Natural anticancer agents: prospection of medicinal and aromatic plants in modern chemoprevention and chemotherapy
Abstract
Natural products obtained from medicinal and aromatic plants are increasingly recognized as promising anticancer agents due to their structural richness, including terpene and flavonoid molecules, which induce apoptosis and modulate gene expression. These compounds offer an alternative to conventional treatments, often costly, which face challenges such as multidrug resistance. This review aims to provide a promising alternative approach to effectively control cancer by consolidating significant findings in identifying natural products and anticancer agent development from medicinal and aromatic plants. It synthesizes the findings of a comprehensive search of academic databases, such as PubMed and Springer, prioritizing articles published in recognized peer-reviewed journals that address the bioprospecting of medicinal and aromatic plants as anticancer agents. The review addresses the anticancer activities of plant extracts and essential oils, which were selected for their relevance to chemoprevention and chemotherapy. Compounds successfully used in cancer therapy include Docetaxel (an antimitotic agent), Etoposide VP-16 (an antimitotic agent and topoisomerase Ⅱ inhibitor), Topotecan (a topoisomerase Ⅰ inhibitor), Thymoquinone (a Reactive Oxygen Species-ROS inducer), and Phenethyl isothiocyanate (with multiple mechanisms). The review highlights natural products such as Hinokitiol, Mahanine, Hesperetin, Borneol, Carvacrol, Eugenol, Epigallocatechin gallate, and Capsaicin for their demonstrated efficacy against multiple cancer types, including breast, cervical, gastric, colorectal, pancreatic, lung, prostate, and skin cancer. Finally, it highlights the need for continued bioprospecting studies to identify novel natural products that can be successfully used in modern chemoprevention and chemotherapy.Graphical Abstract
Keywords
Medicinal and aromatic plants Chemoprevention Chemotherapy Vegetable extracts Essential oils Natural volatiles Cancer cell lines1 Introduction
Cancer is a term that encompasses a diverse group of diseases characterized by the uncontrolled proliferation of abnormal cells, which can affect humans in any part of the body during their life cycle [1]. Under normal conditions, human cells form and multiply to replace cells that age or become damaged; in this sense, aging and cellular dynamics, including cell death and the division of young cells, are crucial for maintaining healthy living. Disruption of this balance can lead to aberrant cell proliferation, resulting in tumors that invade surrounding tissues and spread through the bloodstream and lymphatic system [2]. This process, known as metastasis, is a major cause of cancer-related deaths [1, 2]. The disease is marked by the abnormal expression of the phosphatidylinositol 3-kinase (PI3K) pathway [3], leading to sustained cell proliferation, and the loss of tumor-suppressive functions of the p53 [4], and retinoblastoma protein (pRB) pathways [5].
Despite advancements in anticancer strategies such as surgery, radiotherapy, chemotherapy, and immunotherapy, challenges persist, including immune system suppression, high treatment costs, and drug resistance in tumor cell lines [5, 6]. The significant mortality associated with conventional treatments underscores the need for innovative therapeutic approaches [7].
According to statistics from the World Health Organization (WHO) and the International Agency for Research on Cancer (IARC), cancer represents a significant public health challenge, being the second leading cause of death worldwide [8]. An analysis by Rajappa et al. [9] highlights the uneven distribution of cancer incidence and mortality, evidencing a clear correlation between socioeconomic development and cancer rates in Asian countries, where access to effective anticancer treatments is severely limited due to their high costs.
The most recent update from the Global Cancer Observatory (GCO) (https://gco.iarc.fr/ accessed January 5, 2025) places Latin America and the Caribbean (LAC) in fourth place with 7.8% of new cancer cases in 2022, after Asia (49.2%), Europe (22.4%), and North America (13.4%). According to estimates by the Pan American Health Organization (PAHO)/WHO (https://www.paho.org/es/ accessed January 5, 2025), 40% of cancer cases could be prevented by reducing key risk factors. These risk factors are attributable to infections by viruses: Human papillomavirus (HPV), Hepatitis B virus (HBV), and Hepatitis C virus (HCV); bacteria (Helicobacter pylori), and other microbial agents. Other risk factors are associated with the consumption of alcoholic beverages by the amount of alcohol consumed (moderate, < 20 g/day; risky, 20–60 g/day; heavy, > 60 g/day), obesity (according to anatomical site: breasts, corpus uteri, colon, kidney, gallbladder, pancreas, rectum, oesophageal adenocarcinoma, and ovary), and exposure to ultraviolet radiation.
The strategies proposed by PAHO/WHO include improving access to affordable chemotherapeutic drugs for the population and promoting palliative care policies, as programs for managing symptoms (https://www.paho.org/ accessed January 5, 2025). In response to this global health demand, the study of new natural alternatives for cancer chemoprevention or chemotherapy and its risk factors are linked to PAHO strategies, where scientific knowledge of medicinal and aromatic plants plays a fundamental role in mitigating the damage caused by this disease [10].
Cultural transmission of ethnobotanical knowledge has been globally essential to folk medicine practices [11]. For millennia, medicinal and aromatic herbs have played a crucial role in health rituals conducted by shamans and sorcerers, who incorporated these plants into potions, antidotes, ointments, and cosmetics. Today, these practices form the foundation of traditional medicine in many cultures [12, 13]. This accumulated knowledge, honed through trial and error, continues to thrive, particularly in regions conducive to cultivating these species [14, 15].
Historically, ancient Near Eastern civilizations introduced the traditional use of medicinal and aromatic plants as anticancer agents. Persian, Mesopotamian, Greek, and Roman civilizations greatly influenced these medicinal practices, followed later by influences from Arab and Islamic civilizations [16]. This knowledge subsequently spread globally, and in America, it occurred especially during colonization, where a dynamic and active process of cultural exchange between different groups of people was generated through various types of migratory events [17].
The literature review highlights in vitro studies of the cytotoxic and antiproliferative potential of natural products obtained from plants against cancer cell lines. Several herbs from different origins have been studied for their demonstrated bioactivities in traditional practice. Results of in vivo trials and clinical studies in cancer patients using whole plants, phytochemicals, or their extracts have shown that these exert chemopreventive or chemotherapeutic effects and reduce adverse events of anticancer drugs and disorders associated with this disease [18].
Essential oils (EOs) are noteworthy in anticancer strategy studies due to their chemical nature. The variety of volatile constituents involves different mechanisms to exert biological action, including DNA repair, cell cycle arrest, apoptosis, inhibition of metastasis, and multidrug resistance. This latter is the greatest challenge in cancer treatment [10]. However, medicinal and aromatic plant extracts have shown promising results, especially for obtaining isolated compounds due to their diversity, high availability, and low toxicity in cells in healthy conditions [14].
This review underscores the importance of traditional knowledge and continued research into natural products to identify novel therapeutic agents for cancer prevention and treatment. It aims to highlight the potential of medicinal and aromatic plants in developing innovative anticancer drugs. It focuses on the anticancer properties of chemical compounds, including plant extracts and EOs, which have been evaluated against various cancer cell lines.
2 Methodology
Information was collected through academic search systems for articles published in peer-reviewed journals, including PubMed, Springer, Science Direct, Scopus, Google Scholar, and ResearchGate. The search terms used were "medicinal and aromatic plants", "essential oils", "anticancer", "chemoprevention", "chemotherapy", "phytocompounds", "volatile components" or "natural volatiles" or "cancer cell lines".
2.1 Inclusion criteria
• Priority was given to articles published in recognized peer-reviewed journals that address the topic of Bioprospecting of medicinal and aromatic plants. Additionally, updates in the area of interest published by GCO and PAHO/WHO were included.
• Studies that directly addressed the anticancer activities of plant extracts and essential oils and their applications in chemoprevention and chemotherapy were selected.
• Recent publications were prioritized. However, classical references were also included.
2.2 Exclusion criteria
• Studies that were not directly related to the anticancer properties of plants or topics not addressed in the review were omitted.
• Duplicate publications were excluded.
3 Harnessing nature's defenses: the role of natural products in anticancer drug discovery
During the search for new drugs with anticancer potential, a chemical screening of the extracts and biological activity assays are necessary for rapid progress in obtaining new molecules. Screening of selected plant extracts by their use in traditional medicine can lead to potential anticancer agents to block, retard, or reverse the carcinogenic [19]. However, field observations are pivotal. In this regard, Hostettmann et al. [20] indicated that: "A plant species growing in a hostile environment, such as warm and humid tropical forests, will attempt to protect itself by synthesizing insecticidal, fungicidal, antibacterial, or virucidal constituents. Then, for example, if the leaves of a plant show no signs of aggression, they may contain defensive compounds against insects or microorganisms". That is to say, the plants adjust their physiological states in response to a stressful environment (stress both biotic and abiotic, climate changes, and phenological growth phases, among others) to improve their well-being and survival. These adjustments involve modulation of secondary metabolite production pivotal for the species' survival, mainly antioxidants, including terpenoids, alkaloids, and phenolics [21].
It has been estimated that currently two-thirds of anticancer drugs are obtained from plant extracts, and classification according to the pharmacological effects places them as antimitotics, topoisomerase inhibitors (Topo I and Topo II), ROS inducers, angiogenesis inhibitors, and histone deacetylases (HDAC) inhibitors [22]. In this sense, antimitotic drugs induce cell cycle arrest and tumor cell death [23], topoisomerase inhibitors act via topoisomerase poisoning leading to replication fork arrest and double-strand break formation [24], ROS inducers cause oxidative stress-induced apoptosis in cancer cells [25], angiogenesis inhibitors act on an endothelial cell in the growing vasculature (direct inhibitors) or block the activity of angiogenesis inducers (indirect inhibitors) [26], and HDAC inhibitors (epigenetic therapy) induce cell death in a select subpopulation of cells, restricted to the treatment of hematological malignancies [27, 28].
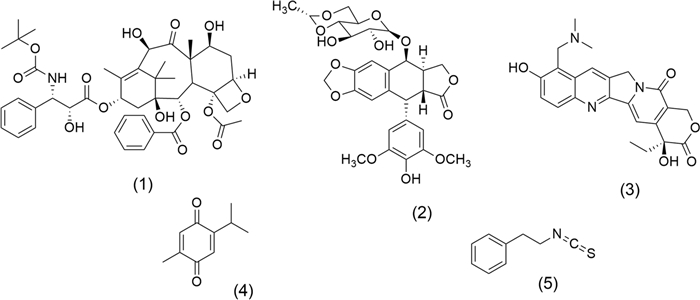
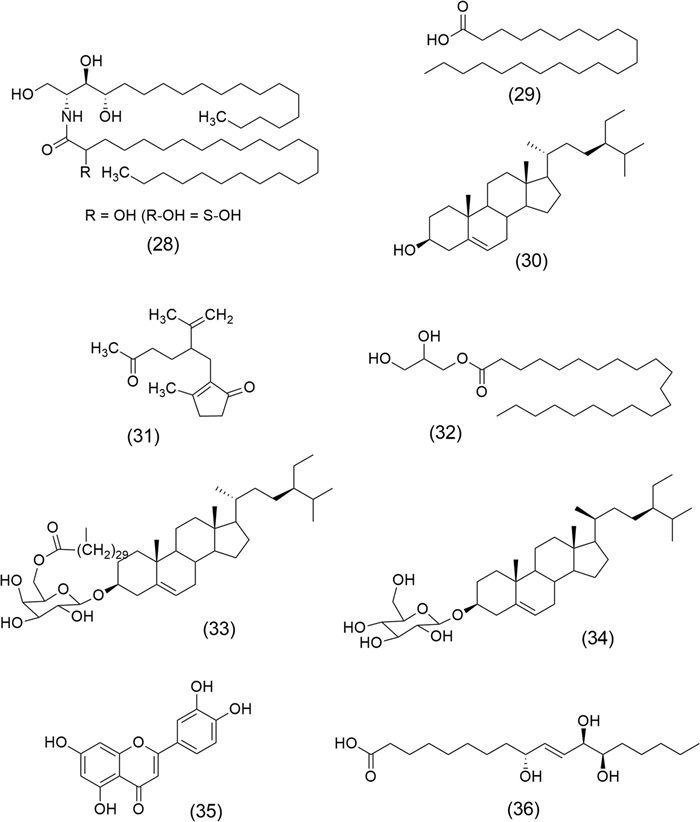
Figure 1 shows the chemical structures of some anticancer drugs derived from secondary metabolites in plant extracts. Docetaxel (1), an antimitotic agent obtained by hemisynthesis of baccatin Ⅲ, extracted from Taxus spp. (Taxaceae family), and a structural analogue of Paclitaxel (Taxol), is used in castration-resistant prostate cancer [29]. Another antimitotic agent and Topo Ⅱ inhibitor recognized is Etoposide, VP-16 (2), obtained from Podophyllum peltatum L. (Berberidaceae family) [30], which is used to manage various types of cancer, including lung, bladder, stomach, testicular, and prostate [31]. Topotecan (3) isolated from Camptotheca acuminata Decne. from Nyssaceae family acts as a potent Topo Ⅰ inhibitor agent [32], Thymoquinone (4) is a ROS inducer obtained from Nigella sativa L. (Ranunculaceae family) [33], and Phenethyl isothiocyanate (5), an anticancer agent whose activity is mediated by various mechanisms, including histone deacetylase inhibitor [34], induction of apoptosis, inhibition of cell proliferation, suppression of angiogenesis, and reduction of metastasis [35]. The latter is isolated from Nasturtium officinale L. (Brassicaceae family) [34].
Chemical structures of recognized anticancer agents: Docetaxel (1), Etoposide VP-16 (2), Topotecan (3), Thymoquinone (4), and Phenethyl isothiocyanate (5)
Today, the antioxidant molecules from nature, including natural products obtained from plants, are remarkable due to their anticancer potential. Some belong to terpenes, alkaloids, flavonoids, tannins, and phenolic groups [36]. The US Food and Drug Administration (US FDA) has approved several of these molecules; others are in the experimental phase. Moreover, some plant species contain bioactive molecules that provide therapeutic benefits synergistically [37].
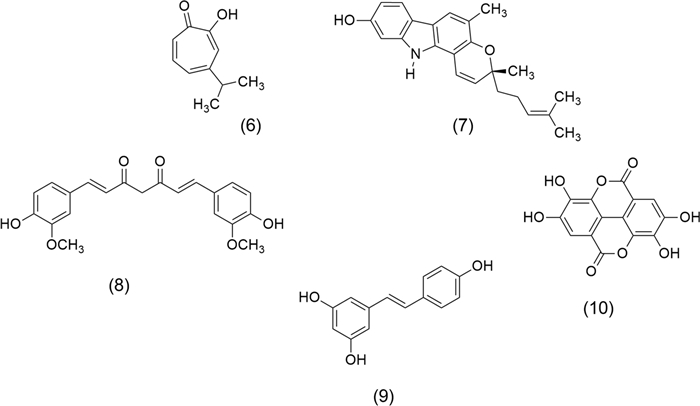
Literature underscores terpenes and alkaloids as key molecules against cancer. Hinokitiol (β-thujaplicin) (6), and Mahanine (7) (Fig. 2) are diterpene and alkaloid, respectively, isolated from Chamaecyparis obtusa (Siebold & Zucc.) Endl. (Cupressaceae) and Murraya koenigii (L.) Spreng. Rutaceae (also called curry tree), have highlighted the importance of plants as a source of biologically active molecules against cancer. Investigations have shown that (6), a natural bioactive monoterpenoid, exerts a potential anticancer effect on metastatic melanoma cells (B16-F10) due to their cell inhibition through downregulation of survivin protein, which activates the extracellular signal-regulated kinases (ERK)/mitogen-activated protein kinase phosphatase-3 (MKP-3)/proteosome pathway [38]. Furthermore, this monoterpene inhibits the heparanase via extracellular signal-regulated kinase and protein kinase B pathway leading to the reduction of tumor metastasis [39]. Recently, Chen et al. [40] showed that (6) has antitumor properties on endometrial cancer since it induces apoptosis mediated by ROS and p53-driven cell-cycle arrest in cell lines of Ishikawa and human endometrial adenocarcinoma (HEC-1A). On the other hand, (7) is a carbazole alkaloid that interferes with tumor growth and metastasis development by stimulating cell cycle arrest and decreasing apoptotic and anti-apoptotic proteins [41]. This compound inhibits the viability of estrogen receptor-positive breast cancer (ER+)/ wild type of p53 (p53WT) MCF-7 and triple-negative/p53Mut MDA-MB-231 cells, by apoptosis and arresting the cells in G0/G1. Also, (7) showed a significant reduction of mammary tumor burden induced by N-Methyl-N-nitrosourea (MNU) in a rat model [42]. Furthermore, (7) disrupts cell migration, invasion, and PI3K/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) signaling pathway in human glioma cell line (HS-683) and inhibits tumor growth in vivo [43].
Chemical structures of terpene, alkaloid, and polyphenolic with anticancer properties: Hinokitiol (6), Mahanine (7), Curcumin (8), Resveratrol (9), and Ellagic acid (10)
Polyphenols such as Curcumin (8) and Resveratrol (9), shown in Fig. 2, are promising anticancer molecules. Curcumin (8) is obtained from Curcuma longa L. (Zingiberaceae). The anticancer activity of (8) is due to antiproliferative and apoptotic effects by modulation of multiple molecular targets, among them, transcription factors (activator protein-1, AP-1; early growth response factor-1, EGR-1; signal transducer and activator of transcription-3 STAT-3; nuclear factor kappa B, NF-кB), cytokines (interleukin IL-2, IL-6, IL-8, macrophage inflammatory proteins MIP, tumor necrosis factor alpha TNF-α), receptors (estrogen receptor alpha, Erα; human epidermal growth factor receptor-2, HER-2; Fas receptor, Fas-R; epidermal growth factor receptor, EGFR), enzymes (enzyme that hydrolyses adenosine triphosphate, ATPase; Glutathione S-transferase, GST; cyclooxygenase 2, COX-2; telomerase), growth factors (epidermal growth factor, EGF; platelet-derived growth factor, PDGF; hepatocyte growth factor, HGF), and kinases (mitogen-activated protein kinase, MAP-K; protein kinase A, PKA; protein tyrosine kinase, PTK; protein kinase B, PKB; p21-activated kinase, PAK) [44]. Resveratrol (9), a stilbenoid isolated from Polygonum cuspidatum Sieb. et Zucc. (Polygonaceae), affects signal-transduction pathways that control cell growth and its division, metastasis, apoptosis, angiogenesis, and inflammation [45], modulates cell signaling molecules, among them cytokines, caspases, matrix metalloproteinases (MMPs), and nuclear factor kappa B (NFκB) [46]. Ellagic acid (10) is another important polyphenol extracted from Punica granatum L. (Punicaceae), which acts by inhibiting tumor-cell migration, invasion through the extracellular matrix, and angiogenesis. It has shown anticancer activity against colorectal, breast, prostate, lung, bladder, and ovarian cell lines [47].
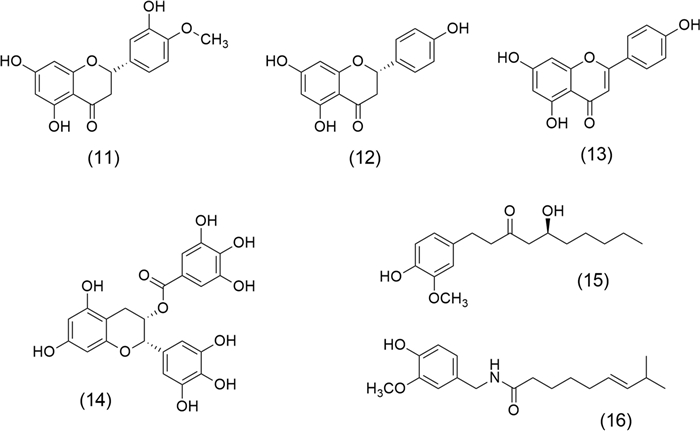
On the other hand, flavonoids are the most abundant polyphenols isolated from aromatic and medicinal plant extracts; these molecules have therapeutic effects in preventing cancer and related chronic diseases. Therefore, they are used by their health-promoting properties as nutraceuticals [48]. Hesperetin (11), Naringenin (12), and Apigenin (13) (Fig. 3) are three flavonoids with anticancer potential. (11) and (12), are found in Origanum acutidens (Hand.-Mazz.) Ietsw. (Lamiaceae) and in citrus fruits, including lemon, and orange. These are two flavonoids with chemotherapeutic and chemosensitising activities. (11), a dihydroflavone, induces G2/M phase cell-cycle arrest and apoptosis in human glioblastoma cells by p38 MAP-K activation [49]. (12) inhibits tumor cell proliferation and angiogenesis in malignant melanoma as B16F10 murine and SK-MEL-28 cells [50]. Combined treatment with (11) and (12) inhibits the proliferation of pancreatic cancer (Miapaca-2 and Panc-1 cells) by induction of caspase-3 cleavage, also acts by inhibition of migration of human pancreatic cancer cells, the phosphorylation of focal adhesion kinase (FAK), and p38 signaling [51]. (13) is a flavone from Origanum vulgare L. (Lamiaceae). This flavone induces apoptosis, autophagy, and immune response. Also, it inhibits cell cycle progress, cell migration, and invasion by modulating the targeting of multiple signaling pathways. Its beneficial effects include colorectal, breast, lung, prostate, ovarian, pancreatic, and cervical cancer, renal cell, adenoid cystic, thyroid, head and neck squamous cell carcinoma, and oral squamous cell. Furthermore, (13) inhibits melanoma growth, leukemia, glioblastoma, mesothelioma, and osteosarcoma [52].
Chemical structures of flavonoid, tannin, and phenolic molecules with anticancer properties: Hesperetin (11), Naringenin (12), Apigenin (13), Epigallocatechin gallate (14), Gingerol (15), and Capsaicin (16)
Epigallocatechin gallate (14) obtained from Camellia sinensis (L.) O. Kuntze. (Theaceae) is the most representative molecule of the tannins; it is an epigallocatechin and gallic acid ester, which induces apoptosis and cell cycle arrest. It has anti-inflammatory effects and modulates epigenetic changes in gene expression and chromatin organization via interaction with deoxyribonucleic acid (DNA) methyltransferase and histone deacetylases (HDACs) [53]. (14) has shown satisfactory results in studies of breast, lung, colorectal cancer, osteosarcomas, and neuroblastoma [54]. Gingerol (15) is an important phenolic compound that modulates NFκB, signal transducer and activator of transcription 3 (STAT3), AP-1, EGFR, vascular endothelial growth factor receptor (VEGFR), MAP-K, TNF-α, and COX-2 [55]. (15) obtained from Zingiber officinale Roscoe (Zingiberaceae), inhibits the proliferation of breast cancer cells (MCF7), suppresses oral cancer cell growth via activation of AMP-activated protein kinase (AMPK), and suppresses the AKT/mTOR signaling pathway [7, 56]. Finally, Capsaicin (16) obtained from Capsicum annuum L., Solanaceae is a phenolic compound that acts as a carcinogen or cancer chemoprevention agent. It treats lung, breast, colorectal, stomach, prostate, pancreatic, bladder cancer, nasopharyngeal carcinoma, cholangiocarcinoma, osteosarcoma, melanoma, fibrosarcoma, and glioblastoma [57].
Currently, computational tools are used with a biological indicative parameter, e.g., methyl thiazolyl tetrazolium (MTT) assay-based cell lines studies [58], which has allowed rapid progress in identifying and obtaining new anticancer molecules. Numerous studies of plant extracts have been evaluated for their cytotoxic, antiproliferative, antiangiogenic, and apoptotic properties. Some of the most significant findings of plant extracts and their phytocompounds (Fig. 4) are those described below.
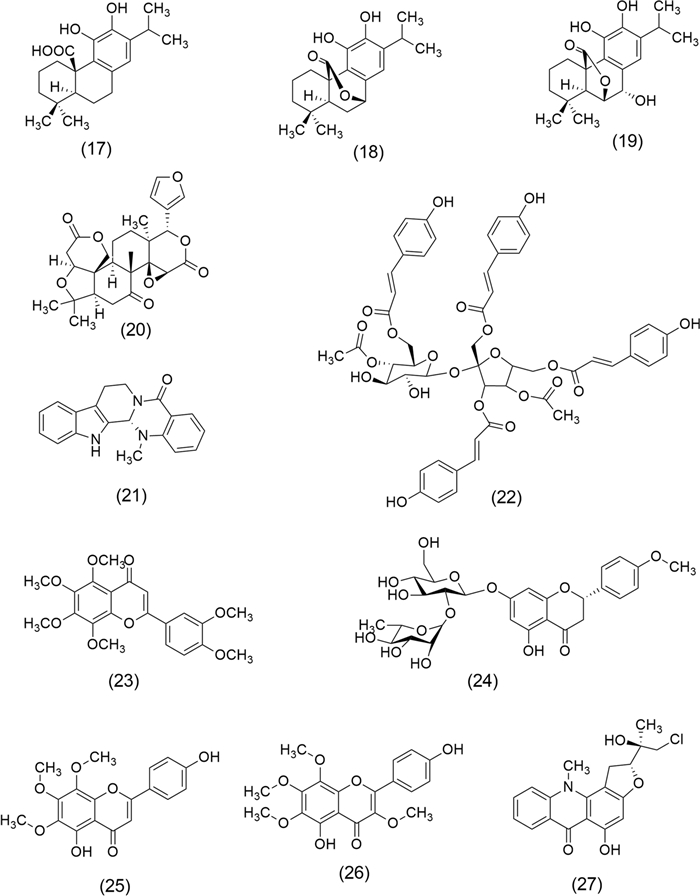
Chemical structures of Carnosic acid (17), Carnosol (18), Rosmanol (19), Limonin (20), Evodiamine (21), Polygonumins A (22), Nobiletin (23), Poncirin (24), Xanthomicrol (25), Calycopterin (26), and Isogravacridonechlorine (27)
Rosmarinus officinalis L. extract (known as rosemary) is a potent antioxidant agent and anti-inflammatory, which has been reported for anticancer properties attributed to diterpenes, including Carnosic acid (17), Carnosol (18), and Rosmanol (19), that act on key signaling pathways [59]. This aromatic plant belongs to the family Lamiaceae and is native to the Mediterranean region. Traditionally, rosemary has been recognized for its culinary uses and medicinal properties [60]. European Union (EU) approved its use as extracts standardized to diterpenes, e.g., (17) and (18). Also, the US FDA granted it the status of Generally Recognized as Safe (GRAS) [61]. Anticancer activities reported include colon cancer (extract showed strong inhibition of proliferation, migration, and colony formation of colon cancer cells regardless of their phenotype) [62], hepatocellular carcinoma (inhibited proliferation of HepG2 Cells) [63], lung cancer (extract decreased the activation of AKT/mTOR/p70S6 kinase (p70S6K) and showed inhibited proliferation and survival of A549 cells) [64], skin cancer (anti-proliferative effect on Human Melanoma A375 Cells) [65], oral cancer (rosemary exerts anti-inflammatory effects, proapoptotic, antiproliferative, and anti-angiogenic potential in buccal pouch carcinogenesis in a hamster model) [66], prostate cancer (extract inhibits prostate cancer cell proliferation and survival by targeting AKT and mTOR) [67], and breast cancer (extract exerts antiproliferative effects, inhibits survival, AKT, and mTOR signaling in triple-negative breast cancer cells) [68].
Evodia rutaecarpa (Juss.) Benth (Rutaceae) also called Euodia or Bee bee tree in the west, is a rich source of anticancer molecules, among them Limonin (20) and Evodiamine (21), which have been studied by their effects on ovarian cancer cells and colon cancer cells, respectively. Euodia extract and (20) showed a significant reduction in ovarian cancer cell lines SKOV-3, A2780, and RMUG-S by inducing apoptosis via activation of the p53 signaling pathway [69]. Furthermore, (21) a quinolone alkaloid exhibited prominent anti-proliferation and apoptosis-inducing effects in HCT-116 cells (colon cancer), mediated by bone morphogenetic protein 9 (BMP9) upregulation, which can activate p53 through upregulation of hypoxia-inducible factor 1-alpha (HIF 1α) [70].
Polygonum minus Huds (Polygonaceae), or kesum, is an aromatic plant that grows in temperate regions in Southeast Asian countries, it is used as a spice in the food industry. Methanol extract of kesum contains Polygonumins A (22), a potential anticancer agent whose structure includes four phenylpropanoid ester units and a sucrose unit, was evaluated on human cancer cell lines, including leukemia cells (K562), breast adenocarcinoma cells (MCF7), and colorectal cancer cells (HCT116) [71].
Citrus reticulate L. (mandarin orange) belongs to the Rutaceae family and is native to Southeast Asia and the Philippines. Mandarin orange peel was evaluated on the DLA cell line (Dalton´s Lymphoma Ascites) showing inducing of apoptosis by G0/G1 phase cell cycle arrest in DLA cells, as well as nuclear condensation, membrane blebbing, formation of apoptotic bodies, and DNA damage. These anticancer effects were attributed to polymethoxy flavones in water extract, e.g., Nobiletin (23) [72]. Furthermore, (23) was reported by suppressing autophagic degradation by over-expressing the AKT pathway, also inducing apoptosis in multidrug-resistant SKOV3/TAX ovarian cancer cells [73]. Another compound flavanone glycoside isolated from mandarin orange is Poncirin (24), which exerts antiproliferative effects on SGC-7901 gastric cancer cells [74].
Dracocephalum kotschyi Boiss (Lamiaceae), is an endemic species of Iran commonly known as Badrandjboie-Dennaie. This plant has been amply used as an anticancer agent, especially in leukemia treatment. Xanthomicrol (25) and Calycopterin (26) are two trimethoxylated hydroxyflavones derived from the leaves of D. kotschyi. These compounds are noted for their immunoinhibitory properties, contributing to their potential therapeutic applications. Studies have demonstrated that (26) exerts antiproliferative effects on HepG2 cells by inhibiting cell cycle progression at the G2/M transition, which leads to growth arrest and apoptosis, increases intracellular levels of ROS, NO, and decreases the expression of mitotic kinase cdc2, mitotic phosphatase cdc25c, mitotic cyclin B1, and apoptotic factors pro-caspases-3 and -9 [75]. On the other hand, (25) inhibits both B16F10 melanoma cell viability and cancer cell growth in an in vivo model by angiogenesis inhibition [76].
Ruta graveolens L. (Rutaceae), popularly called rue, is a species native to southern Europe, recognized for its culinary and medicinal uses. Roots and aerial parts extract of rue contains alkaloids, including Isogravacridonechlorine (27), which exerts a marked effect on the viability of MDA-MB-231 breast cancer cells. (27) disturbs the cell cycle by decreasing the G2/M and G0/G1 and increasing the S phase and the appearance of the subdiploid (sub-G1) population. Furthermore, it activates caspase-3 and -9, but not caspase-8, indicating an activation of an intrinsic apoptotic pathway in MDA-MB-231 cells [77].
Cyperus rotundus L. (Cyperaceae) also known as purple nutsedge is a perennial plant widely used in Traditional Chinese Medicine. Studies have shown anticancer effects on human triple-negative breast cancer (TNBC) cells [78]. Samra et al. [79] showed the anticancer activity of the methanolic extract of C. rotundus and compounds isolated from subfractions: 2′S-[2-Hydroxypentacosanoylamino]-1′,3′S,4′R nonadecanetriol (28), Behenic acid (29), β-Sitosterol (30), Mandassidione (31), Behenic acid monoglyceride (32), Sitosteryl (6`-hentriacontanoyl)-β-D-galactopyranoside (33), β-Sitosterol 3-O-β-D-glucoside (34), Luteolin (35), and Pinellic acid (36) (Fig. 5). Cytotoxicity of these phytocompounds was demonstrated against human hepatocellular carcinoma (HepG2), prostatic adenocarcinoma (PC3), and breast cancer (MCF-7) cell lines using the MTT assay.
Chemical structures of anticancer constituents from Cyperus rotundus L.: 2′S-[2-Hydroxypentacosanoylamino]-1′, 3′S, 4′R nonadecanetriol (28), Behenic acid (29), β-Sitosterol (30), Mandassidione (31), Behenic acid monoglyceride (32), Sitosteryl (6`-hentriacontanoyl)-β-D-galactopyranoside (33), β-Sitosterol 3-O-β-D-glucoside (34), Luteolin (35), and Pinellic acid (36)
Corsican-Sardinian (Santolina corsica Jord. & Fourr., Asteraceae), found only in Monte Albo (Sardinia), exhibits anticancer potential on MDA-MB-231 cells using n-hexane and methanolic extracts. Bonesi et al. [80] showed that both n-hexane and methanolic extracts, trigger apoptosis, and reduce invasive and migratory capacities of MDA-MB-231 cells.
Chenopodium album L. (Chenopodiaceae), commonly known as lamb's quarters, melde, goosefoot, wild spinach, fat-hen, cinzo, or quinhuilla, is amply used in traditional Chinese medicine. Its petroleum ether extract exhibited growth inhibitory effects (dose- and time-dependent) and G1 phase cell cycle arrest, as well as cell apoptosis (exhibited dose-dependent) on A549 human non-small cell lung cancer [81].
Petiveria alliacea L. (Phytolaccaceae) or "anamu" is a perennial herb, widely used by traditional medicine in the Caribbean, and in Central and South America [82]. Leaves and stem extracts of anamu showed anticancer effects in a murine breast cancer model using 4T1 cells. A fraction of the extract affects the glycolytic pathway enzymes and induces cell death and tumor regression in vitro and in vivo models, respectively [83].
Angelica archangelica L. (Apiaceae) or holy ghost root is an aromatic plant used in traditional medicine for its beneficial properties. Oliveira et al. [84] showed that crude extract of holy ghost root was cytotoxic against breast adenocarcinoma cells, especially on 4T1 and MCF-7, but not for human fibroblasts.
Cinnamon (Cinnamomum cassia Presl, Lauraceae), or the eternal tree of tropical medicine, is one of the important spices for health enhancement worldwide [85]. In a study, ethyl acetate, chloroform, and hexane extracts of C. cassia exhibit antiproliferative effects and DNA damage on breast cancer cells (MCF-7) [86]. Park et al. [87] showed cytotoxic activity in human colorectal cancer cells through the suppression of cell proliferation and the induction of apoptosis. Further, apoptotic induction but not autophagic cell death by C. cassia extract was evaluated on human oral cancer cells [88].
In Cymbopogon citratus (DC.) Stapf, a member of the Poaceae family, has several polysaccharides identified in its aqueous extract, which exhibits anticancer activity against human cervical cancer (Siha) and prostate cancer (LNCap) cell lines. The extract activates the intrinsic apoptotic signaling pathway, through the upregulation of caspase 3 and downregulation of Bcl-2 family genes, leading to the release of cytochrome C [89, 90]. Additionally, the essential oil derived from this plant has been shown to induce cell cycle arrest and apoptosis in A549 human lung cancer cells [91].
Recent studies have shown that Dioon rzedowskii (Zamiaceae family) effectively inhibits cell growth in MCF-7 and HepG2 cell lines by suppressing cell proliferation and the induction of apoptosis. The observed anticancer effects were linked to an increased expression of p53 and Bax and a reduction in cyclin D1 levels, which was associated with lower levels of phosphorylated MAPK kinases [92].
Pongamia pinnata Linn, a member of the Fabaceae family, is recognized for its significant role in herb-based traditional medicine. This study investigates the anticancer and antioxidant properties of its leaf extract. The phytochemical analysis was followed by in-silico assessments of anticancer receptors with ligands through molecular docking and simulation. The study revealed incremented phenolic and flavonoid content and anticancer potential against A431 skin cancer cells, suggesting that P. pinnata is a valuable source for drug development targeting melanomas [93].
Saffron (Crocus sativus L.), a traditional medicinal herb of the Iridaceae family, is rich in active compounds known for their anticancer properties. Enrichment analyses indicated that saffron enhances Th17 cell differentiation and IL-17 signaling pathway, effectively suppressing the proliferation of CT26 and HCT116 cells. These findings suggest that the active components of saffron improve the immune microenvironment of tumors, increasing the efficacy of immunotherapy for colorectal cancer [94].
The anticancer activity of an ethanolic extract of leaves from Myrtus communis Linn. (Myrtaceae family) was evaluated against various cancer cell lines, including breast (MCF-7), liver (HepG2), cervical (HeLa), and colon (HCT116) cancers. The extract demonstrated potent cytotoxic effects, primarily through apoptosis and cell cycle arrest in the G1 phase, suggesting a significant potential for developing novel anticancer therapies [95].
4 Essential oils and natural volatiles as promising anticancer therapy
Essential oils exhibit significant antioxidant activity due to their rich content of terpenes, terpenoids, and phenylpropanoids, which have demonstrated anticancer properties in human tumor cell lines and are produced as a mix of secondary metabolites in aromatic plants to attract pollinators and protect against environmental stressors [96]. Research indicates that low doses of EOs can alter cancer cell membrane permeability and metabolism, leading to increased ROS levels that induce apoptosis [97]. EOs target critical cellular pathways involved in growth and proliferation, modulating factors such as NF-κB and AKT, ultimately enhancing apoptotic processes in tumor cells [6]. The literature supports the role of EOs in improving cancer treatment efficacy through their multifaceted mechanisms of action, making them promising candidates for further therapeutic development.
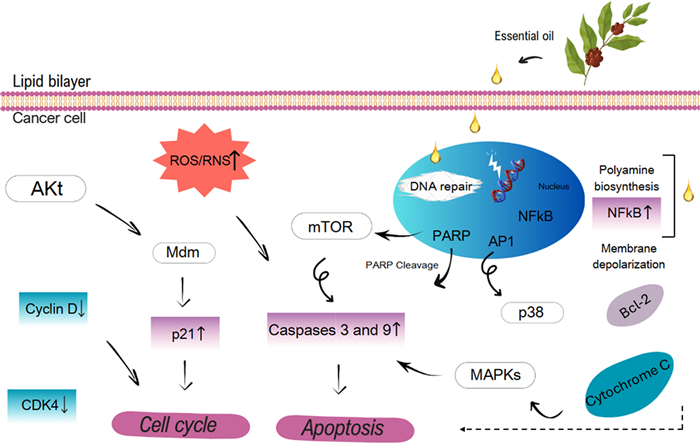
Figure 6 shows cellular targets involved in cell growth, proliferation, and metabolic pathways of EOs. In brief, (1) minimal doses of EOs alter membrane permeability and crosses the cell membrane, (2) increased levels of ROS and reactive nitrogen species (RNS) in cancer cells lead apoptosis, (3) inhibit activities of AKT, mTOR, and MAPK pathways at different steps by EOs leads to corresponding up-/downregulation of various important biomolecules, (4) Altered expression of NF-κB and its further binding to DNA result in apoptosis in cancer cells, (5) dephosphorylation of AKT by the action of EOs results both overexpression of p21 and cell cycle arrest, which induces apoptosis by increasing caspases level or results in binding to cyclins, respectively (6) mitochondrial stress induced by EOs leads both activation of Bcl-2 and membrane depolarisation, these results in enhanced release of cytochrome-C to the cytoplasm, which induces apoptotic cell death in tumour cells, and finally, (7) EOs modulate DNA repair mechanisms due to that they act as DNA polymerase inhibitors and lead to poly (ADP-ribose) polymerases (PARP) cleavage, which promote cell death by apoptosis in cancer cells.
Cellular targets involved in anticancer activity of essential oils. Adapted from Gautam et al. [6]
Currently, EOs from various plant species are recognized as a rich source of potential anticancer agents, particularly from the families Annonaceae, Asteraceae, Lamiaceae, Lauraceae, Myrtaceae, Pinaceae, Poaceae, Rutaceae, and Zingiberaceae. Researchers worldwide have documented the biological effects of these oils, and their anticancer activities summarized in Table 1. The table categorizes the species according to their respective botanical families and presents data on their effects against various cancer cell lines. Among the most commonly evaluated cancer types are breast cancer (including MCF-7, T47D, MCF-10A, MDA-MB-231, SKBR3, BT474), cervical cancer (HeLa, SiHa), colorectal cancer (HCT-116, HT-29, SW48, SW480, 502713, Caco2), gastric cancer (AGS, MGC-803), glioblastoma (M059J, SF763), leukemia (HL-60, K562, Jurkat, THP-1), liver cancer (HepG2, J5), lung cancer (NCI-H358M, A549), nasopharyngeal cancer (KB), neuroblastoma (IMR-32), ovarian cancer (OVCAR-8), oral cancer (OEC-M1, YD-8), prostate cancer (PC3, PC-3M, LNCap), pancreatic cancer (MIA PaCa-2, Panc-28, BxPC3, DANG), renal cancer (ACHN), and skin cancer (A375, A431, MV3, C32, UACC-62). This overview highlights the diverse anticancer potential of EOs across multiple tumor types.
Anticancer properties of essential oils obtained from plant species
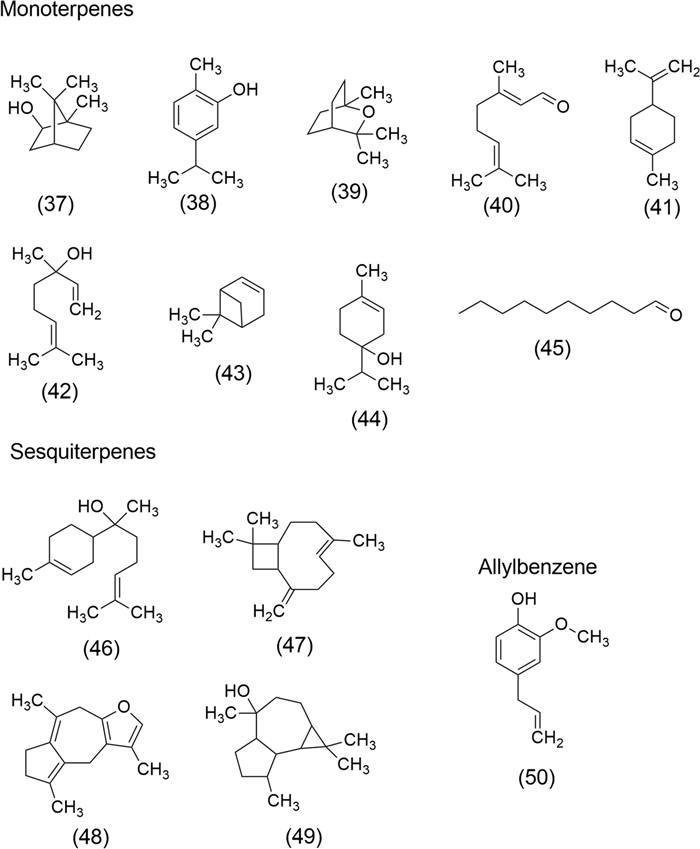
The versatility of EOs has led to the identification of some important volatile compounds with remarkable anticancer activity, including Borneol (37), Carvacrol (38), 1,8-Cineole (39), Citral (40), Limonene (41), Linalool (42), α-Pinene (43), Terpinen-4-ol (44), Decanal (45), α-Bisabolol (46), β-Caryophyllene (47), Isofuranodiene (48), Viridiflorol (49) and Eugenol (50). The chemical structures of these compounds are shown in Fig. 7.
Volatile compounds of EOs with anticancer properties: Borneol (37), Carvacrol (38), 1,8-Cineole (39), Citral (40), Limonene (41), Linalool (42), α-Pinene (43), Terpinen-4-ol (44), Decanal (45), α-Bisabolol (46), β-Caryophyllene (47), Isofuranodiene (48), Viridiflorol (49) and Eugenol (50)
Borneol (37), is a promising monoterpenoid isolated from EOs of many aromatic plants including wormwood (Artemisia iwayomogi Kitam., Asteraceae), camphor bush (Tarchonanthus camphorathus, Asteraceae), and lemongrass (Cymbopogon flexuosus (Nees ex Steud.) Watson., Poaceae), promoting apoptosis in human glioma cells through downregulation of Bcl-2 expression and upregulation of Bax and caspase-3, respectively [108].
Carvacrol (38) is a monoterpene phenol obtained from EOs of an abundant number of plant species, particularly of Lamiaceae and Rutaceae families, including oregano (Origanum vulgare L.), thyme (Thymus vulgaris L.), and wild bergamot (Citrus aurantium L. var. bergamia). Its hydrophobic nature is due to the benzene ring; however, it has been demonstrated that methyl and isopropyl substituents permit the moiety to bind with guanine in DNA [158]. (38) acts as cytotoxic, genotoxic, and proapoptotic in tumor cell lines of the breast, colon, liver, and lung [159].
1,8-Cineole (39) is an achiral volatile component of many plants, including salvia (Salvia officinalis L.) and eucalyptus (Eucalyptus globulus Labill.). This monoterpene induces proapoptotic effects against A2780 ovarian cancer cells [160] and induces G2/M arrest in A431 epidermoid carcinoma cells by upregulation of the p53 apoptotic signaling pathway [161]. Furthermore, apoptotic effects have been observed in Molt 4B acute lymphoblastic leukemia and HL-60 human leukemia cells [162].
Citral (40) or geranial is a common monoterpenoid of oregano (Origanum vulgare L.), aromatic litsea (Litsea cubeba (Lour.) Pers.), lemon verbena (Lippia citriodora Kunth.), and lemon balm (Melissa officinalis L.), which has apoptotic effect by activation of protein procaspase-3, acts on leukemic cell, prostate cancer (LNCaP, PC-3), glioblastoma (SF-767, SF-763) [146], hepatocellular carcinoma (HepG2), breast adenocarcinoma, colon adenocarcinoma (Caco2), and leukemic monocytes (THP-1) [152].
Limonene (41) is a terpenoid widely distributed in aromatic plant species including calabash nutmeg (Monodora myristica (Gaertn.) Dunal.), wild marigold (Tagetes minuta L.), common juniper (Juniperus communis L.), oregano (Origanum vulgare L.), cinnamon (Cinnamomum zeylanicum J. Presl.), aromatic litsea (Litsea cubeba (Lour.) Pers.), bay laurel (Laurus nobilis L.), spearmint (Mentha spicata L.), Japanese red pine (Pinus densiflora Siebold & Zucc.), and lemongrass (Cymbopogon spp.). This monoterpene prevents carcinogen-induced breast cancer at the initiation and the promotion/progression stages and acts as an antiproliferative on prostate cancer (LNCaP), breast cancer (MCF-7), lung cancer (A549) cell lines [99, 138].
Linalool (42), a monoterpene of common aromatic plants such as French lavender (Lavandula stoechas L.), cinnamon (Cinnamomum zeylanicum J. Presl.), bay laurel (Laurus nobilis L.), and ginger (Zingiber striolatum Diels.), acts as an anticancer agent by its cytotoxic and apoptotic properties on amelanotic melanoma and renal cell adenocarcinoma [108, 132], as well as in lung cancer (A549 cell line) [19].
α-Pinene (43), which is an organic compound of the polyphenolic group monoterpene (bicyclic monoterpene), is obtained from EOs from mint (Mentha piperita L. and M. arvensis L.), holy basil (Ocimum sanctum L.), and guava (Psidium guajava L.). (43) exerts an antiproliferative effect in A549 human lung cancer cells [19], it enhances the activity of natural killer cells via ERK/AKT Pathway [163], exerts cytotoxic effects in PA-1 human ovarian teratocarcinoma cells, and suppress the cell sequence progression along with the programmed cell death [164].
Terpinen-4-ol (44) is a monoterpenoid from tea tree (Melaleuca alternifolia (Maiden & Betche) Cheel). (44) showed antiproliferative effects on prostate cancer (LNCaP), and breast cancer (MCF-7) [138]. Shapira et al. [165] demonstrated that this volatile constituent inhibits the growth of cancer cell lines in a dose-dependent manner on colorectal, pancreatic, prostate, and gastric cancer cells.
Decanal (45), is a natural fragrant of the aldehyde group which acts by its antiproliferative properties on lung cancer (A549 cell line) [19].
α-Bisabolol (46), sesquiterpene alcohol, is a volatile compound obtained from lemongrass (Cymbopogon flexuosus (Nees ex Steud.) Watson) and chamomile tea (Matricaria recutita L. and M. chamomilla L.). (46) exerts selective anticancer activity on A549 adenocarcinoma human alveolar basal epithelial cells by cycle arrest, mitochondrial apoptosis, and inhibition of PI3K/AKT signaling pathways [39]. Further, (46) inhibits mammary tumors (4T1cells) in vitro and transplanted [147, 166].
β-Caryophyllene (47) is a bicyclic sesquiterpene common in the EOs of Cannabis sativa L., Cannabaceae family, (Marijuana); it exerts antiproliferative effects on ACHN and C32 cell lines [132]. (47) alter important pathways for cancer development, including mitogen-activated protein kinase (MAPK), PI3K/AKT/mTOR/S6K1, and STAT3 pathways [167].
Isofuranodiene (48) is a volatile constituent of wild celery or Alexander's celery (Smyrnium olusatrum L., Apiaceae). (48) has been shown to induce apoptosis in colon cancer cells (HCT116) in a time- and concentration-dependent manner, indicating its potential as a model for developing chemopreventive agents [168].
Viridiflorol (49), a sesquiterpenoid obtained from the EOs of Cardiopetalum calophyllum Schltdl. (Annonaceae) has shown cytotoxic and apoptotic effects on breast, lung, and brain cancer cell lines [169].
Eugenol (50) is an allylbenzene derived from clove (Syzygium aromaticum L., Myrtaceae), a species recognized for its diverse applications, including its use as a food preservative, antioxidant, anti-inflammatory, antimicrobial, and anticancer agent. Eugenol exerts anticancer effects on the lung, colon, gastric, cervical, breast, and melanoma cells through several mechanisms, including apoptosis, cell cycle arrest, and inhibiting migration, metastasis, and angiogenesis in various tumor cells. Additionally, it is widely used as an adjunct treatment for cancer patients undergoing conventional therapies [170].
5 Anticancer therapy and essential oils: enhancing efficacy and reducing toxicity
In recent years, EOs have been used to enhance the sensitivity of cancer cells [171], reduce the toxicity associated with anticancer drugs, improve the efficacy of radiotherapy [172], and increase cytotoxic effects when combined with herbal treatments [173]. For example, EO from Zataria multiflora Boiss. used in conjunction with doxorubicin, has been shown to enhance the sensitivity of PC3 prostate cancer cells to ROS generation and apoptosis, positioning it as a promising candidate for combinatorial therapy [171]. Furthermore, a nanoemulsion of ginger and frankincense essential oils combined with mitomycin C has enhanced efficacy against malignant cells while reducing individual drug toxicity [174].
Furthermore, encapsulation of green tea EO into nanocarriers has shown the potential to improve therapeutic specificity and efficacy while minimizing toxicity to normal cells [151]. Also, EOs are used as supportive therapies to manage side effects associated with radiotherapy or chemotherapy, such as insomnia and nausea [18]. Finally, combinations of herbal treatments with anticancer properties have shown significant cytotoxic effects on A549 cells compared to cisplatin [173].
These findings represent only a part of recent research in the literature and underline the importance of investigating EOs from aromatic plants as accessible therapeutic strategies for cancer treatment.
6 Conclusions
Medicinal and Aromatic plants exert a pivotal role in the development of new alternatives for the treatment of cancer. Extracts, essential oils, or secondary metabolites obtained from plant species have made it possible to elucidate some of the mechanisms involved in cancer chemoprevention or chemotherapy. Essential oils and extracts have been identified with health-enhancing properties due to the high antioxidant capacity of their phytoconstituents, as well as to the unique properties of essential oils, making their ingredients improve the well-being of cancer patients due to increasing the sensitivity of malignant cells to other therapeutic strategies and reducing the adverse effects generated during radio- or chemotherapy.
Future research should prioritize the elucidation of specific molecular pathways through which bioactive phytochemicals exert their anticancer effects. Investigation of their interactions with cellular targets, including enzymes, receptors, and signaling proteins, is necessary to understand how these compounds induce apoptosis, inhibit proliferation, or modulate the immune response. The development of advanced techniques such as proteomics, metabolomics, and bioinformatics is essential to map these interactions and identify potential biomarkers of efficacy and safety.
Further progress in the identification and isolation of specific bioactive phytochemicals is critical for the development of targeted therapies that minimize side effects and maximize efficacy. The development of targeted delivery systems, such as nanoparticles or liposomes, could further improve the therapeutic index of these compounds by enhancing their accumulation in tumor tissues. As the safety and efficacy of plant-derived compounds are established, their integration into standard chemotherapy regimens is likely to expand.
Finally, exploring undervalued plant species may reveal novel anticancer agents, while sustainable sourcing practices will ensure the ecological integrity and long-term availability of these valuable resources in bioprospecting efforts.
Notes
Abbreviations
AKT
Protein kinase B
AMPK
AMP-activated protein kinase
AP-1
Activator protein-1
ATPase
The enzyme that hydrolyses adenosine triphosphate
BMP9
Bone morphogenetic protein 9
COX-2
Cyclooxygenase 2
DLA
Dalton´s Lymphoma Ascites
DNA
Deoxyribonucleic acid
EGF
Epidermal growth factor
EGFR
Epidermal growth factor receptor
EGR-1
Early growth response factor-1
ER+
Estrogen receptor-positive breast cancer
Erα
Estrogen receptor alpha
ERK
Extracellular signal-regulated kinases
EOs
Essential oils
EU
European Union
FAK
Focal adhesion kinase
Fas-R
Fas receptor
GCO
Global Cancer Observatory
GRAS
Generally Recognized as Safe
GST
Glutathione S-transferase
HEC-1A
Human endometrial adenocarcinoma
HDACs
Histone deacetylases
HGF
Hepatocyte growth factor
HIF 1α
Hypoxia-inducible factor 1-alpha
IARC
International Agency for Research on Cancer
IL
Interleukin
HBV
Hepatitis B virus
HCV
Hepatitis C virus
HDACs
Histone deacetylases
HER-2
Human epidermal growth factor receptor-2
HPV
Human papillomavirus
HS-683
Human glioma cell line
LAC
Latin America and the Caribbean
MAP-K
Mitogen-activated protein kinase
MIP
Macrophage Inflammatory Proteins
MKP-3
Mitogen-activated protein kinase phosphatase-3
MNU
N-Methyl-N-nitrosourea
MMPs
Matrix metalloproteinases
mTOR
Mechanistic target of rapamycin
MTT
Methyl thiazolyl tetrazolium
NFκB
Nuclear factor kappa B
p53WT
Wild type of p53
p70S6K
P70S6 kinase
PAHO
Pan American Health Organization
PAK
P21-activated kinase
PARP
Poly (ADP-ribose) polymerases
PDGF
Platelet-derived growth factor
PI3K
Phosphatidylinositol 3-kinase
PKA
Protein kinase A
PKB
Protein kinase B
pRB
Retinoblastoma protein
PTK
Protein tyrosine kinase
RNS
Reactive nitrogen species
ROS
Reactive oxygen species
TNBC
Triple-negative breast cancer
TNF-α
Tumor necrosis factor alpha
Topo Ⅰ:
Topoisomerase Ⅰ
Topo Ⅱ:
Topoisomerase Ⅱ
STAT-3
Signal transducer and activator of transcription-3
US FDA
United State Food and Drug Administration
VEGFR
Vascular endothelial growth factor receptor
WHO
World Health Organization
Acknowledgements
The authors thank to the Ministry of Science, Technology, and Innovation (MINCIENCIAS) and Francisco José de Caldas National Fund for Science, Technology and Innovation, for supporting P.Q.-R. through the postdoctoral program "Convocatoria Orquídeas: Mujeres en la Ciencia 2024 (No. 948-2024)", Contract 112721-196-2024.
Author contributions
Conceptualization, J.O.-V. and P.Q.-R.; methodology, P.Q.-R., and K.C.-G.; formal analysis, P.Q.-R., K.C.-G., and J.O.-V.; investigation, P.Q.-R.; resources, K.C.-G. and J.O.-V.; data curation, P.Q.-R.; writing—original draft preparation, P.Q.-R.; writing—review and editing, P.Q.-R., K.C.-G., and J.O.-V.; supervision, K.C.-G., and J.O.-V.; project administration, K.C.-G., and J.O.-V.; funding acquisition, K.C.-G., J.O.-V., and P.Q.-R. All authors have read and agreed to the published version of the manuscript." authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technology, and Innovation (Minciencias) from the Francisco José de Caldas Fund (Grant RC-112721-416-2023).
Data availability
The data presented in this study are available in the manuscript.
Declarations
Ethics approval and consent to participate
This article does not contain any studies involving animals performed by any authors. This article contains no studies involving human participants performed by any authors.
Competing interests
The authors declare that there are no conflicts of interest.
References
-
1.de Oliveira MS, Almeida MM, Salazar MLAR, Pires FCS, Bezerra FWF, Cunha VMB, Cordeiro R, Olivo G, Paiva M, Souza A, Holanda R, de Carvalho RN. Potential of medicinal use of essential oils from aromatic plants. In Potential of essential oils. IntechOpen. 2018, 1-21. PubMed Google Scholar
-
2.Wannes WA, Tounsi MS, Marzouk B. A review of Tunisian medicinal plants with anticancer activity. J Complement Integr Med. 2018. CrossRef PubMed Google Scholar
-
3.Nunnery SE, Mayer IA. Targeting the PI3K/AKT/mTOR pathway in hormone-positive breast cancer. Drugs. 2020;80(16): 1685. CrossRef PubMed Google Scholar
-
4.Kadosh E, Snir-Alkalay I, Venkatachalam A, May S, Lasry A, Elyada E, Zinger A, Shaham M, Vaalani G, Mernberger M, Stiewe T, Pikarsky E, Oren M, Ben-Neriah Y. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature. 2020;586(7827): 133. CrossRef PubMed Google Scholar
-
5.Walter DM, Yates TJ, Ruiz-Torres M, Kim-Kiselak C, Gudiel AA, Deshpande C, Wang WZ, Cicchini M, Stokes KL, Tobias JW, Buza E, Feldser DM. RB constrains lineage fidelity and multiple stages of tumour progression and metastasis. Nature. 2019;569(7756): 423. CrossRef PubMed Google Scholar
-
6.Gautam N, Mantha AK, Mittal S. Essential oils and their constituents as anticancer agents: a mechanistic view. Biomed Res Int. 2014;2014: 1. CrossRef PubMed Google Scholar
-
7.Mohammed MS. The molecular activity of gingerol on inhibits proliferation of breast cancer cell line (MCF7) through caspase activity. Ann Roman Soc Cell Biol. 2021;25(4): 11095. PubMed Google Scholar
-
8.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3): 209. CrossRef PubMed Google Scholar
-
9.Rajappa S, Singh M, Uehara R, Schachterle SE, Setia S. Cancer incidence and mortality trends in Asia based on regions and human development index levels: an analyses from GLOBOCAN 2020. Curr Med Res Opin. 2023;39(8): 1127-37. CrossRef PubMed Google Scholar
-
10.Mesquita LSSD, Luz TRSA, Mesquita JWCD, Coutinho DF, Amaral FMMD, Ribeiro MNDS, Malik S. Exploring the anticancer properties of essential oils from family Lamiaceae. Food Rev Intl. 2019;35(2): 105. CrossRef PubMed Google Scholar
-
11.Reyes-García V, Broesch J, Calvet-Mir L, Fuentes-Peláez N, McDade TW, Parsa S, Tanner S, Huanca T, Leonard W, Martínez-Rodríguez M, TAPS Bolivian Study Team. Cultural transmission of ethnobotanical knowledge and skills: an empirical analysis from an Amerindian society. Evol Hum Behav. 2009;30(4): 274. CrossRef PubMed Google Scholar
-
12.Inoue M, Hayashi S, Craker LE. Role of medicinal and aromatic plants: past, present, and future. In Pharmacognosy-medicinal plants. IntechOpen. 2019, 13-27. CrossRef PubMed Google Scholar
-
13.Giannenas I, Sidiropoulou E, Bonos E, Christaki E, Florou-Paneri P. The history of herbs, medicinal and aromatic plants, and their extracts: past, current situation and future perspectives. In Feed additives. Academic Press, 2020: 1-18. DOI:10.1016/B978-0-12-814700-9.00001-7 CrossRef PubMed Google Scholar
-
14.Fitsiou E, Pappa A. Anticancer activity of essential oils and other extracts from aromatic plants grown in Greece. Antioxidants. 2019;8(8): 290. CrossRef PubMed Google Scholar
-
15.Quintero-Rincón P, Pájaro-González Y, Diaz-Castillo F. Maclura tinctoria (L. ) D. Don ex Steud. (Moraceae):a review of the advances in ethnobotanical knowledge, phytochemical composition, and pharmacological potential. Adv Tradit Med. 2024. CrossRef PubMed Google Scholar
-
16.Abu-Darwish MS, Efferth T. Medicinal plants from near east for cancer therapy. Front Pharmacol. 2018;9: 56. CrossRef PubMed Google Scholar
-
17.Albuquerque UP, Patil U, Máthé Á. Medicinal and aromatic plants of South America. Brazil. In Medicinal and aromatic plants of the world. Springer Netherlands. 2018, 17-44. CrossRef PubMed Google Scholar
-
18.Caballero-Gallardo K, Quintero-Rincón P, Olivero-Verbel J. Aromatherapy and essential oils: holistic strategies in complementary and alternative medicine for integral wellbeing. Plants. 2025;14(3): 400. CrossRef PubMed Google Scholar
-
19.Yang C, Chen H, Chen H, Zhong B, Luo X, Chun J. Antioxidant and anticancer activities of essential oil from Gannan navel orange peel. Molecules. 2017;22(8): 1391. CrossRef PubMed Google Scholar
-
20.Hostettmann K, Wolfender JL, Terreaux C. Modern screening techniques for plant extracts. Pharm Biol. 2001;39(sup1): 18. CrossRef PubMed Google Scholar
-
21.Verdeguer M, Sánchez-Moreiras AM, Araniti F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants. 2020;9(11): 1571. CrossRef PubMed Google Scholar
-
22.Hassan B. Plants and cancer treatment. In (Ed. ), Medicinal plants - use in prevention and treatment of diseases. IntechOpen. 2019: 1–11. https://doi.org/10.5772/intechopen.90568 PubMed Google Scholar
-
23.Paier C, Maranhão SS, Carneiro TR, Lima LM, Rocha DD, Santos R, Farias KM, Moraes-Filho MO, Pessoa C. Natural products as new antimitotic compounds for anticancer drug development. Clinics. 2018;73(suppl 1): e813s. CrossRef PubMed Google Scholar
-
24.Delgado JL, Hsieh CM, Chan NL, Hiasa H. Topoisomerases as anticancer targets. Biochem J. 2018;475(2): 373. CrossRef PubMed Google Scholar
-
25.Al-Hayali M, Garces A, Stocks M, Collins H, Bradshaw TD. Concurrent reactive oxygen species generation and aneuploidy induction contribute to thymoquinone anticancer activity. Molecules. 2021;26(17): 5136. CrossRef PubMed Google Scholar
-
26.El-Kenawi AE, El-Remessy AB. Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. Br J Pharmacol. 2013;170(4): 712. CrossRef PubMed Google Scholar
-
27.McClure JJ, Li X, Chou CJ. Advances and challenges of HDAC inhibitors in cancer therapeutics. Adv Cancer Res. 2018;138(2018): 183. CrossRef PubMed Google Scholar
-
28.Ilango S, Paital B, Jayachandran P, Padma PR, Nirmaladevi R. Epigenetic alterations in cancer. Front Biosci. 2020;25: 1058. CrossRef PubMed Google Scholar
-
29.Pilling A, Kim SH, Hwang C. Androgen receptor negatively regulates mitotic checkpoint signaling to induce docetaxel resistance in castration-resistant prostate cancer. Prostate. 2021. CrossRef PubMed Google Scholar
-
30.Huang M, Lu JJ, Ding J. Natural products in cancer therapy: past, present, and future. Nat Prod Bioprospect. 2021;11: 5. CrossRef PubMed Google Scholar
-
31.Reyhanoglu G, Tadi P. Etoposide. In StatPearls. StatPearls Publishing; 2023. PubMed Google Scholar
-
32.Koldysheva EV, Men'Shchikova AP, Lushnikova EL, Popova NA, Kaledin VI, Nikolin VP, Zakharenko A, Luzina O, Salakhutdinov N, Lavrik OI. Antimetastatic activity of combined topotecan and tyrosyl-DNA phosphodiesterase-1 inhibitor on modeled Lewis lung carcinoma. Bull Exp Biol Med. 2019;166(5): 661. CrossRef PubMed Google Scholar
-
33.Jehan S, Zhong C, Li G, Zulqarnain Bakhtiar S, Li D, Sui G. Thymoquinone selectively induces hepatocellular carcinoma cell apoptosis in synergism with clinical therapeutics and dependence of p53 status. Front Pharmacol. 2020;11: 1453. CrossRef PubMed Google Scholar
-
34.Adlravan E, Nejati K, Karimi MA, Mousazadeh H, Abbasi A, Dadashpour M. Potential activity of free and PLGA/PEG nanoencapsulated Nasturtium officinale extract in inducing cytotoxicity and apoptosis in human lung carcinoma A549 cells. J Drug Deliv Sci Technol. 2021;61: 102256. CrossRef PubMed Google Scholar
-
35.Ezzat MS, Merghany MR, Abdel Baki MP, Ali Abdelrahim N, Osman MS, Salem AM, Peña-Corona SI, Cortés H, Kiyekbayeva L, Leyva-Gómez G, Calina D. Nutritional sources and anticancer potential of phenethyl isothiocyanate: molecular mechanisms and therapeutic insights. Mol Nutr Food Res. 2024;68(8): e2400063. CrossRef PubMed Google Scholar
-
36.Jaradat N, Al-Maharik N, Abdallah S, Shawahna R, Mousa A, Qtishat A. Nepeta curviflora essential oil: phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells, and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities. Ind Crops Prod. 2020;158: 112946. CrossRef PubMed Google Scholar
-
37.Asma ST, Acaroz U, Imre K, Morar A, Shah SRA, Hussain SZ, Arslan-Acaroz D, Demirbas H, Hajrulai-Musliu Z, Istanbullugil FR, Soleimanzadeh A, Morozov D, Zhu K, Herman V, Ayad A, Athanassiou C, Ince S. Natural products/bioactive compounds as a source of anticancer drugs. Cancers (Basel). 2022;14(24): 6203. CrossRef PubMed Google Scholar
-
38.Wei KC, Chen RF, Chen YF, Lin CH. Hinokitiol suppresses growth of B16 melanoma by activating ERK/MKP3/proteosome pathway to downregulate survivin expression. Toxicol Appl Pharmacol. 2019;366: 35. CrossRef PubMed Google Scholar
-
39.Wu YJ, Hsu WJ, Wu LH, Liou HP, Pangilinan CR, Tyan YC, Lee CH. Hinokitiol reduces tumor metastasis by inhibiting heparanase via extracellular signal-regulated kinase and protein kinase B pathway. Int J Med Sci. 2020;17(3): 403. CrossRef PubMed Google Scholar
-
40.Chen HY, Cheng WP, Chiang YF, Hong YH, Ali M, Huang TC, Wang KL, Shieh TM, Chang HY, Hsia SM. Hinokitiol exhibits antitumor properties through induction of ROS-mediated apoptosis and p53-driven cell-cycle arrest in endometrial cancer cell lines (Ishikawa, HEC-1A, KLE). Int J Mol Sci. 2021;22(15): 8268. CrossRef PubMed Google Scholar
-
41.Maruthanila VL, Elancheran R, Kunnumakkar AB, Kabilan S, Kotoky J. Pleiotropic effect of mahanine and girinimbine analogs: anticancer mechanism and its therapeutic versatility. Anticancer Agents Med Chem. 2018;18(14): 1983. CrossRef PubMed Google Scholar
-
42.Das M, Kandimalla R, Gogoi B, Dutta KN, Choudhury P, Devi R, Dutta PP, Talukdar NC, Samanta SK. Mahanine, A dietary phytochemical, represses mammary tumor burden in rat and inhibits subtype regardless breast cancer progression through suppressing self-renewal of breast cancer stem cells. Pharmacol Res. 2019;146: 104330. CrossRef PubMed Google Scholar
-
43.Chen M, Yin X, Lu C, Chen X, Ba H, Cai J, Sun J. Mahanine induces apoptosis, cell cycle arrest, inhibition of cell migration, invasion and PI3K/AKT/mTOR signalling pathway in glioma cells and inhibits tumor growth in vivo. Chem Biol Interact. 2019;299: 1. CrossRef PubMed Google Scholar
-
44.Rodrigues FC, Kumar NA, Thakur G. Developments in the anticancer activity of structurally modified curcumin: an up-to-date review. Eur J Med Chem. 2019;177: 76-104. CrossRef PubMed Google Scholar
-
45.Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18(12): 2589. CrossRef PubMed Google Scholar
-
46.Singh AP, Singh R, Verma SS, Rai V, Kaschula CH, Maiti P, Gupta SC. Health benefits of resveratrol: evidence from clinical studies. Med Res Rev. 2019;39(5): 1851. CrossRef PubMed Google Scholar
-
47.Ceci C, Lacal PM, Tentori L, De Martino MG, Miano R, Graziani G. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients. 2018;10(11): 1756. CrossRef PubMed Google Scholar
-
48.Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto E, Novellino E, Antolak H, Azzini E, Setzer W, Martins N. The therapeutic potential of apigenin. Int J Mol Sci. 2019;20(6): 1305. CrossRef PubMed Google Scholar
-
49.Li Q, Miao Z, Wang R, Yang J, Zhang D. Hesperetin induces apoptosis in human glioblastoma cells via p38 MAPK Activation. Nutr Cancer. 2020;72(3): 538. CrossRef PubMed Google Scholar
-
50.Choi J, Lee DH, Jang H, Park SY, Seol JW. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int J Med Sci. 2020;17(18): 3049. CrossRef PubMed Google Scholar
-
51.Lee J, Kim DH, Kim JH. Combined administration of naringenin and hesperetin with optimal ratio maximizes the anti-cancer effect in human pancreatic cancer via down regulation of FAK and p38 signaling pathway. Phytomed Int J Phytother Phytopharmacol. 2019;58: 152762. CrossRef PubMed Google Scholar
-
52.Yan X, Qi M, Li P, Zhan Y, Shao H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7(1): 1-16. CrossRef PubMed Google Scholar
-
53.Aggarwal V, Tuli HS, Tania M, Srivastava S, Ritzer EE, Pandey A, Aggarwal D, Singh T, Jain A, Kaur G, Sak K, Varol M, Bishayee A. Molecular mechanisms of action of epigallocatechin gallate in cancer: recent trends and advancement. Seminars in Cancer Biology. Academic Press, 2020. DOI:10.1016/j.semcancer.2020.05.011 CrossRef PubMed Google Scholar
-
54.Negri A, Naponelli V, Rizzi F, Bettuzzi S. Molecular targets of epigallocatechin—Gallate (EGCG):a special focus on signal transduction and cancer. Nutrients. 2018;10(12): 1936. CrossRef PubMed Google Scholar
-
55.Nafees S, Zafaryab M, Mehdi SH, Zia B, Rizvi MA, Khan MA. Anti-cancer effect of gingerol in cancer prevention and treatment. Anti-Cancer Agents Med Chem. 2021;21(4): 428. CrossRef PubMed Google Scholar
-
56.Zhang H, Kim E, Yi J, Hai H, Kim H, Park S, Lim S, Kim S, Jang S, Kim K, Kim E, Lee Y, Ryoo Z, Kim M. [6]-Gingerol suppresses oral cancer cell growth by inducing the activation of AMPK and suppressing the AKT/mTOR signaling pathway. In Vivo. 2021;35(6): 3193. CrossRef PubMed Google Scholar
-
57.Zhang S, Wang D, Huang J, Hu Y, Xu Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J Clin Pharm Ther. 2020;45(1): 16. CrossRef PubMed Google Scholar
-
58.Rao CH, Prasada MM. Novel series of 1, 5 Benzothiazepine skeleton based compounds as anti-cancer agents–In silico and MTT assay-based study. Journal of PeerScientist. 2018;1(2): e1000008. PubMed Google Scholar
-
59.Allegra A, Tonacci A, Pioggia G, Musolino C, Gangemi S. Anticancer activity of Rosmarinus officinalis L.: mechanisms of action and therapeutic potentials. Nutrients. 2020;12(6): 1739. CrossRef PubMed Google Scholar
-
60.Fard KG, Mokarram M. Investigating the pollution of irrigated plants (Rosmarinus officinalis) with polluted water in different growth stages using spectrometer and K-means method. Environ Sci Pollut Res Int. 2023;30(35): 83903-16. CrossRef PubMed Google Scholar
-
61.Petiwala SM, Johnson JJ. Diterpenes from rosemary (Rosmarinus officinalis):defining their potential for anti-cancer activity. Cancer Lett. 2015;367(2): 93. CrossRef PubMed Google Scholar
-
62.Pérez-Sánchez A, Barrajón-Catalán E, Ruiz-Torres V, Agulló-Chazarra L, Herranz-López M, Valdés A, Cifuentes A, Micol V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci Rep. 2019;9(1): 808. CrossRef PubMed Google Scholar
-
63.Hasei S, Yamamotoya T, Nakatsu Y, Ohata Y, Itoga S, Nonaka Y, Matsunaga Y, Sakoda H, Fujishiro M, Kushiyama A, Asano T. Carnosic acid and carnosol activate AMPK, suppress expressions of gluconeogenic and lipogenic genes, and inhibit proliferation of HepG2 cells. Int J Mol Sci. 2021;22(8): 4040. CrossRef PubMed Google Scholar
-
64.Moore J, Megaly M, MacNeil AJ, Klentrou P, Tsiani E. Rosemary extract reduces Akt/mTOR/p70S6K activation and inhibits proliferation and survival of A549 human lung cancer cells. Biomed Pharmacother. 2016;83: 725. CrossRef PubMed Google Scholar
-
65.Cattaneo L, Cicconi R, Mignogna G, Giorgi A, Mattei M, Graziani G, Ferracane R, Grosso A, Aducci P, Schininà ME, Marra M. Anti-proliferative effect of Rosmarinus officinalis L. extract on human melanoma A375 Cells. PLoS ONE. 2015;10(7): e0132439. CrossRef PubMed Google Scholar
-
66.Rajasekaran D, Manoharan S, Silvan S, Vasudevan K, Baskaran N, Palanimuthu D. Proapoptotic, anti-cell proliferative, anti-inflammatory, and anti-angiogenic potential of carnosic acid during 7, 12 dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Afr J Tradit Complement Altern Med. 2012;10(1): 102. CrossRef PubMed Google Scholar
-
67.Jaglanian A, Termini D, Tsiani E. Rosemary (Rosmarinus officinalis L. xtract inhibits prostate cancer cell proliferation and survival by targeting Akt and mTOR. Biomed Pharmacother. 2020;131: 110717. CrossRef PubMed Google Scholar
-
68.Jaglanian A, Tsiani E. Rosemary extract inhibits proliferation, survival, Akt, and mTOR signaling in triple-negative breast cancer cells. Int J Mol Sci. 2020;21(3): 810. CrossRef PubMed Google Scholar
-
69.Bae JR, Park WH, Suh DH, No JH, Kim YB, Kim K. Role of limonin in anticancer effects of Evodia rutaecarpa on ovarian cancer cells. BMC Complement Med Ther. 2020;20(1): 1-13. CrossRef PubMed Google Scholar
-
70.Li FS, Huang J, Cui MZ, Zeng JR, Li PP, Li L, Deng Y, Hu Y, He BC, Shu DZ. BMP9 mediates the anticancer activity of evodiamine through HIF 1α/p53 in human colon cancer cells. Oncol Rep. 2020;43(2): 415. CrossRef PubMed Google Scholar
-
71.Ahmad R, Sahidin I, Taher M, Low C, Noor NM, Sillapachaiyaporn C, Chuchawankul S, Sarachana T, Tencomnao T, Iskandar F, Rajab N, Baharum SN. Polygonumins A, a newly isolated compound from the stem of Polygonum minus Huds with potential medicinal activities. Sci Rep. 2018;8(1): 1. CrossRef PubMed Google Scholar
-
72.Nair A, Kurup R Sr, Nair AS, Baby S. Citrus peels prevent cancer. Phytomedicine. 2018;50: 231. CrossRef PubMed Google Scholar
-
73.Jiang YP, Guo H, Wang XB. Nobiletin (NOB) suppresses autophagic degradation via over-expressing AKT pathway and enhances apoptosis in multidrug-resistant SKOV3/TAX ovarian cancer cells. Biomed Pharmacother. 2018;103: 29. CrossRef PubMed Google Scholar
-
74.Zhu X, Luo F, Zheng Y, Zhang J, Huang J, Sun C, Li X, Chen K. Characterization, purification of Poncirin from edible citrus Ougan (Citrus reticulate cv. Suavissima) and its growth inhibitory effect on human gastric cancer cells SGC-7901. Int J Mol Sci. 2013;14(5): 8684. CrossRef PubMed Google Scholar
-
75.Esmaeili MA, Farimani MM, Kiaei M. Anticancer effect of calycopterin via PI3K/Akt and MAPK signaling pathways, ROS-mediated pathway, and mitochondrial dysfunction in hepatoblastoma cancer (HepG2) cells. Mol Cell Biochem. 2014;397(1–2): 17. CrossRef PubMed Google Scholar
-
76.Ghazizadeh F, Shafiei M, Falak R, Panahi M, Rakhshani N, Ebrahimi SA, Rahimi-Moghaddam P. Xanthomicrol exerts antiangiogenic and antitumor effects in a mouse melanoma (B16F10) allograft model. Evid Based Complement Altern Med. 2020;2020: 8543872. CrossRef PubMed Google Scholar
-
77.Schelz Z, Ocsovszki I, Bózsity N, Hohmann J, Zupkó I. Antiproliferative effects of various furanoacridones isolated from Ruta graveolens on human breast cancer cell lines. Anticancer Res. 2016;36(6): 2751-8. PubMed Google Scholar
-
78.Wang F, Song X, Ma S, Liu C, Sun X, Wang X, Liu Z, Liang D, Yu Z. The treatment role of Cyperus rotundus L. to triple-negative breast cancer cells. Biosci Rep. 2019;39(6): BSR20190502. CrossRef PubMed Google Scholar
-
79.Samra RM, Soliman AF, Zaki AA, Ashour A, Al-Karmalawy AA, Hassan MA, Zaghloul AM. Bioassay-guided isolation of a new cytotoxic ceramide from Cyperus rotundus L. S Afr J Bot. 2021;139: 210. CrossRef PubMed Google Scholar
-
80.Bonesi M, Brindisi M, Armentano B, Curcio R, Sicari V, Loizzo MR, Cappello MS, Bedini G, Peruzzi L, Tundis R. Exploring the anti-proliferative, pro-apoptotic, and antioxidant properties of Santolina corsica Jord. & Fourr. (Asteraceae). Biomed Pharmacother. 2018;107: 967. CrossRef PubMed Google Scholar
-
81.Zhao T, Pan H, Feng Y, Li H, Zhao Y. Petroleum ether extract of Chenopodium album L. prevents cell growth and induces apoptosis of human lung cancer cells. Exp Ther Med. 2016;12(5): 3301. CrossRef PubMed Google Scholar
-
82.Fiorentino S, Urueña C. La fitoterapia como fuente de medicamentos reguladores del metabolismo tumoral y activadores de la respuesta inmunitaria. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales. 2018;42(163): 132. CrossRef PubMed Google Scholar
-
83.Hernández JF, Urueña CP, Cifuentes MC, Sandoval TA, Pombo LM, Castañeda D, Asea A, Fiorentino S. A Petiveria alliacea standardized fraction induces breast adenocarcinoma cell death by modulating glycolytic metabolism. J Ethnopharmacol. 2014;153(3): 641. CrossRef PubMed Google Scholar
-
84.Oliveira CR, Spindola DG, Garcia DM, Erustes A, Bechara A, Palmeira-Dos-Santos C, Smaili SS, Pereira G, Hinsberger A, Viriato EP, Cristina Marcucci M, Sawaya A, Tomaz SL, Rodrigues EG, Bincoletto C. Medicinal properties of Angelica archangelica root extract: cytotoxicity in breast cancer cells and its protective effects against in vivo tumor development. J Integr Med. 2019;17(2): 132. CrossRef PubMed Google Scholar
-
85.Hariri M, Ghiasvand R. Cinnamon and chronic diseases. In: Gupta S, Prasad S, Aggarwal B, editors. Drug discovery from mother nature. Advances in experimental medicine and biology, vol. 929. Cham: Springer; 2016. https://doi.org/10.1007/978-3-319-41342-6_1. PubMed Google Scholar
-
86.Rad SK, Movafagh A. Study of antioxidant, antiproliferative and DNA damage protecting activities of Cinnamomum cassia extracts obtained by sequential extraction. Recent Pat Food Nutr Agric. 2021;12(1): 45. CrossRef PubMed Google Scholar
-
87.Park GH, Song HM, Park SB, Son HJ, Um Y, Kim HS, Jeong JB. Cytotoxic activity of the twigs of Cinnamomum cassia through the suppression of cell proliferation and the induction of apoptosis in human colorectal cancer cells. BMC Complement Altern Med. 2018;18(1): 28. CrossRef PubMed Google Scholar
-
88.Yu CH, Chu SC, Yang SF, Hsieh YS, Lee CY, Chen PN. Induction of apoptotic but not autophagic cell death by Cinnamomum cassia extracts on human oral cancer cells. J Cell Physiol. 2019;234(4): 5289. CrossRef PubMed Google Scholar
-
89.Thangam R, Sathuvan M, Poongodi A, Suresh V, Pazhanichamy K, Sivasubramanian S, Kanipandian N, Ganesan N, Rengasamy R, Thirumurugan R, Kannan S. Activation of intrinsic apoptotic signaling pathway in cancer cells by Cymbopogon citratus polysaccharide fractions. Carbohyd Polym. 2014;107: 138. CrossRef PubMed Google Scholar
-
90.Gomes LF, Longhi P, Machado L, da Cruz I, Montano M, Martins M, Machado SA, Steffani JA, Cadoná FC. Lemongrass (Cymbopogon citratus (D.C. ) Stapf) presents antitumoral effect and improves chemotherapy activity in prostate cancer cells. Anti-cancer Agents Med Chem. 2021;21(17): 2337. CrossRef PubMed Google Scholar
-
91.Trang DT, Hoang T, Nguyen T, Van Cuong P, Dang NH, Dang HD, Nguyen Quang T, Dat NT. Essential oils of lemongrass (Cymbopogon citratus Stapf) induces apoptosis and cell cycle arrest in A549 lung cancer cells. Biomed Res Int. 2020;2020: 5924856. CrossRef PubMed Google Scholar
-
92.Negm WA, Elekhnawy E, Mahgoub S, Ibrahim HA, Ibrahim Elberri A, Abo Mansour HE, Mosalam EM, Moglad E, Alzahraa Mokhtar F. Dioon rzedowskii: an antioxidant, antibacterial and anticancer plant extract with multi-faceted effects on cell growth and molecular signaling. Int Immunopharmacol. 2024;132: 111957. CrossRef PubMed Google Scholar
-
93.Navyatha Karamala L, Karthik Y, Raghu M, Aditi N, Rachana V, Prasanna A, Narayanappa R, Ramakrishna D, Tidke SA, Mushtaq M, Sayed S, Jafri I, Alsharif G. Exploring the therapeutic potential of Pongamia pinnata plant extract against skin cancer: in-silico and in-vitro study. J Ethnopharmacol. 2025;337(Pt 3): 118964. CrossRef PubMed Google Scholar
-
94.Feng S, Li S, Wu Z, Li Y, Wu T, Zhou Z, Liu X, Chen J, Fu S, Wang Z, Zhong Z, Zhong Y. Saffron improves the efficacy of immunotherapy for colorectal cancer through the IL-17 signaling pathway. J Ethnopharmacol. 2025;337(Pt 2): 118854. CrossRef PubMed Google Scholar
-
95.Mir MA, Memish LA, Elbehairi SE, Bashir N, Masoud FS, Shati AA, Alfaifi MY, Alamri AM, Alkahtani SA, Ahmad I. Antimycobacterial and anticancer properties of Myrtus communis leaf extract. Pharmaceuticals. 2024;17(7): 872. CrossRef PubMed Google Scholar
-
96.Caballero-Gallardo K, Quintero-Rincón P, Stashenko EE, Olivero-Verbel J. Photoprotective agents obtained from aromatic plants grown in Colombia: total phenolic content, antioxidant activity, and assessment of cytotoxic potential in cancer cell lines of Cymbopogon flexuosus L. and Tagetes lucida Cav. essential oils. Plants. 2022;2022(11): 1693. CrossRef PubMed Google Scholar
-
97.Magalhães IFB, Tellis CJM, Calabrese KS, Abreu-Silva AL, Almeida-Souza F. Essential oils' potential in breast cancer treatment: an overview. In: Oliveira MS, Costa WA, Silva SG, editors. Essential oils - bioactive compounds new perspectives and applications. London: IntechOpen; 2020. https://doi.org/10.5772/intechopen.91781. PubMed Google Scholar
-
98.Alves CCF, Oliveira JD, Estevam EBB, Xavier MN, Nicolella HD, Furtado RA, Tavares D, Miranda MLD. Antiproliferative activity of essential oils from three plants of the Brazilian Cerrado: Campomanesia adamantium (Myrtaceae), Protium ovatum (Burseraceae) and Cardiopetalum calophyllum (Annonaceae). Braz J Biol. 2019;80(2): 290. CrossRef PubMed Google Scholar
-
99.Bakarnga-Via I, Hzounda JB, Fokou PVT, Tchokouaha LRY, Gary-Bobo M, Gallud A, Garcia M, Walbadet L, Secka Y, Jazet P, Boyom F, Menut C. Composition and cytotoxic activity of essential oils from Xylopia aethiopica (Dunal) A. Rich, Xylopia parviflora (A. Rich) Benth. ) and Monodora myristica (Gaertn) growing in Chad and Cameroon. BMC Complement Altern Med. 2014;14(1): 1. CrossRef PubMed Google Scholar
-
100.Ferraz RP, Cardoso GM, da Silva TB, Fontes JEDN, Prata APDN, Carvalho AA, Moraes M, Pessoa C, Costa E, Bezerra DP. Antitumour properties of the leaf essential oil of Xylopia frutescens Aubl. (Annonaceae). Food Chem. 2013;141(1): 196. CrossRef PubMed Google Scholar
-
101.Agassi S, Yeh TM, Chang CD, Hsu JL, Shih WL. Potentiation of differentiation and apoptosis in a human promyelocytic leukemia cell line by garlic essential oil and its organosulfur compounds. Anticancer Res. 2020;40(11): 6345. CrossRef PubMed Google Scholar
-
102.Tavakkol Afshari HS, Homayouni Tabrizi M, Ardalan T, Jalili Anoushirvani N, Mahdizadeh R. Anethum Graveolens essential oil nanoemulsions (AGEO-NE) as an exclusive apoptotic inducer in human lung adenocarcinoma (A549) Cells. Nutr Cancer. 2021. CrossRef PubMed Google Scholar
-
103.Cha JD, Jeong MR, Kim HY, Lee JC, Lee KY. MAPK activation is necessary to the apoptotic death of KB cells induced by the essential oil isolated from Artemisia iwayomogi. J Ethnopharmacol. 2009;123(2): 308. CrossRef PubMed Google Scholar
-
104.Ornano L, Venditti A, Sanna C, Ballero M, Maggi F, Lupidi G, Bramucci M, Quassinti L, Bianco A. Chemical composition and biological activity of the essential oil from Helichrysum microphyllum Cambess. Ssp. tyrrhenicum Bacch., Brullo e Giusso growing in La Maddalena Archipelago, Sardinia. J Oleo Sci. 2015;64(1): 19. CrossRef PubMed Google Scholar
-
105.Ali A, Ali A, Warsi MH, Ahmad W. Chemical characterization, antidiabetic and anticancer activities of Santolina chamaecyparissus. Saudi J Biol Sci. 2021;28(8): 4575. CrossRef PubMed Google Scholar
-
106.Shirazi MT, Gholami H, Kavoosi G, Rowshan V, Tafsiry A. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of Tagetes minuta and Ocimum basilicum essential oils. Food Sci Nutr. 2014;2(2): 146. CrossRef PubMed Google Scholar
-
107.Sheashea AA, Ahmed FA, El Zayat E, Ebeed BW, Elberry MH, Hassan ZKM, Hafez MM. In vitro antiviral and anticancer effects of Tanacetum sinaicum essential oil on human cervical and breast cancer. Asian Pac J Cancer Prev. 2024;25(4): 1457-71. CrossRef PubMed Google Scholar
-
108.Nasr FA, Noman OM, Alqahtani AS, Qamar W, Ahamad SR, Al-Mishari AA, Alyhya N, Farooq M. Phytochemical constituents and anticancer activities of Tarchonanthus camphoratus essential oils grown in Saudi Arabia. Saudi Pharm J. 2020;28(11): 1474. CrossRef PubMed Google Scholar
-
109.Li YL, Yeung CM, Chiu LC, Cen YZ, Ooi VE. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytother Res. 2009;23(1): 140. CrossRef PubMed Google Scholar
-
110.Ni X, Suhail MM, Yang Q, Cao A, Fung KM, Postier RG, Woolley C, Young G, Zhang J, Lin HK. Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model. BMC Complement Altern Med. 2012;12: 253. CrossRef PubMed Google Scholar
-
111.Yeo SK, Ali AY, Hayward OA, Turnham D, Jackson T, Bowen ID, Clarkson R. β-Bisabolene, a sesquiterpene from the essential oil extract of Opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother Res. 2016;30(3): 418. CrossRef PubMed Google Scholar
-
112.Islam A, Chang YC, Tsao NW, Wang SY, Chueh PJ. Calocedrus formosana essential oils induce ROS-mediated autophagy and apoptosis by targeting SIRT1 in colon cancer cells. Antioxidants. 2024;13(3): 284. CrossRef PubMed Google Scholar
-
113.Ramadan MM, Ali MM, Ghanem KZ, El-Ghorabe AH. Essential oils from Egyptian aromatic plants as antioxidant and novel anticancer agents in human cancer cell lines. Grasas Aceites. 2015;66(2): e080. CrossRef PubMed Google Scholar
-
114.Maurya AK, Devi R, Kumar A, Koundal R, Thakur S, Sharma A, Kumar D, Kumar R, Padwad YS, Chand G, Singh B, Agnihotri VK. Chemical composition, cytotoxic and antibacterial activities of essential oils of cultivated clones of Juniperus communis and Wild Juniperus Species. Chem Biodivers. 2018;15(9): e1800183. CrossRef PubMed Google Scholar
-
115.Bezerra JJL, Pinheiro AAV. Traditional uses, phytochemistry, and anticancer potential of Cyperus rotundus L. (Cyperaceae):a systematic review. S Afr J Bot. 2022;144: 175. CrossRef PubMed Google Scholar
-
116.Susianti S, Yanwirasti Y, Darwin E. The cytotoxic effects of purple nutsedge (Cyperus Rotundus L. ) tuber essential oil on the HeLa cervical cancer cell line. Pak J Biotechnol. 2018;15(1): 77. PubMed Google Scholar
-
117.Al-Massarani S, Al-Enzi F, Al-Tamimi M, Al-Jomaiah N, Al-amri R, Başer KHC, Tabanca N, Estep A, Becnel J, Bloomquist J, Demirci B. Composition & biological activity of Cyperus rotundus L. tuber volatiles from Saudi Arabia. Nat Volatiles Essential Oils. 2016;3(2): 26-34. PubMed Google Scholar
-
118.Pahore AK, Khan S, Karim N. Illicium verum anticancer activity against MDA-MB-231 cell line. Pak J Med Sci. 2024;40(1Part-Ⅰ): 110-4. CrossRef PubMed Google Scholar
-
119.de Sousa M, Morgan J, Cesca K, Flach A, de Moura NF. Cytotoxic activity of Cunila angustifolia essential oil. Chem Biodivers. 2020;17(2): e1900656. CrossRef PubMed Google Scholar
-
120.Boukhatem MN, Sudha T, Darwish NH, Chader H, Belkadi A, Rajabi M, Houche A, Benkebailli F, Oudjida F, Mousa SA. A new eucalyptol-rich lavender (Lavandula stoechas L. ) essential oil: emerging potential for therapy against inflammation and cancer. Molecules. 2020;25(16): 3671. CrossRef PubMed Google Scholar
-
121.de Sousa AC, Alviano DS, Blank AF, Alves PB, Alviano CS, Gattass CR. Melissa officinalis L. essential oil: antitumoral and antioxidant activities. J Pharm Pharmacol. 2004;56(5): 677. CrossRef PubMed Google Scholar
-
122.Manaharan T, Thirugnanasampandan R, Jayakumar R, Kanthimathi MS, Ramya G, Ramnath MG. Purified essential oil from Ocimum sanctum Linn. triggers the apoptotic mechanism in human breast cancer cells. Pharmacogn Mag. 2016;12(Suppl 3): S327. CrossRef PubMed Google Scholar
-
123.Boonyanugomol W, Rukseree K, Prapatpong P, Reamtong O, Baik SC, Jung M, Shin MK, Kang HL, Lee WK. An in vitro anti-cancer activity of Ocimum tenuiflorum essential oil by inducing apoptosis in human gastric cancer cell line. Medicina. 2021;57(8): 784. CrossRef PubMed Google Scholar
-
124.Athamneh K, Alneyadi A, Alsamri H, Alrashedi A, Palakott A, El-Tarabily KA, Eid AH, Al Dhaheri Y, Iratni R. Origanum majorana essential oil triggers p38 MAPK-mediated protective autophagy, apoptosis, and caspase-dependent cleavage of P70S6K in colorectal cancer cells. Biomolecules. 2020;10(3): 412. CrossRef PubMed Google Scholar
-
125.Sokmen A, Abdel-Baki AAS, Al-Malki ES, Al-Quraishy S, Abdel-Haleem HM. Constituents of essential oil of Origanum minutiflorum and its in vitro antioxidant, scolicidal and anticancer activities. Journal of King Saud University-Science. 2020;32(4): 2377. CrossRef PubMed Google Scholar
-
126.Elshafie HS, Armentano MF, Carmosino M, Bufo SA, De Feo V, Camele I. Cytotoxic activity of Origanum vulgare L. on hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules. 2017;22(9): 1435. CrossRef PubMed Google Scholar
-
127.Rojo-Ruvalcaba BE, García-Cobián TA, Pascoe-González S, Campos-Bayardo TI, Guzmán-García LM, Gil-Gálvez MC, Escobar-Millan Z, Huerta-García E, García-Iglesias T. Dose-dependent cytotoxicity of the Origanum vulgare and carvacrol on triple negative breast cancer cell line. Multidiscipl Digit Publ Inst Proc. 2020;61(1): 6. PubMed Google Scholar
-
128.Jardak M, Elloumi-Mseddi J, Aifa S, Mnif S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017;16(1): 190. CrossRef PubMed Google Scholar
-
129.Guesmi F, Prasad S, Ali MB, Ismail IA, Landoulsi A. Thymus hirtus sp. algeriensis Boiss. and Reut. volatile oil enhances TRAIL/Apo2L induced apoptosis and inhibits colon carcinogenesis through upregulation of death receptor pathway. Aging. 2021;13(18): 21975. CrossRef PubMed Google Scholar
-
130.Najar B, Shortrede JE, Pistelli L, Buhagiar J. Chemical composition and in vitro cytotoxic screening of sixteen commercial essential oils on five cancer cell lines. Chem Biodivers. 2020;17(1): e1900478. CrossRef PubMed Google Scholar
-
131.Kubatka P, Kello M, Kajo K, Samec M, Jasek K, Vybohova D, Uramova S, Liskova A, Sadlonova V, Koklesova L, Murin R, Adamkov M, Smejkal K, Svajdlenka E, Solar P, Mathews S, Kassayova M, Kyu T, Zubor P, Pec M. Chemopreventive and therapeutic efficacy of Cinnamomum zeylanicum L. bark in experimental breast carcinoma: mechanistic in vivo and in vitro analyses. Molecules. 2020;25(6): 1399. CrossRef PubMed Google Scholar
-
132.Loizzo MR, Tundis R, Menichini F, Saab AM, Statti GA, Menichini F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007;27(5A): 3293. PubMed Google Scholar
-
133.Su YC, Ho CL. Composition, in-vitro anticancer, and antimicrobial activities of the leaf essential oil of Machilus mushaensis from Taiwan. Nat Prod Commun. 2013;8(2): 1934578X1300800236. PubMed Google Scholar
-
134.Bardaweel SK, Bakchiche B, ALSalamat HA, Rezzoug M, Gherib A, Flamini G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complement Altern Med. 2018;18(1): 1. CrossRef PubMed Google Scholar
-
135.Ding W, Liping N, Xing H, Wei Z, Zhoua Q, Nong R, Chen J. Essential oil extracted from leaf of Phoebe bournei (Hemsl. ) yang: chemical constituents, antitumor, antibacterial, hypoglycemic activities. Nat Prod Res. 2020;34(17): 2524. CrossRef PubMed Google Scholar
-
136.Döll-Boscardin PM, Sartoratto A, Sales Maia BH, Padilha de Paula J, Nakashima T, Farago PV, Kanunfre CC. In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evid Based Complement Altern Med. 2012;2012: 342652. CrossRef PubMed Google Scholar
-
137.Karunarathne WAHM, Molagoda IMN, Lee KT, Choi YH, Yu SM, Kang CH, Kim GY. Protective effect of anthocyanin-enriched polyphenols from Hibiscus syriacus L. (Malvaceae) against ultraviolet B-induced damage. Antioxidants. 2021;10(4): 584. CrossRef PubMed Google Scholar
-
138.Clark AM, Magawa C, Pliego-Zamora A, Low P, Reynolds M, Ralph SJ. Tea tree oil extract causes mitochondrial superoxide production and apoptosis as an anticancer agent, promoting tumor infiltrating neutrophils cytotoxic for breast cancer to induce tumor regression. Biomed Pharmacother. 2021;140: 111790. CrossRef PubMed Google Scholar
-
139.Manosroi J, Dhumtanom P, Manosroi A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006;235(1): 114. CrossRef PubMed Google Scholar
-
140.Kamal I, Khedr AI, Alfaifi MY, Elbehairi SEI, Elshaarawy RF, Saad AS. Chemotherapeutic and chemopreventive potentials of p-coumaric acid–Squid chitosan nanogel loaded with Syzygium aromaticum essential oil. Int J Biol Macromol. 2021;188: 523. CrossRef PubMed Google Scholar
-
141.Ul-khazir Z, Yatoo GN, Wani H, Shah SA, Zargar MI, Rather MA, Banday JA. Gas chromatographic-mass spectrometric analysis, antibacterial, antioxidant and antiproliferative activities of the needle essential oil of abies pindrow growing wild in Kashmir, India. Microb Pathogenesis. 2021;158: 105013. CrossRef PubMed Google Scholar
-
142.Saab AM, Gambari R, Sacchetti G, Guerrini A, Lampronti I, Tacchini M, El Samrani A, Medawar S, Makhlouf H, Tannoury M, Abboud J, Diab-Assaf M, Kijjoa A, Tundis R, Aoun J, Efferth T. Phytochemical and pharmacological properties of essential oils from Cedrus species. Nat Prod Res. 2018;32(12): 1415. CrossRef PubMed Google Scholar
-
143.Jo JR, Park JS, Park YK, Chae YZ, Lee GH, Park GY, Jang BC. Pinus densiflora leaf essential oil induces apoptosis via ROS generation and activation of caspases in YD-8 human oral cancer cells. Int J Oncol. 2012;40(4): 1238. CrossRef PubMed Google Scholar
-
144.Zhang Y, Xin C, Qiu J, Wang Z. Essential oil from Pinus koraiensis pinecones inhibits gastric cancer cells via the HIPPO/YAP signaling pathway. Molecules. 2019;24(21): 3851. CrossRef PubMed Google Scholar
-
145.Cho SM, Lee EO, Kim SH, Lee HJ. Essential oil of Pinus koraiensis inhibits cell proliferation and migration via inhibition of p21-activated kinase 1 pathway in HCT116 colorectal cancer cells. BMC Complement Altern Med. 2014;14(1): 1. CrossRef PubMed Google Scholar
-
146.Bayala B, Bassole I, Maqdasy S, Baron S, Simpore J, Lobaccaro JA. Cymbopogon citratus and Cymbopogon giganteus essential oils have cytotoxic effects on tumor cell cultures. Identification of citral as a new putative anti-proliferative molecule. Biochimie. 2018;153: 162. CrossRef PubMed Google Scholar
-
147.Sharma PR, Mondhe DM, Muthiah S, Pal HC, Shahi AK, Saxena AK, Qazi GN. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem Biol Interact. 2009;179(2–3): 160. CrossRef PubMed Google Scholar
-
148.Bayala B, Coulibaly AY, Djigma FW, Nagalo BM, Baron S, Figueredo G, Lobaccaro J, Simpore J. Chemical composition, antioxidant, anti-inflammatory and antiproliferative activities of the essential oil of Cymbopogon nardus, a plant used in traditional medicine. Biomol Concepts. 2020;11(1): 86. CrossRef PubMed Google Scholar
-
149.Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, Bouajila J. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem Toxicol. 2013;56: 352. CrossRef PubMed Google Scholar
-
150.Paik SY, Koh KH, Beak SM, Paek SH, Kim JA. The essential oils from Zanthoxylum schinifolium pericarp induce apoptosis of HepG2 human hepatoma cells through increased production of reactive oxygen species. Biol Pharm Bull. 2005;28(5): 802. CrossRef PubMed Google Scholar
-
151.Farrag NS, Shetta A, Mamdouh W. Green tea essential oil encapsulated chitosan nanoparticles-based radiopharmaceutical as a new trend for solid tumor theranosis. Int J Biol Macromol. 2021;186: 811. CrossRef PubMed Google Scholar
-
152.Fitsiou E, Mitropoulou G, Spyridopoulou K, Vamvakias M, Bardouki H, Galanis A, Chlichlia K, Kourkoutas Y, Panayiotidis M, Pappa A. Chemical composition and evaluation of the biological properties of the essential oil of the dietary phytochemical Lippia citriodora. Molecules. 2018;23(1): 123. CrossRef PubMed Google Scholar
-
153.Panyajai P, Viriyaadhammaa N, Tima S, Chiampanichayakul S, Dejkriengkraikul P, Okonogi S, Anuchapreeda S. Anticancer activity of Curcuma aeroginosa essential oil and its nano-formulations: cytotoxicity, apoptosis and cell migration effects. BMC Complement Med Ther. 2024;24(1): 16. CrossRef PubMed Google Scholar
-
154.Xiao Y, Yang FQ, Li SP, Hu G, Lee SM, Wang YT. Essential oil of Curcuma wenyujin induces apoptosis in human hepatoma cells. World J Gastroenterol. 2008;14(27): 4309. CrossRef PubMed Google Scholar
-
155.Santos PA, Avanço GB, Nerilo SB, Marcelino RI, Janeiro V, Valadares MC, Machinski M. Assessment of cytotoxic activity of rosemary (Rosmarinus officinalis L. ), Turmeric (Curcuma longa L. ), and ginger (Zingiber officinale R. ) essential oils in cervical cancer cells (HeLa). Sci World J. 2016;2016: 9273078. CrossRef PubMed Google Scholar
-
156.Al-Otaibi WA, Alkhatib MH, Wali AN. Cytotoxicity and apoptosis enhancement in breast and cervical cancer cells upon coadministration of mitomycin C and essential oils in nanoemulsion formulations. Biomed Pharmacother. 2018;106: 946. CrossRef PubMed Google Scholar
-
157.Tian M, Liu T, Wu X, Hong Y, Liu X, Lin B, Zhou Y. Chemical composition, antioxidant, antimicrobial and anticancer activities of the essential oil from the rhizomes of Zingiber striolatum Diels. Nat Prod Res. 2020;34(18): 2621. CrossRef PubMed Google Scholar
-
158.Mondal, A., Bose, S., Mazumder, K., & Khanra, R. Carvacrol (Origanum vulgare):Therapeutic properties and molecular mechanisms. In Bioactive Natural Products for Pharmaceutical Applications. vol 140. Cham: Springer; 2021:437–462. https://doi.org/10.1007/978-3-030-54027-2_13 PubMed Google Scholar
-
159.Sharifi-Rad M, Varoni EM, Iriti M, Martorell M, Setzer WN, del Mar Contreras M, Salehi B, Soltani-Nejad A, Rajabi S, Tajbakhsh M, Sharifi-Rad J. Carvacrol and human health: a comprehensive review. Phytother Res. 2018;32(9): 1675. CrossRef PubMed Google Scholar
-
160.Abdalla AN, Shaheen U, Abdallah Q, Flamini G, Bkhaitan MM, Abdelhady M, Ascrizzi R, Bader A. Proapoptotic activity of Achillea membranacea essential oil and its major constituent 1,8-cineole against A2780 ovarian cancer cells. Molecules. 2020;25(7): 1582. CrossRef PubMed Google Scholar
-
161.Sampath S, Subramani S, Janardhanam S, Subramani P, Yuvaraj A, Chellan R. Bioactive compound 1,8-Cineole selectively induces G2/M arrest in A431 cells through the upregulation of the p53 signaling pathway and molecular docking studies. Phytomed Int J Phytother Phytopharmacol. 2018;46: 57. CrossRef PubMed Google Scholar
-
162.Moteki H, Hibasami H, Yamada Y, Katsuzaki H, Imai K, Komiya TA. Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines, but not an in human stomach cancer cell line. Oncol Rep. 2002;9(4): 757. PubMed Google Scholar
-
163.Jo H, Cha B, Kim H, Brito S, Kwak BM, Kim ST, Bin BH, Lee MG. α-Pinene enhances the anticancer activity of natural killer cells via ERK/AKT Pathway. Int J Mol Sci. 2021;22(2): 656. CrossRef PubMed Google Scholar
-
164.Hou J, Zhang Y, Zhu Y, Zhou B, Ren C, Liang S, Guo Y. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med Sci Monit. 2019;25: 6631. CrossRef PubMed Google Scholar
-
165.Shapira S, Pleban S, Kazanov D, Tirosh P, Arber N. Terpinen-4-ol: a novel and promising therapeutic agent for human gastrointestinal cancers. PLoS ONE. 2016;11(6): e0156540. CrossRef PubMed Google Scholar
-
166.Krifa M, Meshri SEE, Bentouati N, Pizzi A, Sick E, Chekir-Ghedira L, Rondé P. In vitro and in vivo anti-melanoma effects of Pituranthos tortuosus essential oil via inhibition of FAK and Src activities. J Cell Biochem. 2016;117(5): 1167. CrossRef PubMed Google Scholar
-
167.Fidyt K, Fiedorowicz A, Strządała L, Szumny A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016;5(10): 3007. CrossRef PubMed Google Scholar
-
168.Quassinti L, Maggi F, Barboni L, Ricciutelli M, Cortese M, Papa F, Garulli C, Kalogris C, Vittori S, Bramucci M. Wild celery (Smyrnium olusatrum L.) oil and isofuranodiene induce apoptosis in human colon carcinoma cells. Fitoterapia. 2014;97: 133-41. CrossRef PubMed Google Scholar
-
169.Akiel MA, Alshehri OY, Almuaysib A, Bader A, Al-Asmari AI, Alamri HS, Alrfaei BM, Halwani MA. Viridiflorol induces anti-neoplastic effects on breast, lung, and brain cancer cells through apoptosis. Saudi J Biol Sci. 2021. CrossRef PubMed Google Scholar
-
170.Zari AT, Zari TA, Hakeem KR. Anticancer properties of eugenol: a review. Molecules. 2021;26(23): 7407. CrossRef PubMed Google Scholar
-
171.Zare E, Jamali T, Ardestani SK, Kavoosi G. Synergistic effect of Zataria multiflora essential oil on doxorubicin-induced growth inhibition of PC3 cancer cells and apoptosis. Complement Ther Clin Pract. 2021;42: 101286. CrossRef PubMed Google Scholar
-
172.Alabrahim OAA, Lababidi JM, Fritzsche W, Azzazy HME. Beyond aromatherapy: can essential oil loaded nanocarriers revolutionize cancer treatment? Nanoscale Adv. 2024;6(22): 5511-62. CrossRef PubMed Google Scholar
-
173.Saydam F, Nalkiran HS. Anticancer effects of a novel herbal combination as a potential therapeutic candidate against lung cancer. Eur J Integr Med. 2021;48: 101401. CrossRef PubMed Google Scholar
-
174.Maruca A, Lanzillotta D, Rocca R, Lupia A, Costa G, Catalano R, Moraza F, Gaudio E, Ortuso F, Artese A, Trapasso F, Alcaro S. Multi-targeting bioactive compounds extracted from essential oils as kinase inhibitors. Molecules. 2020;25(9): 2174. CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2025
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.