Therapeutic targeting of ocular diseases with emphasis on PI3K/Akt, and OPRL pathways by Hedera helix L. saponins: a new approach for the treatment of Pseudomonas aeruginosa-induced bacterial keratitis
Abstract
Pseudomonas aeruginosa-induced bacterial keratitis is one of the most sight-threatening corneal infections associated with intense ocular inflammatory reactions that may lead to vision loss. Hence, this study investigated the efficacy of three nanocomposite chitosan-coated penetration enhancer vesicles (PEVs) to augment the ocular delivery of saponin(s), α-hederin (PEVI), hederacoside C (PEVⅡ), or both (PEVⅢ) for treatment of Pseudomonas keratitis and its induced inflammatory response. The three formulations were prepared using the ethanol injection method and comprehensively characterized. In vitro, the antibacterial activity of the three formulations against P. aeruginosa was evaluated using agar well-diffusion method, pyocyanin production inhibition, and swarming and twitching motility inhibition assays. The therapeutic effect of the three formulations has been investigated in P. aeruginosa keratitis by gross lesion monitoring, determination of bacterial bioburden, biochemical markers, histopathological examination, and scoring after 7 days of topical treatment. Data revealed that PEVI, PEVⅡ, and PEVⅢ nanocomposites showed particle size in the nanometer range, high entrapment efficiency, good stability, and sustained release of the saponins throughout 24 h. Among them, PEVⅢ exhibited notably strong in vitro antipseudomonal activity. Additionally, animals treated topically with PEVⅢ showed an appreciable gross lesion reduction, corneal tissue improvement, and formidable bacterial load reduction compared with untreated and gentamicin sulfate eye (GENTAWISE®) ointment-treated groups. Moreover, PEVⅢ treatment showed the most significant reduction in TNF-α, NF-κB, ROS levels, and OPRL virulence gene expression while enhancing PI3K/Akt activation. Therefore, this study offers PEVⅢ as a promising treatment for P. aeruginosa keratitis.Graphical Abstract
Keywords
Hedera helix L. Saponins α-Hederin Hedracoside C Pseudomonas keratitis Eye disease1 Introduction
Bacterial keratitis (corneal infection) is an overwhelming ailment responsible for two million cases yearly. Pseudomonas aeruginosa belongs to ESKAPE pathogens and is considered the most common causative agent of bacterial keratitis, representing a major healthcare problem worldwide [1, 2]. Pseudomonal corneal ulceration and keratitis represent severe and therapeutically challenging ocular pathologies, necessitating a meticulous treatment regimen comprising antimicrobial and anti-inflammatory agents to overcome the risk of irreversible blindness [3, 4].
Yet, hospitalization is obligatory for some cases, like immune-compromised patients with such infections. P. aeruginosa is widely acquiring resistance to different classes of topical broad-spectrum antimicrobials [5], including carbapenem antibiotics, such as imipenem and meropenem, in clinical settings, which is a frightening obstacle in the treatment protocol of P. aeruginosa [6]. Furthermore, P. aeruginosa has a wide panel of virulence traits, including twitching and swarming motility, pyocyanin pigment production, and different virulence genes that aid in bacterial invasion and toxicity during infections [5]. Twitching motility via bacterial pilli is a surface-associated motility not required for initial bacterial adherence but has a key role in tissue penetration or bacterial dissemination [7]. Meanwhile, pyocyanin (1-hydroxy-5-methyl-phenazine) is a blue-green pigment produced only by P. aeruginosa strains, which augments the virulence of the bacterium and produces reactive oxygen species (ROS), leading to tissue damage, proliferation, and blindness [7]. Additionally, the oprL gene (Encoding membrane lipoprotein L) is predominantly involved in P. aeruginosa-related infections and is crucial for maintaining bacterial integrity and protecting against oxidative stress. Although OprL serves as a marker for identifying the genus Pseudomonas, it specifically detects P. aeruginosa [8]. These two issues complicate the treatment process caused by this pathogenic bacterium. Following the invasion of the bacterial pathogens into the corneal tissue, the host's innate immune response is triggered, initiating a cascade of defense mechanisms aimed at pathogen clearance. Several molecular pathways could regulate the host’s response to P. aeruginosa infection. For example, the PI3K/Akt signaling pathway is crucial in modulating inflammation, apoptosis, and proliferation in corneal epithelial cells (CECs) [9]. Besides, reactive oxygen species (ROS) also participate in the regulation of apoptosis and inflammation in CECs in a concentration-dependent manner. For instance, high concentrations of ROS can suppress the PI3K/Akt pathway, thereby promoting apoptosis and inflammation. Conversely, low to moderate ROS levels activate the PI3K/Akt pathway, fostering CEC proliferation and inhibiting apoptotic processes. Nevertheless, uncontrolled PI3K/Akt stimulation can cause inflammation in different circumstances [10]. Several studies validated the contribution of NF-κB to systemic inflammation and keratitis, linking its activation to elevated TNF-α levels and heightened inflammatory responses. For instance, a higher level of the pro-inflammatory cytokine TNF-α was detected in rat models with keratitis, which facilitates inflammatory responses and enhances the activation of NF-κB [11, 12]. Dong et al. [13] reported that persistent NF-κB activation in mice led to severe systemic inflammation and premature mortality [13]. Thus, to lessen the corneal damage caused by P. aeruginosa infection, it may be possible to identify therapy targets by comprehending these pathways.
Hedera helix L., commonly referred to as common ivy, is a woody species in the Araliaceae family [14, 15]. Historically, ivy leaf extracts have been employed to treat inflammatory bronchial diseases, inflammation, burns, cough, neuralgia, and rheumatism [16, 17]. The leaves and fruits of H. helix were also used in Europe to treat gastrointestinal tract-related diseases [18]. Experimentally, ivy extract exhibited antibacterial, antihelmintic, leishmanicidal, and antifungal properties [19]. Moreover, H. helix leaf extract has been approved by the German Commission E for its efficacy against chronic inflammatory bronchial conditions and productive coughs due to its expectorant, bronchodilator, antibacterial, and spasmolytic activities. These effects are partly attributed to its triterpene saponins content, particularly hederacoside C, a key active ingredient and marker of H. helix leaf extract [14, 15]. Several compounds have been isolated from ivy leaves, including α-hederin, hederacoside-C, hederacolchiside-E, and hederacolchiside-F [16, 17], of which α-hederin and hederacoside C have gained considerable attention for their diverse pharmacological activities, including anti-inflammatory, antioxidant, antimicrobial, and anticancer effects [20–22].
Nanotechnology has significantly advanced the field of ocular drug delivery by introducing innovative therapeutic methods that surpass traditional formulations [23]. These advancements enable the penetration of ocular barriers, enhance transcorneal drug permeability, extend drug residence time, minimize degradation, reduce dosing frequency, improve patient adherence, provide controlled and sustained release, and enable precise drug targeting [24, 25]. Therefore, this study utilized and evaluated three novel hybrid nano-vesicular systems: chitosan-coated PEVs containing two isolated ivy saponins: α-hederin (PEVI), hederacoside-C (PEVⅡ), and a combination of both saponins (PEVⅢ). These systems were investigated for their impact on the complex molecular pathways regulating the immune response to P. aeruginosa keratitis, aiming to identify potential therapeutic targets for mitigating corneal damage and guarding against the emergence of antibiotic resistance.
2 Materials and methods
2.1 Instruments
NMR spectra were recorded at 400 (1H) and 100 MHz (13C) on a Bruker NMR spectrometer, Japan. The NMR spectra were recorded in deuterated CD3OD using TMS as an internal standard.
2.2 Isolation of the compounds
Plant material: Hedera helix L. leaves were collected from a private garden in Giza, Egypt, and authenticated by Mrs Teresa Labib, Head of the Taxonomists at Orman Botanic Garden. A voucher specimen was deposited in the Herbarium of the Department of Pharmacognosy, College of Pharmacy, Cairo University, Egypt.
Extraction and fractionation: Two kilograms of the air-dried powder were exhaustively extracted with 70% ethanol using a homogenizer-assisted extraction method. The ethanolic extract was combined and evaporated under reduced pressure to dryness. A portion of the extract (200 g) was dissolved in 500 mL methanol and allowed to precipitate for 1 day at 5 ℃ after the addition of anhydrous acetone (2 L), then filtered. Both filtrate and precipitate were monitored using precoated TLC plates (Fluka, Sigma-Aldrich Chemicals, Germany), where the filtrate was found to be enriched in flavonoids (70 g), and the precipitate was found to be enriched in saponins and further purified by precipitation by acetone to yield saponin-enriched residue (SRF, 130 g). Then, SRF (50 g) was subjected to flash column chromatography on silica gel (0.04–0.063 mm, Merck) and eluted with CHCl3–MeOH–H2O (26: 14: 3) to afford 5 fractions.
Isolation and identification: Fraction 4 (15 g) was further purified on a polyamide column (SC 6 0.07 mm, Merck) with 50% of MeOH then on another silica gel (0.04–0.063 mm, Merck) and eluted with CHCl3–MeOH–H2O (26: 14: 3) to obtain 210 mg of compound (1). Compound 1: Colorless solid; 1H NMR (400 MHz, CD3OD) and 13C NMR (100 MHz, CD3OD, TMS) (Supplementary Figs. S1 & S2). Additionally, Fraction 5 (7 g) was purified on a polyamide column (SC 6 0.07 mm, Merck) with 20% of MeOH, then on RP-18 silica gel (Fluka, Sigma-Aldrich Chemicals, Germany) and eluted with MeOH–H2O (50: 50) to obtain 140 mg of compound (2). Compound 2: Colorless solid; 1H NMR (400 MHz, CD3OD) and 13C NMR (100 MHz, CD3OD, TMS) (Supplementary Figs. S3 & S4).
The structure of saponins was elucidated by 1H and 13C NMR and compared with the literature to be α-hederin (1) and hederacoside C (2) [26].
1H NMR (1) (CD3OD, TMS) δ: 0.72 (3H, s), 0.84 (3H, s), 0.93 (3H, s), 0.96 (3H, s), 0.97 (3H, s), 0.99 (3H, s), 1.0 (1H, m), 1.17 (3H, s), 1.24 (3H, m), 1.28–1.94 (18H, m), 2.88 (1H dd, J = 13.86, 3.84 Hz), 3.29–3.93 (12H, m), 4.57 (1H, d, J = 9.0 Hz), 5.17 (1H, d, J = 1.35 Hz), 5.26 (1H, s).
13C NMR (1) (CD3OD, TMS) δ: 12.3, 14.9, 16.3, 16.5, 17.4, 22.5, 22.7, 23.1, 25.0, 25.0, 27.4, 30.2, 32.0, 32.4, 33.5, 36.2, 38.2, 39.1, 41.3, 41.5, 42.5, 45.8, 46.2, 46.7, 46.9, 63.3, 63.3, 67.7, 68.7, 70.7, 70.7, 72.2, 72.5, 72.5, 75.3, 80.9, 100.5, 102.9, 122.2, 143.8, 180.8.
1H NMR (2) (CD3OD, TMS) δ: 0.72 (3H, s), 0.82 (3H, s), 0.93 (3H, s), 0.97 (3H, s), 1.00 (3H, s), 1.16–2.06 (31H, m), 2.86 (1H dd, J = 14.71, 4 Hz), 3.26–4.09 (29H, m), 4.41 (1H, d, J = 10.62 Hz), 4.56 (1H, d, J = 8.05 Hz), 5.18 (1H, m), 5.27 (1H, m), 5.36 (1H, d, J = 8.05 Hz).
13C NMR (2) (CD3OD, TMS) δ: 12.4, 15.1, 15.1, 16.5, 16.6, 17.3, 22.7, 22.7, 24.9, 25.0, 27.4, 30.1, 32.1, 33.7, 36.2, 38.2, 39.2, 41.1, 41.7, 42.5, 45.8, 46.6, 46.7, 46.7, 47.0, 47.4, 47.7, 47.8, 48.0, 48.3, 48.3, 60.4, 63.2, 62.5, 65.2, 67.9, 68.2, 68.6, 69.2, 69.5, 70.8, 70.8, 71.1, 72.4, 72.4, 73.6, 75.2, 75.4, 76.2, 78.3, 81.2, 94.5, 100.2, 101.5, 102.7, 102.8, 122.4, 143.5, 176.7.
2.3 Formulation and characterization of the prepared nano delivery systems
Composite nanovesicle material: Epikuron 200 was provided by Cargill Texturizing Solutions, Germany. Transcutol was kindly supplied by Gattefosse Company, France. Phosphotungstic acid, low molecular weight chitosan (50 kDa) with 70% acetylation degree, and dialysis membrane with an average flat width of 33 mm were obtained from Sigma Aldrich Company, USA. Absolute ethanol was purchased from Al-Gomhorea pharmaceutical company, Cairo, Egypt. Nanosep centrifuge tubes equipped with an ultra-filter were obtained from Pall Life Sciences, USA.
Preparation of composite nanovesicles using the ethanol injection method: Composite nano-vesicular systems were formulated using the ethanol injection technique, according to Ang et al. [27]. In brief, 5 mL of absolute ethanol comprising 50 mg Epikuron 200 (phospholipid), 25 mg of α-hederin, or hederacoside C, or both were inserted dropwise into 10 mL of a warm 0.6% chitosan acetate buffer solution pH 4.6 encompassing 5% transcutol (acting as a penetration enhancer) using a plastic syringe at an injection rate of 2 mL/min. The dispersions were mechanically stirred at 100 r.p.m. (Lab Tech LMS -1003, Korea) maintained in ambient conditions (25 ℃) till complete evaporation of the employed absolute ethanol. Finally, the obtained dispersions were preserved overnight at refrigeration temperature for further investigations. Table 1 shows the composition of the three vesicular preparations.
Composition and Characterization of composite nanovesicles
Determination of the particle size (P.S.), Polydispersity index (PDI), and zeta potential (ζ-potential): Following appropriate dilution, a Zetasizer (model ZS3600, Malvern Instruments Ltd., Worcestershire, UK) was implemented to assess the aforementioned colloidal properties of the composite vesicles [28].
Determination of entrapment efficiency (EE%): Nanosep centrifugal devices were employed to separate the unentrapped drug from loaded nanoparticles [29]. A volume equivalent to 0.5 mL was transferred to the Nanosep centrifugal devices, then centrifuged in a high-speed cooling centrifuge (SIGMA-3-30KS, Germany) at -4 ℃ and 3000 rpm for 50 min. After dilution with acetate buffer, pH 6.4, the free drug was measured in the filtrate via a reported HPLC method. Isocratic elution was applied using a mobile phase comprising ammonium acetate (pH 8.5) and acetonitrile 70:30 (v/v) at 1.2 mL min-1. Moreover, 1 μl sample volume was injected into a C18 column (Agilent 1290 infinity, Germany) with column effluent being monitored at 220 nm at 30 ℃ [30], using acetate buffer (pH 6.4) serving as the control solution. The following equation was implemented to compute EE% [31]:
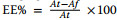
Storage stability study: The P.S., PDI, ζ-potential, and EE% of the three preparations were re-determined after 3 months of storage at 2–8 ℃ to reflect their physical stability [32].
In vitro release study: The present study was assessed utilizing Hanson Franz-type diffusion apparatus (model 60–301–106, CA, Los Angeles, USA) [33]. The cellulose membranes were loaded between the upper and lower chambers of Franz diffusion cells, and the lower chamber comprised 7.2 mL simulated tear fluid (STF), comprising 2 g NaHCO3, 0.08 g CaCl2, and 6.7 g NaCl in 1 L of deionized water, which was agitated at 600 rpm for a period of 24 h at 34 ±0.5 ℃ [34]. A specified volume of the three nano vesicular formulations was introduced to the upper (donor) chamber, with the saponin(s) suspensions in acetate buffer (pH 6.4) serving as control. At specified time intervals (0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 h), a volume equivalent to 2 mL was taken out from the release media and compensated with an equal volume of STF. The amount of released saponin(s) from each formulation was analyzed using a reported HPLC method and measured at ƛmax of 220 nm [35]. In addition, the release data were introduced into several kinetic models to evaluate the mechanism of release of the saponin(s) from the obtained dispersions.
Determination of the surface morphological features of the composite nanosystems employing transmission electron microscope (TEM): The surface morphological features of the formulae were assessed using TEM (JEM—100S, Joel, Tokyo, Japan). Samples were negatively stained imparting 1% phosphotungstic acid and examined under TEM [35].
Sterilization of composite nanosystems by Gamma Irradiation: The three preparations were exposed to gamma radiation at room temperature using a 60 ℃ radiation source at a dosage rate of 10 kGy/hour. After being subjected to gamma rays, the physicochemical properties of the formulae were assessed, and the variations from non-irradiated samples were compared statistically [36]. Furthermore, a sterility test was performed to assess the sterilization efficiency of the gamma radiation dose delivered to the formulae.
2.4 Antipseudomonal activity
2.4.1 Bacterial strain
Three clinical keratitis P. aeruginosa isolates collected from tertiary care hospitals in the greater Cairo area were used in this study. Standard microbiological techniques such as Gram staining and oxidase testing were used as an initial identification. Further confirmation was performed using the Vitek® 2 automated system (bioMérieux, Marcyl’Etoile, France) [37]. The antibiotic susceptibility profile of P. aeruginosa isolates was evaluated using the agar diffusion method against the most clinically used antimicrobials [37, 38]. Results were construed according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2024). Various antibiotic classes were used, such as penicillins (ampicillin, AMP), carbapenems (imipenem, IPM and meropenem, MRP), third-generation cephalosporins (ceftriaxone, CTR and cefotaxime, CTX), and penicillin-like inhibitors with beta-lactamase inhibitors (amoxicillin-clavulanic AMC). Tests were performed twice for confirmation. According to the World Health Organization (WHO), isolates that were resistant to three or more antibiotic classes were considered multidrug-resistant (MDR).
2.4.2 In vitro antimicrobial activity
Agar well diffusion: The antimicrobial activity of α-hederin and hederacoside C was evaluated using the agar well diffusion method. A concentration of 1×108 CFU/mL of P. aeruginosa suspension was streaked on Muller-Hinton agar plates (Oxoid, UK). Six concentrations (100%, 50%, 25%, 12.5%, 6.25%, 3%, 1.5%, and 0.75%) of the investigated compounds were examined. Then, 50 μl of each previous concentration was added to wells before overnight incubation at 37 ℃. Diameters of a zone of inhibition were measured, and the experiments were performed in triplicate [39, 40].
2.4.3 In vitro anti-virulent activity
Pyocyanin production inhibition: In a broth containing MIC and another containing sub-MIC, the P. aeruginosa isolate was grown overnight at room temperature, then centrifuged, and the obtained supernatant was mixed with chloroform, followed by centrifugation. Finally, 0.2 M HCl was added, followed by centrifugation, and the absorbance of pyocyanin was measured at 520 nm [40].
Swarming and twitching motility inhibition: The medium was prepared by the addition of supplementing 0.5% (w/v) casamino acids to Luria–Bertani (LB), while the twitching medium consisted of 1.5% LB agar. Before agar solidification, MIC and sub-MIC concentrations of PEVⅢ were added, and then 2.5 μL of the inoculum was applied to the swarming and twitching agar plates before overnight incubation at room temperature. The migration zones appearing on the agar plates were monitored. All motility experiments were conducted in triplicate [41, 42].
2.4.4 In vivo evaluation of anti-pseudomonal activity
Antimicrobial resistance profiling of the obtained strains: An MDR carbapenem-resistant isolate, which showed resistance to four different classes of antibiotics, was used for the in vivo animal model (Fig. S5).
Animals: The study was approved by the Research Ethics Committee (REC) of the Faculty of Pharmacy, Ahram Canadian University, Giza, Egypt, under permit number REC2524. The study adhered strictly to the Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011). A total of 35 healthy female Sprague–Dawley rats, weighing between 200 and 400 g, were used. All animals were acclimated for 7 days in controlled environmental conditions before the experiment commenced. The rats were randomly assigned to seven groups (n = 5 per group). Group 1 served as the negative control; Group 2 was the positive control (infected, non-treated); Groups 3, 4, and 5 were infected and treated with PEVI, PEVⅡ, and PEVⅢ, respectively; Group 6 was infected and treated with the blank formula; and Group 7 received gentamicin sulphate ophthalmic ointment (GENTAWISE®). At the end of the study, the animals were humanely euthanized with ketamine, followed by cervical dislocation.
Induction of keratitis and grouping of animals: A corneal epithelial defect was created in all rats except for negative control groups (group 1) under anesthesia on the first day, and 0.05 mL per rat was inoculated from a solution containing 1×108 colony-forming units (CFU)/ mL of P. aeruginosa clinical isolate, and those with keratitis at the end of the third day were added to the group. On the 7th day, which was the end of the study, the rats were sacrificed, their globule structures were removed as a whole, and some were stored in 10% formaldehyde for histopathological examination, whereas the rest were frozen for biochemical parameters evaluation [43].
Gross Lesion Monitoring: The mice were examined at 1, 3, 5, and 7 days postinfection (p.i) to monitor disease progression, and digital images of the mice' corneas were taken.
Determination of bacterial bioburden: Ocular bacterial count was estimated after homogenization of samples in 2 mL of phosphate buffer saline (PBS) followed by serial dilution in PBS (1:10, 1:100, 1:1,000, and 1:10,000), then plating on cetrimide agar plates in triplicate and overnight incubation. To ensure reproducibility, the experiment was conducted in triplicate. All colony counts were expressed as log10 CFU per milliliter.
Histopathological examination and scoring: Sacrificed rats were examined microscopically by hematoxylin–eosin (H&E) staining by a pathologist who was unaware of the groups, and the results were evaluated and scored. The eye was placed in the solution immediately after enucleation and trimming. A gauze pad was used to keep the eye submerged. The globe remained in Davidson’s solution for 1–2 days. The eye was then taken out of the solution and placed in 10% paraformaldehyde. Tissue specimens were trimmed off, washed, and dehydrated in ascending grades of alcohol. The dehydrated specimens were then cleared in xylene, embedded in paraffin blocks, and sectioned at 4–6 µm thick. The obtained tissue sections were deparaffinized using xylol and stained using hematoxylin and eosin (H&E) for histopathological examination through the electric light microscope [44]. Davidson's Solution consisted of: glacial acetic acid, 100 mL, 95% ethyl alcohol, 300 mL, 10% neutral buffered formalin, 200 mL, and distilled water, 300 mL. Lesions were scored as follows: (-) for the absence of lesion, (+) for Mild lesion (+ +) for Moderate lesion (+ + +) for Sever Lesion.
Enzyme-linked immunosorbent assays (ELISA): The eye content of tumor necrosis factor-α (TNF-α; SEA133Ra, Cloud-Clone Corp., USA), nuclear factor kappa B (NF-κB; MBS453975, MyBioSource, CA, USA), reactive oxygen species (ROS; MBS039665, MyBioSource, CA, USA), and AKT1 (AKT1; S-F49321, lifespan Biosciences) were measured in the eye homogenate using the corresponding ELISA kit according to the manufacturer’s protocol.
Quantitative real-time PCR (RT-PCR) analysis: The gene expression of PI3K and OPRL was assessed in eye tissues after the extraction of RNA. The Direct-zolTM RNA MiniPrep Plus kit (Cat # R2072, ZYMO Research Corp., CA, USA) was used in the extraction process. The Beckman dual spectrophotometer (USA) was used to assess the purity and concentrations of extracted RNA. As directed by the manufacturer, the SuperScriptTM Ⅳ One-Step RT-PCR kit (Cat # 12594100; Thermo Scientific, MA, USA) was used.
The forward and reverse primers for PI3K were 5'-ACACCACGGTTTGGACTATGG-3' and 5'-GGCTACAGTAGTGGGCTTGG-3', respectively.
For OPRL, the forward primer was 5'-ATGGAAATGCTGAAATTCGGC-3' and the reverse primer was 5'-CTTCTTCAGCTCGACGCGACG-3'.
For GAPDH forward primer was 5'-TGGATTTGGACGCATTGGTC-3' and the reverse primer was 5'-TTTGCACTGGTACGTGTTGAT-3'.
Quantitative real-time PCR reactions were conducted using the Step OneTM system (Applied Biosystems, CA, USA). The relative expression levels of PI3K and OPRL were determined according to the Livak and Schmittgen 2 - ΔΔCt method using GAPDH as an internal standard and presented as fold change from control.
Statistical analysis of data: One-way ANOVA followed by Tukey–Kramer post-test was utilized for data statistical analysis. Bonferroni correction and Cohen’s d were also applied to assess multiple comparisons and effect size, respectively. Data were expressed as mean ±SD, using Graphpad Instat software.
3 Results
3.1 Characterization of the prepared nanocomposite delivery systems
Determination of the particle size (PS), Polydispersity index (PDI), and zeta potential (ζ-potential): As demonstrated in (Table 1), the PS values of the vesicular dispersions (PEVI, PEVⅡ, and PEVⅢ) ranged from 282.6 ±14.83 to 422.5 ±15.96 nm, reflecting the existence of a remarkable relationship between the size of the vesicles and their structure, with recorded PDI values not transcending 0.5, which is considered satisfactory [45]. The formulations imparted positive charges ranging from + 27.36 ±5.69 to + 28.47 ±4.32 mV (Table 1) with insignificant changes between them (P > 0.05), showing the superior stability of the nanoformulations due to the electrostatic repulsive forces between vesicles.
Determination of entrapment efficiency (EE%): As observed from the results of the EE% (Table 1), it was obvious that all the formulae (PEVI-PEVⅢ) displayed high EE% values ranging from 84.61% ±2.54 to 95.98% ±4.87.
Storage stability study: In terms of physical appearance, the three vesicular dispersions remained unchanged after 3 months of storage (data not shown). Upon storage, composite nanoformulations (PEVI-PEVⅢ) displayed a slight but statistically significant increase in PS and PDI (P < 0.05), reflecting vesicles' expansion [24]. Regarding surface charge, the three vesicles showed a slight yet significant decrease (P < 0.05) in ζ-potential values due to the notable increase in the vesicles' PS upon storage [36]. Furthermore, several studies reported their chemical stability, especially when sequestered into nanoparticles, preserving their effectiveness in the treatment of the disease.
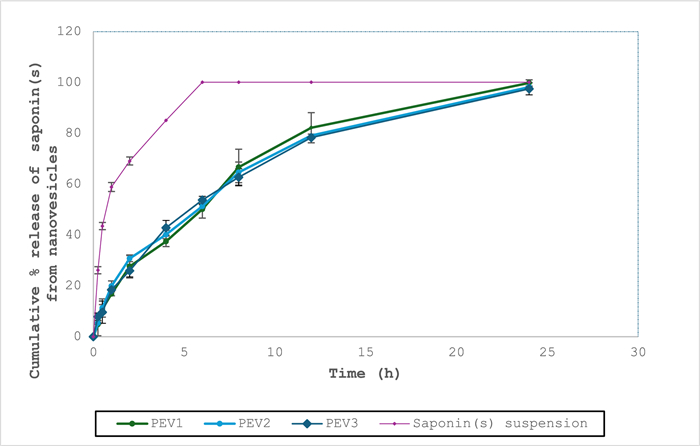
In-vitro release study: The study evaluated the three composite vesicular dispersions (PEVI-PEVⅢ) in comparison to a saponin suspension in acetate buffer (pH 6.4) as a control. The formulations exhibited coherence with Higuchi diffusion kinetics (Table 2), and they were consistent with the findings of Sharma et al. [46]. In addition, fitting the obtained results into the Korsmeyer-Peppas equation demonstrated n values of 0.46, 0.47, and 0.45 for PEVI, PEVⅡ, and PEVⅢ, respectively, confirming the Fickian diffusion of the saponin(s) from the formulations. This was consistent with the outcomes of Lakshmi et al. [47].
Release kinetics data for the prepared formulations PEVI, PEVⅡ, and PEVⅢ
Meanwhile, the three vesicles exhibited a comparable (P > 0.05) sustained release profile of the saponin(s) over a 24 h period compared to the control suspension, in which a rapid release of saponin(s) was observed after 6 h (Fig. 1).
Mean cumulative release of saponin(s) from the formulations (PEVI, PEVⅡ, and PEVⅢ) compared to control drug solutions
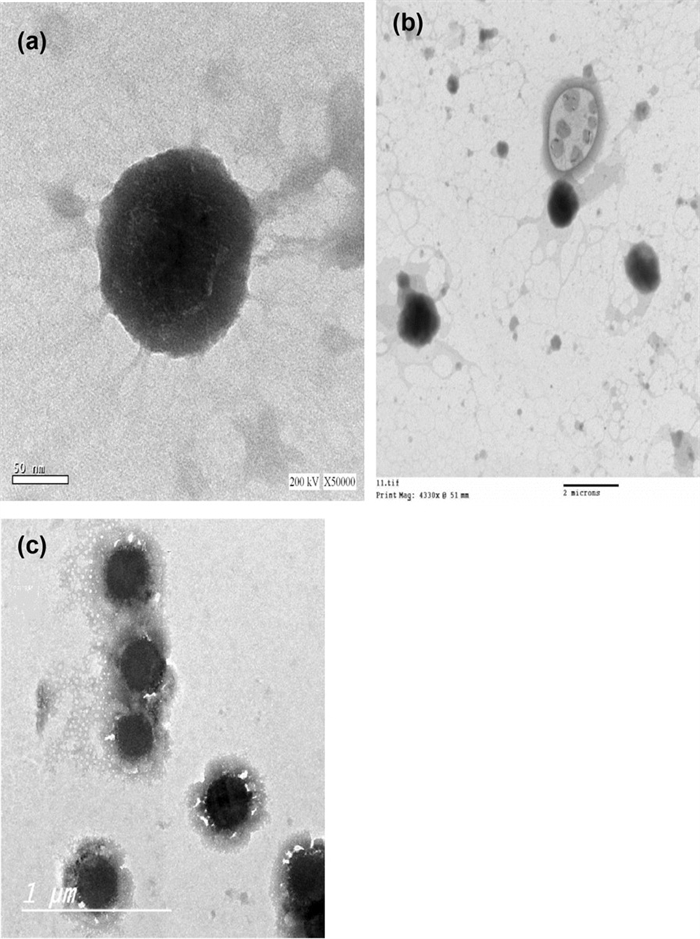
Determination of the surface morphological features of the composite nanosystems employing transmission electron microscope (TEM): The three formulations were selected for photographing using TEM, as displayed in Fig. 2a–c. The photomicrographs revealed well-defined spherical unilamellar nanoparticles with an approximate size of 300–400 nm, which was consistent with the measurements previously obtained using the dynamic light scattering particle size analyzer.
Transmission electron microscope micrographs of the three vesicular systems: a PEVI, b PEVⅡ, and c PEVⅢ) at; a×14,000×and b×50,000x
3.1.1 Sterilization of composite nanosystems by Gamma irradiation
The three prepared formulations (PEVI-PEVⅢ) were sterilized using gamma irradiation with a radiation dose of 10 kGy and then examined for microbiological contamination, either fungal or bacterial, to ensure their sterility. After sterilization, P.S., PDI, ζ-potential, and EE% were evaluated again. At the selected radiation dose, all formulations showed no significant changes in their physical properties (P > 0.05) (data not shown). A confirmatory sterility test was performed to determine the sterility of the prepared formulae. The absence of microbiological growth in any of the tested samples confirms the formula's sterility and the effectiveness of gamma irradiation for sterilization at the prescribed level. These findings agreed with those of Abdel Azim et al. [48].
3.2 In vitro antimicrobial activity
MIC values of PEVI, PEVⅡ, and PEVⅢ were 1024, 512, and 64 μg/mL, respectively.
3.2.1 In vitro anti-virulent activity
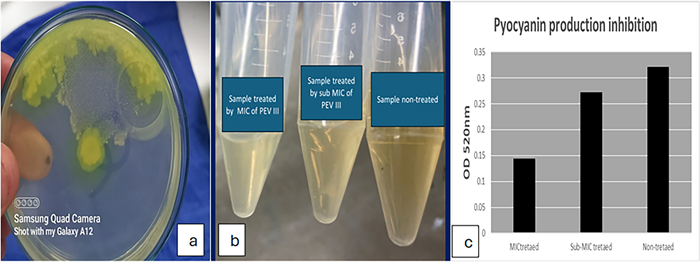
Pyocyanin production inhibition: PEVⅢ significantly reduced absorbance to 0.143 and 0.2715 upon using MIC and sub-MIC concentrations (Fig. 3).
The effect of PEVⅢ on pyocyanin production: a pyocyanin production on cetrimide agar plate, b reduction in pigment production, c reduction in absorbance
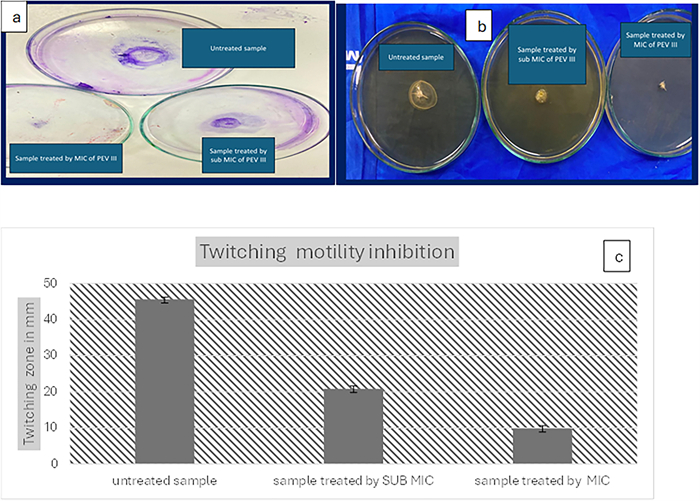
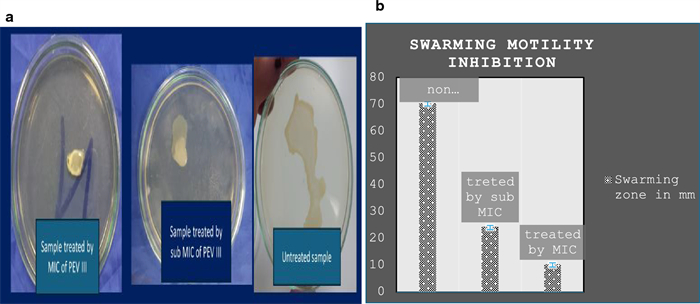
Twitching and swarming motility inhibition: Both swarming and twitching motility were noticeably reduced with increasing nanocomposite concentration compared to the control (absence of the nanocomposite) (Figs. 4 and 5).
The effect of PEVⅢ on twitching motility: a twitching motility on LB agar plate, b twitching motility after CV staining, c reduction in twitching zone
The effect of PEVⅢ on swarming motility: a swarming on LB agar plate, b reduction in swarming zone
3.3 In vivo evaluation of anti-pseudomonal activity
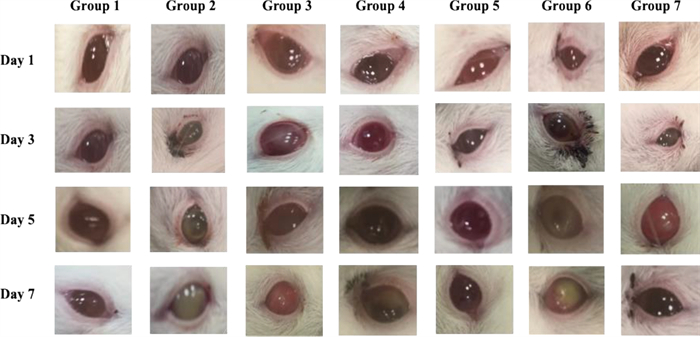
Gross lesion monitoring: The keratitis signs were significantly reduced in the animals treated with PEVⅢ compared to the control group, as shown in Fig. 6.
The effect of nanocomposites treatment lesion gross; Group 1) negative control, group 2) positive control infected nontreated, group 3) Infected then treated by PEV I, group 4) Infected then treated by PEVⅡ, group 5) Infected then treated by PEVⅢ, group 6) Infected then treated by blank formula, group 7) Infected then treated by commercial eye drop (GENTAWISE®)
Determination of bacterial bioburden: Quantification of the bacterial bioburden in each group over time indicates that the P. aeruginosa load in the PEVⅢ groups is lower than that in the infected non-treated group. In the current study, PEVⅢ significantly abridged the bacterial load compared with both untreated and gentamicin sulphate eye ointment (GENTAWISE®) commercial-treated groups Table 3.
The bacterial bioburden of each animal group over time
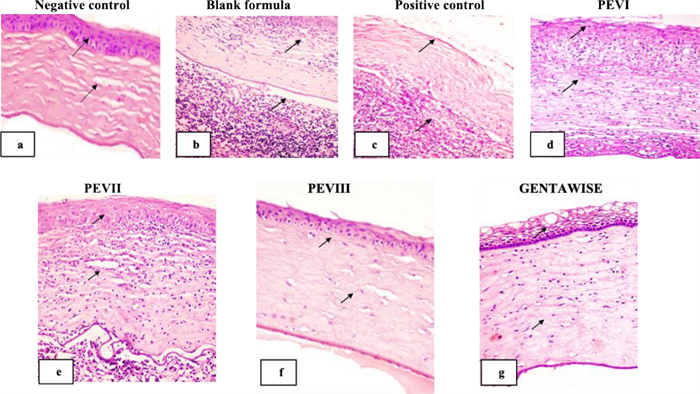
Histopathological examination and scoring: Histological sections of the negative control animal group demonstrated normal cornea histological structure, consisting of epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium. Corna was lined by outer non-keratinized stratified squamous epithelium with intact epithelial basal lamina and Bowman’s layer. The corneal stroma appeared as regularly arranged collagen lamellae with intact Descemet’s membrane, which was lined by a single layer of flat endothelial cells Fig. 7a.
Photomicrograph of corneal tissue section showing: a normal histological architecture of corneal epithelium, Bowman’s layer, stroma, Descemet’s membrane arrow: b, c loss of corneal epithelial lining and massive polymorphonuclear neutrophils infiltration arrow: d, e epithelial loss neutrophils infiltration, oedema and vascularization of corneal stroma: f mild swelling of the outer corneal epithelial lining and oedema of regular arranged collagen bundles arrow: g vacuolar degeneration of outer corneal epithelial layers with moderate oedema of collagen bundles arrow (H&E×200)
The tissue section of the infected eye blank formula & positive control infected non-treated group revealed a disruption of corneal tissue architecture. Loss of the outer epithelial lining of the cornea was seen. Severe corneal damage with intensive polymorphonuclear neutrophil infiltration, degeneration, and necrosis of corneal epithelial layers was also noticed. Disruption of corneal collagen bundles and new vascularization were noticed in the stroma, as shown in Fig. 7b, c.
The tissue section of the infected eye P. aeruginosa treated by PEVI or PEVⅡ displayed similar histological pictures. The corneal tissue section showed mild improvement in comparison with the untreated group. Epithelial loss and moderate to severe inflammatory infiltration, mainly neutrophils and macrophages. Oedema and vascularization of corneal stroma were seen in Fig. 7d, e.
The tissue section of the infected eye P. aeruginosa treated by PEVⅢ showed pronounced improvement in comparison with the previous group. Mild swelling of outer corneal epithelial lining with mild edema of regularly arranged collagen bundles without inflammatory cell infiltration Fig. 7f. On the other hand, P. aeruginosa-infected eyes treated by GENTAWISE® revealed less improvement than the previous group. Vacuolar degeneration of outer corneal epithelial layers with moderate oedema of collagen bundles was seen in Fig. 7g. The histopathological lesions in different treatments are summarized in Table 4.
The histopathological lesions in different treatments
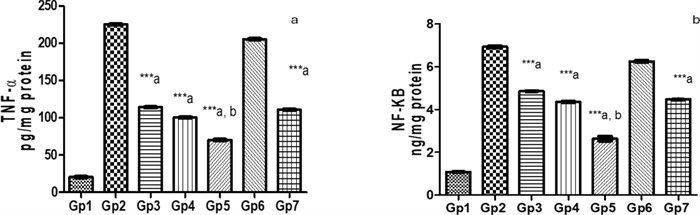
Comparative efficacy of novel saponin treatments in reducing inflammation in Pseudomonas keratitis: insights against standard therapy: In comparing the treated groups with the Pseudomonas keratitis-infected group, PEVⅢ treatment was the most effective in reducing inflammation. The PEVI-treated group showed a modest reduction, with TNF-α decreasing by 49.3% and NF-κB by 30%. The PEVⅡ-treated group showed a greater reduction of 55.5% in TNF-α and 37.2% in NF-κB. However, PEVⅢ treatment had the most significant effect, with a 68.8% and a 62% decrease in TNF-α and NF-κB, respectively, compared to the infected group. On the other hand, GENTAWISE® reduced TNF-α and NF-κB by 50.8% and 35.4%, respectively, highlighting PEVⅢ as the best therapeutic option. Cohen's d analysis revealed significant effect sizes for the differences in TNF-α and NF-κB levels between the treatment groups and the normal group. The Pseudomonas keratitis-infected group exhibited the largest effect sizes, indicating a substantial increase in both cytokines compared to the normal group. PEVⅢ-treated mice and GENTAWISE® -treated mice showed moderate to large reductions in both TNF-α and NF-κB levels, suggesting that these treatments effectively reduce inflammation, though not to the baseline levels observed in the normal group (Fig. 8).
Represents the pro-inflammatory parameters: a Tumor necrosis factor-α (TNF-α) and b nuclear factor kappa B (NF-κB) of infected mice with keratitis that were treated by different composite nanovesicles with saponins (PEVI, PEVⅡ, PEVⅢ) (GP3, Gp4, Gp5), respectively, compared to GENTAWISE® treated mice (Gp7). Where the three groups of prepared saponins show significant differences for the three pro-inflammatory parameters from the positive control (Gp2) (*** p ≤ 0.001 denoted by symbol a). PEVⅢ (Gp5) shows a significant difference also for the two pro-inflammatory parameters from the GENTAWISE®-treated group (Gp7) (*** p ≤ 0.001 denoted by symbol b). One-way ANOVA followed by Bonferroni post-hoc test was performed for multiple comparisons. Statistical significance was set at p < 0.05
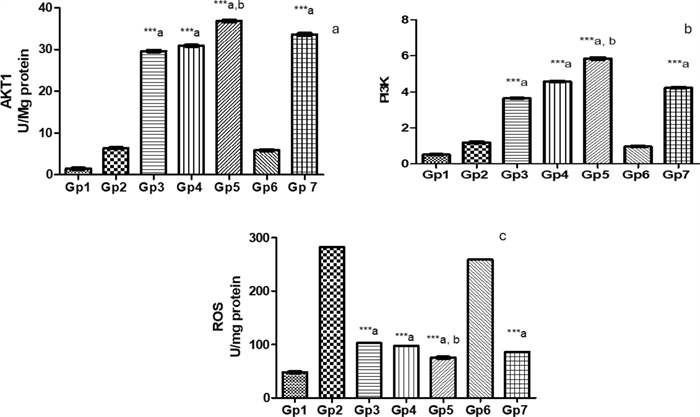
Efficacy of novel treatments against Pseudomonas keratitis: a comparative analysis of protective pathway activation and oxidative stress reduction: The efficacy of various treatments was evaluated by comparing AKT1, PI3K, and ROS levels in the treated groups against those in the Pseudomonas keratitis-infected group. The PEVⅢ-treated group exhibited the highest effectiveness, resulting in an increase in both AKT1 to 5.75-fold and PI3K to 4.97-fold compared to the infected group. Additionally, it demonstrated a significant reduction in ROS levels by 73.3%. In comparison, the GENTAWISE®-treated group showed an increase in AKT1 to 5.25-fold and an increase in PI3K to 3.59-fold, while a reduction in ROS by 69.6%. Although the PEVI and PEVⅡ treatments showed improved outcomes, they were less effective than PEVⅢ and GENTAWISE®. Cohen’s d analysis revealed that the PEVⅢ-treated group had large effect sizes for increased AKT1 and PI3K expression, and a significant reduction in ROS compared to both Pseudomonas infected group and GENTAWISE®-treated group (Fig. 9).
Represents a the survival factors: alpha serine/threonine-protein kinase (AKT1) and b the gene expression of phosphatidylinositol 3-kinases (PI3K) c reactive oxygen species (ROS) of infected mice with keratitis that were treated by different Composite Nanovesicles with Saponins (PEVI, PEVⅡ, PEVⅢ) (GP3, Gp4, Gp5), respectively, compared to GENTAWISE®-treated mice (Gp7). Where the 3 groups of prepared saponins show significant differences for AKT1, PI3K, and ROS levels from the positive control (Gp2) (*** p ≤ 0.001 denoted by symbol a). PEVⅢ (Gp5) shows a significant difference for AKT1, PI3K, and ROS levels from the GENTAWISE®-treated group (Gp7) (*** p ≤ 0.001 denoted by symbol b). One-way ANOVA followed by Bonferroni post-hoc test was performed for multiple comparisons. Statistical significance was set at p < 0.05
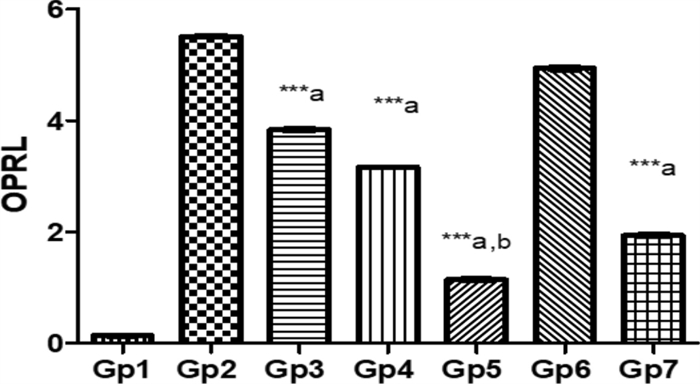
The role of OPRL expression in evaluating treatment efficacy for Pseudomonas keratitis: In this study, the effectiveness of various treatments in reducing the virulence factor OPRL in a Pseudomonas keratitis infection model was assessed. Among the treatments, PEVⅢ was the most effective, leading to a 79.2% reduction in OPRL expression compared to the infected group, highlighting its potential as a strong therapeutic option. GENTAWISE® treatment also significantly reduces OPRL levels, achieving a 64.9% reduction, though it was less effective than PEVⅢ. PEVⅡ showed a moderate reduction in OPRL expression, with a 42.6% decrease, while PEVI had the smallest effect, reducing OPRL levels by 30.3%. Cohen's d analysis revealed significant effect sizes for the difference in OPRL expression levels between groups (Fig. 10).
Represents the OPRL expression in infected mice with keratitis that was treated by different composite nanovesicles with saponins (PEVI, PEVⅡ, PEVⅢ) (Gp3, Gp4, Gp5), respectively, compared to GENTAWISE®-treated mice (Gp7). Where the three groups of prepared saponins show significant differences for OPRL expression from the positive control (Gp2) (*** p ≤ 0.001 denoted by symbol a). PEVⅢ (Gp5) shows a significant difference in the OPRL expression from the GENTAWISE®-treated group (Gp7) (*** p ≤ 0.001 denoted by symbol b). One-way ANOVA followed by Bonferroni post-hoc test was performed for multiple comparisons. Statistical significance was set at p < 0.05
4 Discussion
To advance the development of novel antimicrobial agents targeting P. aeruginosa-induced keratitis, we demonstrated for the first time the antibacterial and anti-inflammatory activities of three nanocomposites, PEVI-PEVⅢ, against P. aeruginosa keratitis in a murine model. The three nanocomposites exhibited high positivity, which could be attributed to the abundance of positively charged amino groups on the surface of the chitosan molecule and the spontaneous formation of densely packed molecules with uniform positive charge [49]. Hederacoside C-containing vesicles (PEVⅡ) exhibited a significantly larger particle size (P.S) compared to α-hederin-containing vesicles (PEVI) (P < 0.05). This difference is attributed to the higher molecular weight of hederacoside C (1221.4 g/mol) relative to α-hederin (751 g/mol), which likely facilitates greater vesicle expansion due to the larger molecular dimensions of hederacoside C. [50]. Co-encapsulation of saponins in PEVⅢ significantly increased particle size (P.S) compared to individual saponin encapsulation in PEVI or PEVⅡ (P < 0.05), probably due to spatial constraints within the nanosystem or saponin aggregation [51]. The encapsulation efficiency (EE%) of α-hederin-containing vesicles (PEVI) was significantly higher than that of hederacoside C-containing vesicles (PEVⅡ) (P < 0.05), attributable to the higher lipophilicity of α-hederin (Log P 3.6) compared to hederacoside C (Log P -2). This disparity likely results from a greater partitioning of hederacoside C into the aqueous phase relative to α-hederin, as supported by their respective Log P values [52, 53].
PEVⅢ, incorporating both saponins, demonstrated a notable increase in EE% (P < 0.05), which can be linked to its larger particle size (P.S), providing additional space for saponin accommodation and enhanced sequestration within the vesicles [54]. Notably, the EE% of the nanocomposites remained stable over 3 months of storage (P > 0.05), reflecting the robust encapsulation properties of the formulations, consistent with findings by Manca et al. [55]. Furthermore, several reports demonstrated the chemical stability of both saponins owing to their distinct molecular structure, which consists of a hydrophobic aglycone (sapogenin) and hydrophilic sugar moieties. Saponins' amphiphilic nature enables them to remain stable in a variety of environments, including aqueous and lipid-based systems. Meanwhile, the glycosidic linkages that link the sugar units to the sapogenin core are generally resistant to hydrolysis under normal physiological conditions, which increases their stability. The presence of numerous hydroxyl groups in their structure also helps them develop stable interactions with other molecules such as lipids and proteins, which can protect them against degradation [48]. Moreover, encapsulating saponins in nanoparticles increased their chemical stability and prevented degradation. Nanoparticles form a protective barrier around drug molecules, shielding them from environmental conditions including light, heat, oxygen, and moisture that can cause chemical degradation. This encapsulation also prevents premature interactions with enzymes or other reactive chemicals in the body, ensuring that the drug remains intact until it reaches its targeted site of action. Furthermore, nanoparticles' controlled release capabilities ensure that the drug is released in a stable and prolonged manner, lowering the possibility of degradation over time [45].
The sustained release of saponin(s) observed in the release profile of all the prepared vesicles could be ascribed to the enhanced saponin(s) encapsulation within the vesicular systems owing to their aforementioned lipophilicity, promoting the sustainment of their diffusion throughout the vesicular structures [56].
In the present study, PEVⅢ demonstrated significant antimicrobial and anti-virulent activity in vitro, with a minimum inhibitory concentration (MIC) of 64 μg/mL. Furthermore, PEVⅢ resulted in a substantial reduction in key virulence factors associated with the induction of keratitis, including a 50% decrease in pyocyanin production and an 80% reduction in twitching motility. These findings highlight the potential of PEVⅢ as an effective therapeutic agent against P. aeruginosa-induced keratitis. Several studies reported that twitching motility is essential for in vivo corneal infection induction to aid bacterial transfer from epithelium to underlying stroma [7]. The mechanisms of PEVⅢ antimicrobial properties may be attributed to the enhanced bacterial cell wall penetration, which is aided by its lipophilic character as well as immune system stimulation [57].
The histological findings from this study provide valuable insights into the efficacy of different therapeutic interventions for P. aeruginosa-induced keratitis. The untreated infected groups demonstrated severe disruption of corneal tissue architecture, characterized by epithelial loss, extensive inflammatory cell infiltration, and stromal neovascularization. These pathological changes highlight the aggressive nature of P. aeruginosa-induced keratitis and the urgent need for effective therapeutic strategies. PEVI and PEVⅡ treatments showed partial improvements, suggesting a degree of efficacy in mitigating inflammatory responses and tissue damage. This partial improvement might be attributed to the individual therapeutic properties of the encapsulated saponins, which may not have been sufficient to completely counteract the aggressive pathogenesis of the infection. In contrast, PEVⅢ treatment resulted in pronounced histological improvement, as evidenced by the preservation of the corneal epithelial lining, minimal stromal edema, and the absence of inflammatory cell infiltration. These results indicate that the co-encapsulation of both α-hederin and hederacoside C in PEVⅢ synergistically enhances therapeutic efficacy. The improved encapsulation efficiency and increased particle size in PEVⅢ likely contributed to prolonged drug release, enhanced bioavailability, and targeted delivery, leading to better therapeutic outcomes. The superior performance of PEVⅢ compared to GENTAWISE®, a widely used antibiotic formulation, further underscores the potential advantages of nanocomposite-based delivery systems. To further validate the anti-inflammatory effects of saponin treatments, we estimated the extent of proinflammatory cytokines expression levels (TNF-α and NF-κB), ROS levels, AKT1 and PI3K, OprL expression levels in corneas infected with P. aeruginosa. The NF-κB signaling pathway plays a pivotal role in regulating inflammatory responses, particularly through tumor necrosis factor-alpha (TNF-α) expression [58]. Saponins directly inhibit NF-κB activity, leading to a reduction in TNF-α production, reducing inflammation, and accelerating the corneal healing process [59, 60]. PEVⅢ demonstrated the most significant therapeutic efficacy in the treatment of P. aeruginosa keratitis. It led to a substantial reduction in inflammatory markers, including a 68.8% decrease in TNF-α and a 62% reduction in NF-κB, surpassing the effects of other treatments, including GENTAWISE®. These findings support the potential of saponins in attenuating the inflammatory cascade associated with Pseudomonas keratitis, as observed in the current study.
One of the key mechanisms by which saponins exert their protective effects is through the reduction of reactive oxygen species (ROS) levels [61, 62]. By reducing ROS, saponins alleviate oxidative stress and its associated cellular damage, especially relevant in conditions like Pseudomonas keratitis. In this study, PEVⅢ exhibited a significant reduction in ROS by 73.3%, indicating enhanced antioxidant capacity. The PI3K/Akt signaling pathway plays a central role in maintaining cellular redox homeostasis through activation of antioxidant responses. Increased activity of this pathway is known to upregulate key antioxidant enzymes such as superoxide dismutase and catalase, facilitating ROS detoxification and protecting cells from oxidative stress. In line with this, we measured both ROS and PI3K/Akt levels to assess the scavenging potential. PEVⅢ displayed the highest activation of protective signaling pathways, with a 5.75-fold increase in AKT1 and a 4.97-fold increase in PI3K, correlating with a significant reduction in ROS. This suggests that the enhanced antioxidant response is at least partially mediated by PI3K/Akt signaling. This finding aligns with previous researchs, including Wang et al. [63] and Wen et al. [64] which highlighted the role of PI3K/Akt activation in promoting redox balance and protecting cell from oxidative damage [63, 64]. Additionally, saponins are reported to stimulate the PI3K/Akt signaling pathway, contributing not only to antioxidant effects but also to improved cell growth, survival, and anti-inflammatory responses [65]. Our results align with the work of Wijesekara et al. [66], demonstrating that saponins enhance Akt production and subsequently suppress the release of pro-inflammatory cytokines [66]. Altogether, these findings suggest that saponins promote tissue healing in PA keratitis through a combined antioxidant and anti-inflammatory mechanism involving PI3K/Akt pathway activation. The outer membrane lipoprotein OprL is a significant virulence factor in P. aeruginosa, contributing to the bacterium's pathogenicity through various mechanisms. OprL is involved in the synthesis of lipopolysaccharide (LPS), a critical component of the bacterial outer membrane that plays a vital role in motility, colonization of host tissues, and the invasion of bacterial active proteins into target cells [44, 67]. By reducing OprL expression, saponins effectively lower bacterial virulence and enhance host immune responses. Interestingly, PEVⅢ treatment demonstrated the strongest reduction in the virulence factor OprL by 79.2% and emerged as the most effective treatment in mitigating the virulence factor OprL, demonstrating its potential for better control of bacterial virulence in Pseudomonas keratitis. Overall, PEVⅢ proved to be the most effective treatment, enhancing tissue protection, reducing inflammation, and controlling bacterial virulence, highlighting its potential as a leading therapeutic option for P. aeruginosa keratitis.
Saponins are a structurally diverse class of amphiphilic glycosides that have garnered increasing interest in ophthalmic drug delivery due to their multifaceted pharmacological properties [68]. Their potential therapeutic benefits in ocular diseases stem from their anti-inflammatory, antioxidant, and immunomodulatory effects. For instance, glycyrrhizin, a triterpenoid saponin, has proven substantial efficacy in ocular treatment of P. aeruginosa Keratitis [69], corneal neovascularization [70], and dry eye disease [71] with good tolerability. Similarly, the ocular application of ginsenosides Rg1 and Rb1 protect against diabetic retinopathy [72, 73], ginsenoside Rh2 attenuates corneal neovascularization [74], and ginsenoside Rg3 safeguards against Age-related Macular Degeneration (AMD) [75]. Despite these promising effects, the clinical translation of saponins for ocular use is limited due to their inherent surfactant-like properties, which can activate nociceptor TRPV1 channels, leading to ocular irritation or discomfort [76]. To overcome these limitations, several formulation strategies have been proposed. For example, nanoencapsulation within biocompatible carriers such as liposomes, solid lipid nanoparticles, or chitosan-coated vesicles can reduce direct contact of saponins with ocular tissues, modulate their release profile and enhance their bioavailability and therapeutic efficacy [77, 78]. In this study, α-hederin and hederacoside C were successfully encapsulated within chitosan-coated penetration enhancer vesicles (PEVs) to improve their ocular delivery. The chitosan's mucoadhesive properties might prolong their corneal residence time and create a protective barrier between the saponins and the ocular surface [79]. Moreover, the anti-inflammatory, antibacterial, wound-healing, and immunomodulatory properties of chitosan further provide ocular surface protection, which in turn reduces the risk of irritation or inflammation that might be triggered by the surfactant-like nature of saponins [80,81,82]. Additionally, the in vivo study revealed no signs of ocular discomforts, such as excessive blinking, redness, or tearing during or after the 7-day treatment. This was further supported by histopathological analysis, which confirmed that none of the PEV formulations caused epithelial damage or elicited an inflammatory response. Notably, the group treated with the saponin-loaded PEVⅢ formulation exhibited remarkable tissue regeneration and significant reduction in inflammatory markers, underscoring the safety and therapeutic potential of the PEVⅢ formulation.
5 Conclusion
Hedera saponins have demonstrated significant therapeutic potential in managing Pseudomonas keratitis. Their multifaceted benefits include reducing oxidative stress, modulating inflammatory pathways, and targeting bacterial virulence factors. Encapsulation of saponins into composite nano vesicular systems has shown enhanced antimicrobial, anti-virulent, antioxidant, and anti-inflammatory activities, posing them as promising candidates for topical ocular applications. Consequently, the topical administration of PEVⅢ represents a promising therapeutic strategy for combating Pseudomonas-induced keratitis. Although the sample size in this study was relatively small (n = 5 per group), we acknowledge that this limitation could influence the generalizability of our findings. The chosen sample size was based on ethical considerations, experimental feasibility, and was consistent with prior studies in similar keratitis models. For instance, Ren and Wu [83] used a comparable sample size in their neutropenic mouse model while demonstrating clear treatment efficacy [83]. Despite the small sample size, we reported large effect sizes, which strongly support the biological relevance of our findings. To mitigate bias, randomization was employed based on baseline health status, and histopathological assessments were conducted by an independent pathologist blinded to group allocations. Given the exploratory nature of our study and its alignment with established models, we consider our methodology to be robust. However, we acknowledge that larger sample sizes would be beneficial in future studies to validate and expand upon our findings. Further futuristic studies using animal tissues and clinical research are recommended to investigate the ex vivo corneal permeation and the pharmacokinetics of these nanosystems to optimize their efficacy and applicability to human health.
Notes
Acknowledgements
Not applicable.
Author contributions
Sherif A. Hamdy, Shymaa Hatem, Heba Elosaily, Abrar Gomaa Abd-Elfattah Hassan, Rana Elshimy, Ahmed H. Osman, Riham A. El-Shiekh: All authors contributed equally throughout the manuscript in investigation, methodology, formal analysis, writing original draft, and reviewing the final version. All authors approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Data available within the article or its supplementary materials.
Declarations
Ethics approval and consent to participate
This experimental design was conducted and approved following the instructions of the institutional animal care and use committee of Ahram Canadian University (Approval number: REC2524). All animal experiments were conducted following the regulations of Directive 2010/63/EU in Europe, and this study is reported under ARRIVE guidelines (https://arriveguidelines.org).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
1.El-Telbany M, Mohamed AA, Yahya G, Abdelghafar A, Abdel-Halim MS, Saber S, et al. Combination of Meropenem and zinc oxide nanoparticles; antimicrobial synergism, exaggerated antibiofilm activity, and efficient therapeutic strategy against bacterial keratitis. Antibiotics 2022;11: 1374. CrossRef PubMed Google Scholar
-
2.De Oliveira DM, Forde BM, Kidd TJ, Harris PN, Schembri MA, Beatson SA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020. https://doi.org/10.1128/cmr.00181-19. PubMed Google Scholar
-
3.Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom 2002;85: 271-8. CrossRef PubMed Google Scholar
-
4.Roshni J, Ahmad SF, Wani A, Ahmed SS. Multi-target effect of Aloeresin-A against bacterial and host inflammatory targets benefits contact lens-related keratitis: a multi-omics and quantum chemical investigation. Molecules 2023;28: 6955. CrossRef PubMed Google Scholar
-
5.Liao C, Huang X, Wang Q, Yao D, Lu W. Virulence factors of Pseudomonas aeruginosa and antivirulence strategies to combat its drug resistance. Front Cell Infect Microbiol 2022;12: 926758. CrossRef PubMed Google Scholar
-
6.Raheema RH, Yasir QD. Molecular detection of carbanemase in gram negative bacteria Isolated from intensive care unit patients Molecular detection of carbanemase in gram negative bacteria Isolated from intensive care unit patients in wasit province, Iraqin wasit province, Iraq. World J Biol Pharm Health Sci 2022;12: 130-9. CrossRef PubMed Google Scholar
-
7.Sánchez-Peña A, Winans JB, Nadell CD, Limoli DH. Pseudomonas aeruginosa surface motility and invasion into competing communities enhances interspecies antagonism. bioRxiv. 2024. https://doi.org/10.1128/mbio.00956-24. PubMed Google Scholar
-
8.Ghazaei C. Pseudomonas aeruginosa: Prevalence of Pathogenic Genes, OprL and ToxA in Human and Veterinary Clinical Samples in Ardabil, Iran. J Adv Biomed Sci. 2022. https://doi.org/10.18502/jabs.v12i4.11443. PubMed Google Scholar
-
9.Kierbel A, Gassama-Diagne A, Mostov K, Engel J. The phosphoinositol-3-kinase–protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol Biol Cell 2005;16: 2577-85. CrossRef PubMed Google Scholar
-
10.Chen K, Li Y, Zhang X, Ullah R, Tong J, Shen Y. The role of the PI3K/AKT signalling pathway in the corneal epithelium: recent updates. Cell Death Dis 2022;13: 513. CrossRef PubMed Google Scholar
-
11.Wu X-Y, Han S-P, Ren M-Y, Chang Y, Yu F-X. The role of NF-κB activation in lipopolysacchavide induced keratitis in rats. Chin Med J 2005;118: 1893-9. PubMed Google Scholar
-
12.Wu X, Chen G, Han S. The expression of nuclear factor-kappa B in lipopolysaccharide-induced keratitis of rats and the effect of pyrrolidine dithiocarbamate on its expression [Zhonghua yan ke za Zhi]. Chin J Ophthalmol 2006;42: 699-703. PubMed Google Scholar
-
13.Dong J, Jimi E, Zeiss C, Hayden MS, Ghosh S. Constitutively active NF-κB triggers systemic TNFα-dependent inflammation and localized TNFα-independent inflammatory disease. Genes Dev 2010;24: 1709-17. CrossRef PubMed Google Scholar
-
14.Kim JM, Yoon JN, Jung JW, Choi HD, Shin YJ, Han CK, et al. Pharmacokinetics of hederacoside C, an active ingredient in AG NPP709, in rats. Xenobiotica 2013;43: 985-92. CrossRef PubMed Google Scholar
-
15.Khdair A, Mohammad MK, Tawaha K, Al-Hamarsheh E, AlKhatib HS, Al-Khalidi B, et al. A validated RP HPLC-PAD method for the determination of hederacoside C in ivy-thyme cough syrup. Int J Anal Chem 2010;2010: 478143. CrossRef PubMed Google Scholar
-
16.Blumenthal M. The complete German commission E monographs. Therapeutic guide to herbal medicines. 1999. PubMed Google Scholar
-
17.Demirci B, Goppel M, Demirci F, Franz G. HPLC profiling and quantification of active principles in leaves of Hedera helix L. Die Pharmazie Int J Pharm Sci 2004;59: 770-4. PubMed Google Scholar
-
18.Hong E-H, Song J-H, Shim A, Lee B-R, Kwon B-E, Song H-H, et al. Coadministration of Hedera helix L. extract enabled mice to overcome insufficient protection against influenza A/PR/8 virus infection under suboptimal treatment with oseltamivir. PloS ONE 2015;10: e0131089. CrossRef PubMed Google Scholar
-
19.Hocaoglu AB, Karaman O, Erge DO, Erbil G, Yilmaz O, Kivcak B, et al. Effect of Hedera helix on lung histopathology in chronic asthma. Iran J Allergy Asthma Immunol 2012;11: 316-23. PubMed Google Scholar
-
20.El-Shiekh R, Atwa AM, Elgindy AM, Ibrahim KM, Senna MM, Ebid N, et al. Current perspective and mechanistic insights on α-Hederin for the prevention and treatment of several non-communicable diseases. Chem Biodivers 2024;22: e202402289. CrossRef PubMed Google Scholar
-
21.Xu H-C, Wu B, Ma Y-M, Xu H, Shen Z-H, Chen S. Hederacoside-C protects against AGEs-induced ECM degradation in mice chondrocytes. Int Immunopharmacol 2020;84: 106579. CrossRef PubMed Google Scholar
-
22.Muhammad A, Aftab S, Arshad Z, Chen Y, Wang Y, Yang M, et al. Hederacoside-C inhibition of staphylococcus aureus-induced mastitis via TLR2 & TLR4 and their downstream Signaling NF-κB and MAPKs pathways in vivo and in vitro. Inflammation 2020;43: 579-94. CrossRef PubMed Google Scholar
-
23.Mazayen ZM, Ghoneim AM, Elbatanony RS, Basalious EB, Bendas ER. Pharmaceutical nanotechnology: from the bench to the market. Future J Pharm Sci 2022;8: 12. CrossRef PubMed Google Scholar
-
24.Wassif RK, Elkheshen SA, Shamma RN, Amer MS, Elhelw R, El-Kayal M. Injectable systems of chitosan in situ forming composite gel incorporating linezolid-loaded biodegradable nanoparticles for long-term treatment of bone infections. Drug Deliv Transl Res 2024;14: 80-102. CrossRef PubMed Google Scholar
-
25.Bisen AC, Biswas A, Dubey A, Sanap SN, Agrawal S, Yadav KS, et al. A review on polymers in ocular drug delivery systems. MedComm Biomater Appl 2024;3: e77. PubMed Google Scholar
-
26.Bajcura M, Lukáč M, Pisárčik M, Horváth B. Study of micelles and surface properties of triterpene saponins with improved isolation method from Hedera helix. Chem Pap 2024;78: 1875-85. CrossRef PubMed Google Scholar
-
27.Ang S-S, Thoo YY, Siow LF. Encapsulation of hydrophobic apigenin into small unilamellar liposomes coated with chitosan through ethanol injection and spray drying. Food Bioprocess Technol 2024;17: 424-39. CrossRef PubMed Google Scholar
-
28.El-Kayal M, Hatem S. A comparative study between nanostructured lipid carriers and invasomes for the topical delivery of luteolin: design, optimization and pre-clinical investigations for psoriasis treatment. J Drug Deliv Sci Technol 2024;97: 105740. CrossRef PubMed Google Scholar
-
29.Hatem S, Elkheshen SA, Kamel AO, Nasr M, Moftah NH, Ragai MH, et al. Functionalized chitosan nanoparticles for cutaneous delivery of a skin whitening agent: an approach to clinically augment the therapeutic efficacy for melasma treatment. Drug Deliv 2022;29: 1212-31. CrossRef PubMed Google Scholar
-
30.Havlíková L, Macáková K, Opletal L, Solich P. Rapid determination of α-hederin and hederacoside C in extracts of Hedera helix leaves available in the Czech Republic and Poland. Nat Prod Commun 2015;10: 1934578X1501000910. PubMed Google Scholar
-
31.Hatem S, Nasr M, Moftah NH, Ragai MH, Geneidi AS, Elkheshen SA. Melatonin vitamin C-based nanovesicles for treatment of androgenic alopecia: design, characterization and clinical appraisal. Eur J Pharm Sci 2018;122: 246-53. CrossRef PubMed Google Scholar
-
32.Hatem S, Nasr M, Moftah NH, Ragai MH, Geneidi AS, Elkheshen SA. Clinical cosmeceutical repurposing of melatonin in androgenic alopecia using nanostructured lipid carriers prepared with antioxidant oils. Expert Opin Drug Deliv 2018;15: 927-35. CrossRef PubMed Google Scholar
-
33.Hatem S, El-Kayal M. Novel anti-psoriatic nanostructured lipid carriers for the cutaneous delivery of luteolin: a comprehensive in-vitro and in-vivo evaluation. Eur J Pharm Sci 2023;191: 106612. CrossRef PubMed Google Scholar
-
34.El-Gendy MA, Mansour M, El-Assal MI, Ishak RA, Mortada ND. Travoprost liquid nanocrystals: an innovative armamentarium for effective glaucoma therapy. Pharmaceutics 2023;15: 954. CrossRef PubMed Google Scholar
-
35.Esposito E, Pozza E, Contado C, Pula W, Bortolini O, Ragno D, et al. Microfluidic fabricated liposomes for Nutlin-3a ocular delivery as potential candidate for proliferative vitreoretinal diseases treatment. Int J Nanomed 2024;19: 3513-36. CrossRef PubMed Google Scholar
-
36.Eltellawy YA, El-Kayal M, Abdel-Rahman RF, Salah S, Shaker DS. Optimization of transdermal atorvastatin calcium–Loaded proniosomes: Restoring lipid profile and alleviating hepatotoxicity in poloxamer 407-induced hyperlipidemia. Int J Pharm 2021;593: 120163. CrossRef PubMed Google Scholar
-
37.Mohamed A, Abdelhamid F. Antibiotic susceptibility of Pseudomonas aeruginosa isolated from different clinical sources. Zagazig J Pharm Sci 2020;28: 10-7. PubMed Google Scholar
-
38.Boongapim R, Ponyaim D, Ponyaim D, Phiwthong T, Rattanasuk S. In vitro antibacterial activity of Capparis sepiaria L. against human pathogenic bacteria. Asian J Plant Sci. 2021. https://doi.org/10.3923/ajps.2021.102.108. PubMed Google Scholar
-
39.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 2008;3: 163-75. CrossRef PubMed Google Scholar
-
40.Jabłońska J, Dubrowska K, Augustyniak A, Wróbel RJ, Piz M, Cendrowski K, et al. The influence of nanomaterials on pyocyanin production by Pseudomonas aeruginosa. Appl Nanosci 2022;12: 1929-40. CrossRef PubMed Google Scholar
-
41.Pham DTN, Khan F, Phan TTV, Park S-K, Manivasagan P, Oh J, et al. Biofilm inhibition, modulation of virulence and motility properties by FeOOH nanoparticle in Pseudomonas aeruginosa. Brazilian J Microbiol 2019;50: 791-805. CrossRef PubMed Google Scholar
-
42.Abdul Hak A, Zedan HH, El-Mahallawy HA, El-Sayyad GS, Zafer MM. In Vivo and in Vitro activity of colistin-conjugated bimetallic silver-copper oxide nanoparticles against Pandrug-resistant Pseudomonas aeruginosa. BMC Microbiol 2024;24: 213. CrossRef PubMed Google Scholar
-
43.Utlu B, Öndaş O, Yıldırım M, Bayrakçeken K, Yıldırım S. Pseudomonas aeruginosa Keratitis in rats: study of the effect of topical 5% hesperidin practice on Healing. Eurasian J Med 2023;55: 64. CrossRef PubMed Google Scholar
-
44.Bancroft JD, Gamble M. Theory and practice of histological techniques. Amsterdam: Elsevier health sciences; 2008. PubMed Google Scholar
-
45.Holsæter AM, Wizgird K, Karlsen I, Hemmingsen JF, Brandl M, Škalko-Basnet N. How docetaxel entrapment, vesicle size, zeta potential and stability change with liposome composition–A formulation screening study. Eur J Pharm Sci 2022;177: 106267. CrossRef PubMed Google Scholar
-
46.Sharma N, Sharma S, Kaushik R. Formulation and evaluation of lornoxicam transdermal patches using various permeation enhancers. Int J Drug Deliv Technol 2019;9: 597-607. PubMed Google Scholar
-
47.Lakshmi P, Mounica V, Manoj KY, Prasanthi D. Preparation and evaluation of curcumin invasomes. Int J Drug Deliv 2014;6: 113. PubMed Google Scholar
-
48.Abdel Azim EA, Elkheshen SA, Hathout RM, Fouly MA, El El Hoffy NM. Augmented in vitro and in vivo profiles of brimonidine tartrate using gelatinized-core liposomes. Int J Nanomed 2022;17: 2753-76. CrossRef PubMed Google Scholar
-
49.Rashki S, Asgarpour K, Tarrahimofrad H, Hashemipour M, Ebrahimi MS, Fathizadeh H, et al. Chitosan-based nanoparticles against bacterial infections. Carbohyd Polym 2021;251: 117108. CrossRef PubMed Google Scholar
-
50.García-Manrique P, Machado ND, Fernández MA, Blanco-López MC, Matos M, Gutiérrez G. Effect of drug molecular weight on niosomes size and encapsulation efficiency. Colloids Surf B 2020;186: 110711. CrossRef PubMed Google Scholar
-
51.Ledezma-Gallegos F, Jurado R, Mir R, Medina LA, Mondragon-Fuentes L, Garcia-Lopez P. Liposomes co-encapsulating cisplatin/mifepristone improve the effect on cervical cancer: in vitro and in vivo assessment. Pharmaceutics 2020;12: 897. CrossRef PubMed Google Scholar
-
52.Budhian A, Siegel SJ, Winey KI. Haloperidol-loaded PLGA nanoparticles: systematic study of particle size and drug content. Int J Pharm 2007;336: 367-75. CrossRef PubMed Google Scholar
-
53.Manchanda R, Fernandez-Fernandez A, Nagesetti A, McGoron AJ. Preparation and characterization of a polymeric (PLGA) nanoparticulate drug delivery system with simultaneous incorporation of chemotherapeutic and thermo-optical agents. Colloids Surf B 2010;75: 260-7. CrossRef PubMed Google Scholar
-
54.El-Kayal M, Nasr M, Elkheshen S, Mortada N. Colloidal (-)-epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: a comprehensive experimental study with preclinical investigation. Eur J Pharm Sci 2019;137: 104972. CrossRef PubMed Google Scholar
-
55.Manca ML, Manconi M, Falchi AM, Castangia I, Valenti D, Lampis S, et al. Close-packed vesicles for diclofenac skin delivery and fibroblast targeting. Colloids Surf, B 2013;111: 609-17. CrossRef PubMed Google Scholar
-
56.Barakat SS, Nasr M, Ahmed RF, Badawy SS, Mansour S. Intranasally administered in situ gelling nanocomposite system of dimenhydrinate: preparation, characterization and pharmacodynamic applicability in chemotherapy induced emesis model. Sci Rep 2017;7: 9910. CrossRef PubMed Google Scholar
-
57.Naik P, Pandey S, Gagan S, Biswas S, Joseph J. Virulence factors in multidrug (MDR) and Pan-drug resistant (XDR) Pseudomonas aeruginosa: a cross-sectional study of isolates recovered from ocular infections in a high-incidence setting in southern India. J Ophthalmic Inflamm Infect 2021;11: 1-11. CrossRef PubMed Google Scholar
-
58.Capece D, Verzella D, Flati I, Arboretto P, Cornice J, Franzoso G. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol 2022;43: 757-75. CrossRef PubMed Google Scholar
-
59.Khan MI, Karima G, Khan MZ, Shin JH, Kim JD. Therapeutic effects of saponins for the prevention and treatment of cancer by ameliorating inflammation and angiogenesis and inducing antioxidant and apoptotic effects in human cells. Int J Mol Sci 2022;23: 10665. CrossRef PubMed Google Scholar
-
60.Passos FRS, Araújo-Filho HG, Monteiro BS, Shanmugam S, de Souza Araújo AA, da Silva Almeida JRG, et al. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: a review of pre-clinical research. Phytomedicine 2022;96: 153842. CrossRef PubMed Google Scholar
-
61.Hu J-N, Yang J-Y, Jiang S, Zhang J, Liu Z, Hou J-G, et al. Panax quinquefolium saponins protect against cisplatin evoked intestinal injury via ROS-mediated multiple mechanisms. Phytomedicine 2021;82: 153446. CrossRef PubMed Google Scholar
-
62.Sun Y, Zhang Y, Qi W, Xie J, Cui X. Saponins extracted by ultrasound from Zizyphus jujuba Mil var. spinosa leaves exert resistance to oxidative damage in Caenorhabditis elegans. J Food Meas Charact 2021;15: 541-54. CrossRef PubMed Google Scholar
-
63.Wang L, Chen Y, Sternberg P, Cai J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest Ophthalmol Vis Sci 2008;49: 1671-8. CrossRef PubMed Google Scholar
-
64.Wen S-Y, Ng S-C, Ho W-K, Huang H-Z, Huang C-Y, Kuo W-W. Activation of PI3K/Akt mediates the protective effect of diallyl trisulfide on doxorubicin induced cardiac apoptosis. Curr Res Toxicol 2023;5: 100136. CrossRef PubMed Google Scholar
-
65.Acosta-Martinez M, Cabail MZ. The PI3K/Akt pathway in meta-inflammation. Int J Mol Sci 2022;23: 15330. CrossRef PubMed Google Scholar
-
66.Wijesekara T, Luo J, Xu B. Critical review on anti-inflammation effects of saponins and their molecular mechanisms. Phytother Res 2024;38: 2007-22. CrossRef PubMed Google Scholar
-
67.Algammal AM, Eidaroos NH, Alfifi KJ, Alatawy M, Al-Harbi AI, Alanazi YF, et al. Opr l gene sequencing, resistance patterns, virulence genes, quorum sensing and antibiotic resistance genes of xdr Pseudomonas aeruginosa isolated from broiler chickens. Infect Drug Resist 2023;16: 853-67. CrossRef PubMed Google Scholar
-
68.Liao Y, Li Z, Zhou Q, Sheng M, Qu Q, Shi Y, et al. Saponin surfactants used in drug delivery systems: a new application for natural medicine components. Int J Pharm 2021;603: 120709. CrossRef PubMed Google Scholar
-
69.Ekanayaka SA, McClellan SA, Barrett RP, Hazlett LD. Topical glycyrrhizin is therapeutic for Pseudomonas aeruginosa keratitis. J Ocul Pharmacol Ther 2018;34: 239-49. CrossRef PubMed Google Scholar
-
70.Shah SL, Wahid F, Khan N, Farooq U, Shah AJ, Tareen S, et al. Inhibitory effects of Glycyrrhiza glabra and its major constituent glycyrrhizin on inflammation-associated corneal neovascularization. Evidence-Based Complementary Altern Med 2018;2018: 8438101. CrossRef PubMed Google Scholar
-
71.Burillon C, Chiambaretta F, Pisella P-J. Efficacy and safety of glycyrrhizin 2.5% eye drops in the treatment of moderate dry eye disease: results from a prospective, open-label pilot study. Clin Ophthalmol 2018;12: 2629-36. CrossRef PubMed Google Scholar
-
72.Ying Y, Zhang Y-L, Ma C-J, Li M-Q, Tang C-Y, Yang Y-F, et al. Neuroprotective effects of ginsenoside Rg1 against hyperphosphorylated tau-induced diabetic retinal neurodegeneration via activation of IRS-1/Akt/GSK3β signaling. J Agric Food Chem 2019;67: 8348-60. CrossRef PubMed Google Scholar
-
73.Wang Y, Sun X, Xie Y, Du A, Chen M, Lai S, et al. Panax notoginseng saponins alleviate diabetic retinopathy by inhibiting retinal inflammation: association with the NF-κB signaling pathway. J Ethnopharmacol 2024;319: 117135. CrossRef PubMed Google Scholar
-
74.Zhang XP, Li KR, Yu Q, Yao MD, Ge HM, Li XM, et al. Ginsenoside Rh2 inhibits vascular endothelial growth factor-induced corneal neovascularization. FASEB J 2018;32: 3782-91. CrossRef PubMed Google Scholar
-
75.Hu R-Y, Qi S-M, Wang Y-J, Li W-L, Zou W-C, Wang Z, et al. Ginsenoside Rg3 improved age-related macular degeneration through inhibiting ROS-mediated mitochondrion-dependent apoptosis in vivo and in vitro. Int J Mol Sci 2024;25: 11414. CrossRef PubMed Google Scholar
-
76.Zhao F, Wang S, Li Y, Wang J, Wang Y, Zhang C, et al. Surfactant cocamide monoethanolamide causes eye irritation by activating nociceptor TRPV1 channels. Br J Pharmacol 2021;178: 3448-62. CrossRef PubMed Google Scholar
-
77.Shree D, Patra CN, Sahoo BM. Applications of nanotechnology-mediated herbal nanosystems for ophthalmic drug. Pharm Nanotechnol 2024;12: 229-50. CrossRef PubMed Google Scholar
-
78.Razavi MS, Ebrahimnejad P, Fatahi Y, D’Emanuele A, Dinarvand R. Recent developments of nanostructures for the ocular delivery of natural compounds. Front Chem 2022;10: 850757. CrossRef PubMed Google Scholar
-
79.Ways TM, Lau WM, Khutoryanskiy VV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018;10: 267. CrossRef PubMed Google Scholar
-
80.Zamboulis A, Nanaki S, Michailidou G, Koumentakou I, Lazaridou M, Ainali NM, et al. Chitosan and its derivatives for ocular delivery formulations: recent advances and developments. Polymers 2020;12: 1519. CrossRef PubMed Google Scholar
-
81.Albarqi HA, Garg A, Ahmad MZ, Alqahtani AA, Walbi IA, Ahmad J. Recent progress in chitosan-based nanomedicine for its ocular application in glaucoma. Pharmaceutics 2023;15: 681. CrossRef PubMed Google Scholar
-
82.Irimia T, Ghica MV, Popa L, Anuţa V, Arsene A-L, Dinu-Pîrvu C-E. Strategies for improving ocular drug bioavailability and corneal wound healing with chitosan-based delivery systems. Polymers 2018;10: 1221. CrossRef PubMed Google Scholar
-
83.Ren M, Wu X. Evaluation of three different methods to establish animal models of Acanthamoeba keratitis. Yonsei Med J 2009;51: 121-7. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.