 µM significantly promoted sub-G1 accumulation of T-47D and MBCDF cells, respectively, indicating cell death [3].
In BJMC3879 and BJMC3879 luc2 breast cancer cells, the treatment with α-mangostin 8 µM and 12 µM, respectively, induced cell cycle arrest in the G1 phase, with reduced populations in the S phase [78, 85].
3.2 α-Mangostin-induced apoptosis in breast cancer cells: mechanism of action
Apoptosis is a tightly regulated process of programmed cell death characterized by morphological changes in cells, chromatin condensation, DNA fragmentation, and caspase activation. It occurs via two primary pathways: the extrinsic and the intrinsic pathways. The extrinsic pathway is initiated by ligand binding, including Tumor Necrosis Factor-alpha (TNF-α), CD95L/FasL, or TRAIL to their corresponding death receptors on the cell surface. This interaction leads to the formation of death-inducing signaling complexes and activation of caspase-8, which initiates the apoptotic cascade. In contrast, the intrinsic pathway, or the mitochondrial pathway, is triggered by cellular stress or DNA damage. This pathway involves mitochondrial membrane depolarization and is regulated by members of the BCL-2 protein family. Disruption of the mitochondrial membrane leads to the release of key apoptotic factors into the cytosol, including the cytochrome c, a caspase-dependent apoptotic factor, and Apoptosis-Inducing Factor (AIF), a caspase-independent apoptotic factor. Both pathways converge on the activation of caspase-3, cleavage of PARP, DNA fragmentation, and, ultimately, apoptotic cell death. Apoptosis is regulated by a variety of signaling pathways, PI3K/AKT, MAPK/ERK1/2, JNK1/2, and p38 play pivotal roles in regulating cellular fate. ERK1/2 promotes cell growth, survival, and differentiation, whereas JNK1/2 and p38 are primarily associated with apoptotic processes. The PI3K/AKT pathway is another significant regulator of apoptosis, with AKT influencing key proteins such as BCL-2, BAX, and caspase-3 [74].
It has been demonstrated that one of the primary mechanisms by which α-mangostin exerts its effects in cancer cells is the induction of apoptosis through distinct pathways. The pro-apoptotic effects of α-mangostin has been predominantly investigated in the human breast cancer cell lines MCF-7, T-47D, and MDA-MB-231 cells, as summarized in Table 3.
Apoptotic effects of α-mangostin or mangosteen extract on human breast cancer cell lines
In MDA-MB-231 cells, prenylated xanthones from mangosteen pericarp, including α-mangostin, γ-mangostin, and garcinone E, target mitochondria by inhibiting mitochondrial respiratory chain complex Ⅱ. This inhibition disrupts oxidative mitochondrial respiration, increases mitochondrial proton leakage, and diminishes ATP production. As a result, mitochondrial superoxide levels rise, leading to membrane permeabilization and trigger apoptosis through caspase-3 and -7 activation. Therefore, the apoptotic effects of this xanthones are mediated through interference with mitochondrial respiration and cellular energy metabolism [87].
Likewise, in SK-BR-3 cells, treatment with crude methanolic extract (CME) from mangosteen pericarp (20–100 µg/mL) resulted in distinct morphological alterations, such as cell retraction, rounding, and membrane blebbing, accompanied by the formation of apoptotic bodies, nuclear fragmentation, and shrinkage. Notably, at 100 µg/mL, CME induced significant DNA fragmentation, further indicating its pro-apoptotic activity [84].
Moreover, α-mangostin induces apoptosis in additional cancer cell models through distinct mechanisms. In BJMC3879 murine mammary adenocarcinoma cells, α-mangostin increased the number of TUNEL-positive cells, elevated caspase activity, and reduced mitochondrial membrane potential [85]. At a concentration of 12 µM for 24 h, α-mangostin triggered apoptosis in BJMC3879 luc2 cells by activating caspases -3, -8, and -9. At 48 h, a significant increase in apoptotic cells was observed via TUNEL assays, and α-mangostin also enhanced the release of cytochrome c into the cytosol [78].
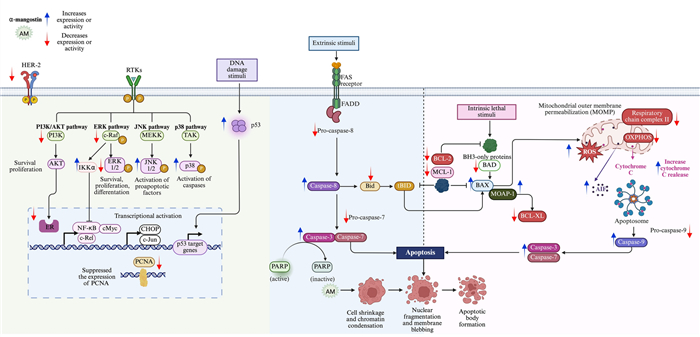
In a general way, α-mangostin induces apoptosis through both caspase-dependent and caspase-independent mitochondrial pathways, and is also associated with the modulation of signaling pathways involved in cell death regulation (Fig. 4).
Antiapoptotic mechanisms of α-mangostin. The xanthone α-mangostin (AM) triggers apoptosis via the extrinsic pathway, initiated by ligand-receptor interactions leading to caspase-8 activation, and the intrinsic pathway, which involves mitochondrial membrane depolarization, cytochrome c release, and caspase-9 activation. Both pathways converge on the activation of caspase-3, cleavage of PARP, and execution of apoptosis. AM regulates BCL-2 family protein, downregulating BCL-2, BID, MCL-1, BCL-XL, and BAD, and upregulating BAX. Also, AM affects apoptosis through an independent caspase pathway, including apoptosis-inducing factor (AIF). Additionally, AM downregulates AKT and ERK1/2 signaling while enhancing JNK1/2 and p38 phosphorylation, favoring apoptotic cell death. AM-induced apoptosis is mediated by the Modulator of Apoptosis 1 (MOAP-1), which interacts with activated BAX while downregulating BCL-XL. AM also increases p53 expression, targets mitochondrial respiratory chain complex Ⅱ, inhibits oxidative mitochondrial respiration (OXPHOS), and increases ROS production. Figure created using BioRender.com
3.3 Mechanisms of α-mangostin-mediated inhibition of cell adhesion, invasion and metastasis in breast cancer
The inhibitory mechanism of α-mangostin in these processes include the suppression of EMT, a biological process in which epithelial cells acquire mesenchymal characteristics, leading to reduced adhesion and enhanced migratory and invasive potential. While EMT plays a crucial role in embryonic development and wound healing, its dysregulation is closely linked to cancer metastasis. Another key mechanism involves the inhibition of FAK, a protein that regulates cell proliferation, survival, adhesion, and migration. The overexpression of FAK in cancer is associated with abnormal cell invasion and metastasis.
3.3.1 Inhibition EMT in breast cancer by α-mangostin
EMT is marked by decreased expression of E-cadherin and increased expression of N-cadherin and vimentin, along with cellular proteases. The primary mediators of the EMT include signaling through Tumor Growth Factor β (TGF-β), Notch, and Wnt, and is also influenced by the tumor microenvironment, such as hypoxia and differential expression of micro RNAs (miRNAs). These signaling mechanisms converge on transcription factors like Snail, Zeb, and Twist, whose differential expression in tumors has been shown to lead to EMT [118].
Interestingly, in MCF-7 cells, the mangosteen rind extract (50 and 100 µg/mL) increased the gene expression of E-cadherin while decreasing the levels of N-cadherin and Snail in a concentration-dependent manner, as well as inhibiting the activity of metalloproteinase-9 (MMP-9) and reducing the migration of these cells [80], strongly supporting its EMT-inhibitory capacity (Table 4).
Mechanisms of α-mangostin inhibiting metastasis and angiogenesis in breast cancer and endothelial cells
Excessive degradation of the extracellular matrix is a hallmark of tumor invasion and migration, often involving overexpression of MMP-2 and MMP-9. In MCF-7 breast cancer cells, at 6 µM, α-mangostin inhibited cell adhesion by 61% and cell motility by 90%. Furthermore, α-mangostin reduced cell invasion and migration by 55% and 61%, respectively, by changing cell shape and reducing their invasive and migratory capacity. α-Mangostin antimetastatic effects are due to the inhibition of MMP-2 and MMP-9 activity, reducing their expression at both mRNA and protein levels, achieving inhibition rates of 88% for MMP-9 and 95% for MMP-3 at 6 µM. Mechanistically, α-mangostin inhibits ERK phosphorylation and reduces the DNA binding activity of NF-kB, AP-1, c-Fos, and c-Jun, key transcription factors involved in TPA-induced MMP expression. α-Mangostin also increases the expression of the kinase inhibitor IkK, blocking phosphorylation of IkBα, which further suppresses MMP expression. Combining α-mangostin with IkK inhibitors demonstrates a synergistic reduction in MMP levels [79]. Additionally, α-mangostin inhibits the TNF-α induced NF-kB translocation into the nucleus [119] (Table 4).
Moreover, the Signal Transducer and Activator of Transcription 3 (STAT3) is a transcription factor that, when activated, promotes EMT by inducing the expression of EMT-related genes. STAT3 is also involved in various signaling pathways that contribute to breast cancer progression, promoting proliferation, inhibiting apoptosis, supporting angiogenesis and metastasis, and conferring resistance to breast cancer therapy [120]. Interestingly, α-mangostin binds to STAT3, inhibiting MCF-7 and MDA-MB-231 cells migration and reducing their invasive capacity [83] (Table 4).
On the other hand, C-X-C- chemokine receptor type 4 (CXCR4) plays a crucial role in cancer cell metastasis. The binding of chemokine stromal cell-derived factor 1 (SDF-1 or CXCL12) to the G-protein coupled receptor CXCR4 activates signaling pathways that lead to the expression of genes involved in EMT, contributing to cancer cell migration, invasion, and metastasis. CXCR4 is highly expressed in the TN breast cancer cells MDA-MB-231, where α-mangostin, at a concentration of 10 μM, significantly suppressed cell migration. Additionally, molecular docking simulation suggested a potential interaction between α-mangostin and CXCR4 [88] (Table 4).
3.3.2 Inhibition of FAK by α-mangostin in breast cancer
Activation of FAK through phosphorylation at the Tyr397 active site is essential for cancer progression and metastasis by modulating these cellular processes. Interestingly, α-mangostin (1–4 µM/24 h) disrupts FAK activation by blocking Tyr397 phosphorylation at the active site, thereby reducing its interaction with extracellular matrix-associated proteins in MCF-7 and MDA-MB-231 breast cancer cells. Furthermore, FAK phosphorylation was downregulated following FAS silencing, suggesting that inhibiting FAS may attenuate FAK activity. Thus, the antimetastatic effect of α-mangostin may be associated with its inhibitory action on FAS [75] (Table 4 and Fig. 5).
Mechanisms by which α-mangostin inhibits adhesion, invasion and metastasis. The α-mangostin (AM) downregulates mesenchymal markers (N-cadherin, vimentin, Snail) while upregulating the epithelial marker E-cadherin, counteracting EMT progression. AM suppresses TPA-induced expression and activity of matrix metalloproteinases (MMP-2 and MMP-9) by inhibiting ERK phosphorylation and reducing c-Fos, c-Jun DNA binding activity and reducing TNF-α-induced NF-κB and AP-1 nuclear translocation. AM increases IκK expression, blocking IκBα phosphorylation and further decreasing MMP expression. AM also inhibits STAT3 activation, reducing EMT-associated gene expression, and impairs CXCR4 signaling, inhibiting migration and invasion. Disrupts FAK activation by inhibiting phosphorylation. Additionally, AM downregulates FAS, which may attenuate FAK activity. Figure created using BioRender.com
3.4 Antiangiogenic effects of α-mangostin in breast cancer
Tumor angiogenesis is essential for the growth and progression of solid tumors, and the VEGF/VEGFR axis is a key driver this process. In mammals, the VEGF family includes VEGF-A, VEGF-B, VEGF-C, and placental growth factor (PLGF), with VEGF-A being the most studied and commonly referred to as VEGF.
The primary receptors for VEGF are VEGFR-1 and VEGFR-2. VEGFR-1 is involved in hematopoiesis, MMPs activation, and the recruitment of monocytes and other immune cells into the tumor microenvironment. VEGFR-2 plays a central role in vasculogenesis and angiogenesis.
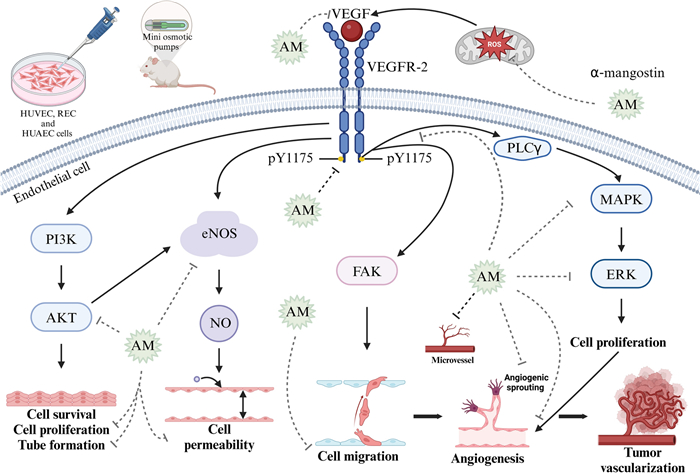
Binding of VEGF to its receptor VEGFR-2 initiates multiple signaling pathways that play essential roles in vascular function. One key pathway involves nitric oxide synthase (NOS), where endothelial NOS (eNOS) and inducible NOS (iNOS) mediates the release of nitric oxide (NO), a potent vasodilator that increases vessel permeability. Another essential signaling pathway is the PI3K/AKT, where PI3K activation initiates downstream activation of PKB, promoting endothelial cell survival, proliferation, and tube formation. Additionally, activation of FAK pathway induces endothelial cell migration, further promoting angiogenesis [124] (Fig. 6). Specifically, VEGF binding to VEGFR-2 induces phosphorylation at key tyrosine residues, including Y951, Y1054, Y1059, Y1175, and Y1214. Among these, phosphorylation of Y1175 is particularly important as it creates binding sites for phospholipase C gamma (PLCγ). This interaction activates the MAPK/ERK1/2 signaling pathway, conducting endothelial cell proliferation, a critical step in angiogenesis [125].
Antiangiogenic effects of α-mangostin in endothelial cells. α-Mangostin (AM) exerts its antiangiogenic effects through multiple mechanisms. AM reduces reactive oxygen species (ROS) production in hypoxic endothelial cells and decreases VEGF protein expression. It also inhibits VEGFR-2 phosphorylation at tyrosine residue Y1175, thereby disrupting key downstream signaling pathways involved in angiogenesis. These include the inhibition of AKT phosphorylation, inhibiting endothelial cell proliferation, and tube formation. AM reduces vessel permeability by reducing endothelial nitric oxide synthase (eNOS) activity. Also, VEGFR-2 inhibition prevents MAPK/ERK1/2 activation, reducing endothelial cell proliferation. Additionally, AM inhibits angiogenic sprouting, endothelial cell migration, and microvessel density. These effects have been demonstrated in both in vitro models (HUVECs, RECs, and HUAECs) and in vivo murine xenograft models. Figure created using BioRender.com
Interestingly, α-mangostin at 10 μM inhibits VEGFR-2 phosphorylation by targeting the Y1175 residue. This inhibition effectively reduces the proliferation of Human Umbilical Vein Endothelial Cells (HUVEC) and Human Umbilical Artery Endothelial Cells (HUAEC), with IC50 values of 1.2 μM and 2.4 μM, respectively. Furthermore, α-mangostin inhibits HUVEC migration with an IC50 value of 0.03 μM and suppresses tubule formation at concentrations of 0.6 μM and 1.2 μM (Fig. 6) [121]. ROS production induces VEGF expression in Bovine Retinal Endothelial Cells (REC) under hypoxic conditions. Notably, α-mangostin at concentrations of 1 μM, 4 μM, and 8 μM inhibited concentration-dependent ROS production, attenuated VEGF-induced endothelial cell hyperpermeability, and suppressed VEGF-induced endothelial cell proliferation, migration, and 3D tube formation. Additionally, α-mangostin blocked angiogenic sprouting in the ex vivo aortic ring assay. Additionally, α-mangostin inhibited VEGF-induced phosphorylation of VEGFR-2 and the downstream activation of MAPK/ERK1/2-signalling, which are key pathways in angiogenesis [122]. Moreover, it has been demonstrated that the ethanol pericarp extract of mangosteen, predominantly composed of α-mangostin, can reduce VEGF protein expression in T47D breast cancer cells [123] (Table 4).
An in vivo study demonstrated that panaxanthone, comprising approximately 80% of α-mangostin and 20% of γ-mangostin, exhibits both antitumoral and antiangiogenic effects. Specifically, panaxanthone administered at 5000 ppm in the diet of mice xenografted with BJMC3879 murine mammary cancer cells, significantly reduced microvessel density within mammary tumors, indicating a marked suppression of tumor angiogenesis [85] (Table 4).
Similarly, another study demonstrated that α-mangostin treatment at 10 and 20 mg/kg/day, administered via mini osmotic pumps to mice xenografted with BJMC3879luc2 cells, reduced microvessel density in mammary tumors [78]. The mechanism by which α-mangostin exerts its antiangiogenic effects may involve a significant decrease in phospho-AKT levels at Thr308 both in vitro (α-mangostin treatment at 20 μM for 3 and 6 h) and in vivo models, suggesting downstream inhibition of AKT signaling pathways [85]. Once activated, AKT phosphorylates various downstream targets involved in critical cellular processes such as cell proliferation, DNA repair, metabolism, cell cycle progression, cell survival, and angiogenesis. In the context of angiogenesis, AKT activates eNOS through phosphorylation, promoting nitric oxide production and facilitating angiogenesis [126]. The inhibition of AKT phosphorylation by α-mangostin may also suppress eNOS activity, further contributing to its anti-angiogenic effects [78] (Table 4).
3.5 Targeting PD-L1: α-mangostin´s potential in breast cancer immunomodulation
The immune checkpoint Programmed Death-Ligand 1 (PD-L1) plays a crucial role in breast cancer, particularly in the interaction between the immune system and cancer cells. PD-L1 is expressed on the surface of some cancer cells, including those in breast cancer, and binds to the PD-1 receptor on T cells. This interaction deactivates the T cells, preventing them from attacking cancer cells, thus facilitating immune evasion. Besides aiding in immune evasion, PD-L1 also promotes tumor growth and progression through various mechanisms [127]. Given this, therapies targeting the PD-1/PD-L1 pathway show great promise for immunotherapy. Drugs that block PD-L1 or PD-1 can reactivate T cells, allowing them to target cancer cells.
In MDA-MB-231 breast cancer cells, which have high levels of PD-L1, both α-mangostin (10 µM) and the mangosteen pericarp ethanol extract (10 µg/mL) significantly inhibit PD-L1 protein expression when treated for 72 h. In silico analysis shows that α-mangostin binds inside PD-L1 dimer pockets, suggesting that α-mangostin stabilized the dimer form, potentially leading to PD-L1 degradation [86].
3.6 Modulation of non-coding RNAs by α-mangostin in breast cancer
The xanthone α-mangostin, also may exert antitumor activity by modulating non-coding RNAs (ncRNAs), which play essential roles in gene expression and chromatin structure regulation. NcRNAs, transcribed from the non-coding DNA in the human genome, are classified into long non-coding RNAs (lncRNAs) and microRNAs (miRNAs). LncRNAs, over 200 nucleotides in length, regulate transcription, influence RNA splicing and stability, and indirectly modulate miRNA activity by acting as molecular sponges. MiRNAs, about 22 nucleotides long, bind to specific mRNA sequences to modulate gene expression, typically affecting mRNA stability or translation. The expression of ncRNAs and miRNAs is tightly regulated; while their dysregulation is linked to diseases, such as cancer, where they act as oncogenes or tumor suppressors [128].
Although no studies have specifically explored the antitumor role of α-mangostin in breast cancer through the direct modulation of lncRNAs or miRNAs, some research has investigated these effects in other cancer models and precursor lesions. In this context, a recent study investigated the antineoplastic effects of α-mangostin in oral submucous fibrosis, a premalignant condition characterized by chronic inflammation, submucosal fibrosis, and myofibroblast activation. This study demonstrated that α-mangostin reduces myofibroblast viability without affecting normal oral cells and inhibits myofibroblast activation, TGF-β/Smad2 signaling, and fibrosis markers through the suppression of LincROR, a lncRNA overexpressed in fibrosis [129]. This lncRNA-mediated mechanism underlies the protective effect of α-mangostin in preventing the progression of oral submucous fibrosis to oral cancer.
Regarding the relationship between miRNAs and the antitumor effects of α-mangostin in cancer, a study conducted on human pancreatic cancer MIA PaCa-2 cells demonstrated that α-mangostin induces autophagy through an AMPK/mTOR and p38-dependent mechanism. Notably, a miRNA PCR array profiled 84 miRNAs in MIA PaCa-2 cells following treatment with α-mangostin. Most miRNAs were found to be downregulated, such as miR-18a, while two, miR-146a and miR-302c, were up-regulated by more than twofold. TaqMan assays further corroborated the reduced expression of miR-18a [130].
Two additional studies conducted in colon cancer cells have investigated the role of α-mangostin as a significant modulator of miRNA expression. In human colon cancer DLD-1 cells, treatment with α-mangostin reduced cell viability by promoting apoptosis. Furthermore, when combined with conventional chemotherapeutic agents such as 5-fluorouracil, α-mangostin enhanced growth inhibition in these cells. Notably, α-mangostin increased the levels of miR-143, a miRNA known to negatively regulate Erk5, a key member of the MAPK family involved in cell proliferation and survival [131]. In a separate study, Kumazaki et al. explored therapeutic strategies to overcome resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in DLD-1 cells and cancer stem-like breast epithelial MCF10A cells. They identified that the expression of death receptor 5 (DR5) is critical for TRAIL sensitivity. Remarkably, α-mangostin reversed TRAIL resistance by upregulating DR5 through the downregulation of miR-133b, a negative regulator of DR5 expression [132].
Overall, these studies underscore the potential significance of miRNAs in the antitumor mechanisms of α-mangostin and emphasize the need for further research in this promising yet underexplored area.
4 α-Mangostin as an adjuvant of conventional therapies in breast cancer
In breast cancer, the antineoplastic effects of α-mangostin have been evaluated in combination with doxorubicin, 5-fluorouracil and tamoxifen, modifying its efficacy and metabolism. In MCF-7 cells, the ethanolic extract of mangosteen, combined with doxorubicin, increases the cytotoxic activity of the chemotherapeutic agent, which could be related to the induction of apoptosis in neoplastic cells and the inhibition of P-glycoprotein expression [133]. Another study reported that in spheroids formed by MCF-7 cells, the combination of α-mangostin and doxorubicin resulted in significant cytotoxicity and notable inhibition of retinaldehyde-dependent aldehyde dehydrogenase (RALDH) isoenzyme activity, which suggests that this combination could be effective in reducing cell stemness [2]. Furthermore, it has been determined that α-mangostin synergizes the antineoplastic effect of the chemotherapeutic agent 5-fluorouracil in breast cancer cells with different phenotypes, allowing for a dose reduction [3]. Additionally, in MCF-7 and T-47D breast cancer cells, the combination of α-mangostin with 4-hydroxy-tamoxifen not only promoted a synergistic antiproliferative effect but also allowed for a dose-reduction of both compounds. Moreover, this combination inhibited, to a greater extent, the gene expression of KCNH1, CCND1, and BIRC5 (survivin), markers of oncogenesis, cell cycle progression, and inhibition of apoptosis, respectively [4].
5 Antitumor effects of α-mangostin in breast cancer murine models
Panaxanthone inhibited tumor growth and metastasis in a mouse model of breast cancer. BJMC3879 cells, a mammary adenocarcinoma cell line highly metastatic to the lungs and lymph nodes with p53 mutation, were subcutaneously (s.c.) inoculated into mice, generating tumors two weeks later. Treatment began at 8 weeks with 2500 ppm and 5000 ppm of panaxanthone. At 2500 ppm, tumor growth and lung metastasis multiplicity were inhibited, with a greater effect observed at 5000 ppm. The antitumor effects of panaxanthone were associated with increased apoptosis, inhibition of cell proliferation (inhibition of PCNA), and anti-angiogenesis (reduced microvasculature density) [85].
In another mouse model study, BJMC3879luc2 cells were s.c. xenografted (2.5 million/0.3 mL of PBS). Three weeks later, with tumor diameters between 0.4 and 0.6 cm, mice were treated with 0, 10 or 20 mg/kg/day of α-mangostin via mini osmotic pumps for 6 weeks. Αt a dose of 20 mg/kg, α-mangostin decreased tumor volume and metastasis to lymph nodes, increased apoptotic cell death, elevated expression of active caspase-3 and -9, and reduced microvasculature density, cell migration via lymphatic vessels, and phosphorylation of AKT [78].
It is widely acknowledged that chronic inflammation, induced by factors such as infections, obesity, and genetic mutations, promotes immunosuppression and contributes to cancer progression as well as aging-related metabolic disorders [134]. A study found that α-mangostin reduces pro-inflammatory cytokines and chemokines in mice, alleviating adiposity, hyperlipidemia, and insulin resistance. It also reduced macrophage content and shifted their polarization, protecting against liver injury by suppressing miRNA-155-5p secretion, making α-mangostin a promising candidate for treating inflammation and aging-related metabolic disorders [135].
In a study using the rat LA7 mammary adenocarcinoma cells model, cells were injected into the mammary fat pads of Sprague–Dawley rats (2.0 million/0.1 mL). Ten days post-implantation, the rats were treated orally via gastric tube with 30 and 60 mg/kg of α-mangostin (from Cratoxylum arborescens) dissolved in Tween 20 and s.c. with 10 mg/kg of tamoxifen dissolved in the same vehicle. Tumors in the control group grew rapidly, reaching an average volume of 1737 ± 563 mm3 by day 28. In contrast, groups treated with 30 mg/kg and 60 mg/kg of α-mangostin showed a significant reduction in tumor volume compared to the control group, with 60 mg/kg of α-mangostin resulting in a greater reduction. Specifically, α-mangostin at 30 mg/kg reduced tumor volume by 74.1%, while the highest inhibition of 79.2% was observed with 60 mg/kg. For comparison, tamoxifen at 10 mg/kg decreased tumor volume by 83.6% [119].
Using the same rat breast cancer model, it was found that α-mangostin not only protects the rat mammary gland from the tumorigenic effects of LA7 cells by increasing apoptosis and suppressing proliferation (as shown by reduced PCNA and increased p53 expression), but also induces antioxidant effects and lowers the serum levels of the cancer biomarkers Carcinoembryonic Antigen and Cancer Antigen 15–3 [136].
6 Clinical trials involving mangosteen
To present date, no clinical trials investigating mangosteen products as anticancer agents have been conducted. Instead, most clinical trials have focused on evaluating their anti-oxidant, anti-inflammatory and anti-obesity effects, as well as their impact on neurological disorders, safety, and tolerability [137-142].
6.1 Safety, bioavailability, antioxidant and anti-inflammatory effects of mangosteen
Few studies have been developed to determine the absorption, bioavailability, antioxidant, and anti-inflammatory effects of mangosteen product consumption, as shown in Table 5.
In vivo studies of bioavailability and antioxidant effects of mangosteen beverages
An additional clinical trial determined that the oral consumption of mangosteen pericarp polar extract capsules (220–280 mg/day) by healthy subjects is safe for up to 24 weeks without liver or kidney dysfunction and exhibited antioxidant effects [148]. In a clinical trial involving patients with acute exacerbation of chronic obstructive pulmonary disease, the addition of mangosteen pericarp extract (1100 mg/twice a day) during hospitalization was safe and significantly decreased the plasma levels of interleukin (IL)-6 and malondialdehyde, shortening the length of stay [149].
7 Adverse effects of α-mangostin both in vitro and in vivo
Regarding the antiproliferative effects of α-mangostin in non-neoplastic cell lines, it was demonstrated that the xanthone exhibits cytotoxicity against the monkey kidney tissue-derived Vero cell line, with a half-maximal cytotoxic concentration (CC50) of 7.5 ± 0.04 µM [19]. Another study also found that α-mangostin inhibited Vero cell proliferation with an IC50 value of 14.26 µM [74].
Interestingly, a study demonstrated that treating isolated rat platelets with α-mangostin (1–10 μM) inhibited platelet aggregation in a concentration-dependent manner and induced platelet shape changes; while at higher concentrations (25 and 50 µM), α-mangostin caused platelet lysis [150].
Unfortunately, in vitro studies have shown that α-mangostin exhibits hemolytic activity in rabbit red blood cells (red blood cells volume/volume of α-mangostin at 4%) and human blood (α-mangostin at 100 µg/mL, equivalent to 242.62 µM), as indicated by the amount of hemoglobin released from erythrocytes [151, 152]. While this effect has only been observed in vitro, it is mitigated by encapsulating α-mangostin into nanoparticles [153]. To our knowledge, such an effect has not been reported with in vivo administration.
On the contrary, some in vivo studies did not report adverse effects. For instance, the consumption of a diet containing 845 mg/kg of α-mangostin showed no adverse effects in mice [154]. However, a pharmacokinetic study in ICR mice determined that the lethal dose (LD50) for intraperitoneal administration of α-mangostin is 150 mg/kg and 231 mg/kg for mangosteen extract [155]. In a murine model of colitis induced by dextran sulfate sodium administration to C57BL/6 J mice, dietary administration of α-mangostin (900 mg of α-mangostin per kg of diet) exacerbated the condition. The treated mice exhibited greater colonic inflammation and injury in the colon compared to the control group, along with intestinal dysbiosis and other signs [156]. In general, in clinical trials no side effects have been reported; however, in a case report described in 2008, a 58-years-old patient showed severe lactic acidosis associated with mangosteen juice consumption during the last 12 months as a dietary supplement to lose weight. The medical history of the patient included pulmonary sarcoidosis, metabolic syndrome, and chronic kidney disease [157].
It is important to note that all signs and symptoms of adverse effects depend on the concentration or dose of α-mangostin, as well as on the administration route.
8 Conclusion
α-Mangostin, an accessible natural antineoplastic compound derived from the mangosteen tree, represents a promising treatment option for breast cancer, whether used independently or alongside conventional therapy, as supported by numerous preclinical studies. The variety of mechanisms of action associated with α-mangostin makes it a powerful therapeutic alternative; however, much remains to be studied, including its in vivo effects in carefully-designed randomized controlled trials. These trials are urgently needed, as none have been conducted on breast cancer so far. Besides helping to determine α-mangostin therapeutic efficacy, clinical trials will outline the adverse effects derived from α-mangostin administration, allowing to define the adequate dose and administration route.
Notes
Acknowledgements
D. A.-M., a student of the Bachelor's degree in Nutrition at the Facultad de Estudios Superiores Zaragoza of the Universidad Nacional Autónoma de México (UNAM), is developing her bachelor´s thesis under the mentorship of J. G.-Q. She receives a scholarship from the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI, México) as a Research Assistant to L. D. Also, M. F.-G., a student of the Bachelor´s degree in Químico Farmacéutico Biólogo at the Unidad Académica de Ciencias Químicas of the Universidad Autónoma de Zacatecas (UAZ), is developing his Social Service under the mentorship of J. G.-Q. R. V.-C., a student of the Maestría en Ciencias Bioquímicas under the mentorship of R. G.-B. at UNAM, is receiving a fellowship from SECIHTI.
Author contributions
D.A.-M. wrote the sections on the taxonomy of mangosteen, antiangiogenic effects of α-mangostin in breast cancer, adverse effects, and clinical trials related to mangosteen. M.F.-G. wrote the sections on xanthones and antitumor effects of α-mangostin in breast cancer murine models. D.A.-M. and M.F.-G. wrote the sections on traditional medicinal applications of mangosteen and its fruit-based supplements, as well as the mechanisms by which α-mangostin inhibits breast cancer cell proliferation. J. G.-Q supervised the overall writing of the article and also contributed to the sections discussing the antineoplastic properties of α-mangostin, its mechanisms of inhibiting cell adhesion, invasion and metastasis, as well as its potential role in breast cancer immunomodulation, and as an adjuvant to conventional therapies. R. V.-C. and R. G.-B. wrote the section on α-mangostin-induced apoptosis, created Figs. 3, 4, 5 and 6 and contributed to the section discussing the mechanisms underlying α-mangostin-mediated inhibition of cell adhesion, invasion and metastasis. E. A. wrote the sections on the xanthones from Garcinia mangostana and the modulation of non-coding RNAs. L.D. was responsible for the abstract, introduction, and conclusion sections. All authors read and gave feedback on all sections, and approved the final version of the review. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received to assist with the preparation of this manuscript.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
References
-
1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3): 229-63. CrossRef PubMed Google Scholar
-
2.Bissoli I, Muscari C. Doxorubicin and α-mangostin oppositely affect luminal breast cancer cell stemness evaluated by a new retinaldehyde-dependent ALDH assay in MCF-7 tumor spheroids. Biomed Pharmacother. 2020;124: 109927. CrossRef PubMed Google Scholar
-
3.Lara-Sotelo G, Díaz L, García-Becerra R, Avila E, Prado-García H, Morales-Guadarrama G, et al. α-mangostin synergizes the antineoplastic effects of 5-fluorouracil allowing a significant dose reduction in breast cancer cells. Processes. 2021;9(3): 458. CrossRef PubMed Google Scholar
-
4.Vargas-Castro R, Garcia-Becerra R, Diaz L, Avila E, Ordaz-Rosado D, Bernadez-Vallejo SV, et al. Enhancing tamoxifen therapy with α-mangostin: synergistic antiproliferative effects on breast cancer cells and potential reduced endometrial impact. Pharmaceuticals. 2023. CrossRef PubMed Google Scholar
-
5.Alam M, Rashid S, Fatima K, Adnan M, Shafie A, Akhtar MS, et al. Biochemical features and therapeutic potential of α-mangostin: mechanism of action, medicinal values, and health benefits. Biomed Pharmacother. 2023;163: 114710. CrossRef PubMed Google Scholar
-
6.Klein-Junior LC, Campos A, Niero R, Correa R, Vander Heyden Y, Filho VC. Xanthones and cancer: from natural sources to mechanisms of action. Chem Biodivers. 2020;17(2): e1900499. CrossRef PubMed Google Scholar
-
7.Majdalawieh AF, Terro TM, Ahari SH, Abu-Yousef IA. Alpha-mangostin: a xanthone derivative in mangosteen with potent anti-cancer properties. Biomolecules. 2024. CrossRef PubMed Google Scholar
-
8.Nauman MC, Johnson JJ. The purple mangosteen (Garcinia mangostana): defining the anticancer potential of selected xanthones. Pharmacol Res. 2022;175: 106032. CrossRef PubMed Google Scholar
-
9.Bi C, Xu H, Yu J, Ding Z, Liu Z. Botanical characteristics, chemical components, biological activity, and potential applications of mangosteen. PeerJ. 2023;11: e15329. CrossRef PubMed Google Scholar
-
10.Díaz-Fuentes VH, Donjuan-Leobardo I, Díaz-Hernández BG. Phenological grown stages of mangostan (Garcinia mangostana L.) in the Soconusco Region, Chiapas Mexico, according to the BBCH extended scale. Horticult Int J. 2023;7(4): 178-86. CrossRef PubMed Google Scholar
-
11.Osman MB, Milan AR. Mangosteen-Garcinia mangostana L. Southampton: Southampton Centre for Underutilised Crops, University of Southampton; 2006. PubMed Google Scholar
-
12.Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol. 2008;46(10): 3227-39. CrossRef PubMed Google Scholar
-
13.Ansori ANM, Fadholly A, Hayaza S, Susilo RJK, Inayatillah B, Winarni D, et al. A review on medicinal properties of mangosteen (Garcinia mangostana L.). Res J Pharm and Tech. 2019;13(2): 9. PubMed Google Scholar
-
14.Sen AK, Sarkar KK, Mazumder PC, Banerji N, Uusvouri R, Haset TA. A xanthone from Garcinia mangostana. Phytochem. 1980;19(10): 2223-5. CrossRef PubMed Google Scholar
-
15.Akao Y, Nakagawa Y, Nozawa Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int J Mol Sci. 2008;9(3): 355-70. CrossRef PubMed Google Scholar
-
16.Gunter NV, Teh SS, Lim YM, Mah SH. Natural xanthones and skin inflammatory diseases: multitargeting mechanisms of action and potential application. Front Pharmacol. 2020;11: 594202. CrossRef PubMed Google Scholar
-
17.Badiali C, Petruccelli V, Brasili E, Pasqua G. Xanthones: biosynthesis and trafficking in plants, fungi and lichens. Plants. 2023. CrossRef PubMed Google Scholar
-
18.Pinto MMM, Palmeira A, Fernandes C, Resende D, Sousa E, Cidade H, et al. From natural products to new synthetic small molecules: a journey through the world of xanthones. Molecules. 2021. CrossRef PubMed Google Scholar
-
19.See I, Ee GCL, Jong VYM, Teh SS, Acuna CLC, Mah SH. Cytotoxic activity of phytochemicals from Garcinia mangostana L. and G. benthamiana (Planch. & Triana) Pipoly against breast cancer cells. Nat Prod Res. 2021;35(24): 6184-9. CrossRef PubMed Google Scholar
-
20.Suksamrarn S, Komutiban O, Ratananukul P, Chimnoi N, Lartpornmatulee N, Suksamrarn A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem Pharm Bull. 2006;54(3): 301-5. CrossRef PubMed Google Scholar
-
21.Chi XQ, Zi CT, Li HM, Yang L, Lv YF, Li JY, et al. Design, synthesis and structure-activity relationships of mangostin analogs as cytotoxic agents. RSC Adv. 2018;8(72): 41377-88. CrossRef PubMed Google Scholar
-
22.Vemu B, Nauman MC, Veenstra JP, Johnson JJ. Structure activity relationship of xanthones for inhibition of cyclin dependent kinase 4 from mangosteen (Garcinia mangostana L.). Int J Nutr. 2019;4(2): 38-45. CrossRef PubMed Google Scholar
-
23.Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. 2006;54(6): 2077-82. CrossRef PubMed Google Scholar
-
24.Mahabusarakam W, Wiriyachitra P, Taylor WC. Chemical constituents of Garcinia mangostana. J Nat Prod. 1987;50(3): 474-8. CrossRef PubMed Google Scholar
-
25.Zarena AS, Udaya Sankar K. Supercritical carbon dioxide extraction of xanthones with antioxidant activity from Garcinia mangostana: characterization by HPLC/LC–ESI-MS. J Supercrit Fluids. 2009;49: 330-7. CrossRef PubMed Google Scholar
-
26.Wittenauer J, Falk S, Schweiggert-Weiz U, Carle R. Characterisation and quantification of xanthones from the aril and pericarp of mangosteens (Garcinia mangostana L.) and a mangosteen containing functional beverage by HPLC–DAD–MSn. Food Chem. 2012;134: 445-52. CrossRef PubMed Google Scholar
-
27.Walker EB. HPLC analysis of selected xanthones in mangosteen fruit. J Sep Sci. 2007;30(9): 1229-34. CrossRef PubMed Google Scholar
-
28.Bumrungpert A, Kalpravidh RW, Suksamrarn S, Chaivisuthangkura A, Chitchumroonchokchai C, Failla ML. Bioaccessibility, biotransformation, and transport of alpha-mangostin from Garcinia mangostana (Mangosteen) using simulated digestion and Caco-2 human intestinal cells. Mol Nutr Food Res. 2009;53(Suppl 1): S54-61. CrossRef PubMed Google Scholar
-
29.Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N, et al. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem Pharm Bull. 2003;51(7): 857-9. CrossRef PubMed Google Scholar
-
30.Verma N, Pandit S, Kumar A, Yadav G, Giri SK, Lahiri D, et al. Recent update on active biological molecules in generating the anticancerous therapeutic potential of Garcinia mangostana. Appl Biochem Biotechnol. 2022;194(10): 4724-44. CrossRef PubMed Google Scholar
-
31.Chen LG, Yang LL, Wang CC. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008;46(2): 688-93. CrossRef PubMed Google Scholar
-
32.Gutierrez-Orozco F, Chitchumroonchokchai C, Lesinski GB, Suksamrarn S, Failla ML. Alpha-mangostin: anti-inflammatory activity and metabolism by human cells. J Agric Food Chem. 2013;61(16): 3891-900. CrossRef PubMed Google Scholar
-
33.Chairungsrilerd N, Furukawa K, Ohta T, Nozoe S, Ohizumi Y. Pharmacological properties of alpha-mangostin, a novel histamine H1 receptor antagonist. Eur J Pharmacol. 1996;314(3): 351-6. CrossRef PubMed Google Scholar
-
34.Sivaranjani M, Prakash M, Gowrishankar S, Rathna J, Pandian SK, Ravi AV. In vitro activity of alpha-mangostin in killing and eradicating Staphylococcus epidermidis RP62A biofilms. Appl Microbiol Biotechnol. 2017;101(8): 3349-59. CrossRef PubMed Google Scholar
-
35.Kaomongkolgit R, Jamdee K, Chaisomboon N. Antifungal activity of alpha-mangostin against Candida albicans. J Oral Sci. 2009;51(3): 401-6. CrossRef PubMed Google Scholar
-
36.Chen SX, Wan M, Loh BN. Active constituents against HIV-1 protease from Garcinia mangostana. Planta Med. 1996;62(4): 381-2. CrossRef PubMed Google Scholar
-
37.Shaneyfelt ME, Burke AD, Graff JW, Jutila MA, Hardy ME. Natural products that reduce rotavirus infectivity identified by a cell-based moderate-throughput screening assay. Virol J. 2006;3: 68. CrossRef PubMed Google Scholar
-
38.Tarasuk M, Songprakhon P, Chimma P, Sratongno P, Na-Bangchang K, Yenchitsomanus PT. Alpha-mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Res. 2017;240: 180-9. CrossRef PubMed Google Scholar
-
39.Sugiyanto Z, Yohan B, Hadisaputro S, Dharmana E, Suharti C, Winarto, et al. Inhibitory effect of alpha-mangostin to dengue virus replication and cytokines expression in human peripheral blood mononuclear cells. Nat Prod Bioprospect. 2019;9(5): 345-9. CrossRef PubMed Google Scholar
-
40.Panda K, Alagarasu K, Patil P, Agrawal M, More A, Kumar NV, et al. In vitro antiviral activity of alpha-mangostin against dengue virus serotype-2 (DENV-2). Molecules. 2021. CrossRef PubMed Google Scholar
-
41.Patil P, Agrawal M, Almelkar S, Jeengar MK, More A, Alagarasu K, et al. In vitro and in vivo studies reveal alpha-Mangostin, a xanthonoid from Garcinia mangostana, as a promising natural antiviral compound against chikungunya virus. Virol J. 2021;18(1): 47. CrossRef PubMed Google Scholar
-
42.Hidayat S, Ibrahim FM, Pratama KF, Muchtaridi M. The interaction of alpha-mangostin and its derivatives against main protease enzyme in COVID-19 using in silico methods. J Adv Pharm Technol Res. 2021;12(3): 285-90. CrossRef PubMed Google Scholar
-
43.Diaz L, Bernadez-Vallejo SV, Vargas-Castro R, Avila E, Gomez-Ceja KA, Garcia-Becerra R, et al. The phytochemical α-mangostin inhibits cervical cancer cell proliferation and tumor growth by downregulating E6/E7-HPV oncogenes and KCNH1 gene expression. Int J Mol Sci. 2023. CrossRef PubMed Google Scholar
-
44.Yongpitakwattana P, Morchang A, Panya A, Sawasdee N, Yenchitsomanus PT. Alpha-mangostin inhibits dengue virus production and pro-inflammatory cytokine/chemokine expression in dendritic cells. Arch Virol. 2021;166(6): 1623-32. CrossRef PubMed Google Scholar
-
45.Upegui Y, Robledo SM, Gil Romero JF, Quinones W, Archbold R, Torres F, et al. In vivo antimalarial activity of alpha-mangostin and the new xanthone delta-mangostin. Phytother Res. 2015;29(8): 1195-201. CrossRef PubMed Google Scholar
-
46.Mekseepralard C, Areebambud C, Suksamrarn S, Jariyapongskul A. Effects of long-term alpha-mangostin supplementation on hyperglycemia and insulin resistance in type 2 diabetic rats induced by high fat diet and low dose streptozotocin. J Med Assoc Thai. 2015;98(Suppl 10): S23-30. PubMed Google Scholar
-
47.Eisvand F, Imenshahidi M, Ghasemzadeh Rahbardar M, Tabatabaei Yazdi SA, Rameshrad M, Razavi BM, et al. Cardioprotective effects of alpha-mangostin on doxorubicin-induced cardiotoxicity in rats. Phytother Res. 2022;36(1): 506-24. CrossRef PubMed Google Scholar
-
48.Devi Sampath P, Vijayaraghavan K. Cardioprotective effect of alpha-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol. 2007;21(6): 336-9. CrossRef PubMed Google Scholar
-
49.Fu T, Li H, Zhao Y, Cai E, Zhu H, Li P, et al. Hepatoprotective effect of α-mangostin against lipopolysaccharide/d-galactosamine-induced acute liver failure in mice. Biomed Pharmacother. 2018;106: 896-901. CrossRef PubMed Google Scholar
-
50.Tiwari A, Khera R, Rahi S, Mehan S, Makeen HA, Khormi YH, et al. Neuroprotective effect of α-mangostin in ameliorating propionic acid-induced experimental model of autism in wistar rats. Brain Sci. 2021. CrossRef PubMed Google Scholar
-
51.Kasemwattanaroj P, Moongkarndi P, Pattanapanyasat K, Mangmool S, Rodpai E, Samer J, et al. Immunomodulatory activities of alpha-mangostin on peripheral blood mononuclear cells. Nat Prod Commun. 2013;8(9): 1257-60. PubMed Google Scholar
-
52.Aukkanimart R, Boonmars T, Sriraj P, Sripan P, Songsri J, Ratanasuwan P, et al. In vitro and in vivo inhibitory effects of alpha-mangostin on cholangiocarcinoma cells and allografts. Asian Pac J Cancer Prev. 2017;18(3): 707-13. CrossRef PubMed Google Scholar
-
53.Sato A, Fujiwara H, Oku H, Ishiguro K, Ohizumi Y. Alpha-mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J Pharmacol Sci. 2004;95(1): 33-40. CrossRef PubMed Google Scholar
-
54.Chao AC, Hsu YL, Liu CK, Kuo PL. Alpha-mangostin, a dietary xanthone, induces autophagic cell death by activating the AMP-activated protein kinase pathway in glioblastoma cells. J Agric Food Chem. 2011;59(5): 2086-96. CrossRef PubMed Google Scholar
-
55.Krajarng A, Nilwarankoon S, Suksamrarn S, Watanapokasin R. Antiproliferative effect of alpha-mangostin on canine osteosarcoma cells. Res Vet Sci. 2012;93(2): 788-94. CrossRef PubMed Google Scholar
-
56.Kaomongkolgit R, Chaisomboon N, Pavasant P. Apoptotic effect of alpha-mangostin on head and neck squamous carcinoma cells. Arch Oral Biol. 2011;56(5): 483-90. CrossRef PubMed Google Scholar
-
57.Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Siddiqui IA, et al. Alpha-mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis. 2012;33(2): 413-9. CrossRef PubMed Google Scholar
-
58.Hung SH, Shen KH, Wu CH, Liu CL, Shih YW. Alpha-mangostin suppresses PC-3 human prostate carcinoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen expression through the JNK signaling pathway. J Agric Food Chem. 2009;57(4): 1291-8. CrossRef PubMed Google Scholar
-
59.Shan T, Cui XJ, Li W, Lin WR, Lu HW, Li YM, et al. Alpha-mangostin suppresses human gastric adenocarcinoma cells in vitro via blockade of Stat3 signaling pathway. Acta Pharmacol Sin. 2014;35(8): 1065-73. CrossRef PubMed Google Scholar
-
60.Ma Y, Yu W, Shrivastava A, Srivastava RK, Shankar S. Inhibition of pancreatic cancer stem cell characteristics by α‐mangostin: molecular mechanisms involving Sonic hedgehog and Nanog. J Cell Mol Med. 2019;23(4): 2719-30. CrossRef PubMed Google Scholar
-
61.Verma RK, Yu W, Shrivastava A, Shankar S, Srivastava RK. Alpha-mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (Kras(G12D), and Kras(G12D)/tp53R270H) mice. Sci Rep. 2016;6: 32743. CrossRef PubMed Google Scholar
-
62.Lei J, Huo X, Duan W, Xu Q, Li R, Ma J, et al. α-Mangostin inhibits hypoxia-driven ROS-induced PSC activation and pancreatic cancer cell invasion. Cancer Lett. 2014;347(1): 129-38. CrossRef PubMed Google Scholar
-
63.Phan TKT, Shahbazzadeh F, Pham TTH, Kihara T. Alpha-mangostin inhibits the migration and invasion of A549 lung cancer cells. PeerJ. 2018;6: e5027. CrossRef PubMed Google Scholar
-
64.Lee CH, Ying TH, Chiou HL, Hsieh SC, Wen SH, Chou RH, et al. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget. 2017;8(29): 47425-39. CrossRef PubMed Google Scholar
-
65.Jo MK, Moon CM, Kim EJ, Kwon JH, Fei X, Kim SE, et al. Suppressive effect of alpha-mangostin for cancer stem cells in colorectal cancer via the Notch pathway. BMC Cancer. 2022;22(1): 341. CrossRef PubMed Google Scholar
-
66.Ittiudomrak T, Puthong S, Roytrakul S, Chanchao C. Alpha-mangostin and apigenin induced cell cycle arrest and programmed cell death in SKOV-3 ovarian cancer cells. Toxicol Res. 2019;35(2): 167-79. CrossRef PubMed Google Scholar
-
67.Yu Y, Fei Z, Qin L. Anticancer effects of alpha-mangostin in OVACAR-3 human ovarian carcinoma cells are mediated via involvement of reactive oxygen species, mitochondrial -mediated apoptosis, suppression of cell migration and invasion and m-TOR/PI3K/AKT signaling pathway. J BUON. 2020;25(5): 2293-300. PubMed Google Scholar
-
68.Wang JJ, Sanderson BJ, Zhang W. Cytotoxic effect of xanthones from pericarp of the tropical fruit mangosteen (Garcinia mangostana Linn.) on human melanoma cells. Food Chem Toxicol. 2011;49(9): 2385-91. CrossRef PubMed Google Scholar
-
69.Chen CM, Hsieh SC, Lin CL, Lin YS, Tsai JP, Hsieh YH. Alpha-mangostin suppresses the metastasis of human renal carcinoma cells by targeting MEK/ERK expression and MMP-9 transcription activity. Cell Physiol Biochem. 2017;44(4): 1460-70. CrossRef PubMed Google Scholar
-
70.Zhu X, Li J, Ning H, Yuan Z, Zhong Y, Wu S, et al. Alpha-mangostin induces apoptosis and inhibits metastasis of breast cancer cells via regulating RXRalpha-AKT signaling pathway. Front Pharmacol. 2021;12: 739658. CrossRef PubMed Google Scholar
-
71.Mardianingrum R, Yusuf M, Hariono M, Mohd Gazzali A, Muchtaridi M. Alpha-mangostin and its derivatives against estrogen receptor alpha. J Biomol Struct Dyn. 2022;40(6): 2621-34. CrossRef PubMed Google Scholar
-
72.Huang W, Liang Y, Ma X. Alpha-mangostin induces endoplasmic reticulum stress and autophagy which count against fatty acid synthase inhibition mediated apoptosis in human breast cancer cells. Cancer Cell Int. 2019;19: 151. CrossRef PubMed Google Scholar
-
73.Scolamiero G, Pazzini C, Bonafe F, Guarnieri C, Muscari C. Effects of alpha-mangostin on viability, growth and cohesion of multicellular spheroids derived from human breast cancer cell lines. Int J Med Sci. 2018;15(1): 23-30. CrossRef PubMed Google Scholar
-
74.Kritsanawong S, Innajak S, Imoto M, Watanapokasin R. Antiproliferative and apoptosis induction of alpha-mangostin in T47D breast cancer cells. Int J Oncol. 2016;48(5): 2155-65. CrossRef PubMed Google Scholar
-
75.Li P, Tian W, Ma X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol Cancer. 2014;13: 138. CrossRef PubMed Google Scholar
-
76.Won YS, Lee JH, Kwon SJ, Kim JY, Park KH, Lee MK, et al. Alpha-mangostin-induced apoptosis is mediated by estrogen receptor alpha in human breast cancer cells. Food Chem Toxicol. 2014;66: 158-65. CrossRef PubMed Google Scholar
-
77.Kurose H, Shibata MA, Iinuma M, Otsuki Y. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with alpha-mangostin extracted from mangosteen pericarp. J Biomed Biotechnol. 2012;2012: 672428. CrossRef PubMed Google Scholar
-
78.Shibata MA, Iinuma M, Morimoto J, Kurose H, Akamatsu K, Okuno Y, et al. Alpha-mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 2011;9: 69. CrossRef PubMed Google Scholar
-
79.Lee YB, Ko KC, Shi MD, Liao YC, Chiang TA, Wu PF, et al. Alpha-mangostin, a novel dietary xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions through the ERK signaling pathway in MCF-7 human breast adenocarcinoma cells. J Food Sci. 2010;75(1): H13-23. CrossRef PubMed Google Scholar
-
80.Muralidharan S, Vellaichamy A. Evaluation of anti-epithelial-mesenchymal transition property of Garcinia mangostana rind extract. Futur J Pharm Sci. 2021;7(222): 1-9. CrossRef PubMed Google Scholar
-
81.Mohamed GA, Al-Abd AM, El-Halawany AM, Abdallah HM, Ibrahim SRM. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J Ethnopharmacol. 2017;198: 302-12. CrossRef PubMed Google Scholar
-
82.Simon SE, Lim HS, Jayakumar FA, Tan EW, Tan KO. Alpha-mangostin activates MOAP-1 tumor suppressor and mitochondrial signaling in MCF-7 human breast cancer cells. Evid Based Complement Alternat Med. 2022;2022: 7548191. CrossRef PubMed Google Scholar
-
83.Nalla LV, Dharavath A, Behera SK, Khairnar A. Alpha-mangostin inhibits proliferation, migration, and invasion of human breast cancer cells via STAT3 inhibition. Adv Cancer Biol-Metastasis. 2023. CrossRef PubMed Google Scholar
-
84.Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol. 2004;90(1): 161-6. CrossRef PubMed Google Scholar
-
85.Doi H, Shibata MA, Shibata E, Morimoto J, Akao Y, Iinuma M, et al. Panaxanthone isolated from pericarp of Garcinia mangostana L. suppresses tumor growth and metastasis of a mouse model of mammary cancer. Anticancer Res. 2009;29(7): 2485-95. PubMed Google Scholar
-
86.Naing S, Sandech N, Maiuthed A, Chongruchiroj S, Pratuangdejkul J, Lomarat P. Garcinia mangostana L. pericarp extract and its active compound α-mangostin as potential inhibitors of immune checkpoint programmed death ligand-1. Molecules. 2023. CrossRef PubMed Google Scholar
-
87.El Gaafary M, Abdel-Baki PM, El-Halawany AM, Mohamed HM, Duweb A, Abdallah HM, et al. Prenylated xanthones from mangosteen (Garcinia mangostana) target oxidative mitochondrial respiration in cancer cells. Biomed Pharmacother. 2024;179: 117365. CrossRef PubMed Google Scholar
-
88.Sarmoko S, Novitasari D, Toriyama M, Fareza MS, Choironi NA, Itoh H, et al. Different modes of mechanism of Gamma-mangostin and alpha-mangostin to inhibit cell migration of triple-negative breast cancer cells concerning CXCR4 downregulation and ROS generation. Iran J Pharm Res. 2023;22(1): e138856. CrossRef PubMed Google Scholar
-
89.Cruz-Gregorio A, Aranda-Rivera AK, Aparicio-Trejo OE, Medina-Campos ON, Sciutto E, Fragoso G, et al. <scp>α‐Mangostin</scp> induces oxidative damage, mitochondrial dysfunction, and apoptosis in a triple‐negative breast cancer model. Phytother Res. 2023;37(8): 3394-407. CrossRef PubMed Google Scholar
-
90.Mal R, Magner A, David J, Datta J, Vallabhaneni M, Kassem M, et al. Estrogen receptor beta (ERbeta): a ligand activated tumor suppressor. Front Oncol. 2020;10: 587386. CrossRef PubMed Google Scholar
-
91.Arifian H, Maharani R, Megantara S, Ikram NKK. Glycine-conjugated α-mangostins as potential estrogen receptor alpha (ERα) antagonists through pharmacophore modeling, docking analysis, and molecular dynamics simulations. Appl Sci. 2024;14(3): 5549. CrossRef PubMed Google Scholar
-
92.Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2): 77-106. CrossRef PubMed Google Scholar
-
93.Li J, Nie X, Panthakarn R, Wu X, Zheng C, Cheng Y, et al. Structure and activity relationship analysis of xanthones from mangosteen: Identifying garcinone E as a potent dual EGFR and VEGFR2 inhibitor. Phytomedicine. 2024;122: 155140. CrossRef PubMed Google Scholar
-
94.Lev S. Targeted therapy and drug resistance in triple-negative breast cancer: the EGFR axis. Biochem Soc Trans. 2020;48(2): 657-65. CrossRef PubMed Google Scholar
-
95.Sieja K, Stanosz S, Glowinska N. Histamine and epidermal growth factor in women with fibrocystic changes of the breast. Breast. 2003;12(2): 99-103. CrossRef PubMed Google Scholar
-
96.von Mach-Szczypinski J, Stanosz S, Sieja K, Stanosz M. Metabolism of histamine in tissues of primary ductal breast cancer. Metabolism. 2009;58(6): 867-70. CrossRef PubMed Google Scholar
-
97.Fernandez-Nogueira P, Bragado P, Almendro V, Ametller E, Rios J, Choudhury S, et al. Differential expression of neurogenes among breast cancer subtypes identifies high risk patients. Oncotarget. 2016;7(5): 5313-26. CrossRef PubMed Google Scholar
-
98.Garcia-Quiroz J, Camacho J. Astemizole: an old anti-histamine as a new promising anti-cancer drug. Anticancer Agents Med Chem. 2011;11(3): 307-14. CrossRef PubMed Google Scholar
-
99.Garcia-Quiroz J, Garcia-Becerra R, Barrera D, Santos N, Avila E, Ordaz-Rosado D, et al. Astemizole synergizes calcitriol antiproliferative activity by inhibiting CYP24A1 and upregulating VDR: a novel approach for breast cancer therapy. PLoS ONE. 2012;7(9): e45063. CrossRef PubMed Google Scholar
-
100.Fernandez-Nogueira P, Noguera-Castells A, Fuster G, Recalde-Percaz L, Moragas N, Lopez-Plana A, et al. Histamine receptor 1 inhibition enhances antitumor therapeutic responses through extracellular signal-regulated kinase (ERK) activation in breast cancer. Cancer Lett. 2018;424: 70-83. CrossRef PubMed Google Scholar
-
101.Garcia-Quiroz J, Garcia-Becerra R, Santos-Martinez N, Barrera D, Ordaz-Rosado D, Avila E, et al. In vivo dual targeting of the oncogenic Ether-a-go-go-1 potassium channel by calcitriol and astemizole results in enhanced antineoplastic effects in breast tumors. BMC Cancer. 2014;14: 745. CrossRef PubMed Google Scholar
-
102.Brodie A, Lu Q, Nakamura J. Aromatase in the normal breast and breast cancer. J Steroid Biochem Mol Biol. 1997;61(3–6): 281-6. CrossRef PubMed Google Scholar
-
103.Balunas MJ, Su B, Brueggemeier RW, Kinghorn AD. Xanthones from the botanical dietary supplement mangosteen (Garcinia mangostana) with aromatase inhibitory activity. J Nat Prod. 2008;71(7): 1161-6. CrossRef PubMed Google Scholar
-
104.Cummings J, Smyth JF. DNA topoisomerase Ⅰ and Ⅱ as targets for rational design of new anticancer drugs. Ann Oncol. 1993;4(7): 533-43. CrossRef PubMed Google Scholar
-
105.Mizushina Y, Kuriyama I, Nakahara T, Kawashima Y, Yoshida H. Inhibitory effects of alpha-mangostin on mammalian DNA polymerase, topoisomerase, and human cancer cell proliferation. Food Chem Toxicol. 2013;59: 793-800. CrossRef PubMed Google Scholar
-
106.Mamun MAA, Zhang Y, Zhao JY, Shen DD, Guo T, Zheng YC, et al. LSD1: an emerging face in altering the tumor microenvironment and enhancing immune checkpoint therapy. J Biomed Sci. 2023;30(1): 60. CrossRef PubMed Google Scholar
-
107.Bennani-Baiti IM. Integration of ERalpha-PELP1-HER2 signaling by LSD1 (KDM1A/AOF2) offers combinatorial therapeutic opportunities to circumventing hormone resistance in breast cancer. Breast Cancer Res BCR. 2012;14(5): 112. CrossRef PubMed Google Scholar
-
108.Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449(7158): 105-8. CrossRef PubMed Google Scholar
-
109.Chen J, Ding J, Wang Z, Zhu J, Wang X, Du J. Identification of downstream metastasis-associated target genes regulated by LSD1 in colon cancer cells. Oncotarget. 2017;8(12): 19609-30. CrossRef PubMed Google Scholar
-
110.Han C, Li Z, Hou J, Wang Z, Xu D, Xue G, et al. Bioactivity evaluation of natural product α-mangostin as a novel xanthone-based lysine-specific demethylase 1 inhibitor to against tumor metastasis. Bioorg Chem. 2018;76: 415-9. CrossRef PubMed Google Scholar
-
111.Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knotgen H, Sanchez A, Rubio ME, et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5: 41. CrossRef PubMed Google Scholar
-
112.Furukawa K, Shibusawa K, Chairungsrilerd N, Ohta T, Nozoe S, Ohizumi Y. The mode of inhibitory action of alpha-mangostin, a novel inhibitor, on the sarcoplasmic reticulum Ca(2+)-pumping ATPase from rabbit skeletal muscle. Jpn J Pharmacol. 1996;71(4): 337-40. CrossRef PubMed Google Scholar
-
113.Kim HJ, Park S, Shin HY, Nam YR, Lam Hong PT, Chin YW, et al. Inhibitory effects of alpha-mangostin on T cell cytokine secretion via ORAI1 calcium channel and K(+) channels inhibition. PeerJ. 2021;9: e10973. CrossRef PubMed Google Scholar
-
114.Mohr CJ, Gross D, Sezgin EC, Steudel FA, Ruth P, Huber SM, et al. K(Ca)3.1 channels confer radioresistance to breast cancer cells. Cancers. 2019. CrossRef PubMed Google Scholar
-
115.Jang SH, Kang KS, Ryu PD, Lee SY. Kv1.3 voltage-gated K(+) channel subunit as a potential diagnostic marker and therapeutic target for breast cancer. BMB Rep. 2009;42(8): 535-9. CrossRef PubMed Google Scholar
-
116.Kim SE, Yin MZ, Roh JW, Kim HJ, Choi SW, Wainger BJ, et al. Multi-target modulation of ion channels underlying the analgesic effects of α-mangostin in dorsal root ganglion neurons. Phytomedicine. 2023;115: 154791. CrossRef PubMed Google Scholar
-
117.Zohny SF, Al-Malki AL, Zamzami MA, Choudhry H. P21(Waf1/Cip1): its paradoxical effect in the regulation of breast cancer. Breast Cancer. 2019;26(2): 131-7. CrossRef PubMed Google Scholar
-
118.Felipe Lima J, Nofech-Mozes S, Bayani J, Bartlett JM. EMT in breast carcinoma-a review. J Clin Med. 2016. CrossRef PubMed Google Scholar
-
119.Ibrahim MY, Hashim NM, Mohan S, Abdulla MA, Kamalidehghan B, Ghaderian M, et al. Alpha-mangostin from Cratoxylum arborescens demonstrates apoptogenesis in MCF-7 with regulation of NF-kappaB and Hsp70 protein modulation in vitro, and tumor reduction in vivo. Drug Des Devel Ther. 2014;8: 1629-47. CrossRef PubMed Google Scholar
-
120.To SQ, Dmello RS, Richards AK, Ernst M, Chand AL. STAT3 signaling in breast cancer: multicellular actions and therapeutic potential. Cancers. 2022. CrossRef PubMed Google Scholar
-
121.Shiozaki T, Fukai M, Hermawati E, Juliawaty LD, Syah YM, Hakim EH, et al. Anti-angiogenic effect of alpha-mangostin. J Nat Med. 2013;67(1): 202-6. CrossRef PubMed Google Scholar
-
122.Jittiporn K, Suwanpradid J, Patel C, Rojas M, Thirawarapan S, Moongkarndi P, et al. Anti-angiogenic actions of the mangosteen polyphenolic xanthone derivative alpha-mangostin. Microvasc Res. 2014;93: 72-9. CrossRef PubMed Google Scholar
-
123.Arifianti L, Rofida S, Sukardiman NCZ. Antiangiogenesis from pericarp of mangosteen on T47D breast cancer. E-J Plant Husada. 2014;2(1): 12-5. PubMed Google Scholar
-
124.Mabeta P, Steenkamp V. The VEGF/VEGFR axis revisited: implications for cancer therapy. Int J Mol Sci. 2022. CrossRef PubMed Google Scholar
-
125.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5): 359-71. CrossRef PubMed Google Scholar
-
126.Khorasani ABS, Hafezi N, Sanaei MJ, Jafari-Raddani F, Pourbagheri-Sigaroodi A, Bashash D. The PI3K/AKT/mTOR signaling pathway in breast cancer: review of clinical trials and latest advances. Cell Biochem Funct. 2024;42(3): e3998. CrossRef PubMed Google Scholar
-
127.Planes-Laine G, Rochigneux P, Bertucci F, Chretien AS, Viens P, Sabatier R, et al. PD-1/PD-L1 targeting in breast cancer: the first clinical evidences are emerging. A literature review. Cancers. 2019. CrossRef PubMed Google Scholar
-
128.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12): 861-74. CrossRef PubMed Google Scholar
-
129.Lee YH, Hsieh PL, Chao SC, Liao YW, Liu CM, Yu CC. α-Mangostin inhibits the activation of myofibroblasts via downregulation of linc-ROR-mediated TGFB1/Smad signaling. Nutrients. 2023. CrossRef PubMed Google Scholar
-
130.Kim M, Chin YW, Lee EJ. Alpha, gamma-mangostins induce autophagy and show synergistic effect with gemcitabine in pancreatic cancer cell lines. Biomol Ther. 2017;25(6): 609-17. CrossRef PubMed Google Scholar
-
131.Nakagawa Y, Iinuma M, Naoe T, Nozawa Y, Akao Y. Characterized mechanism of alpha-mangostin-induced cell death: caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg Med Chem. 2007;15(16): 5620-8. CrossRef PubMed Google Scholar
-
132.Kumazaki M, Shinohara H, Taniguchi K, Ueda H, Nishi M, Ryo A, et al. Understanding of tolerance in TRAIL-induced apoptosis and cancelation of its machinery by α-mangostin, a xanthone derivative. Oncotarget. 2015;6(28): 25828-42. CrossRef PubMed Google Scholar
-
133.Laksmiani NPL. Ethanolic extract of mangosteen (Garcinia mangostana) pericarp as sensitivity enhancer of doxorubicin on MCF-7 cells by inhibiting P-glycoprotein. Nusantara Biosci. 2019;11(1): 49-55. CrossRef PubMed Google Scholar
-
134.Leonardi GC, Accardi G, Monastero R, Nicoletti F, Libra M. Ageing: from inflammation to cancer. Immun Ageing. 2018;15: 1. CrossRef PubMed Google Scholar
-
135.Li D, Liu Q, Lu X, Li Z, Wang C, Leung CH, et al. α-Mangostin remodels visceral adipose tissue inflammation to ameliorate age-related metabolic disorders in mice. Aging. 2019;11(23): 11084-110. CrossRef PubMed Google Scholar
-
136.Ibrahim MY, Hashim NM, Omer FAA, Abubakar MS, Mohammed HA, Salama SM, et al. Potential antitumor effect of alpha-mangostin against rat mammary gland tumors induced by LA7 cells. Int J Mol Sci. 2023. CrossRef PubMed Google Scholar
-
137.Stern JS, Peerson J, Mishra AT, Sadasiva Rao MV, Rajeswari KP. Efficacy and tolerability of a novel herbal formulation for weight management. Obesity (Silver Spring). 2013;21(5): 921-7. CrossRef PubMed Google Scholar
-
138.Watanabe M, Gangitano E, Francomano D, Addessi E, Toscano R, Costantini D, et al. Mangosteen extract shows a potent insulin sensitizing effect in obese female patients: a prospective randomized controlled pilot study. Nutrients. 2018;10(5): 586. CrossRef PubMed Google Scholar
-
139.Turner A, Baker A, Dean OM, Walker AJ, Dodd S, Cotton SM, et al. Adjunctive Garcinia mangostana Linn. (Mangosteen) Pericarp for Schizophrenia: A 24-week double-blind, randomized, placebo controlled efficacy trial: Pericarpe d’appoint Garcinia mangostana Linn (mangoustan) pour la schizophrenie: un essai d’efficacite de 24 semaines, a double insu, randomise et controle par placebo. Can J Psychiatry. 2021;66(4): 354-66. CrossRef PubMed Google Scholar
-
140.Marx W, Skvarc DR, Mohebbi M, Walker AJ, Meehan A, Turner A, et al. The effect of adjunctive mangosteen pericarp on cognition in people with schizophrenia: secondary analysis of a randomized controlled trial. Front Psychiatry. 2021;12: 626486. CrossRef PubMed Google Scholar
-
141.Ashton MM, Berk M, Ng CH, Hopwood M, Dodd S, Turner A, et al. Efficacy of adjunctive Garcinia mangostana Linn (mangosteen) pericarp for bipolar depression: study protocol for a proof-of-concept trial. Braz J Psychiatry. 2019;41(3): 245-53. CrossRef PubMed Google Scholar
-
142.Muangpaisan W, Wiputhanuphongs P, Jaisupa N, Junnu S, Samer J, Moongkarndi P, et al. Effects of water-soluble mangosteen extract on cognitive function and neuropsychiatric symptoms in patients with mild to moderate Alzheimer’s disease (WECAN-AD): a randomized controlled trial. Alzheimers Dement. 2022;8(1): 12292. CrossRef PubMed Google Scholar
-
143.Chitchumroonchokchai C, Riedl KM, Suksumrarn S, Clinton SK, Kinghorn AD, Failla ML. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J Nutr. 2012;142(4): 675-80. CrossRef PubMed Google Scholar
-
144.Kondo M, Zhang L, Ji H, Kou Y, Ou B. Bioavailability and antioxidant effects of a xanthone-rich Mangosteen (Garcinia mangostana) product in humans. J Agric Food Chem. 2009;57(19): 8788-92. CrossRef PubMed Google Scholar
-
145.Xie Z, Sintara M, Chang T, Ou B. Functional beverage of Garcinia mangostana (mangosteen) enhances plasma antioxidant capacity in healthy adults. Food Sci Nutr. 2015;3(1): 32-8. CrossRef PubMed Google Scholar
-
146.Xie Z, Sintara M, Chang T, Ou B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: a randomized, double-blind, placebo-controlled clinical trial. Food Sci Nutr. 2015;3(4): 342-8. CrossRef PubMed Google Scholar
-
147.Udani JK, Singh BB, Barrett ML, Singh VJ. Evaluation of mangosteen juice blend on biomarkers of inflammation in obese subjects: a pilot, dose finding study. Nutr J. 2009;8: 48. CrossRef PubMed Google Scholar
-
148.Suthammarak W, Numpraphrut P, Charoensakdi R, Neungton N, Tunrungruangtavee V, Jaisupa N, et al. Antioxidant-enhancing property of the polar fraction of mangosteen pericarp extract and evaluation of its safety in humans. Oxid Med Cell Longev. 2016;2016: 1293036. CrossRef PubMed Google Scholar
-
149.Baroroh K, Suradi AR. Effects of mangosteen pericarp extract to clinical improvements, the plasma level of IL-6 and malondialdehyde in acute exacerbation of COPD patients. J Respir Indo. 2018;38(3): 164-72. CrossRef PubMed Google Scholar
-
150.Liu Y, Park JM, Chang KH, Chin YW, Lee MY. alpha- and gamma-mangostin cause shape changes, inhibit aggregation and induce cytolysis of rat platelets. Chem Biol Interact. 2015;240: 240-8. CrossRef PubMed Google Scholar
-
151.Koh JJ, Qiu S, Zou H, Lakshminarayanan R, Li J, Zhou X, et al. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim Biophys Acta. 2013;1828(2): 834-44. CrossRef PubMed Google Scholar
-
152.Phuong NTM, Van Quang N, Mai TT, Anh NV, Kuhakarn C, Reutrakul V, et al. Antibiofilm activity of alpha-mangostin extracted from Garcinia mangostana L. against Staphylococcus aureus. Asian Pac J Trop Med. 2017;10(12): 1154-60. CrossRef PubMed Google Scholar
-
153.Pham DT, Saelim N, Tiyaboonchai W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf B Biointerfaces. 2019;181: 705-13. CrossRef PubMed Google Scholar
-
154.Chitchumroonchokchai C, Thomas-Ahner JM, Li J, Riedl KM, Nontakham J, Suksumrarn S, et al. Anti-tumorigenicity of dietary alpha-mangostin in an HT-29 colon cell xenograft model and the tissue distribution of xanthones and their phase Ⅱ metabolites. Mol Nutr Food Res. 2013;57(2): 203-11. CrossRef PubMed Google Scholar
-
155.Choi YH, Han SY, Kim YJ, Kim YM, Chin YW. Absorption, tissue distribution, tissue metabolism and safety of α-mangostin in mangosteen extract using mouse models. Food Chem Toxicol. 2014;66: 140-6. CrossRef PubMed Google Scholar
-
156.Gutierrez-Orozco F, Thomas-Ahner JM, Berman-Booty LD, Galley JD, Chitchumroonchokchai C, Mace T, et al. Dietary alpha-mangostin, a xanthone from mangosteen fruit, exacerbates experimental colitis and promotes dysbiosis in mice. Mol Nutr Food Res. 2014;58(6): 1226-38. CrossRef PubMed Google Scholar
-
157.Wong LP, Klemmer PJ. Severe lactic acidosis associated with juice of the mangosteen fruit Garcinia mangostana. Am J Kidney Dis. 2008;51(5): 829-33. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.